Supplemental Digital Content is available in the text.
Keywords: antipsychotic agents, aripiprazole, dopamine, long-acting injectable, paliperidone palmitate, patient-reported outcomes, prolactin, quality of life, schizophrenia, sexual dysfunction
Abstract
Sexual dysfunction, a common side effect of antipsychotic medications, may be partly caused by dopamine antagonism and elevation of prolactin. In QUALIFY, a randomized study, aripiprazole once-monthly 400 mg (AOM 400), a dopamine D2 receptor partial agonist, showed noninferiority and subsequent superiority versus paliperidone palmitate (PP), a dopamine D2 receptor antagonist, on the Heinrichs–Carpenter Quality-of-Life Scale (QLS) in patients with schizophrenia aged 18–60 years. Sexual dysfunction (Arizona Sexual Experience Scale) and serum prolactin levels were also assessed. Odds for sexual dysfunction were lower with AOM 400 versus PP [week 28 adjusted odds ratio (95% confidence interval), 0.29 (0.14–0.61); P=0.0012] in men [0.33 (0.13–0.86); P=0.023], women [0.14 (0.03–0.62); P=0.0099], and patients aged 18–35 years [0.04 (<0.01–0.34); P=0.003]. Among patients shifting from sexual dysfunction at baseline to none at week 28, there was a trend toward greater improvement in the QLS total score. The mean (SD) prolactin concentrations decreased with AOM 400 [−150.6 (274.4) mIU/l] and increased with PP [464.7 (867.5) mIU/l] in both men and women. Six PP-treated patients experienced prolactin-related adverse events. In addition to greater improvement on QLS, patients had a lower risk for sexual dysfunction and prolactin elevation with AOM 400 versus PP in QUALIFY.
Introduction
Schizophrenia is a chronic disorder characterized by multiple relapses (Emsley et al., 2013). For most patients, maintenance therapy is essential to prevent relapse and maintain or improve functioning (Emsley et al., 2013; Hasan et al., 2013). Long-acting injectable (LAI) antipsychotics may ease the burden of daily adherence to oral antipsychotics, which could be expected to reduce nonadherence and the risk of relapse (Kishimoto et al., 2013). In the setting of maintenance therapy, side effects of antipsychotic medication can pose a considerable patient burden and threat to medication adherence. Side effects may lead some clinicians to consider antipsychotic discontinuation even after successful first-episode treatment (Emsley et al., 2013). Furthermore, patients with schizophrenia who report side effects are less likely to adhere to medication (Dibonaventura et al., 2012).
Sexual dysfunction is a common side effect of antipsychotic medication (Young et al., 2015) experienced by up to 75% of patients with schizophrenia (Harley et al., 2010; Young et al., 2015). It is associated with reduced quality of life (Bushong et al., 2013) and has the potential to increase medication nonadherence (Lambert et al., 2004). The incidence of sexual dysfunction is likely underestimated because patients and physicians may be reluctant to spontaneously discuss and report medication-related sexual side effects, especially if they do not use a specific questionnaire or direct questioning (Serretti and Chiesa, 2011). The development of sexual dysfunction during antipsychotic drug treatment is multifactorial, but suggested mechanisms include dopamine antagonism and associated prolactin elevation (De Hert et al., 2014). Although all antipsychotics are known to affect sexual function, rates of sexual dysfunction may differ from compound to compound (De Hert et al., 2014).
Aripiprazole once-monthly 400 mg (AOM 400) and paliperidone palmitate (PP) are LAIs approved for the treatment of schizophrenia. Although they are both active at central dopamine D2 and serotonin receptors, they have distinct receptor affinities and functions. Aripiprazole shows partial agonism at the dopamine D2 and serotonin 5-HT1A receptors and antagonism at the 5-HT2A receptor (Abilify Maintena US (aripiprazole), 2016), whereas paliperidone is an antagonist at both the D2 and 5-HT2A receptors (Invega Sustenna (paliperidone palmitate), 2015). The differences in the actions of AOM 400 and PP at the dopamine D2 receptor could contribute to different tolerability profiles and result in different effects on prolactin concentrations and sexual function (Kruger et al., 2005; Correll, 2010). In the 26-week naturalistic Schizophrenia Trial of Aripiprazole (STAR) study, which evaluated the effectiveness of aripiprazole in patients in a community health-based or hospital-based setting, aripiprazole was shown to significantly reduce sexual dysfunction at the end of the treatment period compared with standard-of-care antipsychotics olanzapine, quetiapine, or risperidone (Hanssens et al., 2008).
