Abstract
Human-induced ecological change in the open oceans appears to be accelerating. Fisheries, climate change and elevated nutrient inputs are variously blamed, at least in part, for altering oceanic ecosystems. Yet it is challenging to assess the extent of anthropogenic change in the open oceans, where historical records of ecological conditions are sparse, and the geographical scale is immense. We developed millennial-scale amino acid nitrogen isotope records preserved in ancient animal remains to understand changes in food web structure and nutrient regimes in the oceanic realm of the North Pacific Ocean (NPO). Our millennial-scale isotope records of amino acids in bone collagen in a wide-ranging oceanic seabird, the Hawaiian petrel (Pterodroma sandwichensis), showed that trophic level declined over time. The amino acid records do not support a broad-scale increase in nitrogen fixation in the North Pacific subtropical gyre, rejecting an earlier interpretation based on bulk and amino acid specific δ15N chronologies for Hawaiian deep-sea corals and bulk δ15N chronologies for the Hawaiian petrel. Rather, our work suggests that the food web structure in the NPO has shifted at a broad geographical scale, a phenomenon potentially related to industrial fishing.
Keywords: stable isotope biogeochemistry, seabird ecology, oceanography, anthropogenic impact
1. Introduction
Anthropogenic impacts are an increasing threat to marine ecosystems. Warming of marine water alters ocean circulation, contributes to sea-level rise, and has unmistakable impacts on coastal coral reef communities [1]. Rising inorganic nitrogen from rivers, nitrogen fixation, and nitrogen deposition impart eutrophication, promote hypoxia, and alter ecological community structure [2]. The collapse of coastal fisheries is also well known [3]. While anthropogenic disturbance in coastal regions is obvious, human influences on the open oceans are more insidious. Rising temperatures and nutrient-driven increases in primary production are recognized in the open oceans [2,4,5]. Higher in the oceanic food web, fisheries contribute to depletion of top predators and cetaceans, and in the Central Pacific alone, annual catch is ca 1.4 million tonnes [6–8]. While there may be a temptation to consider oceanic food webs, especially in the deep ocean, as relatively insulated from human impacts, this is probably a misperception considering that loss of coastal marine resources and technological advances compel exploitation far beyond continental shelves [9]. Even so, evaluating the state of ecosystems is difficult without historical records of change, a formidable challenge for the vast oceanic realm.
The legacy of climate and certain human impacts is recorded in the ratio of 15N/14N in bulk tissues (δ15Nb) and amino acids (δ15Na) of marine organisms [10,11]. Nitrogen added to the base of the food web by nitrogen fixing diazotrophs is distinguished by a uniquely low δ15N of about 0‰, compared with 5–6‰ for marine nitrate [4,12,13]. Such isotopic differences in source nitrogen are faithfully passed on to organisms higher in the food web, but with approximately a 3‰ increase in δ15Nb per trophic level [14]. While δ15Nb is commonly used to study nitrogen cycling and food web structure, δ15Nb is hard to interpret because it is influenced by source nitrogen and trophic effects.
The difficulty in interpreting δ15Nb underlies a major disagreement between Wiley et al. [15] and Sherwood et al. [11] regarding whether trophic-level shifts or, instead, changes in source nitrogen have influenced food webs in the oceanic realm of the North Pacific Ocean (NPO). Wiley et al. [15] and Sherwood et al. [11] built millennial-scale nitrogen isotope chronologies, each covering a different geographical region of the NPO and using different study organisms. These two studies drew opposing conclusions regarding whether trophic-level shifts or changes in source nitrogen at the base of the food web have influenced food webs in the NPO. To address this controversy, we developed amino-acid-specific δ15N chronologies from two breeding populations of the Hawaiian petrel (Pterodroma sandwichensis), one from the island of Hawaii and the other from Maui.
2. Material and methods
(a). Sample acquisition
Hawaiian petrel bones are from carcasses salvaged from Haleakala National Park (Maui) and Hawaii Volcanoes National Park (Hawaii) between 2001 and 2010 [15]. Subfossil bones from archaeological and palaeontological sites on Hawaii and Maui were also sampled [15]. While adult and hatch year birds had similar δ15Nb values and are included in our Hawaii δ15Na dataset, owing to the disparity between adult and hatch year bird δ15Nb values on the island of Maui, only adult Maui petrels were included in our δ15Na analyses.
