Abstract
Chicken infectious anaemia virus (CIAV) is an economically important and a highly immunosuppressive virus affecting poultry industry worldwide. In this study we assessed the immunomodulatory effects of four herbal preparations namely Withania somnifera, Tinospora cordifolia, Azadirachta indica and E Care Se Herbal in resisting the viral multiplication and immunosuppression inflicted by CIAV in chicks. Day-old chicks (n = 90) were randomly and equally divided into six groups (Groups A–F). Groups A–D were administered with purified extracts of W. somnifera, T. cordifolia, A. indica and E Care Se Herbal, respectively followed by the evaluation of viral load in lymphoid organs by quantitative real-time PCR and cell mediated immune response by flow cytometric analysis of CD4+ and CD8+ T cells. Groups A–D were found to resist CIAV multiplication and pathogenesis with significant reduction of viral load compared with the infected control (P < 0.05). Group A–C chicks showed significantly higher (P < 0.05) CD4+ and CD8+ T cell counts compared to control birds while of E Care Se Herb had minimal effect on T cell count. The findings suggested that the herbal preparations used during the study were effective as both prophylactic and immunomodulatory agents and thus have potential of being used against CIAV induced immunosuppression in poultry.
Keywords: Chicken infectious anaemia virus, Poultry, Herbs, Immunomodulation, Viral load, Quantitative real-time PCR, Flow cytometry, Cell mediated immunity
Chicken infectious anaemia (CIA) is one of the important emerging poultry diseases caused by chicken infectious anaemia virus (CIAV) of the genus Gyrovirus of the family Circoviridae. CIAV contains circular ssDNA genome, encoding three proteins namely, VP1 (major capsid protein), VP2 (scaffold protein) and VP3 (apoptin). The virus has attained the status of an emerging poultry pathogen affecting particularly young chicks and has been reported from many countries worldwide [1, 2]. CIAV is a potent immunosuppressive agent as primarily it affects the lymphoid and haematopoietic tissues, thereby prime the birds highly susceptible to other secondary infections along with depression of vaccinal immunity and production performance in the field situations [2, 3]. Both vertical and horizontal transmission occurs; the most important being vertical transmission from parents to the offspring through the hatching eggs [1, 2].
The treatment and immunomodulatory strategies with haematinics and immunostimulants for checking clinical pathology and improving the immunity in birds have been suggested for its control [1, 2, 4–9]. Herbal based immunomodulation is being traditionally practiced in India and is gaining added importance nowadays throughout the world for its prophylactic and therapeutic efficacies against various diseases including viral infections [10]. Therefore, in this study we assessed the immunomodulatory and prophylactic potential of selected four herbal preparations viz., Withania somnifera, Tinospora cordifolia, Azadirachta indica and E Care Se Herb against CIAV multiplication in experimental chicks through evaluating the viral load in lymphoid tissues using quantitative real-time PCR assay and cell mediated immune (CMI) response by flow cytometry.
Herein, pure extracts of Withania somnifera (or Ashwagandha with active withanaloids @ 3%), Tinospora cordifolia (or Guduchi with active tinosporins @ 1.5%) and Azadirachta indica (or Neem with azadirachtin @ 2.5%) in the powder form (supplied by Natural Remedies Pvt. Ltd., Bangalore, India) and one commercial herbal preparation of poultry named E Care Se Herbal which is a liquid preparation containing extract of Ocimum sanctum (Tulsi) along with vitamin E and selenium (supplied by Provimi Animal Nutrition India Pvt. Ltd., Bangalore, India) were used. A total of ninety (90) clinically healthy day-old specific pathogen free (SPF) chicks of White Leghorn breed were used. The chicks were randomly divided into six groups (A–F) with 15 birds per group. The treatment groups (A, B, C, D) were fed with powdered herbal extracts at the dose rate of W. somnifera (1%), T. cordifolia (1%) and A. indica (0.2%) respectively through feed, while group D was fed with E Care Se Herbal (0.1%) through drinking water. Group E and F were maintained as virus positive and negative controls, respectively, for CIAV infection to be given later at 14th day of age. Powders of W. somnifera, T. cordifolia and A. indica were mixed with feed as per the experimental designs of Yamada et al. [11], Rajkumar et al. [12] and Sadekar et al. [13], respectively. For group D, E Care Se Herbal supplement was mixed with drinking water as indicated in the manufacturer’s product information. All the experimental groups were reared under strict isolated conditions and fed autoclaved feed and water, supplemented with vitamins and minerals, ad libitum. Birds were also given antibiotic Lixen Powder (Cephalexin, Virbac India Ltd.) for five days as prophylactic doses (@ 1.3 g for 100 chicks in drinking water) to prevent secondary bacterial infections. They were regularly monitored for clinical signs of disease and mortality, if any. The experiment on chicks was performed following the recommendations and approval of the Institute Animal Ethics Committee (IAEC) under the guidelines set forth by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
Initially, all the six experimental groups were kept in control experimental shed of Avian Diseases Section, IVRI, Izatnagar, India. Treatment groups (A–D) and virus positive control (group E) were shifted to experimental infection shed at 2 weeks of age and were infected with CIAV—a strain (Accession No. AY583755) intramuscularly by 40 times of the 50% Chicken Infectious Doses (CID50) as per Kaffashi et al. [14]. All the chicks were monitored for any clinical signs of the disease. Three birds from each group were sacrificed humanely for quantifying the viral load in thymus, spleen, and liver. Quantitative real-time polymerase chain reaction (qRT-PCR) was employed to determine the viral load (viral copy number) in these tissues by preparing the standard curve using six 10-fold serial dilutions of the linearised VP2-pTARGET plasmid containing 106–101 copies in triplicates along with non-template controls.