QUALIFY was a randomized study that compared the effectiveness of two atypical LAIs, AOM 400 and PP, using the Heinrichs–Carpenter Quality-of-Life Scale (QLS), a measure of health-related quality of life and functioning, as the primary outcome. The primary effectiveness results of this study have been previously published (Naber et al., 2015). The study fulfilled its primary endpoint of noninferiority and met predefined superiority criteria for AOM 400 versus PP in change from baseline to week 28 on the QLS total score. In adult patients 35 years or younger, the improvement in the QLS total score was significantly greater with AOM 400 than with PP, whereas the treatment difference in patients older than 35 years was numerically but not significantly greater with AOM 400. Significantly better effectiveness versus PP supports initiation of AOM 400 in younger adult patients (Naber et al., 2015), who may be particularly sensitive to sexual dysfunction-related side effects. The selection of 35 years as the age cut-off was derived from the results of an analysis of personal and social performance in an earlier placebo-controlled trial with AOM 400, which showed greater functional improvements in patients 35 years of age or younger (Baker et al., 2013). Furthermore, age analysis was prespecified in QUALIFY and incorporated into randomization as a stratification factor. In addition to effectiveness, serum prolactin concentrations and patient-rated measures of sexual function were also assessed, and have not been reported before. The aim of the present analysis was to evaluate the differential treatment effects of AOM 400 and PP on sexual dysfunction and prolactin levels in QUALIFY for each treatment group as a whole, by sex, and by age strata.
Patients and methods
Study design
This report includes prespecified and post-hoc analyses of data from a 28-week, open-label, randomized trial at 71 centers in 10 countries. Detailed study design and primary study outcomes were reported previously (Naber et al., 2015). Patients were randomized to treatment with AOM 400 (dose reduction to 300 mg permitted for reasons of tolerability) or PP [78–234 mg/month as PP (USA), equivalent to 50–150 mg/month as paliperidone (European Union and Canada)]. After randomization, patients converted from their previous antipsychotic to oral aripiprazole (5–30 mg/day) or paliperidone (3–12 mg/day) over 3 weeks and then received the respective LAI formulation (Naber et al., 2015). The study was carried out in accordance with Good Clinical Practice and the Declaration of Helsinki, and the national coordinating investigator for each country (European Union) or the principal investigator at each site obtained protocol approval from their respective ethics committees (ClinicalTrials.gov: NCT01795547).
Patients
Patients eligible for participation were adults 18–60 years old with schizophrenia (Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision) and stable disease who required a change from current oral antipsychotic treatment because of inadequate response, poor tolerability, or nonadherence, and had the potential to benefit from LAI treatment based on the judgment of the investigator (Naber et al., 2015). The patients or their legal representatives provided written informed consent before participating in any study-related activities. Patients had been treated with oral antipsychotics for at least 3 months before screening and had a Clinical Global Impression-Severity of Illness scale score of 3–5 at both the screening and the baseline visits. To allow for predefined comparisons of changes from baseline on the primary outcome in younger and older adult patients, randomization was stratified by age, and the recruitment target was one-third of adult patients 35 years of age or younger.
Assessments
Sexual dysfunction was assessed at baseline; at weeks 4, 8, and 16; and at week 28 or the last visit using the Arizona Sexual Experience Scale (ASEX) (McGahuey et al., 2000). The ASEX is a patient-reported five-item scale with which men and women rate their sexual experiences over the last week. Each item is rated from 1 to 6, for a possible total score of 5–30, with higher scores indicating greater sexual dysfunction. The predefined criteria for sexual dysfunction were an ASEX total score of at least 19, a score of at least 5 on any item, or a score of at least 4 on at least three items (McGahuey et al., 2000). The QLS (primary outcome measure) was used to assess deficit symptoms of schizophrenia and functioning, administered by a rater blinded to the patient’s treatment assignment. The QLS consists of 21 items divided into four subscales: Interpersonal Relations (eight items), Instrumental Role (four items), Intrapsychic Foundations (seven items), and Common Objects and Activities (two items). Each item is rated on a seven-point scale, from 0 (severe impairment) to 6 (normal or unimpaired functioning). The total score for all items ranges from 0 to 126; higher scores indicate normal or unimpaired functioning (Heinrichs et al., 1984). Serum prolactin samples were collected under fasting conditions at baseline and week 28 or at the last visit and analyzed at a central laboratory. The upper limit of the normal (ULN) range for serum prolactin was 617 mIU/l (29.1 µg/l) for women and 374 mIU/l (17.6 µg/l) for men.
