Abstract
Signal Amplification by Reversible Exchange (SABRE) is a fast and convenient NMR hyperpolarization method that uses cheap and readily available para-hydrogen as a hyperpolarization source. SABRE can hyperpolarize protons and heteronuclei. Here we focus on the heteronuclear variant introduced as SABRE-SHEATH (SABRE in SHield Enables Alignment Transfer to Heteronuclei) and nitrogen-15 targets in particular. We show that 15N-SABRE works more efficiently and on a wider range of substrates than 1H-SABRE, greatly generalizing the SABRE approach. In addition, we show that nitrogen-15 offers significantly extended T1 times of up to 12 minutes. Long T1 times enable higher hyperpolarization levels but also hold the promise of hyperpolarized molecular imaging for several tens of minutes. Detailed characterization and optimization are presented, leading to nitrogen-15 polarization levels in excess of 10% on several compounds.
Introduction
Hyperpolarization techniques enhance nuclear polarization and nuclear magnetic resonance (NMR) signals by 4 to 8 orders of magnitude.1−11 The concomitant increase of signal-to-noise (S/N) has opened new opportunities for in vitro NMR studies such as nanomolar detection12−15 and in vivo MRI with direct access to metabolic activity on the molecular level.16−20
Signal Amplification By Reversible Exchange (SABRE)21 is the nonhydrogenative variant of para-hydrogen induced polarization (PHIP).22−25 A hydrogenation reaction is not required, and substrates can be hyperpolarized continuously or repeatedly. Furthermore, SABRE occurs in the liquid state at room temperature and requires only inexpensive equipment.21,26,27 As depicted in Scheme 1, SABRE uses a transition metal catalyst to establish contact between the polarization source, para-H2, and the target nuclei.
Scheme 1. Reversible Exchange of para-H2 and Substrate on the Polarization Transfer Catalyst Leads to Continuous Buildup of Polarization on the Free Substrate.
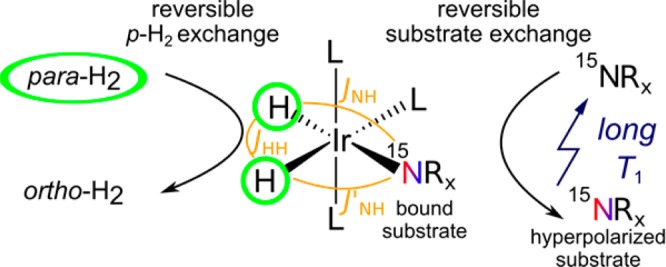
The J-couplings in the polarization transfer complex drive the polarization transfer. L: Ligands. Rx: arbitrary group.
Specifically, magnetic contact is established by the J-coupling of the para-H2-derived hydrides to the target nuclei across the iridium center. Reversible exchange of hydrides and substrate leads to continuous hyperpolarization buildup on the substrate as long as fresh para-H2 is supplied. Accordingly, SABRE has also been referred to as polarization transfer catalysis (PTC).28
Until recently, SABRE was primarily focused on 1H nuclei, where T1 is typically short (several seconds), and high 1H polarization levels (1%) are observed predominantly for Lewis basic N-heterocyclic molecules. On the other hand, heteronuclei (e.g., 15N, 13C, 31P) offer long T1 relaxation times, in particular at low magnetic fields, and allow for large polarization levels for a wider variety of structural motifs. As demonstrated recently, heteronuclei are most efficiently targeted when conducting SABRE at magnetic fields of a few μT inside magnetically shielded environments (SABRE-SHEATH).29−31 Here we focus on the direct hyperpolarization of nitrogen-15 at μT magnetic evolution fields. The relatively simple experimental procedure is shown in the Supporting Information video.
The present contribution presents three major points. First, we demonstrate that the substrate scope is significantly expanded with SABRE-SHEATH. This is because nitrogen is directly bound to iridium, unlike the protons in the R-groups. Second, we show that long T1 lifetimes, in excess of 10 min, can be obtained at relatively low fields in 1 T permanent magnets. This is significant because the combination of low cost hyperpolarization with low cost NMR detection sets the stage for affordable and highly sensitive NMR spectroscopy and molecular magnetic resonance imaging (MRI). Lastly, we optimize the SABRE-SHEATH process in detail leading to nitrogen-15 hyperpolarization in excess of 10%.
Methods
Methanol-d4 solutions with defined concentrations of precatalyst ([IrCl(COD)(IMes)]; COD = 1,5-cyclooctadiene; IMes = 1,3-bis(2,4,6-trimethylphenyl)-imidazol-2-ylidene, substrate (Sub), and coligand (L) were converted to catalytically active solutions of ([Ir(H)2(IMes)LxSub3−x]) by bubbling hydrogen gas through these solutions. The pressure was regulated to 10 bar. The pressure gradient across the sample was negligible (<0.1 bar), just enough to establish the desired flow rate (typically 60 sccm ≈ 6 ccm at 10 bar). The flow rate is set with a needle valve at the outlet after the bubbling stage, directly venting the hydrogen gas into a hood.
The sample volume was 500 μL inside a medium-walled 5 mm pressure NMR tube (Wilmad 524-PV-8). The para-H2 fraction was 80–90% unless noted otherwise. After a suitable activation period (10 min to 1 h), samples were placed in a defined magnetic evolution field Bevo in the μT range. Evolution time in the shield exceeded the build-up time to yield maximal signal intensity. The T1 measurements were performed from a single hyperpolarization process by a sequence of small tip angle (6°) pulses. Measurements at 1 T were performed in a benchtop NMR instrument (Magritek 15N-Spinsolve). Measurements at 8.45 T were carried out on a Bruker Avance DX360 NMR spectrometer. See Supporting Information (SI) for additional experimental details, a theoretical model of SABRE-SHEATH, as well as a video illustrating experiments.
Results and Discussion
I. Expansion of Substrate Range
The generalized substrate scope is illustrated in Table 1. (Full experimental details are provided in the SI). The results suggest that, in general, sp and sp2-hybridized 15N sites can be hyperpolarized. This is significant because sp and sp2 hybridized nitrogens are found in a wide range of metabolites and drugs.32 It is noteworthy that 1H enhancements are negligible, except for Entries 5 and 8, formally imidazole and pyridine derivatives. Table 1 includes T1 values, where available; however, they vary strongly with concentration, solvent, and magnetic field (see below).
Table 1. 15N-SABRE-SHEATH Enhancements (ε) over Thermal Measurements at 8.5 T, Polarization Levels, and T1 in Methanol-d4 for Diverse Molecular Motifsd.
