Abstract
Growing plant stems and shoots exhibit a variety of shapes that embody growth in response to various stimuli. Building on experimental observations, we provide a quantitative biophysical theory for these shapes by accounting for the inherent observed passive and active effects: (i) the active controllable growth response of the shoot in response to its orientation relative to gravity, (ii) proprioception, the shoot's growth response to its own observable current shape, and (iii) the passive elastic deflection of the shoot due to its own weight, which determines the current shape of the shoot. Our theory separates the sensed and actuated variables in a growing shoot and results in a morphospace diagram in terms of two dimensionless parameters representing a scaled local active gravitropic sensitivity, and a scaled passive elastic sag. Our computational results allow us to explain the variety of observed transient and steady morphologies with effective positive, negative and even oscillatory gravitropic behaviours, without the need for ad hoc complex spatio-temporal control strategies in terms of these parameters. More broadly, our theory is applicable to the growth of soft, floppy organs where sensing and actuation are dynamically coupled through growth processes via shape.
Keywords: growth and form, plant shoots, gravitropism, mathematical model
1. Introduction
The ability of plants to control growth movements in response to various environmental cues is well known [1]. These directional movements, called tropisms, enable plants to respond by orienting their growth to external stimuli. One of the most commonly observed of these movements is gravitropism, wherein plant shoots perceive the direction of gravity and regulate their growth to achieve a steady orientation with respect to gravity [2–8]. The perception of gravity occurs within specialized cells called statocytes through the sedimentation of intracellular statoliths [5,8,9] onto sensory structures. This mechanical signal leads to a differential growth across the cross section of a shoot and thence active (sentient) bending of the respective organ [5–8,10]. Thus, plant shoots are ‘negatively gravitropic’, preferring a vertically upward state opposing gravity, while roots are ‘positively gravitropic’, preferring a vertically downward posture.
While shoots of the model organism Arabidopsis thaliana (figure 1b) concur with this expectation leading to vertically upward growing stems, there are many plants which show spatio-temporally complex orientations of their shoots. For example, Impatiens glandulifera [11–13] can have shoots that display oscillatory curving and de-curving before converging to a vertically upward steady orientation, Cerastium tomentosum (figure 1c) and Toxicodendron radicans (figure 1d) have shoots that first grow vertically downwards before turning upwards effectively switching from being positively gravitropic to negatively gravitropic as the plant develops, Salix alba (figure 1e) exhibits an apparent positive gravitropic behaviour by growing in the direction of gravity, and Trandescantia fluminensis and Oplismenus hirtellus [14,15] also display a ‘gravitropic sign reversal’ suggesting a time-dependent preferential orientation.
Figure 1.
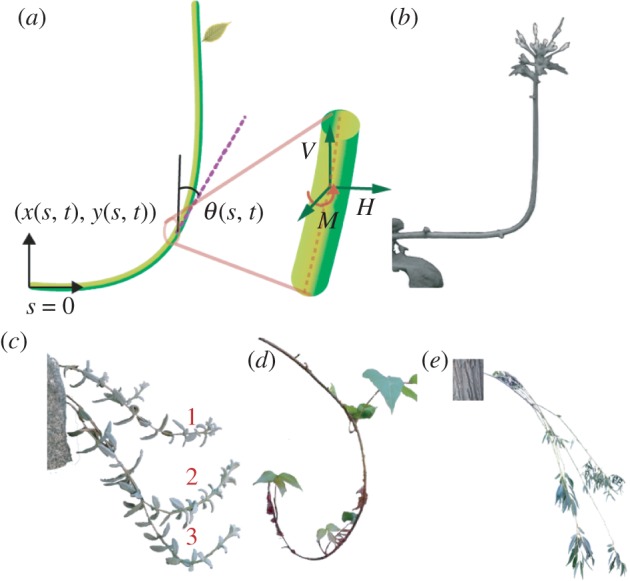
(a) Schematic of our active elastic model used to study the sentient growth of plant shoots. (b–e) Commonly observed gravitropic behaviour of various plant shoots. (b) Negative gravitropic response of Arabidopsis thaliana shoot [11]. (c) Cerastium tomentosum shoots of three different lengths (1–3) with axially varying gravitropic orientation. The local shoot orientation varies as the shoot length increases, suggestive of a complex control strategy. (d) Shoots of Toxicodendron radicans display axially varying gravitropic orientation with curvature reversal. (e) Salix alba shoots displaying apparent positive gravitropism. (Online version in colour.)
Attempts to explain these versatile gravitropic behaviours have led to a variety of hypotheses going back more than a century [1,16]. The simplest of these, Sachs' law, states that the local rate of curvature of the growth zone responds to the orientation of the shoot, leading to a response that is strongest at regions which are horizontal and weakest where it is vertical. However, this does not suffice to build a regulatory framework for stem straightening; indeed, it leads to oscillations [11] similar to those in undamped feedback control systems. Separately, evidence shows that plants and fungi have a proprioceptive sense known as autotropism that causes them to grow straight in the absence of any stimuli [3,17–20]. This straightening mechanism is not specific to the type or direction of induced stimulus and is observed whenever curvature is induced in the plant.
