Abstract
Background:
Retinol-binding protein 4 (RBP4) is known to regulate lipid and glucose metabolism and insulin resistance. The influences of RBP4 on metabolic syndrome (MS) are still unclear. The purpose of this study is to evaluate the association between serum levels of RBP4 and MS components in first-degree relations of type 2 diabetic patients.
Materials and Methods:
This cross-sectional study was performed within the framework of the diabetes prevention project in Isfahan. This study has been conducted during 2012–2013. Seventy-eight subjects participate, with an average age of 43.20 ± 5.29 years. Weight, height, waist and hip circumferences, blood pressure (BP) of participants, fasting plasma glucose, hemoglobin A1c, total cholesterol, high-density lipoprotein cholesterol, triglyceride (TG), and serum RBP4 were measured from fasting blood sample taken from each participant after an overnight fast (12–14 h).
Results:
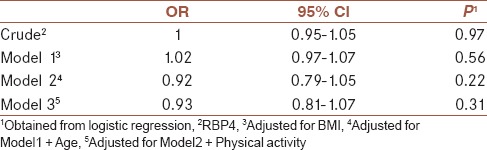
Systolic and diastolic BP were significantly higher in people in top median of RBP4 (11.8 ± 1.5 vs. 11.0 ± 1.2, P = 0.01 and 7.8 ± 1.0 vs. 7.3 ± 0.9, P = 0.03). Moreover, TG in people with high levels of RBP4 was higher compared with those with low levels of RBP4 (177.7 ± 97.6 vs. 138.7 ± 56.9, P = 0.02). People with low levels of RBP4 had significant greater hip circumferences (107.9 ± 7.5 vs. 104.3 ± 8.0, P = 0.04). There was no correlation between RBP4 and MS in crude model (odds ratio [OR]: 1.00, 0.95–1.05, P = 0.97). This null correlation remained after adjustment for body mass index, age, and physical activity (OR: 0.93, 0.91–1.07, P = 0.31).
Conclusion:
Although RBP4 levels were positively association with some risk factors of MS including hip circumference, TG, and systolic and diastolic BP, it does not seem to be a valuable marker for identification of the MS in the first relative degree of diabetic patients.
Keywords: First-degree relations of type 2 diabetic patients, metabolic syndrome, retinol binding protein 4
INTRODUCTION
Metabolic syndrome (MS) is a cluster of metabolic abnormality that increase risks of many chronic diseases, such as cardiovascular diseases and type 2 diabetes mellitus (T2DM).[1] According to ATP III definition for MS (waist circumference [WC] >102 cm for men and >88 cm for women; triglycerides [TGs] ≥150 mg/dl; high-density lipoprotein [HDL]-cholesterol <40 mg/dl for men and <50 mg/dl for women; blood pressure (BP) ≥130/85 mmHg or use of medication for hypertension, and fasting blood glucose (FBS) ≥100 mg/dl or use of medication for hyperglycemia), 24% of the US adults and more than 30% of Qazvin adults estimated to have MS.[2,3] The prevalence of MS in different cities of Iran is as follow: 9% in Zanjan, 23%–27% in Isfahan, 6% in Mashhad, 34% in Tehran, and 22% in Babol.[4] Family members of patients with an established diagnosis of T2DM are theoretically at risk of having the MS and developing T2DM as well.[5] A previous study has been shown that prevalence of MS among Iranian adults particularly women is relatively high rather than men.[6]
Retinol-binding protein 4 (RBP4) is a newly discovered adipokine that produced by hepatocytes and adipocytes, which delivers retinol (Vitamin A) to tissues.[7] Studies have recently been reported that RBP4 is increased in obesity and T2DM and it is associated with insulin resistance (IR) by involved in glucose and lipid metabolism and inhibited insulin signaling in muscle and increased hepatic glucose output.[8,9] Although some studies have been shown that RBP is associated with IR, the association between RBP4 and other components of MS including abdominal obesity, high BP, HDL-cholesterol, FBS, and TG has not been fully investigated.[10,11,12] Furthermore, findings from these studies are inconsistent. While some studies have been shown that serum concentration of RBP4 is associated with some components of MS such as body mass index (BMI) and imbalances in lipid profile, some other studies could not confirm these associations.[12,13] Examining the association between RBP4 and MS components is particularly relevant for this region because most of studies in this area came from the western society. It has been reported that the prevalence of MS among Iranian was more than the world. Oral glucose tolerance test (OGTT) is considered as a diagnostic method of diabetes. Although this method is difficult and time-consuming, it has been identified a suitable method for early detection of diabetes.[14]
According to our knowledge, there are no studies on this topic about first-degree relatives of diabetic patients. On the other hand, these populations are at risk of diabetes and we want to find easy way to diagnose high-risk populations. In this study, we aimed to evaluate the association between serum levels of RBP4 and MS components in first-degree relatives of T2DM.
