Abstract
Climate warming can destabilize interactions between competitors as smaller organisms gain advantages in warmer environments. Whether and how warming-induced effects on competitive interactions are modified by predation remains unknown. We hypothesized that predation will offset the competitive advantage of smaller prey species in warmer environments because of their greater vulnerability to predation. To test this, we assembled a litter arthropod community with two Collembola species (Folsomia candida and Proisotoma minuta) of different body sizes across a temperature gradient (three thermal environments) and in the presence and absence of predatory mites. Predatory mites reduced Collembola coexistence with increasing temperatures. Contradicting our hypothesis, the larger prey species always outperformed the smaller prey species in warmer environments with predators. Larger prey probably benefited as they expressed a greater trait (body length) plasticity to warming. Warming can thus magnify predation effects and reduce the probability of prey coexistence.
Keywords: interspecific competition, body size, species coexistence, trophic interactions, trait plasticity, top-down control
1. Introduction
Temperature is one of the key abiotic forces regulating community dynamics and structure across ecosystems [1–3]. Increasing temperature owing to on-going climate change can potentially alter community dynamics by changing species interactions, such as competition and predation [4–8]. Given that communities are primarily structured by the interactive effects of competition (within trophic groups) and predation (across trophic groups) [9,10], temperature-induced changes in these interactions can have major implications for the organization of biological communities in a warmer environment.
The signature of predation effects on prey competition was emphasized by Paine [11], who showed that predator removal increased local extinction of prey species. Since then, numerous experimental studies have found that the presence of predators, particularly that of generalists, tends to decrease interspecific competition and increase diversity of prey species [12]. This was mainly attributed to predator-induced regulation of dominant prey species—the process known as keystone predation [13,14]. However, some theoretical and empirical works have provided evidence that predators may conversely increase interspecific competition among their prey species [15,16]. Such an effect is argued to emerge from predation of prey species that are relatively inefficient in resource consumption and are rare within communities [15]. Moreover, in a review of the interactive effects between competition and predation, Chase et al. [17] highlighted that predator effects on prey diversity can be positive, neutral or negative, depending on the effects of predators on stabilizing mechanisms within and among prey species (e.g. intra- versus interspecific competition).
Warming effects on competition and predation can be directly linked to how species change their vital rates at elevated temperature [18]. Ectothermic species in particular have greater metabolic demands in warmer environments, which may pose serious constraints on their ability to compete and forage [19–21]. A key response of ectothermic species in a warmer environment is to thus reduce their body size to lessen their metabolic demands and thereby optimize their fitness [22–24]. Cell size and numbers decline in ectotherms as a general physiological response to warming, which eventually reduces their body size and has been shown true for a large group of ectothermic taxa, both aquatic and terrestrial [22,24,25]. Further, warming-induced faster developmental rate (e.g. time to become adult from juvenile) in ectotherms could also result in body size reductions in their adult population [26]. There are, however, also exceptions for temperature-body size rules as warming may also increase body sizes of certain taxa [26]. Besides, traits other than body size could also be important to understand species responses to climate warming [27].
Small body-sized prey species may have a competitive advantage over large-sized prey species in warmer environments [24] due to their lower metabolic costs [18,26] and greater interference among large species in response to warming [28]. However, such size-related competitive advantages in warmer conditions may be offset under predation, since small-sized prey are more vulnerable to predation provided predator body sizes are not too large relative to the prey's [29]. Hence, if predation and warming have countervailing effects on competition among prey species of varying sizes, prey coexistence is a likely outcome. However, predation effects can also get stronger as climate warms within the thermal limits of predators, which has been shown to destabilize communities via predation-induced extinction of prey species [30,31]. Predators are also often more vulnerable to warming than their prey, which may reduce predation effects on prey species at higher temperature [4,32]. It is, therefore, crucial to understand the nature of higher-order interactions among temperature, competition and predation to understand how community structure may change in warmer environments.
Here, we experimentally tested the interactive effects of temperature, predation and competition on the population of two detritivore prey species of different body sizes (small and large). The elevated temperature treatments were established in order to represent moderate to extreme climate warming scenarios, whereas the lowest temperature treatments were based on the climate at which model species were thermally acclimated. We hypothesized that a warmer environment will benefit the smaller prey over the larger prey [24,25]. By contrast, we expected that predators will greatly reduce the population of smaller prey in comparison with larger prey [29]. Consequently, we hypothesized that the presence of predators in a warmer environment will make competition between the two detritivore prey species less asymmetric with a subsequent increase in the coexistence probability of two prey species, i.e. predation will counteract warming-induced asymmetric competition. In contrast with our expectations, our results show that predators promoted prey coexistence only in the lowest temperature treatment. We report a complete exclusion of the smaller prey species in warmer environments in the presence of predators. We also show that the large prey species exhibited greater trait plasticity by reducing their body size in warm environments than the small prey species.
