Abstract
The cilium is an evolutionally conserved apical membrane protrusion that senses and transduces diverse signals to regulate a wide range of cellular activities. The cilium is dynamic in length, structure, and protein composition. Dysregulation of ciliary dynamics has been linked with ciliopathies and other human diseases. The cilium undergoes cell-cycle-dependent assembly and disassembly, with ciliary resorption linked with G1-S transition and cell-fate choice. In the resting cell, the cilium remains sensitive to environmental cues for remodeling during tissue homeostasis and repair. Recent findings further reveal an interplay between the cilium and extracellular vesicles and identify bioactive cilium-derived vesicles, posing a previously unrecognized role of cilia for sending signals. The photoreceptor outer segment is a notable dynamic cilium. A recently discovered protein transport mechanism in photoreceptors maintains light-regulated homeostasis of ciliary length.
Cilia are apical membrane protrusions involved in development, differentiation, homeostasis, and disease. They are constantly changing in composition, shape, and length and may play roles in cell–cell communication.
The primary cilium is a nonmotile cilium. It consists of a ciliary membrane protrusion supported by 9+0 axonemal microtubules anchored on the basal body and extending through the ciliary transition zone. Most cells have a single primary cilium with exception in few cell types that express motile cilia (e.g., airway epithelial cells, ependymal cells, choroid plexus, and sperm) or no cilium (e.g., hematopoietic-lineage cells, oncogenically transformed cells). Although the ciliary membrane is continuous with the plasma membrane, the ciliary membrane–localized components (e.g., receptors, channels) are highly selected through a concerted effort in cargo sorting, targeting, and transport. Human mutations in proteins pivotal for ciliary structure and function have been linked with a family of diseases termed ciliopathies, with wide-ranging clinical manifestations, including cystic kidney, polydactyly, mental retardation, and retinal degeneration. Recently, dysfunction and/or dynamic dysregulation of the cilium have been implicated in an even broader spectrum of clinical conditions, including microcephaly, cancer, diabetes, anosmia, skeletal dysplasia, and obesity. Several recent reviews have detailed the structure, protein trafficking, and signal transduction of the cilium, and its relevance to various disorders (Eggenschwiler and Anderson 2007; Waters and Beales 2011; Christensen et al. 2012; Sung and Leroux 2013).
The primary cilium is a dynamic organelle. The mechanism by which the ciliary axoneme elongates (i.e., ciliogenesis) has been extensively studied and reviewed (Santos and Reiter 2008; Ishikawa and Marshall 2011; Kobayashi and Dynlacht 2011). Once established, the cilium can undergo resorption in various cellular contexts. We will use the term resorption interchangeably with disassembly, shortening, and retraction in this review. We will not discuss deciliation (or deflagellation), that is, cilium detachment from cell body through severing the axoneme distal to the ciliary transition zone. Several comprehensive reviews have also covered the signaling pathways and components key to ciliary disassembly, and its connection to cancer (Plotnikova et al. 2008; Izawa et al. 2015; Keeling et al. 2016; Liang et al. 2016). In this review, we will focus on discussing the biological importance of ciliary dynamics during development, differentiation, homeostasis, and diseases. We will also discuss the turnover of the ciliary plasma membrane and its possible role in cell–cell communication through extracellular vesicles (EVs). Although this review will focus primarily on mammalian cells, we will summarize the ciliary shedding phenomenon recently found in algae and worm.
Finally, we will highlight a notably dynamic cilium, the photoreceptor outer segment (OS). The OS is a popular cilium model for several reasons. First, the OS is large, providing good spatial resolution. Although the typical primary cilium is ∼2- to 3-µm long and ∼0.2 µmdia, the rodent rod OS is ∼25 µm long and ∼1.4 µmdia. Second, the molecular composition of the OS has been detailed, and a large repertoire of ciliary molecules can be tagged to follow OS dynamics. Third, the OSs between photoreceptors are aligned and packed at high density in the outer retina, allowing convenient access to large numbers of cilia in a single histological section. Fourth, retinitis pigmentosa (RP), a common form of rod-predominant retinal degeneration, has been linked with mutations in proteins responsible for rod OS morphogenesis and maintenance (RetNet). This provides leverage for further mechanistic study. Fifth, the OS undergoes constant renewal throughout the animal’s adult life while maintaining a roughly constant length (Young 1967). Scientists have taken advantage of these features and made progress in understanding the dynamics in ciliary protein composition, cytostructure, and turnover of our light-sensing cilium.
CILIARY DYNAMICS IN CELL-CYCLE PROGRESSION
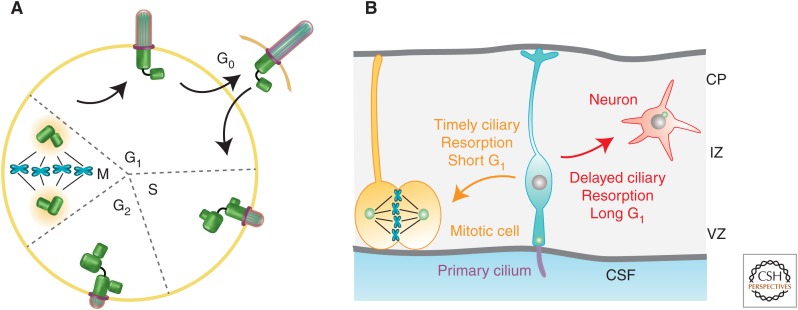
There is an established link between ciliary dynamics and cell-cycle progression (Tucker et al. 1979). This link has been best shown in nontransformed cell cultures (e.g., 3T3, RPE-1, mouse embryonic fibroblast) in which serum starvation can induce concomitant cell quiescence (G1/G0) and ciliogenesis (Fig. 1A). Serum readdition induces biphasic resorption, first at the G1-S transition and then at the G2-M transition. A growing body of evidence put forth by several laboratories suggests that this temporal correlation is not just a coincidence. The cilium plays an active role in regulating the cell cycle, particularly the G1-S transition.
Figure 1.
Ciliary dynamics and cell-cycle progression. (A) Diagram depicts cell-cycle-dependent ciliary assembly and biphasic resorption. (Figure created from data in Bettencourt-Dias and Carvalho-Santos 2008.) (B) Cartoon in which the cell fate choice of a dividing cortical progenitor depends on the time required for ciliary resorption, and, in turn, G1 duration. The primary cilium projecting from the apical endfeet of neural progenitor (in turquoise) faces the ventricle and encounters signal gradient(s) from the cerebral spinal fluid (CSF). VZ, Ventricle zone; IZ, intermediate zone; CP cortical plate.
Pugacheva et al. first showed that the growth factor PDGF-AA is sufficient to trigger ciliary resorption through an Aurora A-HEF1-histone deacetylase 6 (HDAC6) enzymatic cascade that promotes axonemal microtubule disassembly (Pugacheva et al. 2007). Naturally occurring mutations in the inositol-1,4,5-tripsphosphate 5-phosphatase (INPP5E), described in patients with a ciliopathy (Joubert syndrome), cause an acceleration of both ciliary disassembly and S phase reentry (Bielas et al. 2009; Jacoby et al. 2009). Kim et al. showed that cells with longer cilia, via silencing a mother centriole protein Nde1, take a longer time to reenter the cell cycle. Further, Nde1-deficient Zebrafish embryos had longer cilia and fewer proliferating cells in the Kupffer’s vesicle (Kim et al. 2011).