MATERIALS AND METHODS
Study participants
This cross-sectional study was performed within the framework of the diabetes prevention project in Isfahan. During May 2012 until August 2013, 19 males and 59 females with the age range of 35–55 years were selected using available sampling method.[15] Participants were assigned to either case or control groups based on the results of their OGTT. According to a previously performed study, this sample size was adequate to distinguish statistical association of RBP4 levels with WC.[15] Subjects selected from first-degree relatives of T2DM who refer to endocrine metabolic research center. The inclusion criteria for participants were those who had at least one first-degree relative with diagnosed diabetes after 30 years old, nonsmokers, age 35–55 years, not using any hypoglycemic agents. We did not include those with a history of renal failure, cancer, liver or thyroid diseases, or any other diseases. The exclusion criteria were the individuals consuming drug to decrease blood glucose and having any kind of weight reduction diet. Each participant provided written informed consent. This study was ethically approved by the Endocrine and Metabolism Research Center of Isfahan University of Medical Science.
Anthropometric assessment
Weight was measured by a Seca scale while subjects were lightly clothed and without shoes and recorded to the nearest 100 g. Height was measured by Seca stadiometer while subjects were in a standing position without shoes and their shoulders were in the normal position and recorded to the nearest 5 mm. BMI was calculated as weight in kilogram divided by height square in meter. WC was measured in centimeter with an inelastic tape in the minimal WC between the last rib and the iliac crest. Hip circumference was measured in centimeter with a tape at the wide point over the buttocks with an unscratched tape and without any pressure to body surface; measurements were recorded to the nearest 1 cm. Waist to hip ratio (WHR) was calculated by dividing WC (cm) by hip circumference (cm). Skinfold thickness was measured with a standard caliper in biceps, triceps, and subscapular and iliac crest area, and the sum of four skinfold thicknesses was calculated (in percent of fat mass and lean body mass) to estimate the percent of total body fat using the equation of Durnin and Womersley.[16] BP was measured two times in a seated position after 15 min rest from right arm.[17] The average of two measurements was recorded as BP.
Biochemical assessment
FBS, hemoglobin A1c (HbA1c), total cholesterol (TC), HDL-cholesterol, TG, and serum RBP4[18,19] were measured from fasting blood sample taken from each participant after an overnight fast (12–14 h). OGTT was done after consumption of 75 g glucose and 250 cc water. The samples were analyzed using autoanalyzer (Rome, Italy) using commercial kits (Chem Enzyme and Tehran, Iran). Cholesterol and TG levels were measured by enzymatic reagents adapted to the autoanalyzer. HDL levels were measured using available commercial kits (Pars Azmoon, Tehran, Iran). Low-density lipoprotein (LDL)-cholesterol levels were calculated from Friedewald formula. RBP4 was measured by ELISA methods (Hangzhou Eastbiopharm Co., Ltd, USA). Interassay coefficients of variations were 1.25 for TG, 1.2 for TC, 1.25 for glucose, and 9.75% for RBP4. The corresponding intra-assay was 1.97, 1.6, 2.2, and 5, respectively.
Statistical methods
To ensure the normal distribution of variables, we applied Kolmogorov–Smirnov test. Log transformation was applied for nonnormally distributed variables. Descriptive data were expressed as means ± standard deviations or percentages for quantitative and qualitative data, respectively. Distribution of participants in terms of categorical variables across median of RBP4 (median = 31)[20] was examined using independent sample t-test and Chi-square test. To determine the association between RBP4 status and MS, we used logistic regression analysis in different models. BMI was controlled in the first model to determine the obesity-independent association between RBP4 status and MS. Additional controlling was made for age in the second model. We further adjusted for physical activity (no regular physical activity, <3 h/week regular physical activity, and more than 3 h/week regular physical) in the last model. P < 0.05 was considered statistically significant. All statistical analyses were done using the Statistical Package for Social Science version 17 (SPSS Inc., Chicago, Illinois, USA).
