Abstract
Background
The objective was to evaluate the cost-effectiveness of NEPA, an oral fixed combination netupitant (NETU, 300 mg) and palonosetron (PA, 0.5 mg) compared with aprepitant and palonosetron (APPA) or palonosetron (PA) alone, to prevent chemotherapy-induced nausea and vomiting (CINV) in patients undergoing treatment with highly or moderately emetogenic chemotherapy (HEC or MEC) in the UK.
Scope
A systematic literature review and meta-analysis were undertaken to compare NEPA with currently recommended anti-emetics. Relative effectiveness was estimated over the acute (day 1) and overall treatment (days 1–5) phases, taking complete response (CR, no emesis and no rescue medication) and complete protection (CP, CR and no more than mild nausea [VAS scale <25 mm]) as primary efficacy outcomes. A three-health-state Markov cohort model, including CP, CR and incomplete response (no CR) for HEC and MEC, was constructed. A five-day time horizon and UK NHS perspective were adopted. Transition probabilities were obtained by combining the response rates of CR and CP from NEPA trials and odds ratios from the meta-analysis. Utilities of 0.90, 0.70 and 0.24 were defined for CP, CR and incomplete response, respectively. Costs included medications and management of CINV-related events and were obtained from the British National Formulary and NHS Reference Costs. The expected budgetary impact of NEPA was also evaluated.
Findings
In HEC patients, the NEPA strategy was more effective than APPA (quality-adjusted life days [QALDs] of 4.263 versus 4.053; incremental emesis-free and CINV-free days of +0.354 and +0.237, respectively) and was less costly (£80 versus £124), resulting in NEPA being the dominant strategy. In MEC patients, NEPA was cost effective, cumulating in an estimated 0.182 extra QALDs at an incremental cost of £6.65 compared with PA.
Conclusion
Despite study limitations (study setting, time horizon, utility measure), the results suggest NEPA is cost effective for preventing CINV associated with HEC and MEC in the UK.
Keywords: antiemetics, cost-effectiveness, medical oncology, meta-analysis, nausea, netupitant, palonosetron, quality of life, review literature, vomiting
Introduction
Chemotherapy-induced nausea and vomiting (CINV) is among the most common and feared side effects reported by cancer patients and may appear prior to, during or after chemotherapy administration [1]. The development of CINV is a complex process involving the activation of several neurotransmitters, which interact with the central nervous system stimulating a physiological response [2,3]. The incidence of CINV ranges from less than 10% in patients treated with chemotherapy agents with minimal emetic risk, from 30% to 90% in patients with agents with medium risk, to >90% among those whose regimens contain an agent with a high emetic risk [1].
Nausea and emesis, particularly when occurring during the delayed phase (days 2–5 of the chemotherapy cycle), cause significant problems for patients with cancer. CINV can lead to severe clinical conditions such as electrolyte imbalance and dehydration, and can affect a patient’s quality of life and willingness to continue chemotherapy [4]. CINV also exerts a considerable financial burden on the healthcare system. Direct medical costs associated with managing CINV are driven by the cost of rescue medications, unscheduled physician visits, emergency room visits and hospitalisations [5–10].
Several anti-emetic therapies are used to prevent CINV. The Multinational Association of Supportive Care in Cancer (MASCC)/European Society for Medical Oncology (ESMO), American Society of Clinical Oncology (ASCO) and National Comprehensive Cancer Network (NCCN) guidelines are used to direct clinical practice and treatment in the UK. It has been suggested that with the correct use of anti-emetics, CINV could be prevented in 70%–80% of patients; however, suboptimal CINV control remains an issue in clinical practice. Many patients receiving highly or moderately emetogenic chemotherapy (HEC or MEC) still experience acute and delayed nausea, vomiting or both. In particular, current treatments provide insufficient CINV control during the delayed phase [11,12].
While guidelines for preventing CINV are widely available, clinical uptake of guidelines remains low [13,14]. The reasons for non-adherence to clinical guidelines are multifactorial. A key aspect involves individual clinicians disagreeing with the key recommendations of the guidelines [15,16]. Non-adherence may also reflect economic constraints of hospitals and government payers considering the higher costs of branded anti-emetic therapies [13].
NEPA is indicated in Europe for the prevention of the acute and delayed nausea and vomiting associated with HEC and MEC. Two trials have been conducted examining NEPA, including one phase II dose ranging study, one phase III efficacy and one phase III safety trial.
The primary objective of this study was to assess, from the United Kingdom (UK) payer perspective, the cost-effectiveness of NEPA compared to the extemporaneous combination aprepitant plus palonosetron for patients receiving HEC and to palonosetron alone for patients receiving MEC.
Methods
Systematic review
A systematic literature review was undertaken to identify all relevant clinical trials examining the efficacy of NEPA and its comparators in the prevention of CINV for patients undergoing HEC or MEC as treatment for cancer. The systematic literature review focused on English-language publications with the methodological approach in line with established systematic review procedures.
The scope of the literature review was defined in terms of Population, Interventions, Comparators, Outcomes and Study design (PICOS) [17]. Eligible studies included human adult (≥18 years) cancer patients receiving highly or moderately emetogenic chemotherapy that assessed the efficacy or safety of one of the anti-emetics (e.g. 5 HT-3s, NK1s, other) by comparing the intervention with either placebo or an active comparator. Studies were required to contain at least complete response and other outcomes like complete protection, partial response, complete control, total control, time to first emetic episode, time to use of rescue medication or time to treatment failure, and to be blinded, randomised controlled trials (≥ Phase II) with more than 50 patients (Appendix 1). Studies were excluded if they were duplicate, animal or in vitro studies, narrative reviews, editorials, case reports or letters, meta-analyses, were not in English language or if they had the wrong scope, population, intervention, or did not contain the correct study type or outcome of interest. Any disagreements between authors were resolved by a third party.
Appendix 1.
Inclusion criteria.
| Process | Criteria |
|---|---|
| P (Patient Population) | Human adults (≥18 years) cancer patients receiving highly or moderately emetogenic chemotherapy |
| I (Intervention or Exposure) | Studies assessing the efficacy or safety of one of the following antiemetics:
|
| C (Comparators) | Placebo or active comparator |
| O (Outcomes) |
|
| S (Study Design) | Blinded, randomised controlled trials (≥ Phase 2) with more than 50 patients |
5-HT3: 5-HT3 receptor antagonist; NK1: NK1 receptor antagonist.