Li et al. studied Tctex-1 in serum-stimulated cilium disassembly (Li et al. 2011). Tctex-1 is a light chain subunit of cytoplasmic dynein (King et al. 1996). Phosphorylation of Tctex-1 at Thr94 uncouples it from the dynein motor complex (Chuang et al. 2005). Thr94 phosphorylated Tctex-1 is recruited to the transition zone of the cilium preceding ciliary resorption, a process that is required for both ciliary shortening and G1-S transition. Further, ectopic expression of phosphomimic Tctex-1 hastens both ciliary resorption and S phase reentry. Importantly, Tctex-1’s role in S phase reentry is ciliumdependent; it is dispensable in nonciliated cells. These results argue against the idea that cilium resorption is a consequence of cell-cycle reentry. It is a prerequisite instead. These results, together with those above, suggest that the time required for cilium resorption largely determines the length of G1. They also suggest that ciliary resorption is a novel checkpoint for G1-S transition (Kim and Tsiokas 2011; Sung and Li 2011). In support of this view, several molecules important for ciliary resorption, including Aurora A, HDAC6, centrosomal-P4.1-associated protein (CPAP), and FLS1/CKDL5, have been shown to be vital for cell-cycle progression (Li et al. 2011; Hu et al. 2015; Gabriel et al. 2016).
CILIARY DYNAMICS IN CELL-FATE CHOICE DURING BRAIN DEVELOPMENT
In the developing neocortex, the primary cilium is also present on the apical surface of dividing progenitors that face the ventricle (Fig. 1B). Live imaging showed that these cilia are dynamic, undergoing constant extension and retraction (Higginbotham et al. 2013). These cilia can sense extrinsic cues in the cerebral spinal fluid, such as mitogen insulin growth factor (IGF) that accelerates G1-S progression (Hodge et al. 2004; Lehtinen et al. 2011).
A balance between progenitor cell division and cell differentiation into neurons controls brain size. G1 length increases as neural development progresses (Takahashi et al. 1995). Li et al. (2011) used in utero electroporation to ablate Tctex-1 in cortical progenitors. They found that Tctex-1-suppressed cells, which presumably had longer G1, underwent precocious neuronal differentiation at the expense of proliferation. Conversely, overexpression of the phosphomimic Tctex-1 significantly expanded the proliferative progenitor pool by shortening G1. They proposed that timely ciliary resorption of cortical progenitors is key to progress for cell division, whereas perturbation of ciliary resorption drives cells to exit the cell cycle and differentiate (Fig. 1B). This hypothesis was supported by the finding that attenuating other components critical for ciliary resorption (i.e., Aurora A, HDAC6, or CPAP) in cortical progenitors consistently caused precocious neuronal differentiation (Li et al. 2011; Gabriel et al. 2016). The same group further showed that IGF-1 is sufficient for enrichment of phospho-Tctex-1 at the ciliary base and, in fact, it colocalized with phospho-IGF-1 receptor in dividing cortical progenitors (Yeh et al. 2013). The IGF-1-mediated mitogenic effect was dependent on Tctex-1 and the cilium. Cell-autonomous silencing of IGF-1 receptor and its downstream signaling components similarly caused premature cell-cycle exit of the cortical progenitors.
Premature switching from self-renewal to differentiation of cortical progenitors depletes the progenitor population and, ultimately, leads to the development of a smaller brain. Thus, it is logical that some forms of microcephaly could be caused by dysregulated ciliary resorption. Indeed, human mutations in Nde1, INPP5E, CPAP, IGF-1, and IGF-1 receptors have been associated with microcephaly (Walenkamp et al. 2005; Walenkamp and Wit 2006; Bielas et al. 2009; Jacoby et al. 2009; Bakircioglu et al. 2011; Gabriel et al. 2016).
CILIARY DYNAMICS IN TISSUE HOMEOSTASIS
In the “steady state,” the ciliary length is likely to be maintained through similar mechanism(s) used in the cell-cycle-dependent assembly and disassembly. A body of evidence indicates that environmental changes can trigger cilium remodeling. Although most studies were conducted in vitro, cigarette smoke has been linked with shortened cilia in the airway epithelial cells in vivo (Leopold et al. 2009). This results in compromised mucociliary clearance and increased susceptibility to respiratory disorders, including chronic obstructive pulmonary disease. A recent study showed that cigarette smoke–regulated ciliary shortening is caused by increased HDAC6 through epigenetic gene modification (Lam et al. 2013). HDAC6 has a dual role in both ciliary resorption and autophagy degradation. Autophagy-deficient lung epithelia were protected from ciliary shortening. These data underscore autophagy’s roles in removing ciliary components during ciliary shortening and dynamic cilium as a therapeutic target for various respiratory diseases.
Cilia regress as certain tissues terminally differentiate, but regrow under pathophysiological conditions. In auditory hair cells, specialized cilia (kinocilia) are required for proper development of the organ of Corti, and then disappear shortly after birth. These cilia reappear on noise-induced trauma (Engstrom et al. 1983; Sobkowicz et al. 1995). In the eyes, adult corneal endothelial cells have no discernible cilia, but the cilia regrow concomitant with the initiation of repair following wound injury (Blitzer et al. 2011).
Using an acute kidney injury model, it has been shown that the length of renal epithelial cilia first increases and then decreases, following kinetics closely associated with the appearance and disappearance of other histopathologies (Verghese et al. 2009). These investigators proposed that the relatively small increase in cilium length might be sufficient to enhance the sensitivity in sensing cue(s), and thereby circumventing the compromised microenvironment. Intriguingly, adult mice deficient of Kif3 (required for ciliogenesis) had a normal kidney, but these mice were unable to overcome acute kidney injury and developed renal cysts (Patel et al. 2008). Together, these results strongly indicate that the ciliary elongation observed during tissue repair is physiologically relevant. They also imply that the same set of components is used for ciliogenesis and cilium regrowth during tissue homeostasis.
CILIUM MEMBRANE TURNOVER AND INTERACTION OF EXTRACELLULAR VESICLES
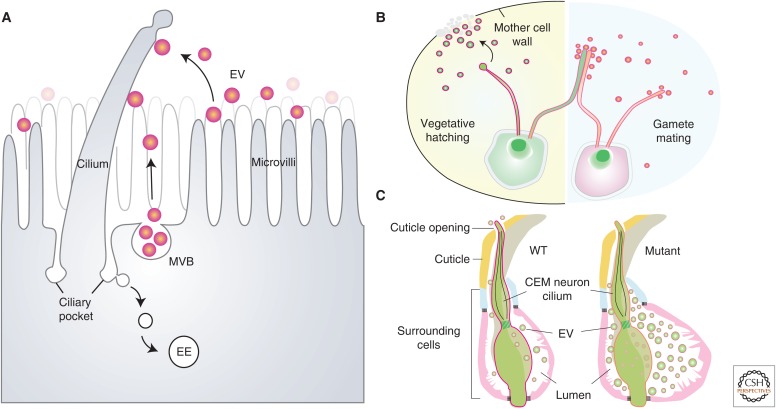
Ciliary resorption represents a concerted effort coordinated by multiple processes: the disassembly of axonemal microtubules, blockage of ciliary (re)assembly, and removal of ciliary membranes and luminal components. Two excellent reviews have highlighted recent progress on the signal pathways and the machinery governing axonemal microtubule dynamics during ciliary resorption (Izawa et al. 2015; Liang et al. 2016). In the next two sections, we will discuss an important but understudied area of ciliary membrane turnover during ciliary resorption. Theoretically, as the axonemal microtubules disassemble, the spent ciliary membranes might be endocytosed, or bud from the ciliary tip, or both (Fig. 2A).