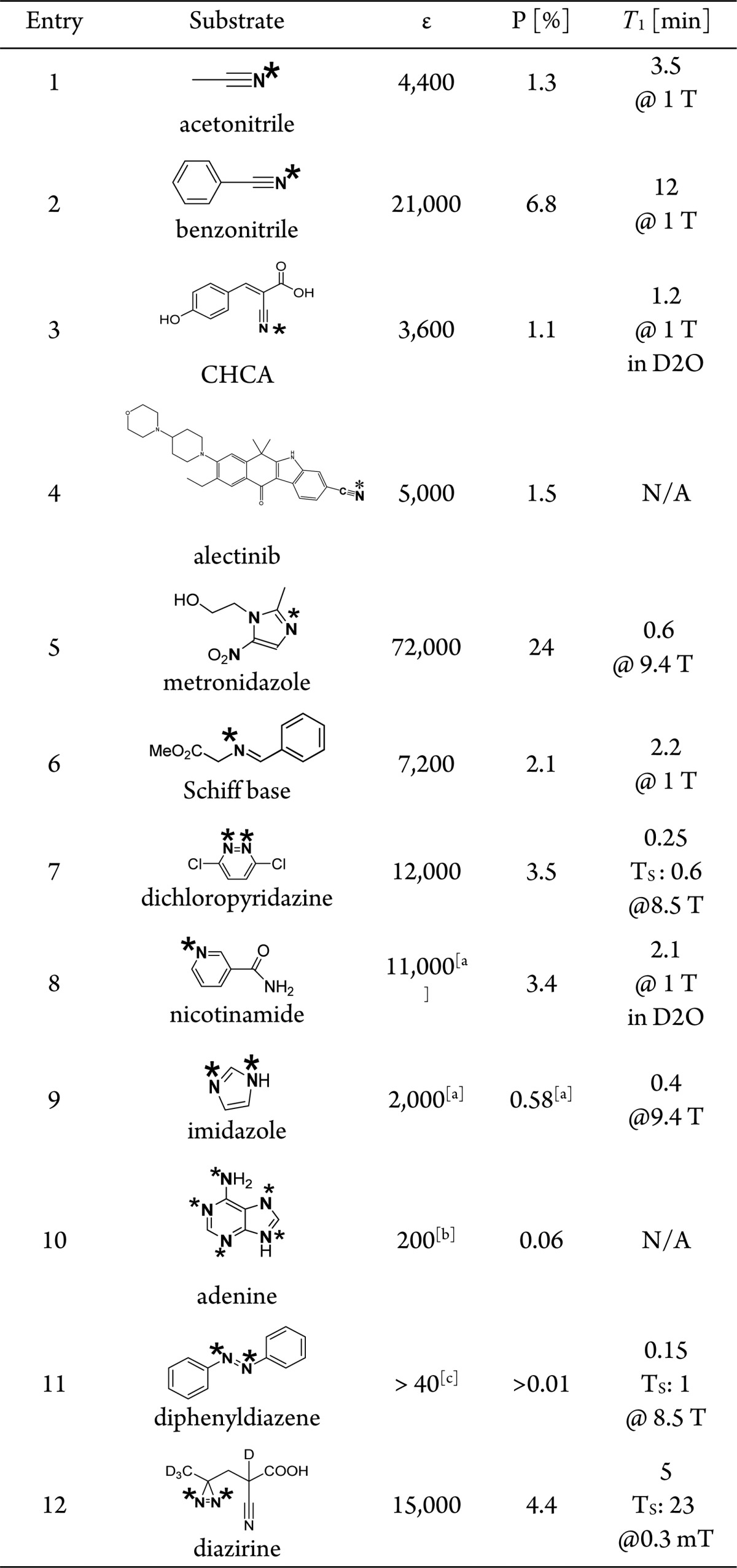
Data obtained with 50% para-H2.
Enhancements averaged over all 15N sites.
SABRE hyperpolarizes the Z-isomer. See SI for additional information.
∗ indicates hyperpolarized site.
The first four entries are nitriles (sp-hybrid 15N), commonly encountered in biologically active molecules and drugs.32 Nitriles have been reported as 1H-SABRE substrates, but enhancements did not exceed ∼60-fold (over 9.4 T).33 In contrast, with SABRE-SHEATH, nitriles consistently show polarization levels of several percent (see Table 1). Nitriles are particularly versatile; polarization levels remain large irrespective of the backbone structure or system composition. Many substituents, including unsaturated bonds, hydroxyl and carboxylic acid groups are tolerated. Examples are α-cyano-4-hydroxycinnamic acid (CHCA, entry 3) with potentiating effects for chemotherapy34 and alectinib (entry 4), a potent inhibitor of anaplastic lymphoma kinase (ALK) also used for the treatment of nonsmall cell lung cancer.
The remaining entries in Table 1 (entries 6−12) all contain sp2-hybridized 15N. As a first example, metronidazole (entry 5) is an antibiotic also used as a hypoxia probe.35−39 Very high polarization levels are observed for this molecule, when nitrogen is at its natural abundance of 0.36%. Next, we found that 15N nuclei in a wide range of Schiff bases (entry 6), which included biologically relevant scaffolds, such as pyridoxal phosphate analogues and retinal with critical roles in visual signaling, hyperpolarize well.40−42
Dichloropyridazine (entry 7) also illustrates the basic principle: the 15N sites hyperpolarize well, whereas the protons do not because they do not directly interact with the catalyst. This molecule also is the first example with two adjacent 15N nuclei, offering the opportunity to store hyperpolarization in long-lived singlet states characterized by extended decay time constants TS.43
A further interesting substrate is nicotinamide (vitamin B3, entry 8) with its important functions in mammalian metabolism.44−47 A significant opportunity in current medical imaging is presented by imidazole (entry 9) which has a pKA of ∼7.1, ideal for in vivo pH sensing.48 Furthermore, nucleobases, such as adenine (entry 10), are also amendable to hyperpolarization. Surprisingly, enhancements can be detected for all nitrogen positions including the amino group, presumably due to the existence of the enamine–imine tautomer, which is structurally similar to Schiff bases (or indirect polarization transfer). Diphenyldiazene (entry 11) exhibits E/Z isomerism induced optically,49,50 or chemically.51 Interestingly the Z form is hyperpolarized exclusively despite the fact that the E form is thermodynamically stable. Enhancements of the Z-form, in presently unknown low concentration, significantly exceed 40-fold (see SI). The last entry, a diazirine (entry 12), stands out as it offers particularly long decay time constants, T1 of 5 min and TS of above 20 min, allowing for detection of enhanced signal for hours after polarization buildup. These diazirine moieties can readily be incorporated in a wide range of drugs or biomolecules replacing CH2– groups.52
The lowest enhancements are recorded for imidazole (entry 9)48 and adenine (entry 10) with exchanging protons at the 15N sites. This causes shorter T1 times and significant polarization losses during transfer to the detection field (∼8 s in our experiments). For substrates with exchanging protons higher pH values are associated with larger enhancements because deprotonated forms bind to the catalyst more easily and because T1 is longer on deprotonated 15N.
II. Extended Relaxation Times
Nitrogen-15 SABRE is particularly appealing because many substrates show large T1 times, which enables the observation of slow processes, such as diffusion, biochemical reactions, or downstream metabolic processes. Table 2 lists T1 times and S/N (signal-to-noise) for various substrates at two different magnetic fields, 8.5 and 1 T. Measurements at 1 T were performed in an inexpensive, portable benchtop NMR instrument (Magritek 15N-Spinsolve), illustrating the potential of low-cost high-sensitivity NMR when combining SABRE hyperpolarization with permanent magnet-based technology. Since most clinical MRI scanners operate in the 1–3 T range, measurements at 1 T are useful for future biomedical translation.
Table 2. Comparison of Spin–Lattice Relaxation Times T1 and Signal-to-Noise in a Single 90°-Acquired Experiment of Dilute (Methanol-d4) and Neat Solutions at 8.5 and 1 Ta.
| compound | conc. [mM] | T1 @ 8.5 T [min] | T1 @ 1 T [min] | S/N @ 8.5 T | S/N @ 1 T |
|---|---|---|---|---|---|
| 15N-pyridine | 50 | 1.05 ± 0.02 | 2.06 ± 0.13 | 5700 | 1500 |
| 15N-acetonitrile | 50 | 2.05 ± 0.02 | 3.73 ± 0.05 | 5000 | 2900 |
| 15N-benzonitrile | 50 | 2.15 ± 0.02 | 11.95 ± 0.35 | 7300 | 1400 |
| 15N2-diazirine | 50 | 0.16 ± 0.002 | 4.35 ± 0.1 | 1200 | 1300 |
| pyridine | neat (12.4 M) | 1.5 ± 0.05 | 3.31 ± 0.3 | 300 | 300 |
| pyridine-d5 | neat (12.4 M) | 1.6 ± 0.02 | 3.68 ± 0.45 | 1300 | 400 |
| acetonitrile | neat (19.1 M) | 2.2 ± 0.05 | 2.3 ± 0.4 | 1000 | 200 |
| acetonitrile-d3 | neat (19.1 M) | 2.36 ± 0.03 | 2.01 ± 0.1 | 1900 | 400 |
| benzonitrile | neat (9.7 M) | 2.88 ± 0.03 | 10.5 ± 1 | 7100 | 2600 |
Neat solutions have 15N at natural abundance (0.36%).
