Abstract
The house mouse Androgen-binding protein (Abp) gene family is comprised of 64 paralogs, 30 Abpa and 34 Abpbg, encoding the alpha (ABPA) and beta-gamma (ABPBG) protein subunits that are disulfide-bridged to form dimers in secretions. Only 14 Abp genes are expressed in distinct patterns in the lacrimal (11) and submandibular glands (3). We created a knockout mouse line lacking two of the three genes expressed in submandibular glands, Abpa27 and Abpbg27, by replacing them with the neomycin resistance gene. The knockout genotype (−/−) showed no Abpa27 or Abpbg27 transcripts in submandibular gland complementary DNA (cDNA) libraries and there was a concomitant lack of protein expression of ABPA27 and ABPBG27 in the −/− genotype saliva, shown by elimination of these two proteins from the saliva proteome and the loss of cross-reactive material in the acinar cells of the submandibular glands. We also observed a decrease in BG26 protein in the −/− animals, suggesting monomer instability. Overall, we observed no major phenotypic changes in the −/− genotype, compared with their +/+ and +/− siblings raised in a laboratory setting, including normal growth curves, tissue histology, fecundity, and longevity. The only difference is that male and female C57BL/6 mice preferred saliva of the opposite sex containing ABP statistically significantly more than saliva of the opposite sex without ABP in a Y-maze test. These results show for the first time that mice can sense the presence of ABP between saliva targets with and without ABPs, and that they spend more time investigating the target containing ABP.
Keywords: androgen-binding protein, knockout mouse, preference testing, sexual selection, label-free quantitative proteomics
PERHAPS the most important theme in biology involves the relationship of structure and function. The difficulties one can encounter in resolving this relationship for a particular structure(s) are illustrated by the proteins that comprise the secretoglobin superfamily. Secretoglobins are defined by a four-helix bundle in a boomerang configuration called the secretoglobin fold [for a review see Mukerjhee and Chilton (2000)]. Even 50 years after the discovery of the first member of this family, uteroglobin [UG, reviewed in Beier (2000)], most superfamily members still do not have unequivocal functions attributed to them. The Androgen-binding proteins (ABPs; Dlouhy and Karn 1984) are a mammalian novelty originally discovered in mouse saliva (Emes et al. 2004; Laukaitis et al. 2008). They are dimers composed of an alpha subunit disulfide-bridged to a beta-gamma subunit, both of which have the secretoglobin fold (Karn and Laukaitis 2003; Laukaitis and Karn 2005). The two mouse salivary ABP dimers share an alpha-subunit encoded by Abpa27 (a27) bound to a second subunit encoded by either Abpbg27 (bg27) or Abpbg26 (bg26). The function(s) of these proteins has been the topic of research for the past 20 years [reviewed in Laukaitis and Karn (2012)].
A great deal of interest has been focused on three rodent pheromone protein families encoded by genes that have undergone extensive gene duplication in mice (Karn and Laukaitis 2009). Some of the proteins encoded by these gene families affect mate selection, thus directly impacting gene exchange and thereby evolution and potentially speciation. These three gene families encode the ABPs, the exocrine gland-secreting peptides (ESPs), and the major urinary proteins (MUPs). ABP-mediated assortative mate selection based on subspecies recognition potentially limits gene exchange between subspecies where they meet (Laukaitis et al. 1997; Talley et al. 2001) and there is evidence that ABP constitutes a system of incipient reinforcement where subspecies make secondary contact, the house mouse hybrid zone in Europe (Bímová et al. 2005; Vošlajerová Bímová et al. 2011). ESPs are small rodent proteinaceous pheromones (Kimoto et al. 2005). Female mice respond to direct facial exposure to an ESP found in male tear fluid by upregulating c-Fos and Egr1 gene expression in vomeronasal sensory neurons (Kimoto and Touhara 2005; Kimoto et al. 2007), and there is evidence that mouse ESP1 enhances female sexual receptive behavior, lordosis, upon male mounting and copulation (Haga et al. 2010). The MUPs are a family of lipocalins shown to mediate female recognition of potential mates [for a review, see Hurst (2009)]. Each adult mouse expresses a pattern of 8–14 different MUP isoforms in its urine, which is determined by its genotype and by its sex, that has been likened to a protein “bar code” (Robertson et al. 1996; Beynon and Hurst 2003; Armstrong et al. 2005; Cheetham et al. 2007; Logan et al. 2008). In addition to causing a female to develop sexual attraction to a male (Roberts et al. 2010), MUPs have been implicated in male–male aggression (Stowers et al. 2002; Chamero et al. 2007) and accelerating puberty in female mice (Clissold et al. 1984; Clark et al. 1985; Mucignat Caretta et al. 1995).
Early studies of the function of ABP drew on population genetic work that suggested that different a27 alleles are fixed in each of the three subspecies of Mus musculus [a27a in M. m. domesticus (western Europe and the Mediterranean basin), a27b in M. m. musculus (eastern Europe to northern China), and a27c in M. m.castaneus (Southeast Asia and Malaysia); (Karn and Dlouhy 1991; Karn et al. 2002)]. This unusual a27 monomorphism in each of the three subspecies and the existence of a relatively narrow house mouse hybrid zone between M. m. domesticus and M. m. musculus in Europe [reviewed in Baird and Macholán (2012)] suggested that ABP might have a role in mediating subspecies recognition. That led to the production of congenic strains, one with a27a from M. m. domesticus and the other with a27b from M. m. musculus, each on a C3H/HeJ background (Laukaitis et al. 1997, 2012). Behavioral analyses compared the preference of various strains of mice for territories, live males, salivas, or urines of the congenic strains. The results showed that house mice of different subspecies could recognize each other on the basis of their salivary ABP phenotype and that they use ABP as a basis for assortative mate choice. An implication of this finding is that sexual isolation based on assortative mate choice might contribute to the stability of the European hybrid zone (Baird and Macholán 2012); therefore, later work extended testing to wild house mice sampled from both allopatric populations and from the hybrid zone itself (Bímová et al. 2005; Vošlajerová Bímová et al. 2011). These studies revealed enhanced choice ratios in wild mice from the tails of the European mouse hybrid zone, providing evidence that ABP-mediated mate preference is a case of reproductive character displacement (Bímová et al. 2005) and that ABP has a role in incipient reinforcement on the house mouse hybrid zone in Europe (Vošlajerová Bímová et al. 2011), reviewed in Baird and Macholán (2012) and Laukaitis and Karn (2012).
In spite of the work described above, the possibility remains that there might be an additional ancestral function, or a completely different function of ABP. Laukaitis et al. (1997) recognized the possibility that ABP’s original function may have more to do with conditioning the mouse’s pelt, resulting in contamination of their environment with their dander, and that subsequent detection by other mice might have been secondary to that function. This suggests the possibility that ABP was preadapted to serve a pheromonal role in mice similar to the observation that long-chain hydrocarbons that protect the cuticle of Drosophila against desiccation have also been shown to act as pheromones involved in sexual isolation (Coyne and Charlesworth 1997). A different study evaluated toxin metabolism in the lateral nasal gland (LNG) of the male Cyp2g1-null/Cyp2a5-low mouse treated with large amounts of acetaminophen (APAP; Zhou et al. 2011). The authors proposed that, through its critical role in testosterone metabolism, CYP2A5 regulates (1) the bioavailability of APAP and APAP-glutathione and (2) the expression of ABP, which can quench reactive APAP metabolites and thereby spare critical cellular proteins from inactivation. In other words, ABP could have a role in toxin metabolism.
