Abstract
Background
Prior findings concerning the use of mirtazapine in the treatment of a variety of substance use disorders and its antagonistic actions at the serotonin 5-HT2A receptor suggest that this drug may have efficacy in the treatment of cocaine dependence in the presence of a depressive disorder.
Methods
Depressed cocaine-dependent subjects received either mirtazapine (target dose 45 mg daily) or placebo for 12 weeks. Urine concentrations of benzoylecgonine and self-report were used to assess cocaine consumption. Depression and sleep quality were evaluated using the Hamilton Depression Rating Scale (HAM-D) and the Pittsburgh Sleep Quality Index, respectively.
Results
Cocaine consumption during the treatment period did not differ significantly between the mirtazapine (n = 11) and placebo (n = 13) groups in this study. In week 4 sleep latency was significantly lower in the active medication than in the placebo group. Positive effects of mirtazapine treatment on early insomnia were suggested by an item analysis of the HAM-D.
Conclusions and Scientific Significance
The results of this study suggest that mirtazapine is superior to placebo in improving sleep in patients with comorbid depression and cocaine dependence, but is not more effective than placebo in reducing cocaine use.
Keywords: insomnia, cocaine, dependence, antidepressant
INTRODUCTION
Depressive disorders occur in frequent association with cocaine abuse and dependence (1,2), with one study showing that 30.5% of treatment seeking cocaine abusers met diagnostic criteria for lifetime major depressive disorder (3). In cocaine-dependent individuals modest associations may exist between depression and functional impairment (4). Comorbid depression may be a risk factor for relapse in cocaine dependence (5).
The use of antidepressant agents to treat cocaine-dependent individuals who also have depressive disorders has been examined in several investigations based on the assumption that depression was a motivating factor for cocaine use by these subjects. Administration of the tricyclic antidepressant desipramine produced a greater reduction in depression than did placebo, but there was no significant difference between these two treatments in their effects on cocaine consumption (6). Treatment with the selective serotonin reuptake inhibitor fluoxetine was found to have no greater effect than placebo on either depression or cocaine use (7). In contrast, urine concentrations of the cocaine metabolite benzoylecgonine (BE) and the strength of cocaine craving declined more rapidly in subjects treated with nefazodone, a novel antidepressant that is a serotonin 5HT2A receptor antagonist, than were those receiving placebo (8). Nefazodone did not differ from placebo in its effects on depression, which was markedly reduced during treatment in both the active medication and control groups.
Mirtazapine is an antidepressant agent that has a unique pharmacological profile that includes the antagonist activity at the α2 noradrenergic receptor and the serotonin 5HT2A and 5HT3 receptors (9,10). Unlike many other antidepressant agents mirtazapine is not a potent inhibitor of monoamine transporter proteins and may act as an inverse agonist at the 5HT2C receptor (11). Given that mirtazapine and nefazodone share antagonistic activity at the 5-HT2A receptor, the possibility exists that mirtazapine may have some efficacy in the treatment of cocaine use disorders. The usefulness of mirtazapine in the treatment of other substance use disorders has been investigated in several studies. In an open-label study mirtazapine administration was found to significantly reduce measures of anxiety, depression, and craving for ethanol in subjects with alcohol dependence and comorbid depressive disorders (12). When administered to subjects undergoing alcohol detoxification, mirtazapine produced a significant improvement of both anxiety and depressive symptoms (13). When used for Amphetamine detoxification, mirtazapine, when compared with placebo, was found in one pilot study to produce significant improvements in withdrawal symptoms including measures of hyperarousal and anxiety (14). In a second study, when compared with the antipsychotic agent pericyazine, mirtazapine administration resulted in a greater decrease in methamphetamine withdrawal symptoms (15). In contrast, in a third study when compared with placebo administration, mirtazapine treatment did not significantly reduce methamphetamine use or improve symptoms of withdrawal from this drug (16). Overall, these studies suggest that mirtazapine might have some value as a medication for the treatment of cocaine dependence in subjects with comorbid depressive disorders.
