Abstract
This is a first report in Mexico of the presence of antibodies against respiratory syncytial virus (RSV) and parainfluenza-3 virus in Mexican sheep in different productive stages. We determine the association of serological positivity with age and production system, and obtain molecular evidence of infection by both virus. RSV prevalence in adult sheep was 47% (49/105) at the tropic and 64% (63/99) at the uplands. A significant difference in RSV seropositivity between animals from the tropic and the uplands was observed (P < 0.05). Seropositivity correlated with production system (P = 0.003, OR = 2.042), with a risk of showing antibodies was 2.042 times higher in sheep under an extensive production system. A significant difference in PI3V seropositivity between animals from either provenance (P = 0.017, OR = 0.475) were also found, with a risk of showing antibodies 0.475 times higher in sheep under an extensive production system. Genetic material from RSV and PI3V was identified by RT-PCR in nasal swab samples from clinically healthy lambs and confirmed by sequencing and phylogenetic analysis. Serological results show that sheep are susceptible to infection by both viruses, and molecular results suggest that the identified antibodies are result of natural infections and reinfections.
Electronic supplementary material
The online version of this article (doi:10.1007/s13337-016-0354-4) contains supplementary material, which is available to authorized users.
Keywords: Paramyxovirus, Respiratory complex, Serology, Seroprevalence, RT-PCR, Phylogeny
Introduction
Small ruminant respiratory complex is one of the main morbidity and mortality causes in sheep flocks as outcome of the exposition to adverse weather conditions, animal displacement, overcrowding, and stress, which increase susceptibility to viral and bacterial infections. It affects animals of all age and breed, but younger animals are more susceptible [22, 33]. Respiratory complex affects cattle all over the world, causing significant economic losses by subclinical manifestations, such as low weight gain, delayed growth, and expensive treatment [4, 35].
Parainfluenza-3 virus (PI3V) and respiratory syncytial virus (RSV) of the family Paramyxoviridae, are prominent and constitutive viruses of the respiratory complex [20], among the most prevalent pathogens in upper and lower respiratory tract infections [11, 12]. Aerosols and direct contact are the main transmission modes [16]. Clinical signs of infection include cough, anorexia, pyrexia, nasal and ocular discharge, and dyspnea [22]. High seroprevalences for RSV (90.8%) and PI3V (85.6%) in cattle of Mexico has been reported [36], which pointed out the importance of implementing control measures such as vaccination, as well as introducing and/or complementing appropriate handling practices [11]. In sheep from other countries, seroprevalence for RSV ranges from 49.3% [1] to 60.86% [2] and for PI3V from 31.3 to 86.5% for PI3V [31]. These data reflect the susceptibility of sheep and cattle to infection by RSV and PI3V due to a species cohabitation and exposition to predisposing factors as weather conditions, inappropriate handling, stress, etc. [43]. To date, no report exists in Mexico on RSV and PI3V circulation among sheep, on their association to factors like age and production system or on the circulating virus strains. This study is aimed to identify the presence of specific antibodies against RSV and PI3V in sheep according to the productive stage, to determine the association of serological results with factors like age and productive system, and to provide molecular evidence of the presence of these viruses.
Materials and methods
Population under study
An observational, transversal study on two sheep production systems was performed. One of these production systems was located at the uplands, Mexico City, with cool, semi-humid climate, summer rains, yearly pluvial precipitation of 800–1200 mm, and average temperature of 19 °C. The other system was located at the tropic, Veracruz, Mexico, with warm, humid climate, average temperature and precipitation of 23.4 °C and 1840 mm, respectively. Two hundred and eighty-five blood samples were taken; 141 were from upland sheep under an intensive production system, mainly of Pelibuey, Suffolk, and Rambouillet breeds; the other 152 samples were from sheep under an extensive production system at the tropic, of Pelibuey and Katahdin breeds. No vaccination against any viral agent is performed in either production system. This study was approved by the Institutional Committee for Care and Use of Test Animals (CICUAE) at Facultad de Medicina Veterinaria y Zootecnia, Universidad Nacional Autónoma de México (FMVZ-UNAM). Sample size was calculated using the formula by Cannon and Roe [42].
Serum samples
Blood samples were collected by direct puncture of the jugular vein with anticoagulant-free Vacutainer® tubes. All samples were labeled and taken to the FMVZ-UNAM Molecular Virology Laboratory; sera were obtained by centrifugation at 1500 rpm for 15 min and kept at −20 °C until processed.
Nasal secretions
Using sterile swabs, nasal secretion samples were collected from lambs and from any other animal with respiratory signs. Swabs were placed in Cryovial® containers with 1 mL of maintenance medium and kept at 4 °C for transportation to the laboratory; then, they were kept at −70 °C until used.
Virus
A bovine RSV strain (ATCC VR-1485 Iowa) was used. It was propagated in VERO cell line culture. A bovine PI3V strain (ATCC VR-281) was used. It was propagated in MDBK cell line culture.
Antibody detection
Antibodies against RSV were detected by serum neutralization test, following the procedure described by Giangaspero et al. [15], with some modifications. Confluent VERO cell monolayers were obtained by placing 100 µL of a 105 cell/mL suspension in sterile, flat-bottom 96-well plates. Twofold serial dilutions were performed, starting with 1:10 until 1:1240 with serum-free MEM. Then, 50 µL of 100-DICC50% virus solution was added. Virus-containing diluted sera were incubated for 1 h at room temperature and supplemented with MEM plus 2% fetal bovine serum. Serum-virus mixture were kept at 37 °C under 5% CO2 atmosphere for 96 h. Any serum capable of protecting the cell monolayer (i.e., no cytopathic effect was observed) equal to or higher than 1:20 was regarded as positive.
