Abstract
Osteoprotective therapies have become an essential component in the management of advanced prostate cancer (PC) patients as bone metastases (BMs) have a major impact on morbidity and mortality. Denosumab is a fully humanized antibody targeting the receptor activator of nuclear factor κB ligand (RANKL), which has been approved by the European Medicines Agency (EMA) in Europe and the United States (US) Food and Drug Administration (FDA) in the US for prevention of skeletal-related events (SREs) in patients with solid tumors and BMs, including PC. The clinical settings in which PC patients should be treated with denosumab are still discussed controversially. In a phase III study, denosumab significantly delayed SREs compared with zoledronic acid (ZA) in patients with metastatic castration-resistant PC (CRPC). In addition, denosumab showed superior effects on pain and health-related quality of life (QoL) in these patients. In patients with nonmetastatic CRPC, denosumab has been proven to significantly increase bone metastases-free survival. However, no significant benefits on cancer-specific and overall survival were observed and denosumab was not approved by the US FDA and EMA in this context. The effectiveness of denosumab in patients with castration-sensitive PC (CSPC) and BMs is also under discussion, as clinical trials with ZA in these patients have not shown significant benefits. Clinical data on the use of denosumab in CSPC are urgently needed.
Keywords: bone metastases, castration-resistant prostate cancer, denosumab, skeletal-related events, zoledronic acid
Introduction
In lethal prostate cancer (PC) the majority of patients develop bone metastases (BMs) [Bubendorf et al. 2000]. The median time between clinical diagnosis of BMs and death is 3–5 years [Pound et al. 1999]. However, 3% of patients already have BMs at the time of initial diagnosis [Nørgaard et al. 2010]. In 86% of patients, metastatic disease is exclusively found in the bone [Hess et al. 2006]. In PC BMs are mainly osteoblastic and involve the axial skeleton, the pelvis and the proximal femur [Wang et al. 2012]. The risk of developing BMs is closely related to known risk factors for disease aggressiveness like TNM stage or prostate-specific antigen (PSA) level [Briganti et al. 2014]. BMs lead to so-called skeletal-related events (SREs). SREs are defined as a pathologic fracture, spinal cord compression, radiation or surgery to bone. The cumulative incidence of SREs within 2 years after diagnosis of metastatic PC is approximately 41.9% [Oster et al. 2013]. Even in patients receiving antiresorptive therapy, 15–20% will develop an SRE within 2 years [Saad et al. 2004]. SREs are linked to pain, immobilization and hospitalization [Weinfurt et al. 2005], which finally result in morbidity and mortality. Overall, SREs are very costly for the health care system [Pereira et al. 2016; Saad et al. 2016].
Recently, the term symptomatic skeletal event (SSE) was introduced to address the clinical value of nonsymptomatic fractures detected during follow-up imaging. Bone turnover markers like bone specific alkaline phosphatase (BSAP, <146 U/l) and collagen type I cross-linked N-telopeptide (NTx, ⩽50 nmol/l), as well as clinical findings like pain or time from initial diagnosis to first bone metastasis, were regarded as significant predictors for survival in PC patients with BMs [Fizazi et al. 2015]. Normalization of bone turnover markers following treatment with antiresorptive drugs correlated with improved outcome [Lipton et al. 2008].
The introduction of zoledronic acid (ZA) in the treatment and prevention of BM has led to the implementation of bone-resorptive agents as a major component in treatment of CRPC. ZA was approved in Europe in 2003 for patients with solid tumors having BMs in prevention of SREs. An intravenous dosage of 4 mg in 15 min, adapted to renal function, is recommended (http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021223s028lbl.pdf, accessed 21 November 2016). As a nitrogenous bisphosphonate it inhibits the mevalonate pathway leading to the induction of apoptosis in osteoclasts [Prentice, 2004].
