Abstract
The haematopoietic stem cells that rejuvenate blood depend on a dietary source of the amino acid valine — a finding that has been exploited to reduce the toxicity of bone-marrow transplantation in mice.
Bone-marrow transplantation is the definitive treatment for many disorders of blood and blood-derived cells, because haematopoietic stem cells (HSCs) that reside in niches within the bone marrow can rejuvenate every blood-cell lineage. Before a transplant, recipients typically receive chemoradiation, a conditioning treatment involving chemotherapy with or without total-body irradiation. This treatment eliminates diseased HSCs, clears bone-marrow niches to render them receptive to donor HSCs, and dampens the immune system to prevent donor-cell rejection. But conditioning can cause profound immunosuppression, tissue damage and even death, and produces long-term side effects such as infertility. Writing in Science, Taya et al.1 report that the simple elimination of the amino acid valine from the diets of healthy mice leads to depletion of native HSCs, pointing to a less toxic way to condition the body for bone-marrow transplantation.
A wealth of evidence2 has demonstrated that non-haematopoietic cells in the bone marrow have essential roles in maintaining the health of HSCs, either by direct contact or through the secretion of soluble factors. Taya et al. investigated whether, by perturbing factors within the HSC niche, they might identify a vulnerability that could be exploited in bone-marrow transplantation. Tipped off by a paper from the 1940s, which reported that blood-forming cells in the bone marrow are particularly sensitive to protein deprivation3, the authors observed marked enrichment of soluble amino acids in the bone marrow compared with peripheral blood, and found that non-haematopoietic cells in the bone marrow release many amino acids.
On the basis of this finding, the researchers asked which, if any, of the 20 amino acids might be required for HSC function. They withheld individual amino acids from HSCs in vitro one by one, and found that valine and cysteine were required for HSC growth.
Valine, but not cysteine, is an ‘essential’ amino acid — there are no metabolic pathways that synthesize valine in humans or mice. Taya et al. therefore investigated the effects of dietary valine restriction on blood formation in vivo. They fed healthy mice a valine-free diet for four weeks, and found that the numbers of white blood cells, red blood cells and HSCs decreased during this period. Next, the authors gauged the fitness of HSCs from valine-deprived mice by measuring the cells’ ability to compete with normal HSCs when engrafted into recipients that had been conditioned with radiation. The authors found that HSCs purified from valine-deprived mice performed poorly in this assay.
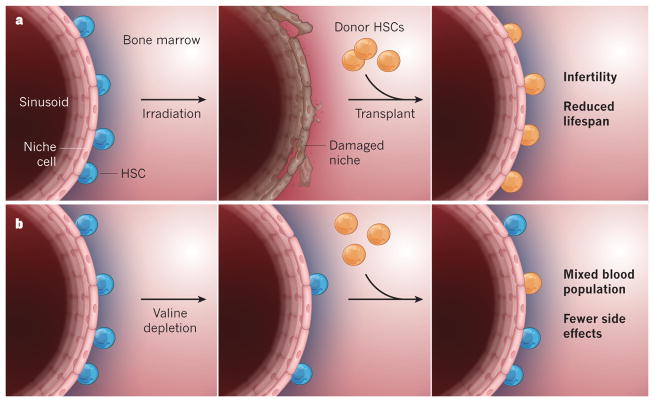
Because valine-deprived HSCs seem to function poorly, the researchers asked whether valine deprivation could impair native HSCs to an extent that would allow engraftment of donor HSCs — in essence, they tested the ability of valine deprivation alone to condition a recipient for a transplant. The researchers fed mice a valine-depleted diet for two weeks, then infused the animals with healthy donor HSCs before gradually returning recipients to a complete diet. Twelve weeks after the transplant, about 10–15% of mature blood cells in recipient mice were derived from donor HSCs, whereas there was no detectable donor HSC engraftment in recipients fed a complete diet. Importantly, mice transplanted following valine deprivation avoided the poor growth, reduced lifespan and infertility that followed conditioning by irradiation (Fig. 1).
