Abstract
Objective:
To summarize published evidence on drug interactions between hormonal contraceptives and antiretrovirals.
Design:
Systematic review of the published literature.
Methods:
We searched PubMed, POPLINE, and EMBASE for peer-reviewed publications of studies (in any language) from inception to 21 September 2015. We included studies of women using hormonal contraceptives and antiretrovirals concurrently. Outcomes of interest were effectiveness of either therapy, toxicity, or pharmacokinetics. We used standard abstraction forms to summarize and assess strengths and weaknesses.
Results:
Fifty reports from 46 studies were included. Most antiretrovirals whether used for therapy or prevention, have limited interactions with hormonal contraceptive methods, with the exception of efavirenz. Although depot medroxyprogesterone acetate is not affected, limited data on implants and combined oral contraceptive pills suggest that efavirenz-containing combination antiretroviral therapy may compromise contraceptive effectiveness of these methods. However, implants remain very effective despite such drug interactions. Antiretroviral plasma concentrations and effectiveness are generally not affected by hormonal contraceptives.
Conclusion:
Women taking antiretrovirals, for treatment or prevention, should not be denied access to the full range of hormonal contraceptive options, but should be counseled on the expected rates of unplanned pregnancy associated with all contraceptive methods, in order to make their own informed choices.
Keywords: antiretroviral therapy, contraceptive implant, depot medroxyprogesterone acetate, HIV, hormonal contraception, systematic review
Introduction
Women living with HIV will likely take combination antiretroviral therapy (cART) for much of their lives [1]. Those at high risk for HIV may also use antiretrovirals for preexposure prophylaxis (PrEP). Contraceptive use among women living with HIV or using antiretrovirals for PrEP is critical, as unintended pregnancy and short interpregnancy intervals can be associated with negative health consequences for both mother and infant [2–4]. Decreasing unintended pregnancies also reduces vertical HIV transmission [5]. Hormonal contraceptives are highly used worldwide, including in areas of high HIV prevalence; they are also among the most effective contraceptive methods [6,7]. Evidence-based guidance for hormonal contraceptives use among women using cART or PrEP is needed to ensure access to a full range of the best contraceptive methods, and therefore increase the likelihood of achieving their reproductive life planning goals.
Concurrent use of hormonal contraceptives and antiretrovirals can lead to drug interactions, predominantly due to effects on liver metabolism (Tables 1 and 2). In the liver, cytochrome P450 (CYP) enzymes catalyze many important reactions, with the most significant for contraceptive metabolism being CYP3A4, which is also expressed in the intestines [8,9]. Antiretrovirals include different classes of drug (Table 2), including nonnucleoside reverse transcriptase inhibitors (NNRTIs), nucleoside analogue reverse transcriptase inhibitors or nucleotide analogue reverse transcriptase inhibitor (NRTIs), protease inhibitors, fusion inhibitors, and integrase inhibitors. The NNRTIs and integrase inhibitors are generally not substrates, inhibitors, nor inducers of cytochrome P450 enzymes [10]. In contrast, both protease inhibitors and NNRTIs are metabolized by CYP3A4 and also inhibit or induce this enzyme, resulting in increases or decreases in the concentration of concomitantly administered drugs [10].
Table 1.
Steroids used in currently available contraceptive methods, their liver metabolism, and effects on liver enzymes.
| Contraceptive steroid | Abbreviation | Contraceptive method type (s) | Metabolism |
| Estrogens | |||
| Ethinyl estradiol | EE | COC, patch, ring | Inhibits CYP2C19, CYP3A4, and CYP2B6 |
| Induces UGTs | |||
| Metabolized by CYP3A4 and CYP 2C9 and UGT | |||
| Estradiol cypionate | E2C | CIC | Inhibits CYP2C19, CYP3A4, and CYP2B6 |
| Induces UGTs | |||
| Metabolized by CYP3A4 and CYP 2C9 and UGT | |||
| Estradiol valerate | E2V | COC | Inhibits CYP2C19, CYP3A4, and CYP2B6 |
| Induces UGTs | |||
| Metabolized by CYP3A4 and CYP 2C9 and UGT | |||
| Progestins | |||
| Ethynodiol diacetate | EDA | COCs | Metabolized to norethindrone |
| Dienogest | DNG | COC | Metabolized by CYP3A4 |
| Nomegestrol acetate | NOMAC | COC | Metabolized by CYP3A3, CYP3A4, and CYP2A6 |
| Drospirenone | DRSP | COC | Metabolized only to a minor extent, by CYP3A4 |
| Gestodene | GES | COC | Metabolized by CYP3A4 |
| Norgestrel | NG | COC | Metabolized by CYP3A4 |
| Norgestimate | NGM | COC | Metabolized by CYP3A4 |
| Desogestrel | DSG | COC, POP | Metabolized by CYP2C9 and CYP3A4 |
| Norethindrone, norethindrone acetate | NET | COC, POI, POP | Metabolized by CYP3A4 |
| Norethisterone enanthate | |||
| Levonorgestrel | LNG | COC, implant, IUD, ECP | Metabolized by CYP3A4 |
| Norelgestromin | NGMN | Patch | Metabolized by CYP3A4 |
| Etonogestrel | ENG | Ring, implant | Metabolized by CYP3A4 |
| Medroxyprogesterone acetate | MPA | CIC, POI | Metabolized by CYP3A4 |
Data from USFDA prescribing information, and review articles summarizing published literature.
CIC, combined injectable contraceptives; COC, combined oral contraceptive; CYP, cytochrome P450 isozyme; ECP, emergency contraceptive pill; IUD, intrauterine device; MPA, medroxyprogesterone acetate; POI, progestin-only injectable; POP, progestin-only pill; UGT, uridine diphosphate glucuronosyltransferase.
Table 2.
Antiretrovirals included in the review, their liver metabolism, and effects on liver enzymes.
| Generic name | Liver metabolism |
| NNRTIs | |
| Efavirenz (EFV) | Induces CYP3A4, CYP2B6, and UGTs |
| Metabolized by CYP2B6 and CYP3A | |
| Etravirine (ETR) | Induces CYP3A and inhibits CYP2C9, CYP2C19 |
| Metabolized by CYP3A, CYP2C9, and CYP2C19 | |
| Nevirapine (NVP) | Induces CYP3A and CYP2B6 |
| Metabolized by CYP3A, CYP2B6, and UGTs | |
| Rilpivirine (RPV) | At higher doses (>3× approved 25 mg dose), induces CYP3A4 |
| Metabolized by CYP3A4, CYP2C19, CYP1A2, and CYP2C | |
| Delavirdine (DLV) | Inhibits CYP3A, CYP3A4, CYP2C9, CYP2D6, and CYP2C19 |
| Metabolized by CYP3A and CYP2D6 | |
| Fosdevirine/GSK GSK2248761a | Weak inhibitor of CYP3A4 and CYP2D6 |
| Metabolized by CYP3A4? | |
| NRTIs | |
| Zidovudine (ZDV) or azidothymidine (AZT) | Does not affect liver enzymes |
| Metabolized by UGTs | |
| Abacavir (ABC) | Does not affect liver enzymes |
| Metabolized by alcohol dehydrogenase and UGTs | |
| Tenofovir disoproxil fumarate (TDF) | Does not affect liver enzymes |
| Minimal liver metabolism; mostly eliminated unchanged in urine | |
| Emtricitabine (FTC) | Does not affect liver enzymes |
| Minimal liver metabolism; mostly eliminated unchanged in urine | |
| Didanosine (DDI) | Does not affect liver enzymes |
| Minimal liver metabolism; mostly eliminated unchanged in urine | |
| Lamivudine (3TC) | Does not affect liver enzymes |
| Minimal liver metabolism; mostly eliminated unchanged in urine | |
| Stavudine (d4T) | Does not affect liver enzymes |
| Minimal liver metabolism; mostly eliminated unchanged in urine | |
| PIs | |
| Ritonavir (RTV) | Induces and inhibits CYP3A |
| Induces CYP1A2, CYP2C9, CYP2C19, CYP2B6, and UGTs | |
| Inhibits CYP2D6 | |
| Metabolized by CYP3A, CYP2D6 | |
| Atazanavir (ATV) | Inhibits CYP3A and UGT1A1, and weak inhibitor of CYP2C8 |
| Mostly metabolized by CYP3A4; other pathways include UGTs | |
| Darunavir (DRV) | Co-administered with ritonavir, inhibits CYP3A and CYP2D6 |
| Mostly metabolized by CYP3A | |
| Fosamprenavir (FOS-APV) | Amprenavir, the active metabolite, induces and inhibits CYP3A4 |
| Metabolized by CYP3A4 | |
| Saquinavir (SQV) | Co-administered with ritonavir, inhibits CYP3A |
| Metabolized by CYP3A | |
| Tipranavir (TPV) | Co-administered with ritonavir, inhibits CYP3A and CYP2D6 |
| Induces CYP1A2 and CYP2C19 at steady state | |
| Metabolized by CYP3A | |
| Indinavir (IDV) | Inhibits CYP3A4 |
| Weak inhibitor of CYP2D6 | |
| Metabolized by CYP3A4 | |
| Nelfinavir (NFV) | Inhibits CYP3A4 |
| Metabolized by CYP3A, CYP2C19 | |
| CCR5 inhibitors | |
| Maraviroc (MVC) | Inhibits CYP2D6 at higher doses |
| Metabolized by CYP3A | |
| Vicriviroc (VCV)a | Does not affect liver enzymes |
| Metabolized by CYP3A | |
| Fusion inhibitors | |
| Enfuvirtide (ENF) | Does not affect liver enzymes |
| Catabolized to constituent amino acids | |
| Integrase inhibitors | |
| Dolutegravir (DTG) | Not an inducer or inhibitor of CYP enzymes |
| Metabolized by UGTs and CYP3A | |
| Elvitegravir (EVG) | Inducer of CYP2C9 |
| Metabolized by CYP3A4 | |
| Raltegravir (RAL) | Not an inducer or inhibitor of CYP enzymes |
| Metabolized by UGTs | |
| Pharmacokinetic enhancers | |
| Cobicistat (COBI) | Inhibits CYP3A, CYP2D6 |
| Metabolized by CYP3A | |
Data from prescribing information, http://medicine.iupui.edu/clinpharm/ddis/main-table, and https://aidsinfo.nih.gov/drugs.
CYP, cytochrome P450 isozyme; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside or nucleotide reverse-transcriptase inhibitor; PI, protease inhibitor; UGT, uridine diphosphate glucuronosyltransferase.
aDevelopment halted due to toxicity or lack of efficacy.
Such interactions could lead to decreased contraceptive effectiveness (increasing risk of unintended pregnancy), decreased cART effectiveness (associated with resistance and/or HIV disease progression), decreased efficacy of PrEP (increasing risk of HIV acquisition), or increased antiretroviral or contraceptive toxicity. Based on theoretical concerns and limited data, women using cART are sometimes offered fewer contraceptive choices than their HIV-negative peers [11]. The objective of this review was to systematically examine published evidence on drug interactions between hormonal contraceptives and antiretrovirals, in order to contribute to improved clinical and policy decision-making.
Methods
We followed the PRISMA and MOOSE guidelines for conducting the review and reporting the results [12,13].
We searched PubMed, POPLINE, and EMBASE from database inception to 21 September 2015 for studies of hormonal contraceptive and antiretroviral drug interactions (Supplement 1). We also hand-searched reference lists of published studies, and contacted topic experts.
Study selection
We included published studies of women using hormonal contraceptives (Table 1), including combined oral contraceptives (COCs), progestin-only pills (POPs), emergency contraceptive pills (ECPs), injectables, vaginal rings, patches, or implants. Studies included women who were either HIV-positive, HIV-negative but at risk of HIV, or healthy, who concurrently used cART, PrEP, or single antiretrovirals and hormonal contraceptives. We included studies reporting on women taking oral contraceptives where the type of oral contraceptive was not specified. We excluded studies evaluating women on cART without comparisons by contraceptive use, those evaluating only genital HIV viral load, and those evaluating only hormonal intrauterine devices (IUDs). We also excluded case or case-series reports, cross-sectional studies, reviews, editorials, and letters.
Outcomes of interest were clinical and pharmacokinetic measures of the contraceptive and the antiretroviral. Clinical outcomes included measures of contraceptive, cART, or PrEP effectiveness, and combined toxicity. Contraceptive effectiveness measures of interest were pregnancy or surrogate measures of pregnancy risk, including ovulation, ovarian activity, or cervical mucus. Because no studies reported on true ovulation as documented by ultrasound, we included studies using serum progesterone alone as a marker of presumed ovulation. For cART effectiveness, we included studies that reported markers of HIV disease progression such as CD4+ cell count or HIV viral load, need for change in cART regimen, or death; for PrEP effectiveness, the relevant outcome was HIV prevention. Pharmacokinetic endpoints were plasma drug concentrations over time, as well as the area under the concentration–time curve (AUC), half-life (t1/2), minimum (Cmin; trough) and maximum (Cmax; peak) concentrations, for both contraceptive steroids and antiretrovirals.
Data abstraction and management
After screening and removal of duplicates, we abstracted relevant data from each included report using a predesigned form. Two authors independently reviewed selected manuscripts, with differences resolved by consensus.
We described strengths, weaknesses, and funding source for each included study (Tables 3 and 4) [14–65], but did not do formal quality assessment because no formal evidence grading system exists for pharmacokinetic studies.
Table 3.