In a pivotal study ZA was tested in 641 patients with BM and castration-resistant PC (CRPC), compared with placebo [Saad et al. 2002]. Treatment was initially planned for 15 months using two dosages of ZA (4 mg and 8 mg) every 3 weeks. Because of several renal adverse events, patient receiving 8 mg were reduced to 4 mg. Saad and colleagues were able to show that men treated with ZA had fewer SREs (38% versus 49%, p = 0.029). Also time to occurrence of the first SRE was prolonged. Moreover, fewer pathological fractures in the ZA group were observed (13.1% versus 22.1%, p = 0.015). Treatment was extended to over 24 months in 122 patients. The annual incidence of SREs was 0.77 for the ZA group versus 1.47 for the placebo group (p = 0.005). Median time to first SRE was 488 days for patients treated with ZA, compared with 321 days in placebo-treated CRPC patients (p = 0.009) [Saad et al. 2004]. Also a reduction in metastases-related pain occurred. In contrast, there were no differences in tumor progression or overall survival between the patients treated with ZA versus the placebo-treated group [Saad et al. 2002].
The introduction of denosumab as an alternative osteoprotective agent has significantly improved therapeutic options in PC [Fizazi et al. 2011]. Denosumab is a fully humanized monoclonal antibody that targets the receptor activator of nuclear factor κB ligand (RANKL) [Hanley et al. 2012]. RANKL is a major contributor to the development and progression of BM and mediates the interaction between tumor cells and bone [Chu and Chung, 2014]. Bone-derived chemokines attract tumor cells [Ibrahim et al. 2010]. Growth factors of PC cells directly stimulate osteoblast activity resulting in an increased expression of RANKL released by osteoblasts and tumor cells that results in increased maturation and differentiation of osteoclast precursor cells. The activity of these osteoclasts leads to further release of substances promoting growth of tumor cells resulting in a vicious cycle of tumor growth and bone destruction [Boyle et al. 2003; Ibrahim et al. 2010]. Denosumab is able to interrupt this vicious cycle by blocking the binding of RANKL to its receptor [Hanley et al. 2012]. This review aims to provide an overview of the use of denosumab in different clinical scenarios of patients with advanced PC.
Denosumab in patients with CRPC and BMs
In a phase III clinical trial using denosumab in patients with metastatic CRPC, patients were randomized to receive either 120 mg denosumab subcutaneously or 4 mg ZA intravenously every 4 weeks [Fizazi et al. 2011]. The study included 1904 CRPC patients having at least one BM and no prior antiresorptive therapy. Randomization was stratified by SREs, PSA and chemotherapy within 6 weeks before induction of therapy. A total of 20% of patients already had an SRE at screening. Regular intake of calcium and vitamin D were strongly recommended, but not mandatory. The primary end point was non-inferiority of denosumab in time to first SRE.
Median time to first SRE was 20.7 months in patients treated with denosumab and 17.1 months in patients with ZA (p = 0.002). Compared with ZA, post-hoc analysis showed the number needed to treat was five for prevention of the first or subsequent SRE [Miller et al. 2011]. Moreover, ZA treatment resulted in a risk reduction of 18% for prevention of the first SRE. Of note, this risk reduction was consistent with the results of studies including other solid malignancies [Stopeck et al. 2010; Henry et al. 2011]. In an additional subgroup analysis, the relevance of SSEs was assessed and showed that denosumab could also significantly reduce the risk of developing first and subsequent SSEs compared with ZA [Smith et al. 2015].
Pain caused by BMs and SREs has a major impact on quality of life (QoL) in patients with metastatic PC. Therefore, assessment of pain and QoL was an important component of the phase III clinical trial [Fizazi et al. 2011]. The Brief Pain Inventory (Short Form) was used to monitor the pain level, while the Functional Assessment of Cancer Therapy (General) was used to measure the QoL throughout the study. Patients with mild to no pain at baseline receiving denosumab showed a lower frequency in pain and a delay in pain worsening compared with patients treated with ZA. These patients also reported better QoL scores [Brown et al. 2011; Patrick et al. 2014]. Of note, overall survival and time to cancer progression was similar in both groups.