Independent of this literature on gravitropism and autotropism, there has also been interest in understanding the growth of plant shoots and roots from a purely mechanical perspective. By treating the growing rod as a one-dimensional continuum that can change its reference length, diameter and stiffness, various authors have formulated a theory for how its shape can vary with time [10,21–24]. A general discussion of possible evolution equations for the natural curvature has also been considered [22,25], with autotropism being the primary driver. These theoretical studies do not consider the role of multiple stimuli that include, for example, autotropic, gravitropic or phototropic responses, nor do they explore the large parameter space of variables to understand the qualitative nature of the solutions or their implications for the observed morphological diversity of plant shoots.
A recent elegant proposal by Bastien et al. [11,26] combines the ideas of autotropism and gravitropism into a single quantitative theory which the authors show is both necessary and sufficient to maintain a steady vertical orientation for shoots, and corroborate using a series of experiments and observations. In the minimal setting considered in [11,26], effects such as elasticity and gravity acting on soft stems, and maturation/lignification are neglected, and this implicitly raises the natural question of how to modify the theory to explain the various complex spatio-temporal morphologies of plant shoots summarized earlier.
Here, we combine these studies on gravitropism and autotropism (the sensing problem) along with the studies on mechanical growth (the actuation problem) in a minimal setting that accounts for multiple stimuli to understand a range of old observations of plant shoots. We begin by questioning the basic assumption inherent in prior models, that (proprioceptive) sensing and actuation (via growth) act on the same variable, the shape of the growth zone. While this is certainly true in a gravity-free environment, this cannot be true in general since the plant droops due to its own weight even as it grows, so that the observable shape associated with a heavy, sagging shoot and the controllable growth rate that determines the intrinsic curvature are fundamentally different objects. Building on this, we propose an observationally rooted biophysical model that combines the effects of the passive elastic deflection of soft plant shoots due to gravity, active gravitropic and proprioceptive growth acting on the deformed shoot, coupled with axial elongation in the growth zone. Our study shows that this model suffices to explain various gravitropic reorientations observed across a range of plant shoots, without the need for ad hoc control strategies for shoot growth rates in space and time.
2. Theoretical model
Gravitropic movements in most plant species are confined to a plane defined by the shoot axis and the gravity vector [1,3]. Therefore, we limit ourselves to a planar description that captures the main characteristics of the observed phenotypes, modelling the plant shoot as a thin inextensible elastic filament. At a given instant of time t, the centreline [x(s, t), y(s, t)] (figure 1a) is parametrized by the arc length s ∈ [0, ℓ(t)], with the initial length ℓ(0) ≡ ℓ0. We assume that the plant shoot has a bending rigidity B(s, t) which can change as the shoot lignifies, and that its weight per unit length is ρg, where ρ is the linear density of the shoot. The shape of the filament is characterized by the angle θ(s, t) between the local tangent and the vertical axis (figure 1a).
We assume that initially the straight filament is oriented along the horizontal axis, with the basal end of the filament (s = 0) being horizontally clamped, with θ(0) =−π/2, x(0) = y(0) = 0, while the apical end (s = ℓ) is free. The primary growth process in shoots, including elongation and tropical bending, takes place only in the growth zone of the shoot, a localized region extending over a finite length ℓg from the apex [3,11] that grows uniformly at a rate 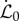 . Then, for an infinitesimal segment of length Δ(s) in this zone, we may write
. Then, for an infinitesimal segment of length Δ(s) in this zone, we may write
| 2.1 |
where
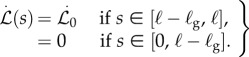 |
2.2 |
In the growth zone, the local intrinsic curvature κ * (s) is modified by active bending of the shoot which responds via local gravitropism and autotropism. A number of empirical studies have verified that the rate of gravitropic curvature change depends on the local orientation of the segment via the relation ∂κ * (s, t)/∂t ∝ sinθ [3,4]. Opposing this curvature change, a proprioceptive mechanism acts to maintain local straightness. Indeed, it has been observed [17,19] that this straightening mechanism acts in response to an induced curvature, irrespective of its cause, i.e. ∂κ * (s, t)/∂t ∝−κ. Then the total rate of change of intrinsic curvature inside the growth zone is given by the sum of these contributions and reads
| 2.3 |
Here the gravitropic sensitivity is β, while the proprioceptive sensitivity is γ. We have rescaled all rates by 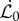 , a parameter that sets the natural (inverse) time scale for growth. The balance between gravitropic sensitivity and proprioception leads to the definition of a characteristic sensitivity length, ℓs = γ/β over which these processes compete with each other [11]. We note that the proprioceptive term acts on the current curvature of the shoot, κ, which is generally different from the intrinsic curvature of the shoot κ* when ρg ≠ 0. It is the presence of this term that couples the current morphology of the shoot which is determined by elasticity and gravity to the sentient growth of the shoot that changes its intrinsic curvature. In the weightless limit κ = κ*, and the dynamics of shoot curvature in the growth zone is entirely determined by (2.3), and we recover the proposal of [11] leading to a single equation for the dynamics of growth. However, for soft, heavy, immature growing shoots, characteristic of real plants, we must account for gravity-induced deformation and the inability of the plant to sense its intrinsic (gravity-free) shape, coupling the physics of the growing, deforming shoot to the control law (2.3).