For qRT-PCR, three birds each from all groups were sacrificed, total DNA was extracted from 25 mg each, of thymus, spleen and liver tissues using the DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany). The isolated samples containing DNA were diluted to 1:100 in order to evade the effects of tissue inhibitory substances on PCR efficiency [14]. For the quantification of CIAV genome (DNA) in various tissues, isolated DNA samples were used for qRT-PCR analysis in a MX 3000P thermal cycler (Stratagene, California, USA). The forward and reverse primers used for qRT-PCR were VP2F (5′-ATGGCAAGACGAGCTCGC-3′) and VP2R (5′-TCACACTATACG TACCGGGG-3′), respectively, that provided an amplicon of 178 bp size [15]. Duplicate sets of each reaction sample were prepared using 10 μl of QuantiFast SYBR Green PCR master mix (Qiagen, Hilden, Germany), 2.5 pmol each of forward and reverse primers, 2 μl of diluted DNA samples and nuclease-free water to make final reaction volume of 20 μl. Non-template controls and efficiency of primer by 10-fold serial dilutions of template DNA were also evaluated and the specificity of the amplified gene products was confirmed by dissociation curve analysis generated by the software at annealing temperatures through 95 °C. Amplification conditions include an initial incubation at 95 °C for 10 min for activation of hot start DNA polymerase, followed by 40 cycles of amplification reaction involving denaturation at 95 °C for 15 s, followed by combined annealing and extension at 60 °C for 30 s.
Cell mediated immune response in each group against CIAV was estimated by flow cytometric analysis of CD4+ and CD8+ peripheral T cells at 10th and 14th DPI using FACS Calibur® instrument (BD Bioscience, USA). The monoclonal antibodies (Mab) used in the study were mouse anti-chicken CD4+ and CD8+ conjugated with RPE and FITC, respectively (AbD Serotech, UK). Where 0.5 ml blood was collected from each six birds in all the six groups, peripheral blood mononuclear cells (PBMCs) were isolated, resuspended in 0.3 ml of PBS, followed by incubating the cells with 6 µl of Mab for 1 h at 4 °C. Samples were acquired taking 10,000 cell counts which were analysed in “Cell quest” software of FACS Calibur (BD). As the monoclonal antibodies were conjugated to dyes (CD4+ conjugated with FITC and CD8+ conjugated with RPE) detected in FL-1 (CD8+) and FL-2 (CD4+) channels, histograms and dot plots were drawn with FL-1 and FL-2 channels. Each sample was analysed twice.