2. Material and methods
(a). Prey species
Two Collembola species were used in this experiment as the prey species: Folsomia candida and Proisotoma minuta. Both prey species had been cultured at 14°C with dry yeast since 2014 at Leipzig University. The adult body length of P. minuta ranges between 600 and 1100 µm [33], whereas the mean adult body length of F. candida ranges from 1500 to 3000 µm [33] (also see the electronic supplementary material, figure S2). Both prey species have short generation times depending on the environmental conditions like temperature and pH [34,35]. The egg hatching time of F. candida is about 7–10 days at 20°C [35]. The egg hatching time of P. minuta also varies from 6 to 10 days in a temperature range of 17–23°C [34]. Studies have reported 15–22°C as the desired temperature for the optimal hatching success of both prey species [34,35]. The time to reach reproductive stage also varies from two to three weeks depending on environmental conditions [32,33]. Both species belong to the Isotomidae family, and species belonging to Folsomia and Proisotoma genera have been reported to co-occur in nature [36]. Our own experiments have shown that F. candida and P. minuta can coexist in experimental set-ups [37,38]. The larger size of F. candida may make it more vulnerable to higher temperature but less vulnerable to predation, compared with the smaller prey P. minuta, which we expected to be more vulnerable to predation and a less inferior competitor in a warmer environment.
(b). Predator species
Predatory mites were used as generalist predators in the experiment. To test the generality of our results, we used two closely related predatory mite species, which were included separately to avoid competition between the two predator species: Hypoaspis aculeifer and Hypoaspis miles. Both predators (purchased from Schneckenprofi in Germany) are reported to feed on Collembola species [39]. We also visually confirmed the predation of Collembola prey species used in this study by the Hypoaspis predators (electronic supplementary material, movie S1).
(c). Experimental design
Prey and predator populations were established in microcosms (Petri dish of 14 cm in diameter with tiny air ventilators to allow air circulation) with the mix of litter and yeast as the main substrate for the prey species (electronic supplementary material, figure S3). Litters were cut into small pieces and sterilized twice (121°C) before being used as a substrate. The C : N ratio of the litter was estimated using a CHNO elemental analyser (Vario EL II, Elementar Analysensysteme GmbH) and found to be 17.60 (±0.35 s.e.). We added 1.5 g dry weight of this sterilized litter material into the Petri dishes. In order to keep the base of Petri dishes moist, litters were added on top of a double layer of filter papers (electronic supplementary material, figure S3). In order to stimulate fungal growth on the litter, we added 10 mg of yeast in 1 ml of deionized water on top of the litter. The litter and yeast mix were made adequately moist before animals were added.
We established prey monocultures of F. candida and P. minuta with initial populations of 20 individuals per microcosm. For the prey mixtures, we applied a substitutive design with 10 individuals each for the two prey species. To both prey monocultures and prey mixtures we added six individuals of either of the predator species.
Three warming treatments were used in this experiment with 8 D : 16 L cycle of temperature: low warming with 12°C (night) and 15°C (day), intermediate warming with 17°C (night) and 20°C (day) and high warming with 22°C (night) and 25°C (day). The low warming treatment is within the prey animal culture temperature (14°C), whereas the intermediate warming treatment represents +5°C increase which corresponds to climate scenarios predicted by the Intergovernmental Panel on Climate Change for the next 100 years at many locations around the globe [40]. The higher warming treatment (+10°C) represents an extreme climate scenario, although some studies have indicated +10°C as a realistic scenario in some regions [41], as heat extremes are getting more common [42]. Note, however, that Collembola may experience temperature differences of 10°C or more in the field owing to seasonal temperature fluctuations. Thermal images of the Petri dishes confirmed that Petri dishes closely resembled the treatment specific temperature levels (electronic supplementary material, figure S4).
The experiment ran in reach-in growth chambers (CLF Plant Climatics GmbH) with a constant air humidity of 70%. On day 3 of the experiment, 10 mg of yeast in 1 ml of deionized water was added again to each microcosm to further stimulate fungal growth on the litter. The microcosms were kept moist by adding 1 ml deionized water every day for the first 7 days of the experiment. After fungal growth had been visually observed after the first week of the experiment (electronic supplementary material, figure S3), we added 1 ml deionized water every third day for the entire period of the experiment. In order to investigate microbial growth at three temperatures, additional microcosms with the same set-up (e.g. litter, amount of yeast and water addition) were established without any animals (electronic supplementary material, figure S5). Competition, predation and warming treatments were fully crossed (competition = prey monocultures and prey mixtures; predation = predator absent and predator present; warming = three levels: 0, 1 and 2) and replicated five times.
(d). Harvest
The experiment ran for a total of 60 days providing an adequate time for several generations for both Collembola species. At the end of the experiment, we extracted animals using heat extraction with a gradual heating of 25°C up to 55°C (5°C increase per day) for 6 days [43]. The animals were collected initially in glycol water solution (1 : 1) and later transferred to 70% ethanol solution. In order to observe the population patterns of Collembola species, additional microcosms were established for another harvest at day 30 using the same experimental set-ups and number of replicates. We also extracted and counted the Collembola population at day 30 from the start of the experiment with the same procedure (e.g. heat extraction; electronic supplementary material, figure S6). Collembola species were counted under a dissecting microscope.
Collembola adult body length was determined for five randomly selected larger individuals (per microcosm) assuming that they had attained reproductive stage. Folsomia individuals greater than 1.5 mm are often considered adult, whereas Proisotoma individuals greater than 0.6 mm are presumably adults [33]. However, Collembola are ametabolous, and body size cannot be easily used to differentiate juvenile and adult individuals [44]. Further, a clearer differentiation between the two prey species, such as in their body size, occurs when they become larger. Body length was measured using the inverted microscope at 40× magnification (electronic supplementary material, figure S2).