The presence of specific antibodies against PI3V was assessed by hemagglutination inhibition (HAI) test, following the procedure described by Gafer et al. [12], with some modifications. Sera were treated with 25% kaolin and 5% heat-inactivated bird red blood cells suspension at 56 °C for 30 min. PI3V was adjusted to 4 hemagglutinant units in 25 µL; 0.5% bovine erythrocytes were used. Two fold serial dilutions were performed, starting with 1:20 until 1:2480 using V-bottom 96-well plates. Sera were regarded as positive when titer was equal to or higher than 40.
Molecular detection of viral RNA in nasal secretion samples
RT-PCR and sequencing
Viral RNA was detected in nasal swabs. RNA was extracted with the QIAamp® Viral RNA purification kit (Qiagen, Dusseldorf, Ger.) following the manufacturer’s instructions. RT-PCR assay was performed with the QIAGEN OneStep RT-PCR Kit® (Qiagen, Dusseldorf, Ger.), using the previously designed primer pairs shown in Table 1. Samples were amplified in 35 cycles starting with a cDNA stage at 50 °C for 30 min and a denaturation stage at 95 °C for 15 min; RT-PCR conditions are summarized in Table 1; a final extension stage at 72 °C for 10 min was used in a Mastercycler Gradient Eppendorf® thermocycler. The amplified fragments were placed on a 2% agarose gel in Tris–acetate-EDTA (TAE 1X) buffer and were run at 89 V in a horizontal electrophoresis chamber for 45 min. Gel was stained with ethidium bromide and viewed in a transilluminator, identifying the expected amplicons with molecular weight markers from 100 through 1000 bp. PCR products from positive samples for both viruses were randomly selected and submitted to the Instituto de Fisiología Celular, UNAM, to be sequenced by the Sanger method.
Table 1.
Primers and temperatures used in RT-PCR assay
| Virus | Gene | Primer | Product size (bp) | Temperature °C | Cycle time (s) |
|---|---|---|---|---|---|
| BPI3 (Bovine PI3) | M | Fw 5′AAAGGAGTAGTTCAGATTCTAGATG3′ | 199 | A 94 | 30 |
| Rw 5′ATATAGAGCCAGTTGCGTT3′ | B 55 | 30 | |||
| BRSV (Bovine SRV)* | N | Fw 5′FGTACCAATGTCAACCAAATTCC3′ | 245 | C 72 | 90 |
| Rw 5′RTTTGCACATCGTAATTGGGTAT3′ |
Each stage temperature was maintained for the time shown in the next column
Temperatures for denaturation (A), annealing (B), and extension (C) PCR stages
* Patent, file # Mx/a/2012/012701
Phylogenetic analysis
The sequence of Mexican PI3V and RSV samples were aligned with sequences from GeneBank by the MUSCLE method [7]; phylogenetic trees were built by the Maximum Likelihood method based on the Kimura-2 model, using the MEGA software ver. 7.0.18 [19]. The robustness of the phylogenetic trees was assessed by bootstrap resampling analysis on 1000 repetitions. The GeneBank access numbers for the sequences used to build phylogenetic trees for RSV are AF188552, AF188550, AF188531, KU159366 y AF295543; and for PI3V are Y00114.1, KP757872.1, JX969001.1, KJ647284.1, KJ647285.1, KJ647287.1, KJ647288.1, KU198929.1, EF108222.1, AF178654.1, KT071671.1, EU277658.1, HQ530157.1, HQ530153.1, AF178655.1, LC000638.1, AB770485.1, LC040886.1, and KT215610.1.
Statistical analysis
Data from either animal production system were collected and then analyzed with contingency tables to determine the association of PI3V or RSV seropositivity in sheep with predisposing factors such as age (>1 year-old, <1 year-old) and production system (extensive, intensive). Tests for independency were performed to determine whether or not association exists between seropositivity and provenance from either production center. The Chi squared statistic was used, and association was regarded as significant when P < 0.05. SPSS statistical software v. 16.0 (SPSS, Inc., Chicago, IL) was used for all analyses.
Results and discussion
From 293 sheep included in this study, 69.6% (204) were older than 1 year and 30.4% (89) were lambs. Among adult sheep, 51.4% (105) were from the tropic (extensive system) and 48.6% (99) were from the uplands (intensive system). Mean age was 3.6 years-old (±2.8 years) at the tropic and 2.4 years-old (±0.75 years) at the uplands.
From 204 adult sheep, 53% (112) were positive to RSV. By production center, seropositivity among adult sheep was 47% (49) for the tropic and 64% (63) for the uplands. From 89 lambs, 61% (54) were positive to RSV. By production center, seropositivity among lambs was 52% (25) for the tropic and 71% (29) for the uplands. On the other side, from 204 adult sheep, 166 (81.4%) were positive to PI3V. By production center, seropositivity among adult sheep was 78.8% (78) for the tropic and 83.9% (88) for the uplands. From 89 lambs, 84.3% (75) were positive to PI3V. By production center, seropositivity among lambs was 95.6% (44) for the tropic and 72.1% (31) for the uplands. The frequency of antibody titers among adult sheep and lambs is shown in Table 2. Lamb seropositivity in both production systems is depicted in Fig. 1, showing prevalence percentage according to age.
Table 2.