Studies reporting clinical measures of contraceptive and/or antiretroviral effectiveness with co-administration of hormonal contraceptives and antiretrovirals.
| Reference; location | Design objective (s) | Number of participants (N); population | Intervention/treatment | Results | Strengths; weaknesses; funding source |
| Scarsi et al. [35]Uganda | Prospective open-label pharmacokinetic studyTo assess the effect of EFV or NVP containing cART on pharmacokinetics of LNG implants in HIV+ women | Sixty HIV+ women >18 years (median age 31), medically eligible for LNG implant, not recently pregnant, and not using other potentially interacting drugs | LNG implantcART-containing NVP or EFV, or no cART | Study stopped early due to unintended pregnancies in EFV group: 3/20 (15%) women in EFV group were pregnant: two at week 48 visit and one at week 42LNG concentrations at last visit before pregnancy (week 36) were 122, 299, and 303 pg/mlZero pregnancies in either no-cART or NVP groupsNo change in CD4+ cell count HIV-RNA in ART groupsNo difference in adverse events | Strengths: clearly described interventions and outcomes; pregnancy confirmed by urine test; HIV+ womenWeaknesses: nonrandomized; not designed to evaluate contraceptive effectiveness; no measurement of progesteroneFunded by government |
| Patel et al. [24]Kenya | Retrospective cohortTo compare pregnancy rates among women using different contraceptives and EFV and NVP containing cart | Twenty-four thousand, five hundred and sixty HIV+ women aged 15–45 years | 2% ENG implant 5% LNG implant 17% DMPA 3% COCs/oral contraceptives 3% IUDs/permanent49% NVP-containing cART 14% EFV-containing 4% LPV/r-containing 32% no cART | 3337 incident pregnancies: overall pregnancy rate of 8.9/100 woman-yearsUnadjusted pregnancy rates by method and antiretroviral type (per 100 woman-years)COCs/oral contraceptives NVP 10.9 EFV 15.4 LPV 15.4 no cART 11.1DMPA NVP 8.4 EFV 9.4 LPV 7.2 no cART 9.8ENG implant NVP 2.3 EFV 5.5 LPV 1.3 no cART 3.4LNG implant NVP 1.9 EFV 7.1 LPV 0 pregnancies no cART 3.3 | Strengths: very large sample size; HIV+; good data on cART use; comparisons made to no contraception; able to separate implant type; pregnancy as main outcomeWeaknesses: retrospective; self-reported pregnancy; high pregnancy rates even with no cART; self-reported contraceptive use; oral contraceptive use included progestin-only pills; no information on contraceptive method insertion/timing; different visit schedule for those on cART and those not on cartFunded by government |
| Pyra et al. [25]Kenya, Uganda | Secondary analysis of HIV prevention trialsTo understand the effect of cART on contraceptive effectiveness | Five thousand, one hundred and fifty-three HIV+ women <50 years; not sterilized and not using IUDs | 9% implants 40% injectables 14% oral contraceptives31% used cART: 23% NVP-containing regimen 5% EFV-containing | Pregnancy rates (per 100 woman-years)Implant no cART 1.4 NVP 0 EFV 6.0Injectable no cART 5.3 NVP 3.3 EFV 3.8oral contraceptives no cART 11.0 NVP 6.4 EFV 12.9 | Strengths: large sample size; prospective data collection; pregnancy diagnosed by urine pregnancy testWeaknesses: self-reported contraceptive and cART use; no information on contraceptive method timing or implant type; few women using EFV-containing regimensFunded by government |
| Callahan et al. [41]Kenya, South Africa | Secondary analysis of HIV prevention trialTo describe contraceptive effectiveness and predictors of pregnancy; and relationship between TDF/FTC and contraceptive effectiveness | Two thousand, one hundred and twenty sexually active women at risk of HIV enrolled in PrEP trial; median age 23; median BMI 23; use of effective contraceptive required at enrollment | ∼50% not using any method or using condoms alone at screening At enrollment: 55% injectables 43% COCs 2% implants, IUDs, or sterilizationTDF/FTC or placebo | Pregnancy rates (per 100 woman-years)Overall 9.6 TDF/FTC 11.4 placebo 7.7Implant 0Injectable: TDF/FTC 2.2 placebo 1.1COCs: TDF/FTC 34.9 placebo 27.9 new users 35.1 prior users 20.7TDF/FTC had no effect on contraceptive effectiveness of COCs in final modelWomen on TDF/FTC and COCs had more nausea or vomiting (8.1%) vs. COC + placebo group (1.4%) | Strengths: large cohort over long period; pregnancy confirmed by urine tests; contraceptive methods dispensed at study clinic; TDF/FTC use verified by blood levels; low loss to follow-upWeaknesses: poor adherence to TDF/FTC; self-reported COC useFunded by government |
| Whiteman et al. [43]Russia | Prospective cohortTo examine the associations between hormonal contraceptive use and HIV progression and cART effectiveness | Seven hundred and nine sexually active HIV+ women; age 16–45 years; not pregnant or breast-feeding; no hysterectomy, infertility, or recent hormonal contraceptive or IUD use | At enrollment: 183 COCs 87 DMPA 156 nonhormonal methodscART-containing either PI or NNRTI | 545 not on cART 161 on cARTThree women discontinued due to unrecognized pregnancy at enrollmentFive pregnancies during study but contraceptive method or cART use not reportedNo significant change in CD4+ cell count or viral suppression between COC or DMPA and nonhormonal usersTwo deaths; no report of which contraceptive method used | Strengths: cART and contraceptive use verified; pregnancy by urine test or medical records; death verified by recordsWeaknesses: very high loss to follow-up; unclear if women becoming pregnant were using contraceptives; limited information on cART regimenFunded by government |
| Song et al. [42]USA | Randomized; double-blind; placebo-controlled; crossover studyTo examine the effect of DTG on pharmacokinetics and pharmacodynamics of EE and NGMN in COC users | Sixteen healthy women; age 18–40; mean age 31 years; 94% white; BMI 19–30 (mean 24.7); normal liver function; women had to use a second nonhormonal contraceptive | COC-containing NGMDTG 50 mg or placebo twice daily for 11 days | Fifteen women completed studySerum pregnancy test 7–14 days after last dose; zero pregnanciesNo difference in LH, FSHProgesterone data not reportedNo participants discontinued due to adverse events; no grade 3 or 4 or serious adverse events | Strengths: randomized; clearly described population and methods; progesterone measured several times during cycleWeaknesses: small sample size; healthy women; single antiretroviral; single cycle; ovulation data not reported and unclear from figureFunded by industry |
| Kasonde et al. [51]Botswana | Secondary analysis of RCTTo investigate the effect of TDF and the interaction of TDF and hormonal contraception on BMD among HIV-uninfected African men and women | One hundred and fourteen sexually active women at risk of HIV, from HIV prevention trial; 18–39 years; nonpregnant and nonbreast-feeding | Injectable or implant oral contraceptivesTDF TDF/FTC placebo | Data not separated for women vs. menBone mineral density with DEXA at distal and ultradistal forearm; lumbar spine; hip3/114 (2.6%) had a low baseline bone mineral density;Changes in bone mineral density for women on either oral or injectable vs. no contraception not significant except for a positive effect of oral contraceptives on spine bone mineral density for women on TDF/FTC | Strengths: used DEXA to measure bone mineral densityWeaknesses: some results not separated by gender or HIV status; no mention of pregnancy or lactation or other medication use; few data on contraceptive use; low adherence to TDF/FTC; unclear if injectable group also included implant usersFunded by government |
| Luque et al. [39]USA | Open-label; multicenter; nonrandomized; steady-state pharmacokinetic studyTo assess the effect of LPV/r on DMPA pharmacokinetics and vice versa; and to assess safety and tolerance of DMPA given concurrent with LPV/r | Twenty nonpregnant; premenopausal HIV+ women; on stable LPV/r for at least 14 days; no DMPA within 180 daysMedian BMI 28 | DMPAcART-containing LPV/r | No pregnanciesProgesterone >5 ng/ml considered presumptive ovulation; zero ovulations notedNo serious adverse events; one grade 3 adverse event (prolonged bleeding).No changes in CD4+ cell count or HIV RNA through week 8At week 12–3/24 women in LPV/r group had detectable HIV RNA; two due to antiretroviral noncompliance | Strengths: clearly described population and methods; HIV+ women; assessed ovulation at several time pointsWeaknesses: small sample size; short durationFunded by government |
| Todd et al. [59]Kenya | Secondary analysis of PrEP HIV prevention trialTo examine PK of LNG with concurrent use of TDF-FTC as PrEP | Twenty-nine sexually active women at risk of HIV, who elected to received LNG implant; ages 18–35TDF/FTC group: N = 17Placebo group: N = 12Mean BMI 22.6 | LNG implantTDF/FTC or placebo | Follow-up 36 weeksNo pregnancies and one implant discontinuation at 7 months, with reason for discontinuation not recorded | Strengths: TDF levels measured to assess for adherenceWeaknesses: Small sample size; percentage retention not statedFunded by government |
| Heffron et al. [50]Kenya, Uganda | Secondary analysis of PrEP RCTTo evaluate TDF/FTC and TDF efficacy among women using DMPA compared with nonhormonal users | One thousand, seven hundred and eighty-five women at risk of HIV; median age 33 years | At enrollment: 486 DMPA usersIn follow-up: additional 415 DMPA usersTDF/FTC, TDF, or placebo | Efficacy of PrEP not different for women using DMPA compared with women using no hormonal contraceptionAmong DMPA users: efficacy 64.7% (PrEP vs. placebo) Among nonhormonal users: efficacy 75.5% (PrEP vs. placebo)P interaction = 0.65No data about pregnancy reported | Strengths: large sample size; high adherenceWeaknesses: secondary analysis; self-reported contraceptive use; adjustment for unprotected sex but unclear whether or how condom use was collectedFunded by government |
| Day et al. [44]Kenya | Prospective cohortTo test the hypothesis that DMPA would be associated with increased detection of HIV-1 RNA in women initiating and continuing cart | One hundred and two HIV+ women starting cART; median age 36; median CD4+ cell count 122 cells/μl | At baseline: 18 (18%) DMPA 5 (5%) implants; 5 (5%) oral contraceptivescART-containing ZDV; d4T; 3TC; and NVP | Seventy two completed ≥33 months follow-upDMPA did not increase plasma HIV RNA | Strengths: long follow-up; adjusted for antiretroviral adherence and CD4+ cell countWeaknesses: self-reported contraceptive and antiretroviral use; large loss to follow-up; 14% changed cART regimen; small number of women using DMPAFunded by government |
| Atrio et al. [16], Atrio et al. [56], Dubois et al. [17]USA | Nonrandomized clinical trialTo evaluate the effect of protease inhibitors on cervical mucus of POP users | Thirty-five HIV+ women, age 18–44 years; no changes in medications; no recent hormonal contraceptives; no immunocompromise; no liver or renal disease; normal ovulation; BMI <40; >30 days postpartum | POPs containing NETIn PI group: 11 taking cART containing ATV (10/11 on ATV/r); 3 DRV/r; 2 LPV/rIn control group: four women not taking cART; 13 taking combinations including ETR, RPV, TDF, FTC, and RAL | Baseline mucus scores similarCervical mucus scores in PI and non-PI groups similar after POPs: median score 3.5 for PI group and four for controls score <10 (unfavorable to sperm penetration): 81% of study group; 60% of comparison group | Strengths: prospective design; blinded assessmentsWeaknesses: no baseline of periovulatory mucus for all women; small sample size; nonrandomized; cART use self-reported; results not separated by antiretroviralFunded by government |
| Murnane et al. [40]Kenya, Uganda | Secondary analysis of PrEP HIV prevention trialTo assess the impact of TDF and TDF/FTC on hormonal contraceptive effectiveness | One thousand, seven hundred and eighty-five sexually active women at risk of HIV, enrolled in HIV prevention trial; median age 33 | At enrollment: 27% injectablesDuring follow-up: 14% initiated oral contraceptives 20% injectables 6% implantsTDF/FTC; TDF; or placebo | Two hundred and eighty eight pregnancies in 267 women (179 TDF or TDF/FTC and 88 placebo); no difference across armsPregnancy rates per 100 women-yearsOral contraceptives TDF or TDF/FTC 17.7 placebo 10.0; no significant differenceInjectablesTDF or TDF/FTC 5.1 placebo 5.3Implants <1% per year in both arms | Strengths: large sample size; verified contraceptive use; good adherence to study product;Weaknesses: nonrandomized; oral contraceptive, implant, and injectable type not specified; contraceptive use and adherence self-reportedFunded by government |
| Perry et al. [36]Switzerland | Retrospective cohortTo evaluate risk of pregnancy in implant users using cART | Five hundred and seventy HIV+ women who had LNG implant | LNG implants347 (61%) using cART at implant insertion: 208 on NVP-containing regimens; 121 on EFV-containing regimens; 18 on LPV/r-containing regimens | Sixteen pregnancies in 570 women15/121 (12.4%) of women on EFV became pregnant; mean time between implant insertion and pregnancy was 16.4 monthsNo women conceived while using NVP or LPV/r, one pregnancy occurred before cART was startedAge, condom use, inserting provider, CD4+ cell count had no association with pregnancy | Strengths: large sample size; HIV+; verified contraceptive use, implant insertion date knownWeaknesses: retrospective study; self-reported cART use; 3/16 women who became pregnant had received antituberculosis treatment; no BMI informationFunded by hospital |
| Vieira et al. [34]Brazil | Prospective cohort with pharmacokinetic analysisTo evaluate the effects of EFV-containing or LPV/r-containing cART on ENG implant pharmacokinetics and to determine the impact of cART on luteal activity | Forty five HIV+ women with regular menstrual cycles, BMI 18–30; excluded women with recent pregnancy or hormonal contraceptive use, acute infections or other opportunistic illnesses, drug or alcohol addiction, use of other potentially interacting drug, chronic diarrhea or malabsorption or noncompliance with cART | ENG implantLPV/r-containing or EFV-containing cART, or no cART | Progesterone measured every 2 weeks; >4.7 ng/ml considered presumptive ovulation; progesterone >3 ng/ml considered luteal activity2.8% of the P samples in EFV group had presumptive ovulation; and 5% had luteal activityNo women in LPV/r or no cART groups had evidence of any luteal activityVL <50 copies/ml in LPV/r and EFV groups | Strengths: clearly described population and methods; frequent progesterone measurementsWeaknesses: Small sample size; nonrandomizedFunded by government |
| Hubacher et al. [45]Kenya | Prospective cohortTo examine how concurrent use of hormonal contraceptive implants and cART might lessen the effectiveness of both medications | Ninty-three sexually active HIV+ nonpregnant women, age 18–44 years; CD4+ cell count ≥ 200 cells/μl; without recent hormonal contraceptive or rifampin use, desire for pregnancy, or contraindications to implant use | LNG implant or nonhormonal contraceptionNVP-containing cart | LNG implant users (60 recruited; 48 analyzed) matched to women not using hormonal contraception (36 recruited; 33 analyzed)CD4+ cell counts for both groups rose slightly but did not differ between groupsNo participants died; six participants (two implant users, four controls) diagnosed with opportunistic infectionsZero pregnancies in implant users | Strengths: large sample size; implant inserted at study siteWeaknesses: method of pregnancy ascertainment not stated; observational study; no non-cART users; six women (10%) of implant group had implant removed within 12 months; cART self-reportedFunded by government |
| Landolt et al. [15,19]Thailand | Prospective; open-label; nonrandomized steady-state clinical trialTo assess risk of ovulation and safety in women taking COCs with cart | Forty-nine HIV+ nonpregnant, nonlactating women; 18–45 years, with regular menses, on EFV-containing or NVP-containing cART; nonsmoking, no recent injectable contraceptive use, no contraindications to COCsFourteen HIV− controls | COC containing DSG for two cyclesNVP-containing or EFV-containing cART | Forty-eight completed study, 15 discontinued, including 13 due to protocol adherence issuesOvulation by serum progesterone:NVP group: All women had progesterone <1.0 ng/mlEFV group: three women had progesterone >3.0 ng/mlMore women in EFV group reported adverse events than NVP group | Strengths: prospective clinical trial; HIV+ womenWeaknesses: nonrandomized; small sample size; single progesterone measurement; no adherence information; high dropout rateFunded by government |
| Nanda et al. [18]Uganda, South Africa | Prospective open-label nonrandomized clinical trialTo compare ovulation and pregnancy rates between two groups of women: those taking COCs concurrently with NVP-containing cART and those taking COCs alone | Four hundred and two sexually active HIV+ women 18–35 years, with regular menses and no contraindications to COC use; median age 29 and median CD4+ cell count 486 cells/μl | COC-containing NGcART group included women on NVP-containing cART; n = 196Control group included women not yet eligible for cART; n = 206 | Ovulation by serum progesterone (>3 ng/ml)cART group: 43/168 (26%) ovulated in cycle 1; 30/163 (18%) in cycle 2; 18/163 (11%) in both cyclesNon-cART group: 26/168 (15%) ovulated in cycle 1; 31/165 (19%) in cycle 2; and 20/165 (12%) in both cyclesNo significant difference in ovulation rates between groupsPregnancy rates (per 100 woman-years): 10. in cART group and 10.1 in non-cART groupAdverse events similar; five serious adverse events, all in non-cART group | Strengths: prospective clinical trial; COCs and antiretrovirals at steady state; multiple progesterone measurements; large sample size; HIV+ women; information on COC adherenceWeaknesses: nonrandomized, self-reported cART and COC adherence; no pharmacokinetic measuresFunded by government |
| Crauwels et al. [27]UK | Open-label, three period pharmacokinetic studyTo evaluate the effect of RPV on COC pharmacokinetics and vice versa, and assess effects on sex hormones and safety of co-administration | Eighteen healthy nonsmoking women, 18–45 years; BMI 18–30 (median 24.6); 67% white; excluded pregnant, breast-feeding, or menopausal women, those with history of drug/alcohol abuse, skin disease, or any significant medical problems; use of concomitant medication | COC-containing NETIn third cycle; RPV 25 mg daily days 1–15 | Thirteen completed trialProgesterone; LH; and FSH on day 1 and 14; 0 ovulations; no effect on FSH; LHNo difference in adverse events | Strengths: clearly described population and methods; directly observed therapyWeaknesses: healthy women; short-term dosing; single antiretroviral; high discontinuation rateFunded by industry |