The reduction of bone turnover markers has been often discussed as a potential surrogate parameter for improved outcome. Bone turnover markers NTx and BSAP were found to be reduced more frequently in the denosumab-treated group compared with the ZA-treated group [Fizazi et al. 2011]. These results match with a previous phase II study in denosumab-treated patients with BMs from several solid tumors after intravenous bisphosphonates switching to denosumab treatment. This study demonstrated that denosumab was more efficient in normalizing bone turnover markers than bisphosphonates. Elevated levels (>50 nmol/l) of NTx decreased in 69% of the denosumab-treated PC patients [Fizazi et al. 2009].
Collectively these studies show that denosumab leads to an improved delay of SREs and has a positive impact on pain and the QoL. Denosumab has been approved as an osteoprotective agent in patients with PC and BM (https://www.cancer.gov/about-cancer/treatment/drugs/fda-denosumab; accessed 21 November 2016; http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002173/human_med_001463.jsp&mid=WC0b01ac058001d124; accessed 21 November 2016). The current European Association of Urology Guidelines (https://uroweb.org/guideline/prostate-cancer/; accessed 21 November 2016) therefore recommend bone protective agents, including denosumab for the treatment of patients with CRPC and skeletal metastases in order to prevent osseous complications.
Due to its broad implementation in the treatment of patients with metastatic CRPC, potential synergisms with other drugs are frequently discussed. The approval of the radionuclide radium-223 has led to a significant improvement of therapeutic options for patients with symptomatic BMs in CRPC [Parker et al. 2013; Fizazi et al. 2011]. In a phase III clinical trial, radium-223 led to a significant improvement in overall survival compared to placebo (14.0 versus 11.2 months). Radium-223 is the first bone-targeting agent that has led to a significant survival benefit. The concomitant use of radium-223, denosumab and calcium has been discussed frequently, because radium-223 is a calcium analog and its activity is highly dependent on a high bone turnover. However, concerns that denosumab or ZA may decrease the effectiveness of radium were countered by a subgroup analysis of the ALSYMPCA (ALpharadin in SYMPtomatic Prostate CAncer) trial showing that the time to symptomatic SREs was significantly longer in patients receiving antiresorptive agents [Coleman et al. 2013]. Due to the risk of hypocalcemia in patients receiving denosumab, the supplementary use of calcium and vitamin D in patients receiving both radium-223 and denosumab is recommended; although a negative impact of calcium on the effectiveness of radium-223 cannot be completely excluded.
Denosumab in high risk CRPC patients without BM
Due to preclinical results indicating a promising effect of antiresorptive agents in the prevention of BMs, several studies have been performed to investigate whether bisphosphonates or RANKL-inhibitors can prevent BMs in patients with nonmetastatic PC and features associated with increased risk of developing BMs. So far, no phase III clinical trial has shown an advantage for bisphosphonates in prevention of BMs.
Results of a recently published phase III trial called the Zometa European Study showed that ZA (4 mg; every 3 months) was ineffective for the prevention of BMs in patients with localized high risk PC [Wirth et al. 2015]. The use of denosumab for prevention of BMs has been recently investigated in a phase III trial including 1432 CRPC patients. The following parameters were indicated as risk factors for developing BMs: PSA ⩾ 8 µg/l or a PSA-doubling time (PSAdt) ⩽ 10 months (or both). Randomized patients received 120 mg denosumab or placebo every 4 weeks. The primary endpoint was BM-free survival. Denosumab showed a significant benefit in this endpoint with a median increase of BM-free survival of 4.2 months [29.5 versus 25.2 months, hazard ratio (HR) 0.85 p=0.028]. Denosumab treatment also increased the median time to first BM by 3.7 months (p=0.032). A risk reduction of 33% for the development of symptomatic BM was shown. Except for osteonecrosis of the jaw (ONJ) and hypocalcemia, the adverse event rates were similar in both groups. ONJ incidence was comparatively high with 4.6%, hypocalcemia occured in 2% of patients. A subgroup analysis showed that metastases prevention was most effective in patients with a short PSAdt. In patients with a PSAdt ⩽ 10 months HR was 0.84 versus 0.71 in patients with a PSAdt ⩽ 4 months [Smith et al. 2012b]. However, progression-free survival and overall survival did not differ between the denosumab and placebo arm of the study [Smith et al. 2012a].