(e). Data analysis
The total number of Collembola individuals for both species at the final harvest were overdispersed (residual deviance >> degrees of freedom when analysed using generalized linear models with Poisson error) owing to numerous zero counts (zero-inflated; electronic supplementary material, figure S7). Models also failed to converge and were still overdispersed with generalized linear models with either negative binomial or quasi-Poisson error structure. Hence, we used zero-inflated regression models (ZIMs), which handles zeroes and ones (animal absence versus presence as a binary response) and counts separately [45]. We used binomial errors (logit link) to account for the binary responses and Poisson errors (log-link) for the prey counts in the ZIMs (also called zero-inflated Poisson model, sensu Zuur et al. [45]). ZIMs were run for total population for both prey species separately. The model structure was: Prey population ∼ (Warming×Predation×Competition)poisson| (Warming×Predation ×Competition)binomial (see Zuur et al. [45]). We used predation and competition as the initial treatments rather than the realized predator and the competitor densities from the end of the experiment. Linearity assumptions for all ZIMs were met, i.e. no correlation between the model residuals versus fitted values from the model was found. ZIMs were run with the ‘pscl’ package [46] built for the R Statistical Software [47].
Further, we also calculated prey coexistence from the prey mixtures as a binary response. The absence of one prey species was scored as ‘0’, whereas ‘1’ was scored for the cases when both prey species (juvenile or adult) were present together. This coexistence measure allowed us to estimate exclusion in the experimental setting (0 being an exclusion event), however, it may not fully resemble the steady-state equilibrium as assumed by theoretical models of species coexistence [48]. Non-steady-state populations have been used to evaluate species coexistence in experimental studies [16,49]. Also note that as no additional resources were added to microcosms (only twice at the starting week of the experiment, see above), the steady-state population would very likely be the total extinction of all species, however, the time to reach this will differ among treatments. Nevertheless, we acknowledge that our study does not estimate the carrying capacity population of prey species as some populations may have still increased after the 60 days of experiment. We used a generalized linear model with binomial error to test warming and predation effects on prey coexistence.
Finally, we also analysed warming and predation effects on the body length of prey species. We were not able to incorporate prey mixtures in this analysis because of lack of samples with an adequate number of individuals of both prey species for body length measurements—particularly in treatments with predators and higher temperature. We used linear mixed models with Gaussian error to predict the effect of warming and predation on the body length of prey species in their monocultures. All regression models met the linearity assumptions. We also used predator identity as a covariate in our models in order to account for potential differences between predator-specific effects on prey density. All statistical analyses were carried out in R Statistical Software [47].
3. Results
(a). Prey density
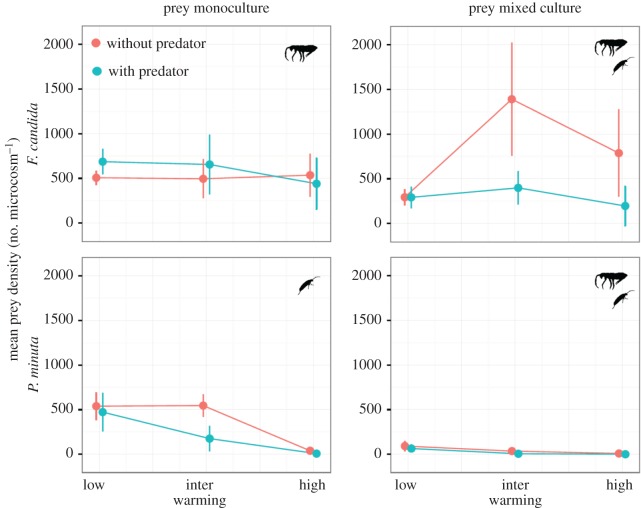
We found a significant three-way interaction effect of warming, predation and competition on the number of F. candida and P. minuta after 60 days (table 1). The negative three-way interaction suggests that predation and competition in a warmer environment generated detrimental conditions for both of the prey species (table 1 and figure 1). We also found that the two prey species were differentially affected by the two predator species (table 1). The density of F. candida was more suppressed by H. miles, whereas that of P. minuta was more suppressed by H. aculeifer (table 1).
Table 1.
Effects of warming, predation and competition (between two prey species) on the population of two prey species based on count data (Poisson's error and log-link function) of zero-inflated model. (The bold values are statistically significant (p < 0.05). The 95% confidence intervals (CIs) that do not overlap zero are statistically significant. coef. stands for regression coefficients of zero-inflated models. Warming was used as a linear term (levels = 0, 1 and 2). HM, Hypoaspis miles; HA, Hypoaspis aculeifer.)
