Abstract
Differentiating arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C) from other cardiomyopathies is clinically important but challenging. Although the modified Task Force Criteria can facilitate diagnosis of ARVD/C according to clinical manifestations, histopathological examination plays a pivotal role in excluding other diseases that can mimic ARVD/C. Here, we report a patient with amyloidosis that initially presented similarly to ARVD/C. The diagnosis was confirmed by endomyocardial biopsy, and catheter ablation eliminated the ventricular tachyarrhythmias through an epicardial approach.
Keywords: Amyloidosis, Arrhythmogenic right ventricular cardiomyopathy-dysplasia, Catheter ablation, Biopsy, Ventricular tachycardia
Introduction
Differential diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C) from other cardiomyopathies is of clinical importance but is challenging. Although the modified Task Force Criteria facilitates the diagnosis of ARVD/C based on clinical manifestations, histopathological examination plays a pivotal role in excluding the possibility of other diseases that can mimic ARVD/C. Here, we report a patient who was confirmed to have amyloidosis-related right ventricular tachycardia/fibrillation (VT/VF) with initial clinical manifestations similar to those in ARVD/C.
Case
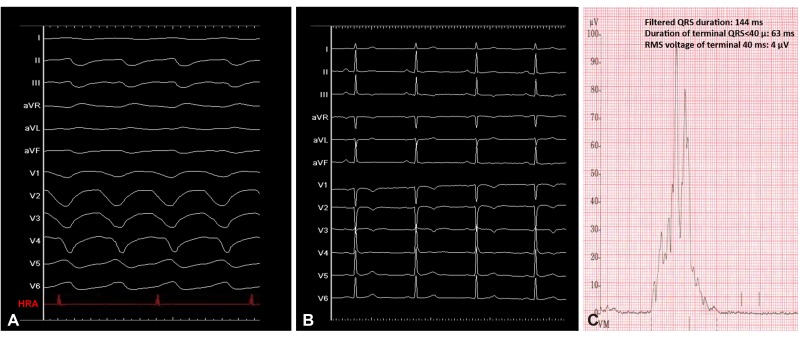
A 53-year-old man presented with syncope, and a wide QRS complex tachycardia with left bundle branch block (LBBB) morphology and superior axis (Fig. 1A, tachycardia cycle length: 321 ms) was documented. The patient denied generalized weakness or fatigue. Table 1 shows results from the initial laboratory examinations and urinalysis. Mild anemia and hypoalbuminemia were noted. Twelve-lead electrocardiogram demonstrated T wave inversion of precordial leads from V1 to V4 (Fig. 1B), while late potentials were detected by signal-averaged electrocardiogram (SAECG; filtered QRS duration: 144 ms, duration of terminal QRS <40 µ: 63 ms, and root means square voltage of terminal 40 ms: 4 µV; Fig. 1C). Echocardiographic examination revealed a mildly dilated right ventricle (RV) and right ventricular outflow tract (parasternal long axis RVOT: 34 mm), regional akinesia of the basal RV with fractional area change of 38%, normal left ventricular wall thickness (septal wall thickness: 9 mm and posterior wall thickness 9 mm), and preserved left ventricular systolic function and diastolic function (lateral e': 17). Intravenous amiodarone and a beta-blocker were prescribed for frequent tachyarrhythmias with unsatisfactory response. Owing to the drug-refractory tachyarrhythmias, a standardized electrophysiological study and catheter ablation were performed in a fasting state with sedation after obtaining informed consent.
Fig. 1. Electrocardiograms of the patient. (A) 12-lead ECG morphology showed a wide QRS complex tachycardia with LBBB morphology and superior axis. Intracardiac tracing demonstrated VA dissociation, which rendered the diagnosis of supraventricular tachycardia less likely. (B) ECG during sinus rhythm showed T wave inversion of precordial leads from V1-V4. (C) SAECG showed positive depolarization abnormalities, including fQRS, duration of terminal QRS <40 µV, and RMS voltage in terminal 40 ms. ECG: electrocardiogram, LBBB: left bundle branch block, VA: ventricular arrhythmia, SAECG: signal-averaged electrocardiogram, RMS: root means square.
