Abstract
Natal dispersal is assumed to be costly. Such costs can be difficult to detect, and fitness consequences of dispersal are therefore poorly known. Because of lower phenotypic quality and/or familiarity with the environment, natal dispersers may be less buffered against a sudden increase in reproductive effort. Consequently, reproductive costs associated with natal dispersal may mostly be detected in harsh breeding conditions. We tested this prediction by comparing lifetime reproductive success between natal dispersers and non-dispersers in a patchy population of collared flycatchers (Ficedula albicollis) when they reared either a non-manipulated brood or an experimentally increased or decreased brood. Natal dispersers achieved lower lifetime reproductive success than non-dispersers only under more stressful breeding conditions (i.e. when brood size was experimentally increased). This was mostly due to a lower number of recruits produced in the year of the increase. Our results suggest a cost associated with natal dispersal paid immediately after an increase in reproductive effort and not subsequently compensated for through increased survival or future offspring recruitment. Natal dispersers adjusted their breeding investment when reproductive effort is as predicted but seemed unable to efficiently face a sudden increase in effort, which could affect the influence of environmental predictability on dispersal evolution.
Keywords: reproductive success, return rate, natal dispersal, reproductive effort, dispersal cost, brood size manipulation
1. Introduction
Natal dispersal is defined as the movement of individuals between the natal site and the site of first breeding [1], and is considered as a crucial life-history trait in many taxa [2,3]. Most often, the life histories of dispersers and non-dispersers are thought to differ because dispersal is assumed to be costly and can carry both direct and deferred costs at each step of the dispersal process (i.e. departure, movement and settlement) [4–6]. Costs and benefits of natal dispersal may lead to opposite selective pressures and dispersal can also be affected by the individual's phenotype (e.g. morphology, behaviour and physiology) in interaction with external conditions (e.g. population density and habitat quality) [7,8]. As a result, the fitness consequences of adopting different natal dispersal strategies are not easy to predict and, not surprisingly, no clear pattern emerges from empirical studies investigating fitness correlates of natal dispersal [9,10]. It is noteworthy, however, that most of these studies focused only on short-term fitness measures (i.e. breeding date and number of young produced) and/or on single fitness components [9,10]. Yet recent studies suggest that natal dispersal costs can also be long-term and affect the individual's life-history or fitness traits long after the dispersal event itself (e.g. [11,12]), and fitness differences may therefore only be detectable at a lifetime scale.
In birds, lifetime fitness measures have been compared between dispersers and non-dispersers in 11 species so far to our knowledge (electronic supplementary material, appendix S1). Overall, these studies do not provide a clearer pattern of the cost–benefit balance of natal dispersal compared with studies based on short-term and/or single fitness measures. Because compensations may occur between different fitness components within breeding events (e.g. between young quantity and quality [13]) or between breeding events across an individual's lifetime [9,14], lifetime differences between natal dispersers and non-dispersers may still be difficult to detect. Nevertheless, the absence of clear evidence for natal dispersal costs suggests that although dispersal is classically assumed to be costly, dispersers may on average perform as well as non-dispersers after settlement in many situations. Importantly, this may reflect that correlative studies addressed mostly situations when dispersers can predict local conditions and adjust their investment in breeding and maintenance accordingly, so that the benefits of natal dispersal may balance costs. However, natal dispersers may not be able to buffer the effects of an unpredictable increase in effort or stress if they are lower-phenotypic-quality individuals compared with non-dispersers before the dispersal event [15], have lower familiarity with the local environment after settlement, preventing them to optimize habitat use (e.g. [16,17]), and/or pay a cost after settlement in terms of time, energy or maladaptation to the local conditions (e.g. [18]). Thus, natal dispersal costs after settlement may only be detected when sudden changes in the environment impose increased effort or stress, while predictable environmental conditions may mask possible costs. This may explain why many correlative studies conducted in predictable environments failed to detect natal dispersal costs and why evidences about dispersal costs can be conflicting in the literature.
Therefore, detecting natal dispersal costs could require an experimental manipulation of the environmental conditions after settlement (i.e. after dispersal decisions are made) to impose an unpredictable increase in effort or stress to individuals. Costs revealed only under experimentally deteriorated and/or stressful situations have been described in different contexts [19,20]. Whether natal dispersers can adjust their investment and life-history strategy as efficiently as non-dispersers in situations of experimentally increased effort or stress, and whether individual quality may influence this ability so that only high-quality natal dispersers may be able to buffer such unpredictable increase, remain open questions. If natal dispersers are unable to buffer the negative impact of an increased effort or stress, exploring whether the fitness differences between dispersers and non-dispersers are only transitory or last long after the natal dispersal event may help identifying the process underlying the apparent cost of dispersal (e.g. a temporary lack of familiarity upon arrival in the new environment versus lifelong differences in phenotypic quality or physiological costs of movement and settlement).
Here, we investigated whether lifetime fitness differences according to natal dispersal behaviour could be detected only in situations of experimentally increased effort or stress in a patchy population of collared flycatchers (Ficedula albicollis). We compared the lifetime reproductive success (LRS; i.e. lifetime number of recruits produced) between natal dispersers and non-dispersers first when breeding effort was not manipulated, and then in response to an increased, decreased and unchanged breeding effort as the result of experimental brood size manipulations. We expected that differences in LRS revealing potential natal dispersal costs may be detected only when individuals experience an unexpected increased breeding effort. To explore the origin of potential differences in LRS in such a stressful situation and possible compensations between fitness components, we further compared the annual number of recruits produced and return rate (as a proxy of local survival) between natal dispersers and non-dispersers when breeding effort was both non-manipulated and manipulated. Finally, we tested whether the potential effect of the brood size manipulation on LRS components in natal dispersers and non-dispersers was observed only when the manipulation occurred in the year of natal dispersal or also later. If differences in LRS components between natal dispersers and non-dispersers are mainly due to a temporary lack of familiarity with the new environment, we expect that they disappear 1 year after the natal dispersal event.
2. Material and methods
(a). Study species and study population
The collared flycatcher is a small, short-lived hole-nesting migratory passerine bird. The data used here have been collected between 1980 and 2005 in a patchy population breeding on the island of Gotland, Southern Baltic, Sweden (57°10′N, 18°20′E), where artificial nest-boxes have been provided in discrete woodland plots. Collared flycatchers typically recruit into the breeding population when either 1 or 2 years old [21], and once they have started breeding, most individuals are thought to attempt to reproduce every year until they die. Old birds (2 years old or more) arrive before yearlings at the breeding grounds; they lay earlier and larger clutches, and produce more surviving offspring than yearlings [22].