| treatments |
Folsomia candida |
|||
|---|---|---|---|---|
| coef. | 95% CI | z-value | p-value | |
| intercept | 6.24 | 6.21, 6.28 | 346.08 | <0.001 |
| warming (W) | 0.13 | 0.11, 0.16 | 10.10 | <0.001 |
| predator (P) | 0.41 | 0.37, 0.45 | 19.95 | <0.001 |
| competitor (C) | 0.16 | 0.14, 0.18 | 13.46 | <0.001 |
| W × P | 0.01 | −0.01, 0.05 | 1.12 | 0.26 |
| W × C | 0.25 | 0.24, 0.27 | 29.06 | <0.001 |
| P × C | −0.56 | −0.60, −0.54 | −35.90 | <0.001 |
| W × P × C | −0.05 | −0.07, −0.03 | −4.57 | <0.001 |
| presence of HM | −0.67 | −0.70, −0.65 | −49.86 | <0.001 |
| presence of HA | <0.01 | −0.01, 0.02 | 0.74 | 0.45 |
|
Proisotoma minuta |
||||
|---|---|---|---|---|
| intercept | 8.19 | 8.08, 8.30 | 142.81 | <0.001 |
| warming (W) | −0.51 | −0.66, −0.36 | −6.84 | <0.001 |
| predator (P) | 0.50 | 0.36, 0.65 | 6.76 | <0.001 |
| competitor (C) | −1.57 | −1.67, −1.48 | −32.41 | <0.001 |
| W × P | 0.37 | 0.04, 0.70 | 2.21 | 0.02 |
| W × C | −0.04 | −0.17, 0.08 | −0.67 | 0.49 |
| P × C | −0.58 | −0.71, −0.45 | −9.08 | <0.001 |
| W × P × C | −0.43 | −0.74, 0.12 | −2.73 | 0.006 |
| presence of HM | 0.21 | 0.17, 0.25 | 11.61 | <0.001 |
| presence of HA | −0.75 | −0.81, −0.70 | −28.00 | <0.001 |
Figure 1.
Effects of warming, predation and competition on Folsomia candida (larger prey) and Proisotoma minuta (smaller prey) mean density (±s.e.). Larger and smaller prey are indicated pictorially in the panels. Details of statistical analyses are provided in table 1. (Online version in colour.)
(b). Prey coexistence
Prey coexistence, within the timeframe of our experiment, was significantly affected by the interaction between warming and predation (table 2 and figure 2). That is, the probability of prey coexistence was promoted by predation in low warming treatments, whereas the probability of prey coexistence was hindered by predation at higher temperature. Predator identity explained no additional variance in prey coexistence (table 2).
Table 2.
Effects of warming and predation on prey coexistence based on generalized linear models (with binomial errors and logit link function). (The bold values indicate statistically significant effects (p < 0.05). coef. stands for regression coefficients of generalized linear models. The 95% confidence intervals (CIs) that do not overlap zero are statistically significant. Warming is used as a linear term (levels = 0, 1 and 2). HM, Hypoaspis miles; HA, Hypoaspis aculeifer.)
| treatments | prey coexistence |
|||
|---|---|---|---|---|
| coef. | 95% CI | z-value | p-value | |
| intercept | −2.59 | −6.94, 1.28 | −1.29 | 0.19 |
| warming (W) | 3.56 | −0.36, 8.10 | 1.70 | 0.08 |
| predator (P) | 2.36 | −0.08, 5.64 | 1.74 | 0.08 |
| W × P | −3.23 | −6.79, −0.55 | −2.12 | 0.03 |
| presence of HM | 0.86 | −1.07, 2.80 | 0.87 | 0.38 |
| presence of HA | 0.52 | −1.89, 3.72 | 0.40 | 0.68 |
Figure 2.
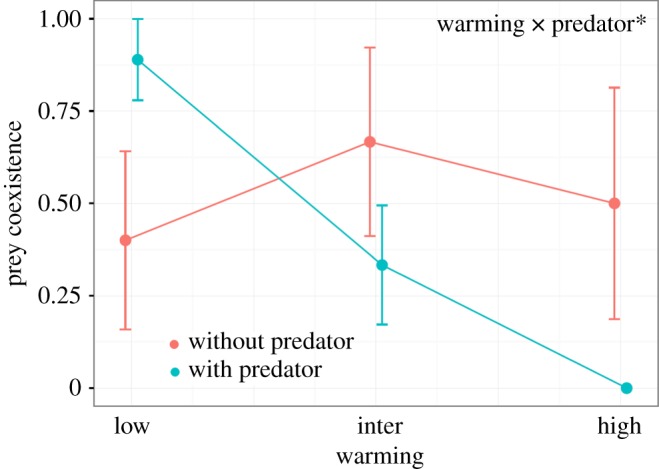
Interactive effects of warming and predation on prey coexistence measured as a binary response in prey mixtures. Each dot represents mean ± s.e. for the warming and predation treatment levels (*p < 0.05). (Online version in colour.)
(c). Prey trait plasticity
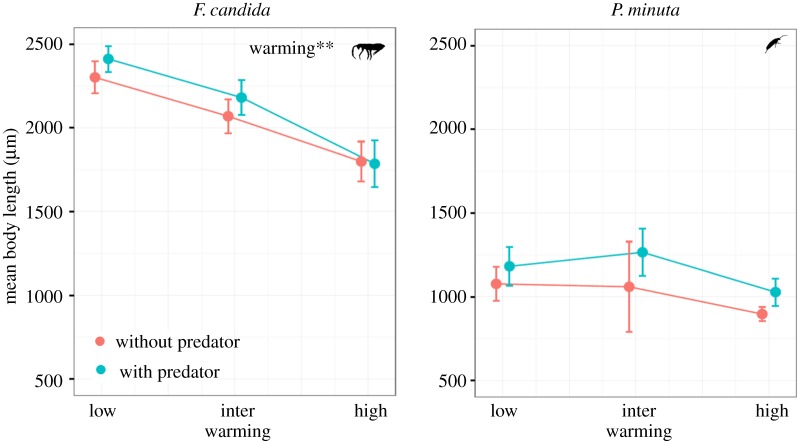
Mean body length of F. candida (the large-sized prey) significantly decreased with warming, whereas no effect of predation was found (table 3, figure 3). Further, we found no interaction effect between warming and predators on the mean body length of F. candida individuals (table 3). Warming and predation treatments did not significantly change the mean body length of P. minuta individuals (table 3 and figure 3). Predator identity also had no effect on changes in body lengths of either of the prey species (table 3).