The electronic search was performed in MEDLINE, EMBASE, the Cochrane Collaboration, and HTA in Cochrane. The search was conducted with limits of ‘English language’ with the date limit from 2000 to 28/08/2013 for all searches except olanzapine, which was included in an updated search which ranged up to 09/01/2014. Although olanzapine was not recommended in ASCO or MASCC as a preferred preventative treatment for CINV, it was later included in the search because of its inclusion in the NCCN guidelines at the time of the updated search. A data table was produced, with key information extracted from the eligible papers including study authors, date, title, study design, treatment strategies, patient characteristics, chemotherapy received, results/outcomes and adverse events. In addition, the quality assessment and evaluation of each study was undertaken using the National Institute for Health and Clinical Excellence (NICE) checklist for randomised controlled trials [18].
Meta-analysis
Following the systematic literature review and a feasibility assessment, which included an evaluation of available evidence and a heterogeneity assessment, it was considered feasible to undertake an indirect comparison within a frequentist framework in the MEC indication and a Bayesian Mixed Treatment Comparison (MTC) in the HEC and AC-MEC indication.
Three efficacy outcomes were analysed: complete response (CR), complete protection (CP) and total control (TC). CR is the efficacy outcome most commonly reported in CINV treatment studies, defined as no nausea, no vomiting, and no use of rescue medication (with studies not defining CR in this way excluded). The definition of CP was no emetic episodes, no use of rescue medication, and no more than mild nausea (defined as Visual Analogue Scale, VAS <25 mm). A subgroup of patients who achieve CR also achieve TC, defined as no emesis, no use of rescue medication, and no nausea (<5 mm on the VAS). For each outcome, where data were available, results were presented for two different time periods: the acute phase (day 1) and the overall phase (days 1–5).
In the MEC population, an indirect treatment comparison of CR was performed using fixed effect models, as the inclusion of random effects was considered not appropriate given the low number of studies available and that CR was the only outcome sufficiently reported. MTC models were performed in the HEC population using both fixed and random effects where the model best fitting the data was selected based on the deviance information criterion (DIC) [19,20]. The MTC allowed the comparative effect size of treatments on a specific outcome scale to be estimated, as well as the treatment-specific probability of being the best for some characteristics. The odds ratio was presented as the primary efficacy outcome measure for each analysis. The uncertainty associated with the measure has been reported via the 95% confidence interval (CI) for the indirect comparison in the MEC population, and the credible intervals (CrI) for the MTC analyses in the HEC population.
Economic evaluation
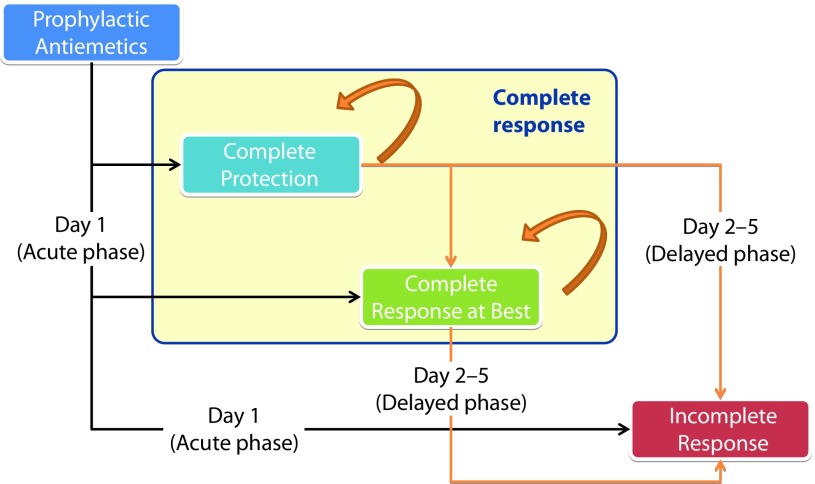
Cost-utility Markov models were developed to estimate the expected cost and health outcomes after HEC or MEC administration, to compare NEPA against aprepitant combined with palonosetron (APPA) in the HEC population and against palonosetron in the MEC population (Figure 1).
Figure 1.
A structure of the Markov cohort model.
The target patient population was cancer patients receiving prophylactic anti-emetics for the management of HEC or MEC. The selection of relevant comparators to NEPA was based on international clinical guidelines [21–23]. The perspective was that of the UK National Health Service (NHS). The time horizon for the model was five days that consist of the first day (acute phase) and from the first to fifth day (overall phase) and was run for one cycle of chemotherapy [11].
All patients entered the Markov model on the first day after the chemotherapy administration, for HEC and MEC regimens, respectively. In the acute phase (day 1), patients had different probabilities of achieving complete response and incomplete response, depending on the efficacy of the administered anti-emetic, obtained from the meta-analysis. Complete response was divided into two subcategories: complete protection and complete response at best (complete response without complete protection). The same approach was taken to assess the patient distribution in each state at the end of day 5 (i.e. response rate in overall phase). To determine the number of patients in each state between the second and the fourth day, the patient flow on days 2–4 was calibrated using linear interpolation between both the acute and overall phase-response rates.
The response rates were obtained from NEPA trials (Table 1). The efficacies of the comparators were obtained by combining the response rates of NEPA and the ORs calculated in the meta-analysis as detailed in the equation below:
Table 1.
Response rates of NEPA (95% CI).
| HEC | MEC | |
|---|---|---|
| NEPA 07–07 (n=135) | NEPA 08–18 (n=724) | |
| RR (95% CI) | RR (95% CI) | |
| Acute phase | ||
| Complete response | 0.985a (0.965–0.999) | 0.884a (0.859–0.905) |
| Complete protection | 0.970a (0.942–0.999) | 0.823 (0.794–0.849) |
| Overall phase | ||
| Complete response | 0.896a (0.845–0.948) | 0.743b (0.71–0.774) |
| Complete protection | 0.830a (0.766–0.893) | 0.638a (0.602–0.672) |
CI: confidence interval; HEC: highly emetogenic chemotherapy; MEC: moderately emetogenic chemotherapy; NA: not available; NEPA: netupitant+ palonosetron+dexamethasone; RR: response rate.
p≤0.05,
p≤0.0001.
where T is a treatment comparator of NEPA and ORNEPAvsT is the odds ratio of NEPA versus this treatment, as obtained from the meta-analysis.
The odds ratios of each considered treatment regimen compared to NEPA are presented in Table 2 for the HEC and MEC populations.
Table 2.
