Abstract
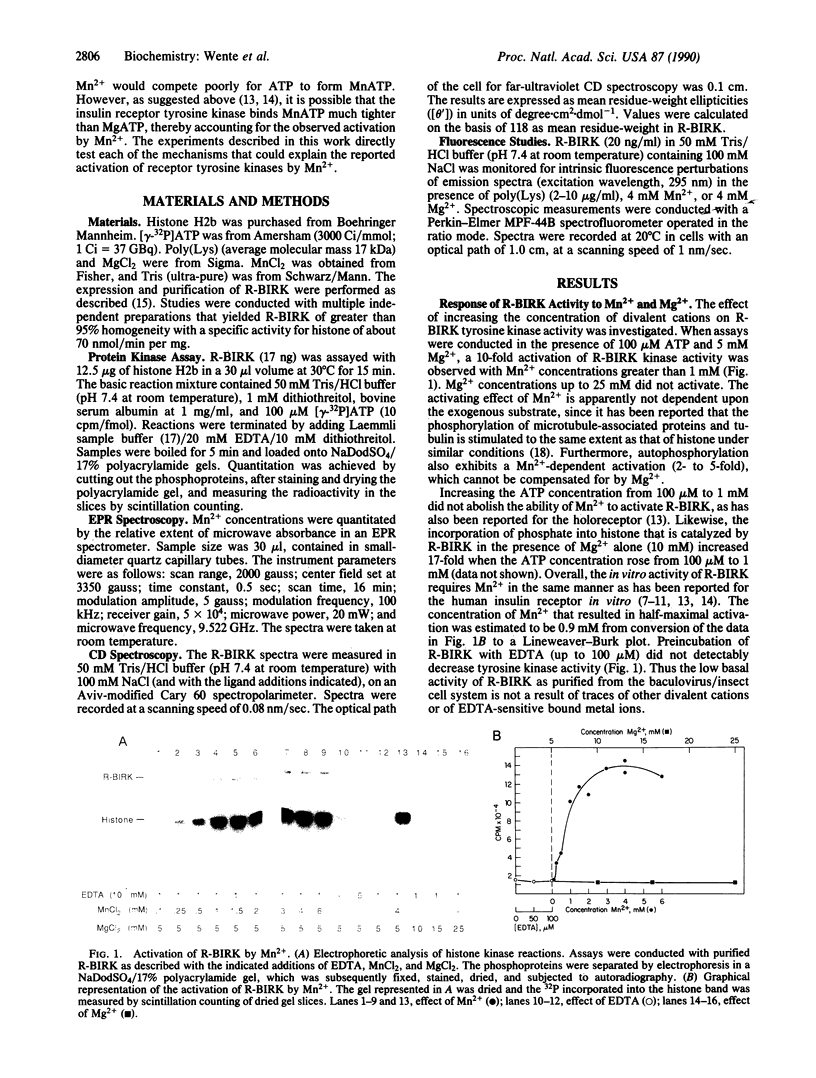
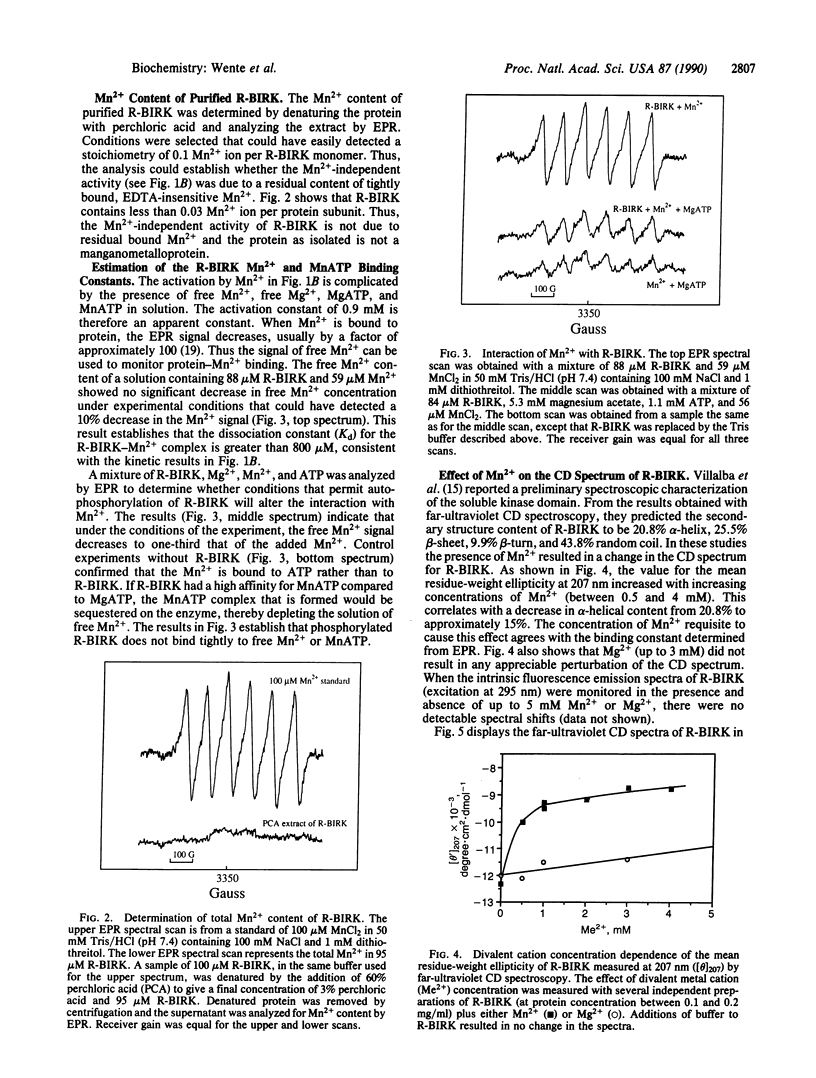
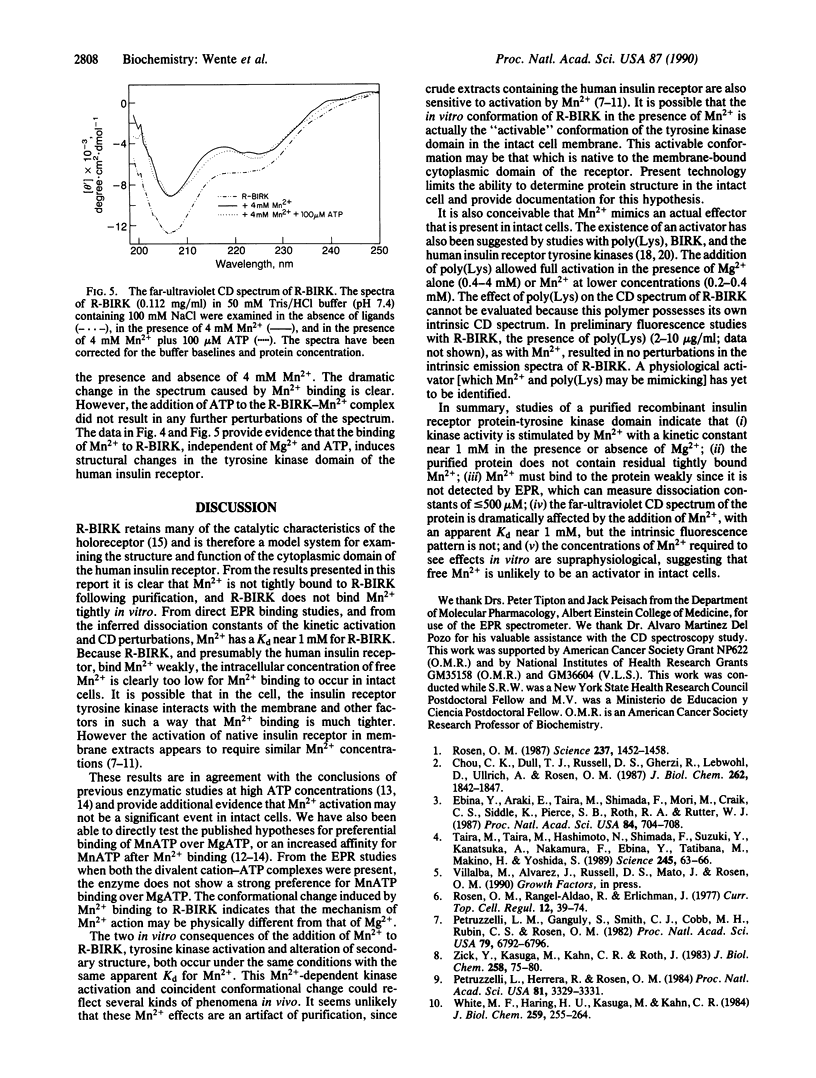
The divalent cation-binding properties of the human insulin receptor tyrosine kinase domain were examined kinetically and by electron paramagnetic resonance and circular dichroic spectroscopy. The protein-tyrosine kinase activity of the purified cytoplasmic domain can be activated nearly 10-fold by 3 mM Mn2+ in the presence or absence of 5 mM Mg2+. Electron paramagnetic resonance spectra of the purified, acid-denatured kinase domain and assays of EDTA-treated kinase show that the purified protein does not possess residual, tightly bound Mn2+. Electron paramagnetic resonance spectroscopy was used to directly measure the binding constant of the kinase domain for Mn2+. The results indicate that the recombinant cytoplasmic domain of the human insulin receptor does not bind Mn2+ tightly in the absence or presence of MgATP (Kd greater than 0.8 mM). Furthermore, the enzyme does not show a strong preference for MnATP binding when both MgATP and MnATP are present. The far-ultraviolet circular dichroic spectrum of this domain is characterized by a negative maximum at 207 nm. In the presence of Mn2+, but not Mg2+, changes in the mean residue-weight ellipticity at 207 nm occur that are consistent with a decrease in alpha-helical content. The addition of ATP to Mn2(+)-bound protein does not further perturb the spectrum. We conclude that Mn2+ ions, although they bind weakly, induce an activating conformational change in the secondary structure of the human insulin receptor cytoplasmic domain. Activation by Mn2+ is unlikely to be significant in intact cells, but it may mimic the action of a physiological activator.