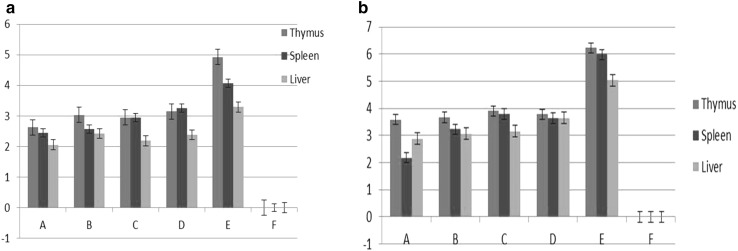
Standard curve for the quantification of viral load in tissues was obtained with a linear relationship between the amount of input plasmid DNA and the Ct values over six log10 dilutions (Fig. 1a). Amplification plot with corresponding Ct values (Fig. 1b) and a single dissociation peak during melting curve analysis through annealing to melting points of the amplified product (Fig. 1c) was observed. Quantification of virus in thymus, spleen and liver indicated more viral load in thymus than in other tissues followed by spleen and liver, at both 7th and 14th DPI. In all the three tissues, the viral load was more on 14th DPI than that of 7th DPI. Among the six groups, maximum viral load was observed in group E from all the tissues, whereas no virus was detected in group F chicks (negative control birds). Treatment groups indicated better resistance to virus multiplication, especially with Ashwagandha, showing log10 2.63 ± 0.2 and 3.59 ± 0.25 for thymus, log10 2.45 ± 0.12 and 2.18 ± 0.28 for spleen, log10 2.06 ± 0.2 and 2.88 ± 0.17 for liver, on 7th and 14th DPI, respectively. Mean viral load for each tissue in six groups are presented in Fig. 1d, e.
Fig. 1.
Absolute viral load quantitation of CIAV in thymus, spleen and liver tissues by qRT-PCR, a viral load represented as mean CIAV copy number log10/g in different tissues at 7th DPI, b viral load represented as mean CIAV copy number log10/g in different tissues at 14th DPI. (Treatment groups: A W. somnnifera, B T. cordifolia, C A. indicduta, D E care Se Herbal, E Positive control, F Negative control)
The number of CD4+ and CD8+ T cells count out of total 10,000 PBMCs in the peripheral blood of all the six groups was estimated on 10th and 14th DPI (refer to Tables 1, 2). Group F showed both highest CD4+ as well as CD8+ counts on 10th and 14th DPI and group E with least counts which is significantly different from group F (P < 0.05). In the four treatment groups (A–D), the CD4+ T cell count at 10th DPI was significantly different from both group E and F (P < 0.05) but on 14th DPI, CD4+ T cell count of group A was comparable with that of uninfected control (group F), whereas groups B, C and D showed values which were significantly different from both infected and uninfected control groups. CD8+ count of group A showed values comparable to that of group F (P > 0.05) on 10th DPI whereas on 14th DPI the count was significantly different from both groups control groups (P < 0.05). For the groups B and C, CD8+ T cell counts on 10th and 14th DPI were significantly different from both group E and group F (P < 0.05) whereas group D was not significantly different from infected control (group E) with respect to CD8+ count.
Table 1.
Mean CD4+ T cell counts at 10th and 14th DPI in different experimental groups of chicks
| DPI | A | B | C | D | E | F |
|---|---|---|---|---|---|---|
| 10th | 1443 ± 11.2b | 1296 ± 8.7b | 1216.5 ± 7.9b | 1212 ± 9.8b | 984.3 ± 11.3c | 1655 ± 9.7a |
| 14th | 1522.4 ± 9a | 1311.3 ± 14b | 1114.7 ± 12.3c | 1116.9 ± 14c | 756.7 ± 10.2d | 1757.5 ± 7.9a |
Values with different superscript along a row differ significantly
Experimental groups: A- Ashwagandha, B- Guduchi, C- Neem, D- E care Se Herbal, E- Positive control, F- Negative control
Table 2.
Mean CD8+ T cell counts at 10th and 14th DPI in different experimental groups of chicks
| DPI | A | B | C | D | E | F |
|---|---|---|---|---|---|---|
| 10th | 667 ± 16.2a | 597.6 ± 15.1b | 512.4 ± 11.4b | 433.3 ± 9.5c | 435 ± 7.8c | 734.7 ± 13.2a |
| 14th | 662.5 ± 13.3b | 498.3 ± 9.7c | 534.3 ± 16.3c | 324 ± 10.6d | 317.8 ± 11.4d | 817.5 ± 9.7a |
Values with different superscript along a row differ significantly
Experimental groups: A- Ashwagandha, B- Guduchi, C- Neem, D- E care Se Herbal, E- Positive control, F- Negative control
The profound immunosuppression induced by CIAV, few limitations of its vaccine usage and lack of any treatment option demands exploration of immunomodulatory and supplementary regimens which could boost immunity of birds to counter this viral infection and ameliorate its pathogenicity and immune depression. Very limited studies are available with regards to the use of supplements and immunomodulators to counter CIAV infection in poultry. The use of immunomodulators and haematinic supplementation has been found effective in amelioration of CIAV induced immunosuppression in chicks suggesting their prophylactic and therapeutic usages and prospective against CIA in poultry [5, 8, 9].