Estimated odds ratios of the comparators in HEC and MEC.
| HEC APPAa |
MEC PAb |
|
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Acute phase | ||
| Complete response | 1.498 (0.217–6.385) | 1.345 (0.879–2.063) |
| Complete protection | 3.501 (1.088–13.263) | 1.084 (0.745–1.577) |
| Overall phase | ||
| Complete response | 2.490 (1.103–6.092) | 1.450 (1.050–2.003) |
| Complete protection | 3.501 (1.088–13.263) | 1.281 (0.947–1.724) |
aprepitant+palonosetron+dexamethasone;
palonosetron+dexamethasone.
CI: confidence interval; HEC: highly emetogenic chemotherapy; MEC: moderately emetogenic chemotherapy; NEPA: netupitant+palonosetron+ dexamethasone; OR: odds ratio.
Reference efficacy was NEPA from NETU 07–07 and NETU 08–18 for HEC and MEC, respectively.
In previously published economic models for CINV, an anchor utility of 0.90 was used for chemotherapy without nausea and vomiting and a utility of 0.20 was used for the ‘incomplete response’ health state, in which emesis and/or nausea are present [24,25]. A value of 0.24 for the incomplete response state was adjusted based on clinical expert feedback. Given that ‘chemotherapy without nausea and vomiting’ anchor value is 0.90, the utility for the mild nausea state from Borjeson et al. (0.752) was assumed to be 0.70 for the complete response at best [26]. The utility value for total control was assumed to be 0.95 in order to linearise between perfect health (1.00) and complete protection (0.90). Finally, utilities of 0.90, 0.70 and 0.24 were defined for CP, CR and incomplete response, respectively [24,25,27].
Treatment costs of the prophylactic anti-emetic comparators were calculated based on recommended doses from international guidelines and local unit costs in the UK as found in the British National Formulary (BNF) 2014, the Personal Social Services Research Unit (PSSRU) 2013 and the NHS reference costs 2012–13 [21–23]. The costs of NEPA were calculated upon the price assumption £69 per package of 300 mg, as published by UK Monthly Index of Medical Specialities (MIMS). The cost per patient per cycle of health resources used due to emetic events were based on a study among cancer patients receiving a broad range of MEC in a trial setting in the UK [28]. The different costs used in the model are detailed in Table 3.
Table 3.
Cost of treatment and healthcare resource use (£, 2013).
| Treatments | PO | IV |
|---|---|---|
| NEPAa | 71.17 | NA |
| APPAb | 105.48 | 106.04 |
| APR+ONDc | 57.09 | 67.24 |
| APR+GRAd | 59.66 | 51.75 |
| OLA+PAe | 57.59 | NA |
| PAf | 56.61 | 58.62 |
| ONDg | 8.23 | 19.82 |
| GRAh | 10.79 | 4.33 |
| Healthcare resource | Cost per patient | |
| Hospitalisation | 68.27 | |
| Rescue medication | 2.05 | |
| Outpatient/physician visit | 13.57 | |
netupitant+palonosetron+dexamethasone;
aprepitant+palonosetron+dexamethasone;
aprepitant+ondansetron+dexamethasone;
aprepitant+granisetron+dexamethasone;
olanzapine+palonosetron+dexamethasone;
palonosetron+dexamethasone;
ondansetron+dexamethasone;
granisetron+dexamethasone;
IV: intravenous; NA: not applicable; PO: per os (by mouth).
The primary outcome measure used in the analysis was the incremental cost-effectiveness ratio (ICER) defined as the ratio of the incremental difference in total cost to the incremental difference in benefits between two treatment strategies. It can be expressed as the cost per life-year gained or by quality-adjusted life years (QALYs) by multiplying the years of life with a weight, measured in utilities, reflecting quality of life.
Sensitivity analyses were conducted in order to assess the impact of parameter and structural uncertainty on results. The Deterministic Sensitivity Analysis (DSA) showed the extent to which the results are affected by sources or assumptions and continuous variables (e.g. efficacy, costs, and utilities) increasing and reducing by 25% of the values. In addition to the DSAs, a probabilistic sensitivity analysis (PSA) was conducted in the model. A PSA allows capturing interactions between several inputs by running the model a large number of times (typically at least 1000 times) as a Monte Carlo simulation, using randomly drawn sets of inputs. The possible inputs are defined by assigning a statistical distribution around the mean value of each uncertain parameter, reflecting the extent and nature of dispersion around the mean. For each run, values are sampled at random from each distribution, and the decision tree is rolled back using these values to obtain a (cost-effectiveness) pair.
Results
Systematic review and meta-analysis
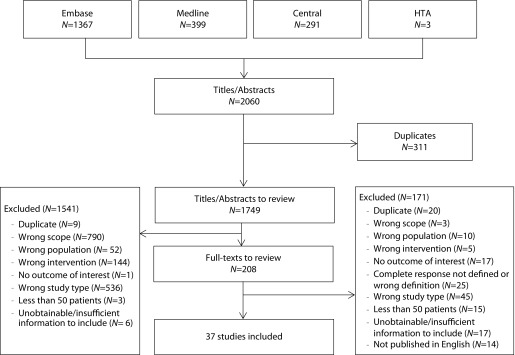
The search of randomised trials examining anti-emetics in adult cancer patients returned a total of 2060 results across the four screened databases. After the removal of duplicates, 1749 abstracts were reviewed and of these 208 full-text articles were evaluated. Titles, abstracts and full texts were screened by two independent reviewers. Discrepancies were resolved by a third reviewer. A total of 37 studies were deemed eligible for inclusion. Details on counts and reasons for exclusion are reported in Figure 2.
Figure 2.
Literature review flow chart.
HTA: health technology assessment.
The results of the meta-analysis were directly used as efficacy inputs for the comparators. They are summarised in Appendixes 2 and 3 for HEC and MEC respectively.
Appendix 2.