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ash D. E., Schramm V. L. Determination of free and bound manganese(II) in hepatocytes from fed and fasted rats. J Biol Chem. 1982 Aug 25;257(16):9261–9264. [PubMed] [Google Scholar]
- Avruch J., Nemenoff R. A., Blackshear P. J., Pierce M. W., Osathanondh R. Insulin-stimulated tyrosine phosphorylation of the insulin receptor in detergent extracts of human placental membranes. Comparison to epidermal growth factor-stimulated phosphorylation. J Biol Chem. 1982 Dec 25;257(24):15162–15166. [PubMed] [Google Scholar]
- Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A., Rosen O. M. Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J Biol Chem. 1987 Feb 5;262(4):1842–1847. [PubMed] [Google Scholar]
- Ebina Y., Araki E., Taira M., Shimada F., Mori M., Craik C. S., Siddle K., Pierce S. B., Roth R. A., Rutter W. J. Replacement of lysine residue 1030 in the putative ATP-binding region of the insulin receptor abolishes insulin- and antibody-stimulated glucose uptake and receptor kinase activity. Proc Natl Acad Sci U S A. 1987 Feb;84(3):704–708. doi: 10.1073/pnas.84.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok Y. C., Nemenoff R. A., Powers A. C., Avruch J. Kinetic properties of the insulin receptor tyrosine protein kinase: activation through an insulin-stimulated tyrosine-specific, intramolecular autophosphorylation. Arch Biochem Biophys. 1986 Jan;244(1):102–113. doi: 10.1016/0003-9861(86)90098-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morrison B. D., Feltz S. M., Pessin J. E. Polylysine specifically activates the insulin-dependent insulin receptor protein kinase. J Biol Chem. 1989 Jun 15;264(17):9994–10001. [PubMed] [Google Scholar]
