Abstract
Foxl2 is essential for mammalian ovary maintenance. Although sexually dimorphic expression of foxl2 was observed in many teleosts, its role and regulative mechanism in fish remained largely unclear. In this study, we first identified two transcript variants of foxl2a and its homologous gene foxl2b in zebrafish, and revealed their specific expression in follicular layer cells in a sequential and divergent fashion during ovary differentiation, maturation, and maintenance. Then, homozygous foxl2a mutants (foxl2a−/−) and foxl2b mutants (foxl2b−/−) were constructed and detailed comparisons, such as sex ratio, gonadal histological structure, transcriptome profiling, and dynamic expression of gonadal development-related genes, were carried out. Initial ovarian differentiation and oocyte development occur normally both in foxl2a−/− and foxl2b−/− mutants, but foxl2a and foxl2b disruptions result in premature ovarian failure and partial sex reversal, respectively, in adult females. In foxl2a−/− female mutants, sox9a-amh/cyp19a1a signaling was upregulated at 150 days postfertilization (dpf) and subsequently oocyte apoptosis was triggered after 180 dpf. In contrast, dmrt1 expression was greater at 105 dpf and increased several 100-fold in foxl2b−/− mutated ovaries at 270 dpf, along with other testis-related genes. Finally, homozygous foxl2a−/−/foxl2b−/− double mutants were constructed in which complete sex reversal occurs early and testis-differentiation genes robustly increase at 60 dpf. Given mutual compensation between foxl2a and foxl2b in foxl2b−/− and foxl2a−/− mutants, we proposed a model in which foxl2a and foxl2b cooperate to regulate zebrafish ovary development and maintenance, with foxl2b potentially having a dominant role in preventing the ovary from differentiating as testis, as compared to foxl2a.
Keywords: Foxl2a, Foxl2b, premature ovarian failure, sex reversal, TALEN
SEX is one of the most intriguing puzzles in biology since antiquity, and has been considered “the queen of problems in evolutionary biology” (Bell 1982; Bachtrog et al. 2014). Most animals reproduce sexually, but the mechanisms of sex determination (SD) and differentiation are remarkably varied (Marín and Baker 1998; Barske and Capel 2008; Bonduriansky 2009; Williams and Carroll 2009; Gui and Zhu 2012; Matson and Zarkower 2012). Sex-determining mechanisms in teleosts are rapidly evolving and highly plastic but usually depend on genetic factors, with interactions between genetic and environmental factors affecting downstream differentiation (Devlin and Nagahama 2002; Volff et al. 2007; Desjardins and Fernald 2009; Mei and Gui 2015; Li et al. 2016). Three distinct SD genes, doublesex and mab-3 related transcription factor 1Y (Dmy/dmrt1bY) (Matsuda et al. 2002), gonadal somatic cell derived factor (gsdf) (Myosho et al. 2012), and sex-determining region Y (SRY)-box 3 (sox3) (Takehana et al. 2014), were respectively identified from three medaka species, Oryzias latipes, O. luzonensis, and O. dancena; suggesting that SD genes are diverse among different teleost species even in the same genus. An additional three SD genes—anti-Mullerian hormone receptor type 2 (amhr2) in tiger pufferfish (Takifugu rubripes) (Kamiya et al. 2012), Y-linked anti-Mullerian hormone (Amhy) in silversides (Odontesthes hatchery) (Hattori et al. 2012), and sexually dimorphic on the Y chromosome (sdY) in rainbow trout (Oncorhynchus mykiss) (Yano et al. 2012)—were also identified as key factors to initiate testicular differentiation. During and after gonadal cell specification, germ cells and somatic cells mutually interact to coordinate sex differentiation of the gonads (Shinomiya et al. 2000; Devlin and Nagahama 2002; Siegfried and Nüsslein-Volhard 2008; Nishimura and Tanaka 2014). In most teleost species, ovarian differentiation begins with the proliferation of somatic cells and oogonia and early oocyte differentiation, while testicular differentiation usually occurs several weeks or months later than ovarian differentiation (Nakamura et al. 1998; Devlin and Nagahama 2002; Saito et al. 2007). In addition, sex in many fishes, even in some with heteromorphic sex chromosomes, is influenced or overrode by a variety of environmental and social cues, such as sex steroids, temperature, pH, salinity, density, hypoxia, and others (Baroiller et al. 2009; Desjardins and Fernald 2009; Mei and Gui 2015).
Zebrafish (Danio rerio), as a vertebrate model animal (Grunwald and Eisen 2002), has been well studied in the contexts of development and disease. Yet, its primary SD and differentiation mechanisms still remain controversial (Nagabhushana and Mishra 2016). Although differentiated sex chromosomes were not found in domesticated zebrafish strains (AB and TU) (Sola and Gornung 2001), a sex-determining region was recently demonstrated in wild zebrafish on a subtelomeric region of the long arm of chromosome 4 (Anderson et al. 2012; Howe et al. 2013; Wilson et al. 2014). Zebrafish sex may be determined by multiple genes, along with the influences of primordial germ cells and environmental factors (Liew and Orban 2013; Nagabhushana and Mishra 2016). The early gonadal development of zebrafish is a complicated process. All gonads initially develop as an undifferentiated “juvenile ovary,” but approximately half of the ovarian tissues subsequently degenerate and transform into testicular tissue (Takahashi 1976). Although several key regulation genes—such as SRY-box 9 (sox9); nuclear receptor subfamily 5 group A (nr5a); cytochrome P450, family 19, subfamily A, polypeptide 1a (cyp19a1a); anti-Mullerian hormone (amh); GATA binding protein 4 (gata4); and Wilms tumor 1 (wt1)—show sexually dimorphic expression patterns and are involved in zebrafish gonad differentiation (Chiang et al. 2001; Trant et al. 2001; Rodríguez-Marí et al. 2005; Von Hofsten and Olsson 2005; Jorgensen et al. 2008; Sreenivasan et al. 2008), it is unclear which of these or others might serve as the initiator(s) or master switch(es) for zebrafish SD and subsequent differentiation.
Forkhead (FKH) box (Fox) proteins which have a highly conserved 100 amino acid FKH DNA-binding domain (FHD), play specialized functions in many key biological processes (Carlsson and Mahlapuu 2002; Benayoun et al. 2011). Named after the Drosophila melanogaster gene fork head (fkh) (Weigel et al. 1989), >2000 family members have been identified in numerous species. Based on similarities in the FHD and unified nomenclature of Fox genes (Kaestner et al. 2000), Fox proteins are divided into 19 subfamilies, from FoxA to FoxS (Fetterman et al. 2008; Hannenhalli and Kaestner 2009). Foxl2, one of the earliest known markers of ovarian differentiation (Cocquet et al. 2002), is required for granulosa cell differentiation (Schmidt 2004) and to prevent transdifferentiation of an adult ovary to a testis by suppressing the SRY target gene Sox9 (Uhlenhaut et al. 2009). FOXL2 was first identified in blepharophimosis-ptosis-epicanthus inversus syndrome (BPES) patients with eyelid and craniofacial malformations (Crisponi et al. 2001). In addition, FOXL2 mutation leads to premature ovarian failure (POF), a reduction in the number of germ cells in some BPES patients (De Baere et al. 2003; Meduri et al. 2010). In the Foxl2 null mouse, granulosa cells fail to undergo the squamous-to-cuboidal transition, which leads to arrest of folliculogenesis and oocyte atresia (Schmidt 2004; Kocer et al. 2008). In teleosts, foxl2 is expressed predominantly during ovary development of several species (Loffler et al. 2003), including Nile tilapia (Oreochromis niloticus) (Wang et al. 2004; Ijiri et al. 2008), rainbow trout (O. mykiss) (Baron 2004), dogfish (Scyliorhinus canicula) (Wotton et al. 2007), medaka (O. luzonensis) (Nakamoto et al. 2006), honeycomb grouper (Epinephelus merra) (Alam et al. 2008), wrasse (Halichoeres trimaculatus) (Kobayashi et al. 2010), African sharptooth catfish (Clarias gariepinus) (Sridevi and Senthilkumaran 2011; Bhat et al. 2016), and sablefish (Anoplopoma fimbria) (Smith et al. 2013). Additional studies have focused on associations of foxl2 with aromatase and sex hormones in the brain-pituitary-gonad axis (Nakamoto et al. 2006; Nakamoto et al. 2007; Wang et al. 2007; Yamaguchi et al. 2007; Von Schalburg et al. 2010; Navarro-Martín et al. 2011; Sridevi and Senthilkumaran 2011; Sridevi et al. 2012; Crespo et al. 2013). Two foxl2 homologous genes, foxl2 and foxl2-like, have been identified in zebrafish (Crespo et al. 2013) and are referred to here as foxl2a and foxl2b, respectively, in accordance with their phylogenetic relationship and current naming conventions. The sexually dimorphic expression pattern of zebrafish foxl2a was already reported (Siegfried and Nüsslein-Volhard 2008; Siegfried 2010; Caulier et al. 2015), however, its functional role and regulative mechanism remained largely unclear and the expression pattern and function of foxl2b were completely unknown. Here, we characterized a sequential and divergent foxl2a and foxl2b expression in zebrafish. Using a transcription activator-like effector nucleases (TALEN) approach (Li et al. 2013; Ota et al. 2013; Huang et al. 2014), we constructed mutants for foxl2a and foxl2b, and identified cooperative roles for these genes in regulating ovary differentiation and maintenance.
Materials and Methods
Maintenance of fish
The wild-type (WT) zebrafish of strain AB was used to produce mutant lines. Embryos were produced by in vitro fertilization according to the protocol described in the zebrafish book (Westerfield 2007). Each group for expression analysis consisted of 30 mutants and 30 WT zebrafish, which was reared in recirculating freshwater tanks from 4 dpf at 28.5° on a 14 hr light/10 hr dark cycle (Westerfield 2007). All procedures were performed with the approval of the Animal Care and Use Committee of the Institute of Hydrobiology, Chinese Academy of Sciences.
Cloning and sequence analysis
Total RNAs were isolated at different stages using SV RNA Isolation Reagent (Promega, Madison, WI) and they were reverse transcribed with M-MLV Reverse Transcriptase (Invitrogen, Carlsbad, CA) at 37° with Oligo (dT) Primer. To ascertain or reexamine full-length complementary DNA (cDNA) sequences of foxl2a and foxl2b, a zebrafish oocyte SMART cDNA library was constructed according to the SMARTer PCR cDNA Synthesis Kit User Manual (Clontech). The foxl2a and foxl2b cDNAs were amplified by 3′ and 5′ RACE with primers (Supplemental Material, Table S1) designed according to the sequences in GenBank (NM_001045252.1 and XM_009298955.1). The complete cDNA sequences of foxl2a-1, foxl2a-2, and foxl2b were deposited in GenBank (accession numbers KR232688, KR232689, and KR232690, respectively).
Multiple amino acid sequence alignment was performed with the Clustal X program and refined with GeneDoc software. Phylogenetic analysis was performed with the Molecular Evolutionary Genetics Analysis (MEGA 5) software (Tamura et al. 2011) and the phylogenetic tree was constructed with neighbor-joining methods and 1000 bootstrap permutations. All the amino acid sequences used in the multiple alignment and phylogenetic analysis were obtained from GenBank (http://www.ncbi.nlm.nih.gov/) and RefSeq (http://www.ncbi.nlm.nih.gov/RefSeq/). The accession numbers are as follows: Homo sapiens FOXL2, NP_075555; Mus musculus FOXL2, NP_036150; Gallus gallus FOXL2, NP_001012630; Chrysemys picta FOXL2, XP_005282573; O. niloticus Foxl2, NP_001266707; O. latipes Foxl2, NP_001098358; Salmo salar Foxl2, XP_014018845; O. mykiss Foxl2, NP_001117957; Cyprinus carpio Foxl2, AKU47820; Gobiocypris rarus Foxl2, ADN38241; D. rerio Foxl2a, NP_001038717; D. rerio Foxl2b, NP_001304690; Kryptolebias marmoratus FoxL2, XP_017277495; Fundulus heteroclitus Foxl2, JAR75273; Dicentrarchus labrax Foxl2, ACW83540; T. rubripes Foxl2, XM_003968745; Xenopus tropicalis Foxl2, XP_004917868; D. rerio Foxl3, AAI62838; T. rubripes Foxl2-like, XP_011620275; O. mykiss Foxl3, NP_001117956; S. salar Foxl2-like, ADJ38819; K. marmoratus Foxl3, APD78564; F. heteroclitus Foxl2-like, XP_012705892; D. labrax Foxl3, AFV13295; O. latipes Foxl2-like, XP_004070713; D. labrax Foxl1, CBN81215; D. rerio Foxl1, NP_957278; O. latipes Foxl1, NP_001116391; X. tropicalis Foxl1, AAI35134; G. gallus FOXL1, XP_001231599; H. sapiens FOXL1, NP_005241; and M. musculus FOXL1, NP_032050. Syntenic analyses were carried out using information extracted from the Ensembl (http://www.ensembl.org) genome and the chromosomal regions around foxl2s genes in H. sapiens chromosome 3, M. musculus chromosome 9, G. gallus chromosome 9, X. tropicalis chromosome 5, and D. rerio chromosome 2 and chromosome 15 were assembled.