Estimated response rates of the comparators in HEC (Reference efficacy was NEPA from NETU 07–07).
| Complete response | Complete protection | |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Acute phase | ||
| APPAa | 1.498 (0.217–6.385) | 3.501 (1.088–13.263) |
| APR+ONDb | 2.100 (0.432–11.787) | 3.714(1.048–15.364) |
| APR+GRAc | 1.917 (0.298–8.741) | 4.973 (0.829–30.877) |
| APR+5-HT3d | 1.851 (0.674–5.376) | 3.501 (1.088–13.263) |
| OLA+PALe | 0.304 (0.024–2.350) | 1.000 (0.500–1.500) |
| PALf | 3.804 (0.989–17.082) | 5.228 (1.478–21.158) |
| ONDg | 4.112 (0.761–25.003) | 7.389 (1.989–31.627) |
| GRAh | 3.494 (0.663–15.863) | 5.601 (1.265–26.816) |
| 5-HT3i | 3.770 (1.361–11.023) | 5.789 (1.813–21.758) |
| Overall phase | ||
| APPAa | 2.490 (1.103–6.092) | 3.501 (1.088–13.263) |
| APR+ONDb | 1.542 (0.627–3.710) | 3.714 (1.048–15.364) |
| APR+GRAc | 3.068 (1.325–7.448) | 4.973 (0.829–30.877) |
| APR+5-HT3d | 1.817 (1.000–3.401) | 3.501 (1.088–13.263) |
| OLA+PALe | 1.970 (0.637–6.606) | 1.000 (0.500–1.500) |
| PALf | 3.394 (1.579–7.301) | 5.228 (1.478–21.158) |
| ONDg | 3.669 (1.377–9.459) | 7.389 (1.989–31.627) |
| GRAh | 5.714 (2.509–13.397) | 5.601 (1.265–26.816) |
| 5-HT3i | 3.931 (2.152–7.382) | 5.789 (1.813–21.758) |
aprepitant+palonosetron+dexamethasone;
aprepitant+ondansetron+dexamethasone;
aprepitant+granisetron+dexamethasone;
aprepitant+5-HT3(pooled)+dexamethasone;
olanzapine+palonosetron+dexamethasone;
palonosetron+dexamethasone;
ondansetron+dexamethasone;
granisetron+dexamethasone;
5-HT3+dexamethasone;
CI: confidence interval; HEC: highly emetogenic chemotherapy; NEPA: netupitant+palonosetron+dexamethasone; OR: odds ratio.
Appendix 3.
Estimated response rates of the comparators in MEC (Reference efficacy was NEPA from NETU 08–18).
| Complete response | Complete protection | |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Acute phase | ||
| APPAa | 0.953 (0.337–2.692) | 1.000 (0.500–1.500) |
| APR+5-HT3b | 0.953 (0.337–2.692) | 1.000 (0.500–1.500) |
| PALc | 1.345 (0.879–2.063) | 1.084 (0.745–1.577) |
| ONDd | 1.807 (0.524–6.238) | 1.084 (0.745–1.577) |
| GRAe | 1.906 (0.135–26.939) | 1.084 (0.745–1.577) |
| 5-HT3f | 1.814 (0.533–6.179) | 1.084(0.745–1.577) |
| Overall phase | ||
| APPAa | 0.868 (0.449–1.68) | 1.000 (0.500–1.500) |
| APR+5-HT3b | 0.868 (0.449–1.68) | 1.000 (0.500–1.500) |
| PALc | 1.450 (1.050–2.003) | 1.281 (0.947–1.724) |
| ONDd | 1.293 (0.435–3.784) | 1.281 (0.947–1.724) |
| GRAe | 1.311 (0.370–3.365) | 1.281 (0.947–1.724) |
| 5-HT3f | 1.474 (0.548–2.43) | 1.281 (0.947–1.724) |
aprepitant+palonosetron+dexamethasone;
aprepitant+5-HT3(pooled)+dexamethasone;
palonosetron+dexamethasone;
ondansetron+dexamethasone;
granisetron+dexamethasone;
5-HT3+dexamethasone;
CI: confidence interval; MEC: moderately emetogenic chemotherapy; NEPA: netupitant+palonosetron+ dexamethasone; OR: odds ratio.
Cost-effectiveness
In the base case analysis, NEPA was evaluated against the relevant comparators. The results of the analyses are presented in Table 4 for HEC and MEC.
Table 4.
Base case analysis of NEPA compared to other treatments in HEC and MEC.
| HEC | MEC | |||
|---|---|---|---|---|
| APPA(PO) | NEPA(PO) | PA(PO) | NEPA(PO) | |
| COSTS (£) | ||||
| Treatment acquisition | 105.48 | 71.17 | 56.61 | 69.72 |
| CINV episode management | 18.81 | 8.72 | 28.02 | 21.56 |
| Inpatient care | 15.31 | 7.10 | 22.80 | 17.55 |
| Rescue medication | 0.46 | 0.21 | 0.68 | 0.53 |
| Outpatient/physician care | 3.04 | 1.41 | 4.53 | 3.49 |
| Cost in acute phase | 1.87 | 1.26 | 12.59 | 9.73 |
| Cost in delayed phase | 16.94 | 7.47 | 15.44 | 11.83 |
| Cost of treated related AEs | 0.00 | 0.00 | 0.00 | 0.00 |
| Indirect costs, other expenses | 0.00 | 0.00 | 0.00 | 0.00 |
| Indirect costs, workday loss | 0.00 | 0.00 | 0.00 | 0.00 |
| Total costs | 124.29 | 79.89 | 84.63 | 91.28 |
| HEALTH OUTCOMES | ||||
| Average emesis-free days | 4.348 | 4.703 | 3.769 | 4.068 |
| Average CINV-free days | 4.273 | 4.500 | 3.428 | 3.653 |
| Emesis-free patients (%) | 77.6 | 89.6 | 66.6 | 74.3 |
| CINV-free patients (%) | 77.6 | 83.0 | 57.9 | 63.8 |
| Quality-adjusted life days | 4.055 | 4.263 | 3.619 | 3.802 |
| Quality-adjusted life years | 0.011 | 0.012 | 0.010 | 0.010 |
| COST/OUTCOMES (£) | ||||
| Cost per emesis-free day | Dominant | 86 | ||
| Cost per CINV-free day | Dominant | 113 | ||
| Cost per QALDs gain | Dominant | 36 | ||
| Cost per QALYs gain | Dominant | 13,318 | ||
| WILLINGNESS-TO-PAY (£30,000) | ||||
| Net monetary benefit | £62 | £8 | ||
| Acceptability | 97% | 88% | ||
AE: adverse event; APPA: aprepitant+palonosetron+dexamethasone; CINV: chemotherapy induced nausea and vomiting; HEC: highly emetogenic chemotherapy; MEC: moderately emetogenic chemotherapy; NEPA: netupitant+palonosetron+ dexamethasone; PA: palonosetron+dexamethasone; PO: per os (by mouth); QALD: quality-adjusted life-days; QALY: quality-adjusted life-years.
