Abstract
The causes and consequences of among-individual variation and covariation in behaviours are of substantial interest to behavioural ecology, but the proximate mechanisms underpinning this (co)variation are still unclear. Previous research suggests metabolic rate as a potential proximate mechanism to explain behavioural covariation. We measured the resting metabolic rate (RMR), boldness and exploration in western stutter-trilling crickets, Gryllus integer, selected differentially for short and fast development over two generations. After applying mixed-effects models to reveal the sign of the covariation, we applied structural equation models to an individual-level covariance matrix to examine whether the RMR generates covariation between the measured behaviours. All traits showed among-individual variation and covariation: RMR and boldness were positively correlated, RMR and exploration were negatively correlated, and boldness and exploration were negatively correlated. However, the RMR was not a causal factor generating covariation between boldness and exploration. Instead, the covariation between all three traits was explained by another, unmeasured mechanism. The selection lines differed from each other in all measured traits and significantly affected the covariance matrix structure between the traits, suggesting that there is a genetic component in the trait integration. Our results emphasize that interpretations made solely from the correlation matrix might be misleading.
Keywords: animal personality, behavioural variation, behavioural syndrome, boldness, exploration, resting metabolic rate
1. Introduction
Among-individual (co)variation in behaviours has been hypothetically explained by a corresponding variation in underlying proximate mechanisms, such as metabolic rate and endocrine profiles [1,2]. According to these hypotheses, a labile behavioural trait covaries with a labile physiological trait, which in turn varies among individuals and thereby leads to among-individual variation in, or covariation between, behaviours.
Although prior research has revealed the above-mentioned covariation patterns in animals [1,2], the proximate mechanisms underpinning behavioural (co)variation still remain unclear [3]. This is because interpreting single pairwise correlations in a multivariate environment is uninformative about what is the most supported structure of the trait associations [4–6]. Structural equation models (SEMs), which estimate the correlation coefficients between two variables when controlling for effects of all other correlations in a multivariate environment, are a powerful tool for comparing which of the hypothesized causal structures underpins the multivariate correlation matrix [4–6]. They thus enable analysis of the potential causal links among traits.
Basal metabolic rate in vertebrates and resting metabolic rate (RMR) in invertebrates are potentially important proximate variables explaining, or covarying with, adaptive individual differences in a range of behaviours, including risky reactions such as boldness or exploration [2,7]. Metabolic rate in its several different forms could explain behavioural variation due to individual-level energetic trade-offs within and between individuals [7–9]. Generally, individuals that are bold or explorative are often predicted to have higher metabolic rates and faster developmental times compared with less bold and less exploratory conspecifics [10–12], leading to a positive correlation between the above-mentioned traits at the individual level due to past correlated selection. At the proximate level, a larger metabolic machinery requires a larger energy input [13], leading to a higher expression of risky behaviours like boldness and exploration which facilitate higher energy input [2,14] but lower survival [15,16]. This results in further divergence in life history traits, like developmental time, to balance the trade-off between survival and the expression of risky behaviours [10]. Nevertheless, empirical evidence for the correlation between metabolic rate and behaviours at the individual level is still mixed [2,7,10,14–18], and the potential proximate mechanisms behind the correlation structure need more research.
The association between RMR and behavioural traits is considered to be underpinned by the same proximate mechanism [7,14], while the metabolic rate itself has also been suggested to be a potential proximate mechanism for behavioural variation or covariation [2,14]. According to the former hypothesis, plastic behavioural traits and RMR might not be directly causally linked; instead, they covary due to the same underlying mechanism [19]. The potential causal patterns behind trait associations cannot, however, be unequivocally drawn from trait correlations [3], even though such correlations have often been applied as ‘empirical support’ for potential causality [2]. This is because traits can also covary with one another through other, unmeasured physiological variables which cause the correlation between traits [4,20]. To test whether RMR potentially causes covariation between behaviours, it is possible to apply SEMs and test which of the alternative trait association structures is best supported by the data [4–6].
Repeatability measures have been suggested as a rough upper proxy for the heritability of traits [21]. Moreover, a recent meta-analysis showed that the among-individual part of the total phenotypic variation in behaviours might have a genetic basis [22], suggesting a potential for the ‘phenotypic gambit’ or ‘Cheverud's conjecture’ on behaviours. This is based on the assumption that individual-level (co)variation in behaviours reflects the patterns of additive genetic variation or covariation [23–25]. Interactions between genetic and environmental effects are likely to be important in the development of individual behavioural differences [26]. However, whether there is a genetic component causing the association between energy metabolism and behavioural variation has not been extensively studied [27]. Importantly, if such covariance structures are underpinned by additive genetic variation, it most probably limits the evolutionary potential for any single trait involved in this trait syndrome [22].
To quantify individual-level relationships between boldness, exploration and RMR, we analysed the hiding behaviour of the western stutter-trilling cricket, Gryllus integer, in familiar and unfamiliar environments and measured their RMR repeatedly. The Gryllus genus has been used earlier as a model to examine the interconnections between behaviours, metabolism and life history traits [17,18,28]. We first ran univariate models for each trait separately in order to study the effects of selection lines for each trait. We then performed the multivariate mixed-effects models in order to study the existence and the direction of among-individual level correlation between behaviours and RMR. Lastly, we applied SEMs for the individual-level correlation matrix in order to test the two competing hypotheses about the potential patterns underpinning the trait covariation: whether RMR acts as a factor explaining behavioural covariation or whether covariation between all three traits is underpinned by another unmeasured mechanism [2,7,14]. To establish whether a genetic component underpins the average expression of measured traits or the covariation structure between measured traits, we used individuals originating from lines selected for fast or slow development. Developmental time is associated with body mass and, indirectly, with boldness, so that faster development to maturity predicts lower body mass and increased boldness [29]. Based on the behavioural syndrome literature [10], we predicted a positive among-individual correlation between boldness and exploration, so that individuals that have short latency times to initiate activity in a familiar environment also have short latency times to resume activity in a novel environment. Empirical evidence from studies on behavioural variation and metabolism shows a remarkable covariation between the traits at the phenotypic level [12,30–34], while there is general agreement that metabolism might be an important predictor of behaviour [10]. As shy behavioural types often have a low RMR compared with bold types [7,14–16,35], we expected a lower RMR in shy, slowly developing and less exploratory individuals compared with bold, rapidly developing and more exploratory ones.