Table 1. Initial laboratory tests and urinary analysis.
| Variables | Values |
|---|---|
| CBC/DC | |
| White blood cell count (/µL) | 5900 |
| Hemoglobin (g/dL) | 12.3 |
| Platelet (/μL) | 205000 |
| Neutrophil/lymphocyte (%/%) | 71.3/24.1 |
| Monocyte (%) | 4.0 |
| Eosinophil (%) | 0.1 |
| Basophil (%) | 0.5 |
| Biochemistry | |
| Total protein (g/dL) | 6.2 |
| Albumin (g/dL) | 3.1 |
| BUN (mg/dL) | 20.0 |
| Creatinine (mg/dL) | 0.95 |
| Sodium (mmol/L) | 135.0 |
| Potassium (mmol/L) | 3.7 |
| Creatinine (mg/dL) | 8.6 |
| Urinalysis | |
| Chemistry strip | |
| Color | Yellow |
| Sugar | - |
| Bilirubin | - |
| Ketone | - |
| Gravity | ≧1.030 |
| Occult blood | +/− |
| pH | 5.5 |
| Protein | +/− |
| Nitrite | - |
| Leukocyte esterase | - |
| RBC | 0–2 |
| White blood cell count/pus | 0–2 |
| Squamous epithelium | 0–2 |
CBC: complete blood count, DC: differential count; BUN: blood urea nitrogen, RBC: red blood cell count
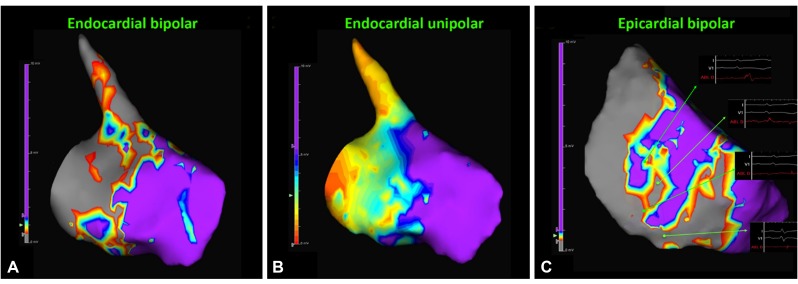
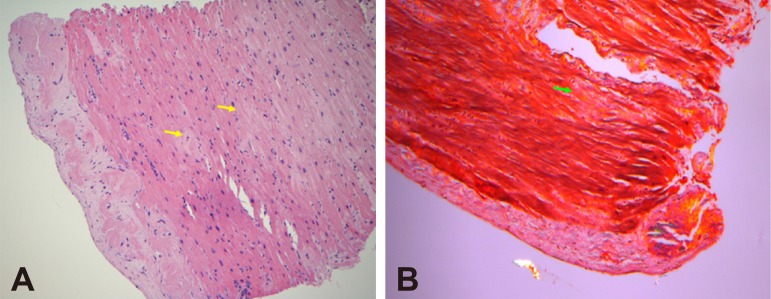
The baseline rhythm was sinus rhythm (cycle length : 620 ms) without identifiable pre-excitation. Ventricular pacing demonstrated ventricular arrhythmia (VA) dissociation. Tachycardia was induced by programmed ventricular stimulation with triple extrastimuli without administration of isoprenaline. Ventricular tachycardia was confirmed by the presence of VA dissociation (Fig. 1A). The arrhythmogenic substrate was evaluated using a 3D mapping system (EnsiteNavX™, St Jude Inc., St Paul, MN, USA). Mapping and ablation were achieved using an irrigated catheter (FlexAbility, St Jude Inc., St Paul, MN, USA). Radiofrequency energy was delivered with a maximal power of 30-40 watts for the endocardium and 20-35 watts for the epicardium, targeting an impedance drop of 10 Ohms and maintaining a minimum of 120 seconds at the site of the disappearance of abnormal potentials at each point for substrate modification. Endocardial bipolar and unipolar voltage mapping demonstrated an extensive scar area extending from the right ventricular outflow tract (RVOT) to basal RV free wall (cutoff value: 0.5-1.5 mV for bipolar voltage mapping and 5.5 mV for unipolar voltage mapping, Fig. 2A, B), while epicardial bipolar voltage mapping depicted scarring within the RVOT, basal RV free wall and apex (cutoff value: 0.5-1 mV, Fig. 2C), which was mimicking the arrhythmogenic substrates in patients with ARVD. Given the entity of hemodynamically unstable VT/VF, radiofrequency catheter ablation (RFCA) based on substrate modification by targeting abnormal electrograms, which were defined by the presence of amplitude <1.5 mV, or associated wide duration (>80 ms), multiple (>3), or delayed components extending beyond the end of the QRS complex,1) located at the inferior endocardial and epicardial RV was performed, which rendered the VT/VF noninducible by the same induction protocol under the infusion of isoprenaline. The total procedure was 3.7 hours and fluoroscopic time was 41 minutes. No periprocedural complication was noted. An implantable cardioverter-defibrillator (ICD) implantation was performed. There was no recurrence of ventricular tachyarrhythmias during the follow-up period of 6 months during which time no antiarrhythmic drugs were administered. Endomyocardial biopsy (EMB) of the RV free wall showed amyloid light-chain (AL) amyloidosis (Fig. 3). Bone marrow biopsy confirmed multiple myeloma was the etiology. After RFCA, the patient received chemotherapy with bortezomib, cyclophosphamide, and dexamethasone, which stabilized his disease status.
