Abstract
Why large and diverse skeletons first appeared ca 550 Ma is not well understood. Many Ediacaran skeletal biota show evidence of flexibility, and bear notably thin skeletal walls with simple, non-hierarchical microstructures of either aragonite or high-Mg calcite. We present evidence that the earliest skeletal macrobiota, found only in carbonate rocks, had close soft-bodied counterparts hosted in contemporary clastic rocks. This includes the calcareous discoidal fossil Suvorovella, similar to holdfasts of Ediacaran biota taxa previously known only as casts and moulds, as well as tubular and vase-shaped fossils. In sum, these probably represent taxa of diverse affinity including unicellular eukaryotes, total group cnidarians and problematica. Our findings support the assertion that the calcification was an independent and derived feature that appeared in diverse groups where an organic scaffold was the primitive character, which provided the framework for interactions between the extracellular matrix and mineral ions. We conclude that such skeletons may have been acquired with relative ease in the highly saturated, high alkalinity carbonate settings of the Ediacaran, where carbonate polymorph was further controlled by seawater chemistry. The trigger for Ediacaran biomineralization may have been either changing seawater Mg/Ca and/or increasing oxygen levels. By the Early Cambrian, however, biomineralization styles and the range of biominerals had significantly diversified, perhaps as an escalating defensive response to increasing predation pressure. Indeed skeletal hardparts had appeared in clastic settings by Cambrian Stage 1, suggesting independence from ambient seawater chemistry where genetic and molecular mechanisms controlled biomineralization and mineralogy had become evolutionarily constrained.
Keywords: Ediacaran, biomineralization, macrobiota, metazoans, carbonate supersaturation
1. Introduction
Sizable calcareous skeletons (1 mm to 1 m) appeared rapidly and globally in the Late Ediacaran, ca 550 Ma [1], marking a step change in the workings of the carbon cycle and marine ecosystem complexity. Many modern metazoan groups with skeletal taxa have non-skeletal close relatives, and Early Cambrian skeletal taxa of varied mineralogy represent a diverse range of phyla [2]. These observations support the hypothesis of convergent or parallel evolution of biomineralization at the phylum level [2], suggesting the operation of an extrinsic trigger in the Ediacaran–Early Cambrian that in turn conferred selective advantage to the acquisition of a skeleton.
The earliest skeletal macrobiota appeared in redox stratified oceans often characterized by a shallow and fluctuating oxic chemocline [3,4]. Possible triggers for biomineralization include the availability of oxygen, changes in seawater chemistry such as an increase in calcium, or the rise of predation [5]. Much uncertainty persists, however, as to the relative importance of these factors, or their potential inter-relationships.
Orthologous genes and their encoded proteins involved in biomineralization are known to have been co-opted and diversified among vertebrates, echinoderms, molluscs, and bilaterians, in general [6,7]. Multiple origins for biomineralization in animals is supported by the appearance of biomineralization after divergence of the major bilaterian clades, and also by the observation that some Cambrian skeletal taxa are resolved as early members of extant phyla rather than stem representatives of larger groups, such as Deuterostomia, Protostomia, Bilateria, or even Metazoa [2].
New data show that the stratigraphic distribution of the so-called Ediacaran and earliest Cambrian skeletal biotas overlap without notable biotic turnover, and hence do not support the contention that mass extinction of the Ediacaran biota facilitated the Cambrian radiation [8]. The relationship between Ediacaran and Early Cambrian skeletal taxa remains, however, unclear.
Here, we consider the nature of the earliest (Ediacaran) macrofossil biomineralization, provide evidence that some of the oldest known skeletal macrobiota have almost morphologically identical soft-bodied counterparts, and finally propose that highly supersaturated and high alkalinity carbonate oxygenated settings promoted early biomineralization. We further highlight broad differences between dominant Ediacaran and Early Cambrian macrobiota biomineralization styles.
2. The nature of the earliest macrofossil biomineralization
Most skeletons combine minerals with structural organic matter, where the physiological cost of producing the mineral is generally small compared with the organic matrix [9]. Several features of Ediacaran skeletal macrofossils suggest the operation of relatively simple biomineralization mechanisms from a pre-existing organic matrix.
Some tubular skeletal biota show evidence of flexibility suggesting the presence of a relatively elastic organic skeleton impregnated with mineral granules [10]. In these forms, the primary wall is also often extremely thin: less than 60 µm in Cloudina [10], less than 90 µm in Sinotubulites [11–13], and less than 40 µm in Suvorovella (below). Such skeletal microstructures are also simple, homogeneous, with no documented retention of preferred crystallographic orientation, and often resemble abiotic cement crusts. Many are either fibrous or microgranular (which may represent diagenetic recrystallization of a primary fibrous microstructure), where individual crystallites are not composed of smaller units, i.e. they are non-hierarchical.
All known Ediacaran skeletal biota produced either aragonite or high-Mg calcite: carbonate polymorphs interpreted to have been favoured by ambient seawater chemistry [14]. Indeed all known Ediacaran skeletal taxa were immobile benthos found exclusively in shallow marine carbonate settings [15]. Finally, we note that Ediacaran skeletal taxa are of diverse affinity, and some possessed a non-mineralized, organic, counterpart, as detailed below.