2. Material and methods
(a). Insects and selection lines
We used crickets from a laboratory stock originating from a wild population (Davis, CA, USA). To start the selection lines, a total of 903 juvenile crickets were placed individually in plastic containers (128 × 98 × 73 mm) with a ventilation hole (30 mm diameter) covered with a plastic net. The crickets were provided a shelter made of cardboard. The crickets were maintained in these containers until they reached sexual maturity and were then mated (12 L : 12 D cycle, 27 ± 1°C, food and water ad libitum).
(b). Creation and maintenance of selection lines
The experimental design consisted of three replicate lines within each main selection line (selection for rapid development, slow development and control). In each generation, offspring were produced using paired fertilizations of at least 45 males and 45 females in each nine replicate lines. This produced, on average, 26 families within each main selection line. For rapid and slow development lines, mated males and females were selected according to their maturation age, with only 16.67% of the most rapidly or slowly maturing individuals used. In the control line, matings were randomized over the whole maturation age range [17,18].
All matings were performed in isolated plastic boxes and were always conducted within each replicate line. Crickets from the same family were never mated with each other. To maximize mating success, males were allowed to stay in these boxes for 1 day, after which they were returned to their housing containers. Females remained in the boxes for 7 days to facilitate successful egg-laying. The eggs were left in the mating boxes until hatching. One and a half months after hatching, random samples of surviving offspring were placed into individual containers in a random order. The same procedure was performed in each of the three generations [17,18]. After two generations of selection, the development time (the average maturation time ± s.d.) for rapidly developing individuals was 101.65 ± 13.36 days (n = 350 crickets), 123.49 ± 27.37 days (n = 346) for the control individuals and 131.79 ± 27.24 days (n = 356) for slowly developing crickets. The development time of all lines generally differed significantly (one-way ANOVA, F2,1050 = 154.753, p < 0.001) and all the lines differed significantly between each other (Tukey post hoc tests, all p < 0.001), while replicates did not differ significantly within each line (all p > 0.05).
(c). Behavioural trials
The study was carried out at the Estonian University of Life Sciences. Measurements of boldness, exploration and RMR were carried out twice for each of the F2 generation crickets as soon as the imago reached 4 weeks of age (n = 146 individuals: 75 slowly developing, 45 rapidly developing and 26 control individuals). All insects were weighed using a Kern analytical balance (Kern & Sohn GmbH, Balingen, Germany) (body mass: 0.528 ± 0.082 g, mean ± s.d., n = 146). Mass-specific values of metabolic rate are used in this study. The observers of the insects' behaviour and metabolic responses were blind to all predictions and treatments [36].
(d). Resuming activity in a familiar environment: boldness tests
Behavioural trials were conducted under constant temperature in a computer-controlled room (25 ± 1°C) and sound-proof conditions. We used a dim red light (a 25 W red incandescent bulb) because Gryllus spp. cannot see long (red) wavelengths properly, which allowed us to observe these nocturnal insects without disturbing them. The crickets were provided with drinking water before the onset of the trials, while food was removed 5 h before the beginning of experimental trials. All trials were performed between 06.00 and 24.00 h.
At the beginning of each trial, the focal cricket was captured in its housing box by hand. After handling the insect for 1 min, it was placed back into its cardboard shelter. Both sides of the shelter were closed with a plexiglass cover and the shelter was transferred to the centre of the insect's housing box. After a 30-s acclimation period the Plexiglass cover was removed, and we started observations of the activity of the focal individual [18].
We recorded the latency to resume activity by observing the time when the cricket started to move its body inside its cardboard shelter after disturbance. We termed this behaviour ‘boldness’ [10,15,18]. We defined boldness as the inverse of the latency to resume activity after being handled, so that bold individuals had the shortest latency periods. We repeated this measurement for each individual 4 days later. The latency to resume activity indicates the duration of freezing, i.e. assuming an immobile state, a widespread anti-predator response used by many taxa [35,37].
(e). Resuming activity under unfamiliar environments: exploration tests
Two days after the boldness test, we handled crickets for 1 min and then gave them the opportunity to escape into a conical plastic Eppendorf test tube (volume 5 ml) kept in dark conditions, which was used as an insect chamber during the measurement of the RMR. This Eppendorf tube acted as a novel environment to the crickets and thus resuming activity resembled the initiation of exploration activity [7]. The insect chamber was connected to the respirometer by means of rubber tubing [38]. Upon the escape to the insect chamber, each of the crickets immediately became completely immobile, as if hiding in a burrow. We waited until all crickets resumed active movements, and recorded the time between entering the tube and resuming activity as a measure of exploration.
(f). Measuring resting metabolic rate
The insects remained in their Eppendorf chambers for 4 h (see above), and we recorded their rates of metabolism at 60 min, 120 min, 180 min and 240 min. This was done in order to find out the point when CO2 emissions reach the lowest rates, and whether all cricket lines reach their RMR simultaneously. As soon as the measurements were over, we returned the crickets back to their plastic housing boxes. We repeated the RMR measurements 5 days later.
We followed several important guidelines while measuring cricket respiration [38–40]. The LI-7000 differential CO2/H2O analyser (LiCor, Lincoln, NE, USA) was calibrated by means of calibration gases with gas injection (Linde AG, Höllriegelskreuth, Germany) [15,16]. While measuring CO2 emissions, the insect chamber was perfused with dry CO2-free air, produced by passing air over Drierite (W. A. Hammond Drierite Co. Ltd, Xenia, OH, USA) and soda-lime granules at a flow rate of 60 ml min−1 controlled by an electronic flowmeter (Agilent Technologies, USA). The humidity and temperature of air entering the insect chamber was continuously measured using a Temperature and Humidity Display Instrument for digital HygroClips probes (Rotronic AG, Germany). The respirometric device was combined with an infrared optical system using IR-emitting and IR-sensor diodes [15,16]. IR diodes made it possible to record CO2 production and to follow the movements of each cricket simultaneously. The baseline drift of the analyser was corrected during analysis from the measurements at the beginning and end of each trial with the respirometer chamber empty [15] and every 30 min by carefully closing the insect chamber and opening the bypass tubing for 2 min. The LI-7000 analyser did not show any important baseline drift when switched on for more than 3 days.
