Abstract
Climate change and humans are proposed as the two key drivers of total extinction of many large mammals in the Late Pleistocene and Early Holocene, but disentangling their relative roles remains challenging owing to a lack of quantitative evaluation of human impact and climate-driven distribution changes on the extinctions of these large mammals in a continuous temporal–spatial dimension. Here, our analyses showed that temperature change had significant effects on mammoth (genus Mammuthus), rhinoceros (Rhinocerotidae), horse (Equidae) and deer (Cervidae). Rapid global warming was the predominant factor driving the total extinction of mammoths and rhinos in frigid zones from the Late Pleistocene and Early Holocene. Humans showed significant, negative effects on extirpations of the four mammalian taxa, and were the predominant factor causing the extinction or major extirpations of rhinos and horses. Deer survived both rapid climate warming and extensive human impacts. Our study indicates that both the current rates of warming and range shifts of species are much faster than those from the Late Pleistocene to Holocene. Our results provide new insight into the extinction of Late Quaternary megafauna by demonstrating taxon-, period- and region-specific differences in extinction drivers of climate change and human disturbances, and some implications about the extinction risk of animals by recent and ongoing climate warming.
Keywords: extinction, range shift, climate warming, human impacts, Late Pleistocene, Holocene
1. Introduction
The extinction of megafauna in the Late Pleistocene to Holocene has long puzzled scientists and the public. During this period, 177 large mammal species went extinct globally or continentally [1]. Northern Eurasia lost nine mammalian megafauna genera (up to 35%) [2]. Climate change, human impacts or a combination of the two have been proposed to explain the massive extinction, with explanations commonly invoking hunting, habitat loss coinciding with expansion of human populations [2–5], and/or landscape modification by human-set fires [6]. However, in some cases megafauna disappeared before the arrival of humans [7,8], and in others, climate change probably drove extinctions by causing range shifts and/or habitat loss [2,9–14]. Evidence from ancient DNA and the distribution of archaeological and palaeontological records highlights the combined impacts of climate and humans as precipitating extinctions [11,15,16].
Most previous studies evaluated the impacts of climate or humans on megafauna extinctions only qualitatively, based on temporal and/or distributional correlations of last occurrence records with the timing of climate change and human arrival [13,17–19]. Recent quantitative investigations of the impacts of climate on herbivore megafauna used climate envelope models, but in several key periods use only a portion of the fossil record [10,11,20]. For example, Varela et al. [16] assessed the impacts of climate and humans by using ecotypes and their overlap with human sites [16]. In general, such analyses are constrained by lack of quantitative and continuous temporal–spatial analysis for both climatic and human impacts.
Here, we use palaeontological and archaeological records to quantitatively assess the impacts of temperature and human activities in causing extirpations on local (within a 100 × 100 km2 grid) and regional (climate-driven range contraction along latitude) scales; we also examine what triggered the extinction or major extirpations of four megafauna mammalian groups (i.e. mammoth, rhinoceros, horse and deer) in the Late Pleistocene and Holocene. We estimated the latitude of taxa and compared those with temperature to evaluate the impacts of climate change. The climate-driven extinction hypothesis predicts positive associations between latitude and temperature. We estimated the extinction time within a spatial grid of 100 × 100 km2 and analysed its relation with human impacts. Extinction time was estimated using the inverse-weighted Gaussian-resampled method (GRIWM) (see Material and methods). Human impacts were assessed with two criteria: the average nearest distance between animal fossil and human sites that are older than animal fossil age (human proximity), and the number of human archaeological sites that are older than animal fossil age (human density) within a spatial grid of 100 × 100 km2. Human proximity represents distance between animal and human sites; while human density represents density of human sites within the grid. According to the human-driven extinction hypothesis, we predict that animals should disappear earlier (or have an older extinction time) if animal fossil sites were closer to human sites or if there were more human sites within the spatial grid of 100 × 100 km2; to disentangle the relative roles of climate and humans in causing extinction or major extirpations from the Late Pleistocene to Holocene when temperatures increased rapidly at the turning point of about 12 kya. We propose the following three assumptions. First, humans would be the predominant factor if many fossils were found in the Holocene but none or very few fossils were seen after the beginning of the Anthropocene (approx. AD 1610) [6]. Second, climate warming would be the predominant factor if none or very few fossils were found after the turning point from Late Pleistocene to Holocene (approx. 12 kya). Third, extinction or major extirpations have been attributed to both climate warming and humans if none or very few fossils were found after the turning point from Late Pleistocene to Holocene, and high-intensity human impacts were found only near the turning point.
2. Material and methods
(a). Species occurrence data
We collected (and edited) the fossil or specimen record data of large mammals during the Late Pleistocene and Holocene from 54.25 kya (thousand years ago; commencement date: 1950) to 32 ya (years ago; commencement date: 1950) using several search engines (e.g. ISI Web of Knowledge, cnki.net and Google Scholar) by using combinations of keywords (e.g. megafauna, mammals, fauna, fossil, remains, Pleistocene, Holocene, Last Galicial Maximum (LGM), glacial, mammoths, rhinos, horse, deer, etc.). We included studies of fossil specimens with radiocarbon dated- or stratigraphic age from 376 officially published literature sources and New and Old Worlds fossil mammal database: http://www.helsinki.fi/science/now/index.html [21] (for details, see the electronic supplementary material, figure S1). Data consisted of species name, age, location, longitude and latitude of fossils. Only data for four large mammal groups with sufficient records in Eurasia from the Late Pleistocene to Holocene were used for statistical analyses: the mammoth (Mammuthus), rhinoceroses (Rhinocerotidae), horses (Equidae) and deer (Cervidae) (electronic supplementary material, figure S1). The dataset consists of 2440 radiocarbon-dated fossils or historical remains records; consisting of 1236 mammoth records (including 1128 records of woolly mammoth Mammuthus primigenius), 137 records for rhinoceroses (including 52 records of genus Rhinoceros), 214 records for horses (including 196 records of genus Equus) and 421 records for deer (including 312 records of genus Cervus). Literature records contained one of two types of fossil dates: dates described as ‘median ± s.d.’ (n = 164 records; s.d., standard deviation, coefficient of variation (CV) = 1.93%) for which we used the median value; a geological layer (n = 2156 records, CV = 8.5% with 95% confidence) for which we used the average age in analyses. Some geographical locations lacked latitude and longitude. For these, we found latitude and longitude using Google Earth (www.google.com/earth/). Based on the data availability and distribution in space and time, we used only records in Eurasia. Because our mammal data were centralized in two parts of Eurasia, we divided Eurasia into two parts: western (mainly in Europe) and eastern Eurasia (mainly in Asia) by the 55° E line (see the electronic supplementary material, figure S1).