Quantitative RT-PCR
Quantitative RT-PCR (qRT-PCR) was performed on a DNA Engine Chromo4 Real Time System (Bio-Rad, Hercules, CA) with SYBR green qRT-PCR master mix (ToYoBo, Osaka, Japan) as described (Huang et al. 2009). All qRT-PCR reactions were performed in 20 μl reactions containing 10 μl 2× SYBR green master mix (Bio-Rad), 0.5 μl (10 μM) of each primer, 1 μl reverse transcriptase RT synthetic cDNA template, and 8 μl double distilled water. Samples were run with the following program parameters: 95° for 1 min, followed by 39 cycles of 95° for 15 sec, 60° for 30 sec, 76° for 5 sec, 79° for 5 sec, and 81° for 5 sec, ending with the melt curve 65–95°, with 0.5°/sec with each temperature lasting for 0.5 sec. Negative control (no-template DNA reaction) was always included. Specificity of amplification for each reaction was analyzed by dissociation curves using CFX manager software (Bio-Rad). To determine the optimal reference genes (Bustin et al. 2009), seven housekeeping genes—including actin, β1 (actb1); glyceraldehyde-3-phosphate dehydrogenase (gapdh); glucose-6-phosphate dehydrogenase (g6pd); TATA box binding protein (tbp); eukaryotic translation elongation factor 1 α1, like 1 (eef1a1l1); polymerase (RNA) II (DNA directed) polypeptide D (polr2d); and succinate dehydrogenase complex, subunit A, flavoprotein (sdha)—were selected to check their expression stability at the developmental stages and in the tissues analyzed. Rank ordering of expression stability using geNorm analysis (Vandesompele et al. 2002) identified actb1 (M value = 1.387 < 1.5) and sdha (1.37) as the most stable normalizers during developmental stages and in the tissues analyzed, while eef1a1l1 (1.805), tbp (1.839), gapdh (2.206), and g6pd (2.59) were the most variable. Therefore, actb1 was selected as the normalizer for qRT-PCR in this study, and the relative expression levels of target genes were calculated using the 2−ΔΔCt method (Livak and Schmittgen 2001). All the samples were analyzed in triplicates.
Probe synthesis and in situ hybridization
For antisense probe synthesis, T7 RNA polymerase promoter was added to the 5′ end of reverse primers and a DIG RNA Labeling Kit (Roche, Mannheim, Germany) was used. In brief, a 775-bp cDNA fragment of foxl2a and a 528-bp cDNA fragment of foxl2b were amplified by specific primers foxl2a probe N, foxl2a probe T7C, foxl2b probe N, and foxl2b probe T7C (Table S1). The ovarian tissues were fixed with 4% paraformaldehyde (PFA) in PBS at 4° overnight. After washing with PBS (pH 7.0) three times, the samples were immersed in 30% saccharose-PBS buffer overnight at 4°, then embedded in Optimal Cutting Temperature compound (Leica, Wetzlar, Germany) and sectioned to 10 μm in thickness using frozen microtomy (Leica, Wetzlar, Germany). After being dried at 37° for 1 hr, the cryostat sections were stored at −70°. Messenger RNA (mRNA) detection by sectioned tissue in situ hybridization (Stahl and Simon 2010) was performed as previously described (Yin et al. 2007). Images were acquired by Zeiss Axio Observer A1 inverted microscope (Leica).
TALEN design and assembly for foxl2a and foxl2b
The first exon sequences of foxl2a and foxl2b genomic loci from the Ensembl zebrafish genome browser were verified by PCR and sequencing. Potential TALEN target sites in the loci were searched using the TAL Effector Nucleotide Targeter (https://tale-nt-.cac.cornell.edu/) with the following parameters: (1) spacer length of 15–20, (2) repeat array length of 12 to 19, (3) T at position 0, and (4) a restriction enzyme cutting site in the spacer. Gene-specific TALEN constructs were assembled using the FastTALE TALEN Assembly Kit (Sidansai Biotechnology). The resulting arrays with TALEN backbones (left, pCS2-PEAS; and right, pCS2-PERR) were confirmed by sequencing.
Establishment of foxl2a and foxl2b mutant zebrafish lines
For in vitro transcription of these TALENs, pCS2-PEAS-foxl2a, pCS2-PERR-foxl2a, pCS2-PEAS-foxl2b, and pCS2-PERR-foxl2b were linearized with NotI and recovered as corresponding transcription templates. The transcriptions were carried out with the mMESSAGE and mMACHINE SP6 Kit (Ambion) and then quantified using NanoDrop-2000 (Thermo Scientific). The left arm and right arm mRNAs of each TALEN pair were mixed at a molar ratio of 1:1 before microinjection, and the mRNA mixtures with 200–600 pg final concentrations were directly microinjected into zebrafish embryos at the one-cell stage.
The injected embryos were hatched at 28.5°. At 24 hr after injection of TALEN mRNAs, genomic DNA (gDNA) was extracted from 20 randomly sampled embryos to evaluate the efficiency of TALEN-induced somatic mutations. The gDNA was obtained by the NaOH method (Meeker et al. 2007). The gDNA fragments including the target site of the TALENs were amplified using foxl2a and foxl2b gene-specific primers (Table S1). After digestion with BstN I (restriction enzyme cutting site in the spacer of foxl2a) or BsrD I (restriction enzyme cutting site in the spacer of foxl2b) (New England Biolabs, Beverly, MA) respectively at 60° and 65° for 2 hr, the uncleaved DNA fragments were separated by gel electrophoresis and cloned into pMD-18T vector (Promega). Sequence alignments were generated to analyze whether or not the gDNA fragments from embryos injected with TALEN mRNAs were mutated.
The F0 founders carrying mosaic mutations were mated with WT zebrafish to generate heterozygous F1 offspring. The heterozygous individuals with frameshift or nonsense sequence alterations in the F1 generation were selected by fin-clip assay. Male and female siblings of the F1 generation carrying the same mutation were mated to generate homozygous F2 mutants as described previously (Xiong et al. 2017).
Sampling and histological examination
All fish sampled were anesthetized with MS-222 (tricaine methanesulfonate, 250 mg/l) (Sigma-Aldrich, St. Louis, MO) to measure body length and body weight. After the measurements, gonads of the fish were collected to calculate the gonadosomatic index (GSI) (gonadal weight/total body weight) values, and fixed in 4% PFA by overnight immersion. For histochemical analyses, 10-μm-thick sections were cut with frozen microtomy (Leica), stained with hematoxylin and eosin (Yulu, Nanchang, China), and mounted in neutral balsam (Sinopharm Chemical Reagent, Shanghai, China). Hematoxylin-eosin staining is performed as previously described (Xia et al. 2007). The slides were imaged by Zeiss Axio Observer A1 inverted microscope (Leica).
Transcriptome analysis
The ovaries from WT, foxl2a−/−, and foxl2b−/− mutants at 150 dpf were sampled to isolate total RNA. Three biological replicates were analyzed per group. The transcriptome analysis, including library construction, sequencing, and bioinformatic analysis were performed by Beijing Genomics Institute, China. Briefly, after quality assessment by Agilent 2100 Bioanalyzer (Agilent Technologies) and agarose gel electrophoresis, the total RNAs were used to construct libraries. A narrow 400–450 bp size range was selected and enriched. The quantification and qualification of libraries were assessed by Agilent 2100 Bioanaylzer (Agilent Technologies) and ABI StepOnePlus Real-Time PCR System (Thermo Scientific). The flow cells amplified from qualified libraries on cBot were sequenced using the Illumina HiSeq 4000 System and 150-bp pair-end reads were generated. The assembly was performed with clean reads to produce the transcripts which were mapped to the Zv10 zebrafish genome for bioinformatic analysis. Differential expression profiles between WT and mutant samples were calculated using RSEM [RNA sequencing (RNAseq) by expectation maximization] (Li and Dewey 2011). The transcripts were classified according to the official annotation of the Gene Ontology (GO) Consortium, and the Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichments were performed using phyper, a function of R. Statistical analysis of differentially expressed genes (DEGs) was performed using a relative change of ≥2 as the threshold to judge the significance of gene expression difference. False discovery rate (FDR) was used to determine the threshold of the P-value and GO or KEGG terms (FDR ≤ 0.01) were considered significantly enriched.
Statistical analysis
Statistical analyses were performed with SPSS (version 13.0) and OriginPro (version 8.0). All quantitative data were expressed as the mean ± SEM. Statistical analyses of the data were performed using an ANOVA, followed by Duncan’s multiple range test. A probability of P < 0.05 was considered statistically significant.
Data availability
The DNA sequences have been deposited at National Center for Biotechnology Information (NCBI), accession numbers from KR232688 to KR232690. The raw data of transcriptomes have been submitted to the NCBI database (accession number: SRR4239959). See Table S2 for gene symbols, full names, RefSeq identifications, and related references. All the Supplemental Table Legends have been shown in File S2.
Results
Molecular characterization of foxl2a and foxl2b in zebrafish
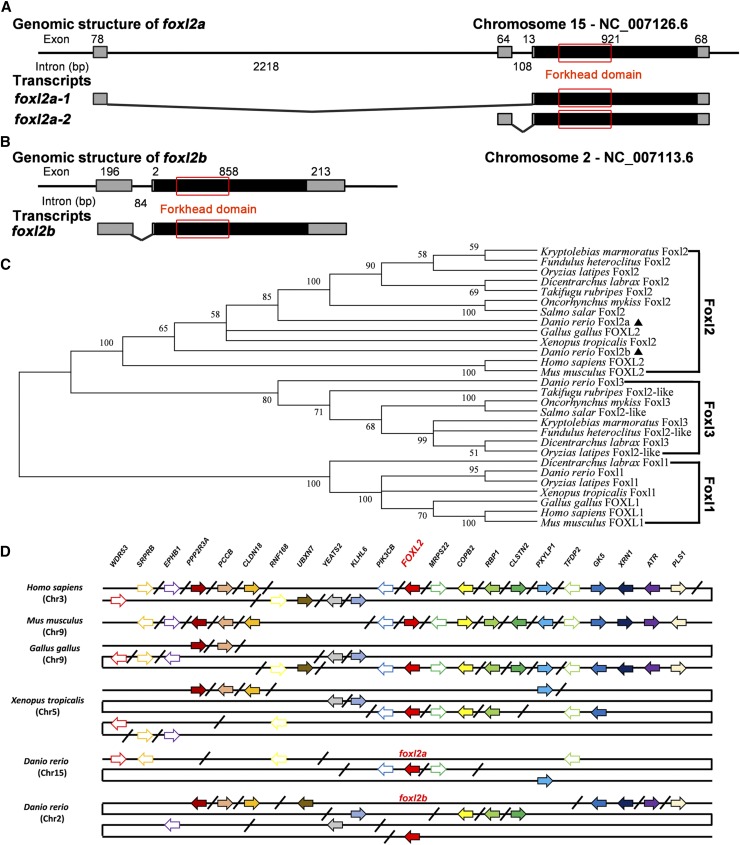
Similar to foxl2a transcripts (OTTDART00000051092 and OTTDART00000051093) in the Vertebrate Genome Annotation database (http://www.sanger.ac.uk/science/tools/vega-genome-browser), two transcript variants, named foxl2a-1 (KR232688) and foxl2a-2 (KR232689), were cloned from zebrafish ovary. Although the 5′ UTR sequences are different, they have an identical ORF (Figure 1A). The 5′ UTRs are transcribed from the first and second exon, respectively, suggesting that they arise from different transcriptional start sites. Moreover, foxl2b (KR232690) was also identified from zebrafish ovary (Figure 1B). The full-length cDNAs of foxl2a-1, foxl2a-2, and foxl2b are 1080, 1066, and 1269 bp, encoding 306, 306, and 285 amino acids, respectively. Similar to other vertebrate Foxl2 proteins, the FHD of zebrafish Foxl2a and Foxl2b are highly conserved, showing 98% amino acid identity, but their full amino acid sequences have only 64% identity (Figure S1 in File S1). Like other nonmammalian Foxl2 proteins, zebrafish Foxl2a and Foxl2b both lack glycine-rich (G), polyalanine tract (A), and proline-alanine-rich domains that are present in mammalian FOXL2 (Figure S1 in File S1). Phylogenetic analysis showed that vertebrate Foxl proteins were divided into three distinctive branches (Foxl1, Foxl2, and Foxl3). As shown in Figure 1, C and D, zebrafish Foxl2a and Foxl2b are both clustered into a Foxl2 grouping, indicating foxl2 is duplicated in the zebrafish genome (Figure 1C) with paralogs on chromosomes 15 and 2, respectively (Figure 1D). Other Foxl2-like genes are grouped with Foxl3, implying that they should be renamed as Foxl3 homologs. Although there is a highly conserved synteny within genomic regions of zebrafish, human, mouse, chicken, and frog around foxl2 genes; a complementary loss/retention pattern exists in the vicinity genes of foxl2a and foxl2b between zebrafish chromosome 15 and chromosome 2 (Figure 1D), indicating that they might undergo divergent evolution after duplication.
Figure 1.
Molecular characterization of zebrafish foxl2a and foxl2b. (A and B) Genomic structure and transcript schematics of zebrafish foxl2a and foxl2b. Exons and introns are shown by boxes and horizontal lines, respectively. ORFs and FHD are highlighted by black boxes and red boxes, respectively. The exon and intron size are indicated above or below as bp. (C) Phylogenetic tree of Foxl proteins in vertebrates. (D) Syntenic alignment of chromosomal regions around vertebrate foxl2 genes. Zebrafish foxl2a and foxl2b are located on chromosome 15 and 2, respectively. Chromosome segments are represented as thick lines. The conserved gene blocks are shown in matching colors and the transcription orientation are indicated by arrows. The oblique line between two genes shows their discontinuity. Gene symbols, full names, RefSeq identifications, and related references are listed in Table S2. Chr, chromosome.