RESULTS
General characteristics of participants divided into non-MS and MS are shown in Table 1. Distribution of participants in terms of sex, age, smoking status, physical activity, and education was not significantly different between people in MS or non-MS group. Mean and standard deviations of MS components across median of RBP4 are shown in Table 2. Comparison of metabolic profiles, anthropometrics measurements, and BP across median of RBP4 revealed no significant differences in FBS, HbA1c, HDL, WC, and cholesterol in people with low levels of RBP4. However, systolic and diastolic BP were significantly higher in people with higher levels of RBP4 (11.8 ± 1.5 vs. 11.0 ± 1.2, P = 0.01 and 7.8 ± 1.0 vs. 7.3 ± 0.9, P = 0.03). Moreover, TG in people with high levels of RBP4 was higher compared with those with low levels of RBP4 (177.7 ± 97.6 vs. 138.7 ± 56.9, P = 0.02). People with low levels of RBP4 had significant greater hip circumferences (107.9 ± 7.5 vs. 104.3 ± 8.0, P = 0.04). The correlation between RBP4 and MS is indicated in Table 3. There was no correlation between RBP4 and MS in crude model (odds ratio [OR]: 1.00, 0.95–1.05, P = 0.97). This null correlation remained after adjustment for BMI, age, and physical activity (OR: 0.93, 0.91–1.07, P = 0.31).
Table 1.
General characteristics of participants
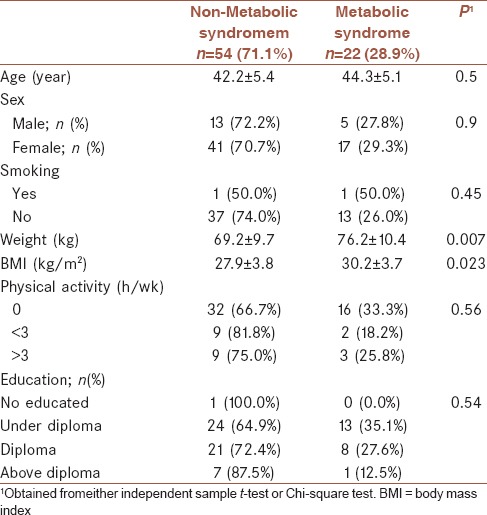
Table 2.
Mean and standard deviations of metabolic syndrome components across median of RBP4
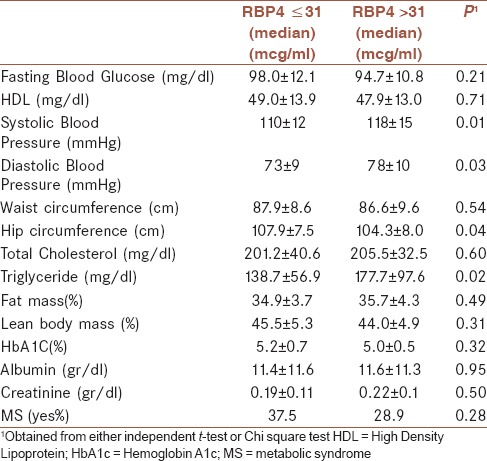
Table 3.
Multiple adjusted OR (95%CI) for the correlation between RBP4 and metabolic syndrome
DISCUSSION
RBP4, as a newly discovered adipokine, has been reported to be associated with obesity, IR, and T2DM.[21] In our study, RBP4 levels were positively associated with some risk factors of MS including TG and systolic and diastolic BP. One previous study has shown that RBP4 levels were not associated with WC and HDL cholesterol.[22] Our finding of this study are in accordance with findings reported by Takashima et al. and Chi-young shim, which showed that serum RBP4 did not correlate with FBS, HDL cholesterol, and WHR but significantly correlated with TG.[23,24] The difference in results may be attributed to a difference in ethnicity or degree of obesity. The lack of association between RBP4 and WHR and HDL cholesterol may be explained by the fact that RBP4 may not be associated with visceral and intramyocellular fat.[25] A previous study revealed a correlation of RBP4 with WHR, suggesting an association between RBP4 levels and abdominal adiposity.[26] In our study, there was correlation between RBP4 levels and hip circumferences. Data demonstrate that changes in adipocyte-derived RBP4 may have systemic effects on insulin sensitivity and glucose homeostasis.