The results clearly show that T1 times strongly depend on the magnetic field and molecular structure. The molecular environment of the respective spin determines which relaxation mechanism is relevant. Usually, NMR relaxation is dominated by dipolar relaxation, caused by other nearby spins, and has a relatively weak field dependence. However, nitrogen-15, which is often far removed from other spins, can be dominated by chemical shift anisotropy (CSA) relaxation. Under these circumstances a strong field dependence is expected because CSA relaxation scales with B02.54
This is best exemplified by 15N-benzonitrile and 15N2-diazirine. In benzonitrile dipolar relaxation is minimized, as protons are far away from the nitrogen nucleus. We observe T1 ≈ 2.15 min at 8.5 T and T1 ≈ 11.95 min at 1 T; CSA dominates relaxation. In the 15N2-diazirine the field dependence is even more pronounced. At 8.5 T we measure T1 of ∼10 s, but at 1 T, T1 ≈ 4.35 min. Note that we recently also reported the relaxation time constant of the long-lived singlet state in diazirine with TS in excess of 20 min at B0 = 0.3 mT.52
Now we focus our attention on the S/N comparison between the investigated magnetic fields. In NMR with thermally polarized spins, signal scales with B02, as, both, polarization and induction are proportional to B0.54,55
The noise, on the other hand (in the coil noise dominated regime), scales with B01/4; hence S/N scales with B07/4.55−57 However, if the polarization source is an external hyperpolarizer, spin polarization is no longer determined by B0 and S/N scales as B03/4. The expected signal loss in our experiments when going from 8.5 to 1 T is thus (8.5/1)3/4 ≈ 5. However, it is evident from Table 2 that measurements at 1 T are on average only 2.8 times less sensitive than at 8.5 T. This can be attributed to different pickup-coil design, where coil sensitivity is higher at 1 T as the inductance in solenoid coils (used at 1 T) is larger than in saddle coils (used at 8.5 T).58 An additional factor is relaxation during sample transfer from the hyperpolarization region to detection fields (∼1 s to 1 T, ∼8 s to 8.5 T). The latter effect is best exemplified by the molecule with the most pronounced field dependence of the relaxation time (15N2 diazirine). In this case, the S/N is better at 1 T than at 8.5 T because of reduced relaxation losses in 1 T experiments. Notice that the S/N comparisons were performed without additional delays before acquisition. With additional delays, the low-field experiments would be more favorable because of extended T1.
For future applications, an important scenario is body-noise-dominated magnetic resonance, as is the case for human MRI. Here body-noise (dielectric loss) dominates, and the S/N with thermal magnetization is proportional to B0.56,59 Accordingly, for hyperpolarized MRI, S/N is expected to be directly proportional to hyperpolarization level and independent of B0.58,60 Therefore, NMR and MRI in low fields are of great general interest as magnet and RF-circuit design is more flexible, and devices are portable and relatively cheap.60,61 For example, recent advances in low-field MRI have already enabled high performance 1H-MRI at fields as low as 6.5 mT, even using thermal magnetization.62 The newest advances, such as “External High-Quality-factor-Enhanced NMR” (EHQE-NMR)63 and others,64 lead to independence of B0, even for spectroscopic applications of hyperpolarized MR.
Both the presented T1 lifetime and S/N results suggest that NMR and MRI with hyperpolarized heteronuclei can benefit from low fields. Especially, the combination of a simple hyperpolarization technique like SABRE with low-field detection appears as a powerful approach. Costs go down, and relaxation times go up without sacrificing S/N. With SABRE-SHEATH, 15N-labeled markers can be hyperpolarized continuously or repeatedly, directly in room-temperature solutions. Thus, low-field NMR and MRI with SABRE may enable high sensitivity NMR and MRI for a large audience at moderate cost with many applications.65,66
III. Analysis of Temperature and Field Dependence
Enhancements and polarization levels reported in Table 1 are not optimized with respect to all variables affecting efficiency, i.e., catalyst and substrate concentrations, coligand concentration, para-H2 pressure/flow rate, temperature, magnetic field, etc. The experimental efforts involved in globally optimizing this multidimensional parameter space would not be practicable for all substrates.
Here we focus on the dependence of polarization on magnetic evolution field Bevo and average complex lifetime τlife using a suitable model system. This model system needs to exhibit large, stable enhancements over many experiments and have a relatively quick polarization buildup (<5 min), and continuous repetition of experiments must be possible. We use a system similar to Mewis et al.,33 i.e., acetonitrile-15N (100 mM), IrCl(IMes) (COD) (5 mM), and pyridine (33 mM) in methanol-d4. (Pyridine is added for stability; without this coligand experiments were inconsistent.) Note that 1H enhancements did not exceed 60-fold (over thermal polarization at 9 T),33 whereas 15N exhibits more than 4000-fold enhancement. This particular system composition yields large S/N and long-term stability, not maximum 15N-polarization (vide infra).
In Figure 1 we show that 15N polarization strongly depends on magnetic field (Figure 1A) and temperature during evolution in the shield (Figure 1B). We observe that the phase of the hyperpolarized signal (with respect to a fixed detector phase) can be controlled by the magnetic evolution field (i.e., 180° phase shift is observed upon Bevo inversion; see Figure 1, A and C). This is a useful feature for hyperpolarization experiments because it allows for simple difference measurements, as well as easy distinction of hyperpolarized signals from the thermal background. This inversion is associated with the existence of the two matching conditions.67,68
Figure 1.
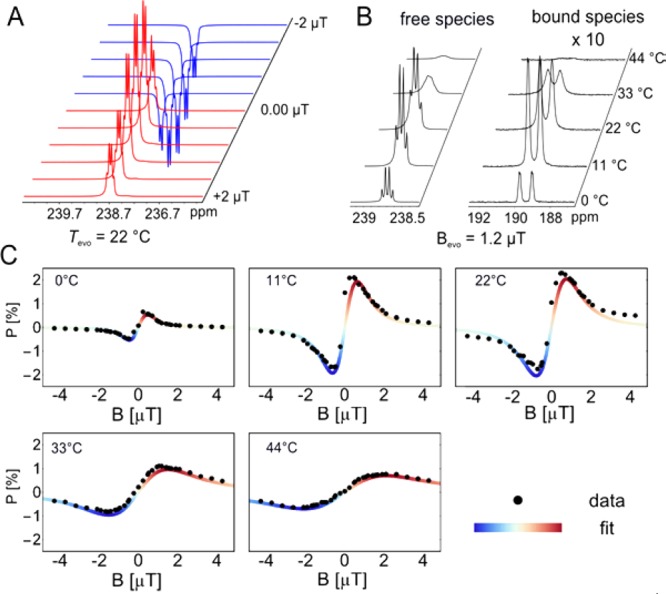
Temperature and field dependence of SABRE-SHEATH. (A) Excerpt of the experimental 15N spectra at different magnetic evolution fields at a temperature of 22 °C. (B) NMR spectra at constant magnetic field but different temperatures. (C) Free substrate 15N polarization at different magnetic evolution fields and temperatures. The data are fit to a simple theoretical model derived from the three-spin model as shown in Scheme 1 and Figure 2A. Red vs blue highlights the 180° phase shift.