Statistical analysis
The all patients treated set (APTS) included all randomized patients who received at least one dose of oral antipsychotic during the study. Safety parameters including prolactin and prolactin-related adverse events (AEs) were analyzed using the APTS. The full analysis set included patients from the APTS who had both a valid baseline and a postbaseline assessment of the QLS total score and was used to analyze effectiveness measures, including QLS and ASEX. Odds ratios (ORs) for the risk of sexual dysfunction at week 28 with AOM 400 or PP treatment were estimated using logistic regression (post-hoc) in the full analysis set and adjusted for the presence/absence of sexual dysfunction at baseline. Similar analyses were carried out in adult patients aged 35 years or younger and older than 35 years, as well as separately in men and women. The adjusted ORs at week 28 were compared between treatment groups with the Wald test. Changes in ASEX total scores, serum prolactin concentrations, and changes in serum prolactin concentrations (all prespecified analyses) are presented using descriptive statistics. To assess a potential relationship between change in QLS and change in sexual dysfunction, partial correlations (post-hoc) between change from baseline in QLS score and change in ASEX total score at week 28, as well as for changes in QLS domains, were calculated for all patients (combining treatment groups) using a mixed model for repeated measures (MMRM). In addition, changes from baseline in the QLS score in sexual dysfunction shift groups were also analyzed using an MMRM. The MMRM measured change from baseline as a function of visit and the interaction between baseline score and visit as a covariate using an unstructured covariance matrix, restricted maximum likelihood, and Kenward-Roger derivation of d.f. Multiplicity corrections were not applied, and all statistical hypotheses were tested on a significance level of 0.05.
Results
Patient disposition and characteristics
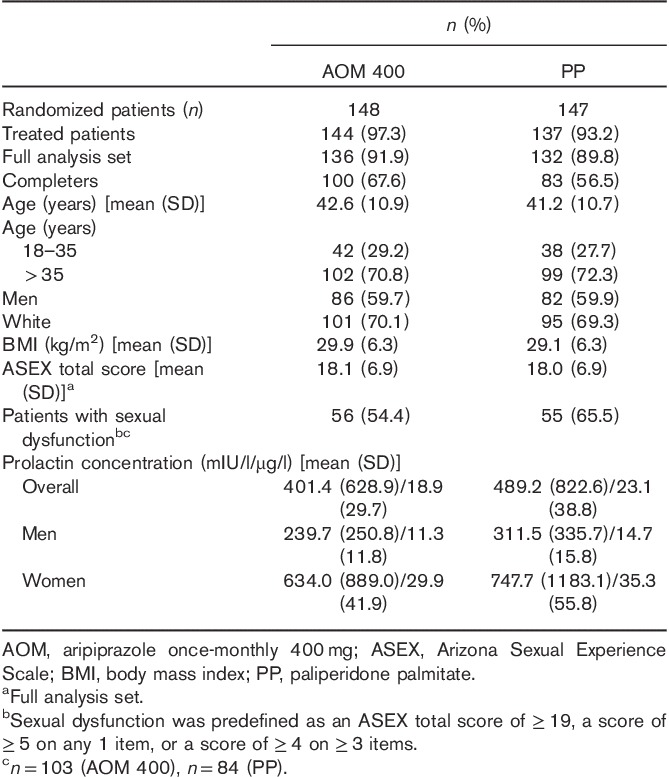
Of 148 patients randomized to AOM 400 and 147 randomized to PP, 100 (67.6%) and 83 (56.5%), respectively, completed 28 weeks of the study. Patient disposition, demographics, and baseline characteristics are shown in Table 1. The mean baseline ASEX total scores were similar between treatment groups and just below the threshold for sexual dysfunction. At baseline, the mean serum prolactin concentrations in men were similar between treatment groups and below the ULN; in women, concentrations were also similar between treatment groups, with mean levels close to or slightly above the ULN.
Table 1.
Patient disposition, demographics, and baseline characteristics
Arizona Sexual Experience Scale
Whereas baseline rates of sexual dysfunction were not significantly different between treatment groups, a smaller proportion of patients reported sexual dysfunction with AOM 400 versus PP at week 28 (37.9 vs. 63.1%; Table 2), and a greater proportion of patients shifted from having sexual dysfunction at baseline to not having sexual dysfunction at week 28 with AOM 400 versus PP (21.4 vs. 11.9%; Fig. 1). At week 28, the odds of sexual dysfunction were significantly lower in the AOM 400 group compared with the PP group {adjusted OR [95% confidence interval (CI)], 0.29 (0.14–0.61), P=0.0012; Table 2}. These results with AOM 400 versus PP were confirmed in men [27.9 vs. 50.0%; 0.33 (0.13–0.86), P=0.023] and in women [52.4 vs. 78.9%; 0.14 (0.03–0.62), P=0.0099; Table 2]. Among adult patients aged 35 years or younger, sexual dysfunction was reported by 16.1% (5/31) of patients in the AOM 400 group and 70.0% (14/20) in the PP group [adjusted OR (95% CI), 0.04 (<0.01–0.34), P=0.0036] at week 28. A similar pattern was observed in patients older than 35 years of age, although the difference in this population was not statistically significant (Table 2).
Table 2.