The potential mechanism for association of RBP4 status and MS components is still unclear. However, increasing RBP4 could act directly to induce the expression of phosphor enolpyruvate carboxykinase (also known as PCK2), increase glucose production, and reduce insulin action to suppress glucose production in hepatocytes. In addition, there is a report explaining that RBP4 attenuates insulin-induced phosphorylation of insulin.
There was no correlation between RBP4 levels with blood glucose and HbA1c, suggesting that RBP4 is not a marker for diagnosis of T2DM. Yang et al. reported no difference in glucose levels between RBP4 transgenic mice and wild-type mice despite elevated RBP4 and insulin levels. This result suggests that RBP4 is not always associated with glucose levels because compensatory elevations of insulin can occur along with increment in glucose.[8] Moreover, Takebayashi et al. did not find any relation between FBS and HbA1c with RBP4.[12]
A previous study revealed a correlation of RBP4 to WHR, suggesting an association between RBP4 levels and percent of body fat.[27] A subsequent study similarly identified a relationship between RBP4 levels and WC but no association to percent of body fat or absolute amount of fat.[28] These results indicate that RBP4 may not uniformly be secreted from all adipose tissues because total body fat did not correlate with RBP4 levels. Furthermore, RBP4 is not predominantly secreted by the trunk fat because trunk fat and WC did not correlate to RBP4. We did not find a correlation between RBP4 levels and WC. One meta-analysis in this regard has shown that several lipid metabolism indices (LDL cholesterol, TC, and TG concentrations) were positively correlated with serum RBP4 concentrations in patients with T2DM.[9] The correlation between TG and RBP4 concentrations may be related to the correlations that we observed between RBP4 concentration and the obesity markers (WHR and WC) which are why TG has been considered a marker for central obesity.[29] These findings were in line with Graham et al. and Takebayashi et al. However, we did not find a positive correlation between serum RBP4 and WC.[11,12] Janke et al. argued that adipose tissue might be a less important source of circulating RBP4 in humans than in animals and that it was possible that the increase in circulating RBP4 in the IR state was not explained by increased RBP4 production in adipose tissue.[26,30] Thus, prospective and biological studies are needed to future explore the association of serum RBP4 with obesity. In line with some studies, we found an association between serum RBP4 and TG.[12,30,31,32,33] It was suggested that RBP4 might have a direct role in the progression of lipogenesis. In a study, RBP4 increased the expression of the gene encoding fatty acid syntheses in adipose tissues in a manner predominantly correlating with visceral fat accumulation.[34] There was positive correlation between the serum RBP4 concentration and both systolic and diastolic BP. This is in accordance with the finding of others, who speculated that RBP4-mediated cellular uptake of retinol activates inflammation and induces arteriosclerosis, resulting in elevated BP.
RBP4 might act through retinol dependent or independent mechanisms. Retinol-dependent mechanisms by which RBP4 may influence insulin action include increased production or altered tissue metabolism of retinoic acid isomers, the active forms of retinol that interact with retinoic acid receptors (RARs) and retinoic acid-X receptors (RXRs) to regulate gene transcription.[35] However, the relationship between retinoic acid and IR is complex as certain retinoic acid isomers cause IR and the MS in humans, whereas others activate the RXR peroxisome proliferator-activated receptor gamma heterodimer and increase insulin sensitivity. RBP4 might also cause IR through a retinol-independent mechanism.[36] Evidence suggests that RBP4 binds with high affinity and high specificity to cell surface receptors. RBP4 might transport and deliver other lipophilic molecules in addition to retinol, as shown by the fact that RBP4 binds a wide range of other retinoid in vitro. Finally, RBP4 could also modulate transthyretin function.[37,38]
This study has several limitations. First, the cross-sectional design of the study represents a limitation, implicating that cause-and-effect relationship cannot be discerned. Second, our study included more women than men because the participation rate was higher among women. In addition, studies are needed to clarify the mechanism for the cross-sectional association of RBP4 with metabolic indices. Furthermore, sample size was small. Moreover, we were unable to measure serum retinol levels associated with Vitamin A status, and we did not assess nutrition intake of participants. Some strengths of this study are that all subjects in this study were healthy and did not use any drug. Moreover, as previous study has shown that levels of RBP4 are influenced by age, we limited our subject to 35–55 years old.