In the absence of chemical exchange we obtain
| 1 |
where the J coupling parameters are defined in Figure 2. The expected optimal field is Bevo = ±0.3 μT. However, the experimental data from Figure 1C clearly show that the maxima are shifted to higher magnetic fields and scale with the temperature. These shifts are caused by broadening of the resonance conditions as a result of chemical exchange. Shorter catalyst/substrate complex lifetimes, at elevated temperatures, cause broader resonance conditions and more overlap. In the SI we develop a theory that includes the exchange and obtains an analytical expression that only depends on the J-coupling parameters, the magnetic field, and the average catalyst lifetime. We use this expression to fit the data as illustrated in Figure 1C and obtain excellent qualitative agreement; however, quantitative agreement with experimental lifetime is not obtained. Experimentally, it is easy to determine the J-couplings and the average lifetime τlife as depicted in Figure 2B. Specifically, the substrate dissociation limited catalyst lifetime69 is monitored by the change in NMR line width Δνi of the catalyst bound and free species70
| 2 |
At the temperature corresponding to maximum enhancement (22 °C), we experimentally determine a lifetime of 44–46 ms. At low temperature of 0 °C line widths are small enough to determine relevant J-coupling constants (2JHH′ = 8 Hz, 3JNH = 1.71 Hz, 3JNH,bound = 1.69 Hz, ΔJNH = 2JNH – 2JNH′ = 24 Hz). The couplings were used as fixed parameters to determine the individual line positions for evaluation of the line widths at higher temperatures. The experimental data also indicate that the major chemical species under our experimental conditions is indeed the system illustrated in Figure 2A because the nonequivalent hydrides indicate different ligands (pyridine and 15N–CH3CN) in the equatorial plane.
Figure 2.
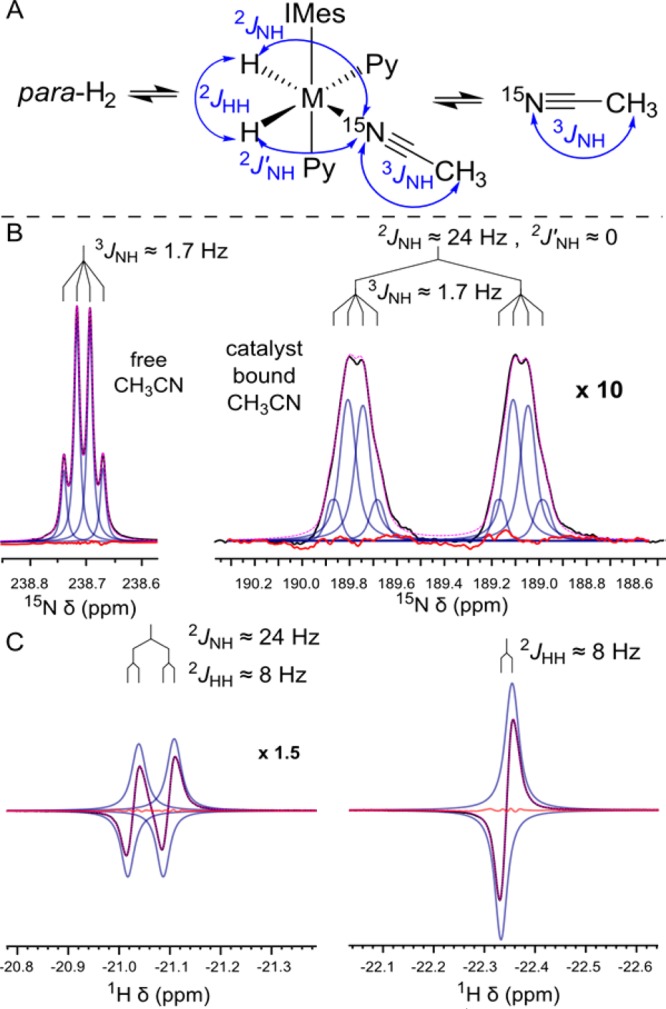
J-coupling and line width analysis. (A) Exchange between bound and free species (15N–CH3CN, H2) and J-coupling definitions. (B) Lineshape analysis of 15N spectra (blue: individual Lorentzians, black: experimental trace, pink: overall fit, red: residual error optimized to rms-noise level). (C) 1H spectra of the hydride region taken under PASADENA conditions.25
IV. Maximizing Polarization
After the above study of hyperpolarization as a function of temperature and magnetic field, further optimization requires inspection of concentrations of catalyst, substrate, and coligand, as well as para-H2 pressure/flow rate. Here we performed a careful study to determine optimum catalyst concentration by conducting a dilution series at fixed catalyst loading (csub/ccat = 20, see Figure 3A). We find maximum enhancement at 0.25 mM [IrCl(IMes)(COD)] and 5 mM 15N–CH3CN in the presence of 0.25 mM pyridine as stabilizing coligand, corresponding to P15N = 7% (enhancement of 23 000-fold at 8.5 T). It is noteworthy that the maximum polarization is observed at the same catalyst concentration experimentally determined for 15N pyridine in methanol30 and neat pyridine.72
Figure 3.
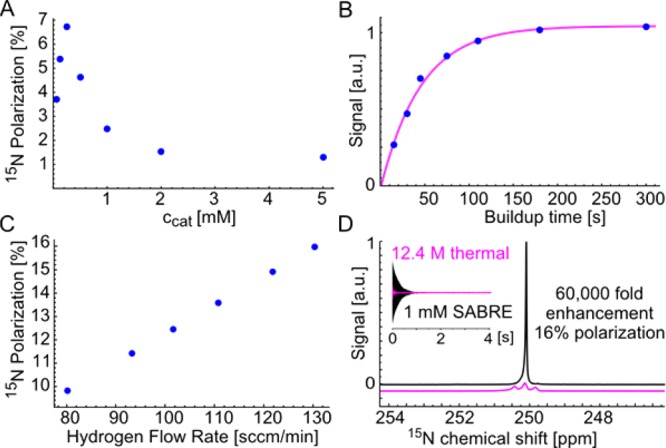
(A) 15N polarization of 15N–CH3CN as a function of the catalyst concentration at fixed catalyst loading of 5 mol%. (B) 15N signal as a function of the buildup time (Tb = 40.8 s). (C) 15N polarization as a function of the hydrogen flow rate (cCatalyst = 0.25 mM, cPyridine = 0.25 mM, cBenzonitrile = 1 mM). (D) Comparison of a neat 15N pyridine reference (c = 12.4 M, pink, thermal 8.45 T, shifted by 63 ppm) and 15N spectrum of hyperpolarized benzonitrile (c = 1 mM, conditions as in C).