The considerations stated above motivated us to produce an Abp knockout mouse so that we could (1) study the general health, fecundity, and longevity of mice lacking any salivary ABP dimer, and (2) determine in preference tests if subjects could tell whether target saliva possessed or lacked ABP and observe what the preference of the subjects would be. To that end, we replaced the genes for two of the three subunits known to be expressed in saliva, a27 and bg27, with the neomycin resistance gene. That left only bg26, encoding a single subunit in an environment lacking an α subunit with which to form a dimer. We studied progeny of the three genotypes from the intercross of mice heterozygous (+/−) for the knocked out region: +/+, +/−, and −/−, and verified that both the transcripts and protein products of the a27 and bg27 genes were eliminated. We report observations on the growth and health of animals of the three genotypes, including their weight gain, fecundity, longevity, and gland/tissue histology. Using a Y-maze two-way preference test system, we also examined the ability of mice of the progenitor C57BL/6 strain to detect ABP in saliva by determining whether they show a preference for +/+ over −/− saliva targets.
Twenty years ago, two laboratories produced targeted disruptions of different regions of the gene encoding mouse UG to study its function [Stripp et al. 1996; Zhang et al. 1997; reviewed in Mukherjee et al. (2007)]. The results of the two knockout studies differed substantially in terms of the overall health of the resulting Ug −/− animals and the effects on specific organs, which alerted us to the importance of evaluating knockout progeny for a variety of potentially subtle manifestations stemming from deletion of the Abp genes. Knockout experiments have sometimes also failed to produce mice with compromised phenotypes due to compensation by another gene(s) upregulated as the result of loss of the target gene (Chen et al. 2010; Smith et al. 2014). Therefore, we also studied the transcript levels of closely-related genes in glands known to express ABP normally in the three genotypes, using conventional and quantitative PCR (qPCR). We discuss the impact of knocking out ABP expression in the salivary and lacrimal glands and the additional perspective it provides on ABP function.
Materials and Methods
The details of the Materials and Methods are described in Supplemental Material, File S1. All animal manipulation except behavioral testing was performed in accordance with University of Arizona IACUC procedures under protocol 08-138. B.V.B. performed all behavioral tests in the breeding facility of the Institute of Animal Physiology and Genetics of the Academy of Sciences of the Czech Republic (IAPG ASCR) in Liběchov and holds license no. CZ 01293 for experimental work on vertebrates in accordance with Czech law. The a27/bg27 knockout mouse strain was created by the Genetically Engineered Mouse Models Core at the University of Arizona. The neomycin resistance gene (MC1NeopA) replaced the entire <bg27-a27> gene module on chromosome 7, which includes the two Abp genes and the intervening sequence (Laukaitis et al. 2008; Karn and Laukaitis 2009) (Figure S1).
Gland harvesting and tissue slide preparation for histological analyses
We euthanized three 45-day-old mice of each genotype and sex and harvested the lacrimal and submandibular glands. Half of each submandibular gland was prepared for RNA extraction and the other half was fixed, embedded, and stained with hematoxylin and eosin (H&E) by standard methods. The lacrimal, sublingual, parotid, harderian, liver, kidney, and ovaries (female only) were treated similarly.
Transcript analysis by qPCR
RNA isolation and complementary DNA (cDNA) library synthesis was performed as previously detailed (Karn et al. 2014). qPCR was performed on an ABI Prism 7000 machine using the Maxima SYBR green qPCR kit from Thermo Scientific (Karn et al. 2014). We generated a standard curve for each primer using male submandibular B6 cDNA templates for three Abp paralog primers (a27, bg26, and bg27) and the remaining standard curves were produced from male lacrimal cDNA template (ABI qPCR manual). Each standard curve was produced using serial dilutions of cDNA template spanning five orders of magnitude. Table S1 shows each primer used in the qPCR analysis along with the efficiency and R2 values (calculated from the standard curves) of each primer.
We calculated the expression of Abp transcripts in each genotype using a relative quantification analysis, where each sample was compared using the standard curves generated for each primer set amplified by the B6 genome mouse cDNA library, described in detail in File S1 (Karn et al. 2014). The ANOVA statistical test was performed to determine interactions between genotype and gender for both salivary and lacrimal glands, and genotype and transcript expression levels in the salivary glands. Confidence intervals for the difference of two means were calculated for lacrimal transcript expression levels because there were too few data to perform ANOVA testing. Significance was assigned when confidence intervals did not cross zero (Essex-Sorlie 1995).
Immunohistochemical analyses
A specific anti-ABP antibody [Dlouhy et al. 1986; see also Figure 6 in Dlouhy et al. (1987)] was used to detect ABP expression by immunostaining sections on slides with a modification of the protocol for transferred nitrocellulose filters (Dlouhy et al. 1986). Detection was done with hydrogen peroxide and DAB chromagen, included in the ImmunoCruz ABC staining system (Santa Cruz BioTechnology). Stained slides were dehydrated before sealing with a mounting medium.
Salivary protein analyses
Nonreducing SDS electrophoresis was performed according to a modification of Dlouhy et al. (1986) using the Mini-PROTEAN II (Bio-Rad, Hercules, CA) system and electroblotting (western blotting) was performed with a Mini Trans-Blot electrophoretic transfer kit onto PVDF membrane (Millipore, Bedford, MA). Immunostaining with a specific anti-ABP antibody was performed as in Dlouhy et al. (1986).
Weighing and litter growth observations
We weighed individual mice from four different intercross (+/− × +/−) litters at ages 14–50 days in a tared plastic weigh boat using an Acculab Vic 612 scale (Sartorius Group). We recorded the weights for a total of 9–18 separate data points for each individual, according to its litter and unique ear tag. The data were evaluated statistically by fitting a least square regression line for weight by day for each mouse and the slope was interpreted as the weight gain rate. The one-way ANOVA model was employed to test the slope, applying the Tukey adjustment for multiple comparisons.
Sexual preference tests
The sexual preferences of 90-day-old female (n = 33) and male (n = 32) B6 mice were tested using a simple two-way choice test in a Y-maze (Figure S2; Bímová et al. 2005; Vošlajerová Bímová et al. 2011). Each tested individual was allowed to choose in 5 min trials between saliva from animals of the opposite sex of the +/+ and −/− genotypes, spotted on the filter paper placed at the bottom of the Y maze. Sexual preferences were analyzed using Observer software (Noldus Technologies, Noldus et al. 2000) in three measured parameters (for details see File S1): (1) a first choice of the Y-maze arm the animal entered after it first traversed the Y-maze stem, (2) the first choice of the signal target the animal sniffed for the first time in the experiment (Laukaitis et al. 1997; Talley et al. 2001), and (3) the coefficient of preference of the signal (RSn) calculated as a difference of total time the animal sniffed each signal target (Smadja and Ganem 2002; Bímová et al. 2005; Vošlajerová Bímová et al. 2011). The data were further analyzed using the χ2 test and t-test after confirmation of normal distribution of the data (see File S1).
Proteomic analyses
We bred three biological replicates of each sex for each of the three genotypes and collected salivas from each of the 18 individual mice. The samples were provided to the Proteomics Core Facility of the University of Arizona where personnel digested 2 μg of each with trypsin. Liquid chromatography-mass spectrometry/mass spectrometry analysis of the digested proteins in the gel pieces (Shevchenko et al. 1996) was carried out using an LTQ Orbitrap Velos mass spectrometer described in Karn et al. (2014) (File S1). Peptides were eluted onto an analytical column and separated using gradients composed of acetonitrile and formic acid.
Raw data were analyzed in Progenesis using both Top3, a label-free quantitation method that calculates the mean of the three highest peptide areas measured for each protein (Fabre et al. 2014) Insert into bibliography: “Fabre, B., T. Lambour, L. Garrigues, M. Ducoux-Petit, F. Amalric et al., 2014 Label-free quantitative proteomics reveals the dynamics of proteasome complexes composition and stoichiometry in a wide range of human cell lines. J Proteome Res 13: 3027–3037.”, and an all-peptide weighted average to reveal protein expression changes. For the three protein subunits of interest, A27, BG27, and BG26, both methods produced the same result. The data output was statistically analyzed with an ANOVA and the P values compared with cutoffs of ≤ 0.05.