3. Contemporary skeletal and non-skeletal counterparts
Here, we document examples of the four contemporary skeletal and non-skeletal taxonomic pairs: Suvorovella-Eoporpita; Cloudina-Conotubus; Sinotubulites-Corumbella; and Protolagena-Sicylagena.
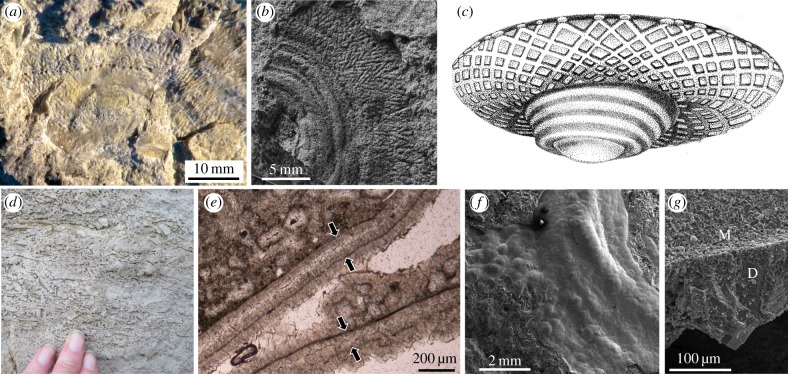
The discoidal skeletal Suvorovella (figures 1 and 2a) occurs in carbonates of the Aim Formation of the southeastern Siberian Platform [16,17], ca 550–543 Ma. Whole Suvorovella shells and broken angular fragments form a thick and persistent shell bed from 1.5 to 2.3 m in thickness, which extends to over 1 km in length, often cross-bedded with coated grains including microbial oncoids (figure 1d). Suvorovella ranges from 10 to 100 mm in diameter and consists of a hollow, flattened disc, with a low conical, slightly eccentrically positioned, an irregularly concentrically folded apex, and an outer flat zone bearing widely separated thin concentric ribs and radiating low relief ridges imparting a diamond-shaped pattern to the surface (figures 1a,b and 2a). These radiating ridges are probably casts of thin, densely anastomosing grooves running from the apex to the periphery, terminating in a distinct rim (figure 1a,b). The lower surface of the shell is smooth.
Figure 1.
Suvorovella aldanica Vologdin and Maslov, 1960, Aim Formation, Ediacaran; Siberian Platform, Republic of Sakha (Yakutia), Russia. (a) Detail of shell inner lower surface showing outer flat zone with diamond-shaped pattern and concentrically arranged chambers. (b) Detail of shell showing folded apex and outer flat zone with diamond-shaped pattern, latex mould of inner lower surface, PIN no. 5119/1063. (c) Reconstruction, diameter 100 mm. (d) Field photograph of shell bed of whole and broken Suvorovella shells. (e) Photomicrograph of thin section showing micritic envelopes (arrowed) around Suvorovella, encrusted by an isopachous crust of early diagenetic radial fibrous dolomite cement. (f) SEM image of detail of outer upper surface of shell at the apex showing concentric ribs and uneven surface. (g) SEM image of broken shell edge showing micrite envelope (M) encrusted by early marine radial fibrous dolomite cement (D). Specimens figured in a, b, f, and g are housed in the Palaeontological Institute named after A.A. Borisyak, Russian Academy of Sciences, Moscow (PIN).
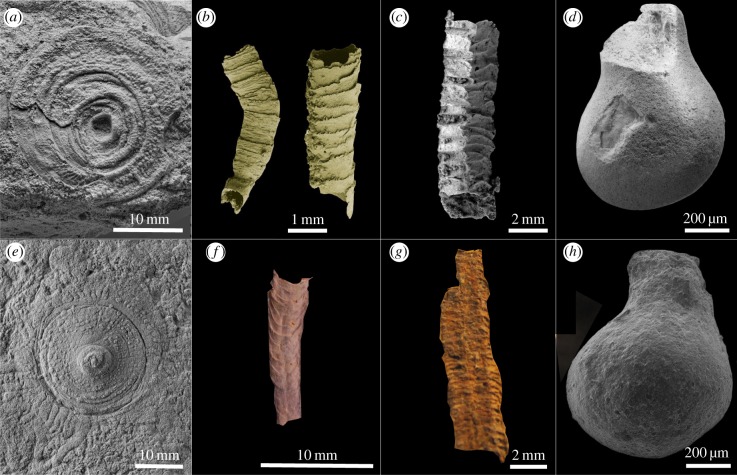
Figure 2.
(a–d) Ediacaran skeletal and (e–h) non-skeletal counterparts. (a) Skeletal Suvorovella. (b) Skeletal Cloudina (Photo: Shuhai Xiao). (c) Skeletal Sinotubulites (Photo: Shuhai Xiao/Yaoping Cai). (d) Skeletal Protolagena (Photo: Shuhai Xiao/Hong Hua). (e) Organic Eoporpita medusa. (f) Organic Conotubus (photo: James Schiffbauer/Yaoping Cai). (g) Organic Corumbella (photo: Lucas Warren). (h) Organic Sicylagena (Photo: Shuhai Xiao/Hong Hua).
Suvorovella specimens are preserved as replicas by an isopachous crust of early diagenetic marine radial fibrous dolomite cement that precipitated upon a micritic envelope [4] (figure 1e,g). Precipitation of such cements required a rigid template capable of preserving fine-scale morphological features. The original shell was probably aragonitic, which was replaced by dolomitizing waters during transgression [4].