Each year, nest-boxes have been monitored throughout the season, allowing detailed breeding data to be recorded and nestlings to be ringed. Breeding adults were trapped inside their nest-box, identified and aged (yearling versus older individuals [23]). Most females were caught during incubation and both parents were subsequently caught while feeding young when the brood survived at least until 5–6 days. Therefore, capture is tightly linked to reproductive activity and success, with a sex bias (capture rate being higher in females than in males because of early breeding failure [24]). For more details on the study species and site, see [25,26].
(b). Natal dispersal status
We defined natal dispersal as a change of plot between the year of birth and the year of first breeding (see [25] for more details). This binary definition (dispersal versus philopatry) has been found to be biologically relevant in previous studies on this population [27], as it depended on both individual (e.g. sex, age) and environmental (e.g. local density and reproductive success) factors. Only adults whose natal site was known (i.e. individuals ringed as nestlings) were included in the analyses: previously unringed adult individuals were discarded because (i) they consisted of a mix of local birds previously missed and true immigrants and could therefore not be assigned a dispersal status with certainty, and (ii) except for yearlings, we lack information about their exact age at first breeding. Indeed, because of frequent breeding dispersal [28], unringed individuals cannot be assumed to be 2-year-old first breeders.
(c). Lifetime reproductive success, annual number of recruits and annual return rate
We computed LRS as the total number of recruits produced by an individual during its life [29]. We restricted analyses to individuals caught as breeders at least once (i.e. for which a natal dispersal status could be determined) and with complete life records (i.e. dead at the end of the study period). An individual was assumed to be dead if it had not been seen for at least three consecutive years (i.e. last breeding record in 2002). For the period 1980–2005, we obtained LRS data for 2301 individuals (1143 females and 1158 males). When an individual was not caught, either at age 1 (n = 833) or later (i.e. with a gap in the breeding history; n = 18), it was assumed to be a non- or failed breeder (i.e. no fledged young, thus no recruit).
We also considered separately the annual number of recruits produced and annual return rate to investigate possible compensations between the two main components of LRS (i.e. reproductive success and survival). We used as a proxy of local survival the annual return rate (i.e. whether the individual was caught as a breeder in the study population in subsequent years). Because a fraction of individuals dispersed beyond the limits of the study area [24] and non- or failed breeders are rarely caught, this measure of survival is underestimated, in particular for dispersal-prone individuals [10]. In this population, local survival rate was estimated (using capture–mark–recapture models accounting for imperfect detection probability) to 55–60% [24,27], while return rate was usually around 40%. This clearly calls for caution when interpreting results based on return rates.
Because mating status strongly impacts reproductive success [30] and thus LRS [31] in this species, we discarded from all analyses all individuals involved in polygynous matings from all analyses as estimated based on males' capture in two different nest-boxes (n = 309 individuals, i.e. 13%; see electronic supplementary material, appendix S2). Because males remained unidentified in 30% of nests on average each year, we may have kept some polygynous males and secondary females in our sample. Because (i) natal dispersers and non-dispersers did not differ in the probability to be a polygynous male (n = 1830, 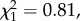 p = 0.368) or a secondary female (n = 1424,
p = 0.368) or a secondary female (n = 1424, 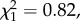 p = 0.366), and (ii) among experimental individuals the brood size manipulation treatment was usually assigned to a nest before the brood status could be known, this was however unlikely to affect our results.
p = 0.366), and (ii) among experimental individuals the brood size manipulation treatment was usually assigned to a nest before the brood status could be known, this was however unlikely to affect our results.
(d). Brood size manipulation
Brood size manipulations have been performed in different years across the long-term study to address in particular the costs of reproduction [21,32]. In all these experiments, pairs or triplets of broods sharing the same hatching date (and in some cases clutches sharing the same laying date) were randomly assigned to one of the three following experimental treatments early during the nestling (resp. incubation) period: (i) they received extra young (resp. eggs), in most cases one or two (increased broods); (ii) they had some young (resp. eggs) removed and placed in another nest, in most cases one or two (decreased broods); or (iii) they had some young (resp. eggs) exchanged with another brood without changing brood (clutch) size (control group).
To standardize the strength of the treatment experienced among individuals, we discarded all individuals that experienced more than one brood size manipulation over their lifetime (n = 95, i.e. 4.1%; see electronic supplementary material, appendix S2, for further details on data selection). According to the treatment an individual experienced during its life, we defined a lifetime brood size manipulation status with individuals having experienced once in their lifetime an increased brood size, a decreased brood size or a control treatment (i.e. three modalities). We used the lifetime brood size manipulation status when comparing LRS between natal dispersers and non-dispersers, and we used the annual brood size manipulation status (i.e. the brood size manipulation treatment at a given breeding attempt) when comparing annual number of recruits and return rate.
(e). Statistical analyses
We first investigated whether LRS, annual number of recruits produced and annual return rate differed between natal dispersers and non-dispersers among individuals whose brood size was not manipulated (n = 1458 remaining individuals). In a second step, we investigated whether natal dispersers and non-dispersers differed in the same fitness measures when reproductive effort was changed as a result of an experimental brood size manipulation, retaining only experimental individuals (n = 683 individuals).
To test whether LRS, annual number of recruits produced and annual return rate differed between natal dispersers and non-dispersers, we used generalized linear mixed models. Because LRS and annual number of recruits show a high number of zero values (i.e. a high number of individuals produced no recruits; electronic supplementary material, appendix S3), we fitted a negative binomial model for these response variables [33], which showed no overdispersion (all ratios of squared Pearson residuals on residual degrees of freedom ranged between 0.92 and 1.10). We used generalized linear mixed models with a binomial error distribution for the analysis of the annual return rate.
For the analyses of LRS, we included in the models as fixed effects the individual's natal dispersal status, as well as sex and age at first breeding (yearling versus older), known to affect LRS in this population [31], and all pairwise interactions with natal dispersal status. To control for possible spatio-temporal effects, we added the plot and year of birth as random factors. In the models analysing the effect of the manipulation of breeding effort, we added the lifetime brood size manipulation status as a fixed effect and the corresponding pairwise interaction with natal dispersal status.