(b). Protein isolation and radiocarbon dating
Gelatin from subfossil bones was previously prepared for radiocarbon and isotope analysis [15]. Radiocarbon dating, reported previously, was conducted at the accelerator mass spectrometry (AMS) facility at the University of California, Irvine (W. M. Keck Carbon Cycle AMS Laboratory) [15]. As described in Wiley et al. [15], conventional radiocarbon ages were calibrated using the programme Calib 6.0 and assigned to time bins based on their median probability dates.
(c). Sample sizes
Bone samples derive from salvaged carcasses and palaeontological and archaeological bones. Samples fall within previously described time bins that mark development of the human population of the Hawaiian Islands: prehuman period (before human colonization of Hawaii; less than 1000 CE or more than 950 yr BP), foundation period (Polynesian colonization, small human population size; 1000–1400 CE; 550–950 yr BP), expansion period (increasing human population size; 1400–1800 CE; 150–550 yr BP) and the modern period (onset of industrialized fishing in the NPO; 1950–2010 CE) [15,16].
Samples from the island of Hawaii were chosen by randomly selecting samples within each of the three time periods (prehuman, late expansion, modern) that were within 1 standard deviation of the sample time-binned mean of our δ15Nb data. This limited the influence of outliers. However, few samples were available from Maui. Consequently, all or the majority of the available samples from Maui were analysed. Sample sizes for our time bins were: modern Hawaii (n = 8), expansion Hawaii (n = 8), prehuman Hawaii (n = 5), modern Maui (n = 7), foundation Maui (n = 5), prehuman Maui (n = 7).
(d). δ15N amino acid analysis and estimates of trophic-level decline
Amino-acid-specific nitrogen isotope analyses were conducted at the Japan Agency for Marine-Earth Science and Technology. Data are expressed as δ15Na = [(15N/14Nsample/15N/14Nstandard) − 1] × 103 relative to the standard atmospheric N2 and units are per mil (‰). δ15N analysis of N-pivaloyl/isopropyl (PV/iPR) amino acid derivatives of gelatin (ca 0.1 mg) hydrolyzates (12 N HCl, 110°C, 12 h) was performed as described by Chikaraishi et al. [17]. Derivatives were analysed on a 6890N GC (Agilent Technologies, USA) fitted with a HP Ultra-2 capillary column (50 m, 0.32 mm i.d., 0.52 µm film thickness) in line with oxidation (950°C) and reduction (550°C) furnaces interfaced to a Delta Plus XP IRMS (Thermo Fisher Scientific, Bremen, Germany). Accuracy was evaluated by analysis of an external standard consisting of PV/iPr derivatives of nine isotopically characterized amino acids (alanine, glycine, leucine, norleucine, aspartic acid, methionine, glutamic acid, phenylalanine and hydroxyproline) [18]. Reproducibility was better than 0.5‰.
The trophic proxy, δ15NGlu–Phe, was used as an indicator of trophic level. In addition, trophic position was calculated using a multi-TDF model [19], necessitated by the observation that the trophic discrimination factor for birds (3.6‰) is lower than that of aquatic and other lower trophic level organisms (7.6‰) [17,19,20]. The model is: TP = (δ15NGlu − δ15NPhe − TDFbird − 3.4)/TDFtypical + 2, where the trophic discrimination factor (TDF) for birds (TDFbird) is 3.6‰, and TDFtypical is the TDF for lower trophic levels = 7.6. We also evaluated our results with a single step model using a TDF = 7.6‰ but, as illustrated in the electronic supplementary material, table S1, this did not alter our conclusions regarding changes in trophic level between ancient and modern time periods.
(e). Diet analysis
Analyses were conducted on regurgitations collected from 12 Hawaiian petrels from Maui in 2007 and 2009. Teleost fishes, primarily lantern fish and flying fish, and ommastrephid squid comprised over 90% of the items in the diet, similar to the findings of Simons [21].
(f). Statistical approach
The population means (and variance) for δ15NPhe and δ15NGlu − δ15NPhe for each time period in each location were estimated using analysis of variance (ANOVA). Data from Hawaii and Maui were analysed separately as there is no reason to believe that changes in δ15NPhe and δ15NGlu − δ15NPhe are the same across time periods in the two locations, especially, because late expansion data (only available from Hawaii) and foundation data (only available from Maui) represent different time periods. Parameter values in the ANOVAs were estimated using a Bayesian approach, which produces unbiased estimates at small sample sizes and allows for the estimation of explicit probability statements [22]. Specifically, we were able to calculate: (i) the probability that δ15NPhe decreased from ancient to modern time periods, and (ii) the probability that δ15NGlu − δ15NPhe (i.e. trophic position) decreased over the same time period. Average time-specific trophic position was calculated as a derived quantity using δ15NGlu − δ15NPhe values according to the formula in the electronic supplementary material, table S1.