In HEC patients, given the price assumption for NEPA, the treatment-acquisition costs and the cost per CINV episode management were lower in the NEPA arm. The quality-adjusted life-days (QALDs) gain of NEPA compared to APPA was 0.21 days (=0.0006 years). Thus NEPA was a cost-saving treatment against APPA. Given the threshold for willingness to pay (WTP) of £30,000, the net benefit (NB) of NEPA against palonosetron was £62 with an acceptability of 97%.
In MEC patients, although the cost per CINV episode was less costly in the NEPA arm, the incremental drug cost resulted in NEPA being more costly overall. Compared to palonosetron (PA), the incremental cost of NEPA was £6.65 while the QALD gain was 0.18 days (=0.0005 QALYs), which gives an ICER of approximately £13,318. Given a threshold of WTP of £30,000, the NB of NEPA against palonosetron was £8 with an acceptability of 88%.
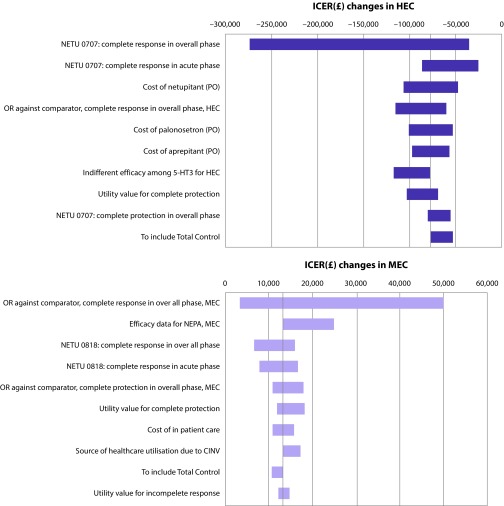
In the one-way sensitivity analysis, the effect of positively and negatively varying the continuous input variables by 25% and selecting alternative sources and assumptions were explored. The results of the sensitivity analyses are presented in a tornado diagram in Figure 3. The considered parameter variations resulted in NEPA always being the dominant strategy with negative incremental costs and positive incremental QALYs.
Figure 3.
Tornado diagram of one-way sensitivity analysis in HEC and MEC.
CINV: chemotherapy induced nausea and vomiting; HEC: highly emetogenic chemotherapy; ICER: incremental cost-effectiveness ratio; MEC: moderately emetogenic chemotherapy; NEPA: netupitant+palonosetron+dexamethasone; OR: odds ratio; PO: per os (by mouth).
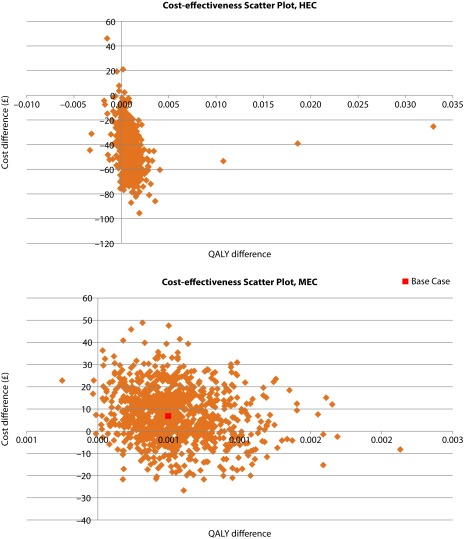
A probabilistic sensitivity analysis (PSA) sampling 1000 simulations was conducted. The results of the PSA are presented as scatterplots of incremental effects and costs (Figure 4) for the HEC and MEC populations. In HEC patients, NEPA was a dominant strategy in 89.2% of simulations against APPA and was cost saving but less effective in 10.4% of cases. Similarly, NEPA was a dominant strategy in 80.4% of simulations against palonosetron and cost saving but less effective in 0.1% of cases in the MEC population.
Figure 4.
Scatterplot of incremental effects and costs in HEC and MEC.
HEC: highly emetogenic chemotherapy; MEC: moderately emetogenic chemotherapy; QALY: quality-adjusted life-years.
Discussion
The primary objective of the analysis was to evaluate the cost-effectiveness of NEPA for the treatment of CINV in terms of the UK payer perspective. The evaluation was implemented by emetogenic level of chemotherapy, that is, HEC and MEC indications. The most relevant comparator to NEPA was APPA for HEC and PA for MEC.
The cost-effectiveness model was developed within a Markov cohort structure, which is well validated and has been used in published CINV models [24,29]. The single-cycle (5-day) structure allows the model to focus on CINV-related consequences and to prevent the outcomes from being influenced by disease characteristics and other toxicities of chemotherapy than emetogenicity. Thus, the results from the analysis can be applicable to CINV management regardless of cancer type, cancer stage and toxicity of chemotherapy except emetogenicity.
Clinical trials have demonstrated that palonosetron is effective for the treatment of CINV as a single agent, especially following cisplatin-based chemotherapy [30–32]. Palonosetron is therefore now the gold standard for treating CINV, either as a single agent in MEC or in combination with an aprepitant-based regimen in HEC [21]. Aprepitant-based regimens have been widely used in combination with 5-HT3 RAs, such as palonosetron, ondansetron or granisetron, for the treatment of CINV in the HEC population and are recommended by evidence-based, European and US consensus guidelines [21–23].
This economic evaluation shows that netupitant forms a better combination with palonosetron compared with aprepitant, in terms of both cost saving and efficacy in the HEC indication. Netupitant plus palonosetron is also more effective in terms of emesis days avoided and increased health-related quality of life, which is an increasingly important outcome for medical interventions, particularly in the area of supportive care. In the MEC indication when NEPA is compared against palonosetron, the current standard of care, the ICER of £13,318 is below the accepted threshold for being considered cost effective in the UK (£20,000–30,000 per QALY gained) [33]. In the UK, the use of NEPA for the treatment of CINV in both MEC and HEC can therefore be considered a cost-effective treatment option. The strength of these results were supported by the consistency of the results across all outcomes and populations in the model and in the sensitivity analyses. Several studies have demonstrated the need for therapies that are effective in preventing both acute and delayed CINV [34]. CINV control in clinical practice remains an issue, especially in the delayed phase; delayed CINV occurred in 58.4% of patients receiving anti-emetic therapy and nausea affected 60.7% of patients treated with current anti-emetics [12]. Owing to the dual component of NEPA in addressing both acute and delayed CINV, this economic evaluation demonstrates that guideline-adherent prophylaxis of CINV may be regarded as a cost-effective investment of resources. Together with the results of clinical trials that demonstrate the efficacy and safety of NEPA, these cost-effectiveness results demonstrate the clinical utility of this combination therapy [35–37]. These findings may therefore enhance clinicians’ individual acceptance of and adherence to guideline recommendations, thereby leading to the optimisation of CINV treatment strategy within clinical practice.