Suvorovella has a similar size range and close overall morphology to many discoidal, soft-bodied Ediacaran taxa [18–20]. But these, by contrast, show stretching, folding, and other plastic deformations, and are always preserved as casts and moulds [18]. In particular, the soft-bodied taxa Eoporpita medusa (figure 2e), Hiemalora stellaris, and Palaeophragmodictya spinosa show both a similar low conical, eccentrically positioned, folded apex and a flatter outer zone with dense radiating branching structures similar to Suvorovella [19,20].
In Suvorovella shells, the very tip of the apex is never preserved, suggesting that the disc may have connected to a non-skeletal structure such as a stem, tube, tentaculate structure, or cone as found in soft-bodied Ediacaran taxa. Of note is that the discoidal soft-bodied taxon Aspidella is found in the sandstones of the Aim Formation [17], within the same sequence immediately underlying the carbonate units that contain Suvorovella [4]. Such discs have been interpreted as holdfasts of taxa of unknown affinity, orientated in life by attachment of the apex to, or within, sea floor sediment [18–20]. We hence tentatively suggest a similar function for Suvorovella (figure 1c).
The globally distributed Cloudina (ca 550–540 Ma) forms a tube of nested funnel-shaped and eccentrically arranged cones with flaring rims, up to 150 mm long and 7 mm in diameter (figure 2b). The Cloudina skeleton is composed of extremely thin (8–12 µm) primary layers of elongated micritic crystals (less than 4 µm) fusing to form secondary lamina (up to 60 µm thick), strengthened by early epitaxial cement crusts which infill the space between walls of successive cones [10,11,21]. The non-skeletal Conotubus (figure 2f) shows a very similar morphology and size range [22,23]. Conotubus occurs just below Cloudina in the same sections of the Dengying Formation on the Yangtze Platform, China, with Conotubus restricted to siltstones while Cloudina is confined to overlying carbonates [22]. Cloudina shows evidence for asexual reproduction including intercalar budding and longitudinal fission. These reproduction styles as well as rapid increase of the tube diameter, a closed apex, occasional tabulae, and hexagonal symmetry of some species, are compatible with a total group cnidarian and, therefore, a crown-eumetazoan affinity [11,21].
The skeletal Sinotubulites (figure 2c) also occurs in the Dengying Formation, as well as Western Laurentia (Mexico and USA), and Spain [11–13]. Sinotubulites possesses a semi-circular to polygonal open thick tube up to 20 mm in length and 4–5 mm in diameter, with multiple, fine concentric slightly eccentric layers (40–50 µm thick) of micritic texture; these are transversely and unevenly corrugated to form incomplete ringlets on the surface merging into each other along the tube length. The organic-walled Corumbella known from upper Ediacaran clastics of South America and Western Laurentia [24,25], resembles Sinotubulites in all fine-scaled detail, including multiple concentric layers, the surface pattern of merging ringlets, and a polygonal cross section (figure 2g). Similar alternating ‘plates’ are present in both Corumbella and Sinotubulites, arranged in a slightly asymmetrical pattern along the midline of the fossils. In the soft-bodied Corumbella, these structures resemble ‘plates’ (figure 2g), while in skeletal Sinotubulites these structures possess a three-dimensional form and hence have a facet-like appearance (figure 2c). Corumbella is slightly longer (up to 34 mm) than the maximum size noted for Sinotubulites, but all documented specimens of the latter are incomplete fragments. In the Tamengo Formation, Brazil, Corumbella occurs within shale units which are overlain by carbonates containing Cloudina waldei, which is morphologically more similar to Sinotubulites rather than to any other Cloudina species [13]. The morphological features of Corumbella are consistent with a total group cnidarian affinity, but the affinity of Sinotubulites is problematic.
Finally, the vase-shaped problematic skeletal taxa Protolagena (figure 2d) from the Dengying Formation, is up to 2.4 mm in diameter with a flaring aperture and a thin (14–40 µm) multi-layered, micritic calcareous wall [26]. It is only mineralization that allows this taxon to be distinguished from its organic-walled counterpart Sicylagena (figure 2h), which is restricted to the clastic beds of the same formation [26]. Protolagena and Sicylagena may represent non-metazoan unicellular eukaryotes (protists).
While it is possible that these skeletal/non-skeletal pairs are taphonomic or diagenetic variants of the same taxa, or different taxa restricted to specific ecological niches or environmental settings, we argue that this is not supported by further observations of differences in preservational style.
Suvorovella, compared to discoidal taxa such as Aspidella, Eoporpita, Hiemalora, and Palaeophragmodictya is preserved as fossils of notably different original rigidity, and the existence of primary biomineralized shells in Suvorovella is confirmed by the presence of multiple broken, angular fragments forming a shell hash (figure 1d). Such a depostional style has never been observed in localities yielding soft-bodied Ediacara biota. In addition, Suvorovella specimens never present features of plastic deformation such as stretching, wrinkling, folding, contraction, or other post-mortem degradational features as noted in soft-bodied discoidal taxa [18]. Even when preserved in carbonates, such as in the Khorbusuonka Formation, northern Siberian Platform, Aspidella, Mawsonites, and Hiemalora lack petrographic evidence for dissolution of original skeletal material [27].