, a parameter that sets the natural (inverse) time scale for growth. The balance between gravitropic sensitivity and proprioception leads to the definition of a characteristic sensitivity length, ℓs = γ/β over which these processes compete with each other [11]. We note that the proprioceptive term acts on the current curvature of the shoot, κ, which is generally different from the intrinsic curvature of the shoot κ* when ρg ≠ 0. It is the presence of this term that couples the current morphology of the shoot which is determined by elasticity and gravity to the sentient growth of the shoot that changes its intrinsic curvature. In the weightless limit κ = κ*, and the dynamics of shoot curvature in the growth zone is entirely determined by (2.3), and we recover the proposal of [11] leading to a single equation for the dynamics of growth. However, for soft, heavy, immature growing shoots, characteristic of real plants, we must account for gravity-induced deformation and the inability of the plant to sense its intrinsic (gravity-free) shape, coupling the physics of the growing, deforming shoot to the control law (2.3).
Since the time scale for elastic equilibrium is much shorter than the time scale for growth [2], elastic equilibrium governs the shape of the filament at all times. Local torque balance yields
| 2.4 |
where M = B(κ − κ* ) and B is the bending rigidity of the filament, κ = ∂θ/∂s is the actual local curvature, κ* is the local intrinsic curvature (in the absence of any external loading). Similarly, local force balance yields
| 2.5 |
where H and V are, respectively, the horizontal and vertical force components (figure 1a), and ρg is the gravitational body force in the y-direction on the filament of linear density ρ due to gravity g. Additionally, we have the kinematic relations
| 2.6 |
associated with the assumption of inextensibility and unshearability of thin filaments. The associated boundary conditions for the shape of the shoot are that the basal end is clamped horizontally with θ(0) =−π/2, x(0) = y(0) = 0, while the apical end is free of forces and torques so that H(ℓ) = V (ℓ) = κ(ℓ) − κ* (ℓ) = 0.
Finally, we note that as stems grow, lignification outside the growth zone [27] leads to an increase in the bending rigidity with time. We model lignification using the simple law
| 2.7 |
where B0 is the uniform bending rigidity in the growth zone, s ∈ [ℓ − ℓg, ℓ], 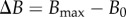 and
and 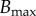 is the maximum value of the bending rigidity of fully lignified wood, δt is the age of a material point outside the growth zone (δt = 0 for s > ℓ − ℓg), and τ−1 is the maturation rate. It may be useful to contrast our model with the many theoretical models for growth-induced morphological changes of plant shoots [21–25] that assume that the actual curvature tries to relax to the natural curvature. We differ by accounting for the experimentally demonstrated facts that a shoot grows to change its curvature in response to its local orientation with respect to the direction of gravity while also trying to maintain straightness [11] noting that the actual (sensed) curvature is different from the (actuated) growth curvature.
is the maximum value of the bending rigidity of fully lignified wood, δt is the age of a material point outside the growth zone (δt = 0 for s > ℓ − ℓg), and τ−1 is the maturation rate. It may be useful to contrast our model with the many theoretical models for growth-induced morphological changes of plant shoots [21–25] that assume that the actual curvature tries to relax to the natural curvature. We differ by accounting for the experimentally demonstrated facts that a shoot grows to change its curvature in response to its local orientation with respect to the direction of gravity while also trying to maintain straightness [11] noting that the actual (sensed) curvature is different from the (actuated) growth curvature.
Four dimensionless parameters characterize the dynamics of the growing filament. (i) The sensitivity parameter 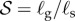 is the ratio of the size of the growth zone ℓg relative to the sensitivity length ℓs that arises from the competition between gravitropism and autotropism. For the majority of terrestrial plants,
is the ratio of the size of the growth zone ℓg relative to the sensitivity length ℓs that arises from the competition between gravitropism and autotropism. For the majority of terrestrial plants, 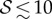 [11]. (ii) The passive elasticity parameter
[11]. (ii) The passive elasticity parameter 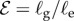 is the ratio of the elasto-gravity length ℓe = (B/ρg)1/3 that arises from the competition between gravity and elasticity, and the size of the growth zone. For a young plant shoot with diameter ≃1 mm, Young's modulus ≃107 Pa, mass density ϱ = 103 kg m−3 [28], ℓg ∼ 10 cm, so that
is the ratio of the elasto-gravity length ℓe = (B/ρg)1/3 that arises from the competition between gravity and elasticity, and the size of the growth zone. For a young plant shoot with diameter ≃1 mm, Young's modulus ≃107 Pa, mass density ϱ = 103 kg m−3 [28], ℓg ∼ 10 cm, so that 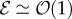 . (iii) The maturation parameter
. (iii) The maturation parameter 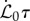 is associated with lignification and controlled by the relative maturation rate. In the absence of precisely quantified maturation rates in plants, we examine our model for
is associated with lignification and controlled by the relative maturation rate. In the absence of precisely quantified maturation rates in plants, we examine our model for 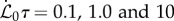 , with
, with 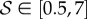 ,
, 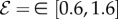 ,
, 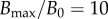 and ℓ(t0) = 0.5ℓg − ℓg. (iv) Finally, the scaled growth zone size is measured relative to the initial length of the shoot ℓg/ℓ(t0). As we will see, only the first two parameters, the growth sensitivity
and ℓ(t0) = 0.5ℓg − ℓg. (iv) Finally, the scaled growth zone size is measured relative to the initial length of the shoot ℓg/ℓ(t0). As we will see, only the first two parameters, the growth sensitivity 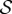 and passive elasticity
and passive elasticity 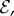 are relevant in determining the qualitative nature of the morphologies of growing shoots. We scale all lengths by ℓg and from now on depict the shape in units of the growth zone length.
are relevant in determining the qualitative nature of the morphologies of growing shoots. We scale all lengths by ℓg and from now on depict the shape in units of the growth zone length.