Separate ANOVAs were run on δ15NPhe and δ15NGlu − δ15NPhe for both Hawaii and Maui, estimating mean values of each quantity in the three time periods (prehuman, late expansion or foundation, modern). Equal variances among time periods within each analysis were assumed, and parameter values for each ANOVA were obtained using Markov chain Monte Carlo (MCMC) executed with the programs R [23] and WinBUGS [24]. Vague priors were used for each of the means (normal distributions centred around 0 with a variance of 1000) and the variance (uniform distribution between 0 and 100). Three chains each for 100 000 iterations and a burn in of 10 000 were used. The remaining samples were thinned by saving only every third value, thus creating a posterior distribution for each parameter that consisted of 90 000 independent values. The derived probabilities (e.g. decreases in δ15NPhe and δ15NGlu − δ15NPhe from ancient to modern times) were calculated by subtracting the estimated mean values in the early time period (prehuman and late expansion or foundation) from the values in the modern time period for each iteration of the MCMC. The probabilities were then calculated as the proportion of those subtracted values that are positive (electronic supplementary material, table S1).
3. Results and discussion
Wiley et al. [15] and Sherwood et al. [11] built millennial-scale nitrogen isotope chronologies, each covering a different geographical region of the NPO. In a study of a wide-ranging oceanic predator, the Hawaiian petrel (figure 1), Wiley et al. [15] found a species-wide decline in δ15Nb in a millennial-scale time series of modern and ancient bones assembled from modern ornithological, archaeological and palaeontological collections, and circumscribed the timing of the decline to the past 100 years. This trend was interpreted as evidence of a significant species-wide decline in trophic level, most likely associated with industrial fishing. Sherwood et al. [11] found a similar decline in δ15Nb values of three long-lived, deep-sea corals (Kulamanamana haumeaae) in the vicinity of the main Hawaiian Islands (figure 1) and interpreted this trend as evidence of a temporal increase in nitrogen fixation. Based on the rough similarity in temporal patterns in the two studies, Sherwood et al. [11] reinterpreted the Hawaiian petrel δ15Nb data as reflecting an increase in nitrogen fixation in the North Pacific subtropical gyre, and argued against a trophic effect associated with fishing, thus laying down a gauntlet for all δ15Nb studies that have claimed to show trophic effects of marine fishing [10,28,29].
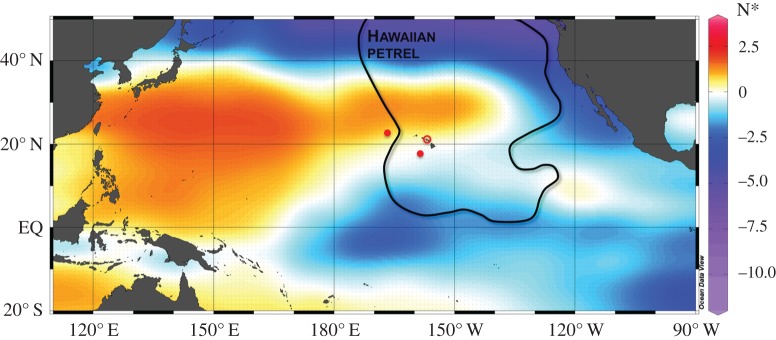
Figure 1.
At-sea distribution of Hawaiian petrel and N* distribution. Curved line delineates Hawaiian petrel distribution [25]. Filled and open red circles are locations of corals with declining δ15Nb ca 1850-present and declining δ15NPhe ca 1850-present, respectively [11]. The anticyclonic North Pacific subtropical gyre extends west of 120° W between 10° N and 40° N [26]. Colour gradient is the distribution of N* defined and gridded by Sherwood et al. [11], where N* = N = 16P + 2.9 µmol kg−1, where P = phosphorus) [27]. Positive values are interpreted as an increase in nitrogen fixation and negative values as net dentrification by Sherwood et al. [11]. (Online version in colour.)