Sexually dimorphic expression between adult gonads and sequential and divergent expression of foxl2a and foxl2b during ovary development
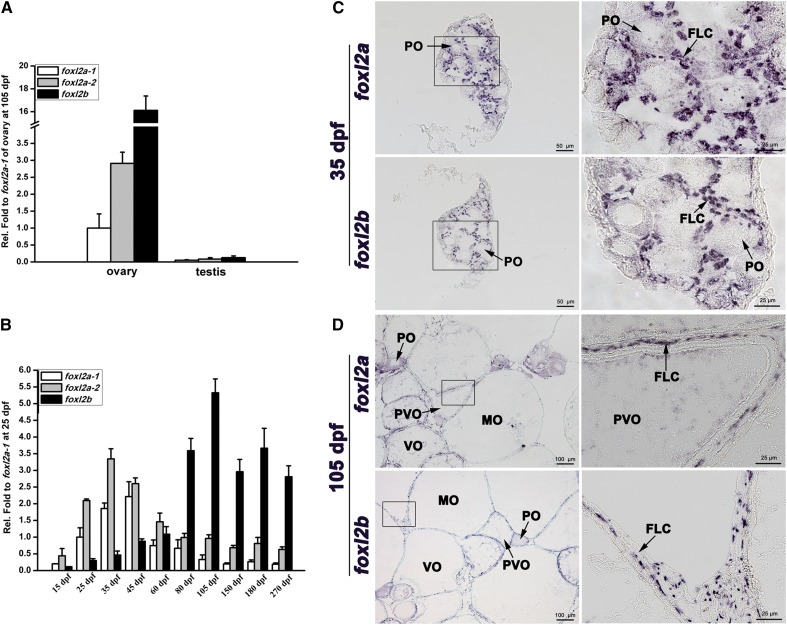
First, sexually dimorphic expression of foxl2a and foxl2b was observed by qRT-PCR in zebrafish mature ovaries and testes. As shown in Figure 2A, foxl2a-1, foxl2a-2, and foxl2b were all expressed in mature ovaries, whereas their expression was almost absent in mature testes. Then, we analyzed their expression levels during ovary differentiation, maturation, and maintenance. As shown in Figure 2B, foxl2a including foxl2a-1 and foxl2a-2 initiated expression early during ovary differentiation, and quickly reached peak values at 45 dpf (foxl2a-1) and 35 dpf (foxl2a-2), respectively. By contrast, foxl2b expression was relatively lower and gradually increased through the early stages of ovary differentiation from 15 to 45 dpf. Then, coinciding with diminishing expression of foxl2a-1 and foxl2a-2, foxl2b expression increased and reached a peak at 105 dpf, and then remained at a relative high level in the mature ovary and stages of oocyte maintenance (Figure 2B).
Figure 2.
Expression characterization of zebrafish foxl2a-1, foxl2a-2, and foxl2b during ovary development and maintenance. (A) Sexually dimorphic expression between mature ovary and testis. (B) Sequential and divergent expression during ovary development and maintenance from 15 to 270 dpf. The qRT-PCR quantification of gene expression was normalized to actb1. The relative expression values were first calculated against actb1 and then against that of foxl2a-1 at 25 dpf. (C and D) Ovary section in situ localization at (C) 35 dpf and (D) 105 dpf. Bars are shown at bottom-right of the images.
The cellular localization and spatio-temporal distribution of foxl2a and foxl2b were assessed in ovaries at 35 and 105 dpf by in situ hybridization on tissue sections. Both foxl2a and foxl2b were expressed in follicular layer cells (FLCs) around the primary-growth oocytes (POs) in 35 dpf ovary, and foxl2a transcript signal was stronger than foxl2b (Figure 2C). At 105 dpf, foxl2a transcript was mainly localized in FLCs around POs, previtellogenic oocytes (PVOs), and vitellogenic oocytes (VOs), and its signal became very weak in mature oocytes (MOs) (Figure 2D). In contrast, foxl2b transcript was ubiquitously localized in FLCs around all oocytes (POs, PVOs, VOs, and MOs), and became stronger in MOs at 105 dpf ovary (Figure 2D). Therefore, foxl2a is expressed in early FLCs and may function early in ovary development, whereas foxl2b may function later.
Establishment of foxl2a and foxl2b knockout mutant lines
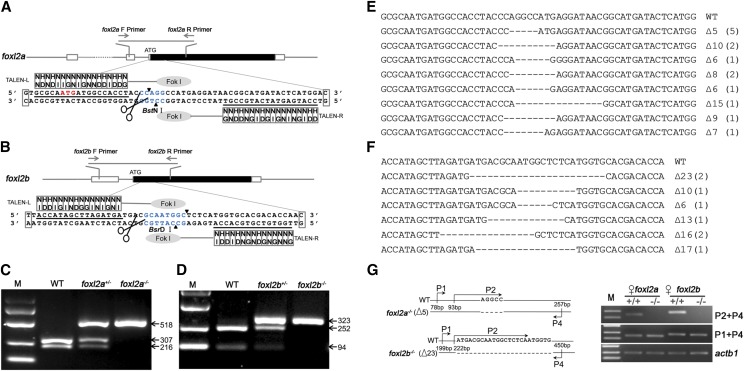
To explore the function of foxl2a and foxl2b in zebrafish SD and differentiation, we constructed two homozygous mutant lines, foxl2a−/− and foxl2b−/−, by using TALEN. The TALEN target sites near the translation start codon were chosen to disrupt the translation of Foxl2a and Foxl2b proteins (Figure 3, A and B). Specific primers were designed to amplify a foxl2a or foxl2b DNA fragment containing the TALEN target site and a cleavage site with BstN I or BsrD I, respectively (Figure 3, A and B). A 523-bp DNA fragment of foxl2a amplified from WT embryos was completely digested by BstN І to produce two DNA fragments (307 and 216 bp), while the fragments from TALEN F0 embryos could not be completely digested (Figure 3C). This suggested that F0 embryos were chimeric for modification of the BstN І site. Indeed, a total of eight different indels modified at the target site were found from 20 embryos (Figure 3E), and the mutation frequency in F0 founders was ∼70% (14/20). Similarly, foxl2b was also successfully disrupted by the TALEN approach (Figure 3, D and F), and the mutation rate was 66.7% (8/12) in the F0 founders (Figure 3F).
Figure 3.
Establishment of foxl2a and foxl2b knockout mutant lines by TALEN. (A and B) The TALEN target sites of zebrafish (A) foxl2a and (B) foxl2b. The coding and untranslated exon regions are depicted as solid and open boxes, respectively. The left and right TALEN binding sites are indicated by underlining. Cleavage sites with BstN I and BsrD I in the spacer are shown by blue color, and forward and reverse primers (F primer and R primer) are indicated in the corresponding sites. (C and D) Detection of (C) foxl2a and (D) foxl2b mutants by BstN I or BsrD I digestion. The amplified fragment sizes (bp) are shown on the right. (E and F) Sequences of different indels of TALEN-induced (E) foxl2a and (F) foxl2b mutants in F0 embryos. A total of eight and six indels (number of embryos are indicated in each bracket) at targeted locus are shown for (E) foxl2a and (F) foxl2b, and the numbers at the right-hand side indicate the number of deleted base pairs. (G) Transcription-level confirmation of foxl2a and foxl2b mutants by RT-PCR. The detected primers are shown on the left, and primer P2 is specific for the deleted sequences. The actb1 was used as control. F, forward; R, reverse; M, marker.
Subsequently, the remaining F0 embryos were raised to adulthood and crossed with WT zebrafish. PCR amplification and digestion were done with gDNA of F1 embryos to test for germ line transmission of induced lesions. Since the 5-bp deletion (Δ5) in foxl2a leads to a frameshift, it was chosen to be crossed with WT zebrafish to obtain F1 offspring. Sibling F1 male and female individuals carrying the same mutation were crossed with each other to produce homozygous F2 mutants (foxl2a−/−) whose genotypes were also confirmed by PCR amplification and digestion. The PCR-amplified fragment from homozygous foxl2a−/− F2 mutants could not be digested by BstN І (Figure 3C). Similarly, a 23-bp deletion (Δ23) in foxl2b leading to frameshifting of the coding sequence was also selected to successfully establish the foxl2b mutant line (foxl2b−/−) (Figure 3, D and F). To confirm successful deletion in foxl2a and foxl2b genes, RT-PCR analyses were performed to ensure no expression of functional transcripts. Indeed, no WT transcripts were detected in the mutant ovaries by using the specific primers over the deleted sequences of foxl2a−/− (Δ5) and foxl2b−/− (Δ23) (Figure 3G).
Foxl2a deficiency leads to POF in adult females
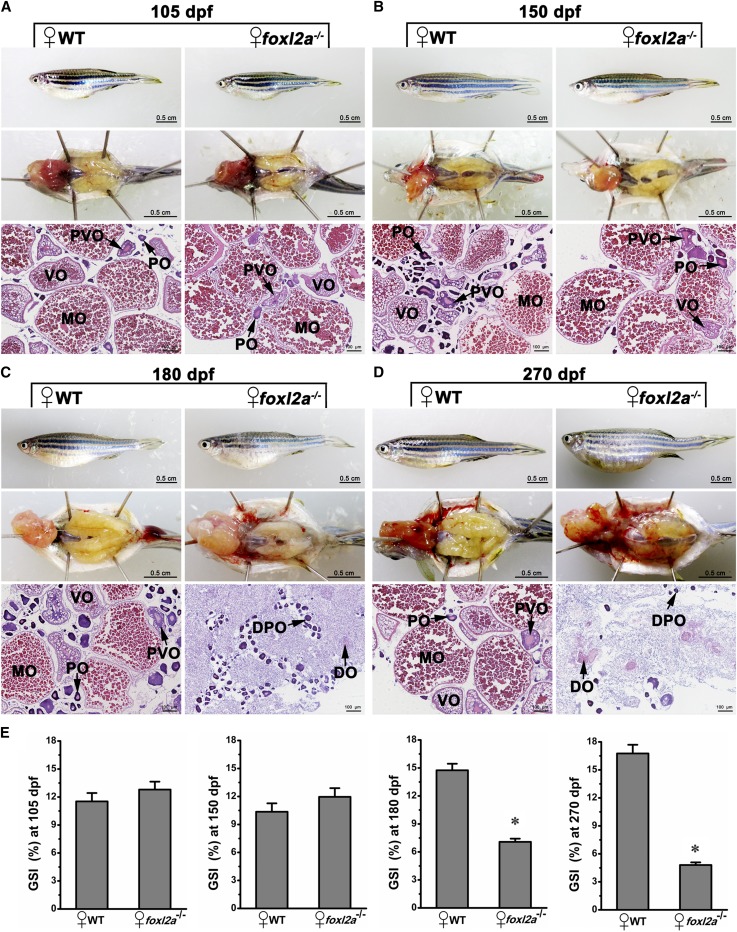
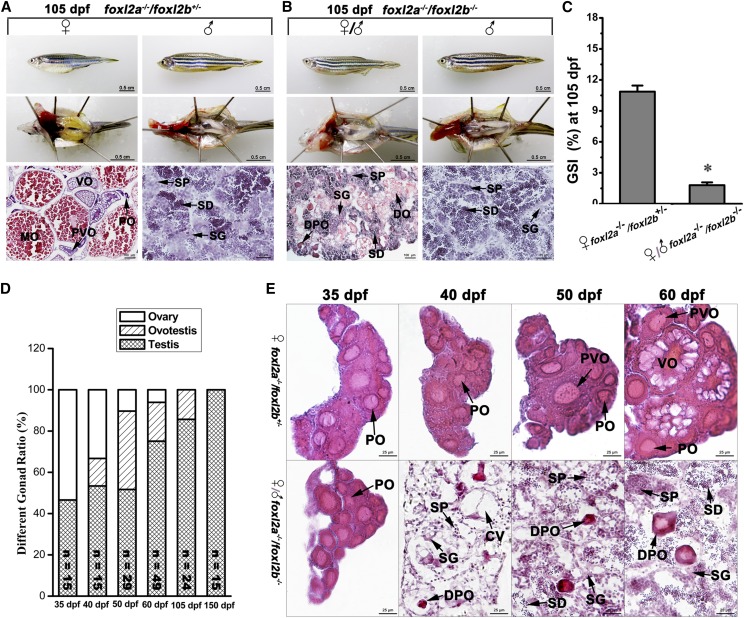
To explore the function of foxl2a and exclude the effects of environmental factors on zebrafish SD and differentiation, we raised to adulthood 30 homozygous foxl2a−/− F3 individuals and 30 control individuals together in one aquarium with 6 replicates. Zebrafish females began to spawn around 3 months after hatching in our aquarium system. The gross morphology and histology of the ovaries were analyzed both in the controls (WT) and homozygous mutants (foxl2a−/−). It was difficult to identify mutants by body size and shape (Figure 4), so we randomly sampled individuals (including mutants and controls) at different ages to identify genotype and sex. As shown in Figure S2 in File S1, foxl2a−/− mutants and controls both had a normal 1:1 sex ratio. At 35 dpf, zebrafish gonads could be distinguished as either an ovary or a testis by morphology under dissection microscope. Histological analyses showed that the gonads of foxl2a−/− female mutants contained numerous POs, similar to controls (Figure S3 in File S1). At 60 dpf, some oocytes of both foxl2a−/− mutants and controls started to enter the PVO and VO stage (Figure S3 in File S1). This indicates that initial ovarian differentiation and oocyte development occur normally in foxl2a−/− mutants. At 105 dpf when adult fishes were ready for spawning, the ovaries from foxl2a−/− mutants and controls both contained large numbers of POs, PVOs, VOs, and MOs (Figure 4A). The results indicate that foxl2a−/− female mutants exhibit normal ovarian differentiation and oocyte development.
Figure 4.
Disruption of foxl2a leads to similar POF in adult females. (A–D) Gross morphology, anatomical, and histological examination of adult ovaries in WT controls and foxl2a mutants (foxl2a−/−) were analyzed at (A) 105 dpf (n = 12), (B) 150 dpf (n = 6), (C) 180 dpf (n = 3), and (D) 270 dpf (n = 6). (E) GSI values in control females and foxl2a mutant females. Data are presented as mean ± SEM * P ≤ 0.05 (Duncan’s multiple range test). Bars are shown at bottom right of the images. ♀, female; ♂, male.