For the analyses of annual number of recruits and annual return rate, we included in the model as fixed effects the individual's natal dispersal status, sex and current age (binary variable), and we added the location of the breeding plot within the study area (plot located on the edge versus in the centre of the study area) to account for a potential edge effect with possible non-random dispersal outside the study area [24,25]. We also included all pairwise interactions with natal dispersal status. To control for spatio-temporal environmental variation in factors affecting reproductive success and/or survival, we included the breeding plot and year as random factors. We also added individual ring number as a random factor to account for multiple observations for a given individual over years (35% of individuals were indeed present at least twice in the dataset). In the models analysing the effect of the manipulation of breeding effort, we added the annual brood size manipulation status as a fixed effect.
To test the influence of the timing of the brood size manipulation (i.e. just after the natal dispersal event or later in life) on the above fitness measures, we focused on the subsample of individuals that bred at least twice (n = 301). We first tested whether any difference in LRS between natal dispersers and non-dispersers in response to brood size manipulation depended on the timing of manipulation by including the three-way interaction between brood size manipulation, natal dispersal status and timing of manipulation (just after the natal dispersal event versus later in life). Second, we compared the annual number of recruits produced during, and return rate following, the first breeding event (i.e. just after the natal dispersal event) and subsequent breeding events between natal dispersers and non-dispersers, for individuals manipulated just after the natal dispersal event, on the one hand, and those manipulated later in life, on the other hand.
When added as a cofactor to the models, the natal dispersal status of the partner was never significant, either alone or in interaction with the dispersal status of the focal individual (results not detailed). This effect is therefore not further considered. We backward-removed non-significant fixed effects starting with interactions. All random factors were kept in all models (see electronic supplementary material, appendix S4 for associated variances). Models were implemented in R v. 3.0.3 [34]. Parameter estimates are presented ±1 s.e.
3. Results
(a). Lifetime reproductive success, annual number of recruits and return rate according to natal dispersal status
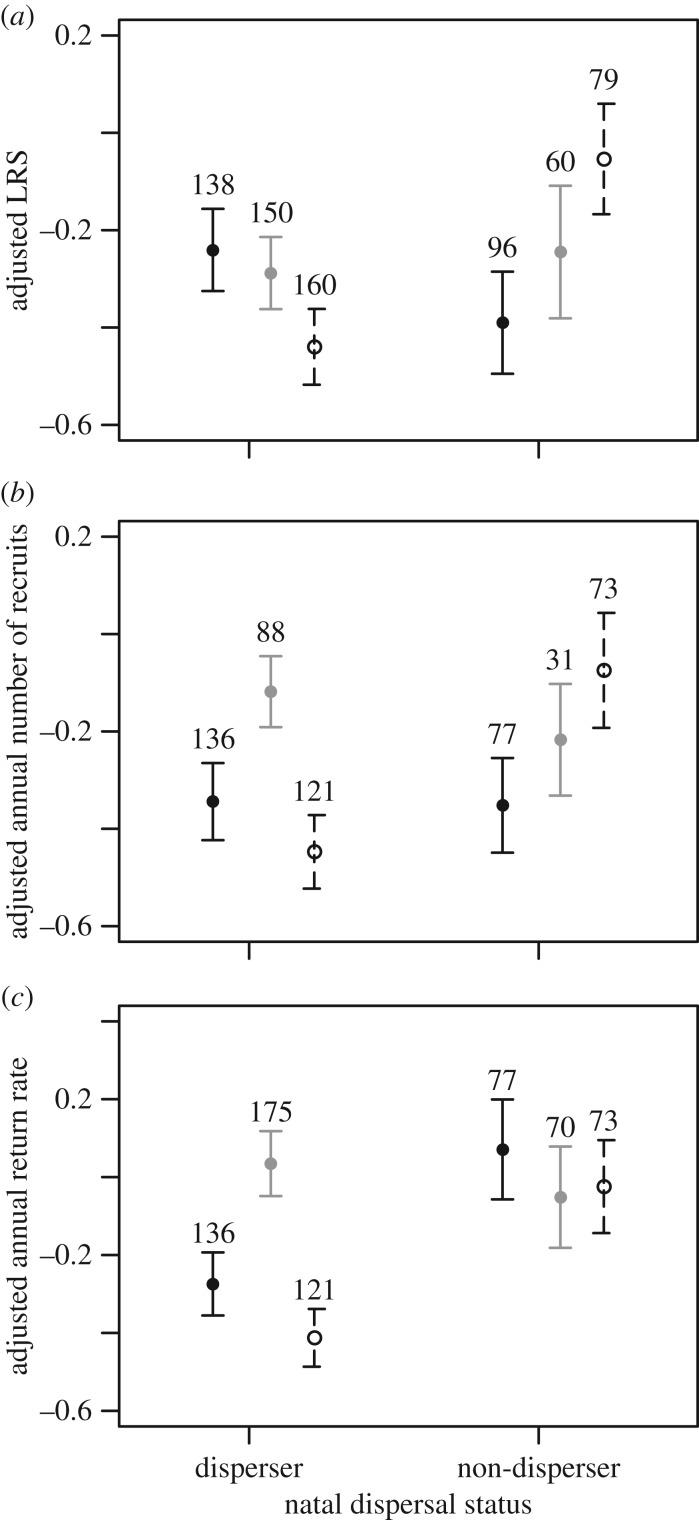
LRS, annual number of recruits produced and return rate did not differ between natal dispersers and non-dispersers when brood size was unmanipulated (table 1a; electronic supplementary material, appendix S4). However, among manipulated birds, the interaction between natal dispersal status and brood size manipulation was significant for all three fitness measures (table 1b; electronic supplementary material, appendix S4). Among birds that experienced a brood size increase, LRS, annual number of recruits and return rate were all lower for natal dispersers when compared with non-dispersers (post hoc tests: LRS: n = 256, 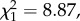 p = 0.003; annual number of recruits: n = 213,
p = 0.003; annual number of recruits: n = 213, 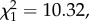 p = 0.001; return rate: n = 213,
p = 0.001; return rate: n = 213, 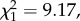 p = 0.002; figure 1). Conversely, no difference was observed among control individuals (post hoc tests: LRS: n = 210,
p = 0.002; figure 1). Conversely, no difference was observed among control individuals (post hoc tests: LRS: n = 210, 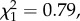 p = 0.376; annual number of recruits: n = 119,
p = 0.376; annual number of recruits: n = 119, 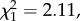 p = 0.146; return rate: n = 119,
p = 0.146; return rate: n = 119, 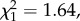 p = 0.201; figure 1), and among individuals with reduced brood size, only annual return rate was lower for natal dispersers compared to non-dispersers (post hoc tests: LRS: n = 217,
p = 0.201; figure 1), and among individuals with reduced brood size, only annual return rate was lower for natal dispersers compared to non-dispersers (post hoc tests: LRS: n = 217, 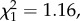 p = 0.280; annual number of recruits: n = 194,
p = 0.280; annual number of recruits: n = 194, 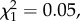 p = 0.830; return rate: n = 194,
p = 0.830; return rate: n = 194, 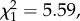 p = 0.018; figure 1).
p = 0.018; figure 1).