| Polis et al. [46]Uganda | Retrospective cohortTo assess the effect of injectable contraceptive use on cART effectiveness and adherence to cart | Four hundred and eighteen pregnant and nonpregnant sexually active HIV+ women initiating cART, without tuberculosis, with information on baseline viral load | 51/418 (12%) used unspecified injectables at baselinecART (not specified) | Failure defined as failure to achieve virologic suppression at 12 months, switch to second-line therapy, or death within 12 months of cART initiationNo difference in treatment failure at 12 months between injectable users and nonusers (11 vs. 12%)Injectables not associated with cART failure in sensitivity analysis restricted to women with complete information who never used pills or implantsNo differences in cART adherence at 6 and 12 months for injectable users and nonusers | Strengths: large sample sizeWeaknesses: retrospective; observational database analysis; self-reported contraceptive use; inconsistent injectable use over time; type of injectable and cART not specifiedFunded by government |
| Carten et al. [52]USA | Open-label two period pharmacokinetic studyTo determine the effect of EFV on the pharmacokinetics of LNG EC and vice versa, and assess safety | Twenty-four healthy women; 18–45; normal BMI (mean BMI 27) with no recent use of hormonal contraceptives or other interacting medications; women were either sterilized or used two nonhormonal contraceptive methods | LNG ECPs (0.75 mg) at 0 and 12 h on days 0 and 17EFV 600 mg 72 h after day 0, for 14 days | Twenty-one women completed studyFollow-up pregnancy test at visit 3 (study day not specified)Pregnancy test results not givenNo grade 3 or 4 treatment-related toxicities. | Strengths: clearly described population and methodsWeaknesses: small sample size; healthy women; single antiretroviral; ovulation not tested; single follow-up pregnancy test but timing and results not givenFunded by industry |
| Piscitelli et al. [29]UK | Randomized crossover pharmacokinetic studyTo examine if GSK2248761 (fosdevirine) interacts with CYP450 substrates, including COCs | Ten healthy women, without hepatitis and not taking any medications | COC containing DRSPGSK228761 200 mg or placebo days 1–11 | No differences in LH/FSHNo serious adverse events or treatment due to adverse events, and no significant laboratory abnormalities | Strengths: randomizedWeaknesses: short term administration; healthy women; single antiretroviral; small sample size; very few study details provided; trials of fosdevirine on hold due to other safety concerns; study terminated early for unknown reasonsFunded by industry |
| Schwartz et al. [21]South Africa | Prospective cohortTo determine the incidence of unplanned pregnancies in HIV+ women on cART; to assess contraceptive use and associations with unplanned pregnancy | Eight hundred and fifty HIV+ women; ages 18–35; on/starting cART; not pregnant, recently pregnant, or breast-feeding; no known infertility | 243 (29%) using HC: Injectables (DMPA + NET-EN; 192); COCs (46); implants (type not stated 4); IUD (1)Multiple antiretrovirals: 52% NVP-containing regimens; 42% EFV-containing | One hundred and seventy pregnancies in 161 women; 105 (62%) unplanned (incidence rate: 16.1/100 woman-yearsNine of 105 unplanned pregnancies were potentially hormonal contraceptive failures; seven on NVP and one on EFV; incidence of unplanned pregnancy 4.4 per 100 woman-yearsOne failure not related to adherence in COC user (5.8/100 woman-years)Seven injectable failures (two DMPA; five NET-EN; (incidence rate 4.2/100 woman-years); 5/7 in last 2 weeks of injection cycle | Strengths: pregnancy by urine hCG; cART confirmed by pharmacy records; contraceptive failures confirmed through records reviewWeaknesses: observational study; contraceptive use self-reported; reported only at baseline; did not report which HC failures were using which antiretroviralFunded by government |
| Kreitchmann et al. [33]Brazil | Prospective cohortTo evaluate the safety and efficacy of ENG implants among HIV+ women | Seventy nine HIV+ women with comorbidities and poor adherence to other contraceptive methods; mean age 29; mean weight 59 kg (range 42–104) | ENG implantAt baseline: 47 used cART; nine began cART during follow-up (PI containing-regimen 31; NNRTI-containing 25) | Women followed up every 6 months over 3 years and 0 pregnancies notedFour women had elevated liver enzymes: all coinfected with hepatitis CENG implant removed in five women: two had tubal ligation; one hysterectomy; two because of excessive bleedingMenstrual irregularity most common adverse event; two unrelated deaths: one of AIDS, and one of cardiac arrest (baseline cardiomyopathy) | Strengths: verified contraceptive use; prospective study; HIV+ womenWeaknesses: self-reported pregnancy; did not specify cART typeFunding source not specified |
| Johnson et al. [47]USA | Retrospective cohortTo examine how use of hormonal contraceptives affects response to cART | One hundred and seven HIV+ adolescent women reporting consistent cART ; median age 17 years | Seventy-two percent oral contraceptivesTwenty eight percent DMPAcART regimens included ZDV and ZDV/3TC | No difference in CD4+ cell counts over timeViral load decreased over time in hormonal users and nonusers; but an interaction was noted: decrease in viral load was slightly slower (1.2 × 10−3; 95% CI: 6.2 × 10−5 to 2.4 × 10−3 copies/ml log viral load per day; P = 0.03) among hormonal contraceptive users | Strengths: HIV+ womenWeaknesses: retrospective; changes in viral load of questionable clinical significance; contraceptive use self-reported; not separate type of contraceptivesFunded by government |
| Stuart et al. [30]Malawi | Prospective nonrandomized clinical trialTo assess the feasibility of measuring anovulation in a pharmacokinetic study of COCs and antiretrovirals | Nine women ages 21–35 (3/group) with similar age and BMI: group 1 included HIV+ women on cART; group 2 included HIV+ women not on cART; and group 3 included HIV− women | COC with NGNVP-containing cART or no cART | Ovulation by serum progesterone (>3.0 ng/ml) on day 14; 0 ovulations | Strengths: Clearly described population and methods; valid assays; included HIV+ womenWeaknesses: very small sample size; nonrandomized; progesterone measured only onceFunding source not reported |
| Sevinsky et al. [26]USA | Open-label 3-period pharmacokinetic studyTo examine effect of EFV on pharmacokinetics of EE and NGMN and vice versa | Twenty-eight healthy women; 18–45 years (median 26); BMI 20–32 (median 25); on COCs for at least 2 months and no baseline safety issues or breakthrough bleeding | COC-containing NGMEFV 600 mg daily for 14 days during third cycle | Nineteen women completed studyPregnancy test day 108; results not reportedProgesterone levels similar and all <1.25 ng/mlNo discontinuations for adverse events; three severe adverse events: headache; anhedonia; and depression; one serious adverse event – suicide attempt after treatment in a woman with prior undisclosed depression | Strengths: clearly described population and methodsWeaknesses: small sample size; single progesterone measurement per cycle; pregnancy testing results not reported; healthy women; single antiretroviral; high discontinuation rateFunded by industry |
| Vogler et al. [37]USA | Open-label; 4-week; nonrandomized; comparative clinical trialTo evaluate pharmacokinetics interactions between LPV/r and contraceptive patch and COCs | Thirty two nonpregnant; premenopausal HIV+ women >13 years either on stable LPV/r-containing regimens for at least 14 days (n = 8) or not on cART or taking NRTIs only (n = 24); nonsmoking; median weight 72 kg; no recent use of injectables or COCs; <198 lb; not taking enzyme inducers | EE/NGMN contraceptive patchSingle-dose COC containing NETLPV/r (400 mg/100 mg twice a day) or no cART/NRTIs only | Zero ovulations by serum progesteroneHIV-1 RNA and CD4+ cell counts measured at 30–45 days prior to entry; at entry and 4 weeksMedian CD4+ ↑15% (LPV group) with maintenance of viral suppressionTreatment arm: single possibly related grade-3 adverse event (generalized aches and pains); 3 patients with 7 w/grade 1–2 adverse events; 14 control pts w/grade 1–2 adverse events; No significant changes in weight; chemical or lab values | Strengths: clearly described population and methods; HIV+Weaknesses: study stopped due to slow accrual; small sample size; single progesterone measurement; single dose COC; high loss to follow-up; not randomisedFunded by government |
| Myer et al. [20]Cote d’Ivoire, Kenya, Rwanda, South Africa, Uganda, Zambia | Secondary analysis of MTCT-Plus Initiative cohortTo examine whether improved health from cART affects pregnancy rates | Four thousand, five hundred and thirty-one HIV+ women who had received PMTCT services; median age = 27 | At baseline 1; 755 (39%) reported contraceptive use: Injectables (15%) oral contraceptives (4%) IUDs (1%)Among women who started cART; 90% used NVP-containing regimens | Five hundred and eighty-nine pregnancies in 4531 women (7.8/100 woman-years)Higher pregnancy rate in women taking cART (9.0/100 woman-years) compared with women not on cART (6.5/100 woman-years)In injectable users pregnancy rates (per 100 woman-years) 1.1 before cART and 2.0 after cartIn oral contraceptive users pregnancy rates (per 100 woman-years) 3.1 before cART and 5.4 after cart | Strengths: large sample size; prospective data collection; long follow-up; HIV+Weaknesses: unclear whether women were actually using contraceptives when pregnancy occurred; study not designed to look at pregnancy; self-reported pregnancy and contraceptive useFunded by private foundation |
| Schöller-Gyüre et al. [28]USA | Open-label three period pharmacokinetic studyTo assess the effect of ETR on COC pharmacokinetics | Twenty-four healthy women; 18–45 years (median 24); BMI 18–30; 97% white; nonsmoking; not pregnant or breast-feeding; no contraindications to hormonal contraceptives; not taking enzyme inducers | COC-containing NETIn cycle 3; 200 mg ETR twice daily from day 1–15 | Serum progesterone; LH; FSH on days 1 and 14 of cycles 2 and 3Zero ovulations; no difference in LH; FSHNine adverse events led to discontinuation: seven grade 2 rashes; one grade 2 pyrexia | Strengths: clearly described population and methods; valid assaysWeaknesses: healthy women; small sample size; few progesterone measurements; single antiretroviralFunded by industry |
| Nanda et al. [32]Brazil | Nonrandomized, controlled, open-label pharmacokinetic studyTo evaluate the effect of EFV-containing cART on pharmacokinetics of MPA, and to evaluate suppression of ovulation and bleeding patterns | Thirty-three HIV+ women aged 19–40 years; with regular menstrual cycles and BMI 18–30 kg/m2; not recently pregnant or breast-feeding | DMPAEFV-containing cART vs. no cart | Progesterone measured every 2 weeks; >3 ng/ml considered evidence of ovulation: one ovulation in non-cART group at 12 weeksNo differences in bleeding patterns between groupsNo serious adverse events | Strengths: Clearly described population and methods; HIV+ women; frequent progesterone measurementsWeaknesses: small sample size, no progesterone levels beyond 12 weeksFunded by government |
| Sekar et al. [38]Belgium | Randomized crossover pharmacokinetic studyTo investigate the effect of DRV/r on COC pharmacokinetics and to examine safety | Twenty-two nonsmoking healthy women, 18–45 years; BMI 18–30; using a second nonhormonal contraceptive method | COC-containing NETDRV/r 600/100 mg twice daily days 1–14 or no treatment | Progesterone; LH; FSH on days 1 and 14 of each cycleNo significant changes in LH or FSH with co-administrationNo serious adverse events or grade 3 or 4 adverse events; five women discontinued due to grade 2 cutaneous reactions with combined treatment | Strengths: clearly described population and methods; randomizedWeaknesses: 17-OH progesterone levels presented instead of progesterone levels; small sample size; healthy women; single antiretroviral; high discontinuation rateFunded by industry |
| Cohn et al., Watts et al. [14,31]USA | Nonrandomized open-label pharmacokinetic studyTo evaluate the effect of various antiretrovirals on pharmacokinetics of MPA and vice versa; and to determine effects on suppression of ovulation and adverse events | Seventy-two HIV+ nonpregnant; premenopausal women, 22–46 years (median 35); median weight 71 kg; with no recent potentially interacting drugs; women required to use second nonhormonal method of contraception | DMPASixteen on no PI or NNRTI (control) 21 on nelfinavir and NRTIs 17 on EFV and NRTIs 16 on NVP and NRTIs | Zero pregnanciesProgesterone every 2 weeks; >5 ng/ml considered presumed ovulation; zero ovulationsNo changes in median CD4+ cell count or proportion with viral load <400 copies/mlNo grade 3 or 4 related adverse events. | Strengths: Clearly described population and methods; HIV+ women; frequent progesterone measurementsWeaknesses: nonrandomized; small sample size; progesterone only measure up to 12 weeksFunded by government |
| Danel et al. [22]Cote d’Ivoire | Prospective cohortTo evaluate the efficacy and tolerance of ZDV; 3TC; and EFV in West African women and men | Five hundred and forty-eight HIV+ women <18 years (median age 23), naive to cART, CD4+ cell count 150–350 cells/ml | Approx. 80 reported using contraceptives: 65% ‘intramuscular progesterone’ 35% COCsEFV-containing cart | Seven pregnancies; incidence 2.6/100 person-years (95% CI 0.67–4.51) | Strengths: HIV+ women; large sample size; prospectiveWeaknesses: study not designed to measure pregnancy; unclear whether women were actually using contraceptives when conception occurred; did not separate injectables from oral contraceptivesFunded by government |
| Chu et al. [48]USA | Retrospective cohortTo determine the effects of hormonal contraceptives on response to cart | Seventy-seven hormonal contraceptive users matched with 77 nonusers from the Women's Interagency HIV Study; all women took cART | Seventy-seven hormonal contraceptive users: 64% COC 27% DMPA 4% LNG implant 4% COCs and DMPASeventy-seven women not using hormonal contraceptivescART containing NRTIs + PI or NNRTI; or NRTIs alone | By fourth visit; 65% stopped hormonal contraceptivesNo significant difference in CD4+ cell count and HIV viral load by hormone use after antiretroviral initiation except in viral load at the third visit after initiationTime-dependent hormonal contraceptive use not associated with changes in CD4+ cell count or undetectable viral load after cART initiation | Strengths: matched comparison group, HIV+ womenWeaknesses: retrospective; low overall use of hormonal methods contraceptive information obtained retrospectively and mainly at baseline (before cART); did not separate COCs from progestin-only methods; high method discontinuation rateFunded by government |
| Clark and Theall [23]USA | Retrospective cohortTo determine the frequency of oral contraceptive failure among HIV+ women on cART | Two thousand and fifty-three women | 86 (4.2%) taking oral contraceptivescART containing various antiretrovirals | Forty one women were pregnant with records showing hormonal contraceptive during the same 6-month periodEleven of 41 women apparently conceived while using hormonal contraceptives (DMPA = 1 or oral contraceptives = 10)Women on NFV-containing regimens more likely to experience oral contraceptive failure | Strengths: large sample size, HIV+ womenWeaknesses: retrospective chart review; unclear whether women actually taking contraceptives at time of pregnancy; difficult to interpret findings; no data on cART use or adherence; no details about pregnancy; very small numbers of cases; timing of HC and cART use not clearFunding source not specified |
| Frohlich et al. [64]Germany | Open-label single period pharmacokinetic studyTo investigate the influence of COCs on SQV pharmacokinetic and to assess the potential contribution of CYP3A4 and P-gp | Eight healthy nonsmoking nonpregnant women with regular menses; mean age 24 years and mean BMI 21; not using any potentially interacting drugs | COC containing GES days 4–25 | Estradiol; progesterone; LH; FSH on days 1 and 22COC use resulted in decreased plasma estradiol levels; progesterone; FSH; and LH; and increased SHBG | Strengths: Clearly described population and methodsWeaknesses: did not evaluate the effect of SQV use on ovarian suppression; not randomized; very small sample size; short course of COCs; healthy women; single antiretroviral only given twiceFunded by government |
| Cejtin et al. [49]USA | Retrospective cohortTo compare HIV-1-RNA and CD4+ cell counts from users and nonusers of hormonal contraception | Premenopausal HIV+ women <50 years of age | One hundred and seventy-seven users of hormonal contraception: 87 oral contraceptives 77 DMPA 13 LNG implantOne thousand, five hundred and forty-four nonusers of hormones40.7% of users and 32.9% of nonusers on cART; most on monotherapy | Hormonal contraceptive use not associated with significant changes in viral load or CD4+ cell count | Strengths: Some longitudinal data and large study sizeWeaknesses: no disaggregation by type of cART or contraceptive method; difficult to interpret findings; self-reported contraceptive useFunded by government |
| Mildvan et al. [53]USA | Open-label, single dose, two period pharmacokinetic studyTo determine the effects of NVP on COC pharmacokinetics and vice versa | Fourteen HIV+ nonpregnant, nonlactating, nonsmoking women; age 18–65 (mean age 37); viral load <400; CD4+ cell count >100; normal renal and hepatic function; no RTV or DLV use | Single dose of COC containing NET on cycle days 1 and 30NVP 200-mg daily on days 2–15; then 200-mg twice daily days 16–29; single dose on day 30cART regimens included IDV; NFV; SQV/RTV | Ten women completed the studyPregnancy test on day 30HIV RNA and T cells measured at screening, day 0, day 30No change in HIV RNA concentration or CD4+ cell count on day 30 compared with baselineEight of 14 had at least one adverse event; no adverse event considered related to COC | Strengths: HIV+ women, well described populationWeaknesses: small study; only single dose COC; NVP added to current cART regiment; included postmenopausal womenFunded by industry |
Abbreviations for antiretrovirals and contraceptive steroids defined in Tables 1 and 2.