Data availability
All data necessary for confirming the conclusions presented in the article are represented fully within the article and Supplemental Material. Strain information is available upon request.
Results and Discussion
Production of an Abp knockout mouse line
Abpa and Abpbg genes most often occur in pairs called modules in 5′–5′ orientation, <Abpa-Abpbg>, where the arrows point in the 3′ direction (Karn and Laukaitis 2009). Here, we report replacing the <a27-bg27> gene module in the B6 mouse strain with the neomycin resistance gene. Figure S1 shows the entire Abp gene region including the genes that were replaced with the target vector by homologous recombination. The knockout mice were backcrossed with the genome mouse, B6, for five generations to produce a line that is essentially B6 lacking the <a27-bg27> gene module. Intercross lines were subsequently bred from fifth-generation mice to produce mice of all three genotypes (+/+, +/−, and −/−) with identical B6 backgrounds. We characterized the knockout mouse line to confirm the absence of a27 and bg27 transcripts and proteins in the submandibular glands and saliva, respectively, before performing functional or behavioral tests.
Inheritance of the knockout haplotype and fecundity
We determined the genotype of each pup from intercrosses (+/− × +/−) of fifth-generation breeders that produced 314 pups from 46 litters, with an average of seven pups per litter. A total of 170 mice were male and 144 mice were female (χ2, P = 0.14). The overall distribution of genotypes was 87 +/+ (28%), 151 +/− (48%), and 76 −/− (24%) (χ2, P = 0.54). The genotype distribution for male mice was 42 +/+ (25%), 92 +/− (54%), and 36 −/− (21%), and the distribution for female mice was 45 +/+ (31%), 59 +/− (41%), and 40 −/− (28%) (χ2, P = 0.45 and 0.08, respectively). Crosses of −/− × −/− breeders produced litters averaging seven −/− pups per litter. That suggests that the fecundity of homozygous animals is essentially the same as +/− heterozygotes and compares favorably to reports of 6.2 ± 0.2 pups per litter for the B6 strain (Nagasawa et al. 1973).
Histological assessment of tissue structure
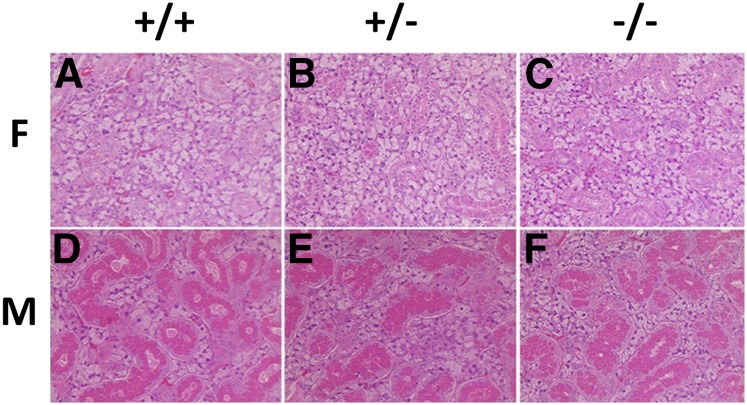
Before analyzing protein and transcript expression in the three genotypes, we performed a general evaluation of tissue structure in the lacrimal and salivary glands (parotid, submandibular, and sublingual) that are known to be major sites of Abp gene expression (Dlouhy et al. 1986; Laukaitis et al. 2005; Karn et al. 2014). In addition, we analyzed the Harderian gland (the source of heteroglobin that may mediate behavior in hamsters) (Dominguez 1995; Alvarez et al. 2002); kidney, which was compromised in a uteroglobin (Ug) knockout (Zhang et al. 1997); and liver, a major site of detoxification that might be affected by toxins that could not be bound and removed in an Abp knockout animal (Zhou et al. 2011). H&E staining revealed no discernable structural differences across tissues, including submandibular gland (Figure 1), sublingual, lacrimal, parotid, Harderian, liver, kidney, and ovaries (female only) (Figure S3), harvested from the three genotypes. Conventional morphology of the secretory granular convoluted tubules (GCT) and the acinar cells was conserved in the submandibular glands of the three genotypes (Figure 1). As observed in genetically unmodified mice, males have elaborated GCT, while females exhibited scant GCT. All other tissues analyzed also had normal structures in the three genotypes of both sexes (Figure S3).
Figure 1.
H&Estaining of the submandibular glands of all three genotypes in both sexes. (A)–(C) are females and (D)–(F) are males, and genotype designations appear at the top of the figure. Males (D–F) show substantially more GCT (red) than seen in females (A–C). GCT, granular convoluted tubules; F, female; H&E, hematoxylin and eosin; M, male.
Our study contrasts significantly with others in which two mouse strains with disrupted Ug were produced and found to have varying phenotypes (Stripp et al. 1996; Zhang et al. 1997). Unlike the profound weight loss of the Ug −/− mouse caused by heavy proteinuria (Zhang et al. 1997; Mukherjee et al. 2007), the Abp knockout (−/−) mice in our study showed normal growth and development. Our −/− mice also showed normal kidney and liver histology, as well as normal histology of salivary and lacrimal glands, suggesting that deleting the <bg27-a27> gene module had no effect on those organs. This is consistent with a role for salivary ABP in an external function such as communication rather than a function affecting internal physiology.
Quantitative analyses of Abp transcripts in submandibular and lacrimal glands
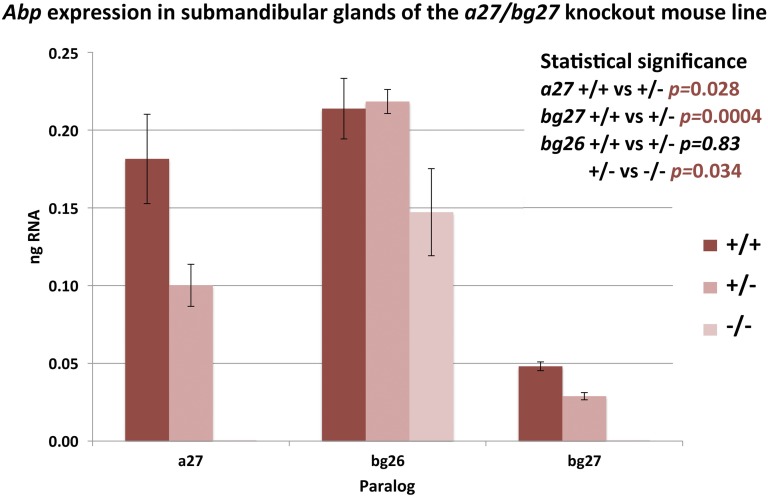
We used qPCR to verify the expression of transcripts that amplified in conventional PCR and to determine the relative expressions of paralogs between different genotypes. Our purpose was to look for possible instances of compensation for the knockout haplotype by upregulation of other Abp paralogs. The expression levels of Abp paralogs in the submandibular glands of all three genotypes can be seen in Figure 2. We did not observe any significant differences in transcript expression between genders for the three salivary Abp paralogs (a27, bg26, and bg27: P = 0.20, 0.23, and 0.15, respectively), thus we were able to pool the female and male data for each paralog. In both males and females, the submandibular glands of the +/− genotype produce significantly lower quantities of a27 and bg27 transcripts compared to that of the +/+ genotype (ANOVA, P = 0.03 and 0.0004, respectively), suggesting haploinsufficiency in the +/− genotype, at least in terms of transcripts.
Figure 2.
Abp transcript expression in submandibular glands of the three genotypes. Expression levels of the three salivary Abp transcripts in the three genotypes were calculated using values obtained from qPCR with standard curves used as the method of optimization and verification for primer sets for each paralog. Male and female expression levels are pooled due to nonsignificant interaction between genotype and gender for the salivary Abp genes. SE bars are shown on the graph and ANOVA P-values comparing the means of the three genotypes are inset on the right, where the significant P-values appear in red typeface. qPCR, quantitative PCR.