Table 1.
Effect of natal dispersal status on LRS, annual number of recruits produced and return rate for (a) individuals whose brood size was not manipulated and (b) experimental individuals (i.e. individuals with increased or decreased brood size and control individuals). For (b), the interaction between natal dispersal status and lifetime brood size manipulation status is shown. χ2 and p-values refer to the LR test. Significant effects are shown in italics.
| fitness measure | variable | χ2 | d.f. | p-values |
|---|---|---|---|---|
| (a) individuals whose brood size was not manipulated | ||||
| LRS | natal dispersal status | 0.95 | 1 | 0.329 |
| annual number of recruits | natal dispersal status | 0.15 | 1 | 0.694 |
| annual return rate | natal dispersal status | 2.01 | 1 | 0.156 |
| (b) experimental individuals | ||||
| LRS | natal dispersal status | 9.97 | 1 | 0.002 |
| brood size manipulation status | 2.45 | 2 | 0.294 | |
| natal dispersal status × brood size manipulation status | 8.67 | 2 | 0.013 | |
| annual number of recruits | natal dispersal status | 15.17 | 1 | <0.001 |
| brood size manipulation status | 22.38 | 2 | <0.001 | |
| natal dispersal status × brood size manipulation status | 12.79 | 2 | 0.002 | |
| annual return rate | natal dispersal status | 8.16 | 1 | 0.004 |
| brood size manipulation status | 16.57 | 2 | <0.001 | |
| natal dispersal status × brood size manipulation status | 9.30 | 2 | 0.009 | |
Figure 1.
(a) LRS, (b) annual number of recruits produced and (c) annual return rate according to natal dispersal status and brood size manipulation. The figure shows mean values (±1 s.e.) adjusted for other significant effects in the model (i.e. residuals from the model without the interaction between natal dispersal status and brood size manipulation). Numbers are sample sizes. Black dots indicate individuals that experienced a brood size decrease; grey dots indicate control individuals (no change in brood size but nestlings/eggs exchanged); white dots indicate individuals that experienced a brood size increase.
(b). Influence of the timing of the brood size manipulation
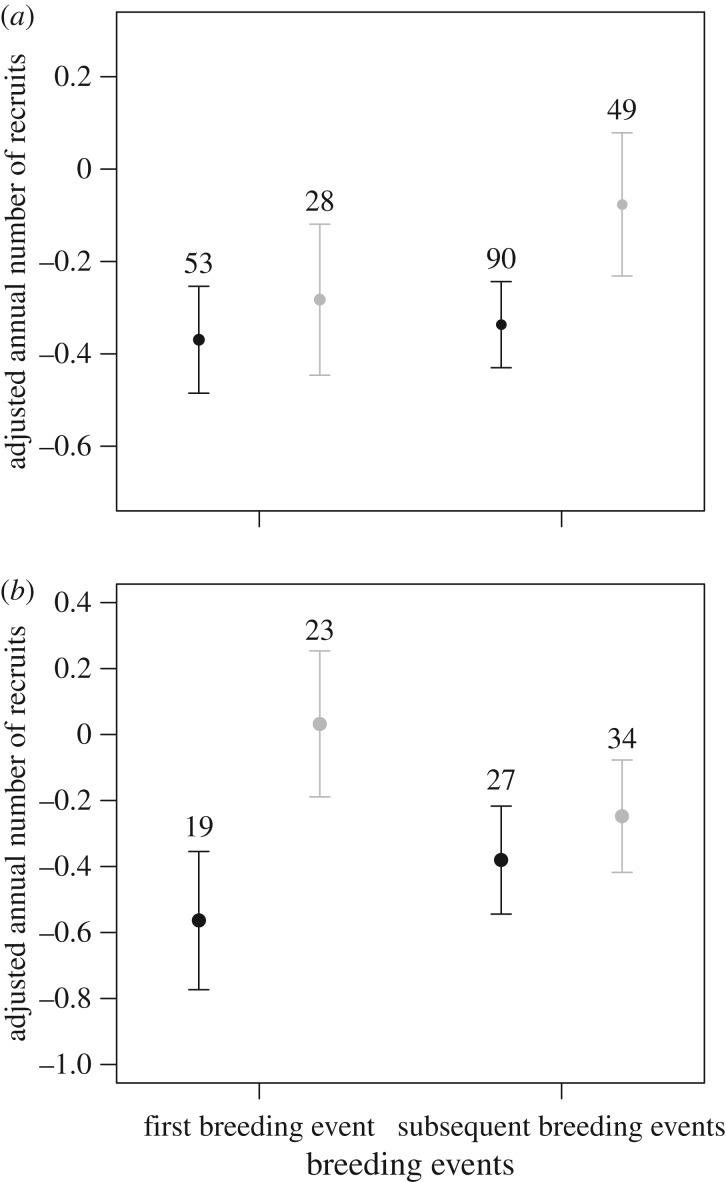
The difference in LRS between natal dispersers and non-dispersers that experienced a brood size increase was not influenced by when the individuals experienced the brood size manipulation (i.e. whether the manipulation occurred just after the natal dispersal event or later in life; three-way interaction between natal dispersal status, brood size manipulation status and timing of manipulation: n = 683, 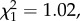 p = 0.600). Thus natal dispersers achieved lower LRS compared with non-dispersers even if the brood size increase occurred with some delay after the natal dispersal event. More specifically, among individuals that experienced a brood size increase 1 year or more after the natal dispersal event, the annual number of recruits produced by natal dispersers did not differ from non-dispersers in the year of the natal dispersal event but it was lower in subsequent years (table 2a and figure 2a; electronic supplementary material, appendix S4). In addition, among individuals that experienced a brood size increase just after the natal dispersal event (i.e. during the first breeding event) and bred again later on, natal dispersers produced less recruits compared with non-dispersers only in the year of the increase, but not in subsequent years (table 2b and figure 2b; electronic supplementary material, appendix S4).
p = 0.600). Thus natal dispersers achieved lower LRS compared with non-dispersers even if the brood size increase occurred with some delay after the natal dispersal event. More specifically, among individuals that experienced a brood size increase 1 year or more after the natal dispersal event, the annual number of recruits produced by natal dispersers did not differ from non-dispersers in the year of the natal dispersal event but it was lower in subsequent years (table 2a and figure 2a; electronic supplementary material, appendix S4). In addition, among individuals that experienced a brood size increase just after the natal dispersal event (i.e. during the first breeding event) and bred again later on, natal dispersers produced less recruits compared with non-dispersers only in the year of the increase, but not in subsequent years (table 2b and figure 2b; electronic supplementary material, appendix S4).