cART, combination antiretroviral therapy; COC, combined oral contraceptive; DEXA, dual energy x-ray absorptiometry; DMPA, depot medroxyprogesterone acetate; ECP, emergency contraceptive pill; FSH, follicle stimulation hormone; HC, hormonal contraceptive; LH, luteinizing hormone; MPA, medroxyprogesterone acetate; NET-EN, norethisterone enanthate; POP, progestin-only pill; PrEP, preexposure prophylaxis; SHBG, sex hormone binding globulin.
Table 4.
Studies reporting pharmacokinetic outcomes with co-administration of hormonal contraceptives and antiretrovirals.
| Reference; location | Design objective (s) | Number of participants (N); Population | Intervention/treatment | Results | Strengths, weaknesses; funding |
| Scarsi et al. [35]Uganda | Prospective open-label pharmacokinetic studyTo assess the effect of EFV-containing or NVP-containing cART on pharmacokinetics of LNG implants in women living with HIV | Sixty HIV+ women >18 years (median age 31), medically eligible for LNG implant, not recently pregnant, and not using other potentially interacting drugs | LNG implantcART-containing NVP or EFV or no cART | Primary endpoint: LNG levels at 24 weeks (compared with no cART group) EFV group ↓47% NVP group ↑35%Secondary: LNG levels at 48 weeks EFV group ↓57% NVP group ↑14%EFV and NVP levels not affected by LNG | Strengths: clearly described interventions and outcomes; valid assays; HIV+ womenWeaknesses: nonrandomized; open label; LNG levels much higher than previously seen in other studiesFunded by government |
| Song et al. [42]USA | Randomized; double-blind; placebo-controlled; crossover studyTo examine the effect of DTG on pharmacokinetic and pharmacodynamic of EE and NGMN in COC users | Sixteen healthy women; age 18–40; mean age 31 years; 94% white; BMI 19–30 (mean 24.7); normal liver function; women had to use a second nonhormonal contraceptive | COC-containing NGMDTG 50 mg or placebo twice daily for 11 days | EE levels unchanged NGMN AUC; Cmin; Cmax unchangedDTG levels similar to historical controls | Strengths: randomized; clearly described methods; valid assaysWeaknesses: healthy women only; single cycleFunded by industry |
| Luque et al. [39]USA | Open-label nonrandomized pharmacokinetic studyTo assess the effect of LPV/r on DMPA pharmacokinetics and vice versa; and to assess safety and tolerability | Twenty-four nonpregnant premenopausal HIV+ women with no recent DMPA; median BMI 28; HIV RNA <400 copies/ml | DMPA; LPV/r containing cARTcART-containing LPV/r | MPA levels compared with historical controls: AUC↑ 46% Cmax ↑66%No changes in LPV or RTV levels after DMPA | Strengths: clearly described population and methods; valid assays; HIV+ womenWeaknesses: small sample size; historical controls; levels only assessed through 12 weeksFunded by government |
| Todd et al. [59]Kenya | Secondary analysis of PrEP HIV prevention trialTo examine pharmacokinetic of LNG with concurrent use of TDF/FTC as PrEP | Twenty-nine healthy women who elected to received LNG implant; ages 18–35TDF/FTC group: N = 17Placebo group: N = 12Mean BMI 22.6 | LNG implantTDF/FTC or placebo | Follow-up 36 weeksLNG levels all above 400 pg/ml; mean LNG levels 469–660 pg/mlLNG levels lower for women randomized to the TDF/FTC arm, but in multivariable analysis TDF/FTC use not associated with changes in LNG levels compared with placebo | Strengths: TDF levels measured to assess for adherenceWeaknesses: Small sample size; percentage retention not stated; LNG concentration measures were missing for 42 time pointsFunded by government |
| Landolt et al. [15,19]Thailand | Open-label; nonrandomized clinical trialTo evaluate EE and ENG levels in women taking EFV-containing and NVP-containing cART and COCs | Forty-eight HIV+ nonpregnant, nonlactating women; 18–45 years, with regular menses, on EFV-containing or NVP-containing cART; no smoking, recent injectable contraceptive use, or contraindications to COCsForteen HIV− controls | COC-containing DSG for two cyclesNVP-containing or EFV-containing cART or no cART | NVP group: EE Cmin ↓58% ENG Cmin↓22%EFV group: EE Cmin ↓9% ENG Cmin↓61%One woman (6%) had NVP level below therapeutic level of 3.1 mg/lThree women had EFV level below therapeutic level of 1.0 mg/l | Strengths: prospective clinical trial; COCs and antiretrovirals at steady state; HIV+ womenWeaknesses: nonrandomized; small sample size; single measurement of ENG levels; high drop out/loss to follow-up rate; unable to measure ENG in 8/16 due to assay interferenceFunded by government |
| Vieira 2014 [34]Brazil | Prospective cohort with pharmacokinetic analysisTo evaluate the effects of EFV-containing or LPV/r-containing cART on ENG implant pharmacokinetics and to determine the impact of cART on luteal activity | Forty-five HIV+ women with regular menstrual cycles, BMI 18–30; excluded women with recent pregnancy or hormonal contraceptive use, acute infections or other opportunistic illnesses, drug or alcohol addiction, use of other potentially interacting drug, chronic diarrhea or malabsorption or noncompliance with cART | ENG implantLPV/r-containing or EFV-containing cART or no cART | ENG levels through 24 weeksEFV group: ENG AUC↓63% Cmax↓54% Cmin ↓70%LPV/r group: ENG AUC↑52% Cmax ↑61% Cmin ↑ 34% | Strengths: Clearly described population and methods; valid assays, HIV+Weaknesses: Small sample size; nonrandomizedFunded by government |
| Atrio et al. [16]Atrio et al. [56]Dubois et al. [17]USA | Open-label; nonrandomized; clinical trialTo compare NET pharmacokinetic in women taking cART with PIs compared with women receiving other cART regimens | Thirty-five HIV+ women age 18–44 years; no changes in medications; no recent hormonal contraceptives; no immunocompromise; no liver or renal disease; normal ovulation; BMI <40; >30 days postpartum; | POPs containing NETIn PI-containing cART group: 11 taking ATV (10/11 on ATV/r); 3 DRV/r; 2 LPV/rIn control group: four women not taking PI-containing cART; 13 taking combinations including ETR, RPV, TDF, FTC, and RAL | PI group: NET AUC ↑50% NET Cmax ↑33% NET Cmin ↑26%In subanalysis limited to women on ATV/r;NETAUC ↑35% NET Cmin ↑39% NET C24 ↑67% | Strengths: clearly described population and methods; valid assays; HIV+ womenWeaknesses: nonrandomized; small sample size; cART use self-reportedFunded by government |
| Crauwels et al. [27]UK | Open-label, three period pharmacokinetic studyTo evaluate the effect of RPV on COC pharmacokinetics and vice versa, and assess effects on sex hormones and safety of co-administration | Eighteen healthy nonsmoking women, 18–45 years; BMI 18–30 (median 24.6); 67% white; excluded pregnant, breast-feeding, or menopausal women, those with history of drug/alcohol abuse, skin disease, or any significant medical problems; use of concomitant medication | COC-containing NETIn third cycle; RPV 25 mg daily days 1–15 | Thirteen completed trialEE Cmin and AUC unchanged EE Cmax ↑17%NET AUC; Cmin; Cmax unchangedRPV pharmacokinetic unchanged from historical controls | Strengths: clearly described population and methods; valid assays; directly observed therapyWeaknesses: healthy women; short-term dosing; single antiretroviral; high discontinuation rateFunded by industry |
| Carten et al. [52]USA | Open-label two period pharmacokinetic studyTo determine the effect of EFV on the pharmacokinetics of LNG EC and vice versa, and assess safety | Twenty-four healthy women; 18–45; normal BMI (mean BMI 27) with no recent use of hormonal contraceptives or other interacting medications; women were either sterilized or used two nonhormonal contraceptive methods | LNG ECPs (0.75 mg) at 0 and 12 h on days 0 and 17EFV 600 mg 72 h after day 0, for 14 days. | Twenty-one women completed studyLNG AUC ↓58% LNG Cmax ↓45% LNG Cmin ↓69% | Strengths: clearly described population and methods; valid assaysWeaknesses: small sample size; healthy women; single antiretroviralFunded by industry |
| Piscitelli et al. [29]UK | Randomized crossover pharmacokinetic studyTo examine if GSK2248761 (fosdevirine) interacts with CYP450 substrates, including COCs | Ten healthy women, without hepatitis and not taking any medications | COC-containing DRSPGSK228761 200 mg or placebo days 1–11 | EE levels unchangedDRSP AUC, Cmax, Cmin ↑18–22% | Strengths: randomizedWeaknesses: short term administration; healthy women; single antiretroviral; small sample size; very few study details provided; trials of fosdevirine on hold due to other safety concerns; study terminated early for unknown reasonsFunded by industry |
| Kasserra et al. [57]USA | Randomized crossover pharmacokinetic studyTo determine the effect of vicriviroc alone or with RTV on COC pharmacokinetics and evaluate safety | Twenty-seven healthy nonpregnant women, 18–40 years; no recent medication use other than acetaminophen or oral contraceptives; BMI 19–32 (median 24.5) | COC-containing NETVicriviroc 75 mg BID for 10 days then vicriviroc 30 mg plus RTV 100 mg daily for next 11 days; group 2: RTV 100 mg daily for first 10 days then plus vicriviroc 30 mg daily for next 11 days | VCV alone: EE AUC and Cmax unchanged NET AUC and Cmax unchangedRTV alone: EE Cmax ↓11% EE AUC ↓29% NET Cmax ↓11% NET AUC ↓7%VCV+RTV: EE Cmax ↓24%% EEAUC ↓29% NET: Cmax ↓11% NET AUC ↓17%No severe or serious AEs | Strengths: randomized; valid assaysWeaknesses: healthy women; small sample size; single antiretroviralFunded by industry |
| Anderson et al. [61]USA | Randomized crossover pharmacokinetic studyTo assess the effect of RAL on COC pharmacokinetic | Twenty healthy nonobese nonpregnant women 18–45 years (mean age 27) using additional barrier contraceptive | COC-containing NGMRaltegravir 400 mg twice daily or placebo days 1–21 | Nineteen women completed the trialEE levels unchangedNGMN AUC ↑ 14% NGMN Cmax ↑29%.No serious clinical or laboratory AEs | Strengths: Randomized; placebo-controlled; clearly described population and methods; valid assaysWeaknesses: evening dose of RAL on day 21 of both periods missed; small sample size; healthy women; single antiretroviralFunded by industry |
| Zhang et al. [55]USA | Open-label three period pharmacokinetic studyTo assess the impact of RTV-boosted ATV on COC pharmacokinetic | Twenty healthy nonpregnant nonbreast-feeding women, 18–45 years (mean age 28); BMI 18–32 (mean 25) | COC containing NGMIn third cycle; ATV/r 300 mg/100 mg daily days 1–14 | EE AUC ↓19% Cmax ↓16% Cmin ↓37%NGMN Cmax ↑ 68% AUC ↑85% Cmin ↑102%Dose normalization estimate magnitude of reduction with lower dose EEATV AUC ↑20% than historical controlsMore AEs with co-administration than with COCs alone (vomiting; headache and abdominal pain); no deaths or SAEs | Strengths: Clearly described population and methods; valid assaysWeaknesses: small sample size; healthy women; single antiretroviralFunded by industry |
| Stuart et al. [30]Malawi | Open label; nonrandomized clinical trialTo assess the feasibility of measuring anovulation in a pharmacokinetic study of COCs and antiretrovirals | Nine women ages 21–35 (3/group) with similar age and BMI: group 1 included HIV+ women on cART; group 2 included HIV+ women not on cART; and group 3 included HIV− women | COC with NGNVP-containing cART or no cART | LNG and EE levels measured by radioimmunoassayLNG AUC 147 in NVP group; 114 no cART group; and 38 in HIV− womenEE AUC; 1384, 1457, 1144, respectivelyAntiretroviral pharmacokinetic similar to historical controls | Strengths: Clearly described population and methods; valid assays; included HIV+ womenWeaknesses: small sample size; nonrandomized; progesterone measured only 1 dayFunding source not reported |
| Sevinsky et al. [26]USA | Open-label three period pharmacokinetic studyTo examine effect of EFV on pharmacokinetics of EE and NGMN and vice versa | Twenty-eight healthy women; 18–45 years (median 26); BMI 20–32 (median 25); on COCs for at least 2 months and no baseline safety issues or breakthrough bleeding | COC-containing NGMEFV 600 mg daily for 14 days during third cycle | Nineteen women completed studyEE AUC, Cmax, Cmin unchangedNGMN Cmax ↓46%; AUC ↓64% Cmin ↓82%Posthoc LNG Cmax; AUC; and Cmin ↓80–86%EFV levels comparable to historical controls | Strengths: clearly described population and methods; valid assaysWeaknesses: small sample size; healthy women; single antiretroviral; high discontinuation rateFunded by industry |