Incidence and odds of Arizona Sexual Experience Scale sexual dysfunctiona (full analysis set)
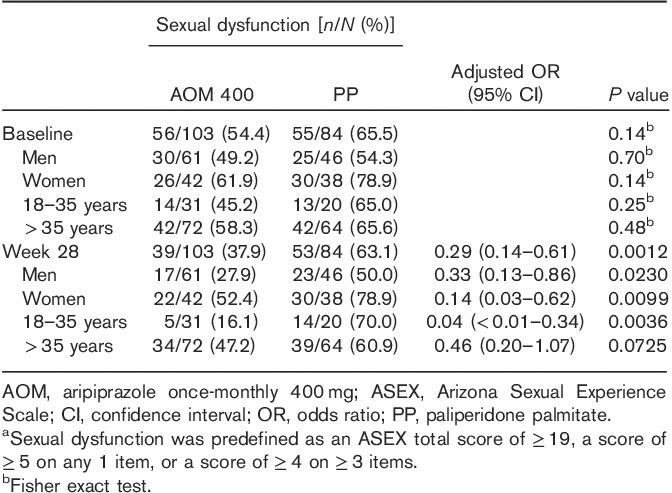
Fig. 1.
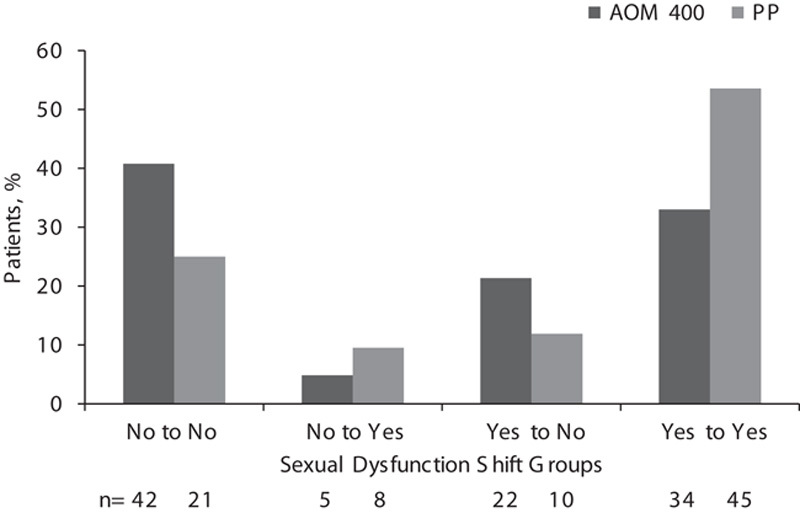
Proportion of patients with or without sexual dysfunction and those showing a sexual dysfunction shift (no sexual dysfunction to having sexual dysfunction; having sexual dysfunction to no sexual dysfunction) from baseline to week 28 by treatment group. AOM 400, aripiprazole once-monthly 400 mg; PP, paliperidone palmitate.
The mean (SD) QLS total scores at baseline were comparable for the AOM 400 and PP groups [66.0 (21.7) vs. 62.9 (21.5) points, respectively]. Partial correlations between change from baseline at week 28 in QLS total and domain scores and ASEX total score were calculated (see Supplementary Table, Supplemental digital content 1, http://links.lww.com/ICP/A22). A modest correlation was observed between the QLS total score and the ASEX total score [correlation estimate (95% CI), –0.21 (–0.35 to –0.06)]. The strongest correlation was between change in the QLS Intrapsychic Foundations domain score and change in the ASEX total score [–0.24 (–0.37 to –0.09)]. Change in QLS scores at week 28 was calculated for patients with or without sexual dysfunction, irrespective of treatment group (Fig. 2). Because of the low number of patients with shifts from ‘No to Yes’ in sexual dysfunction (n=13), these were included in the ‘Yes at Week 28’ group for this analysis. From baseline to week 28, 63 patients did not show sexual dysfunction, and 32 shifted from having sexual dysfunction to no sexual dysfunction (‘Yes to No’). Although differences in QLS change at week 28 by sexual dysfunction status were not statistically significant between sexual dysfunction shift groups, the greatest improvement in QLS total and domain scores occurred in patients who shifted from yes (sexual dysfunction) to no sexual dysfunction at week 28.
Fig. 2.
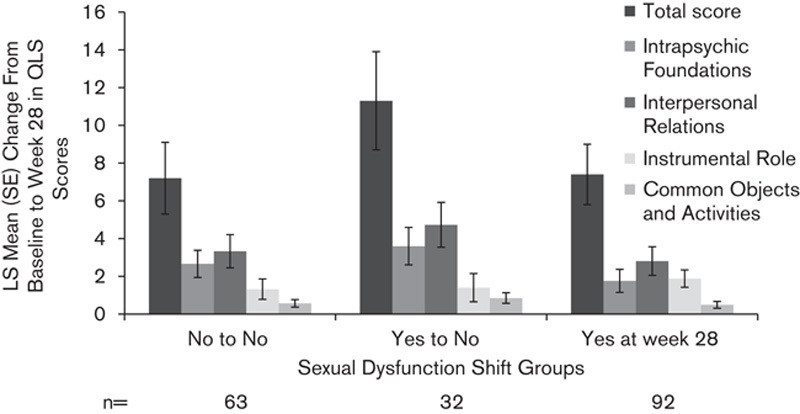
Change from baseline to week 28 in QLS total scores and domains for sexual dysfunction shift groups (in combined treatment groups). ‘Yes at Week 28’ includes patients in ‘Yes to Yes’ and ‘No to Yes’ shift groups. LS mean, least squares; QLS, Heinrichs–Carpenter Quality-of-Life Scale.