(g). Statistical analyses
(i). Univariate models
We used a mixed-effects modelling framework to decompose variation in behaviours and RMR at the within- and among-individual levels [20]. Decomposing variation at these levels enables the analysis of whether individual mean values in a labile trait differ consistently from each other and estimating repeatabilities (i.e. among-individual variance divided by the total phenotypic variance [20]) of the traits of interest to quantify the proportion of the total phenotypic variance explained by the ‘individual’ (used to quantify the presence of animal personality in behavioural ecology). We created a separate univariate model for each of the measured traits with ‘individual identity’ as a random factor. ‘Sex’ (factor: male versus female) and ‘body mass’ (mean centered; covariate) were fitted as fixed effects. To control for effects of the experimental set-up, we included ‘test sequence’ (factor: first versus second trial) and ‘measurement chamber’ (for RMR and activity in a novel environment only: factor; chamber 1 versus chamber 2) as fixed factors. To control for differences between selection lines, we added ‘selection line’ (factor: control as reference category) as a fixed effect. The statistical significance of fixed effects was based on the F-statistics and numerator and denumerator degrees of freedom. The statistical significance of the individual random effect was assessed by comparing the fit of the full model to the model where the individual random effect was removed and using a likelihood ratio test (χ2-test over one degree of freedom). We did not use replicate lines within each selection line in the final models because the preliminary analysis showed no significant differences among replicate lines in any trait.
(ii). Multivariate model
We ran a multivariate model to see if repeatable behaviours and repeatable RMR were associated with each other at the individual level. We used the same fixed-effect structure in the multivariate model as in univariate models, the only difference being the exclusion of ‘test chamber’ from the model, because it was not used when testing activity in a familiar environment. Importantly, ‘selection line’ as a fixed effect in the model provides information on the potential genetics that a trait covariance structure has, i.e. whether selection line significantly explains the trait covariation structure. As we were interested in among-individual covariation of the traits to see if individuals that are consistently more active also have a consistently higher metabolic rate (a priori working hypothesis), we included ‘individual’ as a random factor in the model [20,27]. This enabled us to decompose covariation between the measured traits at the within- and among-individual levels. As the RMR and exploration were recorded in a different sampling event compared to boldness, i.e. the measurements were temporally disparate, we could not estimate within-individual covariation between boldness and any other trait [20]. Therefore the within-individual correlations between boldness and other traits were not estimated. The statistical significance of a trait's covariances (hence, correlations) at each level of variation was calculated by comparing the fit of the full model with the one where the covariance of interest was constrained to zero (using a likelihood ratio test as detailed above).
(iii). Structural equation models
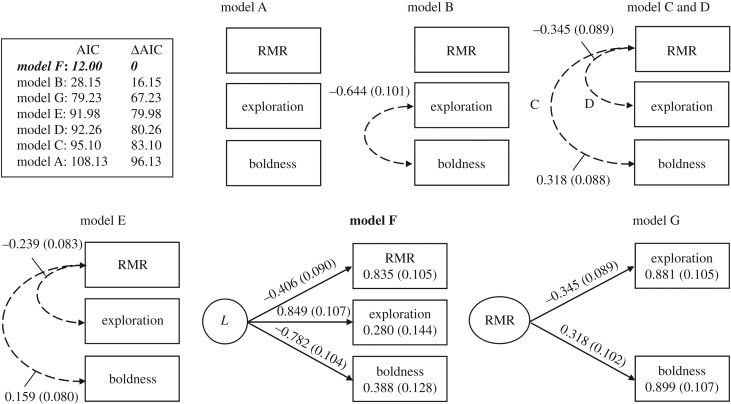
To study which trait association structure was statistically supported, we fitted SEMs to the data at the among-individual level [5,6]. We took the among-individual level covariance matrix forward from the multivariate mixed-effects models (see above) and used that as data for SEMs. We formed seven a priori models and compared the fit of these models using Akaike's information criteria, i.e. AIC. The ΔAIC ≥ 2 between two models is considered to be a statistically significant difference. Model A is a NULL model with traits being independent from each other. In model B, only behaviours are correlated; in model C, RMR correlates only with boldness; in model D, RMR correlates only with exploration; and in model E, RMR correlates with both behaviours. In model F, behaviours are correlated with RMR via a common proximate mechanism (named here as ‘latent variable’). In model G, behavioural correlation is potentially caused by the effects of the RMR on both behaviours.
All traits were analysed using Gaussian error distributions. All traits were log-transformed in order to achieve normality and decrease heteroscedasticity [41], which were both verified by visual inspection. All traits were standardized prior to analysis. The mixed-effects analyses were conducted using the statistical software ASReml 3.0.5. [42], while the SEM analyses were run by using the software R (v. 2.15.3.) [43].