Likewise, Corumbella, when preserved in the carbonate Tagatia Guazu Formation of Paraguay shows no petrographic evidence for the presence of any original skeletal hardparts [28]. Indeed, here Corumbella still shows features of plastic deformations such as bending, twisting, and axial-stretching without any loss of overall integrity [24,25]. Conotubus also shows similar bending, folding, and transverse segment imbrication [23]. Even when ruptured, Corumbella shows irregular tears rather than an angular breakage [24]. Such preservation is in notable contrast to the tubes of Cloudina which, like Suvorovella, can show brittle breakage and form concentrated shell beds [29]. Cloudina can also show both elevated growth of cemented and mutually attached individuals to form substantial reef frameworks [30,31], and possible evidence of predatory boring [32].
Finally, it might be argued that these pairs are merely similar as a consequence of their simplicity of form. But shared, fine-scale features suggest they are indeed the same, or closely related, taxa. Moreover, these Ediacaran skeletal fossils are no simpler than any Cambrian metazoans of the poriferan-cnidarian grade, and each has a unique construction. The Cloudina tube is built of eccentrically nested funnel-like elements with flaring upper margins, and the Sinotubulites tube is polygonal in cross section and consists of concentric nested tubicolous elements with similar alternating plates or facets arranged asymmetrically along the midline. The outer tube surface also has a pattern of incomplete ringlets separated by longitudinal ridges [12,13]. Suvorovella is a hollow disc-like shell with two distinct zones on the upper shell, namely a low conical, eccentrically positioned and tightly concentrically folded apex, and an outer flat zone bearing widely separated thin concentric ribs and braided ridges imparting a diamond-shaped pattern to the surface. Except for the soft-bodied twins of these fossils, no other known Ediacaran or Cambrian fossils share these features.
All described taxa are either of unresolved affinity, unicellular eukaryotes (protists) or possible total group cnidarian affinity (crown-eumetazoans). The affinities of Sinotubulites and Suvorovella are unconstrained but their overall skeletal structures are more complex than known in unicellular testate organisms and are incompatible with the organization of calcified algae.
4. Implications for the origins of biomineralization pathways
Calcareous organisms synthesize calcium-binding and extracellular matrix proteins that provide templates for mineralization as well as macromolecules to act as anti-calcifying inhibitors, so essentially placing diagenetic crystal growth under biological regulation [1,33,34]. This suggests that such biomineralization probably originated from a calcium-regulated extracellular matrix system [33,34].
Our findings support the assertion that the calcification process might be a derived feature in many groups where chitin, collagen, or other organic matrices localized in epithelial cells may be the primitive character predating biomineralization: organic scaffolds provided the framework for interactions between the extracellular matrix and mineral ions [35]. Indeed, we note that calcium signalling pathways underwent a dramatic and unparalleled diversification coincident with the radiation of animals [36].
5. The role of high carbonate supersaturation
Ediacaran skeletal taxa are immobile but of diverse affinity: problematic macrobiota, possible total group Cnidaria, as well as unicellular eukaryotes. Prior to the Late Ediacaran, most microfossils (inferred to be unicellular eukaryotes), despite varied probable affinities, possessed either organic or agglutinated tests only [37] with the exception of phosphatic microfossils of probable algal affinity in the Mid-Proterozoic [38].
All Ediacaran calcareous taxa are found only in carbonate rocks, but have soft-bodied counterparts that occur mainly in clastic rocks, often within the same conformable sequences. This observation suggests the operation of local environmental conditions that promoted calcareous skeletonization in pre-existing soft-bodied biota of diverse affinity but with relatively low metabolic demands.
We propose that the Ediacaran macrobiota biomineralization, including the first possible metazoan calcification, was facilitated by the high carbonate supersaturation and alkalinity of Ediacaran shallow marine carbonate settings. Experimentation confirms that modern carbonate skeleton formation is strongly dependent on carbonate supersaturation [39], and so calcification has a relatively low metabolic cost in the highly saturated surface seawaters of low latitudes. For example, despite divergent skeletal morphologies [1], calcium carbonate skeletons may have appeared at least 20 times among metazoans and as many as eight times within both the Porifera and Cnidaria alone [40].
The influence of unusual seawater chemistry during this interval is further supported by examples of anomalously large carbonate skeletons, grains, and structures that exceed Cambrian equivalents by one to two orders of magnitude: (i) the skeletal taxa Namapokia in the Nama Group, Namibia, which reaches diameters of over 1 m [41], (ii) densely aggregating metazoan reefs with volumetrically significant syn-sedimentary cement more than 10 m in width [31], (iii) Suvorovella shell beds ca 1 km length, and (iv) widespread distribution of giant aragonite and high-Mg calcite ooids [42]. Generally, high alkalinity is also consistent with both the global dominance of carbonate lithologies in terminal Ediacaran successions [43] and the extremely high estimated carbonate sediment accumulation rates for this interval, e.g. more than 650 m/ca 10 Myr for the Dengying Formation [23] and more than 1 000 m/ca 10 Myr for the Nama Group [43].
6. Discussion
Despite the first appearance of stalked, frondose macrobiota at ca 578 Ma and soft-bodied tubular forms at ca 550 Ma, no large biomineralized taxa are known until the terminal Ediacaran (ca 550 Ma). That diverse skeletal taxa appear broadly synchronously supports the hypothesis of a terminal Ediacaran environmentally driven biocalcification event.