At present denosumab has not been approved for prevention of metastases in BM-free patients. The oncologic drug advisory committee voted 12 to 1 against the approval for treatment of these patients as they considered the median delay of BM of almost 4 months to be modest taking into consideration the potential side effects of the drug (http://www.oncologypractice.com/specialty-focus/genitourinary/single-article-page/fda-panel-rejects-denosumab-against-bone-metastasis-in-prostate-cancer/dbab7f1ebb5113e93db285d6b3853b75.html, accessed 12 February 2016). One major concern is the risk of ONJ, which might increase with duration of therapy [Aragon-Ching et al. 2009; Boquete-Castro et al. 2016].
Denosumab in metastatic castration-sensitive PC patients
A significant proportion of patients with BM and PC are still in a castration-sensitive stage of disease (CSPC). Therefore, prevention of SREs in this clinical context is a major challenge.
The phase III trials leading to approval of ZA and denosumab exclusively included patients with CRPC. The discussion on the use of bone protective agents in CSPC patients has been further promoted by the results of two previously published studies showing that ZA has a limited benefit in patients with CSPC. The CALGB 90202 (Alliance) trial investigated ZA versus placebo in patients with hormone-naïve, but metastatic PC. Early ZA treatment was not associated with increased time to first SRE [Smith et al. 2014]. The STAMPEDE trial explored the treatment of metastatic hormone-naïve or locally advanced PC in a multiple-arm design study. In two arms of this trial the effectiveness of ZA was investigated. The addition of docetaxel to standard of care, which was androgen deprivation therapy, resulted in a significant improvement of overall survival. However, adding ZA did not have a significant impact on overall survival. With regard to SREs, time to first event was not prolonged in the SOC/ZA treatment group compared to the SOC group [HR = 0.89; 95% confidence interval 0.73–1.07; p = 0.221]. Frequency of adverse events grade 3–5 were comparable with the SOC group. In conclusion, James and colleagues did not recommend use of ZA in patients with hormone-naïve PC because the primary endpoint of overall survival was not met by adding ZA to SOC [James et al. 2015].
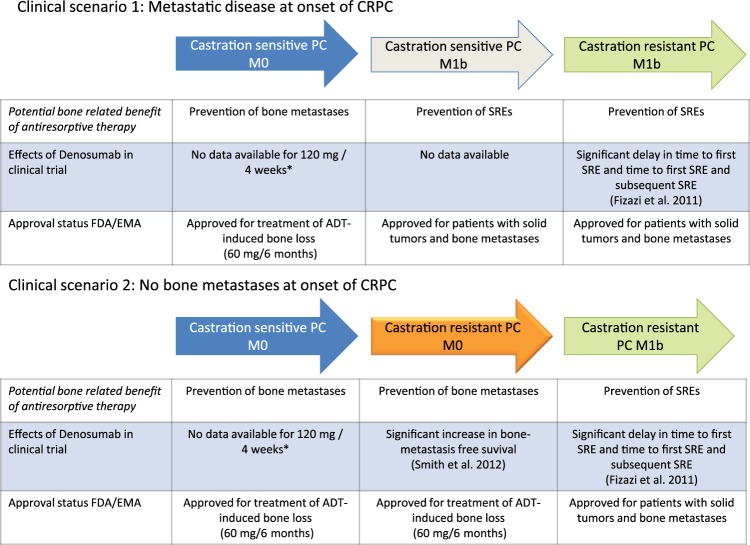
Currently, data on the use of denosumab in patients with metastatic CSPC is missing. Clinical trials are urgently needed to evaluate the potential benefit of denosumab in this setting. Otherwise, there is a high likelihood that due to the negative data on ZA in this setting, no recommendation for the use of antiresorptive drugs can be given. Figure 1 summarizes data from clinical trials and approval status of denosumab in different clinical scenarios in the context of PC.