To bolster their δ15Nb dataset, Sherwood et al. [11] also analysed δ15Na from two corals, yielding one record from about 700 yr BP (AD 1300) to present and another from about 800 yr BP (AD 1400) to 165 yr BP (AD 1850). The δ15Na data offer a means to disentangle the signal of source nitrogen and trophic position from a single sample [17]. Certain amino acids such as phenylalanine are principally influenced by changes in source nitrogen, whereas others such as glutamic acid undergo significant increases in δ15N with each trophic step. Further, the difference between the δ15N values of glutamic acid and phenylalanine, δ15NGlu − δ15NPhe, is a proxy for trophic position. Data from the coral specimen that dated from 700 yr BP to present showed a decline in average δ15N of both source and trophic amino acids, and no change in δ15NGlu − δ15NPhe, supporting the interpretation that the δ15Nb coral data reflect a shift in source nitrogen. Yet, the sinking particles that support the deep-sea corals and the surface particles at the base of the Hawaiian petrel food web differ substantially in δ15Nb [13,30], and no Hawaiian petrel δ15Na data were available to support Sherwood et al.'s [11] interpretation that a temporal trend in nitrogen fixation was widespread across the North Pacific subtropical gyre.
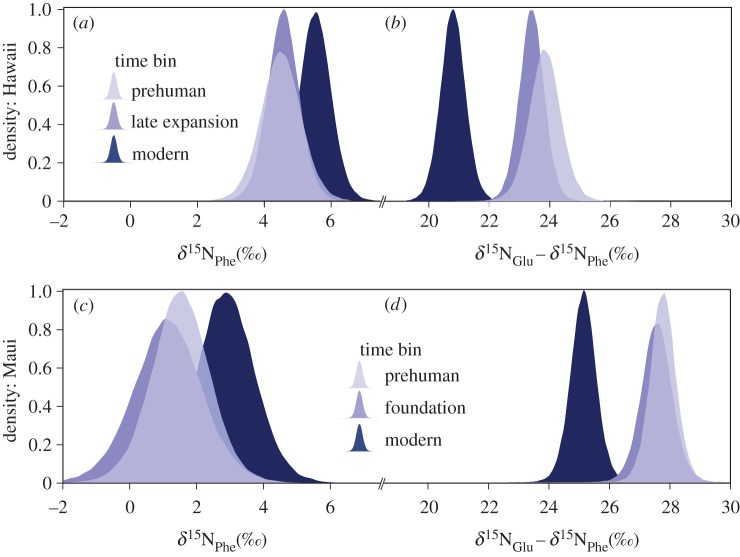
In an independent laboratory from the original study, we generated two separate δ15Na chronologies, representing Hawaiian petrel populations from the islands of Hawaii and Maui, which are known to be genetically distinct and are known to differ in foraging habits [15,31]. We compared the δ15NPhe and δ15NGlu−δ15NPhe of Hawaiian petrel collagen from prehuman, archaeological and modern time periods from Hawaii and Maui using a Bayesian ANOVA, which allowed us to explicitly quantify the probability of changes in δ15N between ancient and modern time periods (figure 2 and the electronic supplementary material, table S2). We also calculated point estimates (means) and 95% credible intervals for both quantities in each time period (electronic supplementary material, table S3). The datasets from each island were analysed separately with strikingly similar results. Between Hawaii's late expansion or prehuman and modern time periods and Maui's foundation or prehuman and modern time period, the probability that δ15NPhe declined was less than or equal to 0.13 for both islands and the probability that δ15NGlu−δ15NPhe, and trophic position declined was more than 0.99 for both islands. While δ15NPhe provides no evidence that a change in nutrient regime (e.g. an increase in nitrogen fixation) influenced the Hawaiian petrel food web, declines in both δ15NGlu and δ15NGlu−δ15NPhe are consistent with a decline in trophic position between prehuman and modern periods of 0.4 and 0.3 for the Hawaiian petrel population on Hawaii and Maui, respectively (electronic supplementary material, table S1).
Figure 2.
Density distribution of δ15NPhe and δ15NGlu−δ15NPhe. (a,b) δ15NPhe and δ15NGlu−δ15NPhe density distributions for Hawaii and (c,d) Maui. Previously described time bins mark development of the human population of the Hawaiian Islands: Prehuman period (before human colonization of Hawaii; <1000 CE or >950 yr BP), foundation period (Polynesian colonization, small human population size; 1000–1400 CE; 550–950 yr BP), late expansion period (increasing human population size; 1400–1800 CE; 150–550 yr BP) and the modern period (onset of industrialized fishing in the NPO; 1950–2010 CE) [16]. Sample sizes: Hawaii prehuman (5), Maui prehuman (7), Hawaii late expansion (8), Maui foundation (5), Hawaii modern (8) and Maui modern (7). The electronic supplementary material, table S1 shows radiocarbon dates and δ15Na data. Collagen preparation, radiocarbon calibration and sample binning into time periods as described previously [15]. Radiocarbon-dated samples were binned using mean probability estimates. (Online version in colour.)