- Nemenoff R. A., Kwok Y. C., Shulman G. I., Blackshear P. J., Osathanondh R., Avruch J. Insulin-stimulated tyrosine protein kinase. Characterization and relation to the insulin receptor. J Biol Chem. 1984 Apr 25;259(8):5058–5065. [PubMed] [Google Scholar]
- Petruzzelli L. M., Ganguly S., Smith C. J., Cobb M. H., Rubin C. S., Rosen O. M. Insulin activates a tyrosine-specific protein kinase in extracts of 3T3-L1 adipocytes and human placenta. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6792–6796. doi: 10.1073/pnas.79.22.6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzzelli L., Herrera R., Rosen O. M. Insulin receptor is an insulin-dependent tyrosine protein kinase: copurification of insulin-binding activity and protein kinase activity to homogeneity from human placenta. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3327–3331. doi: 10.1073/pnas.81.11.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed G. H., Cohn M. Structural changes induced by substrates and anions at the active site of creatine kinase. Electron paramagnetic resonance and nuclear magnetic relaxation rate studies of the manganous complexes. J Biol Chem. 1972 May 25;247(10):3073–3081. [PubMed] [Google Scholar]
- Rosen O. M. After insulin binds. Science. 1987 Sep 18;237(4821):1452–1458. doi: 10.1126/science.2442814. [DOI] [PubMed] [Google Scholar]
- Rosen O. M., Lebwohl D. E. Polylysine activates and alters the divalent cation requirements of the insulin receptor protein tyrosine kinase. FEBS Lett. 1988 Apr 25;231(2):397–401. doi: 10.1016/0014-5793(88)80858-5. [DOI] [PubMed] [Google Scholar]