Figure 2.
Interplay between cilia and extracellular vesicle (EV). (A) Cartoon illustrates several models regulating the homeostasis of the ciliary membrane and its association with EVs. Periciliary membranes can be endocytosed from ciliary pocket and internalized into early endosomes (EEs). EVs can be shed from ciliary tip, microvillar membrane, or multivesicular body (MVB) fused onto the plasma membrane. These vesicles can be from the same cell, neighboring cells, or cells far away. (B) In the vegetative-stage, Chlamydomonas shed lytic enzyme-containing vesicles from their ciliary tips to digest the mother cell wall for hatching (left cartoon). During mating, opposite-sex gametes attach to each other by adhesion between flagella and the flagella-released vesicles bearing receptor-binding signals to promote cell fusion (right cartoon). (C) Cartoon illustrates vesicle secretion from the sensory cilium of a CEM (cephalic male) neuron in Caenorhabditis elegans. (Figure modified from data in Maguire et al. 2015.) In wild-type worms, vesicles can be released to the environment through a narrow opening where the cilium exposes its tip. Some mutants display abnormal vesicles accumulated in the swollen extracellular “lumen” space surrounded by a group of cells, indicating that vesicle release is actively regulated.
The proximal region of the primary cilium is surrounded by a specialized, depressed plasma membrane domain called the ciliary pocket (Fig. 3A). The plasma membrane of the ciliary pocket is decorated by abundant clathrin-coated pits and F-actin filaments, and it shows robust endocytic activity (Molla-Herman et al. 2010; Benmerah 2013; Clement et al. 2013). Further, F-actin polymerization has been implicated in ciliary resorption and cilium length control (Bershteyn et al. 2010; Kim et al. 2010; Li et al. 2011). Thus, internalization of the periciliary membrane at the ciliary base seems to be an attractive mode. This idea, however, remains to be tested.
Figure 3.
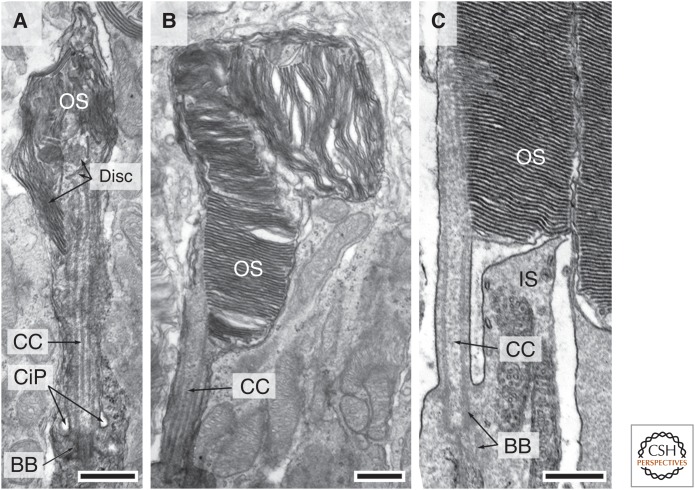
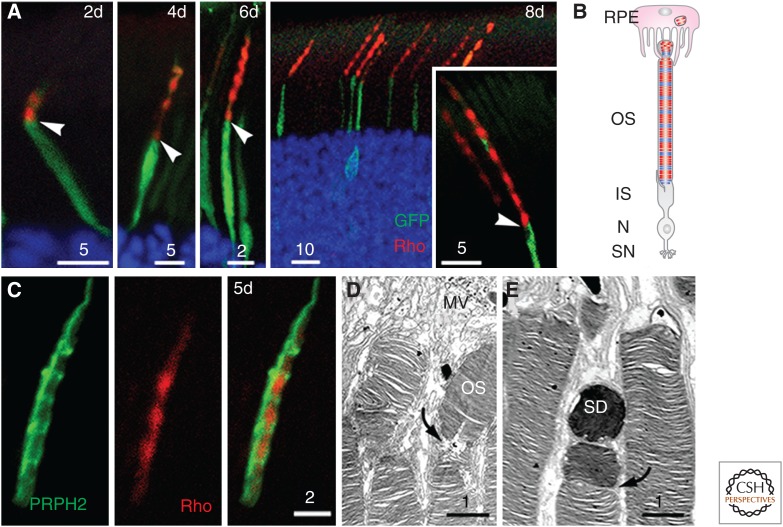
Dynamic cytostructure during outer segment (OS) development. (A,B) Electron micrographs of two developing OSs with distinct morphology from the same postnatal day 10 mouse, highlighting that early OS development is unsynchronized. (A) Shows a rudimentary OS containing flattened discs that are not aligned perpendicular to the ciliary axoneme. (B) Shows a further developed OS expressing more discs, which, however, are oriented in various directions. (C) An electron micrograph shows the proximal region of a mature OS, connecting cilium (CC), and basal body (BB) and its connected apical part of inner segment (IS). CiP, Ciliary pocket. Scale bars, 0.5 µm. (From JZ Chuang and CH Sung, unpubl.)
Using electron microscopy (EM), Marzesco et al. showed the tips of the cilia in cortical progenitors were frequently associated with EVs, particularly during early neurogenesis (Marzesco et al. 2005). These vesicles contained Prominin-1, a stem-cell marker. These investigators suggested the possibility that these vesicles were shed from the distal cilia as a means to reduce the length of cilia, and/or to communicate cell fate choice among the progenitors aligned along the ventricle borders. Although this may be correct, it is also possible that these vesicles are derived from other cells and/or other parts of the same cells (Dubreuil et al. 2007).
In a similar vein, Hirokawa’s group showed that EVs are used to deliver morphogens through leftward rotation of node cilia (a rare form of 9+0 motile cilia). Their results suggested that this process is pertinent to the development of left–right asymmetry. Time-lapse imaging of embryos carrying a lipophilic fluorescent dye showed that these vesicles were released from the apical microvilli (Tanaka et al. 2005). Ward’s group also observed interactions between the EVs and primary cilia (Woollard et al. 2007). They showed that the cilia of biliary epithelial cells of a polycystic kidney disease (PKD) mouse model (Pkhd1del2/del2) had more EVs attached than their wild-type control counterpart (Woollard et al. 2007). The biochemical and EM studies indicated that these vesicles are exosome-like and emerged from the multivesicular body fused onto plasma membranes (Fig. 2A) (Hogan et al. 2009). Regardless of the source of the vesicles, the apparently specific interaction between cilia and EVs has interest in its own right. Are signals transduced through protein–protein cross talk or membrane fusion? Do the cilium-attached vesicles exchange signals between cilia? Future researches should provide details for these questions.