Prolactin
At baseline, the mean (SD) serum prolactin concentrations were similar in the two treatment groups overall and for both men and women (Table 1). From baseline to week 28, the mean (SD) serum prolactin decreased in the AOM 400 group and increased in the PP group [–150.6 (274.4) and 464.7 (867.5) mIU/l, respectively, equivalent to –7.1 (12.9) and 21.9 (40.9) µg/l; Fig. 3]. The same pattern was observed irrespective of sex or age (18–35 vs. >35 years; Fig. 3). At baseline, the mean (SD) prolactin concentrations in patients with sexual dysfunction were higher in both AOM 400 [512.5 (792.3) mIU/l/24.2 (37.4) µg/l] and PP [661.8 (993.8) mIU/l/31.2 (46.9) µg/l] groups compared with concentrations in patients with no sexual dysfunction [AOM, 258.9 (255.8) mIU/l/12.2 (12.1) µg/l; PP, 212.5 (224.6) mIU/l/10.0 (10.6) µg/ml]. Among the patient groups that showed a shift in sexual dysfunction (‘Yes to No’ or ‘No to Yes’) at week 28 with PP treatment, prolactin levels were higher in patients who shifted from no sexual dysfunction to having sexual dysfunction (Fig. 4). The results for AOM 400, however, did not show a similar pattern; the mean prolactin concentrations remained within the normal range at week 28 in all shift groups. Patients in the AOM 400 treatment group shifting from no sexual dysfunction to having sexual dysfunction did not have higher serum prolactin concentrations compared with those who experienced the reverse shift, that is, from yes (sexual dysfunction) to no sexual dysfunction (Fig. 4). Prolactin concentrations of more than two or more than three times the ULN were observed in both treatment groups at baseline; eight patients in the AOM 400 group and nine patients in the PP group had prolactin concentration of more than two times the ULN, whereas two patients in the AOM 400 group and eight patients in the PP group had prolactin concentration of more than three times the ULN. At week 28, there were no patients in the AOM 400 group with prolactin elevations more than twice the ULN, but 25 patients in the PP group had prolactin elevations of more than two or more than three times the ULN in these ranges, including nine women with prolactin of more than three times the ULN.
Fig. 3.
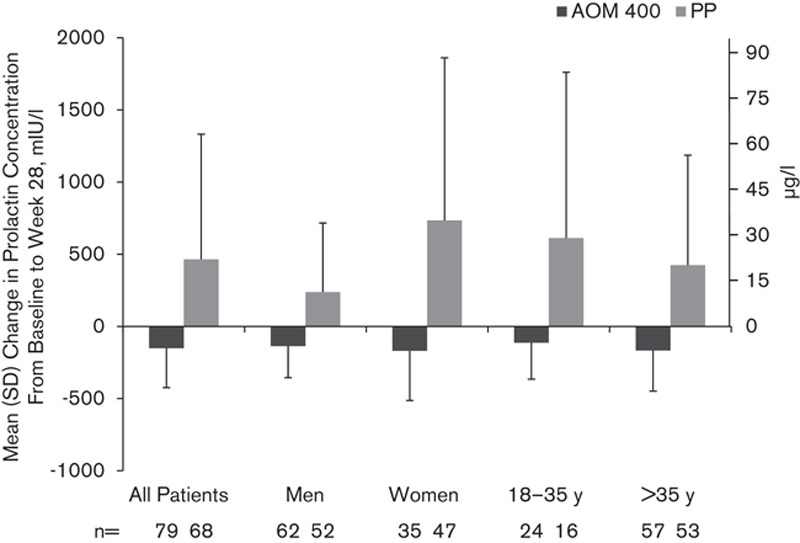
Mean (SD) change from baseline in serum prolactin concentration at week 28 in all patients, and by sex and age. AOM 400, aripiprazole once-monthly 400 mg; PP, paliperidone palmitate.
Fig. 4.
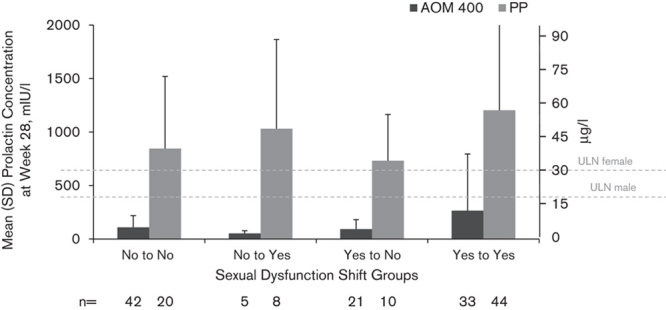
Mean (SD) serum prolactin concentrations at week 28 by sexual dysfunction shift group. Serum prolactin ULN was 617 mIU/l (29.1 µg/l) for women and 374 mIU/l (17.6 µg/l) for men. AOM 400, aripiprazole once-monthly 400 mg; PP, paliperidone palmitate; ULN, upper limit of normal.