Considering POF in some human BPES patients with FOXL2 mutation, we continued to raise foxl2a−/− mutants and controls together after spawning, and examined the status of ovaries during later adult life. Because ovulation occurs asynchronously, zebrafish can spawn continuously after maturation. Oocytes of both foxl2a−/− mutants and controls were recruited into maturation and ovulated again at 150 dpf (Figure 4B). In contrast to controls, for which all females spawned again, ∼10 and 60% of foxl2a−/− female mutants with enlarged abdomens failed to spawn again at 180 and 270 dpf, respectively. Dissection showed that the ovaries of foxl2a−/− mutants became white at 180 dpf, and a large number of somatic cells filled in the lobules of ovarian tissues (Figure 4C). No oocytes at the previtellogenic and vitellogenic stages were observed, and only some degenerating primary-growth oocytes (DPOs) and degenerating oocytes (DOs) existed in the edges of each lobule (Figure 4C). Along with oocyte failure, the ovaries of foxl2a−/− mutants became transparent and aggravated, and only a few DPOs and vestiges of DOs were retained in lobules full of proliferating somatic cells (Figure 4D). In contrast, control individuals could persistently spawn, and the ovaries still contained POs, PVOs, VOs and MOs at 180 and 270 dpf (Figure 4, C and D). Although equal GSI values were observed among all the analyzed females at 105 and 150 dpf, the GSI values of foxl2a−/− females were much lower than those of WT females at 180 and 270 dpf (Figure 4E) (P < 0.05). The accelerated ovarian aging and oocyte failure in foxl2a−/− mutants are very similar to POF in human patients (Pal and Santoro 2002), suggesting that zebrafish foxl2a might also play a similar role in regulating ovarian senescence like its homologous gene in humans.
Foxl2b disruption results in partial sex reversal in adult females
Similarly, we also raised 30 homozygous foxl2b−/− F3 individuals and 30 control individuals together in one aquarium with six repeats. Foxl2b−/− mutants and controls both had normal 1:1 sex ratios from 35 to 150 dpf (Figure S2 in File S1). In contrast to control females that all spawned again, ∼20 and 70% of foxl2b−/− female mutants failed to spawn again at 180 and 270 dpf, respectively (Figure S2 in File S1 and Figure 5). In addition, the secondary sexual characteristics of these foxl2b−/− female individuals progressively transformed from those of males to females. At 105 and 150 dpf, these females still manifested typical secondary feminine characteristics, having a round and bluish body with a large visible urogenital papilla and an indistinct anal fin coloration (Figure 5, A and B). From 180 dpf, some foxl2b−/− females exhibited mixed secondary sexual characteristics, including a slim and reddish body, an anal fin with distinct markings and a feminine urogenital papilla (Figure S4C in File S1). This implies that the homozygous foxl2b−/− female mutants undergo sex change after maturation. To further investigate the sex in foxl2b−/− females, the ovaries were sectioned for histological analyses. The ovaries of these mutants became transparent or white and exhibited partial sex reversal with different proportions of degenerating ovarian tissue and newborn testicular structure (Figure 5, C and D). At 180 dpf, lobules contained numerous somatic cells, with DPOs and vestiges of DOs scattered in the early transitional gonads (Figure 5, C and E). Along with the process of sex reversal, some similar spermatogenic cysts (SCs) containing spermatogonia (SG), spermatocytes (SPs), and spermatids emerged at 270 dpf, and a few of vestiges of DOs were still retained in the lobules (Figure 5, D and F). However, the female foxl2b−/− mutants did not completely change to fertile males, and gradually died off after 270 dpf, likely owing to hyperplasia of testicular and kidney tissues. Meanwhile, the GSI values of foxl2b−/− females in the process of sex reversal decreased more sharply than those of WT females from 180 to 270 dpf (Figure 5G) (P < 0.05). Therefore, foxl2b might play different roles in regulating ovary development and maintenance as compared to foxl2a.
Figure 5.
Partial sex reversal toward testicular tissue in foxl2b-deficient adult females. (A–F) Gross morphology, anatomical, and histological examination of adult ovaries in controls (WT) and foxl2b mutants (foxl2b−/−) were analyzed at (A) 105 dpf (n = 13), (B) 150 dpf (n = 6), (C) 180 dpf (n = 5), and (D) 270 dpf (n = 7). Higher magnifications of the boxed areas in (C) and (D) are shown in (E) and (F). (G) GSI values in control females and foxl2b mutant females. Data are presented as mean ± SEM * P ≤ 0.05 (Duncan’s multiple range test). ♂, female; ♀, male; SD, spermatid. Bars are shown at bottom right of the images.
Transcriptome profiling comparisons of initial transformation ovaries in foxl2a−/− or foxl2b−/− females relative to normal ovaries in control females
To reveal the pathways or genes influenced early by foxl2a or foxl2b deficiency, we performed comparative transcriptome analyses to investigate DEGs between initial transformation ovaries in foxl2a−/− mutants, or foxl2b−/− mutants and normal ovaries at the same age. In mice, Foxl2 cooperates with estrogen receptor 1 (ESR1) to repress Sox9 expression (Uhlenhaut et al. 2009). Before transcriptome analyses, we first tested whether this pathway was conserved in zebrafish by evaluating sox9a expression changes at 150 dpf, because significant changes in gonad histology had occurred at 180 dpf, even though no apparent abnormalities were observed at 150 dpf (Figure 4B and Figure 5B). Indeed, the expression of sox9a was up-regulated both in foxl2a−/− and foxl2b−/− mutant ovaries at 150 dpf (Figure S5 in File S1), indicating that the gonadal transformation process had already been initiated. Therefore, the ovary at 150 dpf is suitable for performing RNAseq analysis. After filtration to remove the reads with low quality or adaptors, ∼30,000,000 clean reads in each sample were obtained and assembled into ∼23,000 transcripts (Table S3). In comparison with control ovaries, a total of 6005 and 6253 DEGs were respectively revealed in foxl2a−/− and foxl2b−/− ovaries at 150 dpf (Table S4), in which 1220 and 1314 DEGs were both upregulated or downregulated in foxl2a−/− and foxl2b−/− mutant ovaries (Figure S6A in File S1 and Table S4). The pathway with most annotated unigenes of commonly upregulated DEGs in KEGG was the “TNF signaling pathway,” followed by “cytokine-cytokine receptor interaction” (Figure S6B in File S1 and Table S5), indicating the pathways involved in apoptosis or necroptosis were activated in both foxl2a−/− and foxl2b−/− mutant ovaries. In contrast, three pathways in “lipid metabolism” were found in the top 10 enriched KEGG pathways of commonly downregulated DEGs, implying the lipid synthesis or secretion was suppressed in both foxl2a−/− and foxl2b−/− mutant ovaries compared to control ovaries (Figure S6C in File S1 and Table S5).
To reveal differential regulation by foxl2a and foxl2b, the DEGs specifically changed in foxl2a−/− and foxl2b−/− mutant ovaries were respectively analyzed. As shown in Figure S6A in File S1, a total of 4471 and 3719 unigenes changed their expression levels in foxl2a−/− and foxl2b−/− mutant ovaries compared to control ovaries. The KEGG mapping analysis showed the differentially enriched pathways between these DEGs (Figure S6, D–G, in File S1; Table S5). Among the top 20 enriched pathways, only one pathway (“neuroactive ligand-receptor interaction”) was shared between specifically upregulated DEGs from foxl2a−/− and foxl2b−/− mutant ovaries. Significantly, several important gonadal development-related genes (e.g., sox9a, wt1, gata4, sox3, and nr5a2) and foxl2b were specifically upregulated in foxl2a−/− mutant ovaries, while dmrt1 and sox9b were upregulated in foxl2b−/− mutant ovaries (Table S4).
Dynamic expression changes of gonadal development-related genes in foxl2a−/− or foxl2b−/− female mutants during gonad transformation
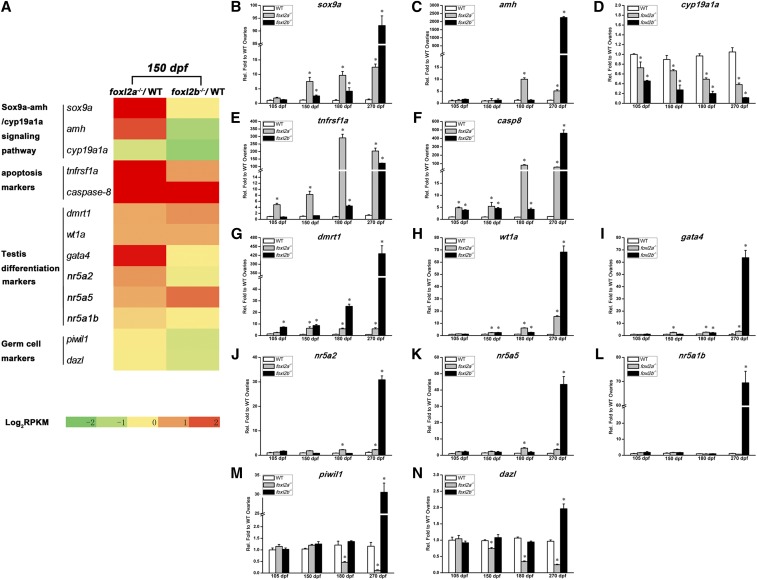
In consideration of the significant gonad transition that occurred in foxl2a−/− or foxl2b−/− female mutants (Figure 4 and Figure 5), we selected several genes involved in gonadal development (Table S4, Table S5, and Figure 6A) to further confirm their dynamic expression changes by using qRT-PCR analyses in transforming gonads from 105 to 270 dpf (Figure 6, B–N). This analysis revealed differential modulation of gene expression between foxl2a−/−/WT and foxl2b−/−/WT ovaries from 105 to 270 dpf. As expected, sox9a expression started to rise after the sexual maturity in foxl2a−/− female mutants at 105 dpf, and gradually increased up to 12-fold at 270 dpf. In contrast, the expression of sox9a rose at 150 dpf and increased up to 92-fold in foxl2b−/− female mutants against that of control ovaries at 270 dpf (Figure 6B). As a downstream target of sox9a, amh expression started to rise after sox9a increase (150 dpf) and reached a peak with a 10-fold increase at 180 dpf in foxl2a−/− mutants, while it maintained a low expression level until 180 dpf and increased dramatically (2255-fold) in expression at 270 dpf in foxl2b−/− female mutants (Figure 6C). cyp19a1a gradually decreased in abundance in both foxl2a−/− and foxl2b−/− female mutants, but the downward trend was more violent in latter (Figure 6D). Consistent with oocyte apoptosis and degeneration in foxl2a−/− and foxl2b−/− mutants, the expression of apoptosis-related genes casp8 and tnfrsf1a gradually increased from 105 dpf. These data support the possibility that the upregulation of sox9a-amh/cyp19a1a signaling triggers oocyte apoptosis and degeneration in both female foxl2a−/− mutants and foxl2b−/− mutants. Interestingly, the dynamic expression changes in foxl2b−/− mutants showed a delayed pattern compared to foxl2a−/− mutants (Figure 6, B–F), suggesting a transition in functional requirements from foxl2a to foxl2b at later stages.
Figure 6.
Transcriptome profiling changes of gonadal development-related genes in ovaries of foxl2a−/− and foxl2b−/− mutants. (A) Relatively transcribed changes of several kinds of gonadal development-related genes between foxl2a−/− and WT ovaries or foxl2b−/− and WT ovaries at 150 dpf. (B–N) Relative expression qRT-PCR detection of the indicated genes in WT, foxl2a−/−, and foxl2b−/− ovaries from 105 to 270 dpf (n = 3). The qRT-PCR quantification of each gene expression is normalized to actb1, and the data are presented as mean ± SEM * P ≤ 0.05 (Duncan’s multiple range test).
Compared to that in corresponding control ovaries, testis differentiation-related genes, such as dmrt1, wt1a, gata4, nr5a2, and nr5a5, maintained a moderately increased expression in ovaries of foxl2a−/− mutants, while their expression in foxl2b−/− mutants increased dramatically from dozens to several 100-fold at 270 dpf (Figure 6, G–K), consistent with the sex change that occurred in foxl2b−/− mutants (Figure 5). Although nr5a1b expression was not significantly different in ovaries between the female controls and foxl2a−/− mutants, it also increased by ∼69-fold at 270 dpf in the ovotestis of foxl2b−/− mutants (Figure 6L). These results suggest that foxl2a and foxl2b might play some divergent roles in regulating ovary development and maintenance, where foxl2b is specific for inhibiting testis development.
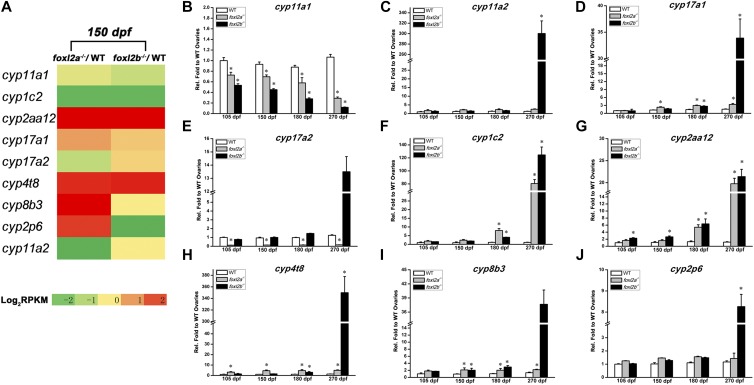
Besides the degeneration of oocytes, the massive increase of somatic cells (Figure 4, C and D) indicates that a POF might have occurred in foxl2a-deficient female zebrafish. Both germ cell marker genes piwil1 and dazl expression levels in foxl2a−/− mutant ovaries were equal to those of control ovaries at 105 and 150 dpf. However, they sharply decreased in abundance to 11 and 24% at 270 dpf, respectively (Figure 6, M and N), indicating that the germ cells largely reduce in foxl2a−/− mutant ovaries from 180 dpf. In contrast, piwil1 and dazl expression in foxl2b−/− female mutants increased in ovotestis at 270 dpf (Figure 6, M and N). This is probably due to a large number of spermatogenic cysts that contain male germ cells generated during gonad transformation from ovary to testis. Additionally, nine cytochrome P450 (cyp) genes also showed differential modulation of gene expression between foxl2a−/−/WT and foxl2b−/−/WT ovaries from 105 to 270 dpf (Figure 7), indicating that both foxl2a and foxl2b regulate the steroidogenesis in zebrafish gonads, and their effects on steroid homeostasis differ.