(b). Environmental and human impact data
A high-resolution (50 years) climate record of the oxygen isotopic composition (δ18O) from the north Greenland ice core project (NGRIP) was used to represent global temperature change (electronic supplementary material, figure S3), which extends back to the last inter-glacial period [9]. This dataset has been widely used for representing temperature change since the last inter-glacial period in the Northern Hemisphere. To represent regional differences and avoid bias by a single curve, we also compared this curve with two other curves from Antarctica and China (electronic supplementary material, figure S3) [22,23]. As far as we know, they are the only two available series covering our study period, but they are uneven temporal resolutions (177–577 years for the China series and 8–56 years for the Antarctica series).
To represent human impacts, we used archaeological sites of anthropological remains (human sites) during the Palaeolithic and Neolithic eras since 50 kya (electronic supplementary material, figure S2). Data from human sites covering mostly western Eurasia (and part of Asia) was extracted from the radiocarbon CONTEXT database (Utz Böhner and Daniel Schyle, http://context-database.uni-koeln.de/), and the radiocarbon palaeolithic Europe database v. 18 [24]. These datasets contain information of geographical co-ordinates and dates (electronic supplementary material, figure S2). Data for human sites covering China were extracted from a Chinese history map published by the Liren Publishing House in 1984 and a book entitled ‘The Chinese Ancient History’ published by Shanghai People's Publishing House in 2000 (electronic supplementary material, figure S2). This dataset only contains location information.
(c). Spatial and temporal distribution analysis
We are interested in the latitudinal distribution changes of animals in latitude–time space so as to study the impacts of temperature on the latitude change of these large mammals. All records were used to estimate the overall latitudinal distribution. Local polynomials were used to estimate the changing trends of these latitudes (figure 1) using the ggplot2 library (v. 1.0.1) in R (v. 3.2.3) [25]. Correlations between the latitude and temperatures in western and eastern Eurasia were conducted.
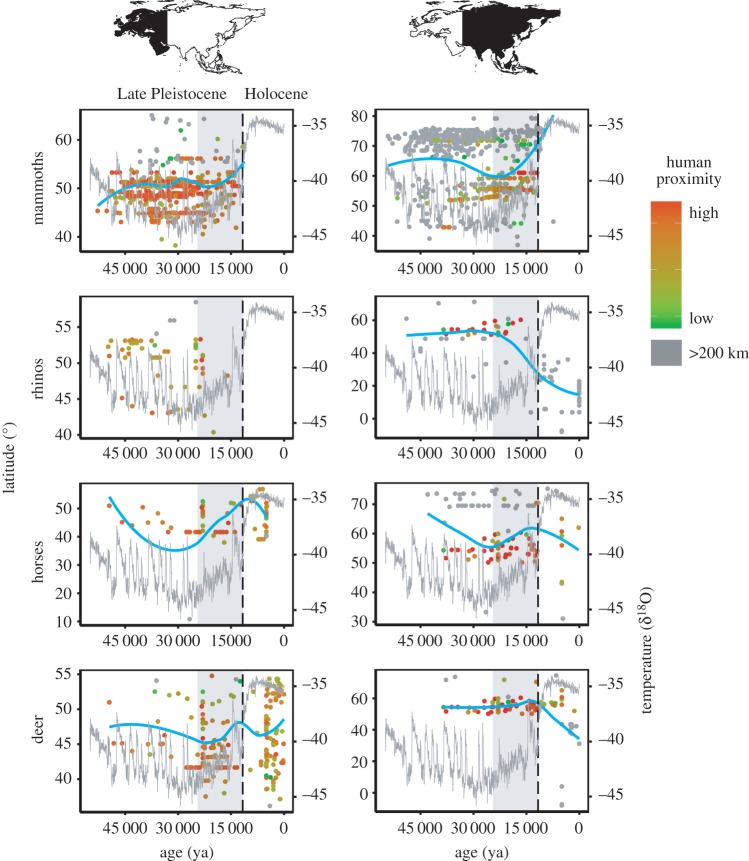
Figure 1.
Disentangling impacts of humans and climate on final or massive extinction of mammoths, rhinos, horses and deer in western and eastern Eurasia. Rapid temperature increase (as measured by δ18O value) from the LGM to Holocene is highlighted by the grey area. The nearest distance of each animal site to human site (as indicated by human proximity; from low to high) is shown in colour (from green to red). The dashed vertical line indicates the Pleistocene–Holocene boundary with the turning point at about 12 kya. Blue lines represent the fitted latitude based on results in table 1.
Spearman's rank correlations (for non-normally distributed data) and Pearson's product–moment correlations (for normally distributed data) [26] were used to detect significant correlations between latitude and temperature.
A non-uniform survey in latitude–longitude space may impose biased estimations of the change of latitude. We used all survey data in our database including archaeological data (n = 17 108 records) and palaeobiological data (n = 46 476 records) to represent the survey effort in space. Latitude–longitude space was divided into 100 × 100 km2 grids. If the number of animal fossils was significantly correlated with the total number of survey sites, we weighted the animal fossil data in calculating the trend of latitude. In fact, we found there were strong, significant positive correlations between the number of animal fossils and the number of all survey sites (ln-transformed values) for all taxa (electronic supplementary material, table S3 and figure S4).