Frequency of antibody titers against SRV and PI3V in adult sheep and lambs by production center
| RSV | PI3V | ||||
|---|---|---|---|---|---|
| Titer | Tropic | Uplands | Titer | Tropic | Uplands |
| n = 152 | n = 141 | n = 152 | n = 141 | ||
| 0 | (37) 24.3% | (10) 7.1% | 0 | (8) 5.3% | (2) 15.5% |
| 10 | (41) 27% | (38) 27% | 20 | (2) 1.3% | (5) 3.5% |
| 20 | (39) 25.7% | (25) 17.7% | 40 | (9) 6% | (6) 4.2% |
| 40 | (13) 8.6% | (28) 19.9% | 80 | (21) 13.9% | (25) 17.6% |
| 80 | (9) 5.9% | (10) 7.1% | 160 | (93) 61.6% | (54) 38% |
| 160 | (5) 3.3% | (13) 9.2% | 320 | (15) 9.9% | (28) 19.7% |
| 320 | (1) 7% | (2) 1.4% | 640 | (3) 2% | (2) 1.4% |
| 640 | 0 | 0 | |||
| 1280 | (7) 4.6% | (15) 10.6% | |||
Titer values are expressed as the inverse of serum dilution
Sera were regarded as RSV positive when titers were ≥ 20
Sera were regarded as PI3V positive when titers were ≥ 40
Fig. 1.
Seropositivity percentage in lambs by age in both animal production system
The association between seropositivity percentage and sheep provenance was assessed to determine whether the risk of showing antibodies against RSV or PI3V was higher in sheep from an extensive or an intensive production system. RSV seropositivity percentage was significantly different between adult animals from the tropic and from the uplands (P < 0.003). A significant association was found between RSV seropositivity and production system (P = 0.003, OR = 2.042), with a risk of showing antibodies 2.042 times higher in sheep under an extensive production system. With respect to PI3V, a significant difference was also found between animals from either provenance (P < 0.05). PI3V seropositivity and production system were also associated (P = 0.017, OR = 0.475), with a risk of showing antibodies 0.475 times higher in sheep under an extensive production system (Table 3).
Table 3.
Association between sheep seropositivity against RSV/PI3V and production center
| Antigen | Variable | Category | No. of seropositive sheep | No. of seronegative sheep | P value |
|---|---|---|---|---|---|
| RSV | Production system | Extensive | 74 | 78 | 0.003* |
| Intensive | 93 | 48 | |||
| PI3V | Extensive | 132 | 19 | 0.017* | |
| Intensive | 109 | 33 |
* Statistically significant, P < 0.05
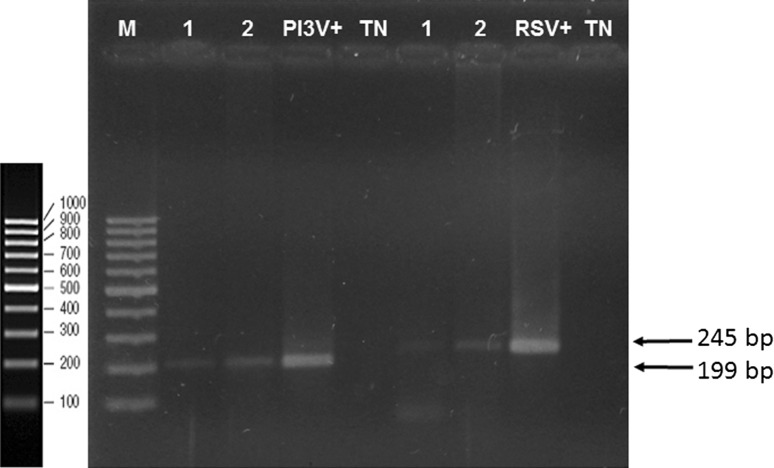
In total, 63 swab samples from lambs between 1 and 2 months of age were collected in winter months. Twelve 5-swab pools and one 3-swab pool were formed, for a total of 13 sample pools. From these 13 samples, 5 (38.4%) were positive to RSV, showing bands of the expected 256-bp size. On the other hand, 9 samples (69.3%) were positive to PI3V, showing bands for the expected 199-bp size (Fig. 2). As positive controls, RSV (ATCC VR-1485 Iowa) propagated in VERO cell line culture and a bovine PI3V strain (ATCC VR-281) propagated in MDBK cell line culture were used, where cytopathic effect (CPE) was observed 72 h post-infection.
Fig. 2.