Table 3.
Results from linear regression models (Gaussian error) for the effects of warming and predation on the body length of two prey species. (The bold values indicate statistically significant effects (p < 0.05). coef. stands for regression coefficients of generalized linear models. The 95% confidence intervals (CIs) that do not overlap zero are statistically significant. Warming is used as a linear term (levels = 0, 1 and 2). HM, Hypoaspis miles; HA, Hypoaspis aculeifer.)
| treatments |
Folsomia candida body length |
|||
|---|---|---|---|---|
| coef. | 95% CI | t-value | p-value | |
| intercept | 2306.90 | 2165.19, 2448.60 | 32.51 | <0.001 |
| warming (W) | −250.35 | −366.04, −134.64 | −4.32 | <0.001 |
| predator (P) | 123.73 | −47.83, 295.29 | 1.44 | 0.15 |
| W × P | −54.60 | −198.28, 89.08 | −0.75 | 0.45 |
| presence of HM | 98.58 | −68.90, 266.07 | 1.17 | 0.24 |
| presence of HA | 11.17 | −154.90, 177.24 | 1.37 | 0.17 |
|
Proisotoma minuta body length |
||||
|---|---|---|---|---|
| intercept | 1095.79 | 882.67, 1308.89 | 10.35 | <0.001 |
| warming (W) | −71.98 | −285.64, 141.68 | −0.68 | 0.50 |
| predator (P) | 115.40 | −156.12, 386.92 | 0.85 | 0.39 |
| W × P | 17.02 | −249.57, 283.61 | 0.12 | 0.89 |
| presence of HM | −14.93 | −291.74, 261.87 | −0.10 | 0.91 |
| presence of HA | 181.14 | −127.85, 490.14 | 1.18 | 0.24 |
Figure 3.
Changes in mean body length (±standard error) of Folsomia candida (larger prey) and Proisotoma minuta (smaller prey) in response to warming and predation in their monocultures (**p < 0.01). (Online version in colour.)
4. Discussion
Our study can be summarized by three key findings. First, under warming, predation and competition had negative interactive effects on the density of both prey species (table 1, figure 1a,b), contradicting our hypothesis that predators would nullify a warming-induced advantage to smaller prey species. Second, the positive effect of predator presence on prey coexistence was only observed in the low warming treatment, but lost in warmer environments where smaller prey species were driven to extinction in the presence of predators (figure 2). Third, the larger prey species always outperformed the smaller prey species in warmer environments, also contradicting our expectations (figure 1b).
The maintenance of species diversity in a community emerges from symmetric interactions, such as trade-offs between competitive ability and vulnerability to predation [9,10]. Our study supports the prediction that coexistence of two prey species is enhanced in the presence of a generalist predator. However, such predator-induced reduction of interspecific competition between two prey species was limited to the temperature regime (lowest temperature treatments) in which both prey species were cultured (separately) for more than 2 years (a number of generations). These results suggest that positive effects of predation on prey coexistence can be realized at temperatures to which prey species are acclimated. Consistent with previous studies [20,30], predation effects became stronger in warmer environments in our study, in particular, in intermediate warming treatments (electronic supplementary material, figure S1), which would be expected to most strongly impact small prey. However, the same prey was also expected to have a competitive advantage over large prey in warmer environments. Nevertheless, we failed to detect an increase in the population of the smaller prey species in the presence of predators in warmer environments. This breakdown of positive predation effects by the presence of predatory mites appears to have resulted from the failure of the small prey to benefit from warming in the absence of predation, and the failure of large prey to suffer a cost from warming in the presence of predation, when the prey species were together (figure 2). This meant the trade-off between warming and predator responses, a requirement of coexistence, was not met in the two prey species.
The poor performance of the smaller prey species in warmer environments suggests that traits other than body size may explain their vulnerability to warming [50]. For instance, the small-sized prey species inhabits the soil subsurface [37,51], which might be cooler than the soil surface, hence making them more vulnerable when exposed to higher temperatures. Thus, even small body-sized species if exposed to temperature near or above their thermal limits, may decline in population in response to climate warming. Accordingly, measuring traits other than body size, such as related to organismal thermal performance traits [52], could enhance our understanding of species performance in a warmer world independent of their body size. It is also noteworthy that the larger prey species, F. candida, have an asexual mode of reproduction providing them an advantage over the smaller prey species [35], despite their comparable growth rates. Further, in order to avoid confounding single versus multi-species and total density, we opted for the substitutive design, but this comes with its own limitation of confounding single versus multi-species with component species' densities. In our previous cultures, populations of both Collembola species have grown very fast in monocultures, when supplied with adequate substrate, and should therefore be minimally affected by the differences in initial densities. Nevertheless, future studies should consider manipulating prey densities across warming and predation gradients to help disentangle density and predation effects.