Figure 7.
Transcriptome profiling changes of cyp genes in the ovaries of foxl2a−/− and foxl2b−/− mutants. (A) Relatively transcribed changes of nine cyp genes between foxl2a−/− and WT ovaries or foxl2b−/− and WT ovaries at 150 dpf. (B–J) Relative expression qRT-PCR detection of nine cyp genes in WT, foxl2a−/−, and foxl2b−/− ovaries from 105 to 270 dpf (n = 3). The qRT-PCR quantification of each gene expression is normalized to actb1, and the data are presented as mean ± SEM * P ≤ 0.05 (Duncan’s multiple range test).
Simultaneous disruption of foxl2a and foxl2b leads to complete sex reversal
Owing to the high identity in amino acid sequence and the sequential and divergent expression between foxl2a and foxl2b, we speculated that their functions would be compensatory in some ways. To address this, zebrafish homozygous foxl2a/foxl2b F2 double mutants (foxl2a−/−/foxl2b−/−) carrying Δ5 in foxl2a and Δ23 in foxl2b were created. One-cell embryos were co-injected as described above with the two TALEN mRNAs which caused foxl2a mutation or foxl2b mutation, respectively. The heterozygous foxl2a+/−/foxl2b+/− double mutant individuals were chosen to cross with each other. In the examined 11 homozygous double mutant individuals with foxl2a−/−/foxl2b−/− genotype from the crossing, interestingly, only mature males with normal testis (Figure S7 in File S1) were found, whereas no adult females were observed. To acquire more homozygous foxl2a−/−/foxl2b−/− mutants, we raised offspring of foxl2a−/−/foxl2b+/− (female) crossed with foxl2a−/−/foxl2b−/− (male) in one aquarium. At 105 dpf, a total of 45 individuals were randomly sampled to examine their genotypes and gonads, and 24 individuals were identified as homozygous foxl2a−/−/foxl2b−/−. In comparison with normal gonads in foxl2a−/−/foxl2b+/− female and male adults (11 females and 10 males) (Figure 8A), again, no mature females with normal ovaries were observed. Only males were detected from the 24 homozygous foxl2a−/−/foxl2b−/− adults, of which three individuals showed dual secondary sexual characteristics, such as feminine urogenital papilla and masculine anal fin (Figure S2 and Figure S4D in File S1), and abnormal ovotestis with DPOs and DOs as well as numerous SCs containing SG, SPs, and spermatids (Figure 8B). Another 21 individuals were found to have normal secondary masculine characteristics, including a nonvisible papillae, a slim and reddish body, and a big anal fin with distinct markings (Figure S4D in File S1), and normally developed testis full of SG, SPs and spermatids (Figure 8B). GSI values of homozygous foxl2a−/−/foxl2b−/− females with sex reversal characteristics were much lower than those of heterozygous foxl2a−/−/foxl2b+/− females at 105 dpf (Figure 8C) (P < 0.05).
Figure 8.
Complete sex reversal in homozygous foxl2a−/−/foxl2b−/− females. (A and B) Gross morphology, anatomical, and gonadal histology examination of (A) heterozygous double mutants (foxl2a−/−/foxl2b+/−) (n = 13) and (B) homozygous double mutants (foxl2a−/−/foxl2b−/−) (n = 3) at 105 dpf. (C) GSI in foxl2a−/−/foxl2b+/− and foxl2a−/−/foxl2b−/− mutants at 105 dpf. Data are presented as mean ± SEM * P ≤ 0.05 (Duncan’s multiple range test). (D) Gonadal development status of homozygous foxl2a−/−/foxl2b−/− individuals from young fishes to adults at 35, 40, 50, 60, 105, and 150 dpf. (E) Gonadal histology examination of heterozygous double mutants (foxl2a−/−/foxl2b+/−) and homozygous double mutants (foxl2a−/−/foxl2b−/−) at 35 dpf (n = 7), 40 dpf (n = 3), 50 dpf (n = 7), and 60 dpf (n = 9). ♀, females; ♂, males; CV, cavity; SD, spermatid. Bars are shown at bottom right of the images.
To investigate when foxl2a/foxl2b deficiency affects ovary development and leads to sex reversal, we checked the gonadal development status of homozygous foxl2a−/−/foxl2b−/− individuals from young to adults at 35, 40, 50, 60, 105, and 150 dpf. As shown in Figure S2 in File S1 and Figure 8D, the ratios of ovary, ovotestis, and testis in foxl2a−/−/foxl2b−/− mutants were very different, changing from seven ovaries:eight testes (∼50%) at 35 dpf to 0 ovaries:15 testes (100% testes), and ∼13–38% of ovotestes existed from 40 to 105 dpf. Moreover, we examined the gonadal histology of these sex reversal individuals from 35, 40, 50, and 60 dpf. As shown in Figure 8E, in comparison with normal ovaries with POs, PVOs, and VOs in heterozygous double mutant (foxl2a−/−/foxl2b+/−) females, the female individuals in homozygous foxl2a−/−/foxl2b−/− mutants began to exhibit extensive oocyte degeneration and initiative sex reversal early from 40 dpf, even though they showed normal ovary differentiation at 35 dpf. At 35 dpf, numerous POs were destined for primitive ovaries, while apoptosis seemed to occur rapidly at 40 dpf, because there were many DPOs and cavities in the gonad tissues. Along with oocyte apoptosis and degeneration from 50 to 60 dpf, testicular tissues began to differentiate and numerously proliferated spermatogenic cells including SG, SPs, and spermatids filled the differentiated testes. After 60 dpf, it became increasingly difficult to identify female individuals, and only three individuals with secondary feminine characteristics and ovaries were discovered (Figure 8D and Figure S2 in File S1). At 150 dpf, all of the homozygous foxl2a−/−/foxl2b−/− individuals examined had histologically normal testes (Figure 8D and Figure S2 in File S1), and were able to produce normal sperm and fertilize eggs. These data indicate that simultaneous disruption of foxl2a and foxl2b leads to complete sex reversal, and sex reversal occurs earlier in foxl2a−/−/foxl2b−/− mutants than in foxl2b−/− mutants. Therefore, the roles of foxl2a and foxl2b might be compensatory in zebrafish ovary development and maintenance.
We also examined secondary sexual characteristics and testis development status of male foxl2a−/−, foxl2b−/−, and foxl2a−/−/foxl2b−/− mutants at 35, 60, and 105 dpf. No significant abnormality in secondary sexual characteristics, testis differentiation and development, or spermatogenesis were observed in the males of three mutants. Similar to controls, some oocyte-like germ cells began to undergo apoptosis as demonstrated by dark-stained cytoplasm, and SCs containing SPs scattered in the gonadal tissues at 35 dpf. At 60 and 105 dpf, the male germ cells at all spermatogenic stages filled the lobules, and numerous free spermatozoa congregated into the lumen of all individuals of the three male mutants (Figure S7 in File S1). Furthermore, all male mutants were fertile because they all successfully spawned with females to produce normal offspring. Therefore, consistent with their sexually dimorphic expression, both foxl2a and foxl2b function specifically in the female gonad.
Zebrafish ovary differentiation and maintenance are cooperatively regulated by foxl2a and foxl2b
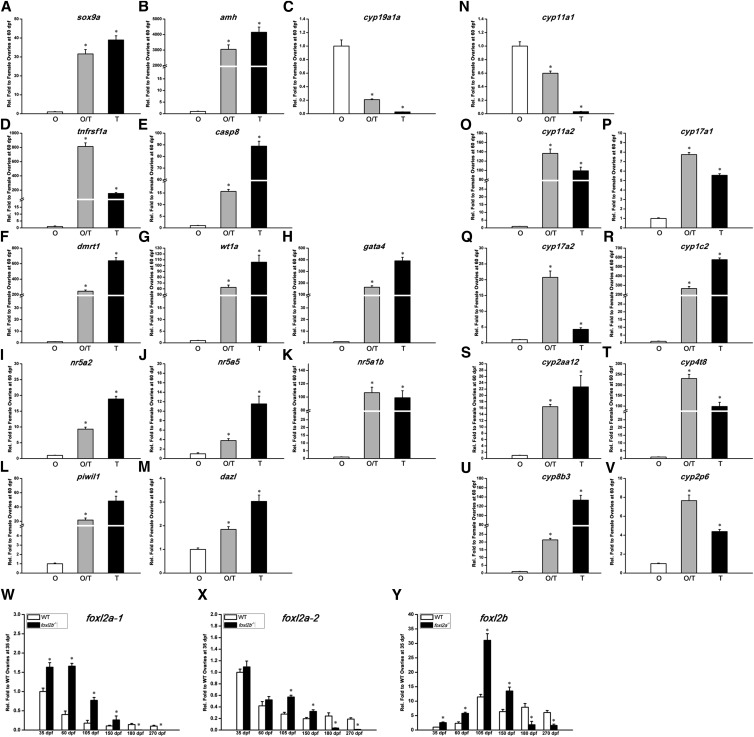
To test the molecular basis of the sex reversal that occurred in homozygous foxl2a−/−/foxl2b−/− mutants, we examined the expression of gonad-related genes selected as above in the gonads from foxl2a−/−/foxl2b−/− mutants at 60 dpf, which were classified into three stages: ovary, ovotestis, and testis. As expected, sox9a, amh, dmrt1, and other genes involved in the male development pathway all increased by several dozen- or even several 100-fold in ovotestis and testis compared to those in ovaries (Figure 9, A, B, and F–K); while ovary-related genes, such as cyp19a1a and cyp11a1, decreased significantly in ovotestis and were hardly detected in testis (Figure 9, C and N). Expression of all other cyp genes examined; as well as tnfrsf1a, caspase-8, piwil1, and dazl (Figure 9, D, E, L, and M); increased sharply in ovotestis and testis compared to those in ovaries (Figure 9, O–V). The altered expression levels in homozygous foxl2a−/−/foxl2b−/− mutants were very similar to those of foxl2b−/− mutants at 270 dpf, implying that foxl2a deficiency enhances sex reversal caused by foxl2b knockout, whereas single foxl2a mutation results in different defects.
Figure 9.
Foxl2a and foxl2b cooperatively regulate ovary differentiation and maintenance in zebrafish. (A–V) Relative expression qRT-PCR detection of the indicated genes in ovary (O), ovotestis (O/T), and testis (T) of homozygous double mutants (foxl2a−/−/foxl2b−/−) at 60 dpf (n = 3). Gene names are indicated at the top. (W–Y) Relative expression qRT-PCR detection of (W) foxl2a-1, (X) foxl2a-2, and (Y) foxl2b in the ovaries of WT and foxl2b mutants (foxl2b−/−) or foxl2a mutants (foxl2a−/−) from 35 to 270 dpf, respectively (n = 3). The qRT-PCR quantification of each gene expression is normalized to actb1, and the data are presented as mean ± SEM * P ≤ 0.05 (Duncan’s multiple range test).
To test whether the functions of foxl2a and foxl2b are compensative in ovary development and maintenance, we assessed the expression changes of foxl2a in foxl2b−/− mutants and vice versa. Interestingly, the mRNA expression levels of both foxl2a-1 and foxl2a-2 were upregulated in the gonads of foxl2b−/− mutants before 180 dpf (Figure 9, W and X), and the reverse was also true (Figure 9Y). It seems that foxl2a and foxl2b can increase in expression to compensate for reduced expression at the other locus, eventually causing normal ovary development in both mutant backgrounds. However, foxl2a and foxl2b transcripts started to decrease in abundance from 180 dpf, when the mutants underwent POF or sex reversal. The pathological changes in the ovary might reduce or destroy the pathways involved in female gonad maintenance. This also suggests that the compensation between foxl2a and foxl2b in this process is partly divergent, causing the different phenotypes in two single mutants. Taken together, these results indicate that foxl2a and foxl2b cooperatively regulate zebrafish ovary differentiation and maintenance, whereas foxl2b plays more specific roles in maintaining female identity and preventing male transformation.