RT-PCR of Parainfluenza-3 Virus and Respiratory Syncytial Virus, 2% agarose gel. Amplicons obtained by RT-PCR (1 and 2) were analyzed by electrophoresis using a molecular weight marker from 100 to 1000 bp. The following controls were included: Bovine Respiratory Syncytial Virus (VRS+), Bovine Parainfluenza-3 Virus (VPI3+), and negative controls (TN)
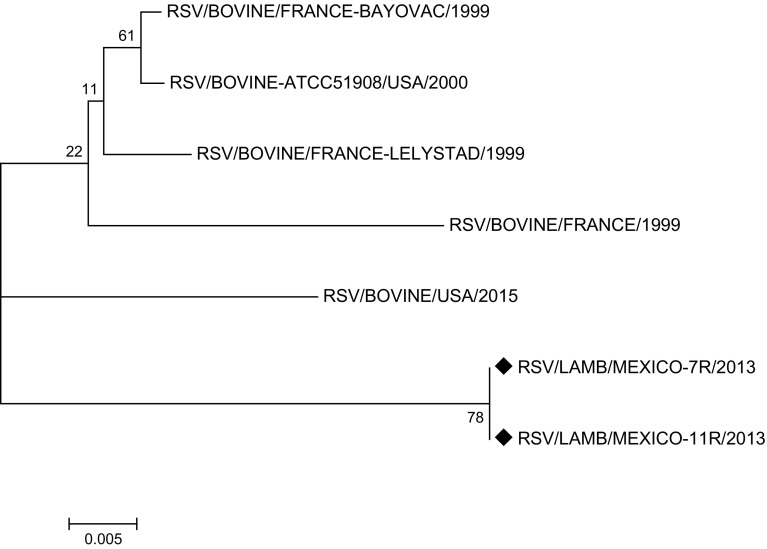
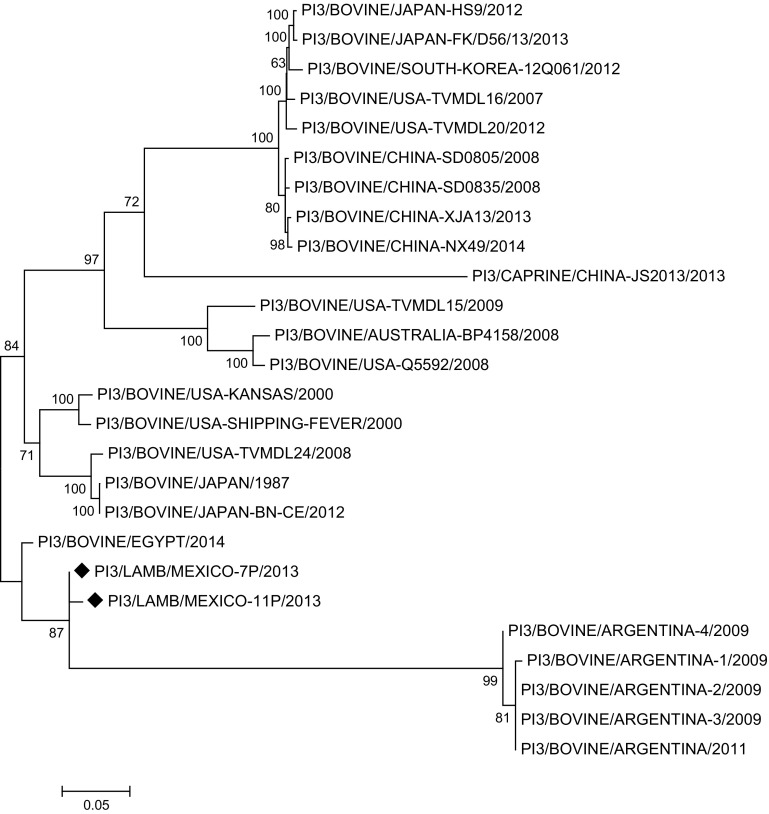
The phylogenetic tree based on the nucleotide sequence of complete genomes, on reported RSV and PI3V sequences, and on randomly selected positive samples (7R and 11R for RSV, 7P and 11P for PI3V), for RSV shows that the RSV/LAMB/MEXICO-7R/2013 and RSV/LAMB/MEXICO-11R/2013 strains are grouped with a bovine-originated strain reported in the USA in 2015; however, the RSV/BOVINE-ATCC51908/USA/2000 reference strain is in another cluster, along with French strains reported in GeneBank (Fig. 3). PI3/LAMB/MEXICO-7P/2013 and PI3/LAMB/MEXICO-11P/2013 are grouped in a cluster with bovine PI3V strains reported to date in Argentina and Egypt (Fig. 4), distant from the US strains, which are grouped in independent clusters with North and South America, Japan, Australia, and China strains, along with a goat-originated strain. The phylogenetic tree based on the nucleotide sequence of complete genomes and on reported sequences.
Fig. 3.
Phylogenetic analysis of RSV/LAMB/MEXICO-7R/2013 and RSV/LAMB/MEXICO-11R/2013. Molecular Phylogenetic analysis by Maximum Likelihood method, phylogenetic trees based on the nucleotide sequences of the genomes of bovine RSV and other RSV strains. Scale bar indicates genetic distance. Numbers at nodes indicate bootstrap percentages obtained after 1000 replicates. Our sequences RSV/LAMB/MEXICO-7R/2013 and RSV/LAMB/MEXICO-11R/2013 were marked with diamond
Fig. 4.
Phylogenetic analysis of PI3/LAMB/MEXICO-7P/2013 and PI3/LAMB/MEXICO-11P/2013. Molecular Phylogenetic analysis by Maximum Likelihood method, phylogenetic trees based on the nucleotide sequences of the genomes of bovine PI3V and other PI3V strains. Scale bar indicates genetic distance. Numbers at nodes indicate bootstrap percentages obtained after 1000 replicates. Our sequences PI3/LAMB/MEXICO-7P/2013 and PI3/LAMB/MEXICO-11P/2013 were marked with diamond
The antibody detection results herein reported demonstrate that RSV and PI3V are circulating in Mexican sheep, and those sheep are seroconverting against both viruses. A RSV seropositivity value of 53% in sheep is similar to that reported for bovines in Colima, Mexico, with a prevalence of 60.8% [10], and also similar to the 49.3% reported for Peruvian sheep in 2006 [1]. A study conducted in Japan in 2013 showed a seropositivity of 8.69% in sheep [15], far below the findings of this study. A PI3V seropositivity of 81.4% is similar to that reported for bovines at Yucatán, Mexico, with 88% [36] and to the 82% reported for Brazilian sheep in 2010 [2], and very different to the findings in Japan, where a recent study showed a PI3V prevalence in sheep of 11.73% [15]. This high antibody detection indicates that exposition to these agents is common, and that both viruses are widely distributed in Mexico, just as it had been demonstrated in other studies with bovine cattle [10, 36]. The serological response reflects a natural infection, since no vaccination against RSV or PI3V was undertaken in this study.