Figure 1.
Data from clinical trials and approval status of denosumab (120 mg every 4 weeks) in different disease contexts of patients with advanced prostate cancer. Green arrows indicate clinical situations with positive data from phase III clinical trials and FDA/EMA approval. The orange arrow indicates positive data from a phase III trial but without FDA/EMA approval. The grey arrow indicates approval but lack of data from a phase III trial. * In patients with ADT-induced bone loss, 60 mg denosumab every 6 months has been shown to improve bone mineral density and to reduce the risk of vertebral fractures.
ADT, androgen deprivation therapy; CRPC, castration-resistant prostate cancer; EMA, European Medicines Agency; FDA, Food and Drug Administration; PC, prostate cancer; SRE, skeletal-related event.
Denosumab in PC patients with ADT-mediated bone loss
ADT is still the first line therapy in patients with primary or recurrent metastatic PC. Moreover, a significant proportion of patients receive ADT in the context of radiation therapy. ADT induces bone loss [Smith et al. 2009] and increases fracture risk rises with duration of therapy [Shahinian et al. 2005]. ADT reduces the activity of osteoblasts and increases bone resorption through osteoclasts [Boyle et al. 2003]. PC patients under hormonal ablation, especially >6 months, are at risk to develop predisposi cancer-treatment induced bone loss (CTIBL). Of tumor cells.
The combination of decreased bone mineral density (BMD) and the risk or having BMs were assumed to synergistically impair bone stability. Interestingly, increased fracture risk is not limited to patients with low BMD, also patients with normal BMD can suffer from pathologic fractures [Sullivan et al. 2011].
In a phase III clinical trial, men undergoing hormonal deprivation for nonmetastatic PC received 60 mg denosumab subcutaneously every 6 months or placebo [Smith et al. 2009]. Results of this so-called HALT trial showed an increase in BMD in the lumbar spine by 5.6% in the denosumab treatment group compared with a decrease of 1.0% in the placebo-treated group. Denosumab-treated patients also showed a decreased incidence of new vertebral fractures (1.5% versus 3.9%). Following these results, the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) approved denosumab for treatment of ADT-induced osteoporosis. However, recent data show that CTIBL is still a highly underestimated clinical problem. In a study including 180 patients with PC receiving ADT, only a minor proportion of patients underwent BMD analysis and received guideline-conforming therapy for treatment of CTIBL [Dhanapal and Reeves, 2012]. Therefore, there is still a high demand for raising awareness of this problem in the urologic community.
Practical aspects and side effects of denosumab
There are common side effects which the treating physician and the patient have to be aware of when using denosumab. One important side effect that can lead to life-threatening complications, if not treated properly, is hypocalcemia.
In the phase III trial including patients with metastatic CRPC, 13% of patients receiving denosumab showed some degree of hypocalcemia with 5% of them developing grade 3 or higher hypocalcemia [Fizazi et al. 2011]. Hypocalcemia tended to occur within the first 6 months of treatment. In the clinical trial assessing the potential of denosumab to prevent BMs in patients with ‘high risk’ CRPC, hypocalcemia occurred in 2% of patients, with grade 3 or higher in 1% of patients [Smith et al. 2012a]. It is therefore of utmost importance to inform the patients treated with denosumab of the necessity of daily calcium and vitamin D intake and regular lab work to check calcium levels [Body et al. 2015].