Figure 2.
Annual number of recruits produced by natal dispersers (black dots) and non-dispersers (grey dots) during their first breeding event (i.e. the natal dispersal event) and subsequent breeding events, depending on when the brood size increase occurred: (a) at least 1 year after the first breeding event (the natal dispersal event) or (b) in the year of the first breeding event (the natal dispersal event). The figure shows mean values (±1 s.e.) adjusted for other significant effects in the model (figure 1). Numbers are sample sizes.
Table 2.
Differences between natal dispersers and non-dispersers in the annual number of recruits produced depending on when the brood size manipulation occurred: (a) at least 1 year after the first breeding event (corresponding to the natal dispersal event) or (b) in the year of the first breeding event. The table shows the test of the interaction between natal dispersal status and lifetime brood size manipulation status. In (a), individuals did not differ in the natal dispersal year; in (b) they did not differ in years following the manipulation. Significant effects are shown in italics.
| period | χ2 | d.f. | p-values |
|---|---|---|---|
| (a) individuals manipulated at least 1 year after the natal dispersal event | |||
| first breeding event | 0.75 | 2 | 0.687 |
| subsequent breeding events | 7.96 | 2 | 0.019 |
| (b) individuals manipulated in the year of the first breeding event | |||
| first breeding event | 6.97 | 2 | 0.030 |
| subsequent breeding events | 3.13 | 2 | 0.209 |
We found no difference in annual return rate between natal dispersers and non-dispersers following brood size manipulation among individuals manipulated in the year of the dispersal event (interaction between natal dispersal and brood size manipulation: n = 496, 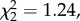 p = 0.537) or later (n = 114,
p = 0.537) or later (n = 114, 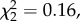 p = 0.920).
p = 0.920).
4. Discussion
We investigated whether LRS and its annual components were linked to natal dispersal behaviour in our patchy population of collared flycatchers. Our results show that, after settlement, natal dispersers and non-dispersers reached the same LRS when breeding effort was not unexpectedly increased. However, when individuals experienced an increased reproductive effort as the result of an experimental brood size increase, natal dispersers reached lower annual number of recruits and annual return rate, and thus ultimately LRS, than non-dispersers. Our results therefore suggest that natal dispersers most often perform as well as non-dispersers after settlement, but natal dispersal can be associated with a reproductive cost under stressful breeding circumstances such as when brood size is increased. Such a cost is expressed when the negative impact of a sudden increase in reproductive effort cannot be buffered against, which may result from a lower phenotypic quality of dispersers and/or reflect a cost of dispersal per se.
(a). Is natal dispersal costly?
Our measures of fitness components are based on the number of recruits locally captured and return rate as a breeder to the study area. Thus, they are at risk of being underestimated in particular for natal dispersers due to non-random dispersal out of the study area by natal dispersers and/or their offspring [35]; yet our results are unlikely to reflect such biases in fitness estimates of natal dispersers, because (i) fitness estimates were similar between brood size manipulation treatments among non-dispersing individuals, (ii) the effect of plot location within the study area on annual recruitment rate did not depend on natal dispersal status, (iii) no difference in return rate was observed depending on plot location (electronic supplementary material, appendix S4), and (iv) results remained unchanged when excluding individuals that were missed for one or more years after the first breeding season and could have temporarily emigrated (see electronic supplementary material, appendix S5). Nevertheless, we cannot exclude here the possibility that natal dispersers experiencing a brood size increase had directly manipulated the dispersal behaviour of their offspring, thereby biasing fitness estimates, in response to the mismatch between expected and realized reproductive success. Further work involving the monitoring of offspring post-fledging movements would be needed to test this hypothesis.
In our study population, as in many others, the capture and identification of parents are biased towards more successful individuals (see [24]), and thus probably higher-quality individuals, which may be more efficient at adjusting breeding decisions when reproductive effort can be predicted. This could reduce our ability to detect fitness differences between natal dispersers and non-dispersers. However, it is likely that, in most situations, natal dispersers are able to reach the same fitness outcome than non-dispersers after settlement, both annually and over a lifetime. Here, natal dispersers achieved lower LRS, annual number of recruits and annual return rate compared with non-dispersers only when facing a considerable and unpredicted increase in reproductive effort, except for the lower annual return rate after a brood size decrease. Because smaller brood sizes were associated with higher dispersal distance [36] and natal dispersers were more likely to disperse again [10,24], the lower return rate of natal dispersers experiencing a brood size decrease compared with non-dispersers may be explained by their higher probability to disperse out of the study area, but this did not translate into lower LRS. Conversely, brood size increase has been shown to strongly increase breeding effort (e.g. [37]). As a consequence, it can result in reduced subsequent parental and offspring fitness [38], as was shown in our study population [21]. Our results thus open the possibility that the costs of reproduction previously reported may be paid only by a fraction of the population, here natal dispersers.
The reduced ability of natal dispersers to cope with an unexpected increase in reproductive effort could result from phenotypic differences between dispersers and non-dispersers that pre-exist in the natal dispersal event. Such phenotypic differences have frequently been reported [7] and are known to affect the balance between the costs and benefits of dispersal [39]. Recent studies have shown that natural selection may favour the associations between dispersal and other phenotypic traits allowing individuals to reduce dispersal costs and thus lead to the evolution of dispersal syndromes (e.g. [2,7]). However, traits favouring settlement success of dispersers may also be traded against future fitness prospects and associated with reduced competitiveness after settlement (e.g. [40]). Moreover, local competition has long been recognized as a main force driving poor-quality, less competitive individuals to disperse [1–3] (e.g. [41,42]). Natal dispersers may then be expected to acquire lower-quality nest sites and/or mates and reach lower mating success and reproductive output compared with non-dispersers, which was indeed previously reported in the collared flycatcher [6]. In our population, natal dispersers may be lower-phenotypic-quality individuals compared with non-dispersers that may be unable to efficiently face the negative effects of increased effort on reproductive output. The observed differences in LRS and its components between natal dispersers and non-dispersers after an increase in breeding effort may therefore reflect phenotype-dependent dispersal rather than a direct dispersal cost. Here, natal dispersers and non-dispersers did not differ in body condition (measured as body mass relative to size, often considered to reflect individual quality [43]), and among experimental individuals, body condition was not related to annual recruitment or return rate, alone or in interaction with natal dispersal status (electronic supplementary material, appendix S6). This suggests that the observed lower LRS of natal dispersers with increased broods compared with non-dispersers did not result from a lower body condition or from a differential cost of natal dispersal depending on the individual's body condition. Nevertheless, the measure of body condition used here may not have captured the relevant phenotypic differences between natal dispersers and non-dispersers. Before we can conclude whether such phenotypic differences can explain the observed differences here, relevant proxies of individual quality may need to be identified.