The lack of both a27 and bg27 transcripts in −/− animals of both sexes validates the successful creation of a knockout line. The reduction of their expression in +/− haplotypes suggests that the remaining a27 and bg27 gene copies are not upregulated to compensate for the loss of one copy of each. A similar decrease was not seen for bg26 transcript expression in the +/− genotype (P = 0.83), probably because its gene is present in the diploid condition, but we did see a significant decrease in transcript expression in the −/− genotype compared to +/− (P = 0.03). This suggests that the absence of a27 and bg27 transcripts in the −/− animals causes a concomitant decrease in expression of the bg26 transcript. Thus, the absence of the other two genes also appears to have downregulated bg26 gene expression, which may be to save the gland from needlessly utilizing resources to make a transcript for a subunit without an alpha counterpart.
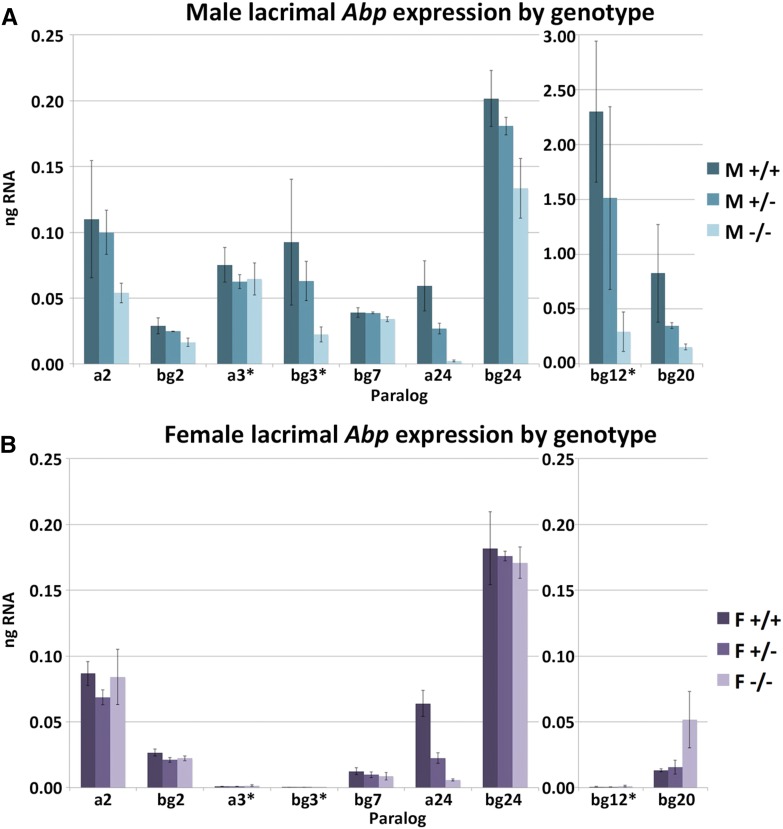
The lacrimal glands in the genome mouse (B6) express a greater number of Abp paralogs than do the salivary glands, and the secretions of the two glands contain no common ABP proteins (Karn et al. 2014). Moreover, expression levels in lacrimal glands are more variable between paralogs than in salivary glands. Figure 3 shows the lacrimal gland expression levels of Abp paralogs in all three genotypes. We did not pool the male and female lacrimal transcript data due to the sex-specific expression of several paralogs. A decrease in transcript expression for male −/− animals compared to either +/+ or +/− genotypes is relatively consistent for the lacrimal paralogs, excluding a3. The confidence intervals of the difference of the means of +/− vs. −/− transcript expression levels do not cross zero for any of the paralogs except a3 (Table S2). The distinct decrease in a24 transcript expression for −/− genotypes compared to +/+ and +/− for both males and females is also noteworthy. It is interesting that previous transcript studies found an appreciable amount of a24 transcript in lacrimal glands whereas proteome studies did not detect any A24 subunit in tears of the genome mouse (Karn et al. 2014). Otherwise, there are no significant differences in expression patterns of the other paralogs expressed in lacrimal glands (Table S2). The paralogs that show male-specific sex-limited expression in the genome mouse (a3, bg3, and bg12; Karn et al. 2014) are still expressed as such (i.e., females express a minimal amount of transcript for paralogs a3, bg3, and bg12, P = 1.3 × 10−9, 0.0032, and 0.0081, respectively; Table S2). Overall, we saw a decrease in transcript expression for male −/− animals for the majority of the lacrimal paralogs, but we did not find any evidence of compensatory upregulation of other Abp paralogs in the knockout mouse.
Figure 3.
Abp transcript expression in lacrimal glands of the three genotypes in both sexes. Lacrimal Abp transcript levels in males (A) and females (B) were calculated using values obtained from qPCR with standard curves used as the method of optimization and verification for primer sets for each paralog. Any values below 10−4 ng RNA are regarded as a nonexpressed paralog. Paralogs with sex-specific expressions are marked with an asterisk. SE bars that show the SD of the means are shown on the graph. F, female; M, male; qPCR, quantitative PCR.
ABP expression in the submandibular gland and saliva
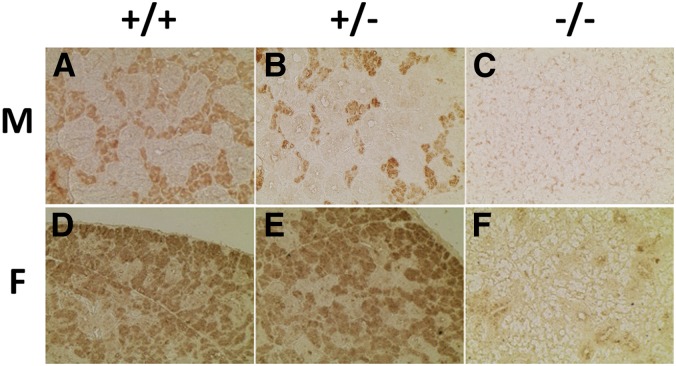
Immunohistochemical staining of submandibular tissues with antibodies specific for salivary ABP (Dlouhy et al. 1986, 1987) provided information about ABP synthesis in submandibular glands between mice of each genotype. Of the two major cell types in mouse submandibular glands, acinar cells, and GCT, the acinar cells are the main source of ABP synthesis and secretion (Dlouhy and Karn 1983). Whereas +/+ and +/− mice exhibited significant, specific staining in the acinar cell regions of the tissue, −/− mice exhibited only very faint, nonspecific staining throughout the tissue, irrespective of acinar location (Figure 4). These results suggest that acinar production of ABP is relatively normal in the +/+ and +/− animals of both sexes but absent in the −/− mice.
Figure 4.
IHC staining of the submandibular glands of the three genotypes in both sexes. Submandibular gland sections were subjected to IHC staining with anti-ABP antibodies. (A)–(C) are males and (D)–(F) are females, and the three genotypes are shown at the top of the figure. Males have substantially more GCT than females, and that tissue stains negatively (note especially (A) and (B) where stained acinar cells are crowded between GCT). ABP, androgen-binding protein; F, female; GCT, granular convoluted tubules; IHC, immunohistochemical; M, male.
To determine the fate of Abp transcripts in salivary glands, we examined ABP protein expression in nonreduced saliva using SDS gel electrophoresis, electroblotting, and immunostaining with a specific anti-ABP antibody (Dlouhy et al. 1986, 1987). We observed that both +/+ and +/− saliva had immunostained bands that migrated in the gel at the expected size of an A:BG dimer [∼21 kDa (Karn and Laukaitis 2003)]. As expected, the −/− genotype did not have a band that corresponded to the dimer but rather a faint band that migrated further down the gel (∼9 kDa), possibly indicating the presence of the smaller ABP BG26 monomer (Figure S4). These results are consistent with both the expression of the bg26 transcript and absence of a27 and bg27 in the −/− genotype; however, the putative BG26 protein appears to be reduced in quantity in the gel analysis compared with what would be expected if BG26 and BG27 are normally expressed equally.