If significant positive correlation between the observed and average survey efforts was found, latitudinal weights were calculated based on the correlation between the observed and average survey efforts within a grid and that between the observed and expected number of animal fossils. For a given latitude–longitude grid, the weighted coefficient r was calculated as follows:
where ln M is the observed total number of survey sites (ln-transformed value) within a grid, c represents the average number of survey sites (ln-transformed value), α represents the differences between the observed (ln M) and the average (c) survey efforts, m is the observed number of animal fossils within a grid, β represents the weighted number of animal fossils (ln-transformed value), m′ is the expected number of animal fossils and r represents the weighted coefficients for the latitudinal value of animal fossils within each grid. Parameter calculations and data normality tests were carried out in the R environment (v. 3.2.3) via the stats library (v. 3.2.2) and plyr library (v. 1.8.3). Weighted correlation between latitude and temperature index of three regions was carried out in the R environment (v. 3.2.3) via weights library (v. 0.85).
(d). Human impact analysis
We divided the latitude–longitude space of our study region into grids with spatial scales of 100 × 100 km2, which approximates the area of influence ancient people had on the environment [16]. We calculated the extinction time within a given space (100 × 100 km2) using GRIWM (Gaussian-resampled, inverse-weighted method) which was developed to estimate probability of true extinction time from the available records [27,28]. The sample size within a grid must be larger than 4, and the standard deviation (s.d.) was not included in GRIWM because we cannot calculate s.d. for most of our data.
We defined two kinds of human impacts on extinction time: the number of human sites that are older than animal fossil age (human density) and the average nearest Euclidean distance of animal fossil sites to human sites that are older than animal fossil age (human proximity). If humans had significant negative impacts on animals, we would have significant and positive associations of extinction time with human density but a negative association with the human proximity within grids (100 × 100 km2). Otherwise (i.e. for non-significant or reverse significant correlations), the negative human impacts were not recognized. Data were ln-transformed to achieve normal distributions. Spearman's rank correlation (for non-normally distributed data) or Pearson's product–moment correlation (for normally distributed data) were used to detect significant correlations between extinction time and human impact criteria (i.e. human proximity, human density). The correlation analyses were carried out in the R environment (v. 3.2.2) via the stats library (v. 3.2.2) and the base library (v. 3.2.2) [25]; human impact criteria were calculated in the R environment (v. 3.2.2) via the FNN (v. 1.1), rgdal (v. 1.1-10) and geosphere (v. 1.5-5) library [29–32].
3. Results
(a). Woolly mammoth
We found that, in eastern Eurasia, all three temperature series showed significant and positive associations with the latitude (all p < 0.001, table 1) from 54.25 kya to 7.38 kya. In western Eurasia, only the Antarctica temperature series showed significant positive correlations with latitude (p < 0.001, table 1). Hence, global warming forced mammoths to move northwards from 52.8 kya to 11.25 kya. Only a few fossil records were reported after the turning point of rapid increase in temperature at about 12 kya.
Table 1.
Correlation coefficients between overall distribution (weighted, measured by latitude) of fossil occurrences of four mammal groups in Eurasia and temperature (measured by δ18O value for the Greenland series, centigrade for the Antarctica series and magnetic susceptibility for the southern Chinese Loess Plateau series) from 55 kya to 0 ya. (n.a. denotes no data; ‘WE’ denotes western Eurasia and ‘EE’ denotes eastern Eurasia. *p < 0.05, **p < 0.01, ***p < 0.001.)
| correlation coefficients |
|||
|---|---|---|---|
| mammal groups | Antarctica | Greenland | China |
| mammoths (WE) | 0.2*** | 0.02 | −0.15 |
| mammoths (EE) | 0.35*** | 0.2*** | 0.29*** |
| rhinoceroses (WE) | −0.08 | 0.17 | n.a. |
| rhinoceroses (EE) | −0.74*** | −0.81*** | n.a. |
| horses (WE) | 0.38*** | 0.37*** | 0.62** |
| horses (EE) | 0.06 | 0.06 | 0.54*** |
| deer (WE) | 0.06 | 0.04 | 0.63*** |
| deer (EE) | −0.29*** | −0.35*** | −0.14 |
We found that the extinction time was positively correlated with the average nearest distance of animal sites to human sites (human proximity), and negatively correlated with the number of human sites (human density) (both p < 0.001) from 52.8 kya to 11.25 kya in western Eurasia (table 2 and figure 2). However, the negative human impact was recognized in eastern Eurasia due to positive correlations of the extinction time with human density (p < 0.001) from 54.25 kya to 7.38 kya (all p < 0.001; table 2 and figure 2; electronic supplementary material, figure S5). Hence, negative human impacts on mammoths were predominant in eastern Eurasia, but not significant in western Eurasia.
Table 2.
Correlation coefficients between the extinction times of four mammal groups within a grid of 100 × 100 km2 in Eurasia and human impact indices. (Indices include the number of human sites that are older than animal fossil age (human density), and the average nearest distance of animal fossil sites to human sites that are older than animal fossil age (human proximity distance ≦ 200 km). ‘WE’ in ‘mammal groups’ denotes western Eurasia; ‘EE’ denotes eastern Eurasia; *p < 0.05, **p < 0.01, ***p < 0.001.)
| mammal groups | human proximity | human density |
|---|---|---|
| mammoths (WE) | 0.27*** | −0.3*** |
| mammoths (EE) | 0.42*** | 0.22***a |
| rhinoceroses (WE) | 0.31 | −0.53*** |
| rhinoceroses (EE) | −0.17 | 0.69***a |
| horses (WE) | −0.67***a | 0.13 |
| horses (EE) | −0.36*a | 0.48***a |
| deer (WE) | −0.26**a | −0.15 |
| deer (EE) | −0.31**a | −0.14 |
aThere is evidence of negative human impacts on the large mammals.
Figure 2.