The efficacy of mirtazapine treatment on cocaine dependence has been examined, thus far, in only one open-label trial in which it had some positive effects on cocaine use in methadone patients (17). In the present study the efficacy of mirtazapine as a medication in cocaine-dependent subjects with comorbid depressive disorders was examined using a placebo-controlled double-blind parallel group design. The effects of this agent on cocaine use and craving, as well as on depression and sleep, were evaluated in this study.
METHODS
Subjects
Men and women aged 18–64 years old were admitted into this study if they had a Diagnostic and Statistical Manual of Mental Disorders-4 diagnosis of cocaine dependence along with a Hamilton Depression Rating Scale (HAM-D) score of 12 or greater and a diagnosis of major depression, dysthymic disorder, or substance-induced mood disorder. Subjects were required to provide at least one urine sample that was positive for the cocaine metabolite BE.
Procedures and Assessments
This 20-week study followed a placebo-controlled double-blind parallel group design. After a 2–4 week long screening period subjects were treated over a period of 12 weeks with mirtazapine, with a target daily maintenance dose of 45 mg or with an equivalent number of matched placebo capsules. One 15 mg capsule of mirtazapine or matched placebo was administered for 3 days, followed by two capsules administered for 5 days with the maintenance dose of three capsules being administered from study day 10 to 78. During week 12 of this study the mirtazapine dose was tapered by 15 mg every 3 days until the drug was discontinued. The dose of study medication could be lowered by the study psychiatrist if necessary to reduce intolerable adverse effects, but subjects needed to receive at least one capsule daily to remain in the study. In addition to medication treatment, subjects attended weekly hour-long sessions involving (manual guided) relapse prevention counseling. All procedures used in this study were approved by the Institutional Review Board of the Boston University Medical Campus, and all subjects were required to provide informed written consent prior to entry into the study.
During screening subjects were evaluated using the Addiction Severity Index-Lite (ASI-Lite) (18), a medical history, a physical examination, and a brief psychiatric evaluation. In the screening session, and throughout the treatment period, cocaine use was assessed using quantitative urine BE measurements and self-reports of daily use recorded on a Substance Use Inventory form. Urine BE was analyzed using reverse phase high-performance liquid chromatography with ultraviolet detection. Urine creatinine concentrations were measured using immunoassay. Depression and anxiety were assessed using the HAM-D (19) and the Hamilton Anxiety Rating Scale (HAM-A) (20), which were administered at screening and weekly during the treatment period. Other screening and weekly assessments included the Cocaine Craving Scale (CCS), the Cocaine Craving Questionnaire (CCQ) (21), Clinical Global Impression-Self Scale (CGI-S), and the Clinical Global Impression -Observer Scale (CGI-O) (22). The Pittsburgh Sleep Quality Index (PSQI) (23) was administered at screening and on weeks 4, 8, and 12 to obtain data on latency of sleep onset and the duration of sleep. Data obtained for each assessment on the PSQI are for sleep occurring during the prior 4 weeks. The motivation of subjects to stop using cocaine was determined in a post hoc assessment by the study psychiatrist using a scale of 1–10, with 1 being extremely poorly motivated and 10 being highly motivated.
Subject medication compliance was evaluated using pill count of returned medication and detection of fluorescence of riboflavin in urine samples under ultraviolet light. Urine samples were considered to be positive for riboflavin if they emitted a yellow fluorescent glow when placed under an ultraviolet light set at 365 nm. Riboflavin at a dose of 25 mg was added to capsules that contained either mirtazapine or a small unmarked oblong white hard candy tablet. Identical capsules were used to encapsulate mirtazapine and placebo.