The present study describes the immunomodulatory and prophylactic efficacy of herbal preparation of four of herb (Withania somnifera, Tinospora cordifolia, Azadirachta indica and E Care Se) against experimentally induced CIA in chicks by assessing CIAV multiplication via viral load estimation in lymphoid tissues by qRT-PCR and CMI by flow cytometry. It was found that the virus concentration in the tissues of positive control birds (group E) was higher than that of herbal treatment groups, with an increase in viral load from 7th to 14th DPI. This pattern of virus multiplication correlates with the observations from other studies in which the virus was inoculated at clinically susceptible age [14, 15]. All the four herbal treated groups showed better response against CIAV infection as evidenced by the viral load measurements by qPCR and CMI responses. Viral load estimation in thymus, spleen and liver at 7th and 14th DPI revealed that the viral genome concentration was more in thymus followed by spleen and liver and that too more on 14th DPI. All the treatment groups, especially the group A treated with W. somnifera, resisted the viral multiplication efficiently followed by other three herbal preparations. This showed the efficiency of these herbs in modulating the immune system especially the thymocytes to defend the viral multiplication in its target organs, thereby preventing its secondary complications.
Cell-mediated immunity has an important role in protection against CIAV since the infection significantly reduces the development of antigen specific cytotoxic T-lymphocytes as well as T-helper cell responses resulting in poor antibody response [1]. So the CMI status of the experimental chicks was assessed by estimating CD4+ and CD8+ T cells using flow cytometry, and it revealed that both CD4+ and CD8+ cell counts were increasingly reduced in the group E, and among the T cells, CD8+cells have increasingly reduced in this study which is in accordance with the findings of Markowski-Grimsrud and Schat [16]. All the treatment groups (A–D) resisted the depletion of CD4+T cell count in respective groups compared to group E, and the first three treatment groups resisted the reduction of CD8+ cells efficiently. This indicates that except group D chicks treated by E Care Se Herbal, all other herbal preparations modulated the CMI response efficiently in limiting the impact of CIAV on lymphocytes.
The ayurvedic herb W. somnifera has been proven to have a wide range of therapeutic potentials including antiviral activity in various studies [17, 18]. The capability of W. somnifera treated chicks (group A) in effectively resisting CIAV infection may be attributed to the withanolides, the major chemical constituent of the herbal plant, which is a triterpenoid that has been reported to have antitumor, antioxidant, anti-inflammatory and antimicrobial properties. Withanolides can selectively skew the immune response towards T-helper1 (Th1) rather than Th2 side by increased IFN γ and IL-2 cytokine production, which is the key factor in immunity against viruses, besides augmenting the CD4+ and CD8+T cell counts, and natural killer (NK) cell activity [17, 18]. Group B chicks fed with T.cordifolia were able to limit the viral pathogenesis/any other term instead of attack which may be attributed to presence of G1-4A, an arabinogalactan polysaccharide present in the plant. G1-4A activates macrophages through TLR6 signaling and NF kB translocation along with resultant production of cytokines that have role in haematopoiesis, thereby influencing the lymphoid tissues [19]. In this regard, T. cordifolia can enhance the CMI response in chicks which may account for the reduced viral load and increased T cell populations in group B compared to that of group E. The chicks in group C treated with A. indica showed marked difference in the viral load and CMI response as compared to positive control (group E). This indicates the immunostimulatory and antiviral activity of A. indica against the pathogens that target lymphoid system which can be attributed to its hepato-stimulatory and hepato-protective nature, resulting in immunopotentiation as it has already been established in various studies [20].
The group D fed with the herbal based (Osimum sanctum) commercial immunomodulatory preparation, E Care Se Herbal, showed improvement in resisting virus load in lymphoid tissues which may due to the functions of its components (O. sanctum, vitamin E and Selenium). Vitamin E provides the first line of defense against oxidative damage, increases lymphoproliferative responses, phagocytic functions and T cell hyper activity [21] and these may be mediated by enhancing the antioxidant activity in the lymphoid tissues [22]. Selenium is an essential nutrient for poultry, which stimulates phagocytosis and chemotaxis of macrophages and neutrophils. It is an essential component of enzyme glutathione peroxidase (GPx), and plays a major role against diseases. The herbal component of E Care Se Herbal, O. sanctum, revealed immunostimulating properties by virtue of increasing heterophils and lymphocyte count, enhancing phagocytic activity and phagocytic index and reducing total bacterial count. Oil from Tulsi seed can mediate GABAergic pathways and by this it can modulate both humoral and cell-mediated immunity [23]. The present study correlates with the above point in case of improved cell mediated immunity but the humoral immunity was not as enhanced as evidenced by the HI titre.