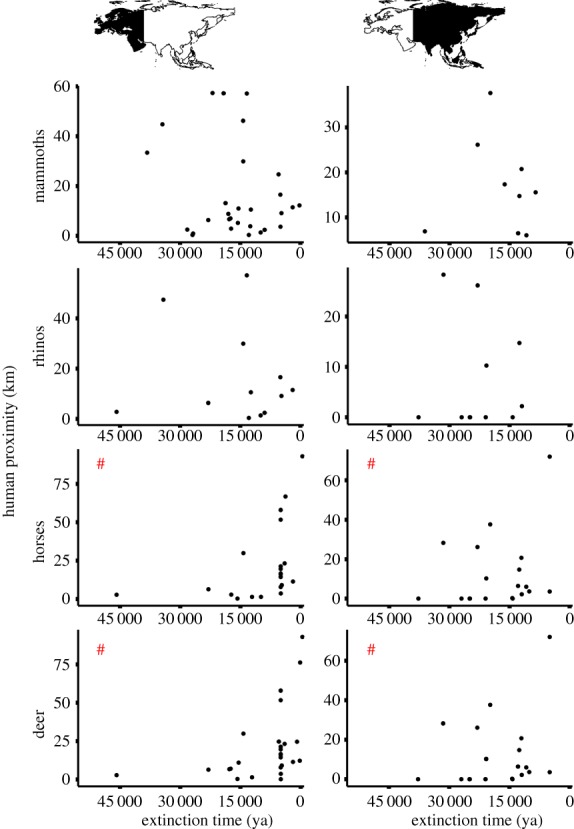
Correlations between the extinction time (ya) and the average nearest distance of the animal fossil site to a human site that is older than animal fossil age (human proximity) for mammoths, rhinos, horses and deer in western and eastern Eurasia. # indicates that the negative human impacts were recognized. For details, see table 2. (Online version in colour.)
(b). Rhinoceroses
In western Eurasia, all three temperature series showed no significant effects on the latitude of rhinos from 49.8 kya to 16 kya (table 1). However, in eastern Eurasia, two temperature series (Antarctica and Greenland series) showed significant and negative associations with latitude of rhinos from 49 kya to 0.05 kya (all p < 0.001, table 1). Hence, global warming caused a distribution shift of rhinos southwards in eastern Eurasia, but not in western Eurasia. After the turning point around 12 kya, no records of rhinos were found in western Eurasia, but many records were found in the southern parts of eastern Eurasia.
We found negative human impacts on rhinos in eastern Eurasia, supported by the positive correlations between the extinction time and human density (p < 0.001) in eastern Eurasia from 49.8 kya to 16 kya (table 2 and figure 2; electronic supplementary material, figure S5). However, in western Eurasia, negative human impacts were not recognized (table 2 and figure 2).
(c). Horses
We found that all three temperature series showed positive and significant associations with the latitudes of horses in western Eurasia from 49.5 kya to 0 ya. In eastern Eurasia, only the China temperature series showed a positive association with the latitude from 43 kya to 0.2 kya (table 1). Hence, global warming forced horses to move northwards. But after the turning point at 12 kya, there are still many fossil records of horses until about 0.7 kya in both regions.
We found negative human impacts on horses in both western and eastern Eurasia, with negative correlations between the extinction time and human proximity in both western and eastern Eurasia (for western: p < 0.001; for eastern: p < 0.05), and positive correlation between the extinction time and human density (p < 0.001) in eastern Eurasia from 49.5 kya to 0.2 kya (table 2, figure 2; electronic supplementary material, figure S5). Hence, humans showed negative effects on horses in both western and eastern Eurasia. Fossil records of horse were very rare after 0.7 kya in both regions.
(d). Deer
In western Eurasia, only the China temperature series showed positive associations with the latitude of deer in western Eurasia from 49.5 kya to 0 ya (table 1). In eastern Eurasia, however, negative associations of temperature on latitude of deer according to latitudinal gradients were found from 38.48 kya to 0 ya (table 1). Hence, global warming forced deer to move northwards in the western part of their range, but move southwards in eastern Eurasia. Fossil records of deer were commonly seen in the Holocene, suggesting that deer succeeded at adapting to the rapid temperature increase.
We found negative human impacts on deer in both western and eastern Eurasia, supported by a negative correlation between the extinction time and human proximity (both p < 0.01) in both western and eastern Eurasia from 49.5 kya to 0.05 kya (table 2, figure 2; electronic supplementary material, figure S5). Fossil records were observed into very recent times, suggesting that deer survived the human impacts in the Holocene.
4. Discussion
Our results indicated that quantitative analysis by using continuous temporal–spatial palaeontological and archaeological records enabled us to distinguish the impacts of climate warming and humans in causing the extinction or major extirpations of four megafauna mammalian groups in the Late Pleistocene and Holocene. We found there were significant and positive associations between temperature and latitude of three mammalian groups (mammoths, horses, deer), which supports the climate-driven extinction hypothesis. We found there were significant and negative effects of humans on all four mammalian groups, which supports the human-driven extinction hypothesis. We found that climate warming was the predominant driver of total extinction of mammoths and rhinos in cold zones from the Late Pleistocene to Holocene, while human impacts were the predominant driver of total extinction of rhinos (taxa living in the cold zones) and horse extinctions in the Anthropocene and Holocene. Most deer taxa survived the rapid climate warming and extensive human impacts from Late Pleistocene to the Holocene and Anthropocene owing to their high fecundity and ecological flexibility to environmental change. Differences in impact of climate and humans on these mammal groups were also found, probably owing to the difference in living habits of humans and climate between western and eastern Eurasia.
(a). Mammoths
Mammoths were herbivores that lived in cool and dry open steppe–tundra. While they were widely distributed in Eurasia by the Late Pleistocene, they disappeared in the Early Holocene. It is still unclear which factor caused the total extinction (global extinction or regional extirpation) of mammoths [11]. In general, our results support the previous observations of range contraction of mammoths during warm periods and southward expansions in cold periods [10,16–19]. They fit with the expectations that climate warming would diminish their habitats in the south, forcing northward movements to areas where suitable herb and grass vegetation remained [17]. It is notable that mammoths moved south before they went extinct in the Early Holocene. This was obviously caused by climate cooling before the Late Pleistocene (figure 1).