With respect to the age of subject animals, the observed RSV seroprevalence of 47% in adult sheep is similar to that reported for Italian sheep in 2000, with 42% [13] and in sheep under a mixed flock system in Peru, with 49.3% [1]. Another study in sheep from Syria reported a higher prevalence, with 63.6% [13]. All these reports were made on sheep under an extensive shepherding system. The found seroprevalence in adult sheep from the uplands is high with respect to the reports from Italy and Peru, but it is similar to that from Syria. There are no prevalence reports on sheep under an intensive production system, but prevalence values over 70% have been reported for bovines under an intensive production system [11]. The presence of antibodies against PI3V in adult sheep from the tropic is high with respect to the reported prevalence in Bulgarian sheep, with 31% [31] or to the reported value for Peruvian sheep, with 49.3% [1]. Antibody prevalence is even higher in sheep from the uplands (83.9%). No previous reports exist on seroprevalence in sheep, but values of 84.4% were reported for Iranian bovines [34] and of 85.6% for bovines from Yucatán, Mexico [36].
In lambs, RSV prevalence was different in both production centers. Lambs from the tropic showed lower prevalence (52%) than lambs from the uplands (71%). PI3V prevalence was also different: a higher prevalence was observed in the tropic (95.6%) than in the uplands (72.1%). Both prevalence values were higher than those reported in feedlot calves from Europe, with 8.4% for bovine RSV (BRSV) and 21.2% for PI3V [27], but similar to those reported in calves from USA, with 75% of seropositivity for BRSV and 50% for PI3V [14].
The difference in antibody prevalence between both production systems could be due to the system itself. Predisposing factors for infection by respiratory complex viruses include coexistence with other species like bovines or goats, confinement, constant exposure to pollutants, sudden temperature changes, and stress [3, 28, 36]. Sheep from the uplands are handled under an intensive-type production system, where they coexist with bovines and goats; overcrowding and the contact between healthy and diseased animals are also favored. In the tropic, on the other side, production system is extensive; no other species coexist directly with sheep. While this could explain a lower seroprevalence, the higher relative humidity at the tropic could favor infection by these viruses [28]. This is the first report on RSV and PI3V seroprevalence in lambs and adult sheep in Mexico. Lambs should gain size and weight in this production stage, but it is clear that animals are becoming infected and could exhibit a drop in production parameters, since it has been demonstrated that respiratory diseases can increase mortality up to 10% in this stage [24] or cause lower food ingestion, and hence a drop in production rates [27].
Antibody titers ranged from 1:10 through 1:1280 for RSV and from 1:20 through 1:160 for PI3V, suggesting that most animals suffer constant reinfections, either by continuous low-level virus circulation or by virus reintroduction [9]. Notwithstanding antibody production, when a challenging virus is highly infectious, the primary immune response fails to prevent reinfection; an effective prevention would require a wide response to multiple viral antigenic sites or to different circulating strains [23, 46].
The presence of serum antibodies against RSV and PI3V in lambs younger than 1 month is due to passive immunity; the antibodies are transferred from mother to lamb by colostrum ingestion, protecting lambs against respiratory disease. Lambs show an increase in anti-RSV antibody titers after 2 months of age, and a decrease by 3 months of age. Anti-PI3V antibodies remain in low but non-zero levels (Fig. 2). This reveals that lambs are in contact with RSV and PI3V and develop a humoral immune response [17]. Passive immunity in calves has been reported to last from 4 months through 6 months, and calves develop their own humoral response only after 12 months of age [8, 45]. Lambs that ingest enough calostrum are protected against diseases. This passive immunity decreases at different rates, lasting up to 12 weeks [41].
Statistically significant differences in RSV and PI3V seropositivity between both production systems were found, Saa et al. [32] suggests that production under an extensive system has a potential risk factor due to the possible proximity to neighboring production facilities or wild animals acting as disease reservoirs [26]. Mentions that flocks are often visited by several persons such as buyers, veterinaries, technicians, and thus have a higher risk of respiratory infections [36]. Sustain that animals at the tropic are more exposed to climatic factors like rain or high relative humidity, or higher temperatures during daytime, with the ensuing stress. Confinement is regarded as one of the main predisposing factors for respiratory disease in ruminants [22], and consequences are the low weight gain and high mortality, which causes economic losses to producers [29]. Best management practices, avoiding adverse weather conditions, proper ventilation of facilities and population density control help to reduce the incidence of respiratory diseases [33, 39].
No relationship was demonstrated between age and the presence of antibodies against both viruses. This could be due to a continuous circulation of the viruses with no disease outburst, being thus all animals equally susceptible [26]. Disregarding the causal agent, the clinical signs of respiratory disease can be very similar. It is often unknown whether it is of viral or bacterial origin. However, the most common bacterial complications have been reported to be with Mannheimia haemolytica and Mycoplasma, which require antibiotic treatment with tilmicosin, florfenicol, amoxicillin-clavulanic acid, ampicilline, oxytetracycline dihydrate or procaine benzylpenicillin hydrochloride [6].
Preventive management includes vaccination three weeks after lamb birth against bacterial diseases such as Mannheimia Haemolytica, Pasteurella or Clostridium, followed by an annual booster vaccination. However, this vaccination regimen only provides passive immunity for lambs up to five weeks after birth [33]. There are bovine P13 attenuated-virus vaccines which are antigenically related to the ovine subtype but they do not reduce the incidence of pneumonia. On the other hand, it has not been reported effective vaccination against RSV [38, 40]; however, lambs eating sufficient colostrum within the first 18 h of life have been reported to be protected against these diseases. This passive immunity slowly decreases and lasts up to 12 weeks [30]. These should be taken into consideration for the control of these diseases in lambs.