Statistical Analysis
Data were analyzed for a sample of subjects that included all randomized subjects who appeared for evaluation during the second treatment week. Mean values were determined for all assessments with the exception of the motivation scale, for which the median value for each group was used. Means are presented along with group SDs. Group demographic and pill count data were compared using either χ2-test or Student’s t-test. Differences between the BE, HAM-D, and PSQI values collected at screening and during the first assessment week were compared using repeated measures analysis of variance. Mixed models repeated measures analysis was used to determine treatment effects, treatment × time interactions, and within-group time effects for urine BE and data for most rating scales for data collected between week 1 and week 11. Assessments obtained during week 11 were the last available from the treatment period which did not include data from subjects for whom study medication was being tapered. Mean BE weekly concentrations were the primary outcome measure in this study. These concentrations were transformed to natural logarithms to reduce skewing. Self-reported substance use was analyzed using general estimating equations (Proc Genmod with logit link SAS 9.2, SAS Institute, Cary NC). Data for sleep measures obtained using the PSQI were analyzed using Student’s t-test for each 4-week assessment period. Satterthwaite p-values were used for t-tests for which there was a significant difference in group variances.
RESULTS
Subjects and Subject Compliance and Motivation
Demographic information on subjects in the mirtazapine (n = 11) and placebo (n = 13) groups included in the analysis is presented in Table 1, as are values of composite scores for the ASI. None of the demographic or ASI composite scores obtained at screening (Table 1) differed significantly between the two treatment groups with the exception of the ASI composite score for medical problems (t = 2.4; degree of freedom (df) = 20; p = .03). In addition, BE urine concentrations (Figure 1) and values for other assessments obtained during screening (Table 2) did not differ significantly between the two treatment groups. Data from nine subjects for each treatment group were available for the week 11 assessment.
Table 1.
Demographic data and mean (SD) values obtained at screening for the ASI for the mirtazapine and placebo treatment groups.
| Treatment | Mirtazapine | Placebo |
|---|---|---|
| N | 11 | 13 |
| Age | 43.8 years (5.6) | 47.2 years (6.7) |
| Female | 3 | 4 |
| Black | 9 | 10 |
| White | 2 | 0 |
| Other | 0 | 3 |
| ASI-ALC | .22 (.17) | .34 (.28) |
| ASI-DRUG | .21 (.07) | .23 (.10) |
| ASI-PSYCH | .39 (.30) | .34 (.15) |
| ASI-MED* | .16 (.26) | .52 (.41) |
| ASI-EMPL | .86 (.19) | .80 (.20) |
| ASI-LEG | .21 (.28) | .12 (.22) |
Notes: ASI, Addiction Severity Index; ASI Domains: ALC, Alcohol; PSYCH, Psychiatric; MED, Medical; EMPL, Employment; LEG; Legal.
p < .05 for between comparison.
Figure 1.
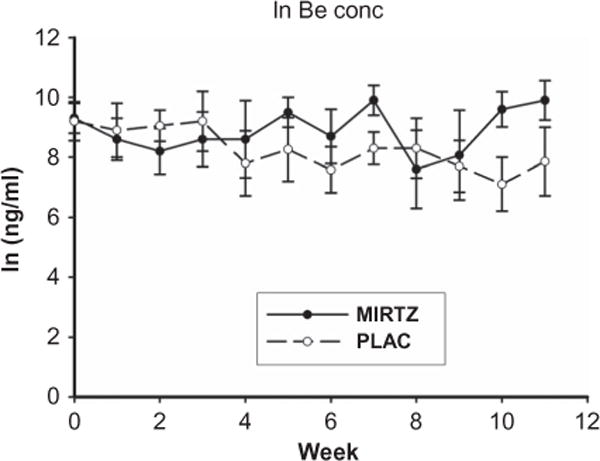
Mean (±SE) ln benzoylecgonine (BE) concentrations obtained for the screening period (week 0) and the treatment period (weeks 1–11) for the mirtazapine (MIRTZ) and placebo (PLAC) groups.
Table 2.