Discussion
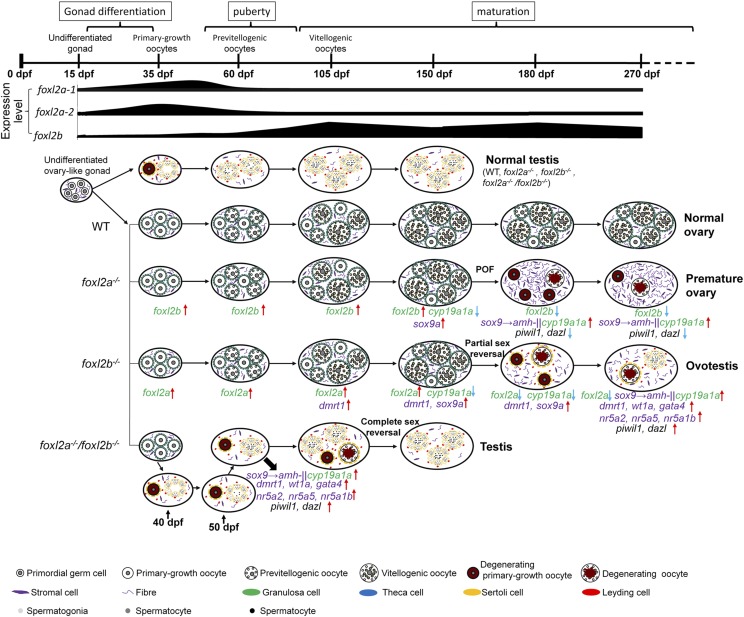
Although SD and differentiation have been well studied in mammals and several sex-determining genes have been identified from different teleost species, this process is still a complicated affair in zebrafish (Liew and Orban 2013; Nagabhushana and Mishra 2016). Here we have revealed a sequential and divergent expression pattern between two zebrafish foxl2 homologs, foxl2a and foxl2b, during oogenesis. Via histological and transcriptomic analyses of the foxl2a−/− and foxl2b−/− mutants, we propose a hypothesized model in which foxl2a and foxl2b cooperatively regulate zebrafish ovary differentiation and maintenance. As shown in Figure 10, unlike normal testis development, ovary development and oogenesis are significantly affected by loss of foxl2a, foxl2b, or both genes. Germ cell marker loci piwil1 and dazl are sharply downregulated and the sox9a-amh/cyp19a1a signaling pathway and foxl2b are upregulated, which thereby results in POF in female foxl2a−/− mutants. In contrast to expression changes in foxl2a−/− premature ovaries, some testis differentiation and androgen-producing genes, such as dmrt1, sox9, amh, wt1a, gata4, nr5a2, nr5a5, nr5a1b, cyp17a, and cyp11a2 are upregulated in female foxl2b−/− mutants and trigger partial sex reversal, although foxl2a expression increases. In homozygous foxl2a−/−/foxl2b−/− females, a complete sex reversal occurs early, and numerous genes in the testis development pathway are sharply activated to lead to complete sex reversal and formation of ovotestis and testis.
Figure 10.
A hypothesized diagram for cooperative regulation between foxl2a and foxl2b in ovary development and maintenance of zebrafish. Different gonad development stages; expression level changes of foxl2a-1, foxl2a-2, and foxl2b; different status gonads including normal testis, normal ovary, premature ovary, and ovotestis; different germ cells and gonad-related somatic cells; different gonadal development-related genes; and their upregulation and downregulation expression are indicated in detail. Red ↑, upregulation; blue ↓, downregulation.
Consistent with other teleosts (Caulier et al. 2015), zebrafish foxl2a1/2 and foxl2b all showed sexual dimorphism. Zebrafish foxl2a transcripts are mainly expressed in the ovary at 35 dpf and decline while maturation proceeds. In mice, Foxl2 is expressed initially at 12.5 days postconception, and occurs in the granulosa cells of early follicles but gradually reduces as oocytes proceed to maturation (Schmidt 2004; Nakamoto et al. 2006). Foxl2 is also highly expressed in Nile tilapia XX gonads as early as 5 days after hatching (dah), increases linearly until 35 dah, and remains high at 70 dah (Ijiri et al. 2008). However, foxl2b increases from immature stages and peaks at the mature and spawning stage (Figure 10), similar to Foxl2 in chicken (Govoroun et al. 2004), Korean rockfish (Mu et al. 2013), and African sharptooth catfish (Sridevi and Senthilkumaran 2011). The sequential and divergent expression patterns of zebrafish foxl2a and foxl2b during oogenesis (Figure 10) indicate that foxl2a and foxl2b may have undergone subfunctionalization after their divergence.
The role of Foxl2 is complicated because of the disparate effects in humans and other mammals, such as mouse and goat (Ottolenghi et al. 2007). Its functions in development were first suggested by studying BPES, which is characterized by eyelid/forehead anomalies associated with POF, an early loss of normal ovarian function (Crisponi et al. 2001; De Baere et al. 2003; Meduri et al. 2010). Foxl2−/− mice showed craniofacial and eyelid defects, a complete failure of follicle formation, and partial sex reversal in females (Schmidt 2004; Uda et al. 2004; Ottolenghi 2005; Ottolenghi et al. 2007). Conditional loss of Foxl2 in the adult ovary resulted in autonomous reprogramming of oocyte-supporting granulosa cells into testis-specific Sertoli-like cells (Uhlenhaut et al. 2009). In goats, FOXL2 mutation led to the polled intersex syndrome, which associated polledness and XX female-to-male sex reversal in a recessive manner (Pailhoux et al. 2001). In our study, zebrafish homozygous foxl2a−/− F3 females showed normal ovarian differentiation and growth at the onset of sexual maturity, but ∼60% of foxl2a−/− females exhibited ovarian histological changes at 270 dpf, with the degeneration of ovarian tissue, presence of atretic follicles, reduction of germ cells, and proliferation of somatic cells (Figure 4 and Figure S2 in File S1), similar to the ovarian senescence that occurred in the 18-month-old female zebrafish (Turola et al. 2015). However, no WT zebrafish exhibited ovarian senescence before 270 dpf, indicating that ovarian senescence was promoted in the foxl2a−/− mutants. The accelerated ovarian aging phenotype in foxl2a−/− mutants is reminiscent of human BPES. Meanwhile, disruption of zebrafish foxl2b led to partial sex reversal from female to male after spawning a few times (Figure 5), similar to the case in mouse and goat. Furthermore, the expression of genes related to gonad development was also different in foxl2a−/− and foxl2b−/− mutants (Figure 6, Figure 7, and Table S4). Therefore, zebrafish foxl2a and foxl2b have evolved functional divergence. On the other hand, the complete sex reversal (Figure 8) and robust increasing expression of testicular differentiation genes occurred earlier (Figure 9) in foxl2a−/−/foxl2b−/− double female mutants than those in any single mutants, suggesting the compensation and redundancy between foxl2a and foxl2b. Indeed, the compensation to a deficiency in either gene was not only observed in the transcriptome profiling comparisons (Table S4), but also confirmed by qRT-PCR (Figure 9, W–Y). Taken together, foxl2a and foxl2b work differentially and cooperate to manipulate zebrafish ovary differentiation and maintenance.
Foxl2 and Sox9, as the yin and yang of sex maintenance, antagonize each other’s actions in the establishment and maintenance of granulosa cells and Sertoli cells (Uhlenhaut et al. 2009). In this case, a significant increase of sox9a transcript takes place both in foxl2a−/− and foxl2b−/− mutated ovaries at 150 dpf (Figure 6, A and B, and Table S4), although no apparent abnormalities of ovarian morphology and histology were observed (Figure 4B). The upregulation of sox9a in foxl2a−/− and foxl2b−/− mutated ovaries suggests that zebrafish sox9a transcription might be directly repressed by Foxl2s in the ovary, similar to mouse. A cis-regulatory element for testis-specific Sox9 expression (TESCO) has been identified in mouse Sox9 (Sekido and Lovell-Badge 2008) and proven to be negatively modulated by FOXL2 (Uhlenhaut et al. 2009). However, the well-conserved 1.4-kb TESCO sequence was only found in placental mammals, not in chicken, Fugu, or zebrafish (Sekido and Lovell-Badge 2008). Further analyses revealed that a 180-bp evolutionarily conserved region (ECR) within the SOX9 testis enhancer containing the conserved FHD binding site was identified in chicken, lizard, and frog, but not in fish (Bagheri-Fam et al. 2010). After searching the zebrafish draft genome by using frog ECR as the query, a 180-bp sequence showing a 43% identity to frog ECR was found in the 68.1 kb upstream of the zebrafish sox9a gene, where seven conserved FHD binding sites occured. This suggests that zebrafish Foxl2s might also inhibit the testis-differentiation program, mainly through repressing sox9a as occurs in mouse (Uhlenhaut et al. 2009). Interestingly, sox9a expression changes corresponding to the disruption of foxl2a and foxl2b are not the same (Figure 6B), implying that a subtle differential regulatory mechanism remains for further investigation in zebrafish. During mammalian male fetal sex differentiation, Sox9 activates the expression of Amh (de Santa Barbara et al. 1998), which induces the degeneration of Müllerian ducts (Behringer et al. 1994) and inhibits Cyp19a1 expression (Rouiller-Fabre et al. 1998). Along with the upregulation of sox9a, amh increased in expression while cyp19a1a decreased in abundance in all three zebrafish mutant ovaries (Figure 6, B–D, and Figure 9, A–C), implying that a sox9a-amh/cyp19a1a pathway should be highly conserved in vertebrates.
Dmrt1 is a central regulator in vertebrate testis determination and differentiation (Xia et al. 2007; Matson and Zarkower 2012; Li et al. 2014). It is required for mammalian postnatal testis differentiation (Raymond et al. 2000), and its homologous genes were identified as SD genes in chicken (Smith et al. 2009), X. laevis (Yoshimoto et al. 2008), and medaka (Matsuda et al. 2002). In conditional Foxl2 deletion mice ovotestis, Dmrt1 is one of the most upregulated genes, indicating that FOXL2 may directly repress Dmrt1 transcription (Uhlenhaut et al. 2009). Interestingly, sox9a and dmrt1 exhibited differentially dynamic expression patterns between foxl2a−/−/WT and foxl2b−/−/WT ovaries (Figure 6, B and G). In accordance with no sex reversal individuals observed in foxl2a−/− mutants, dmrt1 only slightly increased in expression at 270 dpf. However, dmrt1 and other testicular differentiation genes, such as sox9a (Chiang et al. 2001), amh (Schulz et al. 2001), wt1a (Hammes et al. 2001), gata4 (Tevosian et al. 2002), nr5a2, nr5a5, and nr5a1b (Luo et al. 1994; Von Hofsten et al. 2005a,b), were all robustly upregulated in ovotestis of foxl2b−/− mutants along with sex reversal (Figure 6, B, C, and G–L), which indicated that foxl2b might play a more dominant role in suppressing testis-differentiation signaling than foxl2a. Although many key players, such as foxl2a, sox9, amh, dmrt1, and cyp19a1, show sexually dimorphic expression in zebrafish, the interactions within the proposed transcriptional network are unclear. The establishments of the foxl2a−/−, foxl2b−/−, and foxl2a−/−/foxl2b−/− mutants in this study will provide a model to definitively clarify the regulatory interactions among these factors.
The CYP enzymes catalyze oxidation reactions and have diverse functions in vertebrates. It is well known that some cyp family members play essential roles in steroidogenesis, including estrogen production (Stoilov 2001; Payne and Hales 2004; Chang et al. 2005; Baldwin et al. 2009; Huang et al. 2009; Heule et al. 2014). Many studies have been done to reveal the relevance of foxl2, aromatase, and sex hormones in the teleost brain-pituitary-gonad axis, in which foxl2 was found to regulate estrogen synthesis via transcriptional regulation of cyp19 (Baron 2004; Wang et al. 2007; Navarro-Martín et al. 2011; Sridevi et al. 2012; Crespo et al. 2013), and in turn, the ovarian foxl2 was upregulated by estrogen treatment (Baron 2004; Liu et al. 2007; Wu et al. 2008; Jiang et al. 2011; Wang et al. 2012). Zebrafish have a total of 94 cyp genes (Goldstone et al. 2010), in which 59 cyp genes were identified from transcriptome files (Table S4) and 9 cyp genes showing dynamic expression changes in three mutants were confirmed by qRT-PCR (Figure 7 and Figure 9). The differential effects of foxl2a and foxl2b on gonadal steroidogenesis need further investigation to determine the regulatory mechanisms between foxl2s and cyps.
Zebrafish male development undergoes a transformation of juvenile ovary to testis in adolescence (Uchida et al. 2002; Maack and Segner 2003). During this process, the sox9a-amh/cyp19a1a pathway linked to MAPK-p53 signaling precedes oocyte apoptosis and degeneration, and the expression of sox9a increased in the transforming gonads activates stromal cells and increases extracellular matrix (ECM) production (Sun et al. 2013). Similarly, we observed the DOs surrounded by gonadal matrix and a massive increase of somatic cells in the three mutants (Figure 4, Figure 5, and Figure 8). The comparative transcriptome analyses revealed that the TNF signaling pathway, cytokine-cytokine receptor interaction, and “ECM-receptor interaction” pathways were enriched in both foxl2a−/−/WT and foxl2b−/−/WT ovaries (Table S5). Meanwhile, the DOs were dissociated from adjacent cells with a marked vacuolation in the three female mutants (Figure 4, Figure 5, and Figure 8), implying that the oocytes and follicle cells lost their tight junctions with adjacent cells. Consistent with these changes, the top 30 pathways in both foxl2a−/−/WT and foxl2b−/−/WT ovaries include several pathways involved in cell adhesion, such as cell adhesion molecules, focal adhesion, gap junction, and regulation of actin cytoskeleton (Table S5).
In conclusion, this study established foxl2a knockout, foxl2b knockout, and double knockout zebrafish and revealed POF, partial sex reversal, and complete sex reversal in the three mutants, respectively. Based on the comparative transcriptome analyses and dynamic expression patterns of DEGs, we propose that foxl2a and foxl2b cooperate with each other to regulate zebrafish ovary differentiation and maintenance, and that foxl2b plays a dominant role in preventing the ovary from differentiating as testis. In addition, these foxl2-related mutants also provide some model systems to study human POF and the mechanism determining fish sex.
Supplementary Material
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.116.199133/-/DC1.
Acknowledgments
We are grateful to Wenhua Li and Chenyan Mou for helping to construct the transcription activator-like effector nucleases (TALEN) plasmids and to screen the mutants. This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA08030201); the Autonomous Project of the State Key Laboratory of Freshwater Ecology and Biotechnology (2016FBZ01); and the Autonomous Project of the Institute of Hydrobiology, Chinese Academy of Sciences (Y25A171).