RT-PCR assay for BRSV and PI3V detection demonstrated the presence of virus in clinically healthy lambs. All serological test results show that sheep are susceptible to infection by these viruses, and RT-PCR results suggest that the identified antigens are the result of natural infections and probably of reinfections [5, 9]. According to a sequence-based phylogenetic analysis of the complete genome of bovine RSV and PI3V, the genetic material detected in lamb nasal swab samples should be grouped with these previously reported sequences. Since they are located in separate clades, however, it is not possible to state or reject that those isolates are of direct bovine origin; additional samplings and a close follow-up of those herds are required. There are few reports on the circulation of RSV and PI3V in Mexican cattle, and there is no report on the isolation and/or sequencing of these viruses in small ruminants. Virologic isolation and physical–chemical characterizarion of PI3V in bovines has been reported in South America, but no phylogenetic studies were performed on these isolates [21]. PI3V strains have been isolated and characterized from beef cattle in the USA, and the isolation of this agent was reported in China, speculating that it could have derived from North America [47], however, our phylogenetic tree shows that Mexican strains do not come from USA and they are in a different clade to that reported by Wen et al. [47]. PI3 strains found in Mexican lambs are clustered together with bovine virus reported in Argentina in 2009. These viruses come from the PI3/BOVINE/EGYPT/2014 strain and it is unclear how these strains made their way into Mexico; it is very likely that they were introduced in cattle traded from that country.
VRS has also been isolated and sequenced from bovines in countries like Brazil and Norway [18, 37]. A new member of the respirovirus genus, called Caprine Parainfluenza 3 virus, was recently reported in goat herds [48]; however, to date there are no reports on the isolation or detection of PI3V or VRS specific for sheep, even though respiratory diseases in small ruminants proved to be one of the main causes of losses in the herd [25].
This is the first report on BRSV and BPI3V detection in Mexican sheep. BRSV or BPI3V infection can be diagnosed in sheep in a precise and timely manner by using RT-PCR in nasal swab samples collected during the acute phase of a presumed outburst. RT-PCR is potentially useful based on nucleoprotein or the matrix protein, described as the most conserved genes in Pneumovirus [44], besides the use of different bioinformatics tools that help us to confirm the presence of this agents.
The high antibody titers detected in this study reveal that both adult sheep and lambs were exposed to RSV and PI3V by natural infection and this was confirmed by RT-PCR and sequencing. This study revealed seroprevalence difference among production centers, and that these prevalence values are associated to sheep provenance from either production center. This could be due to several factors, such as the climate and handling conditions in each animal production system. Lambs are in contact and infected with RSV and PI3V at an early age and are able to mount a humoral immune response. Statistical analysis of data suggests that extensive-type production system is a risk factor for RSV and PI3V infection.
Herein, the first report on RSV and PI3V seroprevalence in Mexican adult sheep and lambs is presented. This is also the first report on BRSV and PI3V detection in Mexican sheep.
Electronic supplementary material
Below is the link to the electronic supplementary material.
×10 Optical microscopy of infected cells with either RSV or PI3V: a VERO cell control, 72 h post-infection. b VERO cells infected with RSV, 72 h post-infection, showing syncytia (black arrows). c MDBK cell control, 72 h post-infection. d MDBK cells infected with PI3V, 72 h post-infection, showing cell death, formation of intra-cytoplasmic inclusion bodies and cell rounding (black arrows) (JPEG 244 kb)
Acknowledgements
Contreras-Luna M.J. is a recipient of the Consejo Nacional de Ciencia y Tecnología (ID 492430). This study was partially funded by the following Project: PAPIIT IN223514. L.A. and Conacyt CB-2015-01/00254244.
References
- 1.Cabello RK, Rivera GH. Frecuenciadelos virus parainfluenza-3, respiratorio sincitial y diarrea viral bovina en un rebaño mixto de una comunidad campesina de Cusco. Rev Investig Vet Perú. 2006;17(2):167–172. [Google Scholar]
- 2.Calderon GAA. Detection of serum antibodies to parainfluenza type 3 virus, respiratory syncytial virus, bovine viral diarrhea virus, and herpes virus type 1 in sheep in the Region of Botucatu, São Paulo—Brazil. J Vet Med Anim Health. 2011;3(4):1–5. [Google Scholar]
- 3.Collins PL, Graham BS. Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol. 2008;82(5):2040–2055. doi: 10.1128/JVI.01625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortese VS, Cravens RL, Dominguez J. The prevalence of bovine virus diarrhoea and bovine respiratory syncytial virus in Mexico. Bov Pract. 1991;1991(26):159–161. [Google Scholar]
- 5.Crowe JE., Jr Influence of maternal antibodies on neonatal immunization against respiratory viruses. Clin Infect Dis Off Publ Infect Dis Soc Am. 2001;33(10):1720–1727. doi: 10.1086/322971. [DOI] [PubMed] [Google Scholar]
- 6.Duncanson GR; CAB International. Farm animal medicine and surgery: for small animal veterinarians. Wallingford, Oxfordshire; Cambridge, MA: CABI, 2013. ISBN 9781845938826 (hb) 9781845938833 (pb).