Alternatively (but non-exclusively), the lower fitness of natal dispersers in situations of an unexpected increase in breeding effort may also result from a cost of the dispersal event per se. In particular, dispersers may suffer from lower familiarity with the breeding environment [16] (thus more limited knowledge about site quality, food resources, predation risk, etc.) compared with non-dispersers [17,44]. In migratory species, young can acquire knowledge about the natal area by remaining near their birth site before the onset of migration [45,46]. Natal dispersers may therefore lack such knowledge about their settlement area. Recently, personality traits such as exploration and boldness have been shown to relate to natal dispersal behaviour [47,48], and they could alleviate dispersal cost by helping individuals to familiarize more quickly with their new breeding environment. Further work would be needed here to assess how and when dispersing young familiarize with their new environment, how individuals' phenotype may modulate this process and how this may affect lifetime fitness by buffering negative impacts of sudden environmental changes.
(b). Is the difference between natal dispersers and non-dispersers long-lasting?
Importantly, the implications of a reduction in fitness for natal dispersers when faced with an increased reproductive effort may strongly differ depending on when the increase occurs: just after the natal dispersal event (short-term) or along the entire lifetime (long-term). Among studies reporting fitness costs of dispersal, these costs are most often the result of reduced fecundity in the breeding season immediately following dispersal (i.e. short-term costs), which can lead to reduced LRS (e.g. [15,49]). Recent studies nevertheless report long-term costs of dispersal (i.e. affecting life stages after the season immediately following dispersal), in particular increased senescence rate (e.g. [11,12]). Here, even if these analyses should be interpreted with caution due to low sample sizes, we found that among the subsample of individuals breeding at least twice and experiencing a brood size manipulation during their first breeding event, natal dispersers produced fewer recruits only in the year of reproductive effort increase and not later in life. This suggests only a short-term cost of an increased reproductive effort in natal dispersers. Nevertheless, this cost was not compensated for at the lifetime scale, either between breeding events or between fitness components, because natal dispersers achieved lower LRS than non-dispersers even when their reproductive effort was increased early only. Furthermore, the lower number of recruits following an increase in reproductive effort was not compensated by a higher quality of recruits, because natal dispersers also achieved a lower number of grand-offspring after an increase in reproductive effort (post hoc test among individuals with increased brood size: natal dispersal effect: n = 256, 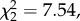 p = 0.023). This suggests that, after the increase in reproductive effort, natal dispersers produced not only fewer recruits but also lower-quality recruits that themselves had lower reproductive success, compared with non-dispersers. Dispersal decisions early in life thus translated into a fitness cost at a lifetime scale that could expand to the next generation when reproductive effort was increased.
p = 0.023). This suggests that, after the increase in reproductive effort, natal dispersers produced not only fewer recruits but also lower-quality recruits that themselves had lower reproductive success, compared with non-dispersers. Dispersal decisions early in life thus translated into a fitness cost at a lifetime scale that could expand to the next generation when reproductive effort was increased.
If the reduction in fitness associated with natal dispersal here was due to a lack of familiarity with the new breeding environment, it could be expected to disappear later in life, once natal dispersers have acquired knowledge about their local environment, unless costs of a higher effort only become apparent later in life (e.g. [50]). Here, among individuals experiencing a brood size increase at least 1 year after the natal dispersal event, natal dispersers produced fewer recruits compared with non-dispersers in the year of the increase, even though most of them (i.e. 107 of 137 natal dispersers; 78%) had not dispersed again as breeders in the year of the increase and therefore had at least one breeding season to get familiar with their new environment. Overall, we showed that the LRS of natal dispersers was lower than non-dispersers following an increase in reproductive effort, had it occurred just after the natal dispersal event or later in life. This lower LRS of natal dispersers is therefore most likely to be explained by phenotypic differences between natal dispersers and non-dispersers rather than by a lack of familiarity with the breeding site.
Identifying the mechanisms underlying the lower LRS observed for natal dispersers following an unexpected increase in reproductive effort deserves deeper investigation. Our results suggest that the lifetime fitness correlates of natal dispersal, possibly linked to differences in individual phenotype in relation to dispersal status, are likely to depend on the spatio-temporal variability of habitat quality, both between and within seasons. This variability may indeed condition the ability of natal dispersers to predict the expected reproductive effort and adjust decisions accordingly after settlement, because of higher sensitivity to stressful situations. If natal dispersers cannot make such adjustments because of unpredictable environmental changes, they are at risk of paying a lifetime fitness cost.
Supplementary Material
Acknowledgements
We thank the many researchers, students and field assistants who have been involved in the long-term monitoring of the collared flycatcher population on Gotland over the years, and the landowners and inhabitants of Gotland for allowing us to work on their properties. We are grateful to L. Crespin and G. Arnqvist for advice and help with statistical analyses and to M. Björklund, L. Cauchard, F. Plard and J. Larroque for comments on previous versions of the manuscript. We also thank A. Chaine and an anonymous referee for their constructive comments on the manuscript.
Ethics
The data upon which this study is based have been obtained following the Swedish guidelines for work on natural populations and under licences and permits from the Swedish Ringing Centre and Swedish National Board for Laboratory Animals, Stockholm.
Data accessibility
Data are available from the Dryad Digital Repository [51].
Authors' contributions
M.G., T.P. and B.D. designed the study and analyses. Data were collected by T.P., L.G. and B.D. with the help of many students and field assistants, and M.G. carried out statistical analyses. The manuscript was written by M.G. and B.D., with editorial input from all authors. All authors gave approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This study has been financially supported by many grants for the long-term study from the Swedish Research Council (VR) and the Swedish Research Council for Environment (FORMAS) (grants to L.G. and T.P.), the CNRS (PICS no. 3054 to B.D.), the French Ministry of Higher Education and Research and Uppsala University (PhD fellowships to M.G.) and the Explora'doc mobility grant from the Région Rhônes-Alpes (to M.G.).