Mean (SD) values obtained at screening and weeks 1 and 11 for subjects in the mirtazapine (MIRTZ) and placebo (PLAC) groups for the mean reported days of cocaine use per week (USE), HAM-D, HAM-A, CCS measure of strength of craving (CCS-Q1), and the total score for the CCQ (CCQ), CGI-O, and CGI-S.
| Screening
|
Week 1
|
Week 11
|
||||
|---|---|---|---|---|---|---|
| MIRTZ | PLAC | MIRTZ | PLAC | MIRTZ | PLAC | |
| USE | 3.1 (2.0) | 2.7 (2.1) | 2.7 (1.4) | 2.6 (1.7) | 2.4 (1.3) | 1.6 (2.3) |
| HAM-D | 18.4 (5.8) | 18.0 (6.6) | 10.6 (5.7) | 10.8 (5.5) | 7.2 (4.7) | 6.7 (6.1) |
| HAM-A | 11.9 (4.9) | 13.4 (6.6) | 7.0 (4.2) | 5.8 (4.7) | 5.3 (3.7) | 4.8 (3.9) |
| CCS-Q1 | 51.1 (22.8) | 52.6 (29.8) | 50.8 (26.3) | 56.7 (20.2) | 45.0 (15.5) | 30.4 (27.3) |
| CCQ | 184.6 (96.0) | 168.6 (56.9) | 177 (30.0) | 143 (45.6) | 156.4 (35.2) | 140 (57.4) |
| CGI-O | 36.2 (5.4) | 34.5 (5.0) | 33.9 (5.5) | 30.7 (4.2) | 29.9 (4.8) | 23.1 (8.5) |
| CGI-S | 4.8 (1.1) | 4.8 (1.5) | 4.3 (1.4) | 4.2 (1.6) | 3.9 (1.6) | 2.6 (1.3) |
Note: HAM-D, Hamilton Depression Rating Scale; HAM-A, Hamilton Anxiety Rating Scale; CCS, Cocaine Craving Scale; CCQ, Cocaine Craving Questionnaire; CGI-O, Clinical Global Impression-Observer Scale; CGI-S, Clinical Global Impression-Self Scale.
With regard to medication compliance, the percentage of dispensed medications used by subjects for each group as determined by pill count was 91% (SD 21) and 91% (SD 15) for the mirtazapine and placebo groups, respectively. For the mirtazapine group a mean value of 93.5 (SD 7.6) of the urine samples collected was positive for riboflavin, while this value for the placebo group was 93.5 (SD 9.5). Missing urine samples for the time in which subjects remained in the study were considered as being negative for riboflavin.
The motivation of subjects to stop using cocaine was determined in a post hoc assessment by the study psychiatrists, who were blinded to their treatment status, using a scale of 1–10, with 1 being extremely poorly motivated and 10 being highly motivated. The median motivation score for the mirtazapine group was 3.0 while for the placebo group it was 6.0. Analysis with the Wilcoxon two-sample test indicated that overall the mirtazapine group was rated as having significantly less motivation to stop using cocaine than did the placebo group (p = .03).
Cocaine Use and Craving
Each subject self-administered cocaine by smoking it in its “crack” form, with two subjects using the nasal route of administration on only a few occasions. The mean log of weekly urine BE concentrations for the screening and treatment periods is shown in Figure 1. BE concentrations declined significantly between the screening period and the first assessment for both the mirtazapine [F(1,8) = 14.3; p = .005] and placebo groups [F(1,11) = 17.0; p = .002]. Both the treatment effect and the treatment × time interaction for BE levels were not significant during the treatment period. There was not a significant time effect for within either treatment group. Similar results were found when BE levels were normalized for urine creatinine concentrations. The values for self-reported cocaine use did not differ significantly for screening period and the treatment effect and treatment × time interaction were not significant for the treatment period.