CILIARY VESICLE SHEDDING IN ALGAE AND WORM
Although the origin of the ciliary-associated vesicles needs further clarification in mammalian cells, emerging evidence from three laboratories argues that vesicle shedding does occur from the tip of the flagella in Chlamydomonas, a unicellular green alga. In the mid-1970s, researchers noticed the presence of membrane vesicles in the culture media of Chlamydomonas gametes and that these vesicles are missing from cultures of flagella-less mutants (Bergman et al. 1975). Because the flagellum of Chlamydomonas is the only part not encased by the cell wall, the flagella were deduced to be the source of the vesicles.
Recently, following pulse-labeling of surface membrane proteins, the flagellar membrane was found to be continuously renewed when the cell is in its vegetative state, with a turnover rate of 6 h (Dentler 2013). Further, flagellar shortening triggered by chemical stress is concomitant with increased protein labeling in the EV pool. Thus, ciliary shedding is an attractive model to explain the reduced cilium length. In the mating stage, the ciliary shedding is even more robust, approximately twice as fast as that of vegetative algae. Cao et al. reported that a mating-related signaling protein rapidly appeared in the cilia during mating, concomitant with ciliary adhesion between gametes of opposite sexes (Fig. 2B, right) (Cao et al. 2015). They showed that the vesicles isolated from the cultures specifically bound to the cilia of the “minus” gametes, but not to the cilia of “minus” vegetative cells.
Wood et al. investigated the postmitotic hatching process of Chlamydomonas (Wood et al. 2013). During hatching, the flagellated daughter cells are trapped within the mother cell wall (Fig. 2B, left). Their live imaging detected the discharge of vesicles from the flagellar tips. The investigators referred to these vesicles as ectosomes with their size (∼100–200 nmdia) larger than a typical exosome (∼30–100 nmdia) (Cocucci and Meldolesi 2015). A key lytic enzyme required for breaking down the mother cell wall for hatching was particularly populated in both the flagellar tips and the shed ectosomes. These investigators showed, remarkably, that although the flagella-less ift88-null mutant alga was unable to hatch, this defect could be rescued by the addition of ectosomes isolated from wild-type algae. Together, these findings point to vesicle shedding as a component regulating the length and homeostasis of cilia. The identification of functional cilium-derived vesicles argues that cilia can also send signals, in addition to their previously recognized role in receiving signals.
Using live imaging, Wang et al. (2014), showed that the PKD-GFP reporter expressed in the specialized ciliated (CEM) sensory neurons of Caenorhabditis elegans can be released into the environment as vesicles (Fig. 2C). This environmental vesicle release was absent in certain mutant worms. Instead, the vesicles were trapped in an expanded extracellular lumen that was encased by a group of supporting cells. A combination of EM and genetics studies led the investigators to conclude that the environmental vesicles had originated from the periciliary membrane near the base of the cilia (rather than the ciliary tips), through a mechanism that warrants further investigation (Maguire et al. 2015).
Together, these findings posit “ciliary shedding” as a novel means to dynamically control ciliary length, and to renew the membrane and ciliary proteins. Vesicles resulting from ciliary shedding could also bring about distinct signal transmission for cell–cell communication. How such selectivity is regulated by extrinsic stimuli and intrinsic regulatory factor(s) remains an interesting topic to be explored.
MEMBRANE DYNAMICS DURING PHOTORECEPTOR CILIARY DEVELOPMENT
Ultrastructural studies showed that the primary cilia in several mouse disease models are normal in length, but not in shape (Davis et al. 2007; Tran et al. 2008; Bielas et al. 2009; Jacoby et al. 2009; Goggolidou et al. 2014; He et al. 2014; Siller et al. 2015). This finding implies that architectural remodeling is also an integral component of ciliary dynamics. Photoreceptor OS is notable for its dynamic structure. Vertebrates have evolved to use a membrane-rich modified cilium to accommodate a large number of photopigment rhodopsin molecules needed for sensitive light detection. Each OS has 10–100 million rhodopsins. They are densely packed in ∼1000 flattened disc membranes that are enwrapped by the enlarged ciliary plasma membrane (Fig. 3C).
In rodents, the rod OS begins to develop postnatally, and it takes almost 3 weeks to mature. For the most part, OS morphogenesis among rods is rather unsynchronized until near the end. Around postnatal day 5–8, the distal region of the primary cilium of the developing rod bulges. A random mixture of membranous vesicles, tubules, and sacs starts to appear (De Robertis 1956, 1960; Besharse et al. 1985). Subsequently, flattened discs emerge, but they are arrayed “vertically” (Fig. 3A). Rods express an increasing number of discs as development progresses. Although many of the distal discs remain misaligned, the basal discs gradually become stacked perpendicular to the axonemal axis (Fig. 3B). In the final stage of OS development (postnatal day 15–21), almost all discs are stacked in an orderly manner, and the OS elongates at a linear rate by adding more nascent discs at the base (LaVail 1973). The mechanism(s) by which the nascent disc is formed and rhodopsin is transported through the ciliary transition zone (i.e., connecting cilium) and incorporated into the disc membranes are currently under debate. A long-time dogma proposes that the discs are formed by pinching off the evaginated ciliary membranes (Steinberg et al. 1980). The data proposed by Chuang et al. (2007, 2015) suggest instead that nascent discs grow by repeated fusion of rhodopsin-bearing vesicles transported through the connecting cilium shafts.
As expected, several animal models deficient or carrying mutations in proteins vital for ciliogenesis (e.g., AHI1/jouberin, OFD1, IFT88, KIF17, NPHP4) fail to commence OS development and undergo early retinal degeneration (Pazour et al. 2002; Insinna et al. 2008; Louie et al. 2010; Westfall et al. 2010; Won et al. 2011). Animal models with mutations of TMEM67/Meckelin, RP1, or Prominin-1 express OSs with disorientated discs, as if they are arrested during development (Liu et al. 2003; Yang et al. 2008; Won et al. 2011; Collin et al. 2012). These studies reveal molecules important for OS disc alignment and/or cytostructural remodeling.
NON-CELL-AUTONOMOUS REGULATION OF PHOTORECEPTOR CILIUM REMOVAL
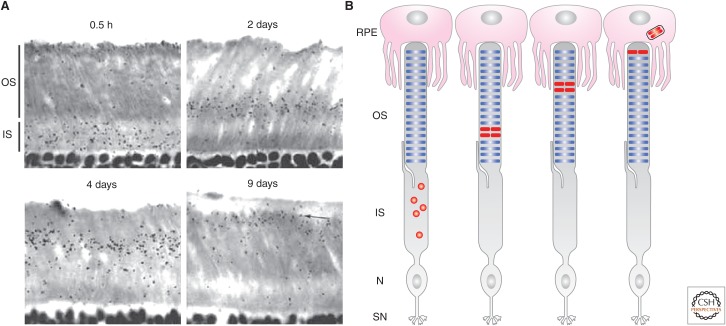
The vertebrate rod OS has evolved an elegant strategy to selectively dispose oldest parts of the cilium and replace them with new components. The concept that the OS undergoes constant renewal in the adult retina was established 50 years ago by Richard Young (Young 1967, 1968). Using autoradiography, Young found that pulse-labeled radioactive amino acids first appeared diffusely in the inner segment and then in the OS (Fig. 4A,B). The radioactive “reaction bands” formed at the base of the OS are displaced progressively toward the retinal pigment epithelium (RPE) juxtaposed to the apex of the OS. In ∼10 days, the distalmost OS discs appeared to be sloughed off in stacks and engulfed by RPE cells through phagocytosis (Young 1967, 1971; LaVail 1983; Anderson et al. 1986). Thus, Young concluded that the mouse rod OS renews every ∼10 days.