3. Results
(a). Univariate models
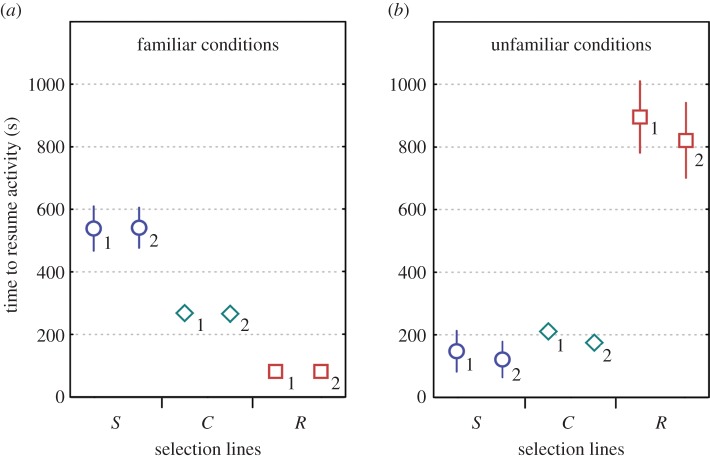
Exploration (activity in a novel environment) (figure 1), boldness (activity in a familiar environment) (figure 1) and RMR (figure 2) expressed significant among-individual variation (repeatabilities; R (s.e.) = 0.858 (0.022), 0.801 (0.030), 0.841 (0.025), respectively) (table 1 and figure 3). The selection line affected the mean expression of the RMR, activity in a novel environment and activity in a familiar environment so that individuals from the slowly maturing selection line had a higher RMR. Slowly developing crickets also resumed activity faster in a novel but more slowly in a familiar environment on average compared with the rapidly maturing selection line (table 1). As the selection line explained the expression of the traits significantly in all univariate models, there might be a genetic component underlying all of the measured traits. Moreover, when individuals were measured for the second time, they resumed activity in a familiar environment more slowly and had higher metabolic rates (table 1). We did not find sex differences in any of the measured traits. Bold, rapidly developing crickets reached the RMR significantly sooner than slowly developing, shy ones (Mann–Whitney test, U = 0, p < 0.0001) (figure 4).
Figure 1.
Average time to resume body movements (±95% CIs) under (a) familiar conditions of cardboard shelter (boldness tests), and (b) under unfamiliar conditions of the insect chamber (exploration tests) in crickets of selected slow development (S, circles, n = 75), rapid development (R, squares, n = 45) and control (C, diamonds, n = 26) lines. Numbers indicate trials. (Online version in colour.)
Figure 2.
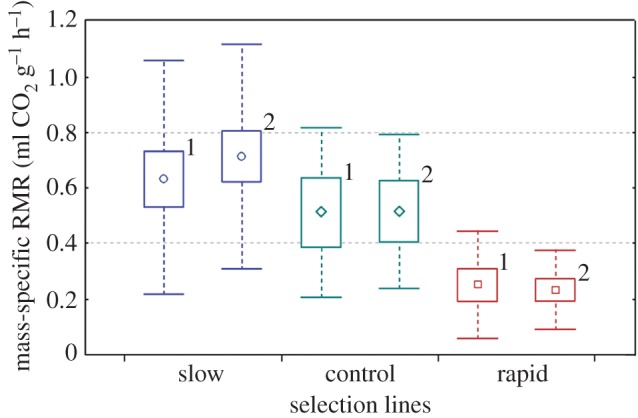
Mass-specific RMR of crickets of selected slow development (circles, n = 75), rapid development (squares, n = 45) and control (diamonds, n = 26) lines. Boxes and whiskers show 95% CIs and s.d., respectively. Numbers indicate trials. (Online version in colour.)
Table 1.
Sources of variation in measured traits; fixed (β) and random (σ2) parameters and repeatabilities (R) are shown with their standard errors, and F-statistics for the fixed- and χ2-values for the random parameters with their respective p-values.
| RMR |
exploration (unfamiliar environment) |
boldness (familiar environment) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| fixed effects | β (s.e.) | F(NUMd.f., DENd.f.) | p-value | β (s.e.) | F(NUMd.f., DENd.f.) | p-value | β (s.e.) | F(NUMd.f., DENd.f.) | p-value |
| intercept | 0.077 (0.173) | 0.001,140.0 | 0.989 | 0.187 (0.129) | 0.011,141.0 | 0.918 | 0.211 (0.147) | 0.001,141.1 | 0.991 |
| sexa | 0.008 (0.138) | 0.001,142.0 | 0.950 | 0.030 (0.103) | 0.081,142.1 | 0.768 | −0.008 (0.117) | 0.001,141.9 | 0.942 |
| sequencea | 0.118 (0.039) | 8.861,144.6 | 0.004 | −0.158 (0.028) | 31.961,145.8 | <0.001 | −0.026 (0.038) | 0.481,145.4 | 0.487 |
| weightb | 1.226 (0.831) | 2.171,160.9 | 0.145 | 0.540 (0.621) | 0.751,163.4 | 0.387 | 0.670 (0.709) | 0.981,156.6 | 0.326 |
| chambera | 0.029 (0.041) | 0.511,145.7 | 0.474 | 0.016 (0.029) | 0.301,145.5 | 0.580 | — | — | — |
| selection linea | 33.852,141.2 | <0.001 | 117.992,141.2 | <0.001 | 76.642,141.2 | <0.001 | |||
| slow | 0.286 (0.185) | −0.818 (0.138) | 0.376 (0.156) | ||||||
| fast | −0.967 (0.201) | 0.941 (0.151) | −1.122(0.171) | ||||||
| random effects | σ2 (s.e.) | 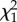 |
p-value | σ2 (s.e.) | 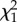 |
p-value | σ2 (s.e.) | 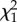 |
p-value |
|---|---|---|---|---|---|---|---|---|---|
| individual | 0.596 (0.078) | 175.69 | <0.001 | 0.339 (0.044) | 190.56 | <0.001 | 0.416 (0.056) | 147.10 | <0.001 |
| residual | 0.113 (0.013) | 0.056 (0.007) | 0.103 (0.012) |
| repeatability | R (s.e.) | 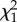 |
p-value | R (s.e.) | 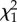 |
p-value | R (s.e.) | 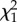 |
p-value |
|---|---|---|---|---|---|---|---|---|---|
| 0.841 (0.025) | 175.69 | <0.001 | 0.858 (0.022) | 190.56 | <0.001 | 0.801 (0.030) | 147.10 | <0.001 |
aCategorical variable: reference category for sex is male; reference category for test sequence is first trial: reference category for selection line is the control line; reference category for the chamber is chamber 1.
bCovariates were centred on their mean value.
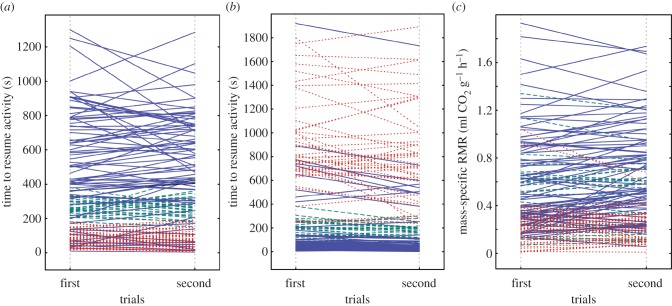
Figure 3.