Although the nonlinear system (2.4)–(2.7) has some specific limiting behaviours that may be deduced analytically in some linearized settings [11], our focus is on broadly defining the phase space of possible morphologies for which a numerical approach is more feasible. This is carried out by discretizing the filament of length ℓ into Nm nodes with position ri connected by Nm − 1 elastic segments labelled by tangent vectors bi = ri +1−ri, such that 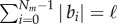 . The growth zone of the filament is marked by the index z such that
. The growth zone of the filament is marked by the index z such that 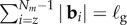 . The tangent angle of the filament, θi, is defined as θi = cos−1(bi.ey/|bi|) and the discretized local curvature is given as κi = (θi+1−θi)/|bi|. The elasticity of the filament is implemented via extensional (Ue) and bending (Ub) potentials, where
. The tangent angle of the filament, θi, is defined as θi = cos−1(bi.ey/|bi|) and the discretized local curvature is given as κi = (θi+1−θi)/|bi|. The elasticity of the filament is implemented via extensional (Ue) and bending (Ub) potentials, where 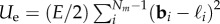 , ℓi being the equilibrium length of segment i, and
, ℓi being the equilibrium length of segment i, and 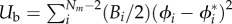 , where ϕi = cos−1((bi.bi+1)/|bi| |bi+1|), and ϕ* is the equilibrium inter-segment angle. The filament is kept practically inextensible by setting E ≫ B. The net force acting on a node i is given by
, where ϕi = cos−1((bi.bi+1)/|bi| |bi+1|), and ϕ* is the equilibrium inter-segment angle. The filament is kept practically inextensible by setting E ≫ B. The net force acting on a node i is given by
| 2.8 |
The filament growth, stiffening and feedback dynamics at discretized time intervals Δt are implemented by changing the equilibrium segment lengths ℓi, the intrinsic curvature κ*i and the bending rigidity Bi using the discretized version of equations (2.1)–(2.7). For each i > z, ℓi and κ*i are updated using the relations 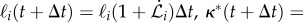
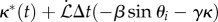 and for every i < z, the bending rigidity
and for every i < z, the bending rigidity 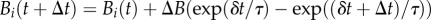 . As the plant shoot is always at an elastic equilibrium during the entire growth process, between each growth time step (Δt), the equilibrium shape of the filament is numerically determined using a damped Verlet method, by including the updated values of κ*i , ℓi and Bi in (2.8). This equilibrium shape provides actual values of local curvature κi and orientation angle θi which are in turn used to calculate the intrinsic shape at the next time step.
. As the plant shoot is always at an elastic equilibrium during the entire growth process, between each growth time step (Δt), the equilibrium shape of the filament is numerically determined using a damped Verlet method, by including the updated values of κ*i , ℓi and Bi in (2.8). This equilibrium shape provides actual values of local curvature κi and orientation angle θi which are in turn used to calculate the intrinsic shape at the next time step.
3. Growth morphologies of a sentient shoot
Although the overall morphology of sentient shoots depends on four dimensionless parameters, we find that changes in the relative maturation rate 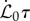 do not significantly influence the qualitative features of the observed growth pattern (electronic supplementary material, section I and figure S1). Likewise, we also find that the observed shapes are qualitatively independent of the scaled growth zone size ℓg/ℓ(t0) (electronic supplementary material sections I–II and figure S2). Therefore, we focus on the results for
do not significantly influence the qualitative features of the observed growth pattern (electronic supplementary material, section I and figure S1). Likewise, we also find that the observed shapes are qualitatively independent of the scaled growth zone size ℓg/ℓ(t0) (electronic supplementary material sections I–II and figure S2). Therefore, we focus on the results for 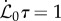 and ℓ(t0) = ℓg in the rest of the paper, and study the dependence of shoot growth on the two remaining parameters: the sensitivity
and ℓ(t0) = ℓg in the rest of the paper, and study the dependence of shoot growth on the two remaining parameters: the sensitivity 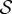 (which can be changed by varying γ) and the elasticity
(which can be changed by varying γ) and the elasticity 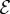 (which can be changed by varying ρg), for the above prescribed values of the relative maturation rates and initial length.
(which can be changed by varying ρg), for the above prescribed values of the relative maturation rates and initial length.