Archaeological evidence from a 50 000-year-old site in south Britain indicates that early humans were highly capable of hunting mammoths [33,34]. Many sites in eastern Europe dating from 15 kya to 44 kya old verify that humans built dwellings using mammoth bones [34]. A large amount of Pleistocene ivory was excavated from the Yana RHS archaeological site [35]. There is evidence of human presence in Siberia at 50–60° N in the Mousterian and Upper Palaeolithic periods (mainly 43 kya–19 kya). Likewise, in the Palaeolithic period, archaeological remains indicate human activity in the Arctic and high-latitude regions [10,36]. Approximately 40% of the European and 35% of the Siberian palaeolithic sites were found to have woolly mammoth remains [11].
In western Eurasia, mammoths successfully survived into the Early Holocene (7.38–11.7 kya), although human sites are very close to the mammoth sites beginning 50 kya (figure 1). They disappeared from Europe and most parts of north Asia by about 12 kya, corresponding well to the rapid climate warming (figure 1). They finally went extinct at Wrangel Island by 3 kya [18]. In eastern Eurasia, many mammoth fossil sites (especially in high latitudes) were far away from human sites, but were closer to human sites in low latitude. Our results indicate that human presence had significant negative impacts on mammoths in eastern Eurasia, but not in western Eurasia. In eastern Eurasia, the human impacts, indicated by human proximity, on mammoths were contemporaneous with climate-triggered contraction of southern range boundaries (figure 1). Hence, both climate warming and human impacts were probably predominant drivers triggering the total extinction of mammoths in this region, with human impacts perhaps providing the coup de grace; the former is consistent with the findings by Varela et al. [16]: cold-adapted species like mammoths are more vulnerable to climate warming. Human impacts clearly caused extirpations (extinction within small grids we defined) in eastern Eurasia but were not the key factor in causing total extinction in both western and eastern Eurasia.
(b). Rhinoceroses
While rhinoceroses (Rhinocerotidae) were widely distributed through Eurasia in the Pleistocene, their current distribution covers only a part of southeastern Asia. Woolly rhinos disappeared at about 14 kya, probably under the driving force of climate warming [11]. We did not find any effect of climate on rhinos in western Eurasia. Instead, a negative association between the latitudes of rhinos and temperature was found in eastern Eurasia, indicating that global warming caused a shift in the distribution of rhinos southwards in eastern Eurasia, which was not consistent with the traditional climate-driven extinction hypothesis. One possible explanation is that the rhinoceroses we studied include taxa adapted to both cold and warm climates. It is notable that no fossil record of rhinos was found in western Eurasia (mainly in the cold region) in the Holocene, indicating that global warming might be the key factor for the total extinction of rhinos in western Eurasia, which is consistent with the previous result [11]. Meanwhile, in eastern Eurasia, very few fossils were found in high latitudes in the Holocene, probably because rapid temperature increases caused extinction of rhinos living in cold regions. The extinction of rhinos (e.g. woolly rhinos) in high latitudes in eastern Eurasia might result in the overall distributional shift to the south, while rhinos in the south (e.g. Javan rhinos) survived the climate warming. Previous studies found that, for ‘cold-adapted’ taxa such as woolly rhinoceros, climate warming, accompanied by increased precipitation, might have led to deeper snow depth in the winter, which caused the extinction of woolly rhinoceros [37]. In China, temperature showed a much deeper decline since the Holocene (China series; electronic supplementary material, figure S3); this might have also caused the retreat of rhinoceroses towards to south. Using historical records, Li et al. found that climate cooling forced the southern expansion of Asian elephants and southward movement of rhinos during the past two millennia [38].
Because rhino horns have been used in traditional medicine of both Europe and Asia, rhinos were heavily hunted by ancient people [38]. Our results indicate that the negative impacts of humans on rhinos were predominant in eastern Eurasia (table 2), but were not significant in western Eurasia. Rhinoceros in western Eurasia went extinct around 12 kya and giant rhinoceros (Elasmotherium) went extinct in the Late Pleistocene. Woolly rhinoceros (Coelodonta) went extinct in the Holocene, suggesting that climate warming is the driving force causing their total extinction. As three rhino species (i.e. the greater one-horned rhinoceros, Rhinoceros unicornis; the Javan rhinoceros, Rhinoceros sondaicus; the Sumatran rhinoceros, Dicerorhinus sumatrensis) are still extant in southeast Asia, this suggests that the rhinos in tropical or subtropical regions survived the effect of climate warming. Large numbers of rhinos became extinct in the Late Holocene in eastern Eurasia (figure 1), suggesting that human impacts were the predominant driving force of major extirpations of rhinos in eastern Eurasia.
(c). Horses
Horses (Equidae) inhabit steppe, semi-desert and desert environments. They were widely distributed across Eurasia but many species became extinct during the Holocene, leaving only a few Equus species [39]. We found that climate warming was positively associated with the northward movement of horses in both western and eastern Eurasia (table 1). Overall, equine distributions moved northwards under climate warming in eastern Eurasia from the LGM to Holocene (grey box in figure 1); this is consistent with the climate-driven hypothesis. These changes were probably caused by spatial changes in habitats: climate warming caused deglaciation in regions of north Europe like Scandinavia and created habitat in the north. In eastern Eurasia, previous studies demonstrated that climate warming increased precipitation in Asia owing to enhanced Southeast and Indian monsoons [40–42], which probably altered the steppe habitats favoured by horses [43]. Despite the effects of climate warming, horses survived to the middle Holocene. Equus capensis went extinct in the Late Pleistocene, but wild horse (Equus przewalskii) and the domestic horse (Equus caballus), donkeys (Equus Asinus) and onager (Equus hemionus) survived. The domestic horse is probably descended from the tarpan and Przewalskii's horses. Our results suggest that climate warming was not the key factor driving the massive extinction of horses.