Time to resume activity in (a) familiar and (b) unfamiliar environments. RMR between first and second trials in individual crickets from different developmental time selection lines is shown in (c) as follows: slow, solid blue lines; rapid, dotted red lines; and control, dashed green lines. The figure is drawn from the raw data. (Online version in colour.)
Figure 4.
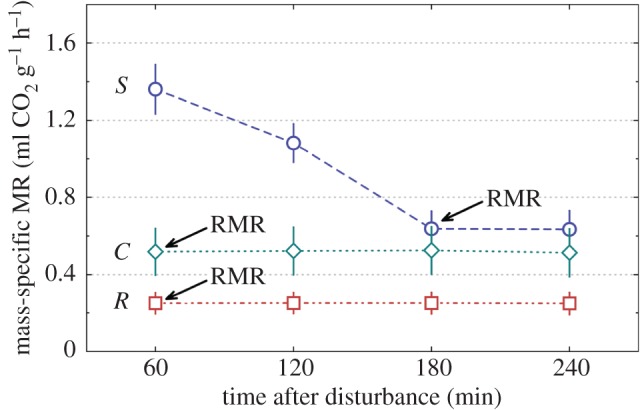
Mass-specific metabolic rate (±95% CIs) of crickets of selected slow development (S, circles, n = 75), rapid development (R, squares, n = 45) and control (C, diamonds, n = 26) lines in periods of 60 min after disturbance. Arrows indicate the time when selected development lines reached their RMR. (Online version in colour.)
(b). Multivariate model
RMR, activity in a novel environment and activity in a familiar environment were all correlated at the among-individual level, which means that there is a behaviour–metabolic rate syndrome between these traits (table 2). The results show that bold individuals (i.e. those with low time values under familiar conditions) are less explorative: they have high time values under unfamiliar conditions. They also have low metabolic rates compared with shy individuals (i.e. those with high time values under familiar conditions) who are more explorative (low time values under unfamiliar conditions) and have high metabolic rates. The best fitting SEM suggests that all measured traits are associated with each other via a common, unmeasured (latent) proximate mechanism (model F, figure 5). The model in which the RMR underpins behavioural covariation was not statistically supported (model G, figure 5). This means that RMR, boldness and exploration are all structurally associated, forming a behaviour–physiology syndrome at the among-individual level. Within-individual correlation between RMR and exploration was non-significant, suggesting an absence of correlated plasticity.
Table 2.
Covariation between the measured traits at within- and among-individual levels: correlations (r) are shown with their standard errors, and χ2-values for the random parameters with their respective p-values.
| correlation | r (s.e.) | 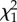 |
p-value |
|---|---|---|---|
| exploration–boldness | |||
| between-individual | −0.664 (0.057) | 63.31 | <0.001 |
| exploration–RMR | |||
| between-individual | −0.345 (0.082) | 14.89 | <0.001 |
| within-individual | −0.079 (0.083) | 0.90 | 0.343 |
| boldness–RMR | |||
| between-individual | 0.318 (0.085) | 12.12 | <0.001 |
Figure 5.
Structural equation models built based on a priori assumptions on how traits might be related to each other. The models were compared with each other using AIC values of each model. The model set is built from the individual-level covariance matrix acquired from the multivariate mixed-effects model. A small AIC value indicates a better fit of the model compared with a large AIC value. Numbers next to the arrows are standardized factor loadings with standard errors (in brackets) for the effects of the underlying syndrome structure on a particular behaviour. Numbers inside the variable boxes are the variance unexplained by the latent variable (L) with standard errors (in brackets) (models F and G).
In the multivariate mixed-effects model, the selection line significantly explained variation, suggesting that a genetic component might partly underpin the trait covariance structure (F6, 184.2 = 43.84, p < 0.001).
4. Discussion
Our analysis of the RMR, boldness and exploration in western stutter-trilling crickets showed that there are among-individual correlations between the measured behaviours and RMR. The correlations, however, were not always in the hypothesized direction. Importantly, our analysis shows that among-individual correlations between RMR and behaviours are caused by an unmeasured proximate mechanism rather than the RMR driving the correlation between the measured behaviours. Our results add to the growing evidence suggesting that the correlation structure among traits associated with a behavioural syndrome may substantially vary depending on the population under investigation [6,44–46], often differing from standard assumptions [10]. Our results are also generally in line with the hypothesis that variation in metabolic rate is associated with variation in behaviours [2,7,14], adding some detail to these theories by taking into account the intrinsic nature of this association.
Two competing energy allocation models have been proposed to predict the direction of the relationships between metabolic rate and behaviour, i.e. whether these traits are positively or negatively associated with each other [2,7,9,14,47]. According to the performance model, a high metabolic rate requires the increased expression of costly behaviours such as activity, boldness or exploration in order to match high energy demands with high energy supply, leading to positive association between RMR and activity. In the compensation model, an animal allocates a fixed amount of energy across competing processes such as RMR or activity: activity and RMR are thus negatively linked because animals with a higher RMR have less energy to expend in activity [14,47]. Our results do not clearly support either model. As measured here, bold crickets were less exploratory and had a lower RMR than shy, more explorative crickets. Therefore, the conservative hypothesis about the sign (i.e. positive or negative) of the relationship between RMR and the most studied and ecologically relevant behaviours such as exploration, boldness and general activity may not be universally valid. Instead, our results suggest that this relationship depends on the behaviour under examination, such as those demonstrated here.
Phenotypic correlations generally follow the direction and magnitude of genetic correlations [24,27,48]. High among-individual variation in behavioural traits might therefore indicate high underpinning additive genetic variation, which explains a part of the among-individual variation in behaviours [22]. According to our results, an artificial selection regime substantially explained trait covariations and the behavioural expression at the average level, which suggests, at least to some extent, that there is a genetic component behind the expression of measured traits and their associations. Even though trait correlations, as discussed above, are informative about the direction of trait association, they cannot fully predict the causal patterns underpinning the trait associations.