When 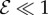 , corresponding to the weightless limit, the morphology of the shoot is dominated by the active bending processes determined by
, corresponding to the weightless limit, the morphology of the shoot is dominated by the active bending processes determined by 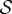 as shown in figure 2a, although the steady state apex orientation is independent of
as shown in figure 2a, although the steady state apex orientation is independent of 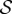 . In this limit, when
. In this limit, when 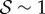 the reorientation process of the apex is monotonic (electronic supplementary material, movie S1, see the appendix for additional details). As
the reorientation process of the apex is monotonic (electronic supplementary material, movie S1, see the appendix for additional details). As 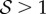 , i.e. when ℓg > ℓs, the filament apex overshoots the vertical, as shown in figure 2a(i). A kymograph of the curvature of the shoot in figure 2a(ii) shows that the filament stays curved only within a limited region near the basal end. If
, i.e. when ℓg > ℓs, the filament apex overshoots the vertical, as shown in figure 2a(i). A kymograph of the curvature of the shoot in figure 2a(ii) shows that the filament stays curved only within a limited region near the basal end. If 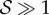 the apex can oscillate about the vertical axis before eventually settling into a straight steady shape, again owing to proprioception. This ‘weightless’ limit recovers the behaviour predicted and measured by Bastien et al. [11] that focuses only on the dynamics of the growth zone, and is commonly observed in a number of plant species [3,11,12].
the apex can oscillate about the vertical axis before eventually settling into a straight steady shape, again owing to proprioception. This ‘weightless’ limit recovers the behaviour predicted and measured by Bastien et al. [11] that focuses only on the dynamics of the growth zone, and is commonly observed in a number of plant species [3,11,12].
Figure 2.
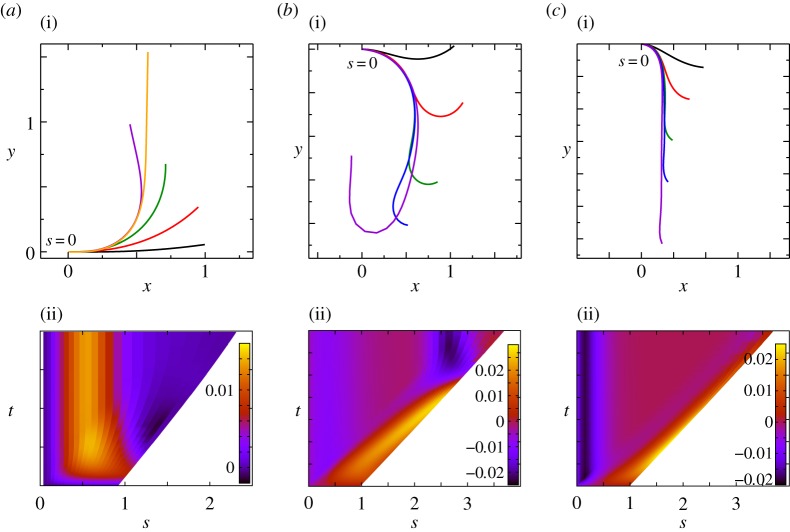
The time evolution of scaled shapes of a growing shoot, with different colours representing shape at different times (i). Kymographs of the local curvature (κ) as a function of arc length (s) and time (t) are shown (ii) for the sensitivity parameter 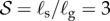 and for various values of the elasticity parameter
and for various values of the elasticity parameter 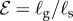 obtained by solving equations (2.1)–(2.7). (a) Vertically upward growth, with negative gravitropism, and
obtained by solving equations (2.1)–(2.7). (a) Vertically upward growth, with negative gravitropism, and 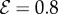 , (b) transitional growth behaviour with an effective gravitropic ‘sign reversal’ and
, (b) transitional growth behaviour with an effective gravitropic ‘sign reversal’ and 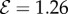 , (c) vertically downward growth, with effective positive gravitropism, and
, (c) vertically downward growth, with effective positive gravitropism, and 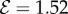 . (Online version in colour.)
. (Online version in colour.)
When 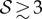 and
and 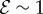 , we observe a qualitatively different gravitropic response shown in figure 2b (electronic supplementary material, movies S2 and S3, see the appendix for additional details). In this regime, the passive elastic deflection of the shoot due to gravity alters the local orientation angle θ(s) and thence its equilibrium shape. Because the gravitropic term in (2.3) depends on the local orientation of the shoot, the rate of curvature of the shoot changes as a result. This leads to an initial response of the shoot that is akin to negative gravitropism, since the apex is oriented vertically upward, and the elastic deflection in the filament is minimal. With time, the region near the base starts to orient towards gravity, while the apex favours an upward orientation (figure 2b(i)), that might be construed as a ‘sign reversal’ in gravitropism. The space–time profile of the curvature in figure 2b(ii) reveals that the zone of positive curvature moves towards the apex as the shoot elongates, disappearing completely as the apex straightens temporarily. Finally, the apex curves in the reverse direction and grows vertically upwards. The observed transient shapes for these intermediate values of
, we observe a qualitatively different gravitropic response shown in figure 2b (electronic supplementary material, movies S2 and S3, see the appendix for additional details). In this regime, the passive elastic deflection of the shoot due to gravity alters the local orientation angle θ(s) and thence its equilibrium shape. Because the gravitropic term in (2.3) depends on the local orientation of the shoot, the rate of curvature of the shoot changes as a result. This leads to an initial response of the shoot that is akin to negative gravitropism, since the apex is oriented vertically upward, and the elastic deflection in the filament is minimal. With time, the region near the base starts to orient towards gravity, while the apex favours an upward orientation (figure 2b(i)), that might be construed as a ‘sign reversal’ in gravitropism. The space–time profile of the curvature in figure 2b(ii) reveals that the zone of positive curvature moves towards the apex as the shoot elongates, disappearing completely as the apex straightens temporarily. Finally, the apex curves in the reverse direction and grows vertically upwards. The observed transient shapes for these intermediate values of 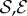 are qualitatively similar to those seen in plant species such as Crassula ovata, Cerastium tomentosum (figure 1c), and Toxicodendron radicans (figure 1d), and the ultimate morphology of such a shoot with reversals is similar to that observed in Trandescantia fluminensis and Oplismenus hirtellus [14,15].
are qualitatively similar to those seen in plant species such as Crassula ovata, Cerastium tomentosum (figure 1c), and Toxicodendron radicans (figure 1d), and the ultimate morphology of such a shoot with reversals is similar to that observed in Trandescantia fluminensis and Oplismenus hirtellus [14,15].