CONCLUSION
RBP4 does not seem to be a valuable marker for identification of the MS in first relative degree, but RBP4 may play a role in lipid profile. This needs to be further investigated in experimental follow-up studies.
Financial support and sponsorship
The study was funded by Isfahan Endocrine and Metabolism Research Center, Isfahan University of Medical Sciences, Isfahan, Iran.
Conflicts of interest
There are no conflicts of interest.
AUTHORS’ CONTRIBUTION
MT: conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
AN: conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
MA: contributed in the conception of thework, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
AF: conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
SSB: conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
MZ: contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
Acknowledgments
We would like to thank all the members of the Isfahan Endocrine and Metabolism Research Center. We also thank Mrs. Kabirzadeh and Azam Khani for her supports in this study.
REFERENCES
- 1.Bonora E. The metabolic syndrome and cardiovascular disease. Ann Med. 2006;38:64–80. doi: 10.1080/07853890500401234. [DOI] [PubMed] [Google Scholar]
- 2.Beltrán-Sánchez H, Harhay MO, Harhay MM, McElligott S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999-2010. J Am Coll Cardiol. 2013;62:697–703. doi: 10.1016/j.jacc.2013.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esmailzadehha N, Ziaee A, Kazemifar AM, Ghorbani A, Oveisi S. Prevalence of metabolic syndrome in qazvin metabolic diseases study (QMDS), Iran: A comparative analysis of six definitions. Endocr Regul. 2013;47:111–20. doi: 10.4149/endo_2013_03_111. [DOI] [PubMed] [Google Scholar]
- 4.Hajian-Tilaki K. Metabolic syndrome and its associated risk factors in Iranian adults: A systematic review. Caspian J Intern Med. 2015;6:51–61. [PMC free article] [PubMed] [Google Scholar]
- 5.Das M, Pal S, Ghosh A. Family history of type 2 diabetes and prevalence of metabolic syndrome in adult Asian Indians. J Cardiovasc Dis Res. 2012;3:104–8. doi: 10.4103/0975-3583.95362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shahbazian H, Latifi SM, Jalali MT, Shahbazian H, Amani R, Nikhoo A, et al. Metabolic syndrome and its correlated factors in an urban population in South West of Iran. J Diabetes Metab Disord. 2013;12:11. doi: 10.1186/2251-6581-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng J, Song ZY, Pu L, Yang H, Zheng JM, Zhang ZY, et al. Retinol binding protein 4 affects the adipogenesis of porcine preadipocytes through insulin signaling pathways. Biochem Cell Biol. 2013;91:236–43. doi: 10.1139/bcb-2012-0112. [DOI] [PubMed] [Google Scholar]
- 8.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–62. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 9.Park H, Green MH, Shaffer ML. Association between serum retinol-binding protein 4 concentrations and clinical indices in subjects with type 2 diabetes: A meta-analysis. J Hum Nutr Diet. 2012;25:300–10. doi: 10.1111/j.1365-277X.2012.01262.x. [DOI] [PubMed] [Google Scholar]
- 10.Tan BK, Chen J, Lehnert H, Kennedy R, Randeva HS. Raised serum, adipocyte, and adipose tissue retinol-binding protein 4 in overweight women with polycystic ovary syndrome: Effects of gonadal and adrenal steroids. J Clin Endocrinol Metab. 2007;92:2764–72. doi: 10.1210/jc.2007-0091. [DOI] [PubMed] [Google Scholar]
- 11.Graham TE, Yang Q, Blüher M, Hammarstedt A, Ciaraldi TP, Henry RR, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354:2552–63. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 12.Takebayashi K, Suetsugu M, Wakabayashi S, Aso Y, Inukai T. Retinol binding protein-4 levels and clinical features of type 2 diabetes patients. J Clin Endocrinol Metab. 2007;92:2712–9. doi: 10.1210/jc.2006-1249. [DOI] [PubMed] [Google Scholar]