Another side effect that is frequently discussed and feared in the context of antiresorptive drugs is ONJ. Although several hypotheses and risk factors have been identified to explain this disorder, the exact mechanism by which ONJ is caused has not been fully elucidated. One identified risk factor is ongoing chemotherapy [Fizazi et al. 2011], others are poor dental hygiene or dental extractions [Boquete-Castro et al. 2016]. In patients with metastatic CRPC taking part in a phase III trial, 2.3% of patients receiving denosumab developed ONJ (versus 1.3% in patients receiving ZA, p = 0.09) [Smith et al. 2012a]. In the AMG 147 trial in patients with nonmetastatic PC, 4.6% of patients developed ONJ [Smith et al. 2012b]. The overall incidence of ONJ in different solid tumor entities receiving denosumab was 1.7% [Boquete-Castro et al. 2016]. In general, incidence and risk factors for ONJ are comparable with ZA [Stopeck et al. 2010; Fizazi et al. 2011].
A major advantage of denosumab from a practical point of view is the subcutaneous administration. Moreover, there is no need for dose adjustments in patients with impaired renal function. Acute phase reactions, a side effect of ZA which is not threatening but bothersome, has not been reported in the context of denosumab.
Conclusion
Denosumab is a drug with significant clinical activity for the prevention of SREs in patients with metastatic CRPC. Thereby, it has a significant positive impact on QoL and pain. Although it has been shown to exhibit efficacy with regards to prevention of BMs, denosumab has not been approved in patients with nonmetastatic PC. Moreover, its use in patients with CSPC is still controversial as critical data from clinical trials are still missing. A prospective clinical trial in patients with CSPC is urgently needed.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Dr Tilman Todenhöfer and Prof. Arnulf Stenzl have served as consultants (Medical Advisory Board, Munich, Germany) for Amgen.
Contributor Information
Miriam Hegemann, Department of Urology, University Hospital, Tuebingen, Germany.
Jens Bedke, Department of Urology, University Hospital, Tuebingen, Germany.
Arnulf Stenzl, Department of Urology, University Hospital, Tuebingen, Germany.
Tilman Todenhöfer, Department of Urology, University Hospital Tuebingen, Hoppe-Seyler-Str. 3, 72076 Tuebingen, Germany.
References
- Aragon-Ching J., Ning Y., Chen C., Latham L., Guadagnini J., Gulley J., et al. (2009) Higher incidence of osteonecrosis of the jaw (ONJ) in patients with metastatic castration resistant prostate cancer treated with anti-angiogenic agents. Cancer Invest 27: 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Body J., Bone H., de Boer R., Stopeck A., Van Poznak C., Damião R., et al. (2015) Hypocalcaemia in patients with metastatic bone disease treated with denosumab. Eur J Cancer 51: 1812–1821. [DOI] [PubMed] [Google Scholar]
- Boquete-Castro A., Gómez-Moreno G., Calvo-Guirado J., Aguilar-Salvatierra A., Delgado-Ruiz R. (2016) Denosumab and osteonecrosis of the jaw: a systematic analysis of events reported in clinical trials. Clin Oral Implants Res 27: 367–375. [DOI] [PubMed] [Google Scholar]
- Boyle W., Simonet W., Lacey D. (2003) Osteoclast differentiation and activation. Nature 423: 337–342. [DOI] [PubMed] [Google Scholar]
- Briganti A., Suardi N., Gallina A., Abdollah F., Novara G., Ficarra V., et al. (2014) Predicting the risk of bone metastasis in prostate cancer. Cancer Treat Rev 40: 3–11. [DOI] [PubMed] [Google Scholar]
- Brown J., Cleeland C., Fallowfield L., Patrick D., Fizazi K., Smith M., et al. (2011) Pain outcomes in patients with bone metastases from castrate-resistant prostate cancer: results form a phase 3 trial of denosumab vs. zoledronic acid. Eur Urol Suppl 10: 336. [Google Scholar]