Our results of better performance (i.e. greater density) of larger prey species in warmer environments contradict studies that have shown a relative advantage of smaller species in warmer environments [24,25,53]. Smaller individuals should have a relative advantage at the cellular level in a warmer environment [23,24]. However, this advantage may be offset by the presence of predators, as small-sized prey tend to be more vulnerable to predation [29]. Indeed, a recent study reported that large phytoplankton species prevailed over smaller phytoplankton species in warmer environments when their grazers were present [54]. The relative advantage of smaller prey may also be undermined if larger prey are able to plastically reduce their body size in order to remain within their metabolic constraints, while staying sufficiently large to reduce predation risk. The larger prey probably showed such an adaptation in our study. Their mean body length decreased significantly in warmer environments. By contrast, we found no statistically significant change in the body length of the smaller prey species with warming (figure 3). Although, these body size results are based on prey monocultures we speculate that the observed warming-induced plasticity of the larger prey population can result in a subsequent exclusion of the smaller prey species. Also note that the prey responses in our study were not owing to resource limitations as microbial availability (resource of prey species) was greater at higher temperature (electronic supplementary material, figure S5), indicating that the observed prey responses were mainly because of interference between the prey species leading to physiological stresses in response to warming and predation. The question that emerges from our results is whether warming will shift communities to smaller prey species, as widely argued [22], when warming simultaneously may enhance predation pressure (electronic supplementary material, figure S1). Both theoretical and empirical research will be required to comprehensively address this question.
The main conclusion of our study is that positive effects of predation on prey coexistence may be lost in warmer environments. Moreover, our results demonstrate that competition and predation effects (presence of predatory mites) got stronger in warmer environments for smaller prey species, particularly where larger-bodied species retained lower vulnerability to predation than smaller prey species. This could be due to a plastic response of the larger prey species in warmer environments, namely by reducing their body length. Future experiments are needed in more open and complex systems to probe the generality of these findings. Our results encourage future studies to incorporate interspecies trait variability in response to warming to predict species coexistence in a changing world.
Supplementary Material
Acknowledgements
We thank Tiffany M. Knight for her comments and suggestions on the results of this study. We thank Minus van Baalen and the two anonymous reviewers for the constructive comments. We also gratefully acknowledge the technical assistance of Alfred Lochner. Further, we thank Christian Ristok for his assistance in body length measurements.
Data accessibility
Available on Dryad: http://dx.doi.org/10.5061/dryad.9ch60 [55].
Authors' contributions
M.P.T. conceived and designed the study. T.K. and M.P.T. performed the experiment. M.P.T. analysed the data with inputs from J.N.G. and wrote the first draft of the paper. J.N.G. and N.E. contributed substantially to revisions.
Competing interests
We declare we have no competing interests.
Funding
M.P.T. and N.E. acknowledge the funding of German Research Foundation (Deutsche Forschungsgemeinschaft; DFG; Ei 862/2). Further support came from the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig, funded by the German Research Foundation (FZT 118). N.E. also acknowledges funding from European Research Council (ERC starting grant 677232).
References
- 1.Humphries MM, McCann KS. 2013. Metabolic Ecology. J. Anim. Ecol. 83, 7–19. ( 10.1111/1365-2656.12124) [DOI] [PubMed] [Google Scholar]
- 2.Yvon-Durocher G, Jones JI, Trimmer M, Woodward G, Montoya JM. 2010. Warming alters the metabolic balance of ecosystems. Phil. Trans. R. Soc. B 365, 2117–2126. ( 10.1098/rstb.2010.0038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houlahan JE, et al. 2007. Compensatory dynamics are rare in natural ecological communities. Proc. Natl Acad. Sci. USA 104, 3273–3277. ( 10.1073/pnas.0603798104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petchey OL, Mcphearson PT, Casey TM, Morin PJ. 1999. Environmental warming alters food-web structure and ecosystem function. Nature 402, 69–72. ( 10.1038/47023) [DOI] [Google Scholar]
- 5.Gilman SE, Urban MC, Tewksbury J, Gilchrist GW, Holt RD. 2010. A framework for community interactions under climate change. Trends Ecol. Evol. 25, 325–331. ( 10.1016/j.tree.2010.03.002) [DOI] [PubMed] [Google Scholar]