Figure 4.
Photoreceptor outer segment (OS) ciliary renewal. (A) Representative radioautographs of mouse photoreceptors pulse labeled for the indicated days (reprinted from Young 1967). (B) Illustrations of the result shown in A. The radioactivity first appears in the inner segment (IS) and then basal OS. The radioactive “reaction bands” (labeled in red) are displaced gradually toward the OS apex before being engulfed by the retinal pigment epithelium (RPE). N, Nucleus; SN, synapse.
By counting the radioactivity that appeared in the RPE cells, it was shown that OS phagocytosis is temporally synchronized with a peak of activity ∼2 h after light onset. This periodicity is regulated by circadian rhythm in rats and by light stimulation in frogs (Basinger et al. 1976; LaVail 1976). Mice deficient in molecules involved in the binding (i.e., αvβ5 integrin, MFG-E8) or engulfment (i.e., MERTK) of the OS during shedding uniformly lack the ability to synchronize OS phagocytosis at dawn, although retaining a basal phagocytic activity (Nandrot et al. 2004, 2007). On the other hand, humans or mice with retinal detachment have abnormally long OSs (Ahlers et al. 2009; Chuang et al. 2010; Zhang et al. 2010; Matsumoto et al. 2011). Retinal detachment describes a clinical symptom associated with several vision-threatening conditions (Yannuzzi et al. 2000). Whether the OS outgrowth is the result of a lack of physical contact between the OS and the RPE phagocytic cells or additional diffusible factor(s) is unclear. These results, nevertheless, point to additional yet-to-be-characterized microenvironmental regulators that influence the distal OS disposal.
CELL-AUTONOMOUS PHOTORECEPTOR CILIUM LENGTH CONTROL
The autoradiography method used by Young, although elegant, has inherent technical limitations (e.g., resolution, sensitivity, lack of protein tagging specificity) in addressing several pertinent questions related to OS homeostasis. Hsu et al. (2015) recently revisited OS renewal using a modern protein synchronization technique. They used the Cre-lox/tamoxifen induction system to express the rhodopsin reporter in transfected rat rods in vivo. This method differs from Young's pulse-labeling experiments, of which the reporter gene is continuously activated and expressed until the harvest.
Using this method, these investigators showed that the newly synthesized rhodopsin indeed first appeared in the inner segment, and then appeared as a single compact “band” ∼1 d after gene induction. This band represented the nascent discs added at the base of the OS. The rhodopsin signals extended distally from the base of the OS as time progressed. The rhodopsin expressed in the OS was alternated with strong and weak signals along the axis (Fig. 5A). Strikingly, the number of rhodopsin-rich “segments” correlated closely with the number of days after the gene induction, and each rod OS had ∼10 segments (Fig. 5B). These results suggested that the rhodopsin produced daily was packed into a single “segment,” which was then shed 10 days after its synthesis.
Figure 5.
Ciliary periodicity and distal disposal of rod outer segment (OS). (A) Rods expressing GFP (green) and human rhodopsin (red) expressed for 2, 4, 6, and 8 days under a 12:12 light:dark cycle. Arrowheads point to the junction between the OS and inner segment (IS). (B) Cartoon depicts the heterogeneous disc stack organization. Each OS has 10 rhodopsin-rich (red) disc stacks, which are formed in darkness, flanked by PRPH2-rich discs (blue), which are formed in light. (C) Rod OS expressing both PRPH2 and human rhodopsin for 5 days. Disc stacks rich for PRPH2 and rhodopsin form an alternating array. (D,E) Electron microscopic images of distal rod OSs contacting the apical microvilli (MV) of retinal pigment epithelium (RPE) cells. Curved arrows show the discs vulnerable to structural changes, premarked for later shedding. SD, shed disc. Scale bars are in μm. N, Nucleus; SN, synapse. (Panels A, C, and D from Hsu et al. 2015; reprinted, with permission, from Elsevier.)
Further, a light regimen experiment showed that the diurnal rhythmic banding pattern of rhodopsin is regulated by cyclic light (not by intrinsic circadian rhythm), and rhodopsin preferentially entered the OS in darkness. Intuitively, these results suggested that the discs synthesized during day and night had different protein compositions. Their follow-up experiment showed that the rhodopsin-less discs are enriched with peripherin-2/rds (PRHP2) (Fig. 5C). PRPH2 is a cysteine-rich tetraspanin OS protein previously localized to the high-curvature motifs (i.e., rim, incisures) of the OS discs (Molday et al. 1987; Kedzierski et al. 1996; Tam et al. 2004). The shape of the OS disc membrane is not a perfect circle; disc incisures are clefts generated by infoldings of the disc rim toward the center of disc lamellae (Pedler and Tilly 1967). PRPH2 is known to form oligomers, remodel lipid bilayers, and participate in OS disposal (i.e., PRPH2 hypomorphic mice shed discs less often) (Wrigley et al. 2000; Molday 2004; Khattree et al. 2013). These observations collectively prompted the investigators to propose that PRPH2-rich discs have a higher curvature, perhaps having deeper or more incisures. Thereby, these discs likely serve as premarked breakpoints, as they are more susceptible to structural changes during disc shedding at the OS tip (Fig. 5D,E).
Taken together, the evidence suggests that the dark–light cycle regulates oscillations in OS protein transport, and thus generates an OS that carries autonomous information knowing how much disc material is made daily and the quantity of the disc to be shed. As a result, the OS is able to maintain the constant length. Thus, the photoreceptor OS presents a vivid example of how the dynamics of ciliary transport can dictate the cilium’s composition and shape and provide an intrinsic mechanism for its homeostasis.
CONCLUDING REMARKS
Owing to its relevance to many diseases, ciliary biology has received immense research interest. A fruitful progression has been witnessed to better appreciate the biology of the ciliary dynamics. As the conceptual framework has been established, we need to fill the gaps by understanding how the cilium achieves the homeostasis of its constant changing composition, shape, and length, and the ability of cell–cell communication. As research in this field continues to flourish, we foresee great promise that our improved knowledge may provide interventions for the many human disorders affected by dysregulated dynamics of cilia.
ACKNOWLEDGMENTS
We apologize to those authors whose works are not cited because of space limitations. Recent findings are referenced. We thank all of the members of Dr. Sung’s laboratory for valuable comments on the manuscript and Junmin Pan and Leonidas Tsiokas for many stimulating discussions. This work is supported by grants from the National Institutes of Health (EY11307, EY016805) and the Starr Stem Cell Foundation. C.-H.S. is a senior Research Preventing Blindness investigator.