Six patients, all of whom were in the PP group, experienced a prolactin-related treatment-emergent AE (TEAE). The severity of these TEAEs was mild (n=3), moderate (n=2), or severe (n=1); none were serious. Of these six patients who experienced a prolactin-related TEAE, five had an ASEX measurement at week 28, and all five fulfilled the criteria for sexual dysfunction. No symptomatic prolactin-related AEs were reported in the AOM 400 group.
Discussion
Sexual dysfunction associated with antipsychotic medication is a troublesome side effect that most patients with schizophrenia are reluctant to spontaneously report and most physicians are reluctant to investigate. Providing patients with a self-rating scale may increase the likelihood that patients will disclose sexual side effects (Serretti and Chiesa, 2011). With awareness of patients’ disclosure of sexual dysfunction secondary to antipsychotic medication, physicians can determine the appropriate action to alleviate side effects and potentially avoid medication nonadherence. The ASEX scale provides patients with an opportunity to self-rate their sexual functioning. These analyses of QUALIFY show that, in addition to superior improvements in health-related quality of life and functioning on the clinician-rated QLS (Naber et al., 2015), a significantly lower risk for patient-reported sexual dysfunction and less prolactin elevation was observed with AOM 400 compared with PP treatment. The significantly lower odds of patient-reported sexual dysfunction and a favorable serum prolactin profile were observed after AOM 400 treatment in both men and women. As reported previously for other outcomes, including QLS, Clinical Global Impression-Severity of Illness scale, and the Investigator’s Assessment Questionnaire (Naber et al., 2015), the treatment effect on sexual dysfunction was most pronounced in adult patients 35 years or younger; in the younger age group, the proportion with sexual dysfunction decreased from baseline to week 28 in the AOM 400, but not the PP group, and the odds of having sexual dysfunction at week 28 were significantly lower with AOM 400 than with PP treatment.
Second-generation antipsychotics are considered to have a more favorable side-effect profile with respect to prolactin and sexual dysfunction compared with first-generation antipsychotics (Hanssens et al., 2008; De Hert et al., 2014). Among the second-generation antipsychotics, paliperidone is known to be prolactin increasing, whereas aripiprazole is known to be prolactin sparing (De Hert et al., 2014; Peuskens et al., 2014). In a meta-analysis of sexual dysfunction in psychiatric patients taking antipsychotics, aripiprazole was associated with a relatively low rate of sexual dysfunction of 27%, and the rate was reduced to zero when restricting the analysis to those studies that did not allow concomitant drugs affecting sexual function (Serretti and Chiesa, 2011).
The current findings with AOM 400 are consistent with the STAR study, a naturalistic, open-label, randomized 26-week study evaluating the effectiveness of oral aripiprazole versus standard of care (choice of olanzapine, quetiapine, or risperidone) in patients with schizophrenia treated in a community health-based or a hospital-based outpatient setting (Hanssens et al., 2008). The ASEX was one of several secondary outcome assessments included in the STAR study. Although both treatment groups showed improved patient-reported sexual function, significantly greater improvement was reported with aripiprazole versus standard of care from week 8 through 26 (mean reduction from baseline at week 26: 1.88 vs. 0.92; P=0.032), and this treatment difference was confirmed in a post-hoc analysis that adjusted for clinical improvement scores. Thus, the present findings from QUALIFY with the LAI formulation of aripiprazole confirm and extend those reported in the STAR study in a setting designed to closely replicate clinical practice.
To address the question of whether patients whose sexual dysfunction decreased also experienced an improvement in quality of life or functioning (or vice versa), we assessed patient-level correlations of change from baseline in ASEX total and QLS (total and domain) scores. Modest correlations were observed between changes in the ASEX total score and both the QLS total score and the Intrapsychic Foundations domain score, whereas there appeared to be little or no correlation between change in the ASEX total score and change in Interpersonal Relations or Instrumental Role domain scores. Importantly, patients who shifted from having sexual dysfunction at baseline to no sexual dysfunction at week 28 showed the largest improvements in total QLS scores. These findings cannot indicate causality in either direction but suggest that, in general, an improvement in patients’ overall functioning was accompanied by a decrease in sexual dysfunction, irrespective of treatment assignment.