- 6.Penuelas J, Filella I. 2001. Responses to a warming world. Science 294, 793–794. ( 10.1126/science.1066860) [DOI] [PubMed] [Google Scholar]
- 7.Kordas RL, Harley CDG, O'Connor MI. 2011. Community ecology in a warming world: the influence of temperature on interspecific interactions in marine systems. J. Exp. Mar. Bio. Ecol. 400, 218–226. ( 10.1016/j.jembe.2011.02.029) [DOI] [Google Scholar]
- 8.Barton BT, Schmitz OJ. 2009. Experimental warming transforms multiple predator effects in a grassland food web. Ecol. Lett. 12, 1317–1325. ( 10.1111/j.1461-0248.2009.01386.x) [DOI] [PubMed] [Google Scholar]
- 9.Terborgh JW. 2015. Toward a trophic theory of species diversity. Proc. Natl Acad. Sci. USA 112, 11 415–11 422. ( 10.1073/pnas.1501070112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chesson P, Kuang JJ. 2008. The interaction between predation and competition. Nature 456, 235–238. ( 10.1038/nature07248) [DOI] [PubMed] [Google Scholar]
- 11.Paine RT. 1966. Food web complexity and species diversity. Am. Nat. 100, 65–75. ( 10.1086/282400) [DOI] [Google Scholar]
- 12.Gurevitch J, Morrison J, Hedges L. 2000. The interaction between competition and predation: a meta-analysis of field experiments. Am. Nat. 155, 435–453. ( 10.1086/303337) [DOI] [PubMed] [Google Scholar]
- 13.Paine RT. 1969. A note on trophic complexity and community stability. Am. Nat. 103, 91–93. ( 10.1086/282586) [DOI] [Google Scholar]
- 14.Navarrete SA, Menge BA. 1996. Keystone predation and interaction strength: interactive effects of predators on their main prey. Ecol. Monogr. 66, 409–429. ( 10.2307/2963488) [DOI] [Google Scholar]
- 15.Holt RD. 1977. Predation, apparent competition, and the structure of prey communities. Theor. Popul. Biol. 229, 197–229. ( 10.1016/0040-5809(77)90042-9) [DOI] [PubMed] [Google Scholar]
- 16.Bonsall MB, Hassell MP. 1997. Apparent competition structures ecological assemblages. Nature 388, 371–373. ( 10.1038/41084) [DOI] [Google Scholar]
- 17.Chase JM, Abrams PA, Grover JP, Diehl S, Chesson P, Holt RD, Richards SA, Nisbet RM, Case TJ. 2002. The interaction between predation and competition: a review and synthesis. Ecol. Lett. 5, 302–315. ( 10.1046/j.1461-0248.2002.00315.x) [DOI] [Google Scholar]
- 18.Brose U, Dunne JA, Montoya JM, Petchey OL, Schneider FD, Jacob U. 2012. Climate change in size-structured ecosystems. Phil. Trans. R. Soc. B 367, 2903–2912. ( 10.1098/rstb.2012.0232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sibly R, Brown J, Kodric-Brown A. 2012. Metabolic ecology: a scaling approach. Chichester, UK: Wiley-Blackwell. [Google Scholar]
- 20.Vucic-Pestic O, Ehnes RB, Rall BC, Brose U. 2011. Warming up the system: higher predator feeding rates but lower energetic efficiencies. Glob. Change Biol. 17, 1301–1310. ( 10.1111/j.1365-2486.2010.02329.x) [DOI] [Google Scholar]
- 21.Gunderson AR, Leal M. 2016. A conceptual framework for understanding thermal constraints on ectotherm activity with implications for predicting responses to global change. Ecol. Lett. 19, 111–120. ( 10.1111/ele.12552) [DOI] [PubMed] [Google Scholar]
- 22.Gardner JL, Peters A, Kearney MR, Joseph L, Heinsohn R. 2011. Declining body size: a third universal response to warming? Trends Ecol. Evol. 26, 285–291. ( 10.1016/j.tree.2011.03.005) [DOI] [PubMed] [Google Scholar]
- 23.Sheridan JA, Bickford D. 2011. Shrinking body size as an ecological response to climate change. Nat. Clim. Change 1, 401–406. ( 10.1038/nclimate1259) [DOI] [Google Scholar]
- 24.Reuman DC, Holt RD, Yvon-Durocher G. 2013. A metabolic perspective on competition and body size reductions with warming. J. Anim. Ecol. 83, 59–69. ( 10.1111/1365-2656.12064) [DOI] [PubMed] [Google Scholar]
- 25.Lindo Z. 2015. Warming favours small-bodied organisms through enhanced reproduction and compositional shifts in belowground systems. Soil Biol. Biochem. 91, 271–278. ( 10.1016/j.soilbio.2015.09.003) [DOI] [Google Scholar]
- 26.Ohlberger J. 2013. Climate warming and ectotherm body size: from individual physiology to community ecology. Funct. Ecol. 27, 991–1001. ( 10.1111/1365-2435.12098) [DOI] [Google Scholar]
- 27.Schmitz OJ. 2013. Global climate change and the evolutionary ecology of ecosystem functioning. Ann. NY Acad. Sci. 1297, 61–72. ( 10.1111/nyas.12181) [DOI] [PubMed] [Google Scholar]
- 28.Lang B, Rall BC, Brose U. 2012. Warming effects on consumption and intraspecific interference competition depend on predator metabolism. J. Anim. Ecol. 81, 516–523. ( 10.1111/j.1365-2656.2011.01931.x) [DOI] [PubMed] [Google Scholar]
- 29.Brose U. 2010. Body-mass constraints on foraging behaviour determine population and food-web dynamics. Funct. Ecol. 24, 28–34. ( 10.1111/j.1365-2435.2009.01618.x) [DOI] [Google Scholar]
- 30.Vasseur DA, McCann KS. 2005. A mechanistic approach for modeling temperature-dependent consumer-resource dynamics. Am. Nat. 166, 184–198. ( 10.1086/431285) [DOI] [PubMed] [Google Scholar]
- 31.Seifert LI, Weithoff G, Gaedke U, Vos M. 2015. Warming-induced changes in predation, extinction and invasion in an ectotherm food web. Oecologia 178, 485–496. ( 10.1007/s00442-014-3211-4) [DOI] [PubMed] [Google Scholar]