- 7.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis JA. Bovine parainfluenza-3 virus. Vet Clin N Am Food Anim Pract. 2010;26(3):575–593. doi: 10.1016/j.cvfa.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Elvander M. Severe respiratory disease in dairy cows caused by infection with bovine respiratory syncytial virus. Vet Rec. 1996;138(5):101–105. doi: 10.1136/vr.138.5.101. [DOI] [PubMed] [Google Scholar]
- 10.Figueroa-Chavez D, Segura-Correa JC, Garcia-Marquez LJ, Pescador-Rubio A, Valdivia-Flores AG. Detection of antibodies and risk factors for infection with bovine respiratory syncytial virus and parainfluenza virus 3 in dual-purpose farms in Colima, Mexico. Trop Anim Health Prod. 2012;44(7):1417–1421. doi: 10.1007/s11250-012-0081-9. [DOI] [PubMed] [Google Scholar]
- 11.Fulton RW, Purdy CW, Confer AW, Saliki JT, Loan RW, Briggs RE, et al. Bovine viral diarrhea viral infections in feeder calves with respiratory disease: interactions with Pasteurella spp., parainfluenza-3 virus, and bovine respiratory syncytial virus. Can J Vet Res. 2000;64(3):151–159. [PMC free article] [PubMed] [Google Scholar]
- 12.Gafer JAM, Hussein HA, Reda IM. Isolation and characterization of PI-3 virus from sheep and goats. Int J Virol. 2009;5(1):28–35. [Google Scholar]
- 13.Gaffuri A, Giacometti M, Tranquillo VM, Magnino S, Cordioli P, Lanfranchi P. Serosurvey of roe deer, chamois and domestic sheep in the central Italian Alps. J Wildl Dis. 2006;42(3):685–690. doi: 10.7589/0090-3558-42.3.685. [DOI] [PubMed] [Google Scholar]
- 14.Gagea MI, Bateman KG, van Dreumel T, McEwen BJ, Carman S, Archambault M, et al. Diseases and pathogens associated with mortality in Ontario beef feedlots. J Vet Diagn Investig. 2006;18(1):18–28. doi: 10.1177/104063870601800104. [DOI] [PubMed] [Google Scholar]
- 15.Giangaspero M, Savini G, Orusa R, Osawa T, Harasawa R. Prevalence of antibodies against Parainfluenza virus type 3, Respiratory syncitial virus and bovine Herpesvirus type 1 in sheep from Northern Prefectures of Japan. Veterinaria Italiana. 2013;49(3):285–289. doi: 10.12834/VetIt.0810.01. [DOI] [PubMed] [Google Scholar]
- 16.Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344(25):1917–1928. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- 17.Keles I, Woldehiwet Z, Murray RD. Replication of bovine respiratory syncytial virus in bovine and ovine peripheral blood lymphocytes and monocytes and monocytic cell lines. Vet Microbiol. 1998;61(4):237–248. doi: 10.1016/S0378-1135(98)00184-9. [DOI] [PubMed] [Google Scholar]
- 18.Klem TB, Rimstad E, Stokstad M. Occurrence and phylogenetic analysis of bovine respiratory syncytial virus in outbreaks of respiratory disease in Norway. BMC Vet Res. 2014;10:15. doi: 10.1186/1746-6148-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maclachlan NJ, Dubovi EJ, Fenner F. Paramyxoviridae. In: Maclachlan N, Dubovi EJ editors. Fenner’s veterinary virology, 4th ed. London: Academic; 2011, pp. 299–325.
- 21.Maidana SS, Lomonaco PM, Combessies G, Craig MI, Diodati J, Rodriguez D, et al. Isolation and characterization of bovine parainfluenza virus type 3 from water buffaloes (Bubalus bubalis) in Argentina. BMC Vet Res. 2012;8:83. doi: 10.1186/1746-6148-8-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin WB. Respiratory infections of sheep. Comp Immunol Microbiol Infect Dis. 1996;19(3):171–179. doi: 10.1016/0147-9571(96)00002-1. [DOI] [PubMed] [Google Scholar]
- 23.Melero JA, Moore ML. Influence of respiratory syncytial virus strain differences on pathogenesis and immunity. Curr Top Microbiol Immunol. 2013;372:59–82. doi: 10.1007/978-3-642-38919-1_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nash ML, Hungerford LL, Nash TG, Zinn GM. Risk factors for respiratory disease mortality in lambs. Small Rumin Res. 1997;26(1–2):53–60. doi: 10.1016/S0921-4488(96)00987-X. [DOI] [Google Scholar]
- 25.Nash ML, Hungerford LL, Nash TG, Zinn GM. Risk factors for respiratory disease mortality in lambs. Small Rumin Res. 1997;26(1):53–60. doi: 10.1016/S0921-4488(96)00987-X. [DOI] [Google Scholar]
- 26.Norstrom M, Skjerve E, Jarp J. Risk factors for epidemic respiratory disease in Norwegian cattle herds. Prev Vet Med. 2000;44(1–2):87–96. doi: 10.1016/S0167-5877(99)00113-0. [DOI] [PubMed] [Google Scholar]
- 27.Pardon B, Hostens M, Duchateau L, Dewulf J, De Bleecker K, Deprez P. Impact of respiratory disease, diarrhea, otitis and arthritis on mortality and carcass traits in white veal calves. BMC Vet Res. 2013;9(1):79. doi: 10.1186/1746-6148-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahal A, Ahmad AH, Prakash A, Mandil R, Kumar AT. Environmental attributes to respiratory diseases of small ruminants. Vet Med Int. 2014;2014:853627. doi: 10.1155/2014/853627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roger PA. The impact of disease and disease prevention on sheep welfare. Small Rumin Res. 2008;76:104–111. doi: 10.1016/j.smallrumres.2007.12.005. [DOI] [Google Scholar]
- 30.(RUMA), T. R. U. O. M. I. A. A. Responsible use of vaccines and vaccination in sheep production. NOAH Compendium of Data Sheets for Animal Medicines. UK. 2006.