The mean serum prolactin concentrations decreased from baseline to week 28 in the AOM 400 group but increased from baseline to week 28 in the PP group. This same pattern (decrease with AOM 400, increase with PP) was observed in both men and women and in younger (18–35 years) and older (>35 years) patients. It should be noted that fewer patients in the AOM 400 group than in the PP group had prolactin levels of more than three times the ULN at baseline. At week 28, however, no patients in the AOM 400 group had prolactin levels of more than two or more than three times the ULN, whereas the number and proportion of patients in the PP group with these levels increased from baseline. Consistent with these findings, prolactin-related TEAEs were limited to the PP group.
Although prolactin levels were higher within treatment groups for the patients who had sexual dysfunction compared with those who did not, a clear pattern between serum prolactin concentration and sexual dysfunction was not evident from the present study. A direct, patient-level comparison of prolactin concentration and sexual dysfunction was not performed in this study, given the multidetermined nature of sexual dysfunction. Indeed, the relationship between prolactin concentrations and sexual dysfunction in patients with schizophrenia is unclear because of an abundance of contradictory data (Montalvo et al., 2013; De Hert et al., 2014). However, it is generally accepted that dopamine receptor blockade and associated increased prolactin levels are influencing factors in the emergence of indicators of sexual dysfunction (e.g. strength of sex drive, ease of sexual arousal, satisfaction with orgasms), along with factors inherent to the disease itself, particularly negative symptoms, and various other factors such as concomitant medications (De Hert et al., 2014). The mechanisms underlying the differential effects of AOM 400 and PP on prolactin and rates of sexual dysfunction cannot be determined on the basis of our study. However, given the established facilitatory role of the dopamine system in sexual behavior and arousal (Kruger et al., 2005; De Hert et al., 2014), a plausible mechanism might involve the different actions of aripiprazole and paliperidone at the dopamine D2 receptor (Invega Sustenna (paliperidone palmitate), 2015; Abilify Maintena US (aripiprazole), 2016).
These analyses have strengths and limitations. Although this was not a purely naturalistic study, inclusion/exclusion criteria were designed to increase generalizability, and the use of a patient-rated scale of sexual function (i.e. the ASEX) may be a more realistic indicator of severity of sexual dysfunction compared with reliance on physician prompting or patients spontaneously reporting sexual side effects (Serretti and Chiesa, 2011). Measurement of sexual dysfunction using patient-rated scales may detect changes in sexual functioning that physicians and patients might not be comfortable discussing otherwise. Further, scales with clearly defined ratings such as the ASEX may detect changes that may not otherwise be apparent to the physician. However, some of our findings should be interpreted with caution because some analyses were post-hoc. Moreover, the study used an open-label design, which could result in uncontrolled baseline differences (e.g. concomitant medications that could affect sexual dysfunction and antipsychotic medication taken before the study) and possible bias resulting from random but unblinded treatment assignment in patients scoring the ASEX scores. It should be noted that QLS scores were rated by clinicians blinded to treatment, and prolactin levels were an objective measure. In addition, ASEX total scores, frequencies of sexual dysfunction, and the mean serum prolactin concentrations were similar between AOM 400 and PP groups at baseline. Nevertheless, although this study was not powered to detect between-treatment differences in indicators of sexual functioning, clear patterns were noted and highlight the importance of addressing sexual dysfunction to avoid potential nonadherence and possible negative influence on quality of life.
Conclusion
Analyses of patient-reported sexual dysfunction and prolactin concentrations in QUALIFY, a randomized, open-label, rater-blinded, head-to-head study, showed a lower risk for patient-reported sexual dysfunction and lower prolactin elevation in men and women with schizophrenia treated with AOM 400 versus PP. Lower sexual dysfunction may have contributed in part to higher scores on the QLS and high study completion rates, underscoring the importance in clinical practice of discussing sexual function with patients and the use of self-rating scales. Prospective trials may help to inform the relative incidence of sexual dysfunction among different antipsychotics as well as the management of this important side effect.
Supplementary Material
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website (www.intclinpsychopharm.com).
Acknowledgements
Editorial support for development of this manuscript was provided by Nicole Strangman, PhD, and Vandana Sharma, PhD, at C4 MedSolutions, LLC (Yardley, Pennsylvania, USA), a CHC Group company, and funded by Otsuka Pharmaceutical Development & Commercialization, Inc., and H. Lundbeck A/S.
Conflicts of interest
Dr Potkin has received research support or speakers’ honoraria from or has served as a consultant to Lundbeck, Otsuka, Sunovion, Roche, FORUM, Alkermes, Eli Lilly, Toyama, Eisai, Novartis, and Forest. Dr Naber has received speakers’ honoraria from or has served as a consultant to Lundbeck, Otsuka, AstraZeneca, Janssen/Cilag, Eli Lilly, and Servier. Jean-Yves Loze is an employee of Otsuka Pharmaceutical Europe Ltd. Ross A. Baker and Timothy Peters-Strickland are employees of Otsuka Pharmaceutical Development & Commercialization, Inc. Christophe Sapin, Maud Beillat, and Karina Hansen are employees of Lundbeck SAS. Anna-Greta Nylander, Peter Hertel, and Henrik Steen Andersen are employees of H. Lundbeck A/S. Carlos Forray and Anna Eramo are employees of Lundbeck LLC.