Proteomic analyses
Table 1 summarizes the statistical analysis of the label-free quantitative saliva ABP proteomics data compared with quantitative submandibular Abp transcriptome data. Transcript levels calculated from qPCR for a27, bg27, and bg26, and quantitation of the proteins from the top three peptides for A27, BG27, and BG26, were averaged and the values submitted to two-way comparisons using a two-tailed t-test (RNA) or an ANOVA (protein; Table S3). The fold-change for one genotype compared with a second genotype was also generated to determine the magnitude of difference between the submandibular transcripts or the saliva protein from animals of different genotypes.
Table 1. Statistical analysis of Abpa27, Abpbg27, and Abpbg26 transcript levels in the submandibular gland and quantitative proteomic data comparing average intensity of peptide peaks in three biological replicates for the ABPA27, ABPBG27, and ABPBG26 proteins.
| ++ vs. – | +− vs. – | ++ vs. +− | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RNA | Protein | RNA | Protein | RNA | Protein | |||||||
| Fold-Change | P-Valuea | Fold-Change | P-Valueb | Fold-Change | P-Valuea | Fold-Change | P-Valueb | Fold-Change | P-Valuea | Fold-Change | P-Valueb | |
| Female | ||||||||||||
| Abpa27/A27 | > 50 | 0.01** | > 50 | 8e−5** | > 50 | 3e−3** | > 50 | 4e−5** | 1.80 | 0.14 | 1.44 | 0.19 |
| Abpbg27/BG27 | > 50 | 6e−5** | > 50 | 1e−5** | > 50 | 3e−6** | > 50 | 4e−7** | 1.61 | 3e−3** | 2.22 | 0.04* |
| Abpbg26/BG26 | 1.58 | 0.19 | 9.81 | 5e−3** | 1.36 | 0.38 | 12.35 | 2e−3** | 1.16 | 0.20 | 0.81 | 0.28 |
| Male | ||||||||||||
| Abpa27/A27 | > 50 | 6e−3** | > 50 | 7e−5** | > 50 | 5e−3** | 48 | 7e−6** | 1.83 | 0.10 | 1.37 | 0.28 |
| Abpbg27/BG27 | > 50 | 2e−5* | > 50 | 2e−3** | > 50 | 6e−3* | > 50 | 2e−3** | 1.74 | 3e−3** | 1.56 | 0.24 |
| Abpbg26/BG26 | 1.30 | 0.21 | 4.93 | 0.02* | 1.63 | 0.03* | 10.55 | 6e−3** | 0.80 | 0.03* | 0.46 | 6e−3** |
Two-tailed Student’s t-test.
ANOVA; * P < 0.05, ** P < 0.01.
The > 50-fold change and statistically significant P-values when comparing a27 and bg27 transcript levels in submandibular glands, and A27 and BG27 protein levels in saliva from −/− mice compared to either +/+ or +/− animals, confirmed our conclusion that we were successful in knocking out the a27 and bg27 genes. This supports the segregation data from +/− intercrosses, transcript data (Figure 2 and Table 1), the immunohistochemistry results for lack of expression in the acinar cells (Figure 4), and the SDS electrophoresis study (Figure S4) of protein subunits in saliva.
There was weak support for haploinsufficiency of BG27 protein levels in the saliva of female +/− compared to that of female +/+, consistent with a significant difference in transcript. In contrast, there was no such statistically significant difference in BG27 in male saliva of +/− compared to +/+, even though the transcript showed a significant drop in the +/− genotype. There was also no significant difference in A27 for either sex in the same +/− vs. +/+ comparison.
We also studied the effect of knocking out the genes for a27 and bg27 on bg26 transcript and BG26 protein expressions. The bg26 gene was not knocked out with the a27 and bg27 genes and so there is no a priori reason to believe that its expression should be affected. However, there was an inconsistent change of bg26 transcript levels in glands missing one or both copies of a27 and bg27. The transcript in male −/− glands decreased slightly but significantly vs. +/−, although the transcript in the +/− genotype actually increased vs. +/+. In the female glands lacking one or both a27 and bg27 haplotypes there were no significant changes in bg26 transcript levels.
Protein levels were a different matter, however, insofar as BG26 levels were significantly lower in −/− vs. +/+ and +/− in both sexes, and this may explain the impression that the putative BG26 subunit protein appears to be reduced in the gel analysis (Figure S4). The BG26 level was significantly elevated in +/− vs. +/+ males and also elevated but not significantly so in +/− females vs. +/+. The explanation for these surprising results is not immediately evident but we suggest that it may be found in the strong similarity of the bg26 and bg27 genes, which may be the products of a recent duplication (Laukaitis et al. 2008) creating similar control mechanisms. The fact that BG26 protein levels decrease significantly in both sexes of the −/− genotype compared to +/+ and +/− while the bg26 transcripts do not suggests that the BG26 monomer is unstable in the absence of an alpha-subunit with which to bind.
Growth and survival of animals of the three genotypes
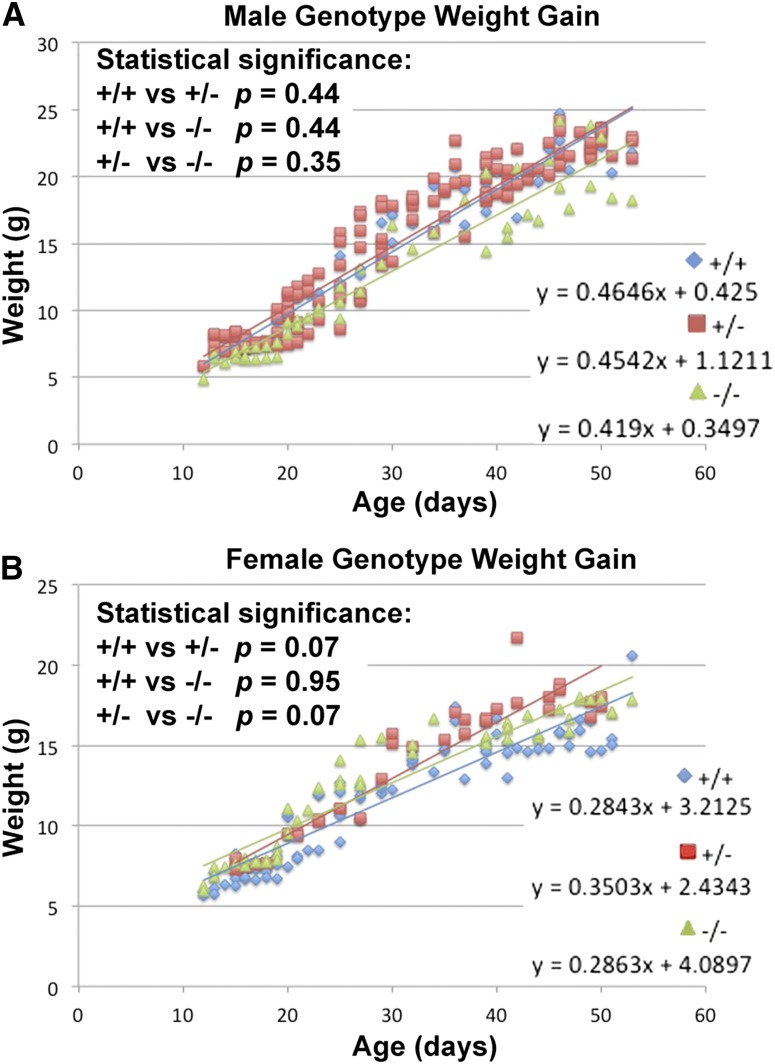
We weighed pups daily to measure the growth of animals of the three different genotypes. Figure 5 shows the growth progression for both male and female pups of each genotype. There were no significant differences in weight gain between the three genotypes in either sex (Table S4). However, marginal differences between curves in females, possibly a consequence of curvilinear data, may represent some sex-specific effect of ABP on growth. Exploring this possibility should be the focus of a future study. Additionally, mice of all three genotypes survived into adulthood and there was no appreciable difference in life span between mice of each genotype up to 1 year of age, which is well beyond the earliest age of reproductive potential.