For further optimization, it is reasonable to expect that large T1, specifically, a T1 that is large at the magnetic evolution field, will increase polarization levels. As reported in Table 2, benzonitrile stands out in terms of long T1. Therefore, we tested benzonitrile at optimized concentrations. Figure 3B shows a buildup curve for benzonitrile where we find a relatively long buildup time constant of 40.8 s. Specifically, for this system we observe the highest hyperpolarization levels at a catalyst loading of 25 mol% (0.25 mM [IrCl(IMes)(COD)] activated in the presence of one catalyst equivalent of pyridine 0.25 mM and four catalyst equivalents 15N-benzonitrile (1 mM)). Note that in absence of stabilizing coligand (pyridine) enhancements drop by ∼50%. Large catalyst loading (csub/ccat = 4) leads to high levels of polarization because a low excess of substrate increases the likelihood of a polarization event per substrate molecule; this is consistent with theoretical predictions by Barskiy et al.68 and experimental findings by Appleby et al.73
On this system, we examined the influence of para-H2 flow rate at maximized pressure (limited to 10 bar by the experimental setup) as depicted in Figure 3C. In the investigated range, hyperpolarization levels are linearly dependent on the flow rate—in line with previous studies.72 At even higher flow rates the sample volume (Vbubbles + Vliquid) is larger than the homogeneous μT magnetic field region, and polarization levels drop. The highest polarization was obtained at 130 sccm at 10 bar of 85% para-H2. We obtain 16% 15N polarization as illustrated in Figure 3D. The results indicate that hyperpolarization levels are limited by the hydrogen exchange from gas to liquid phase; i.e., the interface area between bubbles and solution. We believe that improvements over the reported 16% are within reach by more efficient dispersion of para-H2.
In summary, we propose the following sequence of experiments: First, optimization of magnetic field and temperature, followed by a dilution series at a given csub/ccat ratio to choose the best catalyst concentration ccat. At the identified ccat, substrate concentration should be optimized. Typically, we find that optimal csub is about 4 to 5 times ccat such that the catalysts are coordinatively saturated but an excess of free substrate remains. Finally, para-H2 pressure and flow should be chosen as high as possible for a given setup. We acknowledge that all parameters are interdependent such that multiple cycles through these optimization steps may lead to even higher hyperpolarization level.
Conclusion and Outlook
In this article we have shown that SABRE-SHEATH can hyperpolarize a wide range of nitrogen-containing compounds, often outside the reach of 1H-SABRE. The presented results suggest that sp and sp2 hybridized nitrogens can be hyperpolarized, and 15N polarization of at least several percent can be obtained easily and rapidly (30 s to 1 min) if no impeding factors such as steric hindrance or proton exchange inhibit polarization buildup.42,48,72
Furthermore, we have shown that 15N T1 times may be extraordinarily long and can in special cases exceed 10 min. We obtain extended relaxation times at relatively low magnetic fields of 1 T, using a permanent magnet-based spectrometer. The results are contrasted to measurements at 8.5 T where one may observe only slightly higher S/N (∼3) if relaxation delays are neglected. The extended T1 times at low field quickly compensate for the S/N losses when tracking hyperpolarized species on longer time scales. Moreover, the demonstrated hyperpolarization levels allow for 15N imaging30,74,75 (likely even in low magnetic fields). The cited demonstrations show images with 2 × 2 mm2 resolution acquired in less than a second.30
In addition, polarization transfer strategies from 15N to 1H have been developed avoiding detection on the low gyromagnetic ratio 15N for read-out.76−79 Such polarization transfer strategies may increase S/N by another order of magnitude.
Finally, we present a systematic strategy to optimize SABRE-SHEATH experiments for maximum hyperpolarization. The sequence consists of optimization of temperature and μT magnetic fields, a dilution series to determine the optimal catalyst concentration, and use of high para-H2 pressure and flow.
The present contribution elucidates upon the interplay of kinetic parameters and system compositions, as well as extrinsic parameters on the polarization levels. This is a significant stepping stone for optimization of heterogeneous SABRE,80,81 SABRE in biocompatible aqueous media,82−85 and modes to continuously supply polarized substrates. Such advances, in combination with low-cost, low-field MRI, let us envision hyperpolarized biomolecular MRI in the near future.
Acknowledgments
The authors gratefully acknowledge the NSF (CHE-1363008 and CHE-1416268), NIH 1R21EB018014, U01 CA202229 and 1R21EB020323, DOD CDMRP W81XWH-15-1-0271, and W81XWH-12-1-0159/BC112431, Exxon Mobil Knowledge Build and Duke University, for financial support of this research. In addition, the authors gratefully acknowledge Magritek for constructing and providing the 1 T nitrogen-15 magnet and spectrometer as well as friendly technical assistance.
Glossary
ABBREVIATIONS
- SABRE
Signal Amplification by Reversible Exchange
- SHEATH
Shield Enables Alignment Transfer to Heteronuclei
- para-H2
para-hydrogen
- PHIP
para-hydrogen-induced polarization
- CSA
Chemical Shift Anisotropy
- CHCA
α-Cyano-4-hydroxycinnamic acid
- S/N
signal-to-noise ratio
- SI
Supporting Information
- MRI
Magnetic Resonance Imaging
- NMR
Nuclear Magnetic Resonance
- rms
root-mean-square
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jpcc.6b12097.
Author Contributions
All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Ardenkjaer-Larsen J. H.; Fridlund B.; Gram A.; Hansson G.; Hansson L.; Lerche M. H.; Servin R.; Thaning M.; Golman K. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. U. S. A. 2003, 100, 10158–10163. 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaou P.; Coffey A. M.; Barlow M. J.; Rosen M.; Goodson B. M.; Chekmenev E. Y. Temperature-Ramped 129Xe Spin Exchange Optical Pumping. Anal. Chem. 2014, 86, 8206–8212. 10.1021/ac501537w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin R. G.; Prisner T. F. High field dynamic nuclear polarization-the renaissance. Phys. Chem. Chem. Phys. 2010, 12, 5737–5740. 10.1039/c0cp90019b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardenkjaer-Larsen J.-H.; Boebinger G. S.; Comment A.; Duckett S.; Edison A. S.; Engelke F.; Griesinger C.; Griffin R. G.; Hilty C.; Maeda H.; et al. Facing and Overcoming Sensitivity Challenges in Biomolecular NMR Spectroscopy. Angew. Chem., Int. Ed. 2015, 54, 9162–9185. 10.1002/anie.201410653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T. G.; Happer W. Spin-exchange optical pumping of noble-gas nuclei. Rev. Mod. Phys. 1997, 69, 629–642. 10.1103/RevModPhys.69.629. [DOI] [Google Scholar]