| Vogler et al. [37]USA | Open-label; nonrandomized; clinical trialTo evaluate pharmacokinetic interactions between LPV/r and contraceptive patch and COCs | Thirty-two nonpregnant; premenopausal HIV+ women >13 years either on stable LPV/r regimens for at least 14 days (n = 8) or not on cART or taking NRTIs only (n = 24); nonsmoking; median weight 72 kg; no recent use of injectables or COCs; <198 lb; not taking enzyme inducers | EE/NGMN contraceptive patchSingle-dose COC-containing NETLPV/r (400 mg/100 mg twice a day) or no cART/NRTIs only | Patch EE AUC ↓45% Cmin ↓25%NGMN AUC ↑83% Cmin ↑134%COC EE AUC ↓55%LPV AUC ↓19% Cmin ↓27% Cmax ↓22%RTV AUC↓24% Cmin ↓14% Cmax ↓8% | Strengths: clearly described population and methods; valid assays; HIV infectedWeaknesses: study closed prematurely due to slow accrual; small sample size; single dose COC; high loss to follow-up; not randomizedFunded by government |
| Schöller-Gyüre et al. [28]USA | Open-label three period pharmacokinetic studyTo assess the effect of ETR on COC pharmacokinetics | Twenty-four healthy women; 18–45 years (median 24); BMI 18–30; 97% white; nonsmoking; not pregnant or breast-feeding; no contraindications to hormonal contraceptives; not taking enzyme inducers | COC containing NETIn cycle 3; 200 mg ETR twice daily from day 1 to 15 | EE AUC ↑ 22% Cmax ↑33% Cmin ↑9%NET Cmax; AUC unchanged Cmin ↓22%ETR levels higher than historical controls | Strengths: Clearly described population and methods; valid assaysWeaknesses: small sample size; healthy women; single antiretroviral; not randomizedFunded by industry |
| Kearney and Mathias [58]USA | Open-label; two period pharmacokinetic studyTo evaluate the effect of TDF on the pharmacokinetic of COCs | Twenty healthy nonpregnant; nonlactating women; 19–45 years taking study COC for at least 3 months; without recent medication use or active alcohol or drug use; mean age 25 years; mean weight 64 kg | COC NGMTDF 300 mg/day on days 15–21 of contraceptive cycle 2 | No change in NGMN and EE pharmacokineticTFV levels similar to historical dataNo serious AEs and no discontinuations due to AEs; AEs reported by 10 participants; most commonly headache; rash; dysmenorrhea; nausea; and rhinitis AEs reported by 10 participants; most commonly headache; rash; dysmenorrhea; nausea; and rhinitis | Strengths: clearly described population and methods; valid assaysWeaknesses: healthy women; single antiretroviral; small sample sizeFunded by industry |
| Abel et al. [60]USA | Randomized controlled; crossover pharmacokinetic studyTo evaluate the effect of MVC on COC pharmacokinetic and to assess pharmacokinetic and safety of MVC in women | Fifteen healthy sterilized white women; age 32–45; between 7 and 76 kg (mean 65 kg) and BMI 18–30 | COC LNGMVC 100 mg twice daily or placebo days 1–10 and am of day 11 | EE AUC; Cmax unchangedLNG AUC; Cmax unchangedMVC pharmacokinetic within the range seen in healthy males in previous studiesNo clinically significant abnormalities or severe or serious AEs | Strengths: randomized; placebo controlled; clearly described population and methods; valid assays; 100% retentionWeaknesses: small sample size; healthy women; single antiretroviral; COC only given days 2–8Funded by industry |
| Nanda et al. [32]Brazil | Open-label; nonrandomized; pharmacokinetic studyTo evaluate the effect of EFV-containing cART on pharmacokinetics of MPA and to determine effects on suppression of ovulation and bleeding patterns | Thirty-three HIV+ women aged 19–40 years; with regular menstrual cycles and BMI 18–30 kg/m2; not recently pregnant or breast-feeding | DMPA 150 mg given at enrollmentEFV-containing cART | No difference in MPA levels between groups through 12 weeks | Strengths: Clearly described population and methods; valid assays; HIV+ womenWeaknesses: small sample size; no EFV levels; no MPA levels beyond 12 weeksFunded by government |
| Sekar et al. [38]Belgium | Randomized crossover pharmacokinetic studyTo investigate the effect of DRV/r on COC pharmacokinetics and to examine safety | Twenty-two nonsmoking healthy women, 18–45 years; BMI 18–30; using a second nonhormonal contraceptive method | COC containing NETDRV/r 600/100 mg twice daily days 1–14 or no treatment | Eleven women completed studyEE AUC ↓44% Cmin ↓62% Cmax ↓32%NET AUC ↓14% Cmin ↓30% Cmax ↓10%DRV and RTV levels comparable to historical controls | Strengths: clearly described population and methods; valid assays; randomizedWeaknesses: small sample size; healthy women; single antiretroviral; high discontinuation rateFunded by industry |
| Cohn et al., Watts et al. [14,31]USA | Nonrandomized open-label pharmacokinetic studyTo evaluate the effect of various antiretrovirals on pharmacokinetics of MPA and vice versa; and to determine effects on suppression of ovulation and adverse events | Seventy-two HIV+ nonpregnant; premenopausal women, 22–46 years (median 35); median weight 71 kg; with no recent potentially interacting drugs; women required to use second nonhormonal method of contraception | DMPAAntiretrovirals regimens containing NFV; EFV; NVP; or no antiretroviral/NRTI only | No difference in MPA AUC; Cmax; Cmin; clearance half-life between groupsNVP AUC slightly higher after DMPA. No changes in EFV; nelfinavir pharmacokinetic | Strengths: clearly described population and methods; valid assays; HIV+ womenWeaknesses: nonrandomized; small sample sizeFunded by government |
| Aweeka et al. [65]USA | Open-label two-period pharmacokinetic time series studyTo investigate the effects of sex and contraceptives on ZDV pharmacokinetic and HIV viral load; to evaluate the effect of COCs and DMPA on plasma and genital HIV load among women on stable cART | HIV+: 18 men and 20 women; 22–52 years; on stable ZDV-containing cART | COC containing NET or DMPA begun at second cycle and continued through third cycleParticipants randomized to oral or intravenous ZDV (200 mg)Antiretrovirals included indinavir; nelfinavir; or any NRTI except d4T or tenofovir | Fourteen women (eight DMPA; six COC) provided pharmacokinetic dataNo effect on plasma or cervical HIV viral loadZDV levels and levels of by radioimmunoassay and liquid chromatography/mass spectrometryNo change in ZDV pharmacokinetic after contraceptive useNo differences between COC and DMPA on ZDV pharmacokinetic | Strengths: HIV+; prospective pharmacokinetic study; both PO and IV dosing of ZDVWeaknesses: open-label; nonrandomized; small sample; study stopped early due to slow enrollment; ZDV levels analyzed by two different methods due to discontinuation of reagents; no disaggregation of data for oral contraceptive vs. DMPAFunded by government |
| Burger et al. [62]Netherlands | Single time point retrospective pharmacokinetic analysisTo characterize factors that influence interpatient variability in EFV concentrations | Sixty-six HIV+ women | Eight hormonal contraceptive userscontaining EFV-containing cART | EFV concentration: No HC: 5.0 mg/l HC: 2.7 mg/l | Strengths: HIV+ womenWeaknesses: Study not designed to look at contraceptive effects; retrospective; single time point; very few hormonal users; population not well described; self-reported hormonal contraceptive use, type not specifiedFunding source not specified |
| Muro et al. [63]Netherlands | Open-label; single-period pharmacokinetic studyTo evaluate factors that influence interpatient variability in single dose NVP half-life | Forty-four healthy nonpregnant women age 18–40 (median 26 years); without hepatitis infection; median weight 64 kg | Seventeen oral contraceptive users 27 nonusersSingle dose of 200 mg of NVP | Seventeen women reported COCs; median time to first undetectable NVP plasma level was 21 days; longer than in 27 women not reporting COC use (14 days; P <0.001)Median half-life of NVP in COC users versus nonusers not significantly different (69.7 vs. 52.8 h; P = 0.053). | Strengths: clearly described population and methods; valid assaysWeaknesses: study not designed to look at contraceptive effects; few hormonal users; healthy women; single dose of single antiretroviral; self-reported hormonal contraceptive useFunding source not specified |
| Frohlich et al. [64]Germany | Open-label; two period pharmacokinetic studyTo investigate the influence of COCs on SQV pharmacokinetic and to assess the potential contribution of CYP3A4 and P-glycoprotein | Eight healthy nonsmoking nonpregnant women with regular menses; mean age 24 years and mean BMI 21; not using any potentially interacting drugs | COC containing GES days 4-25600 mg SQV on days 1 and 22 | No effect of COCs on SQV pharmacokinetics | Strengths: Clearly described population and methods; valid assaysWeaknesses: not randomized; very small sample size; short course of COCs; healthy women; single antiretroviral only given twiceFunded by government |
| Mildvan et al. [53]USA | Open-label, single dose, two period pharmacokinetic studyTo determine the effects of NVP on COC pharmacokinetics and vice versa | Fourteen HIV+ nonpregnant, nonlactating, nonsmoking women; age18–65 (mean age 37); viral load <400; CD4+ cell count > 100 cells/μl; normal renal and hepatic function; no RTV or DLV use | Single dose of COC containing NET on cycle day 1 and 30NVP 200-mg daily on days 2–15; then 200-mg twice daily days 16–29; single dose on day 30cART regimens included IDV; NFV; SQV/RTV | Ten women completed the studyEE AUC ↓29% Cmax unchangedNET AUC ↓18% Cmax unchangedNVP levels similar to historical controls | Strengths: HIV+ clearly described population and methods; valid assaysWeaknesses: small study; only single dose COC; NVP added to current cART regiment; included postmenopausal womenFunded by industry |
| Ouellet et al. [54]Canada | Single dose, single period pharmacokinetic studyTo assess the effects of RTV on EE pharmacokinetics | Twenty-three healthy nonpregnant nonlactating women, 18–45, close to ideal weight; women were postmenopausal, sterilized, practiced abstinence, or had a vasectomized partner | Single dose of COC with 50 μg EE + 1 mg ethynodiol diacetate given on cycle days 1 and 29RTV oral solution from day 15–30, 300 mg q12h on Day 15, 400 mg q12h on Day 16, and 500 mg q12h thereafter | EE Cmax ↓32% AUC ↓41% | Strengths: valid assaysWeaknesses: no progestin levels; nonrandomized; single dose COC; postmenopausal healthy women; nonstandard RTV dosesFunded by industry |
Abbreviations for antiretrovirals and contraceptive steroids defined in Tables 1 and 2.
AUC, area under the curve; Cmax, Peak concentration; Cmin, rough concentration; COC, combined oral contraceptive; DMPA, depot medroxyprogesterone acetate; ECP, emergency contraceptive pill; MPA, medroxyprogesterone acetate; POP, progestin-only pill.
Results
Our search identified 1570 records. Fifty published reports from 46 individual studies met the inclusion criteria (Fig. 1, Tables 3 and 4). Four reports were secondary analyses or subsets of the primary studies and are included with the primary study in the tables [14–17]. The results are presented by outcome assessed, focusing first on the most important clinical outcomes (contraceptive effectiveness, antiretroviral effectiveness, toxicity associated with combined administration), then the pharmacokinetic data (for contraceptives and antiretrovirals), in each case by antiretroviral class and by contraceptive method.
Fig. 1.
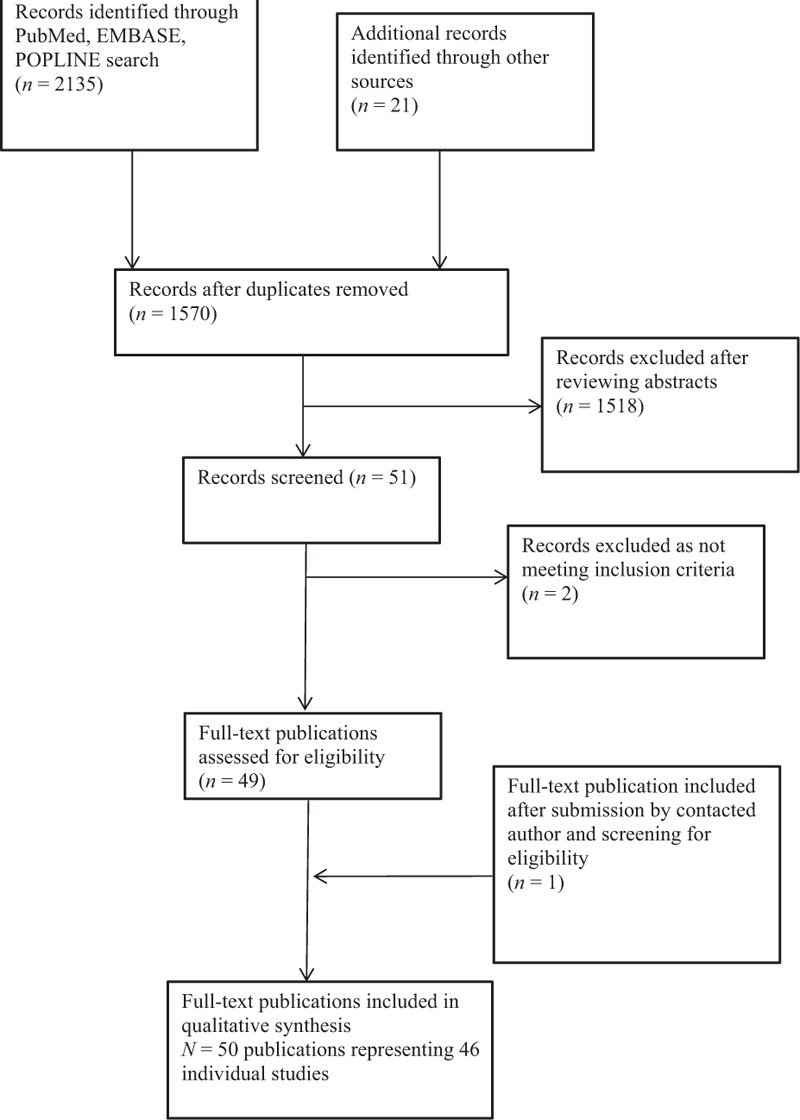
Flow diagram of publication selection for inclusion into the review.
Contraceptive effectiveness
Although pregnancy is the most relevant outcome, few large studies were designed to investigate contraceptive effectiveness. Several secondary analyses helped fill this gap, particularly for women using nevirapine-containing or efavirenz-containing cART. Although some small pharmacokinetic studies of healthy women report on pregnancy, women were generally required to use additional contraception; these studies are included in Table 3 but not summarized here.