Figure 5.
Growth curves of knockout mice of all three genotypes in both sexes. (A) shows the male weight curves and (B) shows the female weight curves. The equations describing the lines of best fit for the weight gains of the three genotypes are shown on the lower right of each graph and the statistical significances are shown in the upper left.
Male and female preference for knockout genotype saliva
The sole function ascribed to salivary ABP up until now is a role in mediating mate choice [Laukaitis et al. 1997; Talley et al. 2001; Bímová et al. 2005; Vošlajerová Bímová et al. 2011; reviewed in Laukaitis and Karn (2012)]. We evaluated the preference of B6 mice for mouse saliva with (+/+ genotype) or without (−/− genotype) A27 and BG27 in two-way choice tests. Table 2 shows that females chose the +/+ genotype male saliva over the −/− genotype male saliva twice as often for the first arm they chose to explore and for the first signal (saliva) they chose to investigate. Males display a similar preference as they chose the signal with the +/+ genotype female saliva in a ratio close to 2:1. The analysis of the total times spent sniffing each signal, analyzed here as coefficient of preference of the signal (RSn) (Table 3), shows that both males and females preferred saliva samples of the +/+ genotype over those of the −/− genotype (RSn, females: t-test: t = 2.178, P = 0.037; males: t-test: t = 2.309, P = 0.028). Stronger preferences displayed in close contact with a signal cue may indicate the role of ABP as a nonvolatile signal similar to MUPs (Bímová et al. 2009). However, based on these data, we cannot speculate about the signaling mechanism of ABPs. Nevertheless, our results show for the first time that subjects can sense the presence of ABP in one saliva target when it is absent in the alternative saliva target and that they prefer to investigate the target containing ABP. These simple but crucial observations could not be made in our previous behavioral testing of preference for congenic strains with the Abpa genotypes of the M. m. domesticus and M. m. musculus subspecies. While these congenics differed only by the presence of different Abpa alleles expressed in saliva, we did not have a source of mice lacking salivary ABP altogether.
Table 2. First choices of the arm and signal of C57BL/6 mice performed in the Y-maze test.
| First Choice of the Arm | First Choice of the Signal | |||||
|---|---|---|---|---|---|---|
| +/+ Choice | −/− Choice | χ2, P-Value | +/+ Choice | −/− Choice | χ2, P-Value | |
| Female | 22 | 11 | 0.056 | 21 | 12 | 0.117 |
| Male | 19 | 13 | 0.288 | 19 | 13 | 0.288 |
Each individual was free to choose between saliva with (+/+ genotype) or without (−/− genotype) A27 and BG27. Results of the χ2 test are presented separately for males and females.
Table 3. The coefficient of preference of the signal (RSn) of C57BL/6 mice performed in the Y-maze test.
| Coefficient of Preference of the Signal (RSn) | ||||
|---|---|---|---|---|
| Mean ± SD | Min/Max | t | P | |
| Female | 0.149 ± 0.392 | −1.000/1.000 | 2.178 | 0.037 |
| Male | 0.128 ± 0.314 | −0.333/0.667 | 2.309 | 0.028 |
Each individual was free to choose between saliva with (+/+ genotype) or without (−/− genotype) A27 and BG27. Mean ± SD, minimum and maximum values, and one-way t-test statistics are presented separately for males and females. Max, maximum; Min, minimum.
Overall impact of knocking out the Abp genes
There is substantial evidence that salivary ABP, represented by the dimers A27:BG27 and A27:BG26, mediates assortative mate preference with implications for sexual selection and incipient reinforcement at the European house mouse hybrid zone [reviewed in Laukaitis and Karn (2012)]. However, there are potential functions for these proteins other than, or in addition to, mouse communication. For example, Dominguez (1995) suggested that most or all secretoglobins have some kind of function involving surface coating, based on the presence of UG/Clara 10 (UG/CC10) secretion in the lungs. Previously, we reported finding salivary ABP on the pelts of mice (Laukaitis et al. 1997); however, in this study, we did not observe any qualitative changes in physical attributes including the condition of the pelts of −/− mice compared to their +/+ or +/− littermates. A visual inspection of all three genotypes suggested that the pelts of the −/− knockout progeny were no less glossy, nor did they show more flakes of dried skin, and they did not seem to lose hair more frequently than those of the other two genotypes. Ultimately, such changes would be expected to lead to denuded skin patches and lesions but those were never observed. Thus, the findings we report here do not support a pelt conditioning function for salivary ABP since we found no evidence that the pelts of −/− genotypes maintained for nearly a year were in any way different from those of the other two sibling genotypes.
Another knockout mouse project produced a Cyp2g1-null/Cyp2a5-low mouse and showed that toxin metabolism in the LNG of the male treated with large amounts of APAP upregulated a27 and bg27 expression (Zhou et al. 2011). This apparently quenched reactive APAP metabolites and thereby spared critical cellular proteins from inactivation. While these observations might suggest that ABP could have a role in toxin metabolism, we remain unconvinced that it is a normal function in nature because, in the process, the ABP dimer apparently dissociated into monomers that subsequently bound the toxic metabolites and this has been observed only in mice treated with extremely large amounts of APAP.
Our preference testing experiments revealed the only effect of knocking out these genes that we were able to find: The B6 strain subjects could distinguish between saliva targets with and without A27 and BG27, and spent more time investigating the saliva of mice with ABP (+/+) over those without ABP (−/−). Indirectly, these results are also congruent with our findings from earlier studies that searched for rodents lacking a protein corresponding to Abp in their saliva (Karn and Dlouhy 1991). All the rodent taxa in that study, even those in the evolutionarily distant Cricetidae and Muridae, had salivary proteins capable of binding testosterone despite the very different nutritional sources of the various rodents evaluated. The finding that they all had a protein(s) capable of binding androgen in their saliva suggested that ABP is not involved in the detoxification of components of any of their varied nutritional sources. These observations prompted the authors to suggest that ABP might serve as a vehicle for oral and/or olfactory detection of androgens in urine and thus mediate recognition, and they suggested that behavioral tests should be done on the basis of their findings.
Only 14 of the 64 Abp genes have been found to be expressed as both transcripts in tissues and proteins in their secretions, and there is a clear partition of these expressions between the two major secretory glands of the face and neck (Laukaitis et al. 2005). The paralogs a27, bg27, and bg26 expressed in submandibular glands and saliva show traces of transcripts in lacrimal glands without concomitant presence of protein in tears, which may represent the vestiges of subfunctionalization (Karn et al. 2014). In contrast, a24 showed higher transcript levels in lacrimal glands but no detectable protein in tears, an observation which has yet to be adequately explained. It is intriguing that there are more Abp paralogs expressed in lacrimal glands than in salivary glands, but thus far there has only been speculation about ABPs in tears influencing aspects of behavior (Laukaitis et al. 2005; Karn et al. 2014).
One of the results of the study we report here is that, in both males and females, the submandibular glands of the +/− genotype produce significantly lower quantities of a27 and bg27 transcripts compared to that of the +/+ genotype, suggesting haploinsufficiency in the +/− genotype. But this downregulation is not limited to Abp genes expressed in salivary glands. Although we were looking for instances of upregulation of other Abp genes in salivary and lacrimal glands of −/− genotype mice to compensate for loss of a27 and/or bg27, we were surprised to find an overall decrease in transcript expression for male −/− genotypes for the majority of the lacrimal paralogs and no evidence of compensatory upregulation of other Abp transcripts in either sex of the −/− mouse. Future studies aimed at elucidating ABP function in lacrimal glands should include not only knocking out other Abp genes expressed in these glands, but also assessing the effects of those knockouts on Abp paralog expression generally in both salivary and lacrimal glands.