Archaeological studies demonstrate that humans commonly preyed on horses in central Germany, indicating heavy hunting pressure [44], and western Europe thus emerged as an extinction hotspot [1]. Our results support negative human impacts on horses in both western and eastern Eurasia. Very few horse fossils were found in the Early Anthropocene or Late Holocene (figure 1), implicating a human impact as the predominant driver of horse extinction.
(d). Deer
Deer (family Cervidae) are large and highly mobile herbivores, and once had a wide geographical distribution in the Late Quaternary period. We found that climate warming was positively associated with northward movement in western Eurasia, but with southward movement in eastern Eurasia. Previous studies indicate that climate warming may have resulted in the change of habitat of deer: steppe–tundra replaced with more productive vegetation like shrubs and some birch trees [45]. Many fossils of deer were found in the Holocene (figure 1), indicating they could safely endure rapid climate warming from LGM to Holocene; during this period many fossil records were reported (figure 1). Similar to rhinos, climate warming might cause extirpations of deer in northern parts of eastern Eurasia, resulting in the negative association between latitudinal distribution of deer and temperature: giant deer (Megaloceros) and stag-moose (Cervalces), which appeared in cold regions, went extinct in the Early Holocene.
Archaeological studies demonstrate that Palaeolithic humans commonly preyed on deer in varied strategies, indicating heavy hunting pressure. Our results support negative human impacts on horses in both western and eastern Eurasia, but many deer fossils were found in the Anthropocene, suggesting that deer are able to adapt to human disturbance to some extent. There are many extant genera, such as moose, elk, roe deer, red deer, fallow deer, caribou and reindeer. Reindeer currently number in the millions across the Holarctic; both deer and reindeer are not currently under threat of extinction. Previous studies have found that deer have high fecundity and ecological flexibility [11]. Thus deer may have evolved behavioural adaptations in response to early hominin hunting strategies, leading to the evolution of a ‘non-naive’ megafauna more resilient to later impacts by modern humans [46].
5. Implications
Our results would give some implications in assessing the animal extinction risk driven by the current climate warming and human impacts. From figure 1, climate warming from the Late Pleistocene (LGM) to the Early Holocene caused extinctions of mammoths, rhinos and horses in western Eurasia, and also mammoths in eastern Eurasia. Previous studies indicated that from the Late Pleistocene (LGM) to Early Holocene temperature showed an increase of about 12°C in China, about 9.8°C in Antarctica and about 12°C in Greenland [23,47,48], thus the southern boundary for mammoths, rhinos and horses in western Eurasia shifted northwards over 2000 km within about 17 000 years (figure 1), resulting in total extinction of these large mammals in temperate and arctic zones. According to the 5th assessment report of the Intergovernmental Panel on Climate Change, the average global surface temperature shows a warming of 0.85°C over the period from 1880 to 2012. Relative to 1850–1900, the global surface temperature change for the end of the 21st century (2081–2100) is projected to probably exceed 1.5°C or even 2°C [49]. If we assume that the temperature will increase 1°C every 100 years, it will take 500–1000 years from now for the temperature to increase 5–10°C which is equivalent to the amplitude of temperature increase during the Late Pleistocene (LGM) to Early Holocene, which took about 10 000–15 000 years. According to a recent study based on data of 1367 species, climate warming caused species to move northwards at the speed of 16.9 km per decade [50], much faster than previously thought. Within 500–1000 years, these animals would move 845–1690 km northward, while the northward retreat of the southern boundary of mammoths was estimated to be about 20–30 degrees of latitude (equal to about 2200–3300 km) from the Late Pleistocene (LGM) to Early Holocene temperature (figure 1). When compared with the period from the Late Pleistocene (LGM) to Early Holocene, both the current speeds of temperature increase and range shift of species towards the poles or higher elevation are even faster today and could accelerate. Our results would help us to assess the future impacts of current global warming on extinctions of our plant flora and animal fauna in temperate and arctic zones, which obviously depends on how many years the current global warming will continue.
Our study suggests that human impacts on species extinction or major extirpation rates were more predominant in the Late Holocene or Anthropocene than during the Late Pleistocene. Currently, we are facing a much faster species extinction rate under the accelerated human impacts. It is obvious that apart from climate change, human disturbance is another major driving force causing species extinction. There is an urgent need to assess the quantitative impacts of humans on extinction or major extirpation rates of modern species, so as to take efficient actions or measures for protecting our endangered species and the biodiversity of the Earth.
Supplementary Material
Acknowledgements
We are grateful to Prof. Anthony D. Barnosky of University of California at Berkeley and Prof. Marcel Holyoak of University of California at Davis for their valuable comments and suggestions on the manuscript.
Authors' contributions
Z.Z. designed the study; X.W. conducted data compilation and analysis; Z.Z. and X.W. wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work is supported by the External Cooperation Program of BIC, Chinese Academy of Sciences (grant no. 152111KYSB20150023), and the scientific program of Biological Consequences of Global Change (BCGC) of International Society of Zoological Sciences (ISZS) and International Union of Biological Sciences (IUBS).