References
- Abilify Maintena US (aripiprazole) (2016). Full prescribing information. Tokyo, Japan: Otsuka Pharmaceutical Co. Ltd. [Google Scholar]
- Baker RA, Eramo A, Carson WH, Perry P, Sanchez R, Zhao J, et al. (2013). Effects of aripiprazole once-monthly vs. placebo on domains of personal and social performance in younger and older patients with schizophrenia. Schizophr Bull 39 (Suppl):S321–S322. [Google Scholar]
- Bushong ME, Nakonezny PA, Byerly MJ. (2013). Subjective quality of life and sexual dysfunction in outpatients with schizophrenia or schizoaffective disorder. J Sex Marital Ther 39:336–346. [DOI] [PubMed] [Google Scholar]
- Correll CU. (2010). From receptor pharmacology to improved outcomes: individualising the selection, dosing, and switching of antipsychotics. Eur Psychiatry 25 (Suppl 2):S12–S21. [DOI] [PubMed] [Google Scholar]
- De Hert M, Detraux J, Peuskens J. (2014). Second-generation and newly approved antipsychotics, serum prolactin levels and sexual dysfunctions: a critical literature review. Expert Opin Drug Saf 13:605–624. [DOI] [PubMed] [Google Scholar]
- Dibonaventura M, Gabriel S, Dupclay L, Gupta S, Kim E. (2012). A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry 12:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley R, Chiliza B, Asmal L, Harvey BH. (2013). The nature of relapse in schizophrenia. BMC Psychiatry 13:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssens L, L’Italien G, Loze JY, Marcus RN, Pans M, Kerselaers W. (2008). The effect of antipsychotic medication on sexual function and serum prolactin levels in community-treated schizophrenic patients: results from the Schizophrenia Trial of Aripiprazole (STAR) study (NCT00237913). BMC Psychiatry 8:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley EW, Boardman J, Craig T. (2010). Sexual problems in schizophrenia: prevalence and characteristics. A cross sectional survey. Soc Psychiatry Psychiatr Epidemiol 45:759–766. [DOI] [PubMed] [Google Scholar]
- Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, et al. (2013). World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry 14:2–44. [DOI] [PubMed] [Google Scholar]
- Heinrichs DW, Hanlon TE, Carpenter WT., Jr (1984). The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull 10:388–398. [DOI] [PubMed] [Google Scholar]
- Invega Sustenna (paliperidone palmitate) (2015). Full prescribing information. Titusville, NJ: Janssen Pharmaceuticals Inc. [Google Scholar]
- Kishimoto T, Nitta M, Borenstein M, Kane JM, Correll CU. (2013). Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry 74:957–965. [DOI] [PubMed] [Google Scholar]
- Kruger TH, Hartmann U, Schedlowski M. (2005). Prolactinergic and dopaminergic mechanisms underlying sexual arousal and orgasm in humans. World J Urol 23:130–138. [DOI] [PubMed] [Google Scholar]
- Lambert M, Conus P, Eide P, Mass R, Karow A, Moritz S, et al. (2004). Impact of present and past antipsychotic side effects on attitude toward typical antipsychotic treatment and adherence. Eur Psychiatry 19:415–422. [DOI] [PubMed] [Google Scholar]
- McGahuey CA, Gelenberg AJ, Laukes CA, Moreno FA, Delgado PL, McKnight KM, et al. (2000). The Arizona Sexual Experience Scale (ASEX): reliability and validity. J Sex Marital Ther 26:25–40. [DOI] [PubMed] [Google Scholar]
- Montalvo I, Ortega L, Lopez X, Sole M, Monseny R, Franch J, et al. (2013). Changes in prolactin levels and sexual function in young psychotic patients after switching from long-acting injectable risperidone to paliperidone palmitate. Int Clin Psychopharmacol 28:46–49. [DOI] [PubMed] [Google Scholar]
- Naber D, Hansen K, Forray C, Baker RA, Sapin C, Beillat M, et al. (2015). Qualify: a randomized head-to-head study of aripiprazole once-monthly and paliperidone palmitate in the treatment of schizophrenia. Schizophr Res 168:498–504. [DOI] [PubMed] [Google Scholar]
- Peuskens J, Pani L, Detraux J, De Hert M. (2014). The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs 28:421–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serretti A, Chiesa A. (2011). A meta-analysis of sexual dysfunction in psychiatric patients taking antipsychotics. Int Clin Psychopharmacol 26:130–140. [DOI] [PubMed] [Google Scholar]
- Young SL, Taylor M, Lawrie SM. (2015). ‘First do no harm.’ A systematic review of the prevalence and management of antipsychotic adverse effects. J Psychopharmacol 29:353–362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website (www.intclinpsychopharm.com).