The analysis had the following limitations. First, the results may vary based on different chemotherapy cycles, since the efficacy of treatments may differ. Second, the analysis was based on a five-day time horizon. Any cost-effectiveness, healthcare consumption, and quality-of-life data, which were acquired beyond the model’s five-day time horizon were, therefore, not evaluated. Third, the generic utility measure used may not be very sensitive to CINV-specific states and therefore might not have comprehensively captured the differences between treatment strategies. Fourth, the results may have to be interpreted with caution when the model is applied to a specific group of patients, for example, young and/or female cancer patients, for whom different response rates may be observed compared to the general cancer population.
Further studies are required to evaluate the use of netupitant in combination with palonosetron in the UK for preventing CINV associated with both HEC and MEC over multiple cycles. Ideally, the allocation of resources should be informed by a comprehensive assessment of all relevant direct healthcare costs and indirect costs. To further assess the impact of CINV from a societal perspective, it would be of interest to also incorporate indirect costs, especially costs associated with missed work days for patients and their caregivers, as well as healthcare consumption data that were captured after the five-day time horizon used in this economic model.
In conclusion, this economic evaluation demonstrates that NEPA is a dominant (in HEC) and cost-effective (in MEC) treatment alternative to current anti-emetic standards of care in the UK during the first five days of chemotherapy treatment in cancer patients.
Abbreviations
- 5-HT3
5-HT3 receptor antagonist
- AE
adverse event
- APPA
aprepitant and palonosetron
- APR
aprepitant
- ASCO
American Society of Clinical Oncology
- BNF
British National Formulary
- CI
confidence interval
- CINV
chemotherapy-induced nausea and vomiting
- CP
complete protection
- CR
complete response
- CrI
credible interval
- DIC
deviance information criterion
- DSA
deterministic sensitivity analysis
- ESMO
European Society for Medical Oncology
- HEC
highly emetogenic chemotherapy
- HTA
health technology assessment
- ICER
incremental cost-effectiveness ratio
- IV
intravenous
- MASCC
The Multinational Association of Supportive Care in Cancer
- MEC
moderately emetogenic chemotherapy
- mg
milligram
- MIMS
Monthly Index of Medical Specialities
- MTC
Mixed Treatment Comparison
- NA
not available
- NCCN
National Comprehensive Cancer Network
- NEPA
netupitant and palonosetron fixed combination
- NETU
netupitant
- NHS
National Health Service
- NICE
National Institute for Health and Clinical Excellence
- NK1
NK1 receptor antagonist
- OR
odds ratio
- PA
palonosetron
- PICOS
Population, Interventions, Comparators, Outcomes and Study design
- PO
per os
- PSA
probabilistic sensitivity analysis
- PSSRU
Personal Social Services Research Unit
- QALD
quality-adjusted life-days
- QALY
quality-adjusted life-years
- RA
receptor antagonist
- RR
response rate
- TC
total control
- UK
United Kingdom
- VAS
visual analogue scale
- WTP
willingness to pay
Footnotes
Contributions: François Bourhis programmed the model and co-drafted the manuscript with Hélène Cawston, who also conducted the targeted literature search in support of the model. All authors were instrumental in the conceptual design and participated extensively in the review and editing of the model and manuscript.
Disclosure and potential conflicts of interest: Hélène Cawston, François Bourhis and Jennifer Eriksson have provided consulting services to Helsinn Healthcare SA. Pierfrancesco Ruffo, Paolo D’Agostino and Marco Turini are employees of Helsinn Healthcare SA. Lee Schwartzberg and Alistair McGuire have provided consulting services to Helsinn Healthcare SA.
The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors are available for download at http://www.drugsincontext.com/wp-content/uploads/2017/03/dic.212298-COI.pdf.
Funding declaration: The study was supported by Helsinn Healthcare SA.
Correct attribution: Copyright © 2017 Cawston H, Bourhis F, Eriksson J, Ruffo P, D’Agostino P, Turini M, Schwartzberg L, McGuire A. https://doi.org/10.7573/dic.212298. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 3.0.
Peer review comments to author: 24 June 2016
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: 14 Weller Street, London, SE1 1QU, UK
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252772009.
For all manuscript and submissions enquiries, contact the Editorial office dic.editorial@bioexcelpublishing.com
For all permissions, rights and reprints, contact Peter Clarke at peter.clarke@bioexcelpublishing.com
References
- 1.Jordan K, Gralla R, Jahn F, Molassiotis A. International antiemetic guidelines on chemotherapy induced nausea and vomiting (CINV): content and implementation in daily routine practice. Eur J Pharmacol. 2014;722:197–202. doi: 10.1016/j.ejphar.2013.09.073. http://dx.doi.org/10.1016/j.ejphar.2013.09.073. [DOI] [PubMed] [Google Scholar]
- 2.Janelsins MC, Tejani MA, Kamen C, Peoples AR, Mustian KM, Morrow GR. Current pharmacotherapy for chemotherapy-induced nausea and vomiting in cancer patients. [Review] Expert Opin Pharmacother. 2013;14(6):757–66. doi: 10.1517/14656566.2013.776541. http://dx.doi.org/10.1517/14656566.2013.776541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia Gomez J, Perez Lopez ME, Garcia Mata J, Isla Casado D SEOM (Spanish Society for Medical Oncology) SEOM clinical guidelines for the treatment of antiemetic prophylaxis in cancer patients receiving chemotherapy. Clin Transl Oncol. 2010;12(11):770–4. doi: 10.1007/s12094-010-0594-5. http://dx.doi.org/10.1007/s12094-010-0594-5. [DOI] [PubMed] [Google Scholar]
- 4.Hesketh P. Comparative review of 5-HT3 receptor antagonists in the treatment of acute chemotherapy-induced nausea and vomiting. Cancer Invest. 2000;18:163–73. doi: 10.3109/07357900009038248. http://dx.doi.org/10.3109/07357900009038248. [DOI] [PubMed] [Google Scholar]