Footnotes
Editor: Keith E. Mostov
Additional Perspectives on Cell Polarity available at www.cshperspectives.org
REFERENCES
- Ahlers C, Geitzenauer W, Stock G, Golbaz I, Schmidt-Erfurth U, Prunte C. 2009. Alterations of intraretinal layers in acute central serous chorioretinopathy. Acta Ophthalmol 87: 511–516. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Fisher SK, Breding DJ. 1986. A concentration of fucosylated glycoconjugates at the base of cone outer segments: Quantitative electron microscope autoradiography. Exp Eye Res 42: 267–283. [DOI] [PubMed] [Google Scholar]
- Bakircioglu M, Carvalho OP, Khurshid M, Cox JJ, Tuysuz B, Barak T, Yilmaz S, Caglayan O, Dincer A, Nicholas AK, et al. 2011. The essential role of centrosomal NDE1 in human cerebral cortex neurogenesis. Am J Hum Genet 88: 523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basinger S, Hoffman R, Matthes M. 1976. Photoreceptor shedding is initiated by light in the frog retina. Science 194: 1074–1076. [DOI] [PubMed] [Google Scholar]
- Benmerah A. 2013. The ciliary pocket. Curr Opin Cell Biol 25: 78–84. [DOI] [PubMed] [Google Scholar]
- Bergman K, Goodenough UW, Goodenough DA, Jawitz J, Martin H. 1975. Gametic differentiation in Chlamydomonas reinhardtii. II: Flagellar membranes and the agglutination reaction. J Cell Biol 67: 606–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershteyn M, Atwood SX, Woo WM, Li M, Oro AE. 2010. MIM and cortactin antagonism regulates ciliogenesis and hedgehog signaling. Dev Cell 19: 270–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besharse JC, Forestner DM, Defoe DM. 1985. Membrane assembly in retinal photoreceptors. III: Distinct membrane domains of the connecting cilium of developing rods. J Neurosci 5: 1035–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettencourt-Dias M, Carvalho-Santos Z. 2008. Double life of centrioles: CP110 in the spotlight. Trends Cell Biol 18: 8–11. [DOI] [PubMed] [Google Scholar]
- Bielas SL, Silhavy JL, Brancati F, Kisseleva MV, Al-Gazali L, Sztriha L, Bayoumi RA, Zaki MS, Abdel-Aleem A, Rosti RO, et al. 2009. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat Genet 41: 1032–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitzer AL, Panagis L, Gusella GL, Danias J, Mlodzik M, Iomini C. 2011. Primary cilia dynamics instruct tissue patterning and repair of corneal endothelium. Proc Natl Acad Sci 108: 2819–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Ning J, Hernandez-Lara CI, Belzile O, Wang Q, Dutcher SK, Liu Y, Snell WJ. 2015. Uni-directional ciliary membrane protein trafficking by a cytoplasmic retrograde IFT motor and ciliary ectosome shedding. eLife 4: e05242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen ST, Clement CA, Satir P, Pedersen LB. 2012. Primary cilia and coordination of receptor tyrosine kinase (RTK) signalling. J Pathol 226: 172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang JZ, Yeh TY, Bollati F, Conde C, Canavosio F, Caceres A, Sung CH. 2005. The dynein light chain Tctex-1 has a dynein-independent role in actin remodeling during neurite outgrowth. Dev Cell 9: 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang JZ, Zhao Y, Sung CH. 2007. SARA-regulated vesicular targeting underlies formation of the light-sensing organelle in mammalian rods. Cell 130: 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang JZ, Chou SY, Sung CH. 2010. Chloride intracellular channel 4 is critical for the epithelial morphogenesis of RPE cells and retinal attachment. Mol Biol Cell 21: 3017–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang JZ, Hsu YC, Sung CH. 2015. Ultrastructural visualization of trans-ciliary rhodopsin cargoes in mammalian rods. Cilia 4: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement CA, Ajbro KD, Koefoed K, Vestergaard ML, Veland IR, Henriques de Jesus MP, Pedersen LB, Benmerah A, Andersen CY, Larsen LA, et al. 2013. TGF-β signaling is associated with endocytosis at the pocket region of the primary cilium. Cell Rep 3: 1806–1814. [DOI] [PubMed] [Google Scholar]
- Cocucci E, Meldolesi J. 2015. Ectosomes and exosomes: Shedding the confusion between extracellular vesicles. Trends Cell Biol 25: 364–372. [DOI] [PubMed] [Google Scholar]
- Collin GB, Won J, Hicks WL, Cook SA, Nishina PM, Naggert JK. 2012. Meckelin is necessary for photoreceptor intraciliary transport and outer segment morphogenesis. Invest Ophthalmol Vis Sci 53: 967–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RE, Swiderski RE, Rahmouni K, Nishimura DY, Mullins RF, Agassandian K, Philp AR, Searby CC, Andrews MP, Thompson S, et al. 2007. A knockin mouse model of the Bardet–Biedl syndrome 1 M390R mutation has cilia defects, ventriculomegaly, retinopathy, and obesity. Proc Natl Acad Sci 104: 19422–19427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentler W. 2013. A role for the membrane in regulating Chlamydomonas flagellar length. PLoS ONE 8: e53366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis E. 1956. Morphogenesis of the retinal rods; an electron microscope study. J Biophys Biochem Cytol 2: 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis E. 1960. Some observations on the ultrastructure and morphogenesis of photoreceptors. J Gen Physiol 43: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil V, Marzesco AM, Corbeil D, Huttner WB, Wilsch-Brauninger M. 2007. Midbody and primary cilium of neural progenitors release extracellular membrane particles enriched in the stem cell marker prominin-1. J Cell Biol 176: 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggenschwiler JT, Anderson KV. 2007. Cilia and developmental signaling. Annu Rev Cell Dev Biol 23: 345–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrom B, Flock A, Borg E. 1983. Ultrastructural studies of stereocilia in noise-exposed rabbits. Hear Res 12: 251–264. [DOI] [PubMed] [Google Scholar]
- Gabriel E, Wason A, Ramani A, Gooi LM, Keller P, Pozniakovsky A, Poser I, Noack F, Telugu NS, Calegari F, et al. 2016. CPAP promotes timely cilium disassembly to maintain neural progenitor pool. EMBO J 35: 803–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goggolidou P, Stevens JL, Agueci F, Keynton J, Wheway G, Grimes DT, Patel SH, Hilton H, Morthorst SK, DiPaolo A, et al. 2014. ATMIN is a transcriptional regulator of both lung morphogenesis and ciliogenesis. Development 141: 3966–3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Subramanian R, Bangs F, Omelchenko T, Liem KF Jr, Kapoor TM, Anderson KV. 2014. The kinesin-4 protein Kif7 regulates mammalian Hedgehog signalling by organizing the cilium tip compartment. Nat Cell Biol 16: 663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginbotham H, Guo J, Yokota Y, Umberger NL, Su CY, Li J, Verma N, Hirt J, Ghukasyan V, Caspary T, et al. 2013. Arl13b-regulated cilia activities are essential for polarized radial glial scaffold formation. Nat Neurosci 16: 1000–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge RD, D’Ercole AJ, O’Kusky JR. 2004. Insulin-like growth factor-I accelerates the cell cycle by decreasing G1 phase length and increases cell cycle reentry in the embryonic cerebral cortex. J Neurosci 24: 10201–10210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan MC, Manganelli L, Woollard JR, Masyuk AI, Masyuk TV, Tammachote R, Huang BQ, Leontovich AA, Beito TG, Madden BJ, et al. 2009. Characterization of PKD protein-positive exosome-like vesicles. J Am Soc Nephrol 20: 278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Chuang JZ, Sung CH. 2015. Light regulates the ciliary protein transport and outer segment disc renewal of mammalian photoreceptors. Dev Cell 32: 731–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Liang Y, He W, Pan J. 2015. Cilia disassembly with two distinct phases of regulation. Cell Rep 10: 1803–1810. [DOI] [PubMed] [Google Scholar]