Nonnucleoside reverse transcriptase inhibitors
Fourteen reports from clinical trials and six secondary analyses described contraceptive effectiveness measures among women using NNRTIs and hormonal contraceptives (Table 3).
Oral contraceptives
Two clinical trials of women using cART and oral contraceptives [18,19], six secondary analyses [20–25] and five pharmacokinetic trials (mostly in healthy women using single antiretrovirals with COCs) [26–30], evaluated pregnancy or ovulation. No pregnancies were found to be associated with nevirapine or efavirenz in the prospective clinical trials.
Pregnancy rates and ovulation rates did not differ between HIV-positive women taking COCs and nevirapine-containing cART and those not yet taking cART [18]. In a small trial of women using COCs with efavirenz-containing cART, three women ovulated (out of 25) but no pregnancies were reported [19]. Five small pharmacokinetic trials of NNRTIs and COCs also demonstrated no ovulation among study participants [26–30].
In large cohort studies, pregnancy rates were slightly higher among women taking efavirenz-containing cART (11–15/100 woman-years) compared with women taking oral contraceptive and nevirapine-containing cART or no cART (pregnancy rates 6–11/100 woman-years) [24,25]. Notably the reported pregnancy rates in the large cohort studies are still lower than an expected pregnancy rate of 40 per 100 woman-years among women not using any modern contraceptive and trying to prevent pregnancy.
Other retrospective cohort studies reported pregnancy rates among oral contraceptive users ranging from 2.6 to 5.8 per 100 woman-years (most, but not all, women were using nevirapine) [20,21].
Depot medroxyprogesterone acetate
In two pharmacokinetic studies, women using depot medroxyprogesterone acetate (DMPA) remained anovulatory when using cART containing either efavirenz or nevirapine [14,31,32]. Five cohort studies also presented pregnancy rates with injectables among cART users. In the largest, pregnancy rates ranged from 8 to 10 per 100 woman-years for injectable users, with higher rates in efavirenz users and those not on cART compared with pregnancy rates in those using nevirapine [24]. Another found women using DMPA had pregnancy rates from 3 to 5 per 100 woman-years, with lower rates among users of nevirapine and efavirenz compared with no cART [25]. Two additional studies reported pregnancy rates from 1.8 to 4.2 per 100 woman-years among DMPA users taking various antiretrovirals (primarily nevirapine) [20,21].
Implants
Pregnancy rates among users of cART and contraceptive implants differed by whether the implant contained levonorgestrel or etonogestrel, and whether women were taking efavirenz or nevirapine. Pregnancy rates were higher among women using the levonorgestrel implant concomitantly with efavirenz. Two prospective studies (N = 79 and N = 45) reported no pregnancies through 3 years and 6 months, respectively, among women using etonogestrel implants and NNRTI-containing cART [33,34], although the second study found that women taking efavirenz-containing cART had a 2.8% presumed ovulation rate over 6 months. In a small pharmacokinetic study of women using levonorgestrel implants and cART containing efavirenz or nevirapine, three pregnancies (3/20; 15%) occurred within 48 weeks, all in women taking efavirenz [35]. Similar findings were seen in a large retrospective study, where 15 of 121 (12.4%) women using levonorgestrel implants and efavirenz-containing cART became pregnant, at a mean duration of 16.4 months; no pregnancies occurred among women taking nevirapine-containing cART [36].
Two secondary analyses described pregnancy rates with implant use in women using efavirenz-containing or nevirapine-containing cART [24,25]. In the first, pregnancy rates for users of efavirenz, nevirapine, and no cART were 5.5, 2.3, and 3.4 per 100 woman-years for etonogestrel implant users, and 7.1, 1.9, and 3.3 per 100 woman-years for levonorgestrel implant users, respectively [24]. In the second, pregnancy rates per 100 woman-years were 1.4 for unspecified implant users not taking cART, 0 for nevirapine users, and 6 for women taking efavirenz [25].
Protease inhibitors
Seven small pharmacokinetic trials described contraceptive effectiveness measures among women using protease inhibitors and hormonal contraceptives (Table 3) [14,16,31,34,37–39].
Combined oral contraceptives/patches
Co-administration of darunavir/ritonavir with COCs resulted in no ovulation [38]. Similarly, coadministration of lopinavir/ritonavir containing cART in women using a contraceptive patch also found no ovulations [37].
Progestin-only pills
One report showed that oral norethindrone thickened cervical mucus and led to similar mucus scores in women using protease inhibitor-containing cART compared with those taking NRTIs alone [16].
Depot medroxyprogesterone acetate
Reports of women using lopinavir/ritonavir-containing or nelfinavir-containing cART with DMPA found that no women ovulated [14,31,39].
Implants
No ovulations nor pregnancies were reported in a pharmacokinetic study of etonogestrel implant users taking lopinavir/ritonavir-containing cART [34].
Nucleoside/nucleotide reverse transcriptase inhibitors
Analyses of two large trials of hormonal contraceptives and NRTIs used for PrEP found that use of tenofovir/emtricitabine did not affect pregnancy rates among users of COCs, injectables, or implants [40,41].
Integrase inhibitors
A small pharmacokinetic study of dolutegravir with COCs resulted in no ovulations [42].
Antiretroviral effectiveness
Eight reports evaluated the effects of hormonal contraceptive use on the effectiveness of NNRTI-containing or protease inhibitor-containing cART, and found no effects on death, CD4+ cell count, or plasma viral load with concurrent use of DMPA, levonorgestrel implants, or oral contraceptives [14,43–49]. Use of DMPA also did not affect the efficacy of PrEP [50].
Toxicity of combined administration
Studies among healthy women using hormonal contraceptives concurrently with single antiretrovirals, or HIV-positive women using cART, generally reported no difference in adverse events of concurrent treatment compared with use of either hormonal contraceptives or antiretrovirals alone (Table 3). One HIV prevention trial evaluated pharmacodynamic interactions between tenofovir-containing PrEP with oral contraceptives or DMPA, and found that bone mineral density was not significantly decreased [51].
Contraceptive pharmacokinetics
Thirty-two reports include contraceptive pharmacokinetic measures among women using antiretrovirals and hormonal contraceptives (Table 4).
Nonnucleoside reverse transcriptase inhibitors
Contraceptive pharmacokinetics among women using NNRTIs and hormonal contraceptives were described in 11 studies (Table 4) [15,26–32,34,52,53].
Combined oral contraceptives
Two studies evaluated efavirenz with COCs: one in women taking only efavirenz, and one in women taking efavirenz-containing cART [15,26]. Ethinyl estradiol concentrations were not significantly changed, but progestin levels decreased by approximately 60%.
Three studies reported the effect of nevirapine on COC pharmacokinetics. Ethinyl estradiol levels varied from being unchanged to being approximately 30–60% lower [15,30,53]. Progestin levels were not significantly affected.
Studies of etravirine and rilpivirine in women taking COCs found minimal effects [27,28]. Similarly, a study of fosdevirine (the development of which was discontinued due to toxicity) found no effect on hormone levels in COC users [29].
Depot medroxyprogesterone acetate
Two studies evaluated the effect of NNRTIs on DMPA, and found no difference in medroxyprogesterone acetate pharmacokinetics through 12 weeks with concurrent use of either efavirenz-containing or nevirapine-containing cART [31,32].
Implants
A study of women using etonogestrel implants found 54–70% lower etonogestrel levels among women taking efavirenz-containing cART compared with women taking no cART [34].
Emergency contraceptive pills
One study in healthy women showed that levonorgestrel levels were 56% lower in ECP users after use of efavirenz [52].
Protease Inhibitors
Ten reports described contraceptive pharmacokinetics among women using protease inhibitors and hormonal contraceptives (Table 3) [17,31,34,37–39,54–57].
Combined oral contraceptives/Patch
Two studies examining concurrent use of ritonavir and COCs found decreased ethinyl estradiol levels, whereas progestin levels were unaffected [54,57]. Another study showed decreased ethinyl estradiol levels, but increased progestin levels, when COCs were used with atazanavir/ritonavir [55]. Similar findings were reported with lopinavir/ritonavir-containing cART and the contraceptive patch [37]. This study also reported lower ethinyl estradiol levels with concurrent use of a single COC pill, but progestin levels were not evaluated. Only darunavir/ritonavir was associated with significantly lower ethinyl estradiol levels as well as slightly lower norethindrone levels [38].
Progestin-only pills
In women receiving protease inhibitor-containing cART, norethindrone levels were higher compared with controls [56]. A subanalysis restricted to women using ritonavir-boosted atazanavir confirmed this finding [17].
Depot medroxyprogesterone acetate
One study showed significantly increased medroxyprogesterone acetate concentrations, compared with historical controls, in women using DMPA and lopinavir/ritonavir-containing cART [39].
Implants
A study evaluating the effect of cART on the pharmacokinetics of the etonogestrel implant found women using lopinavir/ritonavir-containing cART had etonogestrel levels approximately 50% higher than women not taking cART [34].
Nucleoside/nucleotide reverse transcriptase inhibitors
Two studies evaluating NRTIs used for PrEP with COCs or levonorgestrel implants showed no change in hormone levels [58,59].
Chemokine receptor 5 antagonists
Two studies showed that neither maraviroc nor vicriviroc impacted hormone levels when used concurrently with COCs [57,60].
Integrase inhibitors
In two studies, concurrent use of COCs and raltegravir led to small increases in progestin exposure [61], but dolutegravir had no impact on hormone levels [42].
Antiretroviral pharmacokinetics
Fifteen studies described antiretroviral pharmacokinetics among women using antiretrovirals and hormonal contraceptives; most were among healthy women and compared drug concentrations to historical controls (Table 4) [19,26,27,31,37–39,53–55,60,62–65].
Nonnucleoside reverse transcriptase inhibitors
Three studies evaluated the impact of COCs on the pharmacokinetics of efavirenz [19,26,62]. Among women using COCs and efavirenz alone, concentrations were similar to historical controls [26]. However, in a trial of women on efavirenz-containing cART, use of COCs led to efavirenz concentrations lower than historical controls [19]. Another analysis of women taking efavirenz-containing cART found no difference in efavirenz concentrations between hormonal contraceptive users and nonusers [62].
Three studies evaluated the impact of COCs on nevirapine levels. In two reports of women using nevirapine-containing cART, nevirapine levels were not significantly different in women using COCs [19,53]. Time to undetectable nevirapine levels was longer in women receiving single-dose nevirapine and using COCs [63]. Another study found rilpivirine levels in COC users to be similar to historical controls [27].
Among women on various cART regimens, nevirapine levels were slightly higher after administration of DMPA, but no changes in efavirenz levels were noted [31].
Protease inhibitors
Four studies investigated the effects of COCs on the pharmacokinetics of protease inhibitors [38,54,55,64]. Levels of saquinavir were not affected by COC use, but atazanavir levels were slightly increased [55,64]. Co-administration with COCs resulted in darunavir and ritonavir levels comparable to those in historical controls [38,54].
Co-administration of the contraceptive patch with lopinavir/ritonavir-containing cART resulted in slightly decreased levels of both protease inhibitors compared with historical controls [37], whereas DMPA had no effect on protease inhibitor levels in women taking such regimens [39].
Nucleoside/nucleotide reverse transcriptase inhibitors
One study found no effect of hormonal contraceptives (COCs and DMPA) on zidovudine plasma or intracellular pharmacokinetics [65].
Chemokine receptor 5 antagonists
When maraviroc was taken with COCs, levels were similar to those seen in historical controls [60].
Discussion
Few of the 50 reports included in this review provided relevant data that can be applied to clinical practice with certainty (Table 5). The most significant interactions with hormonal contraceptives occurred in women using cART-containing NNRTIs, particularly efavirenz. However, even in these studies, the outcomes reported were often pharmacokinetic rather than clinical, involved small populations, which limited study power, or were derived retrospectively from secondary analyses of existing cohorts.
Table 5.
Summary of included clinical and pharmacokinetic data on HC-antiretroviral drug interactions; PK differences <30% considered no change (↔), blank cells indicate absence of data.
| Antiretroviral | Ethinyl estradiol pharmacokinetics | Progestin pharmacokinetics | Antiretroviral pharmacokinetics | Contraceptive effectiveness (pregnancy rates per 100 woman-years or ovulation %) | Antiretroviral effectiveness | Reported adverse events/toxicity |
| NNRTIs | ||||||
| Efavirenz | ||||||
| COCs | AUC↔ | ENG AUC ENG C24↓61% NGM AUC↓64% | EFV levels <1.0 mg/l in 3/16 | Pregnancy rates 13–15 | Bleeding, nausea, mood change, dizziness | |
| C24↔ | NGM Cmax↓46% | Ovulation rates 0–19 | ||||
| NGM Cmin↓82% | ||||||
| ECPs | LNG AUC ↓58% | |||||
| LNG Cmax ↓45% | ||||||
| LNG Cmin ↓69% | ||||||
| Injectables | NA | MPA ↔ | ↔ | Pregnancy rates 4–9 | No effect on time to virologic suppression, CD4+ cell count, death, viral load, treatment failure | No grade3/4 AEs |
| Ovulation rate 0 | ||||||
| LNG implants | LNG AUC ↓47% | Pregnancy rates 6–15 | No change in CD4+ cell count or HIV viral load | Bleeding | ||
| ENG implants | ENG AUC↓63% | Pregnancy rate 5 | ||||
| ENG Cmax↓54% | Ovulation rate 3 | |||||
| ENG Cmin ↓70% in | ||||||
| Nevirapine | ||||||
| COCs | EE AUC↔ | NET AUC↔ | ↔ | Pregnancy rates 6–10 | Nausea, headache, breast tenderness, mood changes | |
| EE Cmax ↔ | NET Cmax↔ | Ovulation rates 0–30 | ||||
| EE C24 ↓58% | LNG AUC↔ | |||||
| LNG Cmin↔ | ||||||
| ENG C24↔ | ||||||
| Injectables | ↔ | Pregnancy rates 3–8 | No grade 3 or 4 AEs | |||
| LNG implants | Pregnancy rate 0–2 | |||||
| ENG implants | Pregnancy rate 2 | |||||
| Etravirine | ||||||
| COCs | AUC↔ | NET Cmax↔ | ↔ | Ovulation rate 0 | Nine discontinued due to AEs (rash in 7) | |
| EE Cmax ↑ 33%, | NET AUC↔ | |||||
| Cmin↔ | NET Cmin↔ | |||||
| Rilpivirine | ||||||
| COCs | Cmax ↔ | NET Cmax↔ | ↔ | Ovulation rate 0 | No grade 3 or 4 AEs | |
| Cmin ↔ | NET Cmin↔ | |||||
| AUC ↔ | NET AUC↔ | |||||
| Ritonavir-boosted PIs | ||||||
| Lopinavir/ ritonavir | ||||||
| COCs, patch | EE AUC ↓55% | Patch: | ↔ | COC pregnancy rate 15 | One grade 3 AE | |
| Patch: | NGMN AUC ↑ 83%, NGMN Cmin ↑ 134% | Patch ovulation rate 0 | ||||
| EE AUC ↓ 45% | ||||||
| EE Cmin ↔ | ||||||
| Injectables | MPA AUC ↑46% | ↔ | Pregnancy rate 5–7 | No difference in CD4+ cell count or HIV RNA | Menstrual irregularities in 25%, only one considered grade 3 due to persistent bleeding | |
| MPA Cmax ↑66% | Ovulation rate 0 | |||||
| LNG implants | Pregnancy rate 0 | |||||
| ENG implants | AUC ↑ 52% | Pregnancy rate 1 | ||||
| Cmax ↑ 61% | Ovulation rate 0 | |||||
| Cmin ↑ 34% | ||||||
| Atazanavir/ ritonavir | ||||||
| COCs | AUC ↔ | NGM Cmax ↑ 68%, NGM AUC ↑ 85%, NGM Cmin ↑ 102%, | ↔ | incr side-effects | ||
| Cmax ↔ | ||||||
| Cmin ↓37% | ||||||
| POPs | NET Cmax ↑ 39% | Cervical mucus remained thickened | ||||
| NET C24 ↑ 67%, | ||||||
| NET AUC ↑ 50% | ||||||
| Darunavir/ ritonavir | ||||||
| COCs | Cmin ↓62% | NET Cmin ↓30% | DRV and RTV ↔ | 26% discontinued due to grade two AEs | ||
| Cmax ↓32% | NET Cmax ↔ | |||||
| AUC ↓44%; | NET AUC ↔ | |||||
| SQV/ritonavir | ||||||
| COCs | ↔ | |||||
| PIs without ritonavir | ||||||
| Indinavir | ||||||
| COCs | Zero pregnancies | |||||
| Nelfinavir | ||||||
| COCs | Higher failure rate with NFV than other PIs | |||||
| Injectables | MPA ↔ | AUC ↔ | Zero pregnancies | No change in CD4+ cell count or HIV RNA | No grade 3 or 4 AEs | |
| Cmax ↔ | Ovulation rate 0 | |||||
| Cmin ↓46% | ||||||
| CCR5 antagonists | ||||||
| Maraviroc | ||||||
| COCs | ↔ | LNG ↔ | No serious AEs | |||
| Integrase inhibitors | ||||||
| Dolutegravir | ||||||
| COCs | ↔ | ↔ | ||||
| Raltegravir | ||||||
| COCs | ↔ | ↔ | ||||
| NRTIs | ||||||
| Tenofovir disaproxil fumarate, emtricitabine | ||||||
| COCs | ↔ | ↔ | Pregnancy rate 18–35 | |||
| Injectables | Pregnancy rate 2–5 | No difference | ||||
| LNG implants | Pregnancy rate <1 | |||||
| Zidovudine | ||||||
| COCs | ↔ | No change in plasma and cervical HIV viral load | ||||
| Injectables | ↔ | No change in plasma and cervical HIV viral load |
Abbreviations for antiretrovirals and contraceptive steroids defined in Tables 1 and 2.