Footnotes
Communicating editor: D. M. Parichy
Literature Cited
- Alam M. A., Kobayashi Y., Horiguchi R., Hirai T., Nakamura M., 2008. Molecular cloning and quantitative expression of sexually dimorphic markers Dmrt1 and Foxl2 during female-to-male sex change in Epinephelus merra. Gen. Comp. Endocrinol. 157: 75–85. [DOI] [PubMed] [Google Scholar]
- Anderson J. L., Mari A. R., Braasch I., Amores A., Hohenlohe P., et al. , 2012. Multiple sex-associated regions and a putative sex chromosome in zebrafish revealed by RAD mapping and population genomics. PLoS One 7: e40701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtrog D., Mank J. E., Peichel C. L., Kirkpatrick M., Otto S. P., et al. , 2014. Sex determination: why so many ways of doing it? PLoS Biol. 12: e1001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagheri-Fam S., Sinclair A. H., Koopman P., Harley V. R., 2010. Conserved regulatory modules in the Sox9 testis-specific enhancer predict roles for SOX, TCF/LEF, Forkhead, DMRT, and GATA proteins in vertebrate sex determination. Int. J. Biochem. Cell Biol. 42: 472–477. [DOI] [PubMed] [Google Scholar]
- Baldwin W. S., Marko P. B., Nelson D. R., 2009. The cytochrome P450 (CYP) gene superfamily in Daphnia pulex. BMC Genomics 10: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroiller J. F., D’Cotta H., Saillant E., 2009. Environmental effects on fish sex determination and differentiation. Sex Dev. 3: 118–135. [DOI] [PubMed] [Google Scholar]
- Baron D., 2004. An evolutionary and functional analysis of FoxL2 in rainbow trout gonad differentiation. J. Mol. Endocrinol. 33: 705–715. [DOI] [PubMed] [Google Scholar]
- Barske L. A., Capel B., 2008. Blurring the edges in vertebrate sex determination. Curr. Opin. Genet. Dev. 18: 499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behringer R. R., Finegold M. J., Cate R. L., 1994. Müllerian-inhibiting substance function during mammalian sexual development. Cell 79: 415–425. [DOI] [PubMed] [Google Scholar]
- Bell G., 1982. The Masterpiece of Nature: the Evolution of Genetics of Sexuality. University of California Press, Berkeley, CA. [Google Scholar]
- Benayoun B. A., Caburet S., Veitia R. A., 2011. Forkhead transcription factors: key players in health and disease. Trends Genet. 27: 224–232. [DOI] [PubMed] [Google Scholar]
- Bhat I. A., Rather M. A., Dar J. Y., Sharma R., 2016. Molecular cloning, computational analysis and expression pattern of forkhead box l2 (Foxl2) gene in catfish. Comput. Biol. Chem. 64: 9–18. [DOI] [PubMed] [Google Scholar]
- Bonduriansky R., 2009. Reappraising sexual coevolution and the sex roles. PLoS Biol. 7: e1000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin S. A., Benes V., Garson J. A., Hellemans J., Huggett J., et al. , 2009. The miqe guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55: 611–622. [DOI] [PubMed] [Google Scholar]
- Carlsson P., Mahlapuu M., 2002. Forkhead transcription factors: key players in development and metabolism. Dev. Biol. 250: 1–23. [DOI] [PubMed] [Google Scholar]
- Caulier M., Brion F., Chadili E., Turies C., Piccini B., et al. , 2015. Localization of steroidogenic enzymes and Foxl2a in the gonads of mature zebrafish (Danio rerio). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 188: 96–106. [DOI] [PubMed] [Google Scholar]
- Chang K. T., Sefc L., Psenak O., Vokurka M., Necas E., 2005. Early fetal liver readily repopulates B lymphopoiesis in adult bone marrow. Stem Cells 23: 230–239. [DOI] [PubMed] [Google Scholar]
- Chiang E. F. L., Pai C. I., Wyatt M., Yan Y. L., Postlethwait J., et al. , 2001. Two sox9 genes on duplicated zebrafish chromosomes: expression of similar transcription activators in distinct sites. Dev. Biol. 231: 149–163. [DOI] [PubMed] [Google Scholar]
- Cocquet J., Pailhoux E., Jaubert F., Servel N., Xia X., et al. , 2002. Evolution and expression of FOXL2. J. Med. Genet. 39: 916–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo B., Lan-Chow-Wing O., Rocha A., Zanuy S., Gómez A., 2013. Foxl2 and foxl3 are two ancient paralogs that remain fully functional in teleosts. Gen. Comp. Endocrinol. 194: 81–93. [DOI] [PubMed] [Google Scholar]
- Crisponi L., Deiana M., Loi A., Chiappe F., Uda M., et al. , 2001. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat. Genet. 27: 159–166. [DOI] [PubMed] [Google Scholar]
- De Baere E., Beysen D., Oley C., Lorenz B., Cocquet J., et al. , 2003. FOXL2 and BPES: mutational hotspots, phenotypic variability, and revision of the genotype-phenotype correlation. Am. J. Hum. Genet. 72: 478–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Santa Barbara P., Moniot B., Poulat F., Boizet B., Berta P., 1998. Steroidogenic factor-1 regulates transcription of the human anti-mullerian hormone receptor. J. Biol. Chem. 273: 29654–29660. [DOI] [PubMed] [Google Scholar]
- Desjardins J. K., Fernald R. D., 2009. Fish sex: why so diverse? Curr. Opin. Neurobiol. 19: 648–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin R. H., Nagahama Y., 2002. Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture 208: 191–364. [Google Scholar]
- Fetterman C. D., Rannala B., Walter M. A., 2008. Identification and analysis of evolutionary selection pressures acting at the molecular level in five forkhead subfamilies. BMC Evol. Biol. 8: 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone J. V., McArthur A. G., Kubota A., Zanette J., Parente T., et al. , 2010. Identification and developmental expression of the full complement of cytochrome P450 genes in Zebrafish. BMC Genomics 11: 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govoroun M. S., Pannetier M., Pailhoux E., Cocquet J., Brillard J. P., et al. , 2004. Isolation of chicken homolog of the FOXL2 gene and comparison of its expression patterns with those of aromatase during ovarian development. Dev. Dyn. 231: 859–870. [DOI] [PubMed] [Google Scholar]
- Grunwald D. J., Eisen J. S., 2002. Headwaters of the zebrafish emergence of a new model vertebrate. Nat. Rev. Genet. 3: 717–724. [DOI] [PubMed] [Google Scholar]
- Gui J. F., Zhu Z. Y., 2012. Molecular basis and genetic improvement of economically important traits in aquaculture animals. Sci Bull. 57: 1751–1760. [Google Scholar]
- Hammes A., Guo J. K., Lutsch G., Leheste J. R., Landrock D., et al. , 2001. Two splice variants of the Wilms’ tumor 1 gene have distinct functions during sex determination and nephron formation. Cell 106: 319–329. [DOI] [PubMed] [Google Scholar]
- Hannenhalli S., Kaestner K. H., 2009. The evolution of Fox genes and their role in development and disease. Nat. Rev. Genet. 10: 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori R. S., Murai Y., Oura M., Masuda S., Majhi S. K., et al. , 2012. A Y-linked anti-Mullerian hormone duplication akes over a critical role in sex determination. Proc. Natl. Acad. Sci. USA 109: 2955–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heule C., Salzburger W., Bohne A., 2014. Genetics of sexual development: an evolutionary playground for fish. Genetics 196: 579–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe K., Clark M. D., Torroja C. F., Torrance J., Berthelot C., et al. , 2013. The zebrafish reference genome sequence and its relationship to the human genome. Nature 496: 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P., Xiao A., Tong X., Zu Y., Wang Z., et al. , 2014. TALEN construction via “Unit Assembly” method and targeted genome modifications in zebrafish. Methods 69: 67–75. [DOI] [PubMed] [Google Scholar]
- Huang W., Zhou L., Li Z., Gui J. F., 2009. Expression pattern, cellular localization and promoter activity analysis of ovarian aromatase (Cyp19a1a) in protogynous hermaphrodite red-spotted grouper. Mol. Cell. Endocrinol. 307: 224–236. [DOI] [PubMed] [Google Scholar]
- Ijiri S., Kaneko H., Kobayashi T., Wang D. S., Sakai F., et al. , 2008. Sexual dimorphic expression of genes in gonads during early differentiation of a teleost fish, the Nile tilapia Oreochromis niloticus. Biol. Reprod. 78: 333–341. [DOI] [PubMed] [Google Scholar]
- Jiang W., Yang Y., Zhao D., Liu X., Duan J., et al. , 2011. Effects of sexual steroids on the expression of foxl2 in Gobiocypris rarus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 160: 187–193. [DOI] [PubMed] [Google Scholar]
- Jorgensen A., Morthorst J. E., Andersen O., Rasmussen L. J., Bjerregaard P., 2008. Expression profiles for six zebrafish genes during gonadal sex differentiation. Reprod. Biol. Endocrinol. 6: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner K. H., Knochel W., Martinez D. E., 2000. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 14: 142–146. [PubMed] [Google Scholar]
- Kamiya T., Kai W., Tasumi S., Oka A., Matsunaga T., et al. , 2012. A trans-species missense SNP in Amhr2 is associated with sex determination in the tiger pufferfish, Takifugu rubripes (fugu). PLoS Genet. 8: e1002798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., Horiguchi R., Nozu R., Nakamura M., 2010. Expression and localization of forkhead transcriptional factor 2 (Foxl2) in the gonads of protogynous wrasse, Halichoeres trimaculatus. Biol. Sex Differ. 1: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocer A., Pinheiro I., Pannetier M., Renault L., Parma P., et al. , 2008. R-spondin1 and FOXL2 act into two distinct cellular types during goat ovarian differentiation. BMC Dev. Biol. 8: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Dewey C. N., 2011. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12: 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. H., Yang H. H., Li M. R., Sun Y. L., Jiang X. L., et al. , 2013. Antagonistic roles of Dmrt1 and Foxl2 in sex differentiation via estrogen production in tilapia as demonstrated by TALENs. Endocrinology 154: 4814–4825. [DOI] [PubMed] [Google Scholar]
- Li X. Y., Li Z., Zhang X. J., Zhou L., Gui J. F., 2014. Expression characterization of testicular DMRT1 in both Sertoli cells and spermatogenic cells of polyploid gibel carp. Gene 548: 119–125. [DOI] [PubMed] [Google Scholar]
- Li X. Y., Zhang Q. Y., Zhang J., Zhou L., Li Z., et al. , 2016. Extra microchromosomes play male determination role in polyploid gibel carp. Genetics 203: 1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew W. C., Orban L., 2013. Zebrafish sex: a complicated affair. Brief. Funct. Genomics 13: 172–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Wu F., Jiao B., Zhang X., Hu C., et al. , 2007. Molecular cloning of doublesex and mab-3-related transcription factor 1, forkhead transcription factor gene 2, and two types of cytochrome P450 aromatase in Southern catfish and their possible roles in sex differentiation. J. Endocrinol. 194: 223–241. [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D., 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Loffler K. A., Zarkower D., Koopman P., 2003. Etiology of ovarian failure in blepharophimosis ptosis epicanthus inversus syndrome: FOXL2 is a conserved, early-acting gene in vertebrate ovarian development. Endocrinology 144: 3237–3243. [DOI] [PubMed] [Google Scholar]
- Luo X. R., Ikeda Y. Y., Parker K. L., 1994. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual-differentiation. Cell 77: 481–490. [DOI] [PubMed] [Google Scholar]
- Maack G., Segner H., 2003. Morphological development of the gonads in zebrafish. J. Fish Biol. 62: 895–906. [Google Scholar]
- Marín I., Baker B. S., 1998. The evolutionary dynamics of sex determination. Science 281: 1990–1994. [DOI] [PubMed] [Google Scholar]
- Matson C. K., Zarkower D., 2012. Sex and the singular DM domain: insights into sexual regulation, evolution and plasticity. Nat. Rev. Genet. 13: 163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M., Nagahama Y., Shinomiya A., Sato T., Matsuda C., et al. , 2002. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature 417: 559–563. [DOI] [PubMed] [Google Scholar]
- Meduri G., Bachelot A., Duflos C., Bstandig B., Poirot C., et al. , 2010. FOXL2 mutations lead to different ovarian phenotypes in BPES patients: Case Report. Hum Reprod. 25: 235–243. [DOI] [PubMed] [Google Scholar]
- Meeker N. D., Hutchinson S. A., Ho L., Trecle N. S., 2007. Method for isolation of PCR-ready genomic DNA from zebrafish tissues. Biotechniques 43: 610–614. [DOI] [PubMed] [Google Scholar]
- Mei J., Gui J. F., 2015. Genetic basis and biotechnological manipulation of sexual dimorphism and sex determination in fish. Sci. China Life Sci. 58: 124–136. [DOI] [PubMed] [Google Scholar]
- Mu W. J., Wen H. S., Li J. F., He F., 2013. Cloning and expression analysis of Foxl2 during the reproductive cycle in Korean rockfish, Sebastes schlegeli. Fish Physiol. Biochem. 39: 1419–1430. [DOI] [PubMed] [Google Scholar]
- Myosho T., Otake H., Masuyama H., Matsuda M., Kuroki Y., et al. , 2012. Tracing the emergence of a novel sex-determining gene in medaka, Oryzias luzonensis. Genetics 191: 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagabhushana A., Mishra R. K., 2016. Finding clues to the riddle of sex determination in zebrafish. J. Biosci. 41: 145–155. [DOI] [PubMed] [Google Scholar]
- Nakamoto M., Matsuda M., Wang D.-S., Nagahama Y., Shibata N., 2006. Molecular cloning and analysis of gonadal expression of Foxl2 in the medaka, Oryzias latipes. Biochem. Biophys. Res. Commun. 344: 353–361. [DOI] [PubMed] [Google Scholar]