- Insinna C, Pathak N, Perkins B, Drummond I, Besharse JC. 2008. The homodimeric kinesin, Kif17, is essential for vertebrate photoreceptor sensory outer segment development. Dev Biol 316: 160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Marshall WF. 2011. Ciliogenesis: Building the cell’s antenna. Nat Rev Mol Cell Biol 12: 222–234. [DOI] [PubMed] [Google Scholar]
- Izawa I, Goto H, Kasahara K, Inagaki M. 2015. Current topics of functional links between primary cilia and cell cycle. Cilia 4: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby M, Cox JJ, Gayral S, Hampshire DJ, Ayub M, Blockmans M, Pernot E, Kisseleva MV, Compere P, Schiffmann SN, et al. 2009. INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nat Genet 41: 1027–1031. [DOI] [PubMed] [Google Scholar]
- Kedzierski W, Moghrabi WN, Allen AC, Jablonski-Stiemke MM, Azarian SM, Bok D, Travis GH. 1996. Three homologs of rds/peripherin in Xenopus laevis photoreceptors that exhibit covalent and non-covalent interactions. J Cell Sci 109: 2551–2560. [DOI] [PubMed] [Google Scholar]
- Keeling J, Tsiokas L, Maskey D. 2016. Cellular mechanisms of ciliary length control. Cells 5: E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattree N, Ritter LM, Goldberg AF. 2013. Membrane curvature generation by a C-terminal amphipathic helix in peripherin-2/rds, a tetraspanin required for photoreceptor sensory cilium morphogenesis. J Cell Sci 126: 4659–4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Tsiokas L. 2011. Cilia and cell cycle re-entry: More than a coincidence. Cell Cycle 10: 2683–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lee JE, Heynen-Genel S, Suyama E, Ono K, Lee K, Ideker T, Aza-Blanc P, Gleeson JG. 2010. Functional genomic screen for modulators of ciliogenesis and cilium length. Nature 464: 1048–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Zaghloul NA, Bubenshchikova E, Oh EC, Rankin S, Katsanis N, Obara T, Tsiokas L. 2011. Nde1-mediated inhibition of ciliogenesis affects cell cycle re-entry. Nat Cell Biol 13: 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SM, Dillman JFR, Benashski SE, Lye RJ, Patel-King RS, Pfister KK. 1996. The mouse t-complex-encoded protein Tctex-1 is a light chain of brain cytoplasmic dynein. J Biol Chem 271: 32281–32287. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Dynlacht BD. 2011. Regulating the transition from centriole to basal body. J Cell Biol 193: 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam HC, Cloonan SM, Bhashyam AR, Haspel JA, Singh A, Sathirapongsasuti JF, Cervo M, Yao H, Chung AL, Mizumura K, et al. 2013. Histone deacetylase 6-mediated selective autophagy regulates COPD-associated cilia dysfunction. J Clin Invest 123: 5212–5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail MM. 1973. Kinetics of rod outer segment renewal in the developing mouse retina. J Cell Biol 58: 650–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail MM. 1976. Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science 194: 1071–1074. [DOI] [PubMed] [Google Scholar]
- LaVail MM. 1983. Outer segment disc shedding and phagocytosis in the outer retina. Trans Ophthalmol Soc UK 103: 397–404. [PubMed] [Google Scholar]
- Lehtinen MK, Zappaterra MW, Chen X, Yang YJ, Hill AD, Lun M, Maynard T, Gonzalez D, Kim S, Ye P, et al. 2011. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron 69: 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold PL, O’Mahony MJ, Lian XJ, Tilley AE, Harvey BG, Crystal RG. 2009. Smoking is associated with shortened airway cilia. PLoS ONE 4: e8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Saito M, Chuang JZ, Tseng YY, Dedesma C, Tomizawa K, Kaitsuka T, Sung CH. 2011. Ciliary transition zone activation of phosphorylated Tctex-1 controls ciliary resorption, S-phase entry and fate of neural progenitors. Nat Cell Biol 13: 402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Meng D, Zhu B, Pan J. 2016. Mechanism of ciliary disassembly. Cell Mol Life Sci 73: 1787–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Lyubarsky A, Skalet JH, Pugh EN Jr, Pierce EA. 2003. RP1 is required for the correct stacking of outer segment discs. Invest Ophthalmol Vis Sci 44: 4171–4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie CM, Caridi G, Lopes VS, Brancati F, Kispert A, Lancaster MA, Schlossman AM, Otto EA, Leitges M, Grone HJ, et al. 2010. AHI1 is required for photoreceptor outer segment development and is a modifier for retinal degeneration in nephronophthisis. Nat Genet 42: 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JE, Silva M, Nguyen KC, Hellen E, Kern AD, Hall DH, Barr MM. 2015. Myristoylated CIL-7 regulates ciliary extracellular vesicle biogenesis. Mol Biol Cell 26: 2823–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzesco AM, Janich P, Wilsch-Brauninger M, Dubreuil V, Langenfeld K, Corbeil D, Huttner WB. 2005. Release of extracellular membrane particles carrying the stem cell marker prominin-1 (CD133) from neural progenitors and other epithelial cells. J Cell Sci 118: 2849–2858. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Kishi S, Sato T, Mukai R. 2011. Fundus autofluorescence of elongated photoreceptor outer segments in central serous chorioretinopathy. Am J Ophthalmol 151: 617–623.e1. [DOI] [PubMed] [Google Scholar]
- Molday RS. 2004. Molecular organization of rod outer segments. In Photoreceptor cell biology and inherited retinal degenerations (ed. Williams DS), pp. 259–300. World Scientific, London. [Google Scholar]
- Molday RS, Hicks D, Molday L. 1987. Peripherin. A rim-specific membrane protein of rod outer segment discs. Invest Ophthalmol Vis Sci 28: 50–61. [PubMed] [Google Scholar]
- Molla-Herman A, Ghossoub R, Blisnick T, Meunier A, Serres C, Silbermann F, Emmerson C, Romeo K, Bourdoncle P, Schmitt A, et al. 2010. The ciliary pocket: An endocytic membrane domain at the base of primary and motile cilia. J Cell Sci 123: 1785–1795. [DOI] [PubMed] [Google Scholar]