But while locally high carbonate supersaturation appears to have facilitated calcareous skeltonization, globally high supersaturation long predated the appearance of macrobiota hardparts. Archean and Proterozoic seawater is thought to have been highly supersaturated with respect to carbonate, exceeding that of modern oceans [43]: saturation alone was therefore sufficient to alleviate any metabolic barriers on carbonate biomineralization. This suggests that factors other than changes in seawater carbonate saturation provided a broadly synchronous and global trigger for Late Ediacaran biomineralization.
One possibility is that the progressive lowering of seawater Mg/Ca by an increased input of Ca into oceans, may have caused the demise of ‘aragonite–dolomite’ seas so ushering-in an interval of ‘aragonite seas’ [4]. A further, and perhaps related control, is rising oxygenation. Although modern soft-bodied sponge grade animals may tolerate oxygen concentrations as low as 1.25–10 mM [44], skeletonization is hypothesized to have required higher levels of oxygen, more than 13 mM [45], perhaps in part due to the relatively high energetic cost of structural collagen formation and complex skeletal microstructures [46]. The relatively high oxygen requirements of Ediacaran skeletal biota have been confirmed by the identification of low-oxygen, manganous water column conditions in intervals of Ediacaran successions that lack skeletal taxa [47].
The observations presented here support predictions as to the presence of diverse, pre-existing soft-bodied representatives prior to the acquisition of calcareous skeletons, and that the first macrobiota capable of using increased oxygen for extensive collagen fibrogenesis would likely be those with the lowest metabolic demands, in particular those without advanced circulatory-respiratory systems [46]. It has been hypothesized that induced biologically mediated calcification was an attempt to detoxify excess calcium ions [48], or the molecular inhibitors required as anti-calcification defences in soft-bodied ancestors might have been recruited for control over skeleton growth [34]. It is possible that rising oxygen levels may, however, have been necessary to fuel the co-optive selection of proteins from a pre-adaptive state to allow an adaptive breakthrough [46].
Understanding the degree of biological control over Ediacaran macrobiota biomineralization is critical. Biologically induced precipitates form as a result of metabolic activities that affect pH, pCO2, and secretion products where the cell is a causative agent only with no control over mineral type or habit [9,49]. By contrast, in biologically controlled systems, cellular processes direct the nucleation, growth, morphology, and final location of precipitated minerals. Almost all controlled mineralization processes occur in an isolated biological environment, although the degree of control varies considerably. A potentially widespread biomineralization process also involves a transient amorphous calcium carbonate (ACC) precursor phase formed from a highly saturated solution with additions such as Mg or certain proteins that prevent crystallization [49]. Elemental compositional or microstructural heterogeneity is the mark of biologically induced minerals, as the formation of biominerals resulting from induced processes vary according to the environmental settings in which they form [9,49]. Analysis of individual crystal shapes and the stability of faces expressed can also inform the mechanisms of controlled growth, as can the nature of any biologically controlled chemical and isotopic composition (vital effects).
Many, but not all, Ediacaran macrobiota skeletal microstructures are either microgranular or fibrous, with a non-hierarchical organization. This perhaps suggests the operation of biomineralization mechanisms that were not under tight biological control. Indeed we note that the only biomineralized Ediacaran macrobiota to lack a non-mineralized twin possessed more advanced skeletal microstructures constructed of either multiple layers or several different fabrics, with a hierarchical organization where each fabric has specific crystallites composed of sub-units similar to those known in earliest Cambrian skeletal metazoans [11]. But a detailed understanding of Ediacaran biomineralization processes is problematic, as evidence for environmentally induced heterogeneity, the potential role of ACC, original crystal shape and preferred crystal orientation, and the presence of vital effects is difficult to establish given the diagenetic replacement of originally aragonitic or high Mg calcite skeletons and the unknown structure of the organic matrix.
A change in the ecological landscape further created by the rise of predation would have placed a premium on the acquisition of protective hardparts [1]. The Early Cambrian record shows a rapid increase in both diversity of mineralogy and complexity of skeletal microstructures which become conserved within lineages, suggesting that while calcification may first have arisen as a non-selective response to environmental change, it was later co-opted for defence and subsequently evolved under tighter biological control. Indeed, we note also that skeletal hard parts first appeared in clastic environments in Cambrian Stage 1, suggesting independence from ambient seawater chemistry. By the Early Cambrian genetic and molecular mechanisms may have controlled biomineralization and mineralogy had become evolutionarily constrained [2].
7. Conclusion
We report that the oldest known skeletal macrobiota (ca 550 Ma), including unicellular eukaryotes and possible metazoans, found in carbonate settings, have almost morphologically identical soft-bodied counterparts often found in the clastic horizons of the same contemporary stratigraphic sequences. These forms are diverse, and include tubular and vase-shaped taxa, as well as the first example of a skeletonized discoidal fossil, possibly a holdfast, of Ediacaran biota. In sum, they represent problematic macrobiota, possible total group Cnidaria, as well as unicellular eukaryotes. Skeletal microstructures are either microgranular or fibrous, with non-hierarchical organization.
We conclude that the macrobiota biomineralization was first facilitated by the high carbonate saturation of Ediacaran seawater in local shallow, carbonate settings, and that such calcification was potentially under limited biological control in sessile taxa that probably required only modest modification of pre-existing organic templates.