We explored reasons for the disparity in δ15NPhe trends between the coral and petrel data, looking for mechanisms that could explain the differences between the two datasets. The coral data are derived from waters near Hawaii where nitrogen fixation rates are elevated and the nitrogen to phosphorus ratio (N : P) is greater than the expected Redfield stoichiometry of 16 : 1 [4,5]. This results in elevated values for the N* parameter (nitrogen concentration in excess or deficit to phosphorous relative to Redfield stoichiometry N* = N − 16P + 2.9 µmol kg−1) [27]. Elsewhere, anthropogenic atmospheric nitrogen deposition is the major cause of temporal increases in N : P [5]. Even so, large areas of the NPO are characterized by a nitrogen deficit and negative N* values (figure 1). The deep-sea corals studied by Sherwood et al. [11] are centred in waters characterized by neutral to positive N* values. By contrast, Hawaiian petrels obtain source nitrogen from vast expanses of the NPO within their foraging range that include regions where N* is negative, indicative of net denitrification (figure 1). Temporal trends in deep-sea coral δ15NPhe could reflect an increase in nitrogen fixation in water near Hawaii, just as posited by Sherwood et al. [11], whereas the absence of a shift in the δ15NPhe of Hawaiian petrels is indicative of the absence of a pronounced increase in nitrogen fixation or atmospheric nitrogen deposition across their broad foraging range. Thus, the disparity between coral and petrel δ15NPhe trends is not surprising, leading us to ask: what is the significance of the δ15NGlu–Phe decline in the Hawaiian petrel?
Our new data are consistent with our earlier claim that the Hawaiian petrel experienced a trophic shift, a phenomenon that may have broad implications for the NPO. The decrease in δ15NGlu–Phe implies a trophic-level decline but could also reflect a change in diet quality (e.g. variation in protein, fat or carbohydrate content) [19,32]. Considering that our diet analyses and those of others show that Hawaiian petrels are top predators that feed on purely proteinaceous diets of teleost fishes and squid [21], any changes in protein content are likely to be inconsequential. A decline in magnitude of trophic discrimination factors associated with prey might also influence our δ15NGlu–Phe data. Recent literature shows more variation in amino acid-specific trophic discrimination factors among organisms than previously described, probably resulting from differences in feeding ecology, physiology and as discussed earlier, the balance of protein, fat and carbohydrates in an organism's diet [19,32–34]. Owing to the paucity of species-specific trophic discrimination factors in the literature, the extent to which variation in trophic discrimination factors influenced the Hawaiian petrel's prey and our δ15NGlu–Phe data are difficult to assess. If a change in trophic discrimination factors is responsible for the decline in δ15NGlu–Phe between ancient and modern Hawaiian petrels, this would imply that the prey of Hawaiian petrels changed over time. While we do not know that a decline in trophic level was the sole factor driving the observed decrease in Hawaiian petrel δ15NGlu–Phe, independent lines of evidence show a decrease in the mean size of fishes in the NPO, a phenomenon consistent with a trophic-level decline [35]. A trophic-level decline occurring within a vast open ocean basin is indicative of large-scale ecological change. Wiley et al. [15,36] did not find a correlation between climate change indices (e.g. El Niño Southern Oscillation) and our isotope chronologies. Another major agent of ecological change in the NPO is industrial fishing. Ecosystem responses to industrial fishing in the open ocean are complex. Yet we know that, since the 1950s, the central NPO experienced a reduction in catch rates of large fishes while smaller consumers began dominating the catch [35]. Further, the loss of large fishes can increase the abundance of not only the next smaller size class but organisms two orders of magnitude smaller, such that only the micronekton and plankton are unaffected [37]. This offers one scenario where ocean-wide effects of industrial fishing could produce a trophic-level decline in the Hawaiian petrel and probably in other oceanic predators.
Sherwood et al. [11] emphasized changes in nutrient regimes within a large region of the NPO, the North Pacific subtropical gyre. The low average δ15NPhe of 1.9‰ for Hawaiian petrels from Maui is consistent with a substantive contribution of nitrogen fixation to the base of the food web for the population of Hawaiian petrels that breeds on Maui. Yet, even in the Maui population, δ15NGlu − δ15NPhe values are consistent with a trophic decline and not with a temporal increase in nitrogen fixation. Thus, a dietary shift, most likely a trophic-level decline, affected Hawaiian petrels broadly, regardless of where populations fed.