References
- 1.Greenwood PJ, Harvey PH. 1982. The natal and breeding dispersal of birds. Annu. Rev. Ecol. Syst. 13, 1–21. ( 10.1146/annurev.es.13.110182.000245) [DOI] [Google Scholar]
- 2.Clobert J, Baguette M, Benton TG, Bullock JM. 2012. Dispersal ecology and evolution, pp. 1–462. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Clobert J, Danchin E, Dhondt AA, Nichols JD. 2001. Dispersal. Oxford, UK: Oxford University Press. [Google Scholar]
- 4.Bonte D, et al. 2012. Costs of dispersal. Biol. Rev. 87, 290–312. ( 10.1111/j.1469-185X.2011.00201.x) [DOI] [PubMed] [Google Scholar]
- 5.Bensch S, Hasselquist D, Nielsen B, Hansson B. 1998. Higher fitness for philopatric than for immigrant males in a semi-isolated population of great reed warblers. Evolution 52, 877–883. ( 10.2307/2411282) [DOI] [PubMed] [Google Scholar]
- 6.Pärt T. 1994. Male philopatry confers a mating advantage in the migratory collared flycatcher, Ficedula albicollis. Anim. Behav. 48, 401–409. ( 10.1006/anbe.1994.1254) [DOI] [Google Scholar]
- 7.Clobert J, Le Galliard J-F, Cote J, Meylan S, Massot M. 2009. Informed dispersal, heterogeneity in animal dispersal syndromes and the dynamics of spatially structured populations. Ecol. Lett. 12, 197–209. ( 10.1111/j.1461-0248.2008.01267.x) [DOI] [PubMed] [Google Scholar]
- 8.Bowler DE, Benton TG. 2005. Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol. Rev. 80, 205–225. ( 10.1017/s1464793104006645) [DOI] [PubMed] [Google Scholar]
- 9.Belichon S, Clobert J, Massot M. 1996. Are there differences in fitness components between philopatric and dispersing individuals? Acta Oecol. Int. J. Ecol. 17, 503–517. [Google Scholar]
- 10.Doligez B, Pärt T. 2008. Estimating fitness consequences of dispersal: a road to ‘know-where’? Non-random dispersal and the underestimation of dispersers’ fitness. J. Anim. Ecol. 77, 1199–1211. ( 10.1111/j.1365-2656.2008.01446.x) [DOI] [PubMed] [Google Scholar]
- 11.Bouwhuis S, Charmantier A, Verhulst S, Sheldon BC. 2010. Individual variation in rates of senescence: natal origin effects and disposable soma in a wild bird population. J. Anim. Ecol. 79, 1251–1261. ( 10.1111/j.1365-2656.2010.01730.x) [DOI] [PubMed] [Google Scholar]
- 12.Nevoux M, Arlt D, Nicoll M, Jones C, Norris K. 2013. The short- and long-term fitness consequences of natal dispersal in a wild bird population. Ecol. Lett. 16, 438–445. ( 10.1111/ele.12060) [DOI] [PubMed] [Google Scholar]
- 13.Julliard R, Perret P, Blondel J. 1996. Reproductive strategies of philopatric and immigrant blue tits. Acta Oecol. Int. J. Ecol. 17, 487–501. [Google Scholar]
- 14.Lemel JY, Belichon S, Clobert J, Hochberg ME. 1997. The evolution of dispersal in a two-patch system: some consequences of differences between migrants and residents. Evol. Ecol. 11, 613–629. ( 10.1007/s10682-997-1516-z) [DOI] [Google Scholar]
- 15.Hansson B, Bensch S, Hasselquist D. 2004. Lifetime fitness of short- and long-distance dispersing great reed warblers. Evolution 58, 2546–2557. ( 10.1111/j.0014-3820.2004.tb00883.x) [DOI] [PubMed] [Google Scholar]
- 16.Pärt T. 1995. The importance of local familiarity and search costs for age-biased and sex-biased philopatry in the collared flycatcher. Anim. Behav. 49, 1029–1038. ( 10.1006/anbe.1995.0132) [DOI] [Google Scholar]
- 17.Yoder JM, Marschall EA, Swanson DA. 2004. The cost of dispersal: predation as a function of movement and site familiarity in ruffed grouse. Behav. Ecol. 15, 469–476. ( 10.1093/beheco/arh037) [DOI] [Google Scholar]
- 18.Marr AB, Keller LF, Arcese P. 2002. Heterosis and outbreeding depression in descendants of natural immigrants to an inbred population of song sparrows (Melospiza melodia). Evolution 56, 131–142. ( 10.1111/j.0014-3820.2002.tb00855.x) [DOI] [PubMed] [Google Scholar]
- 19.Christe P, Glaizot O, Strepparava N, Devevey G, Fumagalli L. 2012. Twofold cost of reproduction: an increase in parental effort leads to higher malarial parasitaemia and to a decrease in resistance to oxidative stress. Proc. R. Soc. B 279, 1142–1149. ( 10.1098/rspb.2011.1546) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Descamps S, Gilchrist HG, Bety J, Buttler EI, Forbes MR. 2009. Costs of reproduction in a long-lived bird: large clutch size is associated with low survival in the presence of a highly virulent disease. Biol. Lett. 5, 278–281. ( 10.1098/rsbl.2008.0704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gustafsson L, Sutherland WJ. 1988. The costs of reproduction in the collared flycatcher Ficedula albicollis. Nature 335, 813–815. ( 10.1038/335813a0) [DOI] [Google Scholar]
- 22.Pärt T. 1995. Does breeding experience explain increased reproductive success with age? An experiment. Proc. R. Soc. Lond. B 260, 113–117. ( 10.1098/rspb.1995.0067) [DOI] [Google Scholar]
- 23.Svensson L. 1992. Identification guide to European passerines. Stockholm, Sweden: Märstatryck. [Google Scholar]
- 24.Doligez B, Daniel G, Warin P, Pärt T, Gustafsson L, Reale D. 2012. Estimation and comparison of heritability and parent–offspring resemblance in dispersal probability from capture–recapture data using different methods: the Collared Flycatcher as a case study. J. Ornithol. 152, S539–S554. ( 10.1007/s10336-010-0643-4) [DOI] [Google Scholar]
- 25.Doligez B, Gustafsson L, Pärt T. 2009. ‘Heritability’ of dispersal propensity in a patchy population. Proc. R. Soc. B 276, 2829–2836. ( 10.1098/rspb.2009.0454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pärt T. 1990. Natal dispersal in the collared flycatcher—possible causes and reproductive consequences. Ornis Scandinavica 21, 83–88. ( 10.2307/3676802) [DOI] [Google Scholar]