There are two main general hypotheses about the causality behind the correlation between metabolic rate and behaviours. Firstly, RMR could correlate with behaviours because they share a common proximate mechanism [7,14]. Another view suggests that variation in the metabolic rate might be a proximate mechanism for the expression of behavioural (co)variation [14,19]. These causal hypotheses relate to the two energy allocation models (discussed above) so that, in the compensation model, causality is not necessarily present, while, in the performance model, the RMR can act as a causal factor underlying behavioural expression. Our results support the former hypothesis, because the RMR does not generate correlation between boldness and exploration. Instead, the association between all three traits is underpinned by a shared proximate mechanism.
A shared hormonal mechanism is one of the potential proximate explanations for the integration between metabolic rates and behaviours [7,14], as hormones have been found to explain variation in behaviours and metabolic rates independently in correlational studies. For example, circulating hormone levels have been shown to explain among-individual variation in exploration in rats [49] and variation in metabolic rates in birds [50] so that high hormone levels explain high exploration and high metabolic rates within these studies (see [14] for opposite patterns).
Our results are partly contradictory with the results of a recent study conducted with another cricket species showing that metabolic rate and behaviours are not causally related nor integrated due to a shared proximate mechanism, but rather show modularity so that behavioural traits covary separately from metabolic rate and body mass [8]. Contrary to our findings in a cricket, a recently published meta-analysis suggested that metabolic rate acts as a proximate mechanism for expressed individual variation in behavioural traits in birds [51]. This meta-analysis promotes research on proximate mechanisms explaining trait variation and covariation, despite being based on indirect evidence and a bold assumption that variance in any trait must predict variation in all traits that have lower or equal variance compared with a focal trait [51]. All in all, SEMs combined with multivariate mixed-effects models provide a powerful tool to study the potential alternative association patterns between traits [4,20] and to reveal ‘hidden’ biological patterns behind the correlation matrixes. Revealing the potential proximate mechanisms behind trait covariation patterns will help those studying covariation at different levels to take a step forward and make the biological interpretations of the data in a less biased and speculative manner.
5. Conclusion
This study revealed (i) substantial among-individual variation in exploration, boldness and energy metabolism, as well as (ii) covariations among these traits, underpinned by a common, unmeasured proximate mechanism other than the RMR. The results suggest that relationships between the RMR and the behaviour of an organism might be explained by the performance and the allocation model, depending on the type of behaviour being studied. Furthermore, these relationships may be underpinned by additive genetic variation. Our results encourage more research in quantitative genetics of metabolic rate–behavioural syndromes in order to examine the adaptive role(s) of similar trait correlations. Finally, we suggest that caution should be exercised when making interpretations about the potential causal patterns between traits if they are based solely on simple (among-individual) correlation matrixes.
Acknowledgements
We thank Sanni Hietaketo, Yi-Te Lai, Leena Koponen, Leena Pääkkönen, Julia Keronen, Sirpa Kaunisto, Anssi Vainikka, Inese Kivleniece, Jolanta Vrublevska, Sanita Kecko and Ljubova Sivacova for assistance, and Todd M. Freeberg and Sue Bertram for comments on the manuscript.
Data accessibility
The dataset is available upon request from the corresponding author and from the Dryad Data Repository at: http://dx.doi.org/10.5061/dryad.1cb8j [52].
Authors' contributions
I.A.K., Ra.K., M.J.R. and T.K. conceived the study. I.A.K., A.K., Ra.K., T.K., G.M.B., R.K., M.M., M.J.R., J.K., S.L. and I.S. contributed to the design of the study and/or carried out the experiments. I.A.K., P.T.N. and G.T. analysed the data. I.A.K., P.T.N. and S.L. wrote the manuscript. All authors participated in interpreting the results and they edited/approved the final manuscript.
Competing interests
The authors declare that they have no competing financial interests.
Funding
The study was supported by the Academy of Finland, the Latvian Council of Science (grant no. 290/2012), the Estonian Ministry of Education and Science (grant nos. IUT34-8, IUT36-2 and PUT1223), the Estonian Science Foundation (grant no. 9450) and the Fulbright Program (the US Department of State).
References
- 1.Koolhaas JM, De Boer SF, Coppens CM, Buwalda B. 2010. Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Front. Neuroend. 31, 307–321. ( 10.1016/j.yfrne.2010.04.001) [DOI] [PubMed] [Google Scholar]
- 2.Biro PA, Stamps JA. 2010. Do consistent individual differences in metabolic rate promote consistent individual differences in behavior? Trends Ecol. Evol. 25, 653–659. ( 10.1016/j.tree.2010.08.003) [DOI] [PubMed] [Google Scholar]
- 3.Aldrich J. 1995. Correlations genuine and spurious in Pearson and Yule. Stat. Sci. 10, 364–376. [Google Scholar]
- 4.Shipley B. 2000. Cause and correlation in biology: a user’s guide to path analysis, structural equations, and causal inference Cambridge, UK: Cambridge University Press. [Google Scholar]