If indeed a trophic shift occurred in the food web of the Hawaiian petrel, particularly if related to fisheries, the implications could be ecologically and economically far-reaching. Fisheries have been described as a vast uncontrolled experiment with unforetold ecological consequences [1], yet an observation of a trophic-level shift implies changes to trophic structure that can lead to deteriorating ecosystem function [38]. Fishery-related alterations to open ocean food webs are an international issue with implications for food supplies and cultures [39,40]. As human population grows, increased fishery production may spur further trophic-level shifts similar to that observed in our retrospective data. This possibility, coupled with climate change, suggests an uncertain future for the oceanic NPO [41]. Although our millennial-scale data do not decisively implicate fisheries, they offer important evidence of a tropic shift and a rare opportunity to gauge the full magnitude of potential ecological stress by providing a baseline prior to human intervention, a perspective that is critical for predicting the future of marine ecosystems.
Supplementary Material
Acknowledgements
We thank the Bird Division and Palaeobiology Department, National Museum of Natural History, the Natural History Museum of Los Angeles County, the Bernice Bishop Museum, the National Park Service in Hawaii and M. Spitzer, C. Gebhard, K. Garrett, K. Campbell, L. Garetano, C. Kishinami, D. Hu and C. Bailey for assistance with samples. We also thank N. Holmes, S. Judge, K. Swindle, D. Ainley, and J. Adams for sharing expertise, and O. Sherwood for providing the N* gradient in figure 1. We thank H. Gandhi and N. Ostrom for comments on the manuscript.
Data accessibility
The data for this paper are tabulated in the electronic supplementary material. After publication, a digital archive of our data is available in Dryad: http://dx.doi.org/10.5061/dryad.66jd4 [42].
Authors' contributions
P.H.O., A.W. and H.F.J. conceived the project and provided gelatin samples and associated radiocarbon data. The manuscript was written primarily by P.H.O. with contribution from A.E.W. and H.F.J. E.Z. and S.R. designed and conducted statistical analysis and assisted with writing. W.W. identified prey and interpreted prey data. Y.C. prepared and analysed amino acid derivatives, provided intellectual context and assisted with writing. P.H.O., A.W., H.F.J. and Y.C. contributed equally to this work.
Competing interests
We have no competing interests.
Funding
Funding was provided by the National Science Foundation (Division of Environmental Biology-0745604), Geological Society of America, Michigan State University and the National Museum of Natural History, Smithsonian Institution.
References
- 1.Jackson JBC. 2008. Ecological extinction and evolution in the brave new ocean. Proc. Natl Acad. Sci. USA 105, 11 458–11 465. ( 10.1073/pnas.0802812105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duce RA, et al. 2008. Impacts of atmospheric anthropogenic nitrogen on the open ocean. Science 320, 893–897. ( 10.1126/science.1150369) [DOI] [PubMed] [Google Scholar]
- 3.Lotze HK, et al. 2009. Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 312, 1806–1809. ( 10.1126/science.1128035) [DOI] [PubMed] [Google Scholar]
- 4.Karl D, Letelier R, Tupas L, Dore J, Christian J, Hebel D. 1997. The role of nitrogen fixation in biogeochemical cycling in the subtropical North Pacific Ocean. Nature 388, 533–538. ( 10.1038/41474) [DOI] [Google Scholar]
- 5.Kim IN, Lee K, Gruber N, Karl DM, Bullister JL, Yang S, Kim TW. 2014. Increasing anthropogenic nitrogen in the North Pacific Ocean. Science 346, 1102–1106. ( 10.1126/science.1258396) [DOI] [PubMed] [Google Scholar]
- 6.Myers RA, Worm B. 2003. Rapid worldwide depletion of predatory fish communities. Nature 423, 280–283. ( 10.1038/nature01610) [DOI] [PubMed] [Google Scholar]
- 7.Springer AM, Estes JA, Van Vliet GB, Williams TM, Doak DF, Danner EM, Forney KA, Pfister B. 2003. Sequential megafaunal collapse in the North Pacific Ocean: an ongoing legacy of industrial whaling? Proc. Natl Acad. Sci. USA 100, 12223 ( 10.1073/pnas.1635156100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.FAO Fisheries. 2013. FAO global capture production database updated to 2013 summary information Fisheries and Aquaculture Department. 1–5.