- Bubendorf L., Schöpfer A., Wagner U., Sauter G., Moch H., Willi N., et al. (2000) Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol 31: 578–583. [DOI] [PubMed] [Google Scholar]
- Chu G., Chung L. (2014) RANK-mediated signaling network and cancer metastasis. Cancer Metastasis Rev 33: 497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R., Fosså S., Chodacki A., Wedel S., Bruland Ø., Staudacher K., et al. (2013) Time to first symptomatic skeletal event (SSE) with radium-223 dichloride (radium-223) in patients with castration-resistant prostate cancer (CRPC) and bone metastases: ALSYMPCA trial stratification factors analysis. Eur J Cancer 49: S688. [Google Scholar]
- Dhanapal V., Reeves D. (2012) Bone health management in prostate cancer patients receiving androgen deprivation therapy. J Oncol Pharm Pract 18: 84–90. [DOI] [PubMed] [Google Scholar]
- Fizazi K., Carducci M., Smith M., Damião R., Brown J., Karsh L., et al. (2011) Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 377: 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fizazi K., Lipton A., Mariette X., Body J., Rahim Y., Gralow J., et al. (2009) Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol 27: 1564–1571. [DOI] [PubMed] [Google Scholar]
- Fizazi K., Massard C., Smith M., Rader M., Brown J., Milecki P., et al. (2015) Bone-related parameters are the main prognostic factors for overall survival in men with bone metastases from castration-resistant prostate cancer. Eur Urol 68: 42–50. [DOI] [PubMed] [Google Scholar]
- Hanley D., Adachi J., Bell A., Brown V. (2012) Denosumab: mechanism of action and clinical outcomes. Int J Clin Pract 66: 1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry D., Costa L., Goldwasser F., Hirsh V., Hungria V., Prausova J., et al. (2011) Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol 29: 1125–1132. [DOI] [PubMed] [Google Scholar]
- Hess K., Varadhachary G., Taylor S., Wei W., Raber M., Lenzi R., et al. (2006) Metastatic patterns in adenocarcinoma. Cancer 106: 1624–1633. [DOI] [PubMed] [Google Scholar]
- Ibrahim T., Flamini E., Mercatali L., Sacanna E., Serra P., Amadori D. (2010) Pathogenesis of osteoblastic bone metastases from prostate cancer. Cancer 116: 1406–1418. [DOI] [PubMed] [Google Scholar]
- James N., Sydes M., Clarke N., Mason M., Dearnaley D., Spears M., et al. (2015) Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 387: 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton A., Cook R., Saad F., Major P., Garnero P., Terpos E., et al. (2008) Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer 113: 193–201. [DOI] [PubMed] [Google Scholar]
- Miller K., Fizazi K., Smith M., Moroto J., Klotz L., Brown J., et al. (2011) Benefit of denosumab therapy in patients with bone metastases from castrate resistant prostate cancer: a number-needed-to-treat (NNT) analysis. J Urol 185: e262. [Google Scholar]
- Nørgaard M., Jensen A., Jacobsen J., Cetin K., Fryzek J., Sørensen H. (2010) Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007). J Urol 184: 162–167. [DOI] [PubMed] [Google Scholar]
- Oster G., Lamerato L., Glass A., Richert-Boe K., Lopez A., Chung K., et al. (2013) Natural history of skeletal-related events in patients with breast, lung, or prostate cancer and metastases to bone: a 15-year study in two large US health systems. Support Care Cancer 21: 3279–3286. [DOI] [PubMed] [Google Scholar]
- Parker C., Nilsson S., Heinrich D., Helle S., O’Sullivan J., Fosså S., et al. (2013) Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 369: 213–223. [DOI] [PubMed] [Google Scholar]
- Patrick D., Cleeland C., Fallowfield L., Smith M., Klotz R., Oudard S. (2014) Denosumab or zoledronic acid (ZA) therapy on pain interference and cancer-specific quality of life (CSQoL) in patients with castrate-resistant prostate cancer (CRPC) and bone metastases (BM). J Clin Oncol 32(Suppl. 4): abstract 12. [Google Scholar]