- 5.Ballatori E, Roila F, Ruggeri B, Porrozzi S, Iannopollo M, Soru G, Cruciani G, Daniele B, Locatelli MC, Pellissier J, Deuson R, Ballatori E, Roila F, Ruggeri B, Porrozzi S, Iannopollo M, Soru G, Cruciani G, Daniele B, Locatelli MC, Pellissier J, Deuson R. The cost of chemotherapy-induced nausea and vomiting in Italy. Support Care Cancer. 2007;15(1):31–8. doi: 10.1007/s00520-006-0094-x. http://dx.doi.org/10.1007/s00520-006-0094-x. [DOI] [PubMed] [Google Scholar]
- 6.Burke TA, Wisniewski T, Ernst FR, Burke TA, Wisniewski T, Ernst FR. Resource utilization and costs associated with chemotherapy-induced nausea and vomiting (CINV) following highly or moderately emetogenic chemotherapy administered in the US outpatient hospital setting. Support Care Cancer. 2011;19(1):131–40. doi: 10.1007/s00520-009-0797-x. http://dx.doi.org/10.1007/s00520-009-0797-x. [DOI] [PubMed] [Google Scholar]
- 7.Craver C, Gayle J, Balu S, Buchner D, Craver C, Gayle J, Balu S, Buchner D. Clinical and economic burden of chemotherapy-induced nausea and vomiting among patients with cancer in a hospital outpatient setting in the United States. J Med Econ. 2011;14(1):87–98. doi: 10.3111/13696998.2010.547237. http://dx.doi.org/10.3111/13696998.2010.547237. [DOI] [PubMed] [Google Scholar]
- 8.Hamada S, Hinotsu S, Hori K, Furuse H, Oikawa T, Kawakami J, Ozono S, Akaza H, Kawakami K, Hamada S, Hinotsu S, Hori K, Furuse H, Oikawa T, Kawakami J, Ozono S, Akaza H, Kawakami K. The cost of antiemetic therapy for chemotherapy-induced nausea and vomiting in patients receiving platinum-containing regimens in daily practice in Japan: a retrospective study. Support Care Cancer. 2012;20(4):813–20. doi: 10.1007/s00520-011-1155-3. http://dx.doi.org/10.1007/s00520-011-1155-3. [DOI] [PubMed] [Google Scholar]
- 9.Ihbe-Heffinger A, Ehlken B, Bernard R, Berger K, Peschel C, Eichler HG, Deuson R, Thodtmann J, Lordick F, Ihbe-Heffinger A, Ehlken B, Bernard R, Berger K, Peschel C, Eichler HG, Deuson R, Thodtmann J, Lordick F. The impact of delayed chemotherapy-induced nausea and vomiting on patients, health resource utilization and costs in German cancer centers. Ann Oncol. 2004;15(3):526–36. doi: 10.1093/annonc/mdh110. http://dx.doi.org/10.1093/annonc/mdh110. [DOI] [PubMed] [Google Scholar]
- 10.Turini M, Piovesana V, Ruffo P, Ripellino C, Cataldo N. An assessment of chemotherapy-induced nausea and vomiting direct costs in three EU countries. Drugs in context. 2015;4:212285. doi: 10.7573/dic.212285. http://dx.doi.org/10.7573/dic.212285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jordan K, Sippel C, Schmoll HJ. Guidelines for antiemetic treatment of chemotherapy-induced nausea and vomiting: past, present, and future recommendations. Oncologist. 2007;12(9):1143–50. doi: 10.1634/theoncologist.12-9-1143. http://dx.doi.org/10.1634/theoncologist.12-9-1143. [DOI] [PubMed] [Google Scholar]
- 12.Haiderali A, Menditto L, Good M, Teitelbaum A, Wegner J, Haiderali A, Menditto L, Good M, Teitelbaum A, Wegner J. Impact on daily functioning and indirect/direct costs associated with chemotherapy-induced nausea and vomiting (CINV) in a U.S. population. Support Care Cancer. 2011;19(6):843–51. doi: 10.1007/s00520-010-0915-9. http://dx.doi.org/10.1007/s00520-010-0915-9. [DOI] [PubMed] [Google Scholar]
- 13.Aapro M, Molassiotis A, Dicato M, Pelaez I, Rodriguez-Lescure A, Pastorelli D, Ma L, Burke T, Gu A, Gascon P, Roila F, investigators PEER, Aapro M, Molassiotis A, Dicato M, Pelaez I, Rodriguez-Lescure A, Pastorelli D, Ma L, Burke T, Gu A, Gascon P, Roila F, investigators PEER. The effect of guideline-consistent antiemetic therapy on chemotherapy-induced nausea and vomiting (CINV): the Pan European Emesis Registry (PEER) Ann Oncol. 2012;23(8):1986–92. doi: 10.1093/annonc/mds021. http://dx.doi.org/10.1093/annonc/mds021. [DOI] [PubMed] [Google Scholar]
- 14.Molassiotis A, Saunders MP, Valle J, Wilson G, Lorigan P, Wardley A, Levine E, Cowan R, Loncaster J, Rittenberg C, Molassiotis A, Saunders MP, Valle J, Wilson G, Lorigan P, Wardley A, Levine E, Cowan R, Loncaster J, Rittenberg C. A prospective observational study of chemotherapy-related nausea and vomiting in routine practice in a UK cancer centre. Support Care Cancer. 2008;16(2):201–8. doi: 10.1007/s00520-007-0343-7. http://dx.doi.org/10.1007/s00520-007-0343-7. [DOI] [PubMed] [Google Scholar]
- 15.Grunberg SM. Obstacles to the implementation of antiemetic guidelines. J Natl Compr Canc Netw. 2009;7(5):601–5. doi: 10.6004/jnccn.2009.0040. [DOI] [PubMed] [Google Scholar]
- 16.Kaiser R. Antiemetic guidelines: are they being used? Lancet Oncol. 2005;6(8):622–5. doi: 10.1016/S1470-2045(05)70284-9. http://dx.doi.org/10.1016/S1470-2045(05)70284-9. [DOI] [PubMed] [Google Scholar]
- 17.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] Cochrane Database Syst Rev [Internet] 2011. [Accessed May 2014]. [Last accessed: 15 March 2017]. Available at: http://handbook.cochrane.org/
- 18.National Cancer Institute. Chemotherapy Induced Nausea and Vomiting. 2014. [Last accessed: May 2014]. Available at: http://www.cancer.gov/
- 19.Der Simonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. http://dx.doi.org/10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Welton N, Sutton A, Cooper N, Abrams K, Ades A. Evidence synthesis for decision making in healthcare. London: John Wiley & Sons, Ltd; 2012. http://dx.doi.org/10.1002/9781119942986. [Google Scholar]
- 21.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®)—Antiemesis. National Comprehensive Cancer Network®; 2016. [Google Scholar]
- 22.Basch E, Prestrud AA, Hesketh PJ, Kris MG, Feyer PC, Somerfield MR, Chesney M, Clark-Snow RA, Flaherty AM, Freundlich B, Morrow G, Rao KV, Schwartz RN, Lyman GH, Basch E, Prestrud AA, Hesketh PJ, Kris MG, Feyer PC, Somerfield MR, Chesney M, Clark-Snow RA, Flaherty AM, Freundlich B, Morrow G, Rao KV, Schwartz RN, Lyman GH Oncology. ASoC. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. [Review] J Clin Oncol. 2011;29(31):4189–98. doi: 10.1200/JCO.2010.34.4614. http://dx.doi.org/10.1200/JCO.2010.34.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Multinational Association of Supportive Care in Cancer. MASCC/ESMO Antiemetic Guideline 2014. [Last accessed: 15 March 2017]. Available at: http://www.mascc.org/antiemetic-guidelines.