The treatment × time interaction was significant for strength of craving question on the CCS [F(10,155) = 2.04; p = .03], reflecting a greater decrease in strength of craving in the placebo group as compared with the mirtazapine group. The time effect for the strength of craving score was significant for the placebo group [F(10,91.2) = 4.6, p < .0001], but not for the mirtazapine group. For the CCQ total score neither the treatment effect nor the treatment × time interaction was significant, and the within-group time effects were also not significant.
Global Impression, Depression, and Sleep
The treatment effect for the CGI-O approached significance [F(1, 20.9) = 4.3; p = .051]. Time effects were significant for both the mirtazapine group [F(10, 82.9) = 2.8; p = .005] and the placebo group [F(10, 91) = 3.9; p = .0002], indicating a significant reduction in CGI-O scores for both groups. Neither the treatment effect nor the treatment × time interaction was significant for the CGI-S.
There was a significant reduction by week 1 in the HAM-D scores from those obtained at screening for both the mirtazapine [F(1,10) =18.9; p = .002] and the placebo groups [F(1, 12) = 11.8; p = .005]. The treatment effect and treatment × time interaction were not significant for HAM-D obtained during the treatment period. The within-group time effect for the HAM-D approached significance for the mirtazapine group (F(10, 85.2) = 1.89; p = .06), but not for the placebo group. The treatment effect, the treatment × time interaction, and the within-group time effect were not significant for the HAM-A scores. HAM-A scores obtained at screening were significantly higher than were those collected for the week 1 assessment for both the mirtazapine [F(1, 10) = 11.2; p = .007] and placebo groups [F(1,12) = 13.9; p = .003].
The mean latency for sleep onset as assessed using the PSQI did not differ significantly between treatment groups for the month prior to screening (see Figure 2). The assessments of sleep latency obtained in week 4 show a significant difference [t = 3.0, df = 17, p = .008] between the mirtazapine group and the placebo group. The groups did not differ significantly with respect to sleep latency for assessments obtained for weeks 8 and 12. However, the mean value for sleep latency obtained for week 12 was clearly greater in magnitude for the mirtazapine group than for the placebo group. This apparently reflects a very high sleep latency of 4 hours reported by one subject in the mirtazapine group. This subject indicated on item 4 of the HAM-D, which assesses early insomnia, that he had difficulties in falling asleep only in week 12. The treatment effect for this item 4 was significant [F(1, 20) = 5.5; p = .03], with significantly lower scores seen for this item for model-generated least squares means obtained for the mirtazapine group as compared with the placebo group for weeks 3, 4, 10, and 11. Another possible indication that mirtazapine may have sleep-enhancing effects is that the mean number of hours slept per night reported in week 4 was 5.9 (2.0) and 7.3 (.9) hours for the placebo and mirtazapine groups, respectively. These values approached being significantly different (t = 2.0; df = 12.7; p = .06). The mean number of hours slept per night did not differ, however, between the groups for any of the other assessment times.
Figure 2.
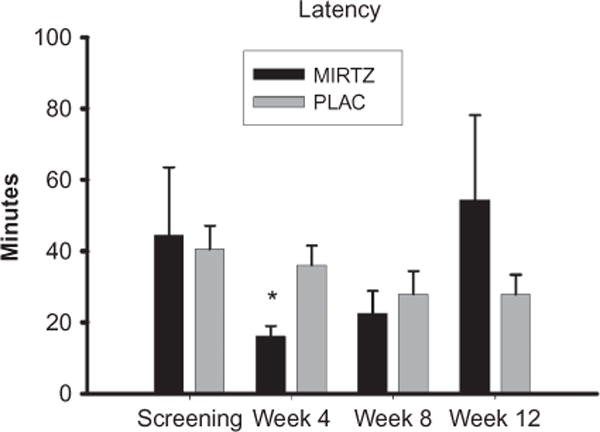
Mean (SE) sleep latency obtained for the mirtazapine (MIRTZ) and placebo (PLAC) groups at screening and weeks 4, 8, and 12.