- Freeman M. S.; Emami K.; Driehuys B. Characterizing and modeling the efficiency limits in large-scale production of hyperpolarized 129Xe. Phys. Rev. A: At., Mol., Opt. Phys. 2014, 90, 023406. 10.1103/PhysRevA.90.023406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. C.; Gentile T. R.; Ye Q.; Walker T. G.; Babcock E. On the limits of spin-exchange optical pumping of 3He. J. Appl. Phys. 2014, 116, 014903. 10.1063/1.4886583. [DOI] [Google Scholar]
- Barskiy D. A.; Coffey A. M.; Nikolaou P.; Mikhaylov D. M.; Goodson B. M.; Branca R. T.; Lu G. J.; Shapiro M. G.; Telkki V.-V.; Zhivonitko V. V.; et al. NMR Hyperpolarization Techniques of Gases. Chem. - Eur. J. 2017, 23, 725–751. 10.1002/chem.201604810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.; Miller G. W.; Tobias W. A.; Cates G. D. A method for imaging and spectroscopy using γ-rays and magnetic resonance. Nature 2016, 537, 652–655. 10.1038/nature19775. [DOI] [PubMed] [Google Scholar]
- Ward H. R.; Lawler R. G. Nuclear magnetic resonance emission and enhanced absorption in rapid organometallic reactions. J. Am. Chem. Soc. 1967, 89, 5518–5519. 10.1021/ja00997a078. [DOI] [Google Scholar]
- Kaptein R. Chemically induced dynamic nuclear polarization in five alkyl radicals. Chem. Phys. Lett. 1968, 2, 261–267. 10.1016/0009-2614(68)85020-1. [DOI] [Google Scholar]
- Hermkens N. K. J.; Eshuis N.; van Weerdenburg B. J. A.; Feiters M. C.; Rutjes F. P. J. T.; Wijmenga S. S.; Tessari M. NMR-Based Chemosensing via pH2 Hyperpolarization: Application to Natural Extracts. Anal. Chem. 2016, 88, 3406–3412. 10.1021/acs.analchem.6b00184. [DOI] [PubMed] [Google Scholar]
- Eshuis N.; van Weerdenburg B. J. A.; Feiters M. C.; Rutjes F. P. J. T.; Wijmenga S. S.; Tessari M. Quantitative Trace Analysis of Complex Mixtures Using SABRE Hyperpolarization. Angew. Chem., Int. Ed. 2015, 54, 1372–1372. 10.1002/anie.201411678. [DOI] [PubMed] [Google Scholar]
- Eshuis N.; Aspers R. L. E. G.; van Weerdenburg B. J. A.; Feiters M. C.; Rutjes F. P. J. T.; Wijmenga S. S.; Tessari M. 2D NMR Trace Analysis by Continuous Hyperpolarization at High Magnetic Field. Angew. Chem., Int. Ed. 2015, 54, 14527–14530. 10.1002/anie.201507831. [DOI] [PubMed] [Google Scholar]
- Eshuis N.; Hermkens N.; van Weerdenburg B. J. A.; Feiters M. C.; Rutjes F. P. J. T.; Wijmenga S. S.; Tessari M. Toward Nanomolar Detection by NMR Through SABRE Hyperpolarization. J. Am. Chem. Soc. 2014, 136, 2695–2698. 10.1021/ja412994k. [DOI] [PubMed] [Google Scholar]
- Rodrigues T. B.; Serrao E. M.; Kennedy B. W.; Hu D. E.; Kettunen M. I.; Brindle K. M. Magnetic resonance imaging of tumor glycolysis using hyperpolarized 13C-labeled glucose. Nat. Med. 2014, 20, 93–7. 10.1038/nm.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S. J.; Kurhanewicz J.; Vigneron D. B.; Larson P. E.; Harzstark A. L.; Ferrone M.; van Criekinge M.; Chang J. W.; Bok R.; Park I.; et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-(13)C]pyruvate. Sci. Transl. Med. 2013, 5, 198ra108. 10.1126/scitranslmed.3006070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshari K. R.; Wilson D. M. Chemistry and biochemistry of 13C hyperpolarized magnetic resonance using dynamic nuclear polarization. Chem. Soc. Rev. 2014, 43, 1627–59. 10.1039/C3CS60124B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshari K. R.; Wilson D. M.; Sai V.; Bok R.; Jen K.-Y.; Larson P.; Van Criekinge M.; Kurhanewicz J.; Wang Z. J. Non-invasive in vivo imaging of diabetes-induced renal oxidative stress and response to therapy using hyperpolarized 13C dehydroascorbate magnetic resonance. Diabetes 2015, 64, 344. 10.2337/db13-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt M. E.; Harrison C.; Sherry A. D.; Malloy C. R.; Burgess S. C. Flux through hepatic pyruvate carboxylase and phosphoenolpyruvate carboxykinase detected by hyperpolarized 13C magnetic resonance. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 19084–9. 10.1073/pnas.1111247108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams R. W.; Aguilar J. A.; Atkinson K. D.; Cowley M. J.; Elliott P. I. P.; Duckett S. B.; Green G. G. R.; Khazal I. G.; Lopez-Serrano J.; Williamson D. C. Reversible Interactions with para-Hydrogen Enhance NMR Sensitivity by Polarization Transfer. Science 2009, 323, 1708–1711. 10.1126/science.1168877. [DOI] [PubMed] [Google Scholar]
- Eisenschmid T. C.; Kirss R. U.; Deutsch P. P.; Hommeltoft S. I.; Eisenberg R.; Bargon J.; Lawler R. G.; Balch A. L. Para Hydrogen Induced Polarization in Hydrogenation Reactions. J. Am. Chem. Soc. 1987, 109, 8089–8091. 10.1021/ja00260a026. [DOI] [Google Scholar]
- Golman K.; Axelsson O.; Johannesson H.; Mansson S.; Olofsson C.; Petersson J. S. Parahydrogen-induced polarization in imaging: Subsecond C-13 angiography. Magn. Reson. Med. 2001, 46, 1–5. 10.1002/mrm.1152. [DOI] [PubMed] [Google Scholar]
- Bowers C. R.; Weitekamp D. P. Parahydrogen and synthesis allow dramatically enhanced nuclear alignment. J. Am. Chem. Soc. 1987, 109, 5541–5542. 10.1021/ja00252a049. [DOI] [Google Scholar]
- Bowers C. R.; Weitekamp D. P. Transformation of Symmetrization Order to Nuclear-Spin Magnetization by Chemical Reaction and Nuclear Magnetic Resonance. Phys. Rev. Lett. 1986, 57, 2645–2648. 10.1103/PhysRevLett.57.2645. [DOI] [PubMed] [Google Scholar]
- Hövener J.-B.; Knecht S.; Schwaderlapp N.; Hennig J.; von Elverfeldt D. Continuous Re-hyperpolarization of Nuclear Spins Using Parahydrogen: Theory and Experiment. ChemPhysChem 2014, 15, 2451–2457. 10.1002/cphc.201402177. [DOI] [PubMed] [Google Scholar]
- Nikolaou P.; Goodson B. M.; Chekmenev E. Y. NMR Hyperpolarization Techniques for Biomedicine. Chem. - Eur. J. 2015, 21, 3156–3166. 10.1002/chem.201405253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley M. J.; Adams R. W.; Atkinson K. D.; Cockett M. C. R.; Duckett S. B.; Green G. G. R.; Lohman J. A. B.; Kerssebaum R.; Kilgour D.; Mewis R. E. Iridium N-Heterocyclic Carbene Complexes as Efficient Catalysts for Magnetization Transfer frompara-Hydrogen. J. Am. Chem. Soc. 2011, 133, 6134–6137. 10.1021/ja200299u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis T.; Truong M. L.; Coffey A. M.; Shchepin R. V.; Waddell K. W.; Shi F.; Goodson B. M.; Warren W. S.; Chekmenev E. Y. Microtesla SABRE Enables 10% Nitrogen-15 Nuclear Spin Polarization. J. Am. Chem. Soc. 2015, 137, 1404–1407. 10.1021/ja512242d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong M. L.; Theis T.; Coffey A. M.; Shchepin R. V.; Waddell K. W.; Shi F.; Goodson B. M.; Warren W. S.; Chekmenev E. Y. 15N Hyperpolarization by Reversible Exchange Using SABRE-SHEATH. J. Phys. Chem. C 2015, 119, 8786–8797. 10.1021/acs.jpcc.5b01799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhivonitko V. V.; Skovpin I. V.; Koptyug I. V. Strong 31P nuclear spin hyperpolarization produced via reversible chemical interaction with parahydrogen. Chem. Commun. 2015, 51, 2506–2509. 10.1039/C4CC08115C. [DOI] [PubMed] [Google Scholar]