- Pereira J., Body J., Gunther O., Sleeboom H., Hechmati G., Maniadakis N., et al. (2016) Cost of skeletal complications from bone metastases in six European countries. J Med Econ 19: 611–618. [DOI] [PubMed] [Google Scholar]
- Pound C., Partin A., Eisenberger M., Chan D., Pearson J., Walsh P. (1999) Natural history of progression after PSA elevation following radical prostatectomy. JAMA 281: 1591–1597. [DOI] [PubMed] [Google Scholar]
- Prentice A. (2004) Diet, nutrition and the prevention of osteoporosis. Public Health Nutr 7: 227–243. [DOI] [PubMed] [Google Scholar]
- Saad F., Fleshner N., So A., Le Lorier J., Perrault L., Poulin-Costello M., et al. (2016) The burden of symptomatic skeletal events in castrate-resistant prostate cancer patients with bone metastases at three Canadian uro-oncology centres. 195(Suppl. 4): e679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad F., Gleason D., Murray R., Tchekmedyian S., Venner P., Lacombe L., et al. (2002) A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst 94: 1458–1468. [DOI] [PubMed] [Google Scholar]
- Saad F., Gleason D., Murray R., Tchekmedyian S., Venner P., Lacombe L., et al. (2004) Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst 96: 879–882. [DOI] [PubMed] [Google Scholar]
- Shahinian V., Kuo Y., Freeman J., Goodwin J. (2005) Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med 352: 154–164. [DOI] [PubMed] [Google Scholar]
- Smith M., Coleman R., Klotz L., Pittman K., Milecki P., Ng S., et al. (2015) Denosumab for the prevention of skeletal complications in metastatic castration-resistant prostate cancer: comparison of skeletal-related events and symptomatic skeletal events. Ann Oncol 26: 368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M., Egerdie B., Toriz N., Feldman R., Tammela T., Saad F., et al. (2009) Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med 361: 745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M., Halabi S., Ryan C., Hussain A., Vogelzang N., Stadler W., et al. (2014) Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (alliance). J Clin Oncol 32: 1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M., Saad F., Coleman R., Shore N., Fizazi K., Tombal B., et al. (2012a) Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet 379: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M., Saad F., Shore N., Oudard S., Miller K., Tombal B. (2012b) Effect of denosumab on prolonging bone-metastasis-free survival (BMFS) in men with nonmetastatic castrate-resistant prostate cancer (CRPC) presenting with aggressive PSA kinetics. J Clin Oncol 30(Suppl. 5): abstract 6. [Google Scholar]
- Stopeck A., Lipton A., Body J., Steger G., Tonkin K., de Boer R., et al. (2010) Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol 28: 5132–5139. [DOI] [PubMed] [Google Scholar]
- Sullivan S., Wagner J., Resnick N., Nelson J., Perera S., Greenspan S. (2011) Vertebral fractures and the misclassification of osteoporosis in men with prostate cancer. J Clin Densitom 14: 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Wu G., Shen M., Cui K., Shen Y. (2012) Comparison of distribution characteristics of metastatic bone lesions between breast and prostate carcinomas. Oncol Lett 5: 391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinfurt K., Li Y., Castel L., Saad F., Timbie J., Glennending D., et al. (2005) The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann Oncol 16: 579–584. [DOI] [PubMed] [Google Scholar]
- Wirth M., Tammela T., Cicalese V., Gomez Veiga F., Delaere K., Miller K., et al. (2015) Prevention of bone metastases in patients with high-risk nonmetastatic prostate cancer treated with zoledronic acid: efficacy and safety results of the Zometa European Study (ZEUS). Eur Urol 67: 482–491. [DOI] [PubMed] [Google Scholar]