For soft heavy shoots with a weak gravitropic response, passive elastic deflection dominates over gravitropic sensitivity, so that we expect the filament to orient progressively towards gravity (electronic supplementary material, movie S4, see the appendix for additional details). In figure 2c, we see this effective ‘positive’ gravitropic response for 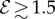 and
and 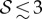 . Many ornamental plant species such as Hedera helix or branches of some medium-sized trees such as Salix alba (figure 1e) show a negative curvature near the base and a relatively straight downward pointing apex, consistent with this scenario.
. Many ornamental plant species such as Hedera helix or branches of some medium-sized trees such as Salix alba (figure 1e) show a negative curvature near the base and a relatively straight downward pointing apex, consistent with this scenario.
In figure 3, we show the steady shapes of shoots for different values of 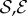 . When
. When 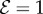 , as
, as 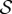 increases, negatively gravitropic shoots show a propensity for decreasing the length over which they turn, as expected, and thus also sag a little less, as seen in figure 3a. Furthermore, as
increases, negatively gravitropic shoots show a propensity for decreasing the length over which they turn, as expected, and thus also sag a little less, as seen in figure 3a. Furthermore, as 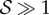 , the steady shape of the shoot has multiple inflection points in the vicinity of the base. When
, the steady shape of the shoot has multiple inflection points in the vicinity of the base. When 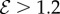 , the orientation of the filament apex is no longer vertically upward. As shown in figure 3b for weak gravitropic sensitivity, i.e.
, the orientation of the filament apex is no longer vertically upward. As shown in figure 3b for weak gravitropic sensitivity, i.e. 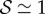 , the shoot grows downwards, while when
, the shoot grows downwards, while when 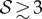 , the shoot eventually turns around and grows upwards.
, the shoot eventually turns around and grows upwards.
Figure 3.
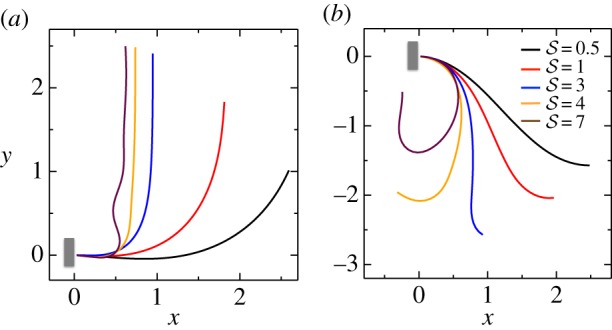
Scaled steady-state shapes of a growing filament for various values of the sensitivity parameter 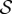 . Different colours represent various values of
. Different colours represent various values of 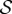 ; from right to left
; from right to left 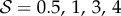 and 7 for (a)
and 7 for (a) 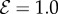 and (b)
and (b) 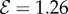 . (Online version in colour.)
. (Online version in colour.)
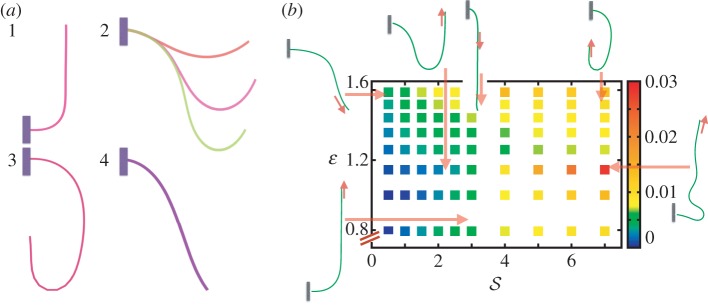
In figure 4, we combine these observations into a shoot morphospace diagram that combines the effects of passive elasticity, active growth, gravitropism and proprioception into just two parameters: the active sensitivity 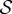 and the passive elasticity
and the passive elasticity 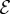 . Shoots display negative gravitropism only when the passive elastic deflection is negligible, i.e.
. Shoots display negative gravitropism only when the passive elastic deflection is negligible, i.e. 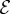 ; an increase in
; an increase in 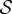 only changes the shoot curvature near base, but does not affect the overall morphology of the shoot. On the other hand, when
only changes the shoot curvature near base, but does not affect the overall morphology of the shoot. On the other hand, when 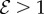 , the shoot shape can display a positive gravitropic trend with a downward orientation of the apex; this region is observed when
, the shoot shape can display a positive gravitropic trend with a downward orientation of the apex; this region is observed when 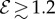 and
and 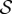 . When
. When 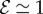 and
and 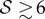 the shoot displays an effective negative gravitropic trend near the apex with multiple inflection points along its length. When both effects are significant, i.e.
the shoot displays an effective negative gravitropic trend near the apex with multiple inflection points along its length. When both effects are significant, i.e. 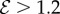 and
and 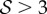 , we observe a more complex regime wherein an effectively graded gravitropic preference of the shoot is seen, with points of inflection in the shape, before the shoot eventually settles into a vertical posture. A simple measure of the integrated curvature, its L2 norm in this morphospace, shows that its maximum value corresponds to intermediate values of
, we observe a more complex regime wherein an effectively graded gravitropic preference of the shoot is seen, with points of inflection in the shape, before the shoot eventually settles into a vertical posture. A simple measure of the integrated curvature, its L2 norm in this morphospace, shows that its maximum value corresponds to intermediate values of 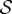 and
and 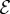 where the shoot exhibits multiple inflection points.
where the shoot exhibits multiple inflection points.