*p < .008 for the between group comparison.
Adverse Effects
None of the subjects experienced a serious adverse event during the study.
Moderately severe adverse events experienced by subjects in the mirtazapine group included sleepiness (two subjects), mood swings (one subject), agitation, and fainting (one subject). One subject in the placebo group had depression that was rated as being a moderately severe adverse event.
DISCUSSION
Differences in the efficacy of mirtazapine and placebo in reducing cocaine use as measured by either urine BE concentrations or self-report were not significant in this study. The strength of cocaine craving as measured by the CCS remained unchanged in the mirtazapine group while it appeared to decline in the placebo group. The CCQ did not reveal any between-group differences in craving. The possibility that mirtazapine treatment resulted in less improvement than placebo is supported by the finding that CGI-O scores were lower in the control group as compared with the mirtazapine group during the treatment period. Consideration, however, needs to be given to the finding that subjects in the mirtazapine group were rated as being significantly less motivated to stop using cocaine than those in the placebo group when interpreting our findings.
Significant differences between mirtazapine and placebo on depression were not observed in this study. A similar finding was reported when fluoxetine (7) was used to treat cocaine-dependent subjects who had comorbid depressive disorders. As was found in our study, Schmitz and colleagues reported a significant decrease in depression during the screening period compared with those observed at the start of the medication treatment period for both the active medication and control groups. Subjects selected for this trial were diagnosed with major depressive disorder, substance-induced mood disorder, or dysthymic disorder based on Structured Clinical Interview for DSM disorders. The rapid improvement in depressive symptoms, as with some other depression studies, can be due to subjects’ expectation to improve with participation in the trial or with nonspecific trial effects such as frequent visits with healthcare providers. In this trial subjects had clinic visits three times per week during the screening period. Early reductions in depression prior to the treatment initiation may limit the ability to detect drug–placebo differences in depression ratings. It may be necessary to include placebo washout periods in future treatment studies of depression and cocaine dependence.
Decreases in latency for sleep onset and increases in the number of hours slept have been seen in prior studies of the effects of mirtazapine in depressed subjects (24,25). In this study the latency for sleep onset was significantly less during the time preceding the week 4 assessment for the mirtazapine group than it was for the placebo group. Analysis of item 4 on the HAM-D indicated that mirtazapine might be more effective than placebo in reducing problems related to early insomnia. Although the group difference was not significant, a marked elevation in the mean latency for sleep onset was seen in the mirtazapine group for the week 12 assessment, which was largely due to an increase in one subject. This increase might be related to some sort of withdrawal effect related to the tapering of the drug. There was a trend for the total number of hours slept to be greater in the mirtazapine group than in the placebo group, which is consistent with the effects of this medication reported in a previous study (24).
A primary limitation of this study was the small number of subjects included in each group. The use of urine BE concentrations obtained for week 11 in this study to determine an appropriate sample size for this study indicated that a sample size of 22 subjects for each group would be needed to detect a large effect size of .8 for a p-value of .05 if the power of the study was .8. The failure to detect a significant decline in cocaine use in the mirtazapine group as measured by urine BE concentrations in this study, however, does not provide support for the conduct of a larger clinical trial of the efficacy of mirtazapine in the treatment of cocaine dependence.
Another limitation of this study was that sleep measures were all dependent on self-report and more objective measures of sleep variables would be needed to confirm any mirtazapine-induced decreases in latency of sleep onset or increases in the number of hours slept. The finding that the adherence to drug/placebo appeared to be higher when urine riboflavin was used as the measure compared with the use of pill count for this purpose indicates that there may have been a problem with the use of riboflavin as a measure of compliance in this study. After the initiation of this study it has become evident that a 50 mg dose of riboflavin is a more reliable marker of medication compliance than the 25 mg dose administered in this study. This is because false positives resulting from the presence of dietary riboflavin in the urine are less likely to occur with the use of 50 mg dose which produces a very discernable yellow glow in urine under ultraviolet light. Overall, however, both pill count and riboflavin marker measures indicated excellent adherence to the medication component of the trial. A final limitation of this investigation was the post hoc impression that subjects in the mirtazapine group were less motivated to stop using cocaine than were the subjects in the placebo group. This, however, is a very difficult factor to measure and we used a novel method to determine motivation. Nevertheless, future cocaine treatment studies should consider incorporating not only baseline motivation assessments, but also study termination motivation evaluations.