- 27.Doncaster CP, Clobert J, Doligez B, Gustafsson L, Danchin E. 1997. Balanced dispersal between spatially varying local populations: an alternative to the source-sink model. Am. Nat. 150, 425–445. ( 10.1086/286074) [DOI] [PubMed] [Google Scholar]
- 28.Doligez B, Danchin E, Clobert J, Gustafsson L. 1999. The use of conspecific reproductive success for breeding habitat selection in a non-colonial, hole-nesting species, the collared flycatcher. J. Anim. Ecol. 68, 1193–1206. ( 10.1046/j.1365-2656.1999.00362.x) [DOI] [Google Scholar]
- 29.Clutton-Brock TH. 1988. Reproductive success. Chicago, IL: University of Chicago Press. [Google Scholar]
- 30.Qvarnström A, Sheldon BC, Pärt T, Gustafsson L. 2003. Male ornamentation, timing of breeding, and cost of polygyny in the collared flycatcher. Behav. Ecol. 14, 68–73. ( 10.1093/beheco/14.1.68) [DOI] [Google Scholar]
- 31.Gustafsson L. 1989. Collared flycatcher. In Lifetime reproduction in birds (ed. Newton I.), pp. 75–88. London, UK: Academic Press. [Google Scholar]
- 32.Doligez B, Clobert J, Pettifor RA, Rowcliffe M, Gustafsson L, Perrins CM, McCleery RH. 2002. Costs of reproduction: assessing responses to brood size manipulation on life-history and behavioural traits using multi-state capture–recapture models. J. Appl. Stat. 29, 407–423. ( 10.1080/02664760120108845) [DOI] [Google Scholar]
- 33.Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White J-SS. 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135. ( 10.1016/j.tree.2008.10.008) [DOI] [PubMed] [Google Scholar]
- 34.R Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.r-project.org/. [Google Scholar]
- 35.Marr AB. 2006. Immigrants and gene flow in small populations. In Conservation and biology of small populations: the song sparrows of Mandarte Island (ed. JNM Smith), pp. 139–153. Oxford, UK: Oxford University Press. [Google Scholar]
- 36.Pärt T, Gustafsson L. 1989. Breeding dispersal in the collared flycatcher (Ficedula albicollis): possible causes and reproductive consequences. J. Anim. Ecol. 58, 305–320. ( 10.2307/5002) [DOI] [Google Scholar]
- 37.Dijkstra C, Bult A, Bijlsma S, Daan S, Meijer T, Zijlstra M. 1990. Brood size manipulations in the kestrel (Falco tinnunculus)—effects on offspring and parent survival. J. Anim. Ecol. 59, 269–285. ( 10.2307/5172) [DOI] [Google Scholar]
- 38.Horak P. 2003. When to pay the cost of reproduction? A brood size manipulation experiment in great tits (Parus major). Behav. Ecol. Sociobiol. 54, 105–112. ( 10.1007/s00265-003-0608-1) [DOI] [Google Scholar]
- 39.Bonte D, De Roissart A, Wybouw N, Van Leeuwen T. 2014. Fitness maximization by dispersal: evidence from an invasion experiment. Ecology 95, 3104–3111. ( 10.1890/13-2269.1) [DOI] [Google Scholar]
- 40.Duckworth RA, Badyaev AV. 2007. Coupling of dispersal and aggression facilitates the rapid range expansion of a passerine bird. Proc. Natl Acad. Sci. USA 104, 15 017–15 022. ( 10.1073/pnas.0706174104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Altwegg R, Ringsby TH, Saether BE. 2000. Phenotypic correlates and consequences of dispersal in a metapopulation of house sparrows Passer domesticus. J. Anim. Ecol. 69, 762–770. ( 10.1046/j.1365-2656.2000.00431.x) [DOI] [PubMed] [Google Scholar]
- 42.Hanski I, Peltonen A, Kaski L. 1991. Natal dispersal and social-dominance in the common shrew Sorex araneus. Oikos 62, 48–58. ( 10.2307/3545445) [DOI] [Google Scholar]
- 43.Clancey E, Byers JA. 2014. The definition and measurement of individual condition in evolutionary studies. Ethology 120, 845–854. ( 10.1111/eth.12272) [DOI] [Google Scholar]
- 44.Jacquot JJ, Solomon NG. 1997. Effects of site familiarity on movement patterns of male prairie voles Microtus ochrogaster. Am. Midland Nat. 138, 414–417. ( 10.2307/2426834) [DOI] [Google Scholar]
- 45.Rappole JH, Ballard K. 1987. Postbreeding movements of selected species of birds in Athens, Georgia. Wilson Bull. 99, 475–480. [Google Scholar]
- 46.Vega Rivera JH, Rappole JH, McShea WJ, Haas CA. 1998. Wood thrush postfledging movements and habitat use in northern Virginia. Condor 100, 69–78. ( 10.2307/1369898) [DOI] [Google Scholar]
- 47.Dingemanse NJ, Both C, van Noordwijk AJ, Rutten AL, Drent PJ. 2003. Natal dispersal and personalities in great tits (Parus major). Proc. R. Soc. Lond. B 270, 741–747. ( 10.1098/rspb.2002.2300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Korsten P, van Overveld T, Adriaensen F, Matthysen E. 2013. Genetic integration of local dispersal and exploratory behaviour in a wild bird. Nat. Commun. 4, 2362 ( 10.1038/ncomms3362) [DOI] [PubMed] [Google Scholar]
- 49.Gienapp P, Merilae J. 2011. Sex-specific fitness consequences of dispersal in Siberian jays. Behav. Ecol. Sociobiol. 65, 131–140. ( 10.1007/s00265-010-1017-x) [DOI] [Google Scholar]
- 50.Boonekamp JJ, Salomons M, Bouwhuis S, Dijkstra C, Verhulst S. 2014. Reproductive effort accelerates actuarial senescence in wild birds: an experimental study. Ecol. Lett. 17, 599–605. ( 10.1111/ele.12263) [DOI] [PubMed] [Google Scholar]
- 51.Germain M, Pärt T, Gustafsson L, Doligez B. 2017. Data from: Natal dispersers pay a lifetime cost to increased reproductive effort in a wild bird population. Dryad Digital Repository. ( 10.5061/dryad.k514v) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Germain M, Pärt T, Gustafsson L, Doligez B. 2017. Data from: Natal dispersers pay a lifetime cost to increased reproductive effort in a wild bird population. Dryad Digital Repository. ( 10.5061/dryad.k514v) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository [51].