Figure 4.
(a) The shapes obtained from the solution of our model described by equations (2.1)–(2.7) which are qualitatively similar to the shapes displayed in figure 1. (1) Negative gravitropism observed for computed shapes with 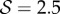 and
and 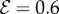 . (2) Axially varying gravitropic orientation with increase in shoot length for
. (2) Axially varying gravitropic orientation with increase in shoot length for 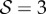 and
and 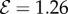 . (3) Axially varying curvature change with curvature reversal, observed in our model for
. (3) Axially varying curvature change with curvature reversal, observed in our model for 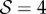 and
and 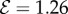 . (4) Apparent positive gravitropism for
. (4) Apparent positive gravitropism for 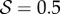 and
and 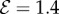 . (b) Morphospace for shoot shapes as a function of the sensitivity parameter
. (b) Morphospace for shoot shapes as a function of the sensitivity parameter 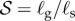 , and the elasticity parameter
, and the elasticity parameter 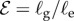 . We note that for
. We note that for 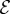 , there are no qualitative differences from what is shown—when
, there are no qualitative differences from what is shown—when 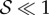 , the shoot turns without any inflection points, while when
, the shoot turns without any inflection points, while when 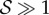 , it can have one or more points of inflection. A comparison with figure 1 shows that we can capture a range of different forms in terms of these two parameters. The colour map indicates the L2 norm of the shoot curvature
, it can have one or more points of inflection. A comparison with figure 1 shows that we can capture a range of different forms in terms of these two parameters. The colour map indicates the L2 norm of the shoot curvature 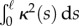 . (Online version in colour.)
. (Online version in colour.)
4. Discussion
Our study shows that the observed diversity in the shape of plant shoots can be explained in terms of their ability to sense themselves (proprioceptively) and their environment (gravitropically) while being constrained by physical law (elasticity and gravity). In particular, our modification of the local gravitropic–proprioceptive model [11] in a simple but critical way allows us to differentiate between the controllable active growth response which acts on the natural curvature and the observable current shape. This allows us to explain effective positive, transient and negative gravitropism in plant shoots [15] in terms of two dimensionless parameters, the scaled sensitivity of growth and the scaled elasticity of the shoots.
Exploration of the solutions in this morphospace allows us to explain various gravitropic reorientations observed across a range of plant shoots, without the need for ad hoc control strategies for shoot growth rates in space and time. Our model can be easily extended to include the phototropic responses associated with shoot reorientation just at the apex. Thus, our theory also naturally begins to set the stage for the study of decision-making in the context of multiple sensory inputs from light, gravity, proprioception, etc., in growing plant shoots. More broadly, our study also highlights how the difference between the observed shape of an organ and its controlled natural shape impacts any control law that acts on the observed shape but changes the natural shape. Indeed, the simple idea of separating the controllable actuation and observable sensing variables in growing biological systems in external fields ought to be relevant for a range of active morphogenetic events in soft growing tissues.
Supplementary Material
Acknowledgments
We thank Renaud Bastien and Haiyi Liang for discussions.
Competing interests
We declare we have no competing interests.
Funding
We thank the MacArthur Foundation for partial financial support.
References
- 1.Darwin C, Darwin F. 1880. The power of movement in plants. London, UK: Murray. [Google Scholar]
- 2.Gilory S, Masson PH. 2008. Plant tropisms. Oxford, UK: Blackwell. [Google Scholar]
- 3.Moulia B, Fournier M. 2009. The power and control of gravitropic movements in plants: a biomechanical and system biology view. J. Exp. Bot. 60, 461–486. ( 10.1093/jxb/ern341) [DOI] [PubMed] [Google Scholar]
- 4.Galland P. 2002. Tropisms of Avena coleoptiles: sine law for gravitropism, exponential law for photogravitropic equilibrium. Planta 215, 779–784. ( 10.1007/s00425-002-0813-6) [DOI] [PubMed] [Google Scholar]