Figure 1. Reducing toxicity in bone-marrow transplantation.
a, In conventional bone-marrow transplantation in mice, radiation is used to ablate haematopoietic stem cells (HSCs), but this treatment also damages the niche in which the HSCs grow in close proximity to small bone-marrow blood vessels called sinusoids. Healthy donor HSCs are then transplanted into the bone marrow, and completely reconstitute the blood. This approach is effective in replacing the recipient’s blood-forming system, but causes infertility and diminished lifespan. b, Taya et al.1 describe an alternative approach. Depletion of the amino acid valine from the diet causes a degree of native HSC depletion and dysfunction that enables the engraftment of healthy donor HSCs, while apparently preserving the niche. The engrafted HSCs partially contribute to the blood-forming system, and some recipient HSCs recover when mice are put back on a normal diet. Mice transplanted using this strategy have normal lifespans and fertility.
Finally, Taya et al. performed analogous experiments using human cells. They found that human HSCs require not only valine but also another essential amino acid, leucine, for growth in culture and for maintenance when engrafted into mice that have a compromised immune system.
Overall, this study shows that simple depletion of a key dietary factor can profoundly impair HSC function and permit engraftment of healthy donor HSCs. These findings provide an alternative strategy for conditioning that could prove useful in studies of HSC biology, because standard approaches for bone-marrow transplantation in model organisms use irradiation, which disrupts the HSC niche4. Furthermore, the data beg the tantalizing question of how amino-acid depletion might be leveraged to lower the toxicity of human bone-marrow transplants.
But we should note that, relative to techniques that use irradiation, valine depletion in transplant recipients has so far achieved only a low level of repopulation by donor HSCs. This would be adequate for treating some blood disorders, such as β-thalassaemia, but not all. Furthermore, the utility of valine or leucine depletion in bone-marrow transplants for leukaemia remains in question, because leukaemia stem cells have different metabolic requirements from normal HSCs (ref. 5), and may not depend on these amino acids. It is also unclear how the third objective of conditioning — blunting the recipient’s immune system — could be achieved with this method alone.
Mice deprived of valine for four weeks develop signs of protein malnutrition and, on reintroduction of valine, sometimes succumb to a toxic metabolic reaction called refeeding syndrome. Perhaps an ideal approach to conditioning would combine a shorter period of valine deprivation with other emerging methods for non-toxic conditioning. These include the use of anti-HSC antibodies6 and antibody–drug conjugates7 to clear native HSCs, and modulation of the immune system by varying signalling through cytokine molecules to permit donor engraftment8.
Minimizing the toxicity of conditioning regimens could render bone-marrow transplantation accessible to people with leukaemia, who often are too ill to receive conditioning by chemoradiation. Moreover, it could open up the possibility of using transplants to treat milder blood disorders, in which current chemoradiation produces more complications than the underlying disease. Only time will tell whether these non-toxic approaches live up to the promise they show in mice, by proving safe and effective in humans.
Contributor Information
R. Grant Rowe, Division of Pediatric Hematology/Oncology, Boston Children’s Hospital and Dana-Farber Cancer Institute, Boston, Massachusetts 02115, USA.
George Q. Daley, Howard Hughes Medical Institute, Boston
References
- 1.Taya Y, et al. Science. 2016;354:1152–1155. doi: 10.1126/science.aag3145. [DOI] [PubMed] [Google Scholar]
- 2.Yu VWC, Scadden DT. Curr Top Dev Biol. 2016;118:21–44. doi: 10.1016/bs.ctdb.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kornberg A. J Biol Chem. 1946;164:203–212. [PubMed] [Google Scholar]
- 4.Mendelson A, Frenette PS. Nature Med. 2014;20:833–846. doi: 10.1038/nm.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Signer RAJ, Magee JA, Salic A, Morrison SJ. Nature. 2014;509:49–54. doi: 10.1038/nature13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czechowicz A, Kraft D, Weissman IL, Bhattacharya D. Science. 2007;318:1296–1299. doi: 10.1126/science.1149726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palchaudhuri R, et al. Nature Biotechnol. 2016;34:738–745. doi: 10.1038/nbt.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McIntosh BE, et al. Stem Cell Rep. 2015;4:171–180. doi: 10.1016/j.stemcr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]