- 13.Li F, Yang T, Zhao Z, Xia K. Plasma level of RBP4 in patients with coronary heart disease and the effect of hyperinsulinemia. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2012;37:1177–82. doi: 10.3969/j.issn.1672-7347.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Salmasi AM, Dancy M. The glucose tolerance test, but not HbA (1c), remains the gold standard in identifying unrecognized diabetes mellitus and impaired glucose tolerance in hypertensive subjects. Angiology. 2005;56:571–9. doi: 10.1177/000331970505600508. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Li N. Correlation of retinol binding protein 4 with metabolic indexes of glucose and lipid, bile cholesterol saturation index. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2015;40:657–65. doi: 10.11817/j.issn.1672-7347.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Davidson LE, Wang J, Thornton JC, Kaleem Z, Silva-Palacios F, Pierson RN, et al. Predicting fat percent by skinfolds in racial groups: Durnin and Womersley revisited. Med Sci Sports Exerc. 2011;43:542–9. doi: 10.1249/MSS.0b013e3181ef3f07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhie YJ, Choi BM, Eun SH, Son CS, Park SH, Lee KH. Association of serum retinol binding protein 4 with adiposity and pubertal development in Korean children and adolescents. J Korean Med Sci. 2011;26:797–802. doi: 10.3346/jkms.2011.26.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng S, Zhu Y, Yan C, Wang Y, Zhang Z. Retinol binding protein 4 correlates with and is an early predictor of carotid atherosclerosis in type 2 diabetes mellitus patients. J Biomed Res. 2015;29 doi: 10.7555/JBR.29.20140087. doi: 10.7555/JBR.29.20140087 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ottobelli Chielle E, de Souza WM, da Silva TP, Moresco RN, Moretto MB. Adipocytokines, inflammatory and oxidative stress markers of clinical relevance altered in young overweight/obese subjects. Clin Biochem. 2016;49:548–53. doi: 10.1016/j.clinbiochem.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Christou GA, Tellis CC, Elisaf MS, Tselepis AD, Kiortsis DN. The relationship between retinol-binding protein 4 and apolipoprotein B-containing lipoproteins is attenuated in patients with very high serum triglycerides: A pilot study. Hormones. 2016;15:99–105. doi: 10.14310/horm.2002.1663. [DOI] [PubMed] [Google Scholar]
- 21.Pu L, Cheng J, Wu G, Yang H, Qiu Y, Zhang Z, et al. Effects of retinol binding protein 4 knockdown on the PI3K/Akt pathways in porcine adipocytes. Sheng Wu Gong Cheng Xue Bao. 2013;29:447–57. [PubMed] [Google Scholar]
- 22.Suh JB, Kim SM, Cho GJ, Choi KM, Han JH, Taek Geun H. Elevated serum retinol-binding protein 4 is associated with insulin resistance in older women. Metabolism. 2010;59:118–22. doi: 10.1016/j.metabol.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 23.Takashima N, Tomoike H, Iwai N. Retinol-binding protein 4 and insulin resistance. N Engl J Med. 2006;355:1392. doi: 10.1056/NEJMc061863. [DOI] [PubMed] [Google Scholar]
- 24.Su YX, Hong J, Yan Q, Xu C, Gu WQ, Zhang YF, et al. Increased serum retinol-binding protein-4 levels in pregnant women with and without gestational diabetes mellitus. Diabetes Metab. 2010;36(6 Pt 1):470–5. doi: 10.1016/j.diabet.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Bajzová M, Kováciková M, Vítková M, Klimcáková E, Polák J, Kovácová Z, et al. Retinol-binding protein 4 expression in visceral and subcutaneous fat in human obesity. Physiol Res. 2008;57:927–34. doi: 10.33549/physiolres.931379. [DOI] [PubMed] [Google Scholar]