- 24.Annemans L, Strens D, Lox E, Petit C, Malonne H, Annemans L, Strens D, Lox E, Petit C, Malonne H. Cost-effectiveness analysis of aprepitant in the prevention of chemotherapy-induced nausea and vomiting in Belgium. Support Care Cancer. 2008;16(8):905–15. doi: 10.1007/s00520-007-0349-1. http://dx.doi.org/10.1007/s00520-007-0349-1. [DOI] [PubMed] [Google Scholar]
- 25.Lordick F, Ehlken B, Ihbe-Heffinger A, Berger K, Krobot KJ, Pellissier J, Davies G, Deuson R, Lordick F, Ehlken B, Ihbe-Heffinger A, Berger K, Krobot KJ, Pellissier J, Davies G, Deuson R. Health outcomes and cost-effectiveness of aprepitant in outpatients receiving antiemetic prophylaxis for highly emetogenic chemotherapy in Germany. Eur J Cancer. 2007;43(2):299–307. doi: 10.1016/j.ejca.2006.09.019. http://dx.doi.org/10.1016/j.ejca.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 26.Borjeson S, Hursti TJ, Peterson C, Fredikson M, Furst CJ, Avall-Lundqvist E, Steineck G. Similarities and differences in assessing nausea on a verbal category scale and a visual analogue scale. Cancer Nurs. 1997;(4):260–6. doi: 10.1097/00002820-199708000-00005. http://dx.doi.org/10.1097/00002820-199708000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Grunberg SM, Boutin N, Ireland A, Miner S, Silveira J, Ashikaga T. Impact of nausea/vomiting on quality of life as a visual analogue scale-derived utility score. Support Care Cancer. 1996;1996(6):435–9. doi: 10.1007/BF01880641. http://dx.doi.org/10.1007/BF01880641. [DOI] [PubMed] [Google Scholar]
- 28.Humphreys S, Pellissier J, Jones A. Cost-effectiveness of an aprepitant regimen for prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer in the UK. Cancer Manag Res. 2013;5(1):215–24. doi: 10.2147/CMAR.S44539. http://dx.doi.org/10.2147/CMAR.S44539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore S, Tumeh J, Wojtanowski S, Flowers C, Moore S, Tumeh J, Wojtanowski S, Flowers C. Cost-effectiveness of aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with highly emetogenic chemotherapy. Value in health: the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2007;10(1):23–31. doi: 10.1111/j.1524-4733.2006.00141.x. http://dx.doi.org/10.1111/j.1524-4733.2006.00141.x. [DOI] [PubMed] [Google Scholar]
- 30.Eisenberg P, Figueroa-Vadillo J, Zamora R, Charu V, Hajdenberg J, Cartmell A, Macciocchi A, Grunberg S Palonosetron Study G. Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single-dose trial versus dolasetron. Cancer. 2003;98(11):2473–82. doi: 10.1002/cncr.11817. http://dx.doi.org/10.1002/cncr.11817. [DOI] [PubMed] [Google Scholar]
- 31.Likun Z, Xiang J, Yi B, Xin D, Tao ZL. A systematic review and meta-analysis of intravenous palonosetron in the prevention of chemotherapy-induced nausea and vomiting in adults. Oncologist. 2011;16(2):207–16. doi: 10.1634/theoncologist.2010-0198. http://dx.doi.org/10.1634/theoncologist.2010-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aapro MS, Grunberg SM, Manikhas GM, Olivares G, Suarez T, Tjulandin SA, Bertoli LF, Yunus F, Morrica B, Lordick F, Macciocchi A. A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol. 2006;17(9):1441–9. doi: 10.1093/annonc/mdl137. http://dx.doi.org/10.1093/annonc/mdl137. [DOI] [PubMed] [Google Scholar]
- 33.National Institute for Health and Care Excellence. Guide to the methods of technology appraisal. 2013. [Last accessed: 15 March 2017]. Available at: https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781. [PubMed]
- 34.de Boer-Dennert M, de WR, Schmitz PI, Djontono J, Beurden V, Stoter G, Verweij J. Patient perceptions of the side-effects of chemotherapy: the influence of 5HT3 antagonists. BrJ Cancer. 1997;76(8):1055–61. doi: 10.1038/bjc.1997.507. http://dx.doi.org/10.1038/bjc.1997.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aapro M, Rugo H, Rossi G, Rizzi G, Borroni ME, Bondarenko I, Sarosiek T, Oprean C, Cardona-Huerta S, Lorusso V, Karthaus M, Schwartzberg L, Grunberg S. A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy. Ann Oncol. 2014;25(7):1328–33. doi: 10.1093/annonc/mdu101. http://dx.doi.org/10.1093/annonc/mdu101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gralla RJ, Bosnjak SM, Hontsa A, Balser C, Rizzi G, Rossi G, Borroni ME, Jordan K. A phase III study evaluating the safety and efficacy of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting over repeated cycles of chemotherapy. Ann Oncol. 2014;25(7):1333–9. doi: 10.1093/annonc/mdu096. http://dx.doi.org/10.1093/annonc/mdu096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hesketh PJ, Rossi G, Rizzi G, Palmas M, Alyasova A, Bondarenko I, Lisyanskaya A, Gralla RJ. Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: a randomized dose-ranging pivotal study. Ann Oncol. 2014;25(7):1340–6. doi: 10.1093/annonc/mdu110. http://dx.doi.org/10.1093/annonc/mdu110. [DOI] [PMC free article] [PubMed] [Google Scholar]