The results of this study do not support the efficacy of mirtazapine in the treatment of cocaine dependence and depressive symptoms. Whether this medication might have greater efficacy in the treatment of cocaine dependence in a more motivated group of subjects remains open to question. This medication has been reported to be effective in the treatment of both alcohol and methamphetamine withdrawal and might have value in the management of subjects who are withdrawing from cocaine (12–15). In this study, mirtazapine administration did appear to decrease the sleep latency. Finally, the failure to find effects of mirtazapine on depression in this study indicates the need in future studies of comorbid depression in substance users to exclude subjects who exhibit spontaneous reductions in depressive symptoms.
Acknowledgments
This work was supported by funds received from the Gennaro Acampora Charitable Trust Fund.
Footnotes
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- 1.Brady KT, Grice DE, Dustan L, Randall C. Gender differences in substance use disorders. Am J Psychiatry. 1993;150(11):1707–1711. doi: 10.1176/ajp.150.11.1707. [DOI] [PubMed] [Google Scholar]
- 2.López A, Becoña E. Depression and cocaine dependence. Psychol Rep. 2007;100(2):520–524. doi: 10.2466/pr0.100.2.520-524. [DOI] [PubMed] [Google Scholar]
- 3.Rounsaville BJ, Anton SF, Carroll K, Budde D, Prusoff BA, Gawin F. Psychiatric diagnoses of treatment-seeking cocaine abusers. Arch Gen Psychiatry. 1991;48(1):43–51. doi: 10.1001/archpsyc.1991.01810250045005. [DOI] [PubMed] [Google Scholar]
- 4.Conner KR, Pinquart M, Holbrook AP. Meta-analysis of depression and substance use and impairment among cocaine users. Drug Alcohol Depend. 2008;98(1–2):13–23. doi: 10.1016/j.drugalcdep.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poling J, Kosten TR, Sofuoglu M. Treatment outcome predictors for cocaine dependence. Am J Drug Alcohol Abuse. 2007;33(2):191–206. doi: 10.1080/00952990701199416. [DOI] [PubMed] [Google Scholar]
- 6.McDowell D, Nunes EV, Seracini AM, Rothenberg J, Vosburg SK, Ma GJ, Petkova E. Desipramine treatment of cocaine-dependent patients with depression: A placebo-controlled trial. Drug Alcohol Depend. 2005;80(2):209–221. doi: 10.1016/j.drugalcdep.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 7.Schmitz JM, Averill P, Stotts AL, Moeller FG, Rhoades HM, Grabowski J. Fluoxetine treatment of cocaine-dependent patients with major depressive disorder. Drug Alcohol Depend. 2001;63(3):207–214. doi: 10.1016/s0376-8716(00)00208-8. [DOI] [PubMed] [Google Scholar]
- 8.Ciraulo DA, Knapp C, Rotrosen J, Sarid-Segal O, Ciraulo AM, LoCastro J, Greenblatt DJ, Leiderman D. Nefazodone treatment of cocaine dependence with comorbid depressive symptoms. Addiction. 2005;100(Suppl. 1):23–31. doi: 10.1111/j.1360-0443.2005.00984.x. [DOI] [PubMed] [Google Scholar]
- 9.Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7(3):249–264. doi: 10.1111/j.1527-3458.2001.tb00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kent JM. SNaRIs, NaSSAs, and NaRIs: New agents for the treatment of depression. Lancet. 2000;355(9207):911–918. doi: 10.1016/S0140-6736(99)11381-3. [DOI] [PubMed] [Google Scholar]