- 26.Castro AP, Cândido AP, Nicolato RL, Caldas IS, Machado-Coelho GL. Retinol-binding protein 4 and insulin resistance are related to body fat in primary and secondary schoolchildren: The ouro preto study. Eur J Nutr. 2014;53:433–40. doi: 10.1007/s00394-013-0543-5. [DOI] [PubMed] [Google Scholar]
- 27.Gavi S, Stuart LM, Kelly P, Melendez MM, Mynarcik DC, Gelato MC, et al. Retinol-binding protein 4 is associated with insulin resistance and body fat distribution in nonobese subjects without type 2 diabetes. J Clin Endocrinol Metab. 2007;92:1886–90. doi: 10.1210/jc.2006-1815. [DOI] [PubMed] [Google Scholar]
- 28.Godoy-Matos AF, Moreira RO, MacDowell R, Bendet I, Mory PB, Moises RS. Serum retinol binding protein 4 in patients with familial partial lipodystrophy. Clin Biochem. 2009;42:1183–6. doi: 10.1016/j.clinbiochem.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Yamaaki N, Yagi K, Kobayashi J, Nohara A, Ito N, Asano A, et al. Impact of serum retinol-binding protein 4 levels on regulation of remnant-like particles triglyceride in type 2 diabetes mellitus. J Diabetes Res 2013. 2013:143515. doi: 10.1155/2013/143515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janke J, Engeli S, Boschmann M, Adams F, Böhnke J, Luft FC, et al. Retinol-binding protein 4 in human obesity. Diabetes. 2006;55:2805–10. doi: 10.2337/db06-0616. [DOI] [PubMed] [Google Scholar]
- 31.von Eynatten M, Lepper PM, Liu D, Lang K, Baumann M, Nawroth PP, et al. Retinol-binding protein 4 is associated with components of the metabolic syndrome, but not with insulin resistance, in men with type 2 diabetes or coronary artery disease. Diabetologia. 2007;50:1930–7. doi: 10.1007/s00125-007-0743-8. [DOI] [PubMed] [Google Scholar]
- 32.Xu M, Li XY, Wang JG, Wang XJ, Huang Y, Cheng Q, et al. Retinol-binding protein 4 is associated with impaired glucose regulation and microalbuminuria in a Chinese population. Diabetologia. 2009;52:1511–9. doi: 10.1007/s00125-009-1386-8. [DOI] [PubMed] [Google Scholar]
- 33.Sopher AB, Gerken AT, Blaner WS, Root JM, McMahon DJ, Oberfield SE. Metabolic manifestations of polycystic ovary syndrome in nonobese adolescents: Retinol-binding protein 4 and ectopic fat deposition. Fertil Steril. 2012;97:1009–15. doi: 10.1016/j.fertnstert.2012.01.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aeberli I, Biebinger R, Lehmann R, L’allemand D, Spinas GA, Zimmermann MB. Serum retinol-binding protein 4 concentration and its ratio to serum retinol are associated with obesity and metabolic syndrome components in children. J Clin Endocrinol Metab. 2007;92:4359–65. doi: 10.1210/jc.2007-0468. [DOI] [PubMed] [Google Scholar]
- 35.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–54. [PubMed] [Google Scholar]
- 36.Koistinen HA, Remitz A, Gylling H, Miettinen TA, Koivisto VA, Ebeling P. Dyslipidemia and a reversible decrease in insulin sensitivity induced by therapy with 13-cis-retinoic acid. Diabetes Metab Res Rev. 2001;17:391–5. doi: 10.1002/dmrr.222. [DOI] [PubMed] [Google Scholar]
- 37.Rodondi N, Darioli R, Ramelet AA, Hohl D, Lenain V, Perdrix J, et al. High risk for hyperlipidemia and the metabolic syndrome after an episode of hypertriglyceridemia during 13-cis retinoic acid therapy for acne: A pharmacogenetic study. Ann Intern Med. 2002;136:582–9. doi: 10.7326/0003-4819-136-8-200204160-00007. [DOI] [PubMed] [Google Scholar]
- 38.Chiba M, Saitoh S, Ohnishi H, Akasaka H, Mitsumata K, Furukawa T, et al. Associations of metabolic factors, especially serum retinol-binding protein 4 (RBP4), with blood pressure in Japanese – the Tanno and Sobetsu study. Endocr J. 2010;57:811–7. doi: 10.1507/endocrj.k10e-054. [DOI] [PubMed] [Google Scholar]