- Fleming F. F.; Yao L.; Ravikumar P. C.; Funk L.; Shook B. C. Nitrile-Containing Pharmaceuticals: Efficacious Roles of the Nitrile Pharmacophore. J. Med. Chem. 2010, 53, 7902–7917. 10.1021/jm100762r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mewis R. E.; Green R. A.; Cockett M. C. R.; Cowley M. J.; Duckett S. B.; Green G. G. R.; John R. O.; Rayner P. J.; Williamson D. C. Strategies for the Hyperpolarization of Acetonitrile and Related Ligands by SABRE. J. Phys. Chem. B 2015, 119, 1416–1424. 10.1021/jp511492q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Lanks K. W. 2-Cyanocinnamic Acid Sensitization of L929 Cells to Killing by Hyperthermia. Cancer research 1986, 46, 5349–5352. [PubMed] [Google Scholar]
- Barskiy D. A.; Shchepin R. V.; Coffey A. M.; Theis T.; Warren W. S.; Goodson B. M.; Chekmenev E. Y. Over 20% 15N Hyperpolarization in Under One Minute for Metronidazole, an Antibiotic and Hypoxia Probe. J. Am. Chem. Soc. 2016, 138, 8080–8083. 10.1021/jacs.6b04784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P.; Denton R. M. Specific inhibition of pyruvate transport in rat liver mitochondria and human erythrocytes by α-cyano-4-hydroxycinnamate. Biochem. J. 1974, 138, 313–316. 10.1042/bj1380313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais-Santos F.; Miranda-Goncalves V.; Pinheiro S.; Vieira A. F.; Paredes J.; Schmitt F. C.; Baltazar F.; Pinheiro C. Differential sensitivities to lactate transport inhibitors of breast cancer cell lines. Endocr.-Relat. Cancer 2014, 21, 27–38. 10.1530/ERC-13-0132. [DOI] [PubMed] [Google Scholar]
- McKeage K. Alectinib: A Review of Its Use in Advanced ALK-Rearranged Non-Small Cell Lung Cancer. Drugs 2015, 75, 75–82. 10.1007/s40265-014-0329-y. [DOI] [PubMed] [Google Scholar]
- Santarpia M.; Altavilla G.; Rosell R. Alectinib: a selective, next-generation ALK inhibitor for treatment of ALK-rearranged non-small-cell lung cancer. Expert Rev. Respir. Med. 2015, 9, 255–268. 10.1586/17476348.2015.1009040. [DOI] [PubMed] [Google Scholar]
- Conti P.; Tamborini L.; Pinto A.; Blondel A.; Minoprio P.; Mozzarelli A.; De Micheli C. Drug Discovery Targeting Amino Acid Racemases. Chem. Rev. 2011, 111, 6919–6946. 10.1021/cr2000702. [DOI] [PubMed] [Google Scholar]
- Eliot A. C.; Kirsch J. F. Pyridoxalphophateenzymes: Mechanistic, Structural, and Evolutionary Considerations. Annu. Rev. Biochem. 2004, 73, 383–415. 10.1146/annurev.biochem.73.011303.074021. [DOI] [PubMed] [Google Scholar]
- Logan A. W.; Theis T.; Colell J. F.; Warren W. S.; Malcolmson S. J. Hyperpolarization of Nitrogen-15 Schiff Bases by Reversible Exchange Catalysis with para-Hydrogen. Chem. - Eur. J. 2016, 22, 10777–81. 10.1002/chem.201602393. [DOI] [PubMed] [Google Scholar]
- Feng Y.; Theis T.; Wu T.-L.; Claytor K.; Warren W. S. Long-lived polarization protected by symmetry. J. Chem. Phys. 2014, 141, 134307. 10.1063/1.4896895. [DOI] [PubMed] [Google Scholar]
- Ellinger P.; Kader M. M. A. Nicotinamide metabolism in mammals. Biochem. J. 1949, 44, 77–87. 10.1042/bj0440077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C.; Houtkooper; Riekelt H.; Pirinen E.; Youn; Dou Y.; Oosterveer; Maaike H.; Cen Y.; Fernandez-Marcos; Pablo J.; Yamamoto H.; Andreux; Pénélope A.; Cettour-Rose P.; et al. The NAD+ Precursor Nicotinamide Riboside Enhances Oxidative Metabolism and Protects against High-Fat Diet-Induced Obesity. Cell Metab. 2012, 15, 838–847. 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauve A. A. NAD+ and vitamin B3: from metabolism to therapies. J. Pharmacol. Exp. Ther. 2008, 324, 883–893. 10.1124/jpet.107.120758. [DOI] [PubMed] [Google Scholar]
- Shchepin R. V.; Barskiy D. A.; Mikhaylov D. M.; Chekmenev E. Y. Efficient Synthesis of Nicotinamide-1–15N for Ultrafast NMR Hyperpolarization Using Parahydrogen. Bioconjugate Chem. 2016, 27, 878–882. 10.1021/acs.bioconjchem.6b00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shchepin R. V.; Barskiy D. A.; Coffey A. M.; Theis T.; Shi F.; Warren W. S.; Goodson B. M.; Chekmenev E. Y. 15N Hyperpolarization of Imidazole-15N2 for Magnetic Resonance pH Sensing Via SABRE-SHEATH. ACS Sens. 2016, 1, 640. 10.1021/acssensors.6b00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley G. S. The Cis-form of Azobenzene. Nature 1937, 140, 281–281. 10.1038/140281a0. [DOI] [Google Scholar]
- Merino E.; Ribagorda M. Control over molecular motion using thecis–transphotoisomerization of the azo group. Beilstein J. Org. Chem. 2012, 8, 1071–1090. 10.3762/bjoc.8.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohle D. S.; Rosadiuk K. A. Nitric Oxide Catalysis of Diazene E/Z Isomerization. Inorg. Chem. 2015, 54, 7145–7151. 10.1021/acs.inorgchem.5b00476. [DOI] [PubMed] [Google Scholar]
- Theis T.; Ortiz G. X.; Logan A. W. J.; Claytor K. E.; Feng Y.; Huhn W. P.; Blum V.; Malcolmson S. J.; Chekmenev E. Y.; Wang Q.; et al. Direct and cost-efficient hyperpolarization of long-lived nuclear spin states on universal 15N2-diazirine molecular tags. Sci. Adv. 2016, 2, e1501438. 10.1126/sciadv.1501438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abragam A.The principles of nuclear magnetism; Clarendon Press: Oxford, 1961. [Google Scholar]
- Hoult D. I.; Richards R. E. The signal-to-noise ratio of the nuclear magnetic resonance experiment. J. Magn. Reson. 1976, 24, 71–85. 10.1016/0022-2364(76)90233-X. [DOI] [PubMed] [Google Scholar]
- Hoult D. I.; Lauterbur P. C. The sensitivity of the zeugmatographic experiment involving human samples. J. Magn. Reson. 1979, 34, 425–433. 10.1016/0022-2364(79)90019-2. [DOI] [Google Scholar]
- Hoult D. I.Sensitivity of the NMR Experiment. In eMagRes.; John Wiley & Sons, Ltd: 2007. [Google Scholar]
- Hoult D. I.; Richards R. E. The signal-to-noise ratio of the nuclear magnetic resonance experiment. J. Magn. Reson. 1976, 24, 71–85. 10.1016/0022-2364(76)90233-X. [DOI] [PubMed] [Google Scholar]
- Hoult D. I. Sensitivity of Whole Body MRI Experiments. Enc. Magn. Reson. 2007, 10.1002/9780470034590.emrstm0491. [DOI] [Google Scholar]
- Minard K. R.; Wind R. A. Solenoidal microcoil design—Part II: Optimizing winding parameters for maximum signal-to-noise performance. Concepts Magn. Reson. 2001, 13, 190–210. 10.1002/cmr.1008. [DOI] [Google Scholar]