References
- 1.Sandom C, Faurby S, Sandel B, Svenning JC. 2014. Global Late Quaternary megafauna extinctions linked to humans, not climate change. Proc. R. Soc. B 281, 20133254 ( 10.1098/rspb.2013.3254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koch PL, Barnosky AD. 2006. Late Quaternary extinctions: state of the debate. In Annual review of ecology evolution and systematics, pp. 215–250. Palo Alto, CA: Annual Reviews. [Google Scholar]
- 3.Martin PS, Wright HE. 1967. Pleistocene extinctions. The search for a cause. In Proc. 7th Congr. INQUA Int. Ass. Quaternary Research, vol. 6, 453p New Haven, CT: Yale University Press. [Google Scholar]
- 4.Barnosky AD, et al. 2011. Has the Earth's sixth mass extinction already arrived? Nature 471, 51–57. ( 10.1038/nature09678) [DOI] [PubMed] [Google Scholar]
- 5.Gilmour DM, Butler VL, O'Connor JE, Davis EB, Culleton BJ, Kennett DJ, Hodgins G. 2015. Chronology and ecology of Late Pleistocene megafauna in the northern Willamette Valley, Oregon. Quat. Res. 83, 127–136. ( 10.1016/j.yqres.2014.09.003) [DOI] [Google Scholar]
- 6.Lewis SL, Maslin MA. 2015. Defining the Anthropocene. Nature 519, 171–180. ( 10.1038/nature14258) [DOI] [PubMed] [Google Scholar]
- 7.Barnosky AD. 1986. Big game extinction caused by Late Pleistocene climatic-change—Irish elk (Megaloceros giganteus) in Ireland. Quat. Res. 25, 128–135. ( 10.1016/0033-5894(86)90049-9) [DOI] [Google Scholar]
- 8.Zazula GD, et al. 2014. American mastodon extirpation in the Arctic and Subarctic predates human colonization and terminal Pleistocene climate change. Proc. Natl Acad. Sci. USA 111, 18 460–18 465. ( 10.1073/pnas.1416072111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen KK, et al. 2004. High-resolution record of Northern Hemisphere climate extending into the last interglacial period. Nature 431, 147–151. ( 10.1038/nature02805) [DOI] [PubMed] [Google Scholar]
- 10.Nogues-Bravo D, Rodiguez J, Hortal J, Batra P, Araujo MB. 2008. Climate change, humans, and the extinction of the woolly mammoth. PLoS. Biol. 6, 685–692. ( 10.1371/journal.pbio.0060079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorenzen ED, et al. 2011. Species-specific responses of Late Quaternary megafauna to climate and humans. Nature 479, 359 ( 10.1038/nature10574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCain CM, King SRB. 2014. Body size and activity times mediate mammalian responses to climate change. Glob. Change Biol. 20, 1760–1769. ( 10.1111/gcb.12499) [DOI] [PubMed] [Google Scholar]
- 13.Cooper A, Turney C, Hughen KA, Brook BW, McDonald HG, Bradshaw CJA. 2015. Paleoecology abrupt warming events drove Late Pleistocene Holarctic megafaunal turnover. Science 349, 602–606. ( 10.1126/science.aac4315) [DOI] [PubMed] [Google Scholar]
- 14.Holyoak M, Heath SK. 2016. The integration of climate change, spatial dynamics, and habitat fragmentation: a conceptual overview. Integr. Zool. 11, 40–59. ( 10.1111/1749-4877.12167) [DOI] [PubMed] [Google Scholar]
- 15.Crees JJ, Turvey ST. 2014. Holocene extinction dynamics of Equus hydruntinus, a late-surviving European megafaunal mammal. Quat. Sci. Rev. 91, 16–29. ( 10.1016/j.quascirev.2014.03.003) [DOI] [Google Scholar]
- 16.Varela S, Lima-Ribeiro MS, Diniz JAF, Storch D. 2015. Differential effects of temperature change and human impact on European Late Quaternary mammalian extinctions. Glob. Change Biol. 21, 1475–1481. ( 10.1111/gcb.12763) [DOI] [PubMed] [Google Scholar]
- 17.Stuart AJ. 2005. The extinction of woolly mammoth (Mammuthus primigenius) and straight-tusked elephant (Palaeoloxodon antiquus) in Europe. Quat. Int. 126, 171–177. ( 10.1016/j.quaint.2004.04.021) [DOI] [Google Scholar]
- 18.Stuart AJ, Sulerzhitsky LD, Orlova LA, Kuzmin YV, Lister AM. 2002. The latest woolly mammoths (Mammuthus primigenius Blumenbach) in Europe and Asia: a review of the current evidence. Quat. Sci. Rev. 21, 1559–1569. ( 10.1016/s0277-3791(02)00026-4) [DOI] [Google Scholar]
- 19.Markova AK, Puzachenko AY, van Kolfschoten T, van der Plicht J, Ponomarev DV. 2013. New data on changes in the European distribution of the mammoth and the woolly rhinoceros during the second half of the Late Pleistocene and the early Holocene. Quat. Int. 292, 4–14. ( 10.1016/j.quaint.2012.11.033) [DOI] [Google Scholar]
- 20.Ye J, Xiao Z, Li C, Wang F, Liao J, Fu J, Zhang Z. 2015. Past climate change and recent anthropogenic activities affect genetic structure and population demography of the greater long-tailed hamster in northern China. Integr. Zool. 10, 482–496. ( 10.1111/1749-4877.12150) [DOI] [PubMed] [Google Scholar]
- 21.The NOW Community 2017. New and Old Worlds database of fossil mammals (NOW) Licensed under CC BY 4.0. See http://www.helsinki.fi/science/now/.
- 22.Qin XG, Mu Y, Ning B, Yin ZQ. 2009. Climate effect of dust aerosol in southern Chinese Loess Plateau over the last 140,000 years. Geophys. Res. Lett. 36, L02707 ( 10.1029/2008gl036156) [DOI] [Google Scholar]
- 23.Jouzel J, et al. 2007. Orbital and millennial Antarctic climate variability over the past 800,000 years. Science 317, 793–796. ( 10.1126/science.1141038) [DOI] [PubMed] [Google Scholar]
- 24.Vermeersch PM.2015. Radiocarbon Palaeolithic Europe Database, Version 18. See http://ees.kuleuven.be/geography/projects/14c-palaeolithic/index.html .
- 25.Team RC. 2015. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See https://www.R-project.org/. [Google Scholar]
- 26.Shieh G. 2010. Estimation of the simple correlation coefficient. Behav. Res. Methods 42, 906–917. ( 10.3758/brm.42.4.906) [DOI] [PubMed] [Google Scholar]
- 27.Bradshaw CJA, Cooper A, Turney CSM, Brook BW. 2012. Robust estimates of extinction time in the geological record. Quat. Sci. Rev. 33, 14–19. ( 10.1016/j.quascirev.2011.11.021) [DOI] [Google Scholar]
- 28.Saltre F, Brook BW, Rodriguez-Rey M, Cooper A, Johnson CN, Turney CSM, Bradshaw CJA. 2015. Uncertainties in dating constrain model choice for inferring extinction time from fossil records. Quat. Sci. Rev. 112, 128–137. ( 10.1016/j.quascirev.2015.01.022) [DOI] [Google Scholar]
- 29.Environmental Systems Research Institute (ESRI). 2014. ArcGIS release 10.3 for Desktop. See http://www.esri.com/.