- 32.Fussmann KE, Schwarzmüller F, Brose U, Jousset A, Rall BC. 2014. Ecological stability in response to warming. Nat. Clim. Change 4, 1–5. ( 10.1038/NCLIMATE2134) [DOI] [Google Scholar]
- 33.Hopkin S. 2007. A key to the Collembola (Springtails) of Britain and Ireland. Telford, UK: FSC Publications. [Google Scholar]
- 34.Park E-K. 2007. Effect of laboratory culture conditions on population growth of Proisotoma minuta (Tullberg) (Collembola: Isotomidae). Entomol. Sci. 10, 135–140. ( 10.1111/j.1479-8298.2007.00207.x) [DOI] [Google Scholar]
- 35.Fountain MT, Hopkin SP. 2005. Folsomia candida (Collembola): a ‘standard’ soil arthropod. Annu. Rev. Entomol. 50, 201–222. ( 10.1146/annurev.ento.50.071803.130331) [DOI] [PubMed] [Google Scholar]
- 36.Sabais ACW, Scheu S, Eisenhauer N. 2011. Plant species richness drives the density and diversity of Collembola in temperate grassland. Acta Oecol. 37, 195–202. ( 10.1016/j.actao.2011.02.002) [DOI] [Google Scholar]
- 37.Thakur MP, Eisenhauer N. 2015. Plant community composition determines the strength of top-down control in a soil food web motif. Sci. Rep. 5, 9134 ( 10.1038/srep09134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thakur MP, Herrmann M, Steinauer K, Rennoch S, Cesarz S, Eisenhauer N. 2015. Cascading effects of belowground predators on plant communities are density-dependent. Ecol. Evol. 5, 4300–4314. ( 10.1002/ece3.1597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koehler HH. 1999. Predatory mites (Gamasina, Mesostigmata). Agric. Ecosyst. Environ. 74, 395–410. ( 10.1016/S0167-8809(99)00045-6) [DOI] [Google Scholar]
- 40.IPCC. 2014. Synthesis report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Geneva, Switzerland: IPCC.
- 41.Seneviratne SI, Donat MG, Pitman AJ, Knutti R, Wilby RL. 2016. Allowable CO2 emissions based on regional and impact-related climate targets. Nature 1870, 1–7. ( 10.1038/nature16542) [DOI] [PubMed] [Google Scholar]
- 42.Buckley LB, Huey RB. 2016. Temperature extremes: geographic patterns, recent changes, and implications for organismal vulnerabilities. Glob. Change Biol. 22, 3829–3842. ( 10.1111/gcb.13313) [DOI] [PubMed] [Google Scholar]
- 43.Macfadyen A. 1961. Improved funnel-type extractors for soil arthropods. J. Anim. Ecol. 30, 171–184. ( 10.2307/2120) [DOI] [Google Scholar]
- 44.Testerink GJ. 1982. Strategies in energy consumption and partitioning in Collembola. Ecol. Entomol. 7, 341–351. ( 10.1111/j.1365-2311.1982.tb00675.x) [DOI] [Google Scholar]
- 45.Zuur A, Ieno E, Walker N, Saveliev A, Smith G. 2009. Mixed effects models and extensions in ecology with R. Berlin, Germany: Springer. [Google Scholar]
- 46.Jackman S. 2015. pscl: Classes and Methods for R Developed in the Political Science Computational Laboratory, Stanford University. Department of Political Science, Stanford University, Stanford, California. R package version 1.4.9. See http://pscl.stanford.edu/.
- 47.R Development Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- 48.Chesson P. 2000. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 31, 343–366. ( 10.1146/annurev.ecolsys.31.1.343) [DOI] [Google Scholar]
- 49.Schoener TW, Spiller DA, Losos JB. 2001. Predators increase the risk of catastrophic extinction of prey populations. Nature 412, 183–186. ( 10.1038/35084071) [DOI] [PubMed] [Google Scholar]
- 50.Twomey M, Brodte E, Jacob U, Brose U, Crowe TP, Emmerson MC. 2012. Idiosyncratic species effects confound size-based predictions of responses to climate change. Phil. Trans. R. Soc. B 367, 2971–2978. ( 10.1098/rstb.2012.0244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hopkin SP. 1997. Biology of Springtails (Insecta: Collembola). New York, NY: Oxford University Press. [Google Scholar]
- 52.Schulte PM, Healy TM, Fangue NA. 2011. Thermal performance curves, phenotypic plasticity, and the time scales of temperature exposure. Integr. Comp. Biol. 51, 691–702. ( 10.1093/icb/icr097) [DOI] [PubMed] [Google Scholar]
- 53.Falkowski PG, Oliver MJ. 2007. Mix and match: how climate selects phytoplankton. Nat. Rev. Microbiol. 5, 813–819. ( 10.1038/nrmicro1792) [DOI] [PubMed] [Google Scholar]
- 54.Yvon-Durocher G, et al. 2015. Five years of experimental warming increases the biodiversity and productivity of phytoplankton. PLoS Biol. 13, e1002324 ( 10.1371/journal.pbio.1002324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thakur MP, Künne T, Griffin JN, Eisenhauer N. 2017. Data from: Warming magnifies predation and reduces prey coexistence in a model litter arthropod system. Dryad Digital Repository. ( 10.5061/dryad.9ch60) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Thakur MP, Künne T, Griffin JN, Eisenhauer N. 2017. Data from: Warming magnifies predation and reduces prey coexistence in a model litter arthropod system. Dryad Digital Repository. ( 10.5061/dryad.9ch60) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Available on Dryad: http://dx.doi.org/10.5061/dryad.9ch60 [55].