- 5.Dochtermann NA, Jenkins SH. 2007. Behavioural syndromes in Merriam's kangaroo rats (Dipodomys merriami): a test of competing hypotheses. Proc. R. Soc. B 274, 2343–2349. ( 10.1098/rspb.2007.0622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dingemanse NJ, Dochterman N, Wright J. 2010. A method for exploring the structure of behavioural syndromes to allow formal comparison within and between data sets. Anim. Behav. 79, 439–450. ( 10.1016/j.anbehav.2009.11.024) [DOI] [Google Scholar]
- 7.Careau V, Thomas D, Humphries MM, Réale D. 2008. Energy metabolism and animal personality. Oikos 117, 641–653. ( 10.1111/j.0030-1299.2008.16513.x) [DOI] [Google Scholar]
- 8.Royaute R, Greenlee K, Baldwin M, Dochtermann NA. 2015. Behavior, metabolism, and size: phenotypic modularity or integration in Acheta domesticus? Anim. Behav. 110, 163–169. ( 10.1016/j.anbehav.2015.09.027) [DOI] [Google Scholar]
- 9.Mathot KJ, Dingemanse NJ. 2015. Energetics and behavior: unrequited needs and new directions. Trends Ecol. Evol. 30, 199–206. ( 10.1016/j.tree.2015.01.010) [DOI] [PubMed] [Google Scholar]
- 10.Réale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio PO. 2010. Personality and the emergence of the pace-of-life syndrome concept at the population level. Phil. Trans. R. Soc. B 365, 4051–4063. ( 10.1098/rstb.2010.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Careau V, Thomas D, Pelletier F, Turki L, Landry F, Garant D, Réale D. 2011. Genetic correlation between resting metabolic rate and exploratory behaviour in deer mice (Peromyscus maniculatus). J. Evol. Biol. 24, 2153–2163. ( 10.1111/j.1420-9101.2011.02344.x) [DOI] [PubMed] [Google Scholar]
- 12.Mathot KJ, Nicolaus M, Araya-Ajoy YG, Dingemanse NJ, Kempenaers B. 2015. Does metabolic rate predict risk-taking behaviour? A field experiment in a wild passerine bird. Funct. Ecol. 29, 239–249. ( 10.1111/1365-2435.12318) [DOI] [Google Scholar]
- 13.Hammond KA, Diamond J. 1997. Maximal sustained energy budgets in humans and animals. Nature 386, 457–462. ( 10.1038/386457a0) [DOI] [PubMed] [Google Scholar]
- 14.Careau V, Garland T Jr. 2012. Performance, personality, and energetics: correlation, causation, and mechanism. Physiol. Biochem. Zool. 85, 543–571. ( 10.1086/666970) [DOI] [PubMed] [Google Scholar]
- 15.Krams I, Kivleniece I, Kuusik A, Krama T, Freeberg TM, Mand R, Vrublevska J, Rantala MJ, Mand M. 2013. Predation selects for low resting metabolic rate and consistent individual differences in anti-predator behavior in a beetle. Acta Ethol. 16, 163–172. ( 10.1007/s10211-013-0147-3) [DOI] [Google Scholar]
- 16.Krams I, Kivleniece I, Kuusik A, Krama T, Mand R, Rantala MJ, Znotina S, Freeberg TM, Mand M. 2013. Predation promotes survival of beetles with lower resting metabolic rates. Entomol. Exp. Appl. 148, 94–103. ( 10.1111/eea.12079) [DOI] [Google Scholar]
- 17.Niemelä PT, Vainikka A, Lahdenperä S, Kortet R. 2012. Nymphal density, behavioral development, and life history in a field cricket. Behav. Ecol. Sociobiol. 66, 645–652. ( 10.1007/s00265-011-1312-1) [DOI] [Google Scholar]
- 18.Niemelä PT, Vainikka A, Hedrick AV, Kortet R. 2012. Integrating behaviour with life history: boldness of the field cricket, Gryllus integer, during ontogeny. Funct. Ecol. 26, 450–456. ( 10.1111/j.1365-2435.2011.01939.x) [DOI] [Google Scholar]
- 19.Biro PA, Stamps JA. 2008. Are animal personality traits linked to life-history productivity? Trends Ecol. Evol. 23, 361–368. ( 10.1016/j.tree.2008.04.003) [DOI] [PubMed] [Google Scholar]
- 20.Dingemanse NJ, Dochtermann NA. 2013. Quantifying individual variation in behaviour: mixed-effect modeling approaches. J. Anim. Ecol. 82, 39–54. ( 10.1111/1365-2656.12013) [DOI] [PubMed] [Google Scholar]
- 21.Boake CRB. 1989. Repeatability: its role in evolutionary studies of mating behavior. Evol. Ecol. 3, 173–182. ( 10.1007/BF02270919) [DOI] [Google Scholar]
- 22.Dochtermann NA, Schwab T, Sih A. 2015. The contribution of additive genetic variation to personality variation: heritability of personality. Proc. R. Soc. B 282, e20142201 ( 10.1098/rspb.2014.2201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheverud JM. 1988. A comparison of genetic and phenotypic correlations. Evolution 42, 958–968. ( 10.2307/2408911) [DOI] [PubMed] [Google Scholar]
- 24.Dochtermann NA. 2011. Testing Cheverud's conjecture for behavioral correlations and behavioral syndromes. Evolution 65, 1814–1820. ( 10.1111/j.1558-5646.2011.01264.x) [DOI] [PubMed] [Google Scholar]
- 25.Brommer JE, Kluen E. 2012. Exploring the genetics of nestling personality traits in a wild passerine bird: testing the phenotypic gambit. Ecol. Evol. 2, 3032–3044. ( 10.1002/ece3.412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dingemanse NJ, Dochtermann NA. 2014. Individual behaviour: behavioural ecology meets quantitative genetics. In Quantitative genetics in the wild (eds Charmantier A, Garant D, Kruuk LEB), pp. 54–67. Oxford, UK: Oxford University Press. [Google Scholar]
- 27.Dingemanse NJ, Baerber I, Wright J, Brommer JE. 2012. Quantitative genetics of behavioural reaction norms: genetic correlations between personality and behavioural plasticity vary across stickleback populations. J. Evol. Biol. 25, 485–496. ( 10.1111/j.1420-9101.2011.02439.x) [DOI] [PubMed] [Google Scholar]
- 28.Kralj-Fiser S, Schuett W. 2014. Studying personality variation in invertebrates: why bother? Anim. Behav. 91, 41–52. ( 10.1016/j.anbehav.2014.02.016) [DOI] [Google Scholar]
- 29.Niemelä PT, Dingemanse NJ, Alioravainen N, Vainikka A, Kortet R. 2013. Personality pace-of-life hypothesis: testing genetic associations among personality and life history. Behav. Ecol. 24, 935–941. ( 10.1093/beheco/art014) [DOI] [Google Scholar]