- 30.Hijmans RJ. 2016. Geosphere: spherical trigonometry. R package version 1.5-5. See https://CRAN.R-project.org/package=geosphere.
- 31.Beygelzimer A, Kakadet S, Langford J, Arya S, Mount D, Li S. 2013. FNN: fast nearest neighbor search algorithms and applications. R package version 1.1. See https://CRAN.R-project.org/package=FNN.
- 32.Bivand R, Keitt T, Rowlingson B. 2016. rgdal: bindings for the Geospatial Data Abstraction Library. R package version 1.1-10. See https://CRAN.R-project.org/package=rgdal.
- 33.Sapsted D.2002. Woolly mammoth butchery site unearthed. The Telegraph. See http://www.telegraph.co.uk/news/science/science-news/3296348/Woolly-mammoth-butchery-site-unearthed.html .
- 34.Gray R.2011. Neanderthals built homes with mammoth bones. Telegraph. See http://www.telegraph.co.uk/news/science/science-news/8963177/Neanderthals-built-homes-with-mammoth-bones.html .
- 35.Basilyan AE, Anisimov MA, Nikolskiy PA, Pitulko VV. 2011. Wooly mammoth mass accumulation next to the Paleolithic Yana RHS site, Arctic Siberia: its geology, age, and relation to past human activity. J. Archaeol. Sci. 38, 2461–2474. ( 10.1016/j.jas.2011.05.017) [DOI] [Google Scholar]
- 36.Kuzmin YV, Orlova LA. 1998. Radiocarbon chronology of the Siberian Paleolithic. J. World Prehist. 12, 1–53. ( 10.1023/a:1022431307503) [DOI] [Google Scholar]
- 37.Boeskorov GG. 2012. Some specific morphological and ecological features of the fossil woolly rhinoceros (Coelodonta antiquitatis Blumenbach 1799). Biol. Bull. 39, 692–707. ( 10.1134/S106235901208002x) [DOI] [Google Scholar]
- 38.Li X, Jiang G, Tian H, Xu L, Yan C, Wang Z, Wei F, Zhang Z. 2015. Human impact and climate cooling caused range contraction of large mammals in China over the past two millennia. Ecography 38, 74–82. ( 10.1111/ecog.00795) [DOI] [Google Scholar]
- 39.MacFadden BJ. 1992. Fossil horses: systematics, paleobiology, and evolution of the family equidae. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 40.Fleitmann D, Burns SJ, Mudelsee M, Neff U, Kramers J, Mangini A, Matter A. 2003. Holocene forcing of the Indian monsoon recorded in a stalagmite from Southern Oman. Science 300, 1737–1739. ( 10.1126/science.1083130) [DOI] [PubMed] [Google Scholar]
- 41.Porter SC, An ZS. 1995. Correlation between climate events in the north-Atlantic and China during last glaciation. Nature 375, 305–308. ( 10.1038/375305a0) [DOI] [Google Scholar]
- 42.Xu L, Liu QY, Stige LC, Ben Ari T, Fang XY, Chan KS, Wang SC, Stenseth NC, Zhang ZB. 2011. Nonlinear effect of climate on plague during the third pandemic in China. Proc. Natl Acad. Sci. USA 108, 10 214–10 219. ( 10.1073/pnas.1019486108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prado JL, Alberdi MT. 2014. Global evolution of Equidae and Gomphotheriidae from South America. Integr. Zool. 9, 434–443. ( 10.1111/1749-4877.12064) [DOI] [PubMed] [Google Scholar]
- 44.Diedrich CG. 2012. Late Pleistocene Crocuta crocuta spelaea (Goldfuss, 1823) clans as prezewalski horse hunters and woolly rhinoceros scavengers at the open air commuting den and contemporary Neanderthal camp site Westeregeln (central Germany). J. Archaeol. Sci. 39, 1749–1767. ( 10.1016/j.jas.2012.01.013) [DOI] [Google Scholar]
- 45.Stuart AJ, Kosintsev PA, Higham TFG, Lister AM. 2004. Pleistocene to Holocene extinction dynamics in giant deer and woolly mammoth. Nature 431, 684–689. ( 10.1038/nature02890) [DOI] [PubMed] [Google Scholar]
- 46.Turvey ST, Tong H, Stuart AJ, Lister AM. 2013. Holocene survival of Late Pleistocene megafauna in China: a critical review of the evidence. Quat. Sci. Rev. 76, 156–166. ( 10.1016/j.quascirev.2013.06.030) [DOI] [Google Scholar]
- 47.Peterse F, Prins MA, Beets CJ, Troelstra SR, Zheng HB, Gu ZY, Schouten S, Damste JSS. 2011. Decoupled warming and monsoon precipitation in East Asia over the last deglaciation. Earth Planet. Sci. Lett. 301, 256–264. ( 10.1016/j.epsl.2010.11.010) [DOI] [Google Scholar]
- 48.Grootes PM, Stuiver M, White JWC, Johnsen S, Jouzel J. 1993. Comparison of oxygen-isotope records from the GISP2 and GRIP Greenland ice cores. Nature 366, 552–554. ( 10.1038/366552a0) [DOI] [Google Scholar]
- 49.IPCC. 2014. Climate Change 2014: Synthesis report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (Core Writing Team RK Pachauri, LA Meyer) Geneva, Switzerland: IPCC. [Google Scholar]
- 50.Chen IC, Hill JK, Ohlemuller R, Roy DB, Thomas CD. 2011. Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026. ( 10.1126/science.1206432) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.