- Danieli E.; Perlo J.; Blümich B.; Casanova F. Highly Stable and Finely Tuned Magnetic Fields Generated by Permanent Magnet Assemblies. Phys. Rev. Lett. 2013, 110, 180801. 10.1103/PhysRevLett.110.180801. [DOI] [PubMed] [Google Scholar]
- Sarracanie M.; LaPierre C. D.; Salameh N.; Waddington D. E. J.; Witzel T.; Rosen M. S. Low-Cost High-Performance MRI. Sci. Rep. 2015, 5, 15177. 10.1038/srep15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suefke M.; Liebisch A.; Blumich B.; Appelt S. External high-quality-factor resonator tunes up nuclear magnetic resonance. Nat. Phys. 2015, 11, 767–771. 10.1038/nphys3382. [DOI] [Google Scholar]
- Coffey A. M.; Truong M.; Chekmenev E. Y. Low-field MRI can be more sensitive than high-field MRI. J. Magn. Reson. 2013, 237, 169–174. 10.1016/j.jmr.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata R.; Nonaka H.; Takakusagi Y.; Ichikawa K.; Sando S. Design of a hyperpolarized 15N NMR probe that induces a large chemical-shift change upon binding of calcium ions. Chem. Commun. 2015, 51, 12290–12292. 10.1039/C5CC04597E. [DOI] [PubMed] [Google Scholar]
- Clavijo Jordan M. V.; Lo S.-T.; Chen S.; Preihs C.; Chirayil S.; Zhang S.; Kapur P.; Li W.-H.; De Leon-Rodriguez L. M.; Lubag A. J. M.; et al. Zinc-sensitive MRI contrast agent detects differential release of Zn(II) ions from the healthy vs. malignant mouse prostate. Proc. Natl. Acad. Sci. U. S. A. 2016, 113, E5464–E5471. 10.1073/pnas.1609450113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S.; Pravdivtsev A. N.; Hövener J.-B.; Yurkovskaya A. V.; Ivanov K. L. Quantitative description of the SABRE process: rigorous consideration of spin dynamics and chemical exchange. RSC Adv. 2016, 6, 24470–24477. 10.1039/C5RA28059A. [DOI] [Google Scholar]
- Barskiy D. A.; Pravdivtsev A. N.; Ivanov K. L.; Kovtunov K. V.; Koptyug I. V. Simple analytical model for Signal Amplification by Reversible Exchange (SABRE) process. Phys. Chem. Chem. Phys. 2016, 18, 89–93. 10.1039/C5CP05134G. [DOI] [PubMed] [Google Scholar]
- Cowley M. J.; Adams R. W.; Atkinson K. D.; Cockett M. C. R.; Duckett S. B.; Green G. G. R.; Lohman J. A. B.; Kerssebaum R.; Kilgour D.; Mewis R. E. Iridium N-Heterocyclic Carbene Complexes as Efficient Catalysts for Magnetization Transfer from para-Hydrogen. J. Am. Chem. Soc. 2011, 133, 6134–6137. 10.1021/ja200299u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther H.NMR Spectroscopy: Basic Principles, Concepts and Applications in Chemistry, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Shchepin R. V.; Truong M. L.; Theis T.; Coffey A. M.; Shi F.; Waddell K. W.; Warren W. S.; Goodson B. M.; Chekmenev E. Y. Hyperpolarization of “Neat” Liquids by NMR Signal Amplification by Reversible Exchange. J. Phys. Chem. Lett. 2015, 6, 1961–1967. 10.1021/acs.jpclett.5b00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleby K. M.; Mewis R. E.; Olaru A. M.; Green G. G. R.; Fairlamb I. J. S.; Duckett S. B. Investigating pyridazine and phthalazine exchange in a series of iridium complexes in order to define their role in the catalytic transfer of magnetisation from para-hydrogen. Chem. Sci. 2015, 6, 3981–3993. 10.1039/C5SC00756A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W.; Lumata L.; Chen W.; Zhang S.; Kovacs Z.; Sherry A. D.; Khemtong C. Hyperpolarized 15N-pyridine Derivatives as pH-Sensitive MRI Agents. Sci. Rep. 2015, 5, 9104. 10.1038/srep09104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudalbu C.; Comment A.; Kurdzesau F.; van Heeswijk R. B.; Uffmann K.; Jannin S.; Denisov V.; Kirik D.; Gruetter R. Feasibility of in vivo 15N MRS detection of hyperpolarized 15N labeled choline in rats. Phys. Chem. Chem. Phys. 2010, 12, 5818–5823. 10.1039/c002309b. [DOI] [PubMed] [Google Scholar]
- Sarkar R.; Comment A.; Vasos P. R.; Jannin S.; Gruetter R.; Bodenhausen G.; Hall H.; Kirik D.; Denisov V. P. Proton NMR of 15N-Choline Metabolites Enhanced by Dynamic Nuclear Polarization. J. Am. Chem. Soc. 2009, 131, 16014–16015. 10.1021/ja9021304. [DOI] [PubMed] [Google Scholar]
- Mishkovsky M.; Cheng T.; Comment A.; Gruetter R. Localized in vivo hyperpolarization transfer sequences. Magn. Reson. Med. 2012, 68, 349–352. 10.1002/mrm.23231. [DOI] [PubMed] [Google Scholar]
- Truong M. L.; Coffey A. M.; Shchepin R. V.; Waddell K. W.; Chekmenev E. Y. Sub-second proton imaging of 13C hyperpolarized contrast agents in water. Contrast Media Mol. Imaging 2014, 9, 333–341. 10.1002/cmmi.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chekmenev E. Y.; Norton V. A.; Weitekamp D. P.; Bhattacharya P. Hyperpolarized 1H NMR Employing Low γ Nucleus for Spin Polarization Storage. J. Am. Chem. Soc. 2009, 131, 3164–3165. 10.1021/ja809634u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F.; Coffey A. M.; Waddell K. W.; Chekmenev E. Y.; Goodson B. M. Heterogeneous Solution NMR Signal Amplification by Reversible Exchange. Angew. Chem., Int. Ed. 2014, 53, 7495–7498. 10.1002/anie.201403135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F.; Coffey A. M.; Waddell K. W.; Chekmenev E. Y.; Goodson B. M. Nanoscale Catalysts for NMR Signal Enhancement by Reversible Exchange. J. Phys. Chem. C 2015, 119, 7525–7533. 10.1021/acs.jpcc.5b02036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F.; He P.; Best Q.; Groome K. A.; Truong M. L.; Coffey A. M.; Zimay G.; Shchepin R. V.; Waddell K. W.; Chekmenev E. Y.; et al. Aqueous NMR Signal Enhancement by Reversible Exchange in a Single Step Using Water-Soluble Catalysts. J. Phys. Chem. C 2016, 120, 12149. 10.1021/acs.jpcc.6b04484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H.; Xu J.; McMahon M. T.; Lohman J. A. B.; van Zijl P. C. M. Achieving 1% NMR polarization in water in less than 1 min using SABRE. J. Magn. Reson. 2014, 246, 119–121. 10.1016/j.jmr.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hövener J.-B.; Schwaderlapp N.; Borowiak R.; Lickert T.; Duckett S. B.; Mewis R. E.; Adams R. W.; Burns M. J.; Highton L. A. R.; Green G. G. R.; et al. Toward Biocompatible Nuclear Hyperpolarization Using Signal Amplification by Reversible Exchange: Quantitative in Situ Spectroscopy and High-Field Imaging. Anal. Chem. 2014, 86, 1767–1774. 10.1021/ac403653q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong M. L.; Shi F.; He P.; Yuan B.; Plunkett K. N.; Coffey A. M.; Shchepin R. V.; Barskiy D. A.; Kovtunov K. V.; Koptyug I. V.; et al. Irreversible Catalyst Activation Enables Hyperpolarization and Water Solubility for NMR Signal Amplification by Reversible Exchange. J. Phys. Chem. B 2014, 118, 13882–13889. 10.1021/jp510825b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