Herbal therapy has its relevance all the time because, there exist a great potential for herbal and alternative medicine in veterinary as well human practices where antiviral therapy is still a mirage. In our recent studies, these four herbal preparations (W. somnifera, A. indica, T. cordifolia and E Care Se) revealed significant ameliorative effects on the pathology, pathogenesis and haematological changes as well as improving live body weights of chicks experimentally infected with CIAV [6, 7].
All the four herbal preparations evaluated in the study proved to be useful to resist the CIAV multiplication in target tissues and modulated the cell mediated immune response of chicks against CIAV. Use of these herbs reduced CIAV load in body tissues and significantly increased the CD4+ and CD8+ T lymphocytes. Thus, herbal immunomodulation could represent a safe and economical prophylactic and treatment regimen for CIAV in resource poor countries, and would help prevent huge economic losses being suffered to poultry industry by this highly immunosuppressive virus as well as simultaneously may be from other pathogens. Further studies are suggested for exploiting the potent and proven herbal remedies for their full potential to be used commercially in poultry production system and ameliorating harmful effects of infectious agents.
Acknowledgements
All the authors acknowledge the research support from ICAR—Indian Veterinary Research Institute, Izatnagar.
References
- 1.Dhama K, Mahendran M, Somavanshi R, Chawak MM. Chicken infectious anaemia virus: an immunosuppressive pathogen of poultry—a review. Ind J Vet Pathol. 2008;32(2):158–167. [Google Scholar]
- 2.Umar S, Ullah S, Yaqoob M, Shah MAA, Ducatez M. Chicken infectious anaemia, an immunosuppressive disease of poultry birds. World’s Poult Sci J. 2014;70:759–766. doi: 10.1017/S0043933914000828. [DOI] [Google Scholar]
- 3.Wani MY, Dhama K, Malik YPS. Impact of virus load on immunocytological and histopathological parameters during clinical chicken anemia virus (CAV) infection in poultry. Microb Pathog. 2016;96:42–51. doi: 10.1016/j.micpath.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Sawant PM, Dhama K, Rawool DB, Wani MY, Tiwari R, Singh SD, Singh RK. Development of a DNA vaccine for chicken infectious anemia and its immunogenicity studies using high mobility group box 1 protein as a novel immunoadjuvant indicated induction of promising protective immune responses. Vaccine. 2015;33:333–340. doi: 10.1016/j.vaccine.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Bhatt P, Shukla SK, Wani MY, Tiwari R, Dhama K. Amelioration of chicken infectious anaemia virus induced immunosuppression by immunomodulator and haematinic supplementation in chicks. Vet Arch. 2013;83:639–652. [Google Scholar]
- 6.Latheef SK, Dhama K, Wani MY, Samad HA, Barathidasan R, Tiwari R, Singh SD, Rai RB. Ameliorative effects of four herbs (Withania somnifera, Azadirachta indica, Tinospora cordifolia and E Care Se Herbal) on the pathogenesis of chicken infectious anaemia virus. Int J Curr Res. 2013;5(8):2327–2331. [Google Scholar]
- 7.Latheef SK, Dhama K, Wani MY, Samad HA, Tiwari R, Singh SD. Ameliorative effects of Withania somnifera, Azadirachta indica, Tinospora cordifolia and E care Se herbal preparations on chicken infectious anaemia virus induced haematological changes in chicks and their live body weights. South Asian J Exp Biol. 2013;3(4):172–182. [Google Scholar]
- 8.Krishan G, Shukla SK, Bhatt P, Kumar R, Tiwari R, Malik YPS, Dhama K. Immunomodulatory and therapeutic prospective of a protein supplement with vitamins and selenium (Multimune) against chicken infectious anaemia in broiler chicks. Adv Anim Vet Sci. 2015;3(3S):1–8. doi: 10.14737/journal.aavs/2015/3.3s.1.8. [DOI] [Google Scholar]