- Nakamoto M., Wang D. S., Suzuki A., Matsuda M., Nagahama Y., et al. , 2007. Dax1 suppresses P450arom expression in medaka ovarian follicles. Mol. Reprod. Dev. 74: 1239–1246. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Kobayashi T., Chang X. T., Nagahama Y., 1998. Gonadal sex differentiation in teleost fish. J. Exp. Zool. 281: 362–372. [Google Scholar]
- Navarro-Martín L., Viñas J., Ribas L., Díaz N., Gutiérrez A., et al. , 2011. DNA methylation of the gonadal aromatase (cyp19a) promoter is involved in temperature-dependent sex ratio shifts in the European sea bass. PLoS Genet. 7: e1002447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T., Tanaka M., 2014. Gonadal development in fish. Sex Dev. 8: 252–261. [DOI] [PubMed] [Google Scholar]
- Ota S., Hisano Y., Muraki M., Hoshijima K., Dahlem T. J., et al. , 2013. Efficient identification of TALEN-mediated genome modifications using heteroduplex mobility assays. Genes Cells 18: 450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolenghi, C., 2005 Foxl2 is required for commitment to ovary differentiation. Hum. Mol. Genet. 14: 2053–2062. [DOI] [PubMed] [Google Scholar]
- Ottolenghi, C., E. Pelosi, J. Tran, M. Colombino, E. Douglass et al., 2007 Loss of Wnt4 and Foxl2 leads to female-to-male sex reversal extending to germ cells. Hum. Mol. Genet. 16: 2795–2804. [DOI] [PubMed] [Google Scholar]
- Pailhoux E., Vigier B., Chaffaux S., Servel N., Taourit S., et al. , 2001. A 11.7-kb deletion triggers intersexuality and polledness in goats. Nat. Genet. 29: 453–458. [DOI] [PubMed] [Google Scholar]
- Pal L., Santoro N., 2002. Premature ovarian failure (POF): discordance between somatic and reproductive aging. Ageing Res. Rev. 1: 413–423. [DOI] [PubMed] [Google Scholar]
- Payne A. H., Hales D. B., 2004. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 25: 947–970. [DOI] [PubMed] [Google Scholar]
- Raymond C. S., Murphy M. W., O’Sullivan M. G., Bardwell V. J., Zarkower D., 2000. Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation. Genes Dev. 14: 2587–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Marí A., Yan Y. L., BreMiller R. A., Wilson C., Cañestro C., et al. , 2005. Characterization and expression pattern of zebrafish anti-Müllerian hormone (amh) relative to sox9a, sox9b, and cyp19a1a, during gonad development. Gene Expr. Patterns 5: 655–667. [DOI] [PubMed] [Google Scholar]
- Rouiller-Fabre V., Carmona S., Abou Merhi R., Cate R., Habert R., et al. , 1998. Effect of anti-mullerian hormone on sertoli and leydig cell functions in fetal and immature rats. Endocrinology 139: 1213–1220. [DOI] [PubMed] [Google Scholar]
- Saito D., Morinaga C., Aoki Y., Nakamura S., Mitani H., et al. , 2007. Proliferation of germ cells during gonadal sex differentiation in medaka: insights from germ cell-depleted mutant zenzai. Dev. Biol. 310: 280–290. [DOI] [PubMed] [Google Scholar]
- Schmidt D., 2004. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development 131: 933–942. [DOI] [PubMed] [Google Scholar]
- Schulz R. W., Vischer H. F., Cavaco J. E. B., Santos E. M., Tyler C. R., et al. , 2001. Gonadotropins, their receptors, and the regulation of testicular functions in fish. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 129: 407–417. [DOI] [PubMed] [Google Scholar]
- Sekido R., Lovell-Badge R., 2008. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature 453: 930–934. [DOI] [PubMed] [Google Scholar]
- Shinomiya A., Tanaka M., Kobayashi T., Nagahama Y., Hamaguchi S., 2000. The vasa-like gene, olvas, identifies the migration path of primordial germ cells during embryonic body formation stage in the medaka, Oryzias latipes. Dev. Growth Differ. 42: 317–326. [DOI] [PubMed] [Google Scholar]
- Siegfried K. R., 2010. In search of determinants: gene expression during gonadal sex differentiation. J. Fish Biol. 76: 1879–1902. [DOI] [PubMed] [Google Scholar]
- Siegfried K. R., Nüsslein-Volhard C., 2008. Germ line control of female sex determination in zebrafish. Dev. Biol. 324: 277–287. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Roeszler K. N., Ohnesorg T., Cummins D. M., Farlie P. G., et al. , 2009. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature 461: 267–271. [DOI] [PubMed] [Google Scholar]
- Smith E. K., Guzmán J. M., Luckenbach J. A., 2013. Molecular cloning, characterization, and sexually dimorphic expression of five major sex differentiation-related genes in a Scorpaeniform fish, sablefish (Anoplopoma fimbria). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 165: 125–137. [DOI] [PubMed] [Google Scholar]
- Sola L., Gornung E., 2001. Classical and molecular cytogenetics of the zebrafish, Danio rerio (Cyprinidae, Cypriniformes): an overview. Genetica 111: 397–412. [DOI] [PubMed] [Google Scholar]
- Sreenivasan R., Cai M. N., Bartfai R., Wang X. G., Christoffels A., et al. , 2008. Transcriptomic analyses reveal novel genes with sexually dimorphic expression in the zebrafish gonad and brain. PLoS One 3: e1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridevi P., Senthilkumaran B., 2011. Cloning and differential expression of FOXL2 during ovarian development and recrudescence of the catfish, Clarias gariepinus. Gen. Comp. Endocrinol. 174: 259–268. [DOI] [PubMed] [Google Scholar]
- Sridevi, P., R. K. Chaitanya, A. Dutta-Gupta, and B. Senthilkumaran, 2012 FTZ-F1 and FOXL2 up-regulate catfish brain aromatase gene transcription by specific binding to the promoter motifs. Biochim. Biophys. Acta 1819: 57–66. [DOI] [PubMed] [Google Scholar]
- Stahl Y., Simon R., 2010. mRNA detection by whole mount in situ hybridization (WISH) or sectioned tissue in situ hybridization (SISH) in Arabidopsis. Methods Mol. Biol. 655: 239–251. [DOI] [PubMed] [Google Scholar]
- Stoilov I., 2001. Cytochrome P450s: coupling development and environment. Trends Genet. 17: 629–632. [DOI] [PubMed] [Google Scholar]
- Sun D., Zhang Y., Wang C., Hua X., Zhang X. A., et al. , 2013. Sox9-related signaling controls zebrafish juvenile ovary-testis transformation. Cell Death Dis. 4: e930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehana Y., Matsuda M., Myosho T., Suster M. L., Kawakami K., et al. , 2014. Co-option of Sox3 as the male-determining factor on the Y chromosome in the fish Oryzias dancena. Nat. Commun. 5: 4157. [DOI] [PubMed] [Google Scholar]
- Takahashi H., 1976. Juvenile hermaphroditism in the zebrafish, Brachydanio rerio. J. Phys. Soc. Jpn. 41: 958–964. [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., et al. , 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevosian S. G., Albrecht K. H., Crispino J. D., Fujiwara Y., Eicher E. M., et al. , 2002. Gonadal differentiation, sex determination and normal Sry expression in mice require direct interaction between transcription partners GATA4 and FOG2. Development 129: 4627–4634. [DOI] [PubMed] [Google Scholar]
- Trant J. M., Gavasso S., Ackers J., Chung B. C., Place A. R., 2001. Developmental expression of cytochrome P450 aromatase genes (CYP19a and CYP19b) in zebrafish fry (Danio rerio). J. Exp. Zool. 290: 475–483. [DOI] [PubMed] [Google Scholar]
- Turola E., Petta S., Vanni E., Milosa F., Valenti L., et al. , 2015. Ovarian senescence increases liver fibrosis in humans and zebrafish with steatosis. Dis. Model. Mech. 8: 1037–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida D., Yamashita M., Kitano T., Iguchi T., 2002. Oocyte apoptosis during the transition from ovary-like tissue to testes during sex differentiation of juvenile zebrafish. J. Exp. Biol. 205: 711–718. [DOI] [PubMed] [Google Scholar]
- Uda, M., C. Ottolenghi, L. Crisponi, J. E. Garcia, M. Deiana et al., 2004 Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum. Mol. Genet. 13: 1171–1181. [DOI] [PubMed] [Google Scholar]
- Uhlenhaut N. H., Jakob S., Anlag K., Eisenberger T., Sekido R., et al. , 2009. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell 139: 1130–1142. [DOI] [PubMed] [Google Scholar]
- Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., et al. , 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3: RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volff J. N., Nanda I., Schmid M., Schartl M., 2007. Governing sex determination in fish: regulatory putsches and ephemeral dictators. Sex Dev. 1: 85–99. [DOI] [PubMed] [Google Scholar]
- Von Hofsten J., Olsson P. E., 2005. Zebrafish sex determination and differentiation: involvement of FTZ-F1 genes. Reprod. Biol. Endocrinol. 3: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Hofsten J., Larsson A., Olsson P. E., 2005a Novel steroidogenic factor-1 homolog (ff1d) is coexpressed with anti-mullerian hormone (amh) in zebrafish. Dev. Dyn. 233: 595–604. [DOI] [PubMed] [Google Scholar]
- Von Hofsten J., Modig C., Larsson A., Karlsson J., Olsson P. E., 2005b Determination of the expression pattern of the dual promoter of zebrafish fushi tarazu factor-1a following microinjections into zebrafish one cell stage embryos. Gen. Comp. Endocrinol. 142: 222–226. [DOI] [PubMed] [Google Scholar]
- Von Schalburg K. R., Yasuike M., Davidson W. S., Koop B. F., 2010. Regulation, expression and characterization of aromatase (cyp19b1) transcripts in ovary and testis of rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 155: 118–125. [DOI] [PubMed] [Google Scholar]
- Wang D. S., Kobayashi T., Zhou L., Nagahama Y., 2004. Molecular cloning and gene expression of Foxl2 in the Nile tilapia, Oreochromis niloticus. Biochem. Biophys. Res. Commun. 320: 83–89. [DOI] [PubMed] [Google Scholar]
- Wang D. S., Kobayashi T., Zhou L. Y., Paul-Prasanth B., Ijiri S., et al. , 2007. Foxl2 up-regulates aromatase gene transcription in a female-specific manner by binding to the promoter as well as interacting with Ad4 binding protein/steroidogenic factor 1. Mol. Endocrinol. 21: 712–725. [DOI] [PubMed] [Google Scholar]
- Wang H., Wu T., Qin F., Wang L., Wang Z., 2012. Molecular cloning of Foxl2 gene and the effects of endocrine-disrupting chemicals on its mRNA level in rare minnow, Gobiocypris rarus. Fish Physiol. Biochem. 38: 653–664. [DOI] [PubMed] [Google Scholar]
- Weigel D., Jurgens G., Kuttner F., Seifert E., Jackle H., 1989. The homeotic gene fork head encodes a nuclear-protein and is expressed in the terminal regions of the drosophila embryo. Cell 57: 645–658. [DOI] [PubMed] [Google Scholar]
- Westerfield M., 2007. The Zebrafish Book: A Guide for the Laboratory use of Zebrafish (Danio rerio), Ed. 5. University of Oregon Press, Eugene, OR. [Google Scholar]
- Williams T. M., Carroll S. B., 2009. Genetic and molecular insights into the development and evolution of sexual dimorphism. Nat. Rev. Genet. 10: 797–804. [DOI] [PubMed] [Google Scholar]
- Wilson C. A., High S. K., McCluskey B. M., Amores A., Yan Y. L., et al. , 2014. Wild sex in zebrafish: loss of the natural sex determinant in domesticated strains. Genetics 198: 1291–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotton K. R., French K. E. M., Shimeld S. M., 2007. The developmental expression of foxl2 in the dogfish Scyliorhinus canicula. Gene Expr. Patterns 7: 793–797. [DOI] [PubMed] [Google Scholar]
- Wu G. C., Tomy S., Nakamura M., Chang C. F., 2008. Dual roles of cyp19a1a in gonadal sex differentiation and development in the protandrous black porgy, Acanthopagrus schlegeli. Biol. Reprod. 79: 1111–1120. [DOI] [PubMed] [Google Scholar]
- Xia W., Zhou L., Yao B., Li C. J., Gui J. F., 2007. Differential and spermatogenic cell-specific expression of DMRT1 during sex reversal in protogynous hermaphroditic groupers. Mol. Cell. Endocrinol. 263: 156–172. [DOI] [PubMed] [Google Scholar]
- Xiong S., Wu J., Jing J., Huang P., Li Z., et al. , 2017. Loss of stat3 function leads to spine malformation and immune disorder in zebrafish. Sci. Bull. 62: 185–196. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Yamaguchi S., Hirai T., Kitano T., 2007. Follicle-stimulating hormone signaling and Foxl2 are involved in transcriptional regulation of aromatase gene during gonadal sex differentiation in Japanese flounder, Paralichthys olivaceus. Biochem. Biophys. Res. Commun. 359: 935–940. [DOI] [PubMed] [Google Scholar]
- Yano A., Guyomard R., Nicol B., Jouanno E., Quillet E., et al. , 2012. An immune-related gene evolved into the master sex-determining gene in rainbow trout, Oncorhynchus mykiss. Curr. Biol. 22: 1423–1428. [DOI] [PubMed] [Google Scholar]
- Yin J., Xia J. H., Du X. Z., Liu J., Zhou L., et al. , 2007. Developmental expression of CagMdkb during gibel carp embryogenesis. Int. J. Dev. Biol. 51: 761–769. [DOI] [PubMed] [Google Scholar]
- Yoshimoto S., Okada E., Umemoto H., Tamura K., Uno Y., et al. , 2008. A W-linked DM-domain gene, DM-W, participates in primary ovary development in Xenopus laevis. Proc. Natl. Acad. Sci. USA 105: 2469–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The DNA sequences have been deposited at National Center for Biotechnology Information (NCBI), accession numbers from KR232688 to KR232690. The raw data of transcriptomes have been submitted to the NCBI database (accession number: SRR4239959). See Table S2 for gene symbols, full names, RefSeq identifications, and related references. All the Supplemental Table Legends have been shown in File S2.