- 30.Timonin ME, Carrière CJ, Dudych AD, Latimer JGW, Unruh ST, Willis CKR. 2011. Individual differences in the behavioural responses of meadow voles to an unfamiliar environment are not correlated with variation in resting metabolic rate. J. Zool. 284, 198–205. ( 10.1111/j.1469-7998.2011.00792.x) [DOI] [Google Scholar]
- 31.Killen SS, Marras S, Ryan MR, Domenici P, McKenzie DJ. 2012. A relationship between metabolic rate and risk-taking behaviour is revealed during hypoxia in juvenile European sea bass. Funct. Ecol. 26, 134–143. ( 10.1111/j.1365-2435.2011.01920.x) [DOI] [Google Scholar]
- 32.Le Galliard JF, Paquet M, Cisel M, Montes-Poloni L. 2013. Personality and the pace-of-life syndrome: variation and selection on exploration, metabolism and locomotor performances. Funct. Ecol. 27, 136–144. ( 10.1111/1365-2435.12017) [DOI] [Google Scholar]
- 33.Gifford ME, Clay TA, Careau V. 2014. Individual (co)variation in standard metabolic rate, feeding rate, and exploratory behavior in wild-caught semiaquatic salamanders. Physiol. Biochem. Zool. 87, 384–396. ( 10.1086/675974) [DOI] [PubMed] [Google Scholar]
- 34.Šíchová K, Koskela E, Mappes T, Lantová P, Boratynński Z. 2014. On personality, energy metabolism and mtDNA introgression in bank voles. Anim. Behav. 92, 229–237. ( 10.1016/j.anbehav.2014.04.011) [DOI] [Google Scholar]
- 35.Krams I, Kivleniece I, Kuusik A, Krama T, Freeberg TM, Mand R, Sivacova L, Rantala MJ, Mand M. 2014. High repeatability of anti-predator responses and resting metabolic rate in a beetle. J. Insect Behav. 27, 57–66. ( 10.1007/s10905-013-9408-2) [DOI] [Google Scholar]
- 36.Burghardt GM, Bartmess-LeVasseur JN, Browning SA, Morrison KE, Stec CL, Zachau CE, Freeberg TM. 2012. Perspectives—minimizing observer bias in behavioral studies: a review and recommendations. Ethology 118, 511–517. ( 10.1111/j.1439-0310.2012.02040.x) [DOI] [Google Scholar]
- 37.Chelini MC, Willemart RH, Hebets EA. 2009. Costs and benefits of freezing behavior in the harvestman Eumesosoma roeweri (Arachnida, Opiliones). Behav. Process. 82, 153–159. ( 10.1016/j.beproc.2009.06.001) [DOI] [PubMed] [Google Scholar]
- 38.Lighton JRB. 2008. Measuring metabolic rate: a manual for scientists. Oxford, UK: Oxford University Press. [Google Scholar]
- 39.Lighton JRB, Halsey LG. 2011. Flow-through respirometry applied to chamber systems: pros and cons, hints and tips. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 158, 265–275. ( 10.1016/j.cbpa.2010.11.026) [DOI] [PubMed] [Google Scholar]
- 40.Lighton JRB. 2015. Metabolic measurement techniques: baselining, mathematical correction of water vapour dilution and response correction. In Indirect calorimetry: techniques, computations and applications (eds Gerrits W, Labussière E), pp. 57–72. Wageningen, The Netherlands: Wageningen Academic Publishers. [Google Scholar]
- 41.Cleasby IR, Nakagawa S. 2011. Neglected biological patterns in the residuals. Behav. Ecol. Sociobiol. 65, 2361–2372. ( 10.1007/s00265-011-1254-7) [DOI] [Google Scholar]
- 42.Gilmour AR, Gogel BJ, Cullis BR, Thompson R. 2009. ASReml user guide release 3.0. Hemel Hempstead, UK: VSN International Ltd, See www.vsni.co.uk.
- 43.R Core Team. 2016. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 44.Burghardt GM, Schwartz JM. 1999. Geographic variations on methodological themes in comparative ethology: a natricine snake perspective. In Geographic variation in behavior: perspectives on evolutionary mechanisms (eds Foster SA, Endler JA), pp. 69–94. Oxford, UK: Oxford University Press. [Google Scholar]
- 45.Bell AM. 2007. Future directions in behavioural syndromes research. Proc. R. Soc. B 274, 755–761. ( 10.1098/rspb.2006.0199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dingemanse NJ, Wright J, Kazem AJN, Thomas DK, Hickling R, Dawnay N. 2007. Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J. Anim. Ecol. 76, 1128–1138. ( 10.1111/j.1365-2656.2007.01284.x) [DOI] [PubMed] [Google Scholar]
- 47.Speakman JR. 1997. Doubly labelled water: theory and practice. London, UK: Chapman & Hall. [Google Scholar]
- 48.Dochtermann NA, Roff DA. 2010. Applying a quantitative genetics framework to behavioural syndrome research. Phil. Trans. R. Soc. B 365, 4013–4020. ( 10.1098/rstb.2010.0129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Helmreich DL, Tylee D. 2011. Thyroid hormone regulation by stress and behavioral differences in adult male rats. Horm. Behav. 60, 284–291. ( 10.1016/j.yhbeh.2011.06.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chastel O, Lacroix A, Kersten M. 2003. Pre-breeding energy requirements: thyroid hormone, metabolism and the timing of reproduction in house sparrows Passer domesticus. J. Avian. Biol. 34, 298–306. ( 10.1034/j.1600-048X.2003.02528.x) [DOI] [Google Scholar]
- 51.Holtmann B, Lagisz M, Nakagawa S. 2017. Metabolic rates, and not hormone levels, are a likely mediator of between-individual differences in behaviour: a meta-analysis. Funct. Ecol. 31, 685–696. ( 10.1111/1365-2435.12779) [DOI] [Google Scholar]
- 52.Krams IA, et al. 2017. Data from: Metabolic rate associates with, but does not generate covariation between, behaviours in western stutter-trilling crickets, Gryllus integer. Dryad Digital Repository. ( 10.5061/dryad.1cb8j) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Krams IA, et al. 2017. Data from: Metabolic rate associates with, but does not generate covariation between, behaviours in western stutter-trilling crickets, Gryllus integer. Dryad Digital Repository. ( 10.5061/dryad.1cb8j) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The dataset is available upon request from the corresponding author and from the Dryad Data Repository at: http://dx.doi.org/10.5061/dryad.1cb8j [52].