Fig. 2. Endocardial and epicardial RV voltage mapping. (A) Bipolar voltage mapping demonstrated the scar/low voltage zone extending from RVOT to RV free wall. (B) Unipolar voltage mapping showed a larger abnormal substrate extending from RVOT to RV free wall compared to those identified by bipolar voltage mapping. (C) Epicardial voltage mapping also revealed extensive scarring involving RVOT, basal RV free wall and RV apex. Examples of abnormal electrograms, including fractionated and isolated late potentials, were recorded surrounding the scar/LVZ. RV: right ventricular, RVOT: right ventricular outflow tract, LVZ: low voltage zone.
Fig. 3. Histopathological examination of amyloid heart tissue by endomyocardial biopsy. (A) H&E staining (×400) of endomyocardial biopsy obtained from right ventricular free wall. (B) Congo red staining showed light-chain right ventricular amyloidosis (yellow and green arrows).
Discussion
In our case, VT was confirmed by the dissociation of VA conduction during wide QRS complex tachycardia. Furthermore, pace-mapping for the potential exit of VT demonstrated the best pace-mapping site as the RV free wall rather than the RV septum. The above findings made the diagnosis of bundle branch reentrant VT (BBRVT) less likely. Endocardial and epicardial substrate modification of the RV free wall that resulted in the noninducibility of ventricular tachyarrhythmia also provided indirect evidence for substrate VT rather than BBRVT in the present case. Currently, modified Task Force criteria2) helps with the early and accurate diagnosis of ARVD/C. Distinguishing ARVD from other mimicking diagnoses such as myocarditis, sarcoidosis, or endomyocardial fibrosis, is warranted.3) Dominant right ventricular involvement with AL amyloidosis and initial manifestations similar to ARVD/C has not been previously reported. The clinical characteristics of this patient fulfilled 2 major criteria and 2 minor criteria, which could easily result in a misdiagnosis of ARVD/C. EMB serves as an important value in differentiating these two different etiologies. Furthermore, given the poor outcomes and advanced treatment options with bortezomib and lenalidomide, recognition of cardiac involvement in patients with multiple myeloma-associated AL amyloidosis is clinically relevant.4) The heterogeneous deposition of amyloid within myocardial tissue could contribute to non-uniform conduction properties and development of potential critical isthmuses that could lead to the development of ventricular arrhythmogenicity. Radiofrequency catheter ablation through the application of heat energy provides the interruption and destruction of potential isthmuses, which could result in ventricular tachyarrhythmia noninducibility. Aside from the above, previous studies also demonstrated the importance of substrate modification and noninducibility as reasonable endpoints for scar-related ventricular tachyarrhythmias,5),6) as shown in the present case. A noteworthy aspect about this case is that it also demonstrates the promising results of RFCA in eliminating VT/VF arising from the arrhythmogenic substrate in patients with cardiac amyloidosis.
In conclusion, our case highlights the pivotal value of endomyocardial biopsy and histopathological examinations for the differentiation of ARVD/C from other cardiomyopathies in patients with ventricular tachycardia arising from the right ventricle. In certain unusual situations, ventricular tachyarrhythmias caused by cardiac amyloidosis with right ventricular involvement, which could be eliminated by RFCA, can display similar clinical features that mimic ARVD/C.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Chung FP, Lin YJ, Chang SL, et al. Long-term follow-up of catheter ablation of ventricular arrhythmias: experiences from a tertiary referral center in Taiwan. Acta Cardiol Sin. 2015;31:8–17. doi: 10.6515/ACS20140721A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Eur Heart J. 2010;31:806–814. doi: 10.1093/eurheartj/ehq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung FP, Lin YJ, Chang SL, et al. Current and state of the art on the electrophysiologic characteristics and catheter ablation of arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Cardiol. 2015;65:441–450. doi: 10.1016/j.jjcc.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 4.Mori M, Kitagawa T, Sasaki Y, et al. Long-term survival of a patient with multiple myeloma-associated severe cardiac AL amyloidosis after implantation of a cardioverter-defibrillator. Rinsho Ketsueki. 2014;55:450–455. [PubMed] [Google Scholar]
- 5.Frankel DS, Mountantonakis SE, Zado ES, et al. Noninvasive programmed ventricular stimulation early after ventricular tachycardia ablation to predict risk of late recurrence. J Am Coll Cardiol. 2012;59:1529–1535. doi: 10.1016/j.jacc.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 6.Sacher F, Lim HS, Derval N, et al. Substrate mapping and ablation for ventricular tachycardia: the LAVA approach. J Cardiovasc Electrophysiol. 2015;26:464–471. doi: 10.1111/jce.12565. [DOI] [PubMed] [Google Scholar]