The first appearance of large skeletal taxa may have been facilitated by changing seawater Mg/Ca and/or rise of oxygen. We hypothesize that from Ediacaran beginnings, macrobiota biomineralization diversified in complexity and had become under tighter biological control by the Early Cambrian, including occupation of clastic environments suggesting independence from ambient seawater chemistry, probably fuelled by an escalating defensive response to increasing predation pressure and other feedbacks.
Metazoans probably originated in the Cryogenian, diversified in the Neoproterozoic, and came to ecological dominance in the Cambrian. But molecular clock estimates predict the existence of only stem members of extant phyla in the Late Ediacaran, with crown groups of most animal phyla originating in the Cambrian [50]. Complex hierarchical Cambrian skeletal fabrics are notably different from the extremely thin, simple, Ediacaran skeletal organizations and represent a more advanced step in the evolution of biomineralization.
Our findings support recent molecular evidence that the first appearance of fossil skeletons faithfully reflects their independent origins and further suggests that developmental and molecular architecture for biomineralization evolved prior to the divergence of the metazoan phyla, providing a mechanism for synchronous, multiple origins of biomineralization through exaptation of existing genes [2]. This is also compatible with the assertion that the ‘Cambrian Explosion’ is a biological signal of the diversification of preservable skeletal fossils, not of animals.
Acknowledgements
We thank the Director of Biological Resources and Protected Natural Territories of the Ministry of Nature Protection of the Republic of Sakha (Yakutia), the administration of the Ust′-Maya region, Nikolay Atlasov, Elena Aleksandrova and Mike Hall. We are grateful to Shuhai Xiao, Yaoping Cai, Hong Hua, Maoyan Zhu, Miriam Lisa Pacheco, Lucas Warren and James Schiffbauer for specimen photographs, Alina Konovalenko for the reconstruction of Suvorovella, and Alex Lui and Phil Donoghue for reviewing the manuscript.
Ethics
This work has not involved any living subjects and conforms to the Ethics guidelines of the School of GeoSciences, University of Edinburgh.
Data accessibility
All data used in this study are presented in the text.
Authors' contributions
R.W. and A.Z. designed the study; A.I. re-found the original Suvorovella fieldsite, undertook initial fieldwork and collected most material. A.I. and A.Z. carried out the morphological description, undertook SEM analysis and reconstructed Suvorovella; R.W. undertook the petrographic analysis of Suvorovella; R.W. and A.Z. drafted the manuscript; all authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
A.Z. acknowledges funding from the University of Edinburgh.
References
- 1.Knoll AH. 2003. Biomineralization and evolutionary history. Rev. Mineral. Geochem. 54, 329–356. ( 10.2113/0540329) [DOI] [Google Scholar]
- 2.Murdock DJE, Donoghue PCJ. 2011. Evolutionary origins of animal skeletal biomineralization. Cells Tissues Organs 194, 98–102. ( 10.1159/000324245) [DOI] [PubMed] [Google Scholar]
- 3.Wood RA, et al. 2015. Dynamic redox controls Ediacaran metazoan communities in the Nama Group, Namibia. Precambrian Res. 261, 252–271. ( 10.1016/j.precamres.2015.02.004) [DOI] [Google Scholar]
- 4.Wood R, Zhuravlev AYu, Sukhov SS, Zhu M, Zhao F. 2017. Demise of Ediacaran dolomitic seas marks widespread biomineralization on the Siberian Platform. Geology 45, 27–30. ( 10.1130/G38367.1) [DOI] [Google Scholar]
- 5.Peters SE, Gaines RR. 2012. Formation of the ‘Great Unconformity’ as a trigger for the Cambrian explosion. Nature 484, 363–366. ( 10.1038/nature10969) [DOI] [PubMed] [Google Scholar]
- 6.Livingston BT, et al. 2006. A genome-wide analysis of biomineralization-related proteins in the sea urchin Strongylocentrotus purpuratus. Dev. Biol. 300, 335–348. ( 10.1016/j.ydbio.2006.07.047) [DOI] [PubMed] [Google Scholar]
- 7.Jackson DJ, et al. 2010. Parallel evolution of nacre building gene sets in molluscs. Mol. Biol. Evol. 27, 591–608. ( 10.1093/molbev/msp278) [DOI] [PubMed] [Google Scholar]
- 8.Zhu M, Zhuravlev AYu, Wood R, Zhao F, Sukhov SS. In press A deep root for the Cambrian Explosion: implications of new bio- and chemostratigraphy from the Siberian Platform. Geology. [Google Scholar]
- 9.Lowenstam HA, Weiner S. 1989. On Biomineralization. New York, NY: Oxford University Press. [Google Scholar]
- 10.Grant SWF. 1990. Shell structure and distribution of Cloudina, a potential index fossil for the terminal Proterozoic. Am. J. Sci. 290-A, 261–294. [PubMed] [Google Scholar]