The loss-of-function results we report here complement our previous preference tests. Not only can mice distinguish between other mice by the subspecies-specific type of the ABP in their saliva (Laukaitis et al. 1997; Talley et al. 2001; Bímová et al. 2005; Vošlajerová Bímová et al. 2011), but they can also recognize when saliva is devoid of ABP altogether. Since the knockout strain is derived from a classical inbred mouse strain, we remain cautious in extrapolating these results to situations in wild animals. It is important to emphasize that we developed this knockout mouse in the B6 mouse strain, whose genome sequence is thoroughly understood, to use it to refine our understanding of ABP-mediated mouse behavior. Our earlier work with congenic strains already established a putative connection between the fixed a27 alleles in mouse subspecies and a role for ABP in assortative mate selection. In this study, we used B6 test subjects because their genetic backgrounds are identical with those of the knockout mice. Overall, our observations suggest that, if a function of Abp other than one involving communication exists, it was not evident in these mice.
Supplementary Material
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.116.194571/-/DC1.
Acknowledgments
The authors thank Corina Mauss, Anastasia Miramontes, Georgina Linton-Smart, and a number of summer students who assisted in performing mouse husbandry and genotyping; Petr Šolc at the Institute of Animal Physiology and Genetics, Academy of Sciences of the Czech Republic who kindly provided C57BL/6 mice for behavioral testing; and Rob Beynon and Philip Brownridge for quantifying the data produced by the University of Arizona (UA) Proteomics Core. They gratefully acknowledge the Genetically Engineered Mouse Modeling Core, BIO5 Institute, UA who produced the knockout mouse with funding from the National Cancer Institute at the National Institutes of Health (NCI/NIH) grant #P30 CA23074 and from the Department of Medicine, UA. A.G.C. was supported in part by grant #52005889 to UA from the Howard Hughes Medical Institute. P.M.B. was funded by NCI/NIH grant #CA143924 to the Partnership for Native American Cancer Prevention. B.V.B. was supported in part by grant #15-13265S from the Czech Science Foundation. C.M.L. received funding from NCI/NIH grant #PCA23074 and from the Vice President for Health Sciences, UA. Mass spectrometry and proteomics data were acquired by the Arizona Proteomics Consortium supported by National Institute of Environmental Health Sciences grant #ES06694 to the Southwest Environmental Health Sciences Center, NIH/NCI grant # CA023074 to the UA Cancer Center, and by the BIO5 Institute of UA. The Thermo Fisher LTQ Orbitrap Velos mass spectrometer was provided by grant #1S10 RR028868-01 from the NIH National Center for Research Resources.
Footnotes
Communicating editor: G. Bosco
Literature Cited
- Alvarez J., Vinas J., Alonso J. M., Albar J. P., Ashman K., et al. , 2002. Characterization and cloning of two isoforms of heteroglobin, a novel heterodimeric glycoprotein of the secretoglobin-uteroglobin family showing tissue-specific and sex differential expression. J. Biol. Chem. 277: 233–242. [DOI] [PubMed] [Google Scholar]
- Armstrong S. D., Robertson D. H., Cheetham S. A., Hurst J. L., Beynon R. J., 2005. Structural and functional differences in isoforms of mouse major urinary proteins: a male-specific protein that preferentially binds a male pheromone. Biochem. J. 391: 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird S. J., Macholán M., 2012. What can the Mus musculus musculus/M. m. domesticus Hybrid Zone Tell us about Speciation? Cambridge University Press, Cambridge, UK. [Google Scholar]
- Beier H., 2000. The discovery of uteroglobin and its significance for reproductive biology and endocrinology. Ann. N. Y. Acad. Sci. 923: 9–24. [DOI] [PubMed] [Google Scholar]
- Beynon R. J., Hurst J. L., 2003. Multiple roles of major urinary proteins in the house mouse, Mus domesticus. Biochem. Soc. Trans. 31: 142–146. [DOI] [PubMed] [Google Scholar]
- Bímová B., Karn R. C., Pialek J., 2005. The role of salivary androgen-binding protein in reproductive isolation between two subspecies of house mouse: Mus musculus musculus and Mus musculus domesticus. Biol. J. Linn. Soc. Lond. 84: 349–361. [Google Scholar]
- Bímová B., Albrecht T., Macholán M., Pialek J., 2009. Signalling components of the house mouse mate recognition system. Behav. Processes 80: 20–27. [DOI] [PubMed] [Google Scholar]
- Chamero P., Marton T. F., Logan D. W., Flanagan K., Cruz J. R., et al. , 2007. Identification of protein pheromones that promote aggressive behaviour. Nature 450: 899–902. [DOI] [PubMed] [Google Scholar]
- Cheetham S. A., Thom M. D., Jury F., Ollier W. E., Beynon R. J., et al. , 2007. The genetic basis of individual-recognition signals in the mouse. Curr. Biol. 17: 1771–1777. [DOI] [PubMed] [Google Scholar]
- Chen X., Shu S., Schwartz L. C., Sun C., Kapur J., et al. , 2010. Homeostatic regulation of synaptic excitability: tonic GABA(A) receptor currents replace I(h) in cortical pyramidal neurons of HCN1 knock-out mice. J. Neurosci. 30: 2611–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J., Ghazal P., Bingham R. W., Barrett D., Bishop J. O., 1985. Sequence structures of a mouse major urinary protein gene and pseudogene compared. EMBO J. 4: 3159–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clissold P. M., Hainey S., Bishop J. O., 1984. Messenger RNAs coding for mouse major urinary proteins are differentially induced by testosterone. Biochem. Genet. 22: 379–387. [DOI] [PubMed] [Google Scholar]
- Coyne J. A., Charlesworth B., 1997. Genetics of a pheromonal difference affecting sexual isolation between Drosophila mauritiana and D. sechellia. Genetics 145: 1015–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlouhy S. R., Karn R. C., 1983. The tissue source and cellular control of the apparent size of androgen binding protein (Abp), a mouse salivary protein whose electrophoretic mobility is under the control of sex-limited saliva pattern (Ssp). Biochem. Genet. 21: 1057–1070. [DOI] [PubMed] [Google Scholar]
- Dlouhy S. R., Karn R. C., 1984. Multiple gene action determining a mouse salivary protein phenotype: identification of the structural gene for androgen binding protein (Abp). Biochem. Genet. 22: 657–667. [DOI] [PubMed] [Google Scholar]
- Dlouhy S. R., Nichols W. C., Karn R. C., 1986. Production of an antibody to mouse salivary androgen binding protein (ABP) and its use in identifying a prostate protein produced by a gene distinct from Abp. Biochem. Genet. 24: 743–763. [DOI] [PubMed] [Google Scholar]
- Dlouhy S. R., Taylor B. A., Karn R. C., 1987. The genes for mouse salivary androgen-binding protein (ABP) subunits alpha and gamma are located on chromosome 7. Genetics 115: 535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez P., 1995. Cloning of a Syrian hamster cDNA related to sexual dimorphism: establishment of a new family of proteins. FEBS Lett. 376: 257–261. [DOI] [PubMed] [Google Scholar]
- Emes R. D., Riley M. C., Laukaitis C. M., Goodstadt L., Karn R. C., et al. , 2004. Comparative evolutionary genomics of androgen-binding protein genes. Genome Res. 14: 1516–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex-Sorlie D., 1995. Medical Biostatistics and Epidemiology. Appleton & Lange, Norwalk, CT. [Google Scholar]
- Fabre B., Lambour T., Garrigues L., Ducoux-Petit M., Amalric F., et al. , 2014. Label-free quantitative proteomics reveals the dynamics of proteasome complexes composition and stoichiometry in a wide range of human cell lines. J Proteome Res 13: 3027–3037. [DOI] [PubMed] [Google Scholar]