COC, combined oral contraceptive; DMPA, depot medroxyprogesterone acetate; ECP, emergency contraceptive pill; POP, progestin-only pill.
AUC, area under the curve; Cmax, peak concentration; Cmin, trough concentration.
The most important outcome for contraceptive drug interactions is method failure resulting in pregnancy, but few studies reported this outcome (Table 5). Changes in contraceptive hormone levels do not necessarily translate into reduced efficacy or increased toxicity, as levels vary greatly within and between individuals and populations [66,67]. Further, the contraceptive threshold, or minimum steroid hormone level required to maintain contraceptive effectiveness, is difficult to determine [68,69]. However, when pharmacokinetic data show no or minimal changes, clinical effects are unlikely. Ovulation is used in many drug–drug interaction studies to indicate risk of pregnancy, but ovulation is also a surrogate marker. The occurrence of ovulation does not always result in pregnancy. For example, many women ovulate during levonorgestrel implant use, yet contraceptive effectiveness remains high [70]. Additionally, no included studies evaluated true ovulation; rather, ovulation was presumed based on serum progesterone measurements alone, which can be inaccurate [70].
The most clinically significant drug–drug interactions identified in our systematic review were reported in women using efavirenz-containing cART and COCs or progestin-containing subdermal implants. Although studies show DMPA is not impacted by efavirenz use, studies of women using efavirenz and contraceptive implants reported pregnancy rates ranging from 5 to 15 per 100 woman-years, and COC users taking efavirenz had pregnancy rates ranging from 13 to 15 per 100 woman-years [24,25,35]. For COCs, because contraceptive effectiveness relies on user adherence, potential additional reductions in effectiveness from a drug interaction, if confirmed, are concerning. Conversely, studies that reported on women using contraceptives with nevirapine-containing cART were generally reassuring. None of the studies that enrolled women using a number of different hormonal contraceptives with nevirapine reported increases in pregnancy or ovulation rates [18,19,21,24,25,30,35,36,45].
The many other studies included in this review that evaluated hormonal contraceptives and antiretrovirals other than efavirenz reported no results that should change clinical practice. Antiretrovirals used in PrEP do not affect hormonal contraceptive effectiveness. Concurrent use of protease inhibitors with COCs does not alter contraceptive effectiveness despite the observed decreased ethinyl estradiol plasma levels found, as the progestin component is primarily responsible for contraceptive effectiveness. Minimal to no changes in progestin levels were reported in multiple studies of concurrent protease inhibitor and hormonal contraceptive use. Although concomitant use of a few protease inhibitors led to increased progestin levels in some studies, these changes are unlikely to impact safety given the variable doses and wide safety margin of contraceptive progestins.
Despite the small number of reports in our review, studies were also reassuring with regard to the effect of hormonal contraceptives on cART or PrEP effectiveness, or antiretroviral pharmacokinetics. Pharmacokinetic studies were limited because they either only reported antiretroviral pharmacokinetics compared with historical controls or presented data from healthy women taking single antiretrovirals.
Concurrent antiretroviral and hormonal contraceptive use also does not appear to lead to increased toxicity, though most studies were of short duration (1–28 days). Short-term pharmacokinetic studies may not accurately reflect adverse effects that may occur during use of long-term cART or PrEP with hormonal contraceptives. The only study to evaluate long-term toxicity showed little impact of concurrent use of DMPA and tenofovir on bone mineral density over 1 year [51]. Pharmacokinetic effects may also be time-dependent, further limiting the utility of short-term evaluations.
Strengths of our review included a comprehensive search strategy, systematic review of study inclusion by all authors, and dual data abstraction. Limitations are generally due to lack of studies evaluating relevant clinical outcomes. In addition, few studies are available regarding whether implants containing levonorgestrel or etonogestrel have different contraceptive effectiveness when used with efavirenz. Furthermore, with both implants, it is possible that interactions with enzyme inducers such as efavirenz are time-dependent, because hormone levels decrease over time after implantation [71]. Other data gaps are whether injectable contraceptives other than intramuscular DMPA, such as the lower-dose subcutaneous DMPA or injectable norethisterone enanthate, might be susceptible to drug interactions. Questions also remain regarding any impact of lower-dose efavirenz regimens [72] on hormonal contraceptive effectiveness, which cannot be predicted. Another limitation is that intracellular antiretroviral concentrations are likely a better predictor of clinical effectiveness than plasma levels, but pharmacokinetic studies only reported the latter. Finally, women on cART may also take other drugs that can alter liver metabolism, such as rifampin, and the combined effect of multiple enzyme-inducing medications on contraceptive hormone levels remains poorly characterized.
Our review highlights the dearth of studies designed to provide meaningful clinical data to guide contraceptive choices for HIV-positive women taking cART. Studies should be designed to report clinical outcomes such as pregnancy and HIV disease progression during long-term administration. Currently, incomplete data are being used to limit contraceptive choices for HIV-positive women. In the absence of well conducted prospective clinical trials, data from pharmacokinetic studies and secondary analyses have been used to make clinical judgments on medication effectiveness and inform contraceptive policy. For example, in October 2014 the South African authorities recommended that women using efavirenz or other enzyme-inducing drugs should not use etonogestrel implants [11]. In May 2016, the European Medicines Agency recommended that women taking hepatic enzyme inducing drugs, including efavirenz, be offered double doses of oral levonorgestrel for postcoital emergency contraception [73]. When such guidance is developed, the absolute risk of pregnancy should be considered and addressed in the guidance publications, as well as other considerations such as availability and contraceptive effectiveness of the alternatives proposed. Even if a particular contraceptive method is potentially less effective than usual in a woman using a concomitant antiretroviral, it may still be more effective than many alternative contraceptive methods [74]. Although nonhormonal methods such as copper IUDs are not affected by drug interactions, their use remains very low in many settings worldwide, and efforts to increase IUD use have had limited success [7]. If access to implants is restricted, in many settings DMPA would be the primary option available to women, virtually eliminating woman-centered decision making.
In summary, current published data do not support limiting women's access to any hormonal contraceptives. Women taking antiretrovirals for HIV treatment (in the form of cART) or prevention (in the form of PrEP) should have access to the full range of hormonal contraceptive options, and be enabled to make informed decisions about their options. Contraceptive efficacy is only one of many factors that an individual may consider when choosing a contraceptive method, and some women who are motivated to use the etonogestrel implant may wish to do so even if there is concern for decreased efficacy when used with efavirenz. National or regional restrictions on contraceptive method access, while well intentioned, supersede women's personal decisions, which may actually increase risk of unintended pregnancy if remaining contraceptive options are unacceptable or inaccessible. More well designed prospective studies are needed to examine potential drug interactions between antiretrovirals and all contraceptive methods, to better inform guidelines and counseling for the more than 16 million women living with HIV.
Acknowledgements
This manuscript is made possible by the generous support of the American people through the United States Agency for International Development (USAID), provided to FHI 360 through cooperative agreement number AID-OAA-A-15-00045 and consolidated grant number GHA-G-00-09-00003 provided to the WHO. We also thank Drs Margaret Doherty, Marco De Avila Vitoria, and Shaffiq Essajee for their expert advice.
Roles of the authors: The WHO (MLG) initiated the idea to update this systematic review. K.N. led the conduct of the systematic review, conducted the literature searches, and coordinated review procedures and drafting of the manuscript. All authors participated in framing the study questions, developing eligibility criteria, reviewing identified studies for eligibility, abstracting study information, interpreting the data, and contributing to the writing, and editing of the manuscript. All authors reviewed and approved the final manuscript before submission.
Source of funding: K.N. has led previous systematic reviews on this topic, and authored a few of the studies included in the review. G.S.S. authored one of the studies included in the review. The WHO and USAID provided support for the writing of this systematic review and for the writing group to attend a working meeting in Geneva, Switzerland, in 2015.
Disclaimer: The contents are the responsibility of the authors and do not necessarily reflect represent the official positions of FHI360, USAID, the Centers for Disease Control and Prevention, the WHO, or other institutions with which the authors are affiliated.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
References
- 1.Gunthard HF, Saag MS, Benson CA, del Rio C, Eron JJ, Gallant JE, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society – USA panel. JAMA 2016; 316:191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Obare F, van der Kwaak A, Birungi H. Factors associated with unintended pregnancy, poor birth outcomes and postpartum contraceptive use among HIV-positive female adolescents in Kenya. BMC Womens Health 2012; 12:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeFranco EA, Seske LM, Greenberg JM, Muglia LJ. Influence of interpregnancy interval on neonatal morbidity. Am J Obstet Gynecol 2015; 212:386.e381–386.389. [DOI] [PubMed] [Google Scholar]
- 4.Ngo AD, Roberts CL, Figtree G. Association between interpregnancy interval and future risk of maternal cardiovascular disease – a population-based record linkage study. BJOG 2016; 123:1311–1318. [DOI] [PubMed] [Google Scholar]
- 5.Wilcher R, Petruney T, Cates W. The role of family planning in elimination of new pediatric HIV infection. Curr Opin HIV AIDS 2013; 8:490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trussell J. Contraceptive failure in the United States. Contraception 2011; 83:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.United Nations Department of Social and Economic Affairs, Population Division. World Contraceptive Use; 2015. http://www.un.org/en/development/desa/population/publications/dataset/contraception/wcu2015.shtml [Accessed 1 December 2016]. [Google Scholar]
- 8.Shen DD, Kunze KL, Thummel KE. Enzyme-catalyzed processes of first-pass hepatic and intestinal drug extraction. Adv Drug Deliv Rev 1997; 27:99–127. [DOI] [PubMed] [Google Scholar]
- 9.Edelman AB, Cherala G, Stanczyk FZ. Metabolism and pharmacokinetics of contraceptive steroids in obese women: a review. Contraception 2010; 82:314–323. [DOI] [PubMed] [Google Scholar]
- 10.Tseng A, Hills-Nieminen C. Drug interactions between antiretrovirals and hormonal contraceptives. Expert Opin Drug Metab Toxicol 2013; 9:559–572. [DOI] [PubMed] [Google Scholar]
- 11.Department of Health, Republic of South Africa. Circular: changes in the prescription of progestin subdermal implants (Implanon) in women who are taking enzyme inducing drugs such as efavirenz for HIV, rifampicin for TB, and certain drugs used for epilepsy (carbamazepine, phenytoin, and phenobarbital); 2014. Available at: http://www.sahivsoc.org/upload/documents/Circular – Changes in the Prescription of Progestin Subdermal Implants.pdf [Accessed 1 December 2016]. [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 14.Watts DH, Park JG, Cohn SE, Yu S, Hitti J, Stek A, et al. Safety and tolerability of depot medroxyprogesterone acetate among HIV-infected women on antiretroviral therapy: ACTG A5093. Contraception 2008; 77:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landolt NK, Phanuphak N, Ubolyam S, Pinyakorn S, Kerr S, Ahluwalia J, et al. Significant decrease of ethinylestradiol with nevirapine, and of etonogestrel with efavirenz in HIV-positive women. J Acquir Immune Defic Syndr 2014; 66:e50–e52. [DOI] [PubMed] [Google Scholar]
- 16.Atrio J, Stek A, Vora H, Sanchez-Keeland L, Zannat F, Natavio M. The effect of protease inhibitors on the cervical mucus of HIV-positive women taking norethindrone contraception. Eur J Contracept Reprod Healthcare 2015; 20:149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DuBois BN, Atrio J, Stanczyk FZ, Cherala G. Increased exposure of norethindrone in HIV+ women treated with ritonavir-boosted atazanavir therapy. Contraception 2015; 91:71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nanda K, Delany-Moretlwe S, Dube K, Lendvay A, Kwok C, Molife L, et al. Nevirapine-based antiretroviral therapy does not reduce oral contraceptive effectiveness. AIDS 2013; 27 suppl 1:S17–S25. [DOI] [PubMed] [Google Scholar]
- 19.Landolt NK, Phanuphak N, Ubolyam S, Pinyakorn S, Kriengsinyot R, Ahluwalia J, et al. Efavirenz, in contrast to nevirapine, is associated with unfavorable progesterone and antiretroviral levels when coadministered with combined oral contraceptives. J Acquir Immune Defic Syndr 2013; 62:534–539. [DOI] [PubMed] [Google Scholar]