- Rosen O. M., Rangel-Aldao R., Erlichman J. Soluble cyclic AMP-dependent protein kinases: review of the enzyme isolated from bovine cardiac muscle. Curr Top Cell Regul. 1977;12:39–74. [PubMed] [Google Scholar]
- Taira M., Taira M., Hashimoto N., Shimada F., Suzuki Y., Kanatsuka A., Nakamura F., Ebina Y., Tatibana M., Makino H. Human diabetes associated with a deletion of the tyrosine kinase domain of the insulin receptor. Science. 1989 Jul 7;245(4913):63–66. doi: 10.1126/science.2544997. [DOI] [PubMed] [Google Scholar]
- Vicario P. P., Saperstein R., Bennun A. Role of divalent metals in the kinetic mechanism of insulin receptor tyrosine kinase. Arch Biochem Biophys. 1988 Mar;261(2):336–345. doi: 10.1016/0003-9861(88)90349-9. [DOI] [PubMed] [Google Scholar]
- Villalba M., Wente S. R., Russell D. S., Ahn J. C., Reichelderfer C. F., Rosen O. M. Another version of the human insulin receptor kinase domain: expression, purification, and characterization. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7848–7852. doi: 10.1073/pnas.86.20.7848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. F., Haring H. U., Kasuga M., Kahn C. R. Kinetic properties and sites of autophosphorylation of the partially purified insulin receptor from hepatoma cells. J Biol Chem. 1984 Jan 10;259(1):255–264. [PubMed] [Google Scholar]
- Zick Y., Kasuga M., Kahn C. R., Roth J. Characterization of insulin-mediated phosphorylation of the insulin receptor in a cell-free system. J Biol Chem. 1983 Jan 10;258(1):75–80. [PubMed] [Google Scholar]