- 9.McCauley DJ, Pinsky ML, Palumbi SR, Estes JA, Joyce FH, Warner RR. 2015. Marine defaunation: animal loss in the global ocean. Science 347, 236–238. ( 10.1126/science.1255641) [DOI] [PubMed] [Google Scholar]
- 10.Christensen JT, Richardson K. 2008. Stable isotope evidence of long-term changes in the North Sea food web structure. Mar. Ecol. Prog. Ser. 368, 1–8. ( 10.3354/meps07635) [DOI] [Google Scholar]
- 11.Sherwood OA, Guilderson TP, Batista FC, Schiff JT, McCarthy MD. 2014. Increasing subtropical North Pacific Ocean nitrogen fixation since the Little Ice Age. Nature 505, 78–81. ( 10.1038/nature12784) [DOI] [PubMed] [Google Scholar]
- 12.Sigman DM, Mccorkle DC, Francois R, Fischer G. 2000. The δ15N of nitrate in the Southern Ocean: nitrogen cycling and circulation in the ocean interior. J. Geophys. Res. 105, 19 599–19 614. ( 10.1029/2000JC000265) [DOI] [Google Scholar]
- 13.Casciotti KL, Trull TW, Glover DM, Davies D. 2008. Constraints on nitrogen cycling at the subtropical North Pacific Station ALOHA from isotopic measurements of nitrate and particulate nitrogen. Deep. Res. Part II Top. Stud. Oceanogr. 55, 1661–1672. ( 10.1016/j.dsr2.2008.04.017) [DOI] [Google Scholar]
- 14.Post DM. 2002. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83, 703–718. ( 10.1890/00129658(2002)083%5B0703:USITET%5D2.0.CO;2) [DOI] [Google Scholar]
- 15.Wiley AE, et al. 2013. Millennial-scale isotope records from a wide-ranging predator show evidence of recent human impact to oceanic food webs. Proc. Natl Acad. Sci. USA 110, 8972–8977. ( 10.1073/pnas.1300213110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirch PV. 1990. The evolution of sociopolitical complexity in prehistoric Hawaii: an assessment of the archaeological evidence. J. World Prehistory 4, 311–345. ( 10.1007/BF00974883) [DOI] [Google Scholar]
- 17.Chikaraishi Y, Ogawa NO, Kashiyama Y, Takano Y, Suga H, Tomitani A, Miyashita H, Kitazato H, Ohkouchi N. 2009. Determination of aquatic food-web structure based on compound-specific nitrogen isotopic composition of amino acids. Limnol. Oceanogr. Methods 7, 740–750. ( 10.4319/lom.2009.7.740) [DOI] [Google Scholar]
- 18.Sato R, Kawanishi H, Schimmelmann A, Suzuki Y, Chikaraishi Y. 2014. New amino acid reference materials for stable nitrogen isotope analysis. Bunseki Kagaku 63, 399–403. ( 10.2116/bunsekikagaku.63.399) [DOI] [Google Scholar]
- 19.McMahon KW, Thorrold SR, Elsdon TS, Mccarthy MD. 2015. Trophic discrimination of nitrogen stable isotopes in amino acids varies with diet quality in a marine fish. Limnol. Oceanogr. 60, 1076–1087. ( 10.1002/lno.10081) [DOI] [Google Scholar]
- 20.Lorrain A, Graham B, Menard F, Popp B, Bouillon S, van Breugel P, Cherel Y. 2009. Nitrogen and carbon isotope values of individual amino acids: a tool to study foraging ecology of penguins in the Southern Ocean. Mar. Ecol. Prog. Ser. 391, 293–306. ( 10.3354/meps08215) [DOI] [Google Scholar]
- 21.Simons TR. 1985. Biology and behavior of the endangered Hawaiian dark-rumped petrel. Condor 87, 229–245. ( 10.2307/1366887) [DOI] [Google Scholar]
- 22.Gelman A, Hill J. 2006. Data analysis using regression and multilevel/hierarchical models. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 23.R Development Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 24.Spiegelhalter D, Thomas A, Best N, Lunn D. 2003. WinBUGS user manual See https://www.mrc-bsu.cam.uk/wp-content/uploads/manual14.pdf.