- 11.Zhuravlev AYu, Liñán E, Gámez Vintaned JA, Debrenne F, Fedorov AB. 2012. New finds of skeletal fossils in the terminal Neoproterozoic of the Siberian Platform and Spain. Acta Palaeontol. Polonica 57, 205–224. ( 10.4202/app.2010.0074) [DOI] [Google Scholar]
- 12.Chen Z, Bengtson S, Zhou CM, Hua H, Yue Y. 2008. Tube structure and original composition of Sinotubulites: shelly fossils from the late Neoproterozoic in southern Shaanxi, China. Lethaia 41, 37–45. ( 10.1111/j.1502-3931.2007.00040.x) [DOI] [Google Scholar]
- 13.Cai Y, Xiao S, Hua H, Yuan X. 2015. New material of the biomineralizing tubular fossil Sinotubulites from the late Ediacaran Dengying Formation, South China. Precambrian Res. 261, 12–24. ( 10.1016/j.precamres.2015.02.002) [DOI] [Google Scholar]
- 14.Zhuravlev AYu, Wood RA. 2008. Eve of biomineralization: controls on skeletal mineralogy. Geology 36, 923–926. ( 10.1130/G25094A.1) [DOI] [Google Scholar]
- 15.Wood R. 2011. Paleoecology of early skeletal metazoans: insights into biomineralization. Earth-Sci. Rev. 106, 184–190. ( 10.1016/j.earscirev.2011.01.011) [DOI] [Google Scholar]
- 16.Vologdin AG, Maslov AB. 1960. On a new group of fossil organisms from the lower Yudoma Formation of the Siberian Platform. Doklady Akad. nauk SSSR 134, 691–693. [In Russian.] [Google Scholar]
- 17.Ivantsov AYu. 2017. On the finds of typical Ediacaran fossils in the Yudoma Group of the Vendian in eastern Siberia. Doklady Akad. nauk 472, 1–4. [In Russian]. [Google Scholar]
- 18.Gehling JG, Narbonne GM, Anderson MM. 2000. The first named Ediacaran body fossil, Aspidella terranovica. Palaeontology 43, 427–456. ( 10.1111/j.0031-0239.2000.00134.x) [DOI] [Google Scholar]
- 19.Zakrevskaya M. 2014. Paleoecological reconstruction of the Ediacaran benthic macroscopic communities of the White Sea (Russia). Palaeogeogr. Palaeoclim. Palaeoecol. 410, 27–38. ( 10.1016/j.palaeo.2014.05.021) [DOI] [Google Scholar]
- 20.Serezhnikova EA. 2013. Attachments of Vendian fossils: preservation, morphology, morphotypes, and possible morphogenesis. Paleontol. J. 47, 231–243. ( 10.1134/S0031030113030088) [DOI] [Google Scholar]
- 21.Vinn O, Zaton B. 2012. Inconsistencies in proposed annelid affinities of early biomineralized organism Cloudina (Ediacaran): structural and ontogenetic evidences. Carnets Géol. 2012/03, 39–47. ( 10.4267/2042/46095) [DOI] [Google Scholar]
- 22.Cui H, et al. 2016. Environmental context for the terminal Ediacaran biomineralization of animals. Geobiology 14, 344–363. ( 10.1111/gbi.12178) [DOI] [PubMed] [Google Scholar]
- 23.Cai Y, Schiffbauer JD, Hua H, Xiao S. 2011. Morphology and paleoecology of the late Ediacaran tubular fossil Conotubus hemiannulatus from the Gaojiashan Lagerstätte of southern Shaanxi Province, South China. Precambrian Res. 191, 46–57. ( 10.1016/j.precamres.2011.09.002) [DOI] [Google Scholar]
- 24.Babcock LE, Grunow AM, Sadowski GR, Leslie SA. 2005. Corumbella, an Ediacaran-grade organism from the Late Neoproterozoic of Brazil. Palaeogeogr. Palaeoclim. Palaeoecol. 220, 7–18. ( 10.1016/j.palaeo.2003.01.001) [DOI] [Google Scholar]
- 25.Pacheco MLAF, et al. 2015. Insights into the skeletonization, lifestyle, and affinity of the unusual ediacaran fossil Corumbella. PLoS ONE 10, e0114219 ( 10.1371/journal.pone.0114219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hua H, Chen Z, Yuan XL, Xiao SH, Cai YP. 2010. The earliest foraminifera from southern Shaanxi, China: Sci. China. Earth Scis. 42, 1753–1764. ( 10.1007/s11430-010-4085-x) [DOI] [Google Scholar]
- 27.Nagovitsin KE, Rogov KI, Marusin VV, Grazhdankin D. 2015. Revised Neoproterozoic and Terreneuvian stratigraphy of the Lena-Anabar Basin and north-western slope of the Olenek Uplift, Siberian Platform. Precambrian Res. 270, 226–245. ( 10.1016/j.precamres.2015.09.012) [DOI] [Google Scholar]
- 28.Warren LV, Pacheco MLAF, Fairchild TR, Simões MG, Riccomini C, Boggiani PC. 2012. The dawn of animal skeletogenesis: ultrastructural analysis of the Ediacaran metazoan Corumbella werneri. Geology 40, 691–694. ( 10.1130/G33005.1) [DOI] [Google Scholar]
- 29.Warren LV, Simões MG, Fairchild TR, Riccomini CR, Gaucher C, Anelli LE, Freitas BT, Boggiani PC, Quaglio F. 2013. Origin and impact of the oldest metazoan bioclastic sediments. Geology 41, 507–512. ( 10.1130/G33931.1) [DOI] [Google Scholar]
- 30.Penny AM, Wood R, Curtis A, Bowyer F, Tostevin R, Hoffman KH. 2014. Ediacaran metazoan reefs from the Nama Group, Namibia. Science 344, 1504–1506. ( 10.1126/science.1253393) [DOI] [PubMed] [Google Scholar]