- 31.Rusenova N. Comparison of the seroprevalence against some respiratory viruses in mixed sheep-goat herds in two regions of Bulgaria. Trakia J Sci. 2009;7(4):58–62. [Google Scholar]
- 32.Saa LR, Perea A, Jara DV, Arenas AJ, Garcia-Bocanegra I, Borge C, et al. Prevalence of and risk factors for bovine respiratory syncytial virus (BRSV) infection in non-vaccinated dairy and dual-purpose cattle herds in Ecuador. Trop Anim Health Prod. 2012;44(7):1423–1427. doi: 10.1007/s11250-012-0082-8. [DOI] [PubMed] [Google Scholar]
- 33.Scott PR. Treatment and control of respiratory disease in sheep. Vet Clin N Am Food Anim Pract. 2011;27(1):175–186. doi: 10.1016/j.cvfa.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Shirvani E, Lotfi M, Kamalzadeh M, Noaman V, Bahriari M, Morovati H, et al. Seroepidemiological study of bovine respiratory viruses (BRSV, BoHV-1, PI-3V, BVDV, and BAV-3) in dairy cattle in central region of Iran (Esfahan province) Trop Anim Health Prod. 2012;44(1):191–195. doi: 10.1007/s11250-011-9908-z. [DOI] [PubMed] [Google Scholar]
- 35.Solis-Calderon JJ, Segura-Correa VM, Segura-Correa JC. Bovine viral diarrhoea virus in beef cattle herds of Yucatan, Mexico: seroprevalence and risk factors. Prev Vet Med. 2005;72(3–4):253–262. doi: 10.1016/j.prevetmed.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Solis-Calderon JJ, Segura-Correa JC, Aguilar-Romero F, Segura-Correa VM. Detection of antibodies and risk factors for infection with bovine respiratory syncytial virus and parainfluenza virus-3 in beef cattle of Yucatan, Mexico. Prev Vet Med. 2007;82(1–2):102–110. doi: 10.1016/j.prevetmed.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 37.Spilki FR, Almeida RS, Domingues HG, D’Arce RC, Ferreira HL, Campalans J, et al. Phylogenetic relationships of Brazilian bovine respiratory syncytial virus isolates and molecular homology modeling of attachment glycoprotein. Virus Res. 2006;116(1–2):30–37. doi: 10.1016/j.virusres.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Stubbs A, Abud G, Bencini R. Dairy sheep manual. Farm management guidelines. Barton: Rural Industries Research and Development Corporation; 2009. [Google Scholar]
- 39.Sweiger SH, Nichols MD. Control methods for bovine respiratory disease in stocker cattle. Vet Clin N Am Food Anim Pract: 2010; 26(2):261–71. ISSN 1558-4240. (Electronic) 0749-0720 (Linking). Disponível em: <http://www.ncbi.nlm.nih.gov/pubmed/20619183>. [DOI] [PubMed]
- 40.Thonney Ml SM, Rg Mateescu, et al. Vaccination of ewes and lambs against parainfluenza 3 to prevent lamb pneumonia. Small Rumin Res. 2008;74:30–36. doi: 10.1016/j.smallrumres.2007.03.002. [DOI] [Google Scholar]
- 41.Thonney ML, Smith MC, Mateescu RG, Heuer C. Vaccination of ewes and lambs against parainfluenza3 to prevent lamb pneumonia. Small Rumin Res. 2008;74(1–3):30–36. doi: 10.1016/j.smallrumres.2007.03.002. [DOI] [Google Scholar]
- 42.Thrusfield MV. Veterinary epidemiology. 3. Oxford: Blackwell Science; 2007. [Google Scholar]
- 43.Trigo TF. El complejo respiratorio infecciosos de los bovinos y ovinos. Ciencia Veterinaria. 1987;4:1–37. [Google Scholar]
- 44.Vainionpaa R, Hyypia T. Biology of parainfluenza viruses. Clin Microbiol Rev. 1994;7(2):265–275. doi: 10.1128/CMR.7.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valarcher JF, Taylor G. Bovine respiratory syncytial virus infection. Vet Res. 2007;38(2):153–180. doi: 10.1051/vetres:2006053. [DOI] [PubMed] [Google Scholar]
- 46.van Wyke Coelingh KL, Winter CC, Tierney EL, Hall SL, London WT, Kim HW, et al. Antibody responses of humans and nonhuman primates to individual antigenic sites of the hemagglutinin-neuraminidase and fusion glycoproteins after primary infection or reinfection with parainfluenza type 3 virus. J Virol. 1990;64(8):3833–3843. doi: 10.1128/jvi.64.8.3833-3843.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wen YJ, Shi XC, Wang FX, Wang W, Zhang SQ, Li G, et al. Phylogenetic analysis of the bovine parainfluenza virus type 3 from cattle herds revealing the existence of a genotype A strain in China. Virus Genes. 2012;45(3):542–547. doi: 10.1007/s11262-012-0810-1. [DOI] [PubMed] [Google Scholar]
- 48.Yang L, Li W, Mao L, Hao F, Wang Z, Zhang W, et al. Analysis on the complete genome of a novel caprine parainfluenza virus 3. Infect Genet Evol J Mol Epidemiol Evol Genet Infect Dis. 2016;38:29–34. doi: 10.1016/j.meegid.2015.11.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
×10 Optical microscopy of infected cells with either RSV or PI3V: a VERO cell control, 72 h post-infection. b VERO cells infected with RSV, 72 h post-infection, showing syncytia (black arrows). c MDBK cell control, 72 h post-infection. d MDBK cells infected with PI3V, 72 h post-infection, showing cell death, formation of intra-cytoplasmic inclusion bodies and cell rounding (black arrows) (JPEG 244 kb)