- 25.Birdlife International. 2012. Pterodroma sandwichensis . The IUCN red list of threatened species 2012: e.T22698017A40208872. See 10.2305/IUCN.UK.2012-1.RLTS.T22698017A40208872.en. [DOI]
- 26.Karl DM. 1999. A sea of change: biogeochemical variability in the North Pacific subtropical gyre. Ecosystems 2, 181–214. ( 10.1007/s100219900068) [DOI] [Google Scholar]
- 27.Deutsch C, Gruber N, Key RM, Sarmiento JL, Ganachaud A. 2001. Denitrification and N2 fixation in the Pacific Ocean. Glob. Biogeochem. Cycles 15, 483–506. ( 10.1029/2000GB001291) [DOI] [Google Scholar]
- 28.Becker BH, Beissinger SR. 2006. Centennial decline in the trophic level of an endangered seabird after fisheries decline. Conserv. Biol. 20, 470–479. ( 10.1111/j.1523-1739.2006.00379.x) [DOI] [PubMed] [Google Scholar]
- 29.Farmer RG, Leonard ML. 2011. Long-term feeding ecology of great black-backed gulls (Larus marinus) in the northwest Atlantic: 110 years of feather isotope data. Can. J. Zool. 89, 123–133. ( 10.1139/Z10-102) [DOI] [Google Scholar]
- 30.Kaiser K, Benner R. 2012. Organic matter transformations in the upper mesopelagic zone of the North Pacific: chemical composition and linkages to microbial community structure. J. Geophys. Res. Oceans 117, 1–12. ( 10.1029/2011JC007141) [DOI] [Google Scholar]
- 31.Welch A, et al. 2012. Population divergence and gene flow in an endangered and highly mobile seabird. Heredity 109, 19–28. ( 10.1038/hdy.2012.7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chikaraishi Y, Steffan SA, Takano Y, Ohkouchi N. 2015. Diet quality influences isotopic discrimination among amino acids in an aquatic vertebrate. Ecol. Evol. 5, 2048–2059. ( 10.1002/ece3.1491) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielsen JM, Popp BN, Winder M. 2015. Meta-analysis of amino acid stable nitrogen isotope ratios for estimating trophic position in marine organisms. Oecologia 178, 631–642. ( 10.1007/s00442-015-3305-7) [DOI] [PubMed] [Google Scholar]
- 34.Bradley CJ, Wallsgrove NJ, Choy CA, Drazen JC, Hetherington ED, Hoen DK, Popp BN. 2015. Trophic position estimates of marine teleosts using amino acid compound specific isotopic analysis. Limnol. Oceanogr. Methods 13, 476–493. ( 10.1002/lom3.10041) [DOI] [Google Scholar]
- 35.Ward P, Myers RA. 2005. Shifts in open-ocean fish communities coinciding with the commencement of commercial fishing. Ecology 86, 835–847. ( 10.1890/03-0746) [DOI] [Google Scholar]
- 36.Wiley A, et al. 2012. Foraging segregation and genetic divergence between geographically proximate colonies of a highly mobile seabird. Oecologia 168, 119–130. ( 10.1007/s00442-011-2085-y) [DOI] [PubMed] [Google Scholar]
- 37.Polovina JJ, Woodworth-Jefcoats PA. 2013. Fishery-induced changes in the subtropical Pacific pelagic ecosystem size structure: observations and theory. PLoS ONE 8 e62341 ( 10.1371/journal.pone.0062341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Estes JA, et al. 2011. Trophic downgrading of planet Earth. Science 333, 301–306. ( 10.1126/science.1205106) [DOI] [PubMed] [Google Scholar]
- 39.Worm B, et al. 2006. Impacts of biodiversity loss on ocean ecosystem services. Science 314, 787–790. ( 10.1126/science.1132294) [DOI] [PubMed] [Google Scholar]
- 40.Worm B, et al. 2009. Rebuilding global fisheries. Science 325, 578–585. ( 10.1126/science.1173146) [DOI] [PubMed] [Google Scholar]
- 41.Halpern BS, et al. 2008. A global map of human impact on marine ecosystems. Science 319, 948–952. ( 10.1126/science.1149345) [DOI] [PubMed] [Google Scholar]
- 42.Ostrom PH, Wiley AE, James HF, Rossman S, Walker WA, Zipkin EF, Chikaraishi Y. 2017. Data from: Broad-scale tropic shift in the pelagic North Pacific revealed by an oceanic seabird. Dryad digital repository. ( 10.5061/dryad.66jd4) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Ostrom PH, Wiley AE, James HF, Rossman S, Walker WA, Zipkin EF, Chikaraishi Y. 2017. Data from: Broad-scale tropic shift in the pelagic North Pacific revealed by an oceanic seabird. Dryad digital repository. ( 10.5061/dryad.66jd4) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data for this paper are tabulated in the electronic supplementary material. After publication, a digital archive of our data is available in Dryad: http://dx.doi.org/10.5061/dryad.66jd4 [42].