- Haga S., Hattori T., Sato T., Sato K., Matsuda S., et al. , 2010. The male mouse pheromone ESP1 enhances female sexual receptive behaviour through a specific vomeronasal receptor. Nature 466: 118–122. [DOI] [PubMed] [Google Scholar]
- Hurst J. L., 2009. Female recognition and assessment of males through scent. Behav. Brain Res. 200: 295–303. [DOI] [PubMed] [Google Scholar]
- Karn R. C., Dlouhy S. R., 1991. Salivary androgen-binding protein variation in Mus and other rodents. J. Hered. 82: 453–458. [DOI] [PubMed] [Google Scholar]
- Karn R. C., Laukaitis C. M., 2003. Characterization of two forms of mouse salivary androgen-binding protein (ABP): implications for evolutionary relationships and ligand-binding function. Biochemistry 42: 7162–7170. [DOI] [PubMed] [Google Scholar]
- Karn R. C., Laukaitis C. M., 2009. The mechanism of expansion and the volatility it created in three pheromone gene clusters in the mouse (Mus musculus) genome. Genome Biol. Evol. 1: 494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn R. C., Orth A., Bonhomme F., Boursot P., 2002. The complex history of a gene proposed to participate in a sexual isolation mechanism in house mice. Mol. Biol. Evol. 19: 462–471. [DOI] [PubMed] [Google Scholar]
- Karn R. C., Chung A. G., Laukaitis C. M., 2014. Did androgen-binding protein paralogs undergo neo- and/or subfunctionalization as the Abp gene region expanded in the mouse genome? PLoS One 9: e115454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimoto H., Touhara K., 2005. Induction of c-Fos expression in mouse vomeronasal neurons by sex-specific non-volatile pheromone(s). Chem. Senses 30(Suppl. 1): i146–i147. [DOI] [PubMed] [Google Scholar]
- Kimoto H., Haga S., Sato K., Touhara K., 2005. Sex-specific peptides from exocrine glands stimulate mouse vomeronasal sensory neurons. Nature 437: 898–901. [DOI] [PubMed] [Google Scholar]
- Kimoto H., Sato K., Nodari F., Haga S., Holy T. E., et al. , 2007. Sex- and strain-specific expression and vomeronasal activity of mouse ESP family peptides. Curr. Biol. 17: 1879–1884. [DOI] [PubMed] [Google Scholar]
- Laukaitis C., Karn R. C., 2012. Recognition of subspecies status mediated by androgen-binding protein (ABP) in the evolution of incipient reinforcement on the European house mouse hybrid zone, in Evolution of the House Mouse, edited by Macholan M., Munclinger P., Baird S. J., Pialek J. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Laukaitis C. M., Karn R. C., 2005. Evolution of the secretoglobins: a genomic and proteomic view. Biol. J. Linn. Soc. Lond. 84: 493–501. [Google Scholar]
- Laukaitis C. M., Critser E. S., Karn R. C., 1997. Salivary androgen-binding protein (ABP) mediates sexual isolation in Mus musculus. Evolution 51: 2000–2005. [DOI] [PubMed] [Google Scholar]
- Laukaitis C. M., Dlouhy S. R., Emes R. D., Ponting C. P., Karn R. C., 2005. Diverse spatial, temporal, and sexual expression of recently duplicated androgen-binding protein genes in Mus musculus. BMC Evol. Biol. 5: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukaitis C. M., Heger A., Blakley T. D., Munclinger P., Ponting C. P., et al. , 2008. Rapid bursts of androgen-binding protein (Abp) gene duplication occurred independently in diverse mammals. BMC Evol. Biol. 8: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukaitis C. M., Mauss C., Karn R. C., 2012. Congenic strain analysis reveals genes that are rapidly evolving components of a prezygotic isolation mechanism mediating incipient reinforcement. PLoS One 7: e35898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan D. W., Marton T. F., Stowers L., 2008. Species specificity in major urinary proteins by parallel evolution. PLoS One 3: e3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucignat Caretta C., Caretta A., Cavaggioni A., 1995. Pheromonally accelerated puberty is enhanced by previous experience of the same stimulus. Physiol. Behav. 57: 901–903. [DOI] [PubMed] [Google Scholar]
- Mukerjhee A., Chilton B., 2000. The uteroglobin/clara cell protein family. Ann. N. Y. Acad. Sci. 932: 1–358. [Google Scholar]
- Mukherjee A. B., Zhang Z., Chilton B. S., 2007. Uteroglobin: a steroid-inducible immunomodulatory protein that founded the Secretoglobin superfamily. Endocr. Rev. 28: 707–725. [DOI] [PubMed] [Google Scholar]
- Nagasawa H., Miyamoto M., Fujimoto M., 1973. Reproductivity in inbred strains of mice and project for their efficient production. Exp. Animals (Japan) 22: 119–126. [DOI] [PubMed] [Google Scholar]
- Noldus L. P., Trienes R. J., Hendriksen A. H., Jansen H., Jansen R. G., 2000. The Observer Video-Pro: new software for the collection, management, and presentation of time-structured data from videotapes and digital media files. Behav. Res. Methods Instrum. Comput. 32: 197–206. [DOI] [PubMed] [Google Scholar]
- Roberts S. A., Simpson D. M., Armstrong S. D., Davidson A. J., Robertson D. H., et al. , 2010. Darcin: a male pheromone that stimulates female memory and sexual attraction to an individual male’s odour. BMC Biol. 8: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. H., Cox K. A., Gaskell S. J., Evershed R. P., Beynon R. J., 1996. Molecular heterogeneity in the Major Urinary Proteins of the house mouse Mus musculus. Biochem. J. 316(Pt. 1): 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko A., Wilm M., Vorm O., Jensen O. N., Podtelejnikov A. V., et al. , 1996. A strategy for identifying gel-separated proteins in sequence databases by MS alone. Biochem. Soc. Trans. 24: 893–896. [DOI] [PubMed] [Google Scholar]
- Smadja C., Ganem G., 2002. Subspecies recognition in the house mouse: a study of two populations from the border of a hybrid zone. Behav. Ecol. 13: 312–320. [Google Scholar]
- Smith M. L., Souza F. G., Bruce K. S., Strang C. E., Morley B. J., et al. , 2014. Acetylcholine receptors in the retinas of the alpha7 nicotinic acetylcholine receptor knockout mouse. Mol. Vis. 20: 1328–1356. [PMC free article] [PubMed] [Google Scholar]
- Stowers L., Holy T. E., Meister M., Dulac C., Koentges G., 2002. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science 295: 1493–1500. [DOI] [PubMed] [Google Scholar]
- Stripp B. R., Lund J., Mango G. W., Doyen K. C., Johnston C., et al. , 1996. Clara cell secretory protein: a determinant of PCB bioaccumulation in mammals. Am. J. Physiol. 271: L656–L664. [DOI] [PubMed] [Google Scholar]
- Talley H. M., Laukaitis C. M., Karn R. C., 2001. Female preference for male saliva: implications for sexual isolation of Mus musculus subspecies. Evolution 55: 631–634. [DOI] [PubMed] [Google Scholar]
- Vošlajerová Bímová B., Macholán M., Baird S. E. B., Munclinger P., Laukaitis C. M., et al. , 2011. Reinforcement selection acting on the European house mouse hybrid zone. Mol. Ecol. 20: 2403–2424. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Kundu G. C., Yuan C. J., Ward J. M., Lee E. J., et al. , 1997. Severe fibronectin-deposit renal glomerular disease in mice lacking uteroglobin. Science 276: 1408–1412. [DOI] [PubMed] [Google Scholar]
- Zhou X., Wei Y., Xie F., Laukaitis C. M., Karn R. C., et al. , 2011. A novel defensive mechanism against acetaminophen toxicity in the mouse lateral nasal gland: role of CYP2A5-mediated regulation of testosterone homeostasis and salivary androgen-binding protein expression. Mol. Pharmacol. 79: 710–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data necessary for confirming the conclusions presented in the article are represented fully within the article and Supplemental Material. Strain information is available upon request.