- Nandrot EF, Kim Y, Brodie SE, Huang X, Sheppard D, Finnemann SC. 2004. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking αvβ5 integrin. J Exp Med 200: 1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandrot EF, Anand M, Almeida D, Atabai K, Sheppard D, Finnemann SC. 2007. Essential role for MFG-E8 as ligand for αvβ5 integrin in diurnal retinal phagocytosis. Proc Natl Acad Sci 104: 12005–12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V, Li L, Cobo-Stark P, Shao X, Somlo S, Lin F, Igarashi P. 2008. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet 17: 1578–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Baker SA, Deane JA, Cole DG, Dickert BL, Rosenbaum JL, Witman GB, Besharse JC. 2002. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J Cell Biol 157: 103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedler CM, Tilly R. 1967. The fine structure of photoreceptor discs. Vision Res 7: 829–836. [DOI] [PubMed] [Google Scholar]
- Plotnikova OV, Golemis EA, Pugacheva EN. 2008. Cell cycle-dependent ciliogenesis and cancer. Cancer Res 68: 2058–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. 2007. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell 129: 1351–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos N, Reiter JF. 2008. Building it up and taking it down: The regulation of vertebrate ciliogenesis. Dev Dyn 237: 1972–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siller SS, Burke MC, Li FQ, Takemaru K. 2015. Chibby functions to preserve normal ciliary morphology through the regulation of intraflagellar transport in airway ciliated cells. Cell Cycle 14: 3163–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobkowicz HM, Slapnick SM, August BK. 1995. The kinocilium of auditory hair cells and evidence for its morphogenetic role during the regeneration of stereocilia and cuticular plates. J Neurocytol 24: 633–653. [DOI] [PubMed] [Google Scholar]
- Steinberg RH, Fisher SK, Anderson DH. 1980. Disc morphogenesis in vertebrate photoreceptors. J Comp Neurol 190: 501–508. [DOI] [PubMed] [Google Scholar]
- Sung CH, Leroux MR. 2013. The roles of evolutionarily conserved functional modules in cilia-related trafficking. Nat Cell Biol 15: 1387–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung CH, Li A. 2011. Ciliary resorption modulates G1 length and cell cycle progression. Cell Cycle 10: 2825–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness VS Jr. 1995. The cell cycle of the pseudostratified ventricular epithelium of the embryonic murine cerebral wall. J Neurosci 15: 6046–6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam BM, Moritz OL, Papermaster DS. 2004. The C terminus of peripherin/rds participates in rod outer segment targeting and alignment of disk incisures. Mol Biol Cell 15: 2027–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Okada Y, Hirokawa N. 2005. FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left–right determination. Nature 435: 172–177. [DOI] [PubMed] [Google Scholar]
- Tran PV, Haycraft CJ, Besschetnova TY, Turbe-Doan A, Stottmann RW, Herron BJ, Chesebro AL, Qiu H, Scherz PJ, Shah JV, et al. 2008. THM1 negatively modulates mouse Sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia. Nat Genet 40: 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker RW, Pardee AB, Fujiwara K. 1979. Centriole ciliation is related to quiescence and DNA synthesis in 3T3 cells. Cell 17: 527–535. [DOI] [PubMed] [Google Scholar]
- Verghese E, Ricardo SD, Weidenfeld R, Zhuang J, Hill PA, Langham RG, Deane JA. 2009. Renal primary cilia lengthen after acute tubular necrosis. J Am Soc Nephrol 20: 2147–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walenkamp MJ, Wit JM. 2006. Genetic disorders in the growth hormone—Insulin-like growth factor-I axis. Horm Res 66: 221–230. [DOI] [PubMed] [Google Scholar]
- Walenkamp MJ, Karperien M, Pereira AM, Hilhorst-Hofstee Y, van Doorn J, Chen JW, Mohan S, Denley A, Forbes B, van Duyvenvoorde HA, et al. 2005. Homozygous and heterozygous expression of a novel insulin-like growth factor-I mutation. J Clin Endocrinol Metab 90: 2855–2864. [DOI] [PubMed] [Google Scholar]
- Wang J, Silva M, Haas LA, Morsci NS, Nguyen KC, Hall DH, Barr MM. 2014. C. elegans ciliated sensory neurons release extracellular vesicles that function in animal communication. Curr Biol 24: 519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AM, Beales PL. 2011. Ciliopathies: An expanding disease spectrum. Pediatr Nephrol 26: 1039–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall JE, Hoyt C, Liu Q, Hsiao YC, Pierce EA, Page-McCaw PS, Ferland RJ. 2010. Retinal degeneration and failure of photoreceptor outer segment formation in mice with targeted deletion of the Joubert syndrome gene, Ahi1. J Neurosci 30: 8759–8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won J, Marin de Evsikova C, Smith RS, Hicks WL, Edwards MM, Longo-Guess C, Li T, Naggert JK, Nishina PM. 2011. NPHP4 is necessary for normal photoreceptor ribbon synapse maintenance and outer segment formation, and for sperm development. Hum Mol Genet 20: 482–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood CR, Huang K, Diener DR, Rosenbaum JL. 2013. The cilium secretes bioactive ectosomes. Curr Biol 23: 906–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollard JR, Punyashtiti R, Richardson S, Masyuk TV, Whelan S, Huang BQ, Lager DJ, vanDeursen J, Torres VE, Gattone VH, et al. 2007. A mouse model of autosomal recessive polycystic kidney disease with biliary duct and proximal tubule dilatation. Kidney Int 72: 328–336. [DOI] [PubMed] [Google Scholar]
- Wrigley JD, Ahmed T, Nevett CL, Findlay JB. 2000. Peripherin/rds influences membrane vesicle morphology. Implications for retinopathies. J Biol Chem 275: 13191–13194. [DOI] [PubMed] [Google Scholar]
- Yang Z, Chen Y, Lillo C, Chien J, Yu Z, Michaelides M, Klein M, Howes KA, Li Y, Kaminoh Y, et al. 2008. Mutant prominin 1 found in patients with macular degeneration disrupts photoreceptor disk morphogenesis in mice. J Clin Invest 118: 2908–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannuzzi LA, Freund KB, Goldbaum M, Scassellati-Sforzolini B, Guyer DR, Spaide RF, Maberley D, Wong DW, Slakter JS, Sorenson JA, et al. 2000. Polypoidal choroidal vasculopathy masquerading as central serous chorioretinopathy. Ophthalmology 107: 767–777. [DOI] [PubMed] [Google Scholar]
- Yeh C, Li A, Chuang JZ, Saito M, Caceres A, Sung CH. 2013. IGF-1 activates a cilium-localized noncanonical Gβγ signaling pathway that regulates cell-cycle progression. Dev Cell 26: 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW. 1967. The renewal of photoreceptor cell outer segments. J Cell Biol 33: 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW. 1968. Passage of newly formed protein through the connecting cilium of retinal rods in the frog. J Ultrastruct Res 23: 462–473. [DOI] [PubMed] [Google Scholar]
- Young RW. 1971. Shedding of discs from rod outer segments in the rhesus monkey. J Ultrastruct Res 34: 190–203. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Stanton JB, Wu J, Yu K, Hartzell HC, Peachey NS, Marmorstein LY, Marmorstein AD. 2010. Suppression of Ca2+ signaling in a mouse model of Best disease. Hum Mol Genet 19: 1108–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]