- 20.Myer L, Carter RJ, Katyal M, Toro P, El-Sadr WM, Abrams EJ. Impact of antiretroviral therapy on incidence of pregnancy among HIV-infected women in Sub-Saharan Africa: a cohort study. PLoS Med 2010; 7:e1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz SR, Rees H, Mehta S, Venter WD, Taha TE, Black V. High incidence of unplanned pregnancy after antiretroviral therapy initiation: findings from a prospective cohort study in South Africa. PLoS One 2012; 7:e36039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Danel C, Moh R, Anzian A, Abo Y, Chenal H, Guehi C, et al. Tolerance and acceptability of an efavirenz-based regimen in 740 adults (predominantly women) in West Africa. J Acquir Immune Defic Syndr 2006; 42:29–35. [DOI] [PubMed] [Google Scholar]
- 23.Clark RA, Theall K. Population-based study evaluating association between selected antiretroviral therapies and potential oral contraceptive failure. J Acquir Immune Defic Syndr 2004; 37:1219–1220. [DOI] [PubMed] [Google Scholar]
- 24.Patel RC, Onono M, Gandhi M, Blat C, Hagey J, Shade SB, et al. Pregnancy rates in HIV-positive women using contraceptives and efavirenz-based or nevirapine-based antiretroviral therapy in Kenya: a retrospective cohort study. Lancet HIV 2015; 2:e474–e482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pyra M, Heffron R, Mugo NR, Nanda K, Thomas KK, Celum C, et al. Effectiveness of hormonal contraception in HIV-infected women using antiretroviral therapy. AIDS 2015; 29:2353–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sevinsky H, Eley T, Persson A, Garner D, Yones C, Nettles R, et al. The effect of efavirenz on the pharmacokinetics of an oral contraceptive containing ethinyl estradiol and norgestimate in healthy HIV-negative women. Antivir Ther 2011; 16:149–156. [DOI] [PubMed] [Google Scholar]
- 27.Crauwels HM, Van Heeswijk RPG, Buelens A, Stevens M, Hoetelmans RMW. Lack of an effect of rilpivirine on the pharmacokinetics of ethinylestradiol and norethindrone in healthy volunteers. Int J Clin Pharmacol Ther 2014; 52:118–128. [DOI] [PubMed] [Google Scholar]
- 28.Scholler-Gyure M, Kakuda TN, Woodfall B, Aharchi F, Peeters M, Vandermeulen K, et al. Effect of steady-state etravirine on the pharmacokinetics and pharmacodynamics of ethinylestradiol and norethindrone. Contraception 2009; 80:44–52. [DOI] [PubMed] [Google Scholar]
- 29.Piscitelli S, Kim J, Gould E, Lou Y, White S, de Serres M, et al. Drug interaction profile for GSK2248761, a next generation nonnucleoside reverse transcriptase inhibitor. Br J Clin Pharmacol 2012; 74:336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stuart GS, Moses A, Corbett A, Phiri G, Kumwenda W, Mkandawire N, et al. Combined oral contraceptives and antiretroviral PK/PD in Malawian women: pharmacokinetics and pharmacodynamics of a combined oral contraceptive and a generic combined formulation antiretroviral in Malawi. J Acquir Immune Defic Syndr 2011; 58:e40–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohn SE, Park JG, Watts DH, Stek A, Hitti J, Clax PA, et al. Depo-medroxyprogesterone in women on antiretroviral therapy: effective contraception and lack of clinically significant interactions. Clin Pharmacol Ther 2007; 81:222–227. [DOI] [PubMed] [Google Scholar]
- 32.Nanda K, Amaral E, Hays M, Viscola MA, Mehta N. Pharmacokinetic interactions between depot medroxyprogesterone acetate and combination antiretroviral therapy. Fertil Steril 2008; 90:965–971. [DOI] [PubMed] [Google Scholar]
- 33.Kreitchmann R, Innocente AP, Preussler GM. Safety and efficacy of contraceptive implants for HIV-infected women in Porto Alegre, Brazil. Int J Gynaecol Obstet 2012; 117:81–82. [DOI] [PubMed] [Google Scholar]
- 34.Vieira C, Bahamondes MV, Souza R, Brito M, Prandini T, Amaral E, et al. Effect of antiretroviral therapy including lopinavir/ritonavir or efavirenz on the etonogestrel-releasing implant pharmacokinetics in HIV-infected women. Eur J Contracept Reprod Healthcare 2014; 19:S72–S73. [Google Scholar]
- 35.Scarsi KK, Darin KM, Nakalema S, Back DJ, Byakika-Kibwika P, Else LJ, et al. Unintended pregnancies observed with combined use of the levonorgestrel contraceptive implant and efavirenz-based antiretroviral therapy: a three-arm pharmacokinetic evaluation over 48 weeks. Clin Infect Dis 2016; 62:675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perry SH, Swamy P, Preidis GA, Mwanyumba A, Motsa N, Sarero HN. Implementing the Jadelle implant for women living with HIV in a resource-limited setting: concerns for drug interactions leading to unintended pregnancies. AIDS 2014; 28:791–793. [DOI] [PubMed] [Google Scholar]
- 37.Vogler MA, Patterson K, Kamemoto L, Park JG, Watts H, Aweeka F, et al. Contraceptive efficacy of oral and transdermal hormones when co-administered with protease inhibitors in HIV-1-infected women: pharmacokinetic results of ACTG trial A5188. J Acquir Immune Defic Syndr 2010; 55:473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sekar VJ, Lefebvre E, Guzman SS, Felicione E, De Pauw M, Vangeneugden T, et al. Pharmacokinetic interaction between ethinyl estradiol, norethindrone and darunavir with low-dose ritonavir in healthy women. Antivir Ther 2008; 13:563–569. [PubMed] [Google Scholar]
- 39.Luque AE, Cohn SE, Park JG, Cramer Y, Weinberg A, Livingston E, et al. Depot medroxyprogesterone acetate in combination with a twice-daily lopinavir-ritonavir-based regimen in HIV-infected women showed effective contraception and a lack of clinically significant interactions, with good safety and tolerability: results of the ACTG 5283 study. Antimicrob Agents Chemother 2015; 59:2094–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murnane PM, Heffron R, Ronald A, Bukusi EA, Donnell D, Mugo NR, et al. Preexposure prophylaxis for HIV-1 prevention does not diminish the pregnancy prevention effectiveness of hormonal contraception. AIDS 2014; 28:1825–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Callahan R, Nanda K, Kapiga S, Malahleha M, Mandala J, Ogada T, et al. Pregnancy and contraceptive use among women participating in the FEM-PrEP trial. J Acquir Immune Defic Syndr 2015; 68:196–203. [DOI] [PubMed] [Google Scholar]
- 42.Song IH, Borland J, Chen S, Wajima T, Peppercorn AF, Piscitelli SC. Dolutegravir has no effect on the pharmacokinetics of oral contraceptives with norgestimate and ethinyl estradiol. Ann Pharmacother 2015; 49:784–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whiteman MK, Jeng G, Samarina A, Akatova N, Martirosyan M, Kissin DM, et al. Associations of hormonal contraceptive use with measures of HIV disease progression and antiretroviral therapy effectiveness. Contraception 2015; 93:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Day S, Graham SM, Masese LN, Richardson BA, Kiarie JN, Jaoko W, et al. A prospective cohort study of the effect of depot medroxyprogesterone acetate on detection of plasma and cervical HIV-1 in women initiating and continuing antiretroviral therapy. J Acquir Immune Defic Syndr 2014; 66:452–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hubacher D, Liku J, Kiarie J, Rakwar J, Muiruri P, Omwenga J, et al. Effect of concurrent use of antiretroviral therapy and levonorgestrel sub-dermal implant for contraception on CD4 counts: a prospective cohort study in Kenya. J Int AIDS Soc 2013; 16:18448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polis CB, Nakigozi G, Ssempijja V, Makumbi FE, Boaz I, Reynolds SJ, et al. Effect of injectable contraceptive use on response to antiretroviral therapy among women in Rakai, Uganda. Contraception 2012; 86:725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson D, Kempf MC, Wilson CM, Shrestha S. Hormonal contraceptive use and response to antiretroviral therapy among adolescent females. HIV AIDS Rev 2011; 10:65–69. [Google Scholar]
- 48.Chu JH, Gange SJ, Anastos K, Minkoff H, Cejtin H, Bacon M, et al. Hormonal contraceptive use and the effectiveness of highly active antiretroviral therapy. Am J Epidemiol 2005; 161:881–890. [DOI] [PubMed] [Google Scholar]
- 49.Cejtin HE, Jacobson L, Springer G, Watts DH, Levine A, Greenblatt R, et al. Effect of hormonal contraceptive use on plasma HIV-1-RNA levels among HIV-infected women. AIDS 2003; 17:1702–1704. [DOI] [PubMed] [Google Scholar]
- 50.Heffron R, Mugo N, Were E, Kiarie J, Bukusi E, Mujugira A, et al. PrEP is efficacious for HIV prevention among women using DMPA for contraception. Topics Antivir Med 2014; 22:498. [Google Scholar]
- 51.Kasonde M, Niska RW, Rose C, Henderson FL, Segolodi TM, Turner K, et al. Bone mineral density changes among HIV-uninfected young adults in a randomised trial of preexposure prophylaxis with tenofovir-emtricitabine or placebo in Botswana. PLoS One 2014; 9:e90111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carten ML, Kiser JJ, Kwara A, Mawhinney S, Cu-Uvin S. Pharmacokinetic interactions between the hormonal emergency contraception, levonorgestrel (Plan B), and Efavirenz. Infect Dis Obstet Gynecol 2012; 2012:137192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mildvan D, Yarrish R, Marshak A, Hutman HW, McDonough M, Lamson M, et al. Pharmacokinetic interaction between nevirapine and ethinyl estradiol/norethindrone when administered concurrently to HIV-infected women. J Acquir Immune Defic Syndr 2002; 29:471–477. [DOI] [PubMed] [Google Scholar]
- 54.Ouellet D, Hsu A, Qian J, Locke CS, Eason CJ, Cavanaugh JH, et al. Effect of ritonavir on the pharmacokinetics of ethinyl oestradiol in healthy female volunteers. Br J Clin Pharmacol 1998; 46:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang J, Chung E, Yones C, Persson A, Mahnke L, Eley T, et al. The effect of atazanavir/ritonavir on the pharmacokinetics of an oral contraceptive containing ethinyl estradiol and norgestimate in healthy women. Antivir Ther 2011; 16:157–164. [DOI] [PubMed] [Google Scholar]
- 56.Atrio J, Stanczyk FZ, Neely M, Cherala G, Kovacs A, Mishell DR., Jr Effect of protease inhibitors on steady state pharmacokinetics of oral norethindrone contraception in HIV infected women. J Acquir Immune Defic Syndr 2014; 65:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kasserra C, Li J, March B, O’Mara E. Effect of vicriviroc with or without ritonavir on oral contraceptive pharmacokinetics: a randomized, open-label, parallel-group, fixed-sequence crossover trial in healthy women. Clin Ther 2011; 33:1503–1514. [DOI] [PubMed] [Google Scholar]
- 58.Kearney BP, Mathias A. Lack of effect of tenofovir disoproxil fumarate on pharmacokinetics of hormonal contraceptives. Pharmacotherapy 2009; 29:924–929. [DOI] [PubMed] [Google Scholar]
- 59.Todd CS, Deese J, Wang M, Hubacher D, Steiner MJ, Otunga S, et al. Sino-implant (II)(R) continuation and effect of concomitant tenofovir disoproxil fumarate-emtricitabine use on plasma levonorgestrel concentrations among women in Bondo, Kenya. Contraception 2015; 91:248–252. [DOI] [PubMed] [Google Scholar]
- 60.Abel S, Russell D, Whitlock LA, Ridgway CE, Muirhead GJ. Effect of maraviroc on the pharmacokinetics of midazolam, lamivudine/zidovudine, and ethinyloestradiol/levonorgestrel in healthy volunteers. Br J Clin Pharmacol 2008; 65 suppl 1:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anderson MS, Hanley WD, Moreau AR, Jin B, Bieberdorf FA, Kost JT, et al. Effect of raltegravir on estradiol and norgestimate plasma pharmacokinetics following oral contraceptive administration in healthy women. Br J Clin Pharmacol 2011; 71:616–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burger D, van der Heiden I, la Porte C, van der Ende M, Groeneveld P, Richter C, et al. Interpatient variability in the pharmacokinetics of the HIV nonnucleoside reverse transcriptase inhibitor efavirenz: the effect of gender, race, and CYP2B6 polymorphism. Br J Clin Pharmacol 2006; 61:148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muro E, Droste JA, Hofstede HT, Bosch M, Dolmans W, Burger DM. Nevirapine plasma concentrations are still detectable after more than 2 weeks in the majority of women receiving single-dose nevirapine: implications for intervention studies. J Acquir Immune Defic Syndr 2005; 39:419–421. [DOI] [PubMed] [Google Scholar]
- 64.Frohlich M, Burhenne J, Martin-Facklam M, Weiss J, von Wolff M, Strowitzki T, et al. Oral contraception does not alter single dose saquinavir pharmacokinetics in women. Br J Clin Pharmacol 2004; 57:244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aweeka FT, Rosenkranz SL, Segal Y, Coombs RW, Bardeguez A, Thevanayagam L, et al. The impact of sex and contraceptive therapy on the plasma and intracellular pharmacokinetics of zidovudine. Aids 2006; 20:1833–1841. [DOI] [PubMed] [Google Scholar]
- 66.Fotherby K, Akpoviroro J, Abdel-Rahman HA, Toppozada HK, de Souza JC, Coutinho EM, et al. Pharmacokinetics of ethynyloestradiol in women for different populations. Contraception 1981; 23:487–496. [DOI] [PubMed] [Google Scholar]
- 67.Fotherby K. Variability of pharmacokinetic parameters for contraceptive steroids. J Steroid Biochem 1983; 19:817–820. [DOI] [PubMed] [Google Scholar]
- 68.Lobo RA, Stanczyk FZ. New knowledge in the physiology of hormonal contraceptives. Am J Obstet Gynecol 1994; 170:1499–1507. [DOI] [PubMed] [Google Scholar]
- 69.Cherala G, Edelman A, Dorflinger L, Stanczyk FZ. The elusive minimum threshold concentration of levonorgestrel for contraceptive efficacy. Contraception 2016; 94:104–108. [DOI] [PubMed] [Google Scholar]
- 70.Alvarez F, Brache V, Faundes A, Tejada AS, Thevenin F. Ultrasonographic and endocrine evaluation of ovarian function among Norplant implants users with regular menses. Contraception 1996; 54:275–279. [DOI] [PubMed] [Google Scholar]
- 71.Sivin I, Wan L, Ranta S, Alvarez F, Brache V, Mishell DR, Jr, et al. Levonorgestrel concentrations during 7 years of continuous use of Jadelle contraceptive implants. Contraception 2001; 64:43–49. [DOI] [PubMed] [Google Scholar]
- 72.Carey D, Puls R, Amin J, Losso M, Phanupak P, Foulkes S, et al. Efficacy and safety of efavirenz 400 mg daily versus 600 mg daily: 96-week data from the randomised, double-blind, placebo-controlled, noninferiority ENCORE1 study. Lancet Infect Dis 2015; 15:793–802. [DOI] [PubMed] [Google Scholar]
- 73.European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP). CHMP assessment report: Levonelle 1500 mcg tablets and associated names; 26 May 2016. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Levonelle_13/WC500211776.pdf [Accessed 1 December 2016]. [Google Scholar]
- 74.Steiner MJ, Kwok C, Dominik R, Byamugisha JK, Chipato T, Magwali T, et al. Pregnancy risk among oral contraceptive pill, injectable contraceptive, and condom users in Uganda, zimbabwe, and Thailand. Obstet Gynecol 2007; 110:1003–1009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