- 9.Krishan G, Shukla SK, Bhatt P, Kumar R, Tiwari R, Malik YPS, Dhama K. Immunomodulatory and protective effects of a polyherbal formulation (immon) against infectious anemia virus infection in broiler. Int J Pharmacol. 2015;11(5):470–476. doi: 10.3923/ijp.2015.470.476. [DOI] [Google Scholar]
- 10.Dhama K, Latheef SK, Mani S, Samad HA, Karthik K, Tiwari R, Khan R, Alagawany M, Farag MR, Alam GM, Laudadio V, Tufarelli V. Multiple beneficial applications and modes of action of herbs in poultry health and production—a review. Int J Pharmacol. 2015;11:152–176. doi: 10.3923/ijp.2015.152.176. [DOI] [Google Scholar]
- 11.Yamada K, Hung P, Park TK, Park PJ, Lim BO. A comparison of the immunostimulatory effects of the medicinal herbs Echinacea, Ashwagandha and Brahmi. J Ethnopharmacol. 2011;137:231–235. doi: 10.1016/j.jep.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 12.Rajkumar RS, Yadav AS, Kirupasanker M, Saxena VK, Singh S. Effect of Tinospora cordifolia supplementation on immunity of broiler chicks. Indian Vet J. 2009;86:1244–1245. [Google Scholar]
- 13.Sadekar RD, Kolte AY, Barmase BS, Desai VF. Immunopotentiating effects of Azadirachta indica (Neem) dry leaves powder in broilers, naturally infected with IBD virus. Indian J Exp Biol. 1998;36(11):1151–1153. [PubMed] [Google Scholar]
- 14.Kaffashi A, Noormohammadi AH, Allott ML, Browning GF. Viral load in 1-day-old and 6-week-old chickens infected with chicken anaemia virus by the intraocular route. Avian Pathol. 2006;35(6):471–474. doi: 10.1080/03079450601028837. [DOI] [PubMed] [Google Scholar]
- 15.Wani MY, Dhama K, Latheef SK, Singh SD, Tiwari R. Correlation between cytokine profile, antibody titre and viral load during sub-clinical chicken anaemia virus infection. Vet Med. 2014;59(1):33–43. [Google Scholar]
- 16.Markowski-Grimsrud CJ, Schat KA. Infection with chicken anemia virus impairs the generation of antigen-specific cytotoxic T lymphocytes. Immunology. 2003;109:283–294. doi: 10.1046/j.1365-2567.2003.01643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan B, Ahmad SF, Bani S. Augmentation and proliferation of T lymphocytes and Th-1 cytokines by Withania somnifera in stressed mice. Int Immunopharmacol. 2006;6:1394–1403. doi: 10.1016/j.intimp.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Malik F, Singh J, Khajuria A, Suri KA, Satti NK, Singh S, Kaul MK, Kumar A, Bhatia A, Qazi GN. A standardized root extract of Withania somnifera and its major constituent withanolide-A elicit humoral and cell-mediated immune responses by up regulation of Th1-dominant polarization in BALB/c mice. Life Sci. 2007;80(16):1525–1538. doi: 10.1016/j.lfs.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 19.Ahmad W, Jantan I, Kumolosasi E, Abbas Bukhari SN. Immunostimulatory effects of the standardized extract of Tinospora crispa on innate immune responses in Wistar Kyoto rats. Drug Des Dev Ther. 2015;9:2961–2973. doi: 10.2147/DDDT.S85405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ansari J, Khana SH, Haqb A, Yousaf M. Effect of the levels of Azadirachta indica dried leaf meal as phytogenic feed additive on the growth performance and haemato biochemical parameters in broiler chicks. J Appl Anim Res. 2012;9:1–10. [Google Scholar]
- 21.Da Silva ICM, Ribeiro AML, Canal CW, Trevizan L, Macagnan M. The impact of organic and inorganic selenium on the immune system of growing broiler submitted to immune stimulation and heat stress. Rev Bras Cienc Avic. 2010;12:247–254. doi: 10.1590/S1516-635X2010000400005. [DOI] [Google Scholar]
- 22.Payne RL, Southern L. Changes in glutathione peroxidase and tissue selenium concentrations of broilers after consuming a diet adequate in selenium. Poult Sci. 2005;84:1268–1276. doi: 10.1093/ps/84.8.1268. [DOI] [PubMed] [Google Scholar]
- 23.Das R, Raman RP, Saha H, Singh R. Effect of Ocimum sanctum Linn. (Tulsi) extract on the immunity and survival of Labeo rohita (Hamilton) infected with Aeromonas hydrophila. Aquac Res. 2015;46(5):1111–1121. doi: 10.1111/are.12264. [DOI] [Google Scholar]