- 31.Wood RA, Curtis A. 2015. Extensive Ediacaran metazoan reefs from the Nama Group, Namibia: the rise of suspension feeding. Geobiology 13, 112–122. ( 10.1111/gbi.12122) [DOI] [PubMed] [Google Scholar]
- 32.Bengtson S, Yue Z. 1992. Predatorial borings in late Precambrian mineralized exoskeletons. Science 257, 367–369. ( 10.1126/science.257.5068.367) [DOI] [PubMed] [Google Scholar]
- 33.Helman Y, Natale F, Sherrell RM, LaVigne M, Starovoytov V, Gorbunov MY, Falkowski PG. 2008. Extracellular matrix production and calcium carbonate precipitation by coral cells in vitro. Proc. Natl Acad. Sci. USA 105, 54–58. ( 10.1073/pnas.0710604105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marin F, Smith M, Isa Y, Muyzer G, Westbroek P. 1996. Skeletal matrices, muci, and the origin of invertebrate calcification. Proc. Natl Acad. Sci. USA 93, 1554–1559. ( 10.1073/pnas.93.4.1554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sugawara A, Nashimura T, Yamamoto Y, Inoue H, Nagasawa H, Kato T. 2006. Self-organization of oriented calcium carbonate/polymer composites: effects of a matrix peptide isolated from the exoskeleton of a crayfish. Angew. Chem. Int. Ed. 45, 2876–2879. ( 10.1002/anie.200503800) [DOI] [PubMed] [Google Scholar]
- 36.Marchadier E, Oates ME, Fang H, Donoghue PC, Hetherington AM, Gough J. 2016. Evolution of the calcium-based intracellular signaling system. Genome Biol. Evol. 8, 2118–2132. ( 10.1093/gbe/evw139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bosak T, Lahr DJG, Pruss SB, Macdonald FA, Dalton L, Matys E. 2011. Agglutinated tests in post-Sturtian cap carbonates of Namibia and Mongolia. Earth Planet. Sci. 308, 29–40. ( 10.1016/j.epsl.2011.05.030) [DOI] [Google Scholar]
- 38.Cohen PA, Schopf JW, Butterfield NJ, Kudryavtsev AB, Macdonald FA. 2011. Phosphatic biomineralization in mid-Neoproterozoic protists. Geology 39, 539–542. ( 10.1130/G31833.1) [DOI] [Google Scholar]
- 39.Gattuso JP, Allemand D, Frankignoulle M. 1999. Photosynthesis and calcification at cellular, organismal and community levels in coral reefs: a review on interactions and control by carbonate chemistry. Integr. Comp. Biol. 39, 160–183. ( 10.1093/icb/39.1.160) [DOI] [Google Scholar]
- 40.Romano SR, Palumbi SR. 1997. Molecular evolution of a portion of the mitochondrial 16S ribosomal gene region in scleractinian corals. J. Mol. Evol. 45, 397–411. ( 10.1007/PL00006245) [DOI] [PubMed] [Google Scholar]
- 41.Wood RA, Grotzinger JP, Dickson JAD. 2002. Proterozoic modular biomineralized metazoan from the Nama Group, Namibia. Science 296, 2383–2386. ( 10.1126/science.1071599) [DOI] [PubMed] [Google Scholar]
- 42.Trower EJ, Grotzinger JP. 2010. Sedimentology, diagenesis, and stratigraphic occurrence of giant ooids in the Ediacaran Rainstorm Member, Johnnie Formation, Death Valley region, California. Precambrian Res. 180, 113–124. ( 10.1016/j.precamres.2010.03.007) [DOI] [Google Scholar]
- 43.Grotzinger JP, James NP. 2000. In Carbonate sedimentation and diagenesis in the evolving Precambrian world (ed. Grotzinger JP, James NP). Tulsa, OK: SEPM, SEPM Special Publication. [Google Scholar]
- 44.Mills DB, Canfield DN. 2014. Oxygen requirement of the earliest animals. Proc. Natl Acad. Sci. USA 111, 4168–4172. ( 10.1073/pnas.1400547111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levin LA, Gage JD, Martin C, Lamont PA. 2000. Macrobenthic community structure within and beneath the oxygen minimum zone, NW Arabian Sea. Deep Sea Res. II 47, 189–226. ( 10.1016/S0967-0645(99)00103-4) [DOI] [Google Scholar]
- 46.Towe K. 1970. Oxygen-collagen priority and the early metazoan fossil record. Proc. Natl Acad. Sci. USA 65, 781–788. ( 10.1073/pnas.65.4.781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tostevin R, et al. 2016. Low-oxygen waters limited habitable space for early animals. Nature Comms. 7, 12818 ( 10.1038/ncomms12818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simkiss K. 1977. Biomineralization and detoxification. Calc. Tissue Res. 24, 199–200. ( 10.1007/BF02223316) [DOI] [PubMed] [Google Scholar]
- 49.Weiner S, Dove PM. 2003. An overview of biomineralization processes and the problem of the vital effect. Biomineralization 54, 1–31. ( 10.2113/0540001) [DOI] [Google Scholar]
- 50.dos Reis M, Thawornwattana Y, Angelis K, Telford MJ. 2015. Uncertainty in the timing of origin of animals and the limits of precision in molecular timescales. Curr. Biol. 25, 2939–2950. ( 10.1016/j.cub.2015.09.066) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in this study are presented in the text.