- 5.Morita MT. 2010. Directional gravity sensing in gravitropism. Annu. Rev. Plant Biol. 61, 705–720. ( 10.1146/annurev.arplant.043008.092042) [DOI] [PubMed] [Google Scholar]
- 6.Morita MT, Tasaka M. 2004. Gravity sensing and signaling. Curr. Opin. Plant Biol. 7, 712–718. ( 10.1016/j.pbi.2004.09.001) [DOI] [PubMed] [Google Scholar]
- 7.Tasaka M, Kato T, Fukaki H. 1999. The endodermis and shoot gravitropism. Trends Plant Sci. 4, 103–107. ( 10.1016/S1360-1385(99)01376-X) [DOI] [PubMed] [Google Scholar]
- 8.Hashiguchi Y, Tasaka M, Morita MT. 2013. Mechanism of higher plant gravity sensing. Am. J. Bot. 100, 91–100. ( 10.3732/ajb.1200315) [DOI] [PubMed] [Google Scholar]
- 9.Kolesnikov YS, Kretynin SV, Volotovsk ID, Kordyum EL, Ruelland E, Kravet VS. 2016. Molecular mechanisms of gravity perception and signal transduction in plants. Protoplasma 253, 987–1004. ( 10.1007/s00709-015-0859-5) [DOI] [PubMed] [Google Scholar]
- 10.Silk WK. 1984. Quantitative descriptions of development. Annu. Rev. Plant Physiol. 35, 479–518. ( 10.1146/annurev.pp.35.060184.002403) [DOI] [Google Scholar]
- 11.Bastien R, Bohr T, Moulia B, Douady S. 2013. Unifying model of shoot gravitropism reveals proprioception as a central feature of posture control in plants. Proc. Natl Acad. Sci. USA 110, 755–760. ( 10.1073/pnas.1214301109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfeffer W. 1904. Pflanzenphysiologie, vol. 2 Leipzig, Germany: W. Engelmann. [Google Scholar]
- 13.Kutschera U. 2001. Gravitropism of axial organs in multicellular plants. Adv. Space Res. 27, 851–860. ( 10.1016/S0273-1177(01)00148-X) [DOI] [PubMed] [Google Scholar]
- 14.Myers AB, Firn R, Digby J. 1994. Gravitropic sign reversal—a fundamental feature of the gravitropic perception or response mechanisms in some plant organs. J. Exp. Bot. 45, 77–83. ( 10.1093/jxb/45.1.77) [DOI] [Google Scholar]
- 15.Digby J, Firn R. 1995. The gravitropic set-point angle (gsa): the identification of an important developmentally controlled variable governing plant architecture. Plant Cell Environ. 18, 1434–1440. ( 10.1111/j.1365-3040.1995.tb00205.x) [DOI] [PubMed] [Google Scholar]
- 16.Sachs JV. 1887. Lectures on physiology of plants. Oxford, UK: Oxford University Press. [Google Scholar]
- 17.Firn R, Digby J. 1979. A study of the autotropic straightening reaction of a shoot previously curved during geotropism. Plant Cell Physiol. 38, 1346–1353. ( 10.1111/j.1365-3040.1979.tb00786.x) [DOI] [Google Scholar]
- 18.Tarui Y, Iino M. 1997. Gravitropism of oat and wheat coleoptiles: dependence on the stimulation angle and the involvement of autotropic straightening. Plant Cell Environ. 2, 149–154. ( 10.1093/oxfordjournals.pcp.a029128) [DOI] [PubMed] [Google Scholar]
- 19.Meskauskas A, Moore D, Novak Frazer L. 1998. Mathematical modelling of morphogenesis in fungi: spatial organization of the gravitropic response in the mushroom stem of Coprinus cinereus. New Phytol. 140, 111–123. ( 10.1046/j.1469-8137.1998.00252.x) [DOI] [PubMed] [Google Scholar]
- 20.Stankovic B, Volkmann D, Sack FD. 1998. Autotropism, automorphogenesis, and gravity. Physiol. Plant. 102, 328–335. ( 10.1034/j.1399-3054.1998.1020222.x) [DOI] [PubMed] [Google Scholar]
- 21.Fourcaud T, Lac P. 2003. Numerical modelling of shape regulation and growth stresses in trees: 1. An incremental static finite element formulation. Trees 17, 23–30. ( 10.1007/s00468-002-0202-6) [DOI] [Google Scholar]
- 22.Goldstein RE, Goriely A. 2006. Dynamic buckling of morphoelastic filaments. Phys. Rev. E 74, 010901(R) ( 10.1103/PhysRevE.74.010901) [DOI] [PubMed] [Google Scholar]
- 23.Senan NAF, O'Reilly OM, Tresierras TN. 2008. Modeling the growth and branching of plants: a simple rod-based model. J. Mech. Phys. Solids 56, 3021–3036. ( 10.1016/j.jmps.2008.06.005) [DOI] [Google Scholar]
- 24.Moulton DE, Lessinnes T, Goriely A. 2013. Morphoelastic rods. Part 1: a single growing elastic rod. J. Mech. Phys. Solids 61, 398–427. ( 10.1016/j.jmps.2012.09.017) [DOI] [Google Scholar]
- 25.O'Reilly OM, Tresierras TN. 2011. On the evolution of intrinsic curvature in rod-based models of growth in long slender plant stems. Int. J. Solids Struct. 48, 1239–1247. ( 10.1016/j.ijsolstr.2010.12.006) [DOI] [Google Scholar]
- 26.Bastien R, Douady S, Moulia B. 2014. A unifying modeling of plant shoot gravitropism with an explicit account of the effects of growth. Front. Plant Sci. 5, 136 ( 10.3389/fpls.2014.00136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cosgrove DJ. 1997. Assembly and enlargement of the primary cell wall in plants. Annu. Rev. Cell Dev. Biol. 13, 171–201. ( 10.1146/annurev.cellbio.13.1.171) [DOI] [PubMed] [Google Scholar]
- 28.Niklas KJ. 1992. Plant biomechanics: an engineering approach to plant form and function. Chicago, IL: University of Chicago Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.