- 11.Chanrion B, Mannoury la Cour C, Gavarini S, Seimandi M, Vincent L, Pujol JF, Bockaert J, Marin P, Millan MJ. Inverse agonist and neutral antagonist actions of antidepressants at recombinant and native 5-hydroxytryptamine2C receptors: Differential modulation of cell surface expression and signal transduction. Mol Pharmacol. 2008;73(3):748–757. doi: 10.1124/mol.107.041574. [DOI] [PubMed] [Google Scholar]
- 12.Yoon SJ, Pae CU, Kim DJ, Namkoong K, Lee E, Oh DY, Lee YS, Shin DH, Jeong YC, Kim JH, Choi SB, Hwang IB, Shin YC, Cho SN, Lee HK, Lee CT. Mirtazapine for patients with alcohol dependence and comorbid depressive disorders: A multicentre, open label study. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(7):1196–1201. doi: 10.1016/j.pnpbp.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 13.Liappas J, Paparrigopoulos T, Tzavellas E, Rabavilas A. Mirtazapine and venlafaxine in the management of collateral psychopathology during alcohol detoxification. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(1):55–60. doi: 10.1016/j.pnpbp.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Kongsakon R, Papadopoulos KI, Saguansiritham R. Mirtazapine in amphetamine detoxification: A placebo-controlled pilot study. Int Clin Psychopharmacol. 2005;20(5):253–256. doi: 10.1097/01.yic.0000166815.83017.d8. [DOI] [PubMed] [Google Scholar]
- 15.McGregor C, Srisurapanont M, Mitchell A, Wickes W, White JM. Symptoms and sleep patterns during inpatient treatment of methamphetamine withdrawal: A comparison of mirtazapine and modafinil with treatment as usual. J Subst Abuse Treat. 2008;35(3):334–342. doi: 10.1016/j.jsat.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Cruickshank CC, Montebello ME, Dyer KR, Quigley A, Blaszczyk J, Tomkins S, Shand D. A placebo-controlled trial of mirtazapine for the management of methamphetamine withdrawal. Drug Alcohol Rev. 2008;27(3):326–333. doi: 10.1080/09595230801935672. [DOI] [PubMed] [Google Scholar]
- 17.Zueco Pereez PL. Mirtazapine in the treatment of cocaine-dependence in patients with methadone. Actas Esp Psiquiatr. 2002;30(6):337–342. [PubMed] [Google Scholar]
- 18.McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, O’Brien CP. New data from the Addiction Severity Index. Reliability and validity in three centers. J Nerv Ment Dis. 1985;173(7):412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 21.Tiffany ST, Singleton E, Haertzen CA, Henningfield JE. The development of a cocaine craving questionnaire. Drug Alcohol Depend. 1993;34(1):19–28. doi: 10.1016/0376-8716(93)90042-o. [DOI] [PubMed] [Google Scholar]
- 22.Guy W, editor. Clinical Global Impression (CGI) ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Health, Education, and Welfare; 1976. [Google Scholar]
- 23.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 24.Winokur A, DeMartinis NA, 3rd, McNally DP, Gary EM, Cormier JL, Gary KA. Comparative effects of mirtazapine and fluoxetine on sleep physiology measures in patients with major depression and insomnia. J Clin Psychiatry. 2003;64(10):1224–1229. doi: 10.4088/jcp.v64n1013. [DOI] [PubMed] [Google Scholar]
- 25.Winokur A, Sateia MJ, Hayes JB, Bayles-Dazet W, MacDonald MM, Gary KA. Acute effects of mirtazapine on sleep continuity and sleep architecture in depressed patients: A pilot study. Biol Psychiatry. 2000;48(1):75–78. doi: 10.1016/s0006-3223(00)00882-9. [DOI] [PubMed] [Google Scholar]


