Abstract
Objective
Evaluate the cost-effectiveness of incorporating tramadol or oxycodone into knee osteoarthritis (OA) treatment.
Methods
We used the Osteoarthritis Policy model (OAPol) to evaluate long-term clinical and economic outcomes of knee OA patients with mean age 60 with persistent pain despite conservative treatment. We evaluated three strategies: 1) opioid-sparing (OS); 2) tramadol (T); and 3) tramadol followed by oxycodone (T+O). We obtained estimates of pain reduction and toxicity from published literature and annual costs for tramadol ($600) and oxycodone ($2,300) from Red Book. Based on published data, in the base-case we assumed a 10% reduction in TKA effectiveness in opioid-based strategies. Outcomes included quality-adjusted life years (QALYs), lifetime cost, and incremental cost-effectiveness ratios (ICERs) and were discounted 3%/year.
Results
In the base case, T and T+O strategies delayed TKA by 7 and 9 years and led to reduction in TKA utilization by 4% and 10% respectively. Both opioid-based strategies increased cost and decreased QALYs compared to the OS strategy. Tramadol’s ICER was highly sensitive to its effect on TKA outcomes. Reduction in TKA effectiveness by 5% (compared to base case 10%) resulted in an ICER for T strategy of $110,600/QALY; with no reduction in TKA effectiveness, the ICER was $26,900/QALY. When TKA was not considered a treatment option, the ICER for T was $39,600/QALY.
Conclusion
Opioids do not appear to be cost-effective in OA patients without comorbidities, principally because of their negative impact on pain relief after TKA. The influence of opioids on TKA outcomes should be a research priority.
INTRODUCTION
Knee osteoarthritis (OA) affects over 9.3 million American adults1 and contributes more than $27 billion in healthcare expenditures annually.2 It manifests as progressive joint destruction associated with substantial pain and activity limitations. In the absence of disease modifying regimens, effective non-operative pain management is crucial for preserving functional status and quality of life (QoL) for knee OA patients.
Guideline-based knee OA treatment begins with acetaminophen and progresses to non-steroidal anti-inflammatory drugs (NSAIDs) and intra-articular corticosteroid injections. Although many patients eventually require total knee arthroplasty (TKA), they spend an average of 13 years2 exhausting pharmacologics before undergoing surgery. The addition of opioids involves trade-offs between better pain relief and higher toxicities and the need for more frequent monitoring. Further, risks of illicit use may discourage some clinicians from prescribing these agents. OA treatment guidelines provide ambiguous and conflicting suggestions concerning the role of opioids in the course of knee OA treatment. The American College of Rheumatology (ACR) suggests using opioids in patients who have failed all other pharmacologic treatments and are unwilling to undergo or are contraindicated for TKA,3 while the guidelines of Osteoarthritis Research Society International (OARSI) and the American Academy of Orthopaedic Surgeons (AAOS) are inconclusive regarding the use of opioids in knee OA patients without additional chronic conditions.
The question of appropriateness of opioids in the treatment of knee OA is complicated by the possibility of poorer TKA outcomes in patients who take opioids prior to TKA. Long-term use or high doses of opioids are associated with development of hyperalgesia and opioid-dependence, which may contribute to persistent pain following surgery.4 A growing body of literature documents greater pain and poorer functional outcomes in persons with opioid use before TKA.5
Despite concerns about their appropriateness, the prescription of opioids for knee OA patients has increased substantially over the past decade, with approximately 40% of knee OA patients in the US receiving at least one opioid prescription in 2009,6 suggesting an annual national outlay of $1.5 billion.1,6–9 Concurrent with the increased utilization of prescription opioids, illicit opioid use and overdose deaths have risen substantially. Nonmedical use of opioid analgesics costs the US over $72 billion annually in healthcare costs10 and has led to a 300% increase in overdose deaths since 1999.11
Our objective was to weigh the benefits of opioid use in this setting, including enhanced analgesia and delay or elimination of the need for costly TKA surgery and revision against the downside risks of opioids including toxicities, frequent monitoring, reduced efficacy of eventual TKA, and risk of illicit use. We address this by performing a formal cost-effectiveness analysis.
METHODS
Analytic Overview
We used the Osteoarthritis Policy (OAPol) Model, a validated computer micro-simulation2,12 to assess the cost-effectiveness of tramadol and oxycodone in persons with knee OA and no major comorbidities. Primary outcomes included quality-adjusted life years (QALYs), lifetime costs, and delay and reduction of utilization of primary and revision TKA. The cost-effectiveness of opioid-based treatment strategies was evaluated as recommended by the US Panel on Cost-Effectiveness in Health and Medicine.13 Incremental cost-effectiveness ratios (ICERs) were defined as the ratio of change in costs to change in QALYs of two strategies. Annual QoL utilities represent preference-based measures of health states and were evaluated from 0.0 to 1.0, where 0.0 represents death and 1.0 represents perfect health. We adopted a societal perspective, discounting costs and QALYs by 3% annually.13 Strategies that increased costs while decreasing QALYs relative to an alternative strategy were deemed Dominated. We assumed a willingness to pay (WTP) threshold of $100,000/QALY.14 In conformity with accepted practice, strategies with ICERs below WTP were labeled cost-effective.
The OAPol Model
The OAPol Model is a computer simulation model of the natural history and management of knee OA.2,12 Using Monte Carlo simulation, the model generates cohorts of patients based on user-defined distributions of demographic and clinical characteristics, including body mass index (BMI), knee OA structural severity, and pain.15,16 The model tracks each subject until death, allowing annual transitions among health states (defined by structural knee OA and pain severity, obesity, and comorbidities) and accumulation of OA-related and non-OA-related medical costs and QoL decrements. Details of these transitions have been previously published.16,17 Medical costs not attributable to knee OA are assigned based on a subject’s accumulated number of comorbidities and pain severity. Each treatment regimen is defined by its pain reduction, toxicity profile, and annual cost. Toxicities, classified as either major or minor, contribute additional costs and QoL decrements. Major toxicities carry a probability of death and lead to treatment discontinuation.
Treatment Strategies
We evaluated three treatment strategies in persons whose pain persisted after conservative treatment with NSAIDs, physical therapy (PT), and corticosteroid injections: 1) opioid-sparing (OS); 2) tramadol (T); and 3) tramadol followed by oxycodone (T+O) for those in whom tramadol did not lead to sustained, sufficient pain relief. Following conservative therapy, patients in the OS strategy could take acetaminophen for intermittent pain relief until they either became eligible and willing to undergo TKA or they died. Subjects on opioid-based strategies proceeded to tramadol after conservative therapy, and those on the T+O strategy could progress to oxycodone if tramadol did not provide sufficient pain relief during any year of treatment. For both opioid-based strategies, once opioids failed to relieve symptoms, patients could take acetaminophen as needed for pain relief until either they became eligible for and agreed to undergo TKA or they died. In all strategies some patients could be eligible and opt to undergo TKA immediately following the failure of the prior analgesic regimen (Figure 1).
Figure 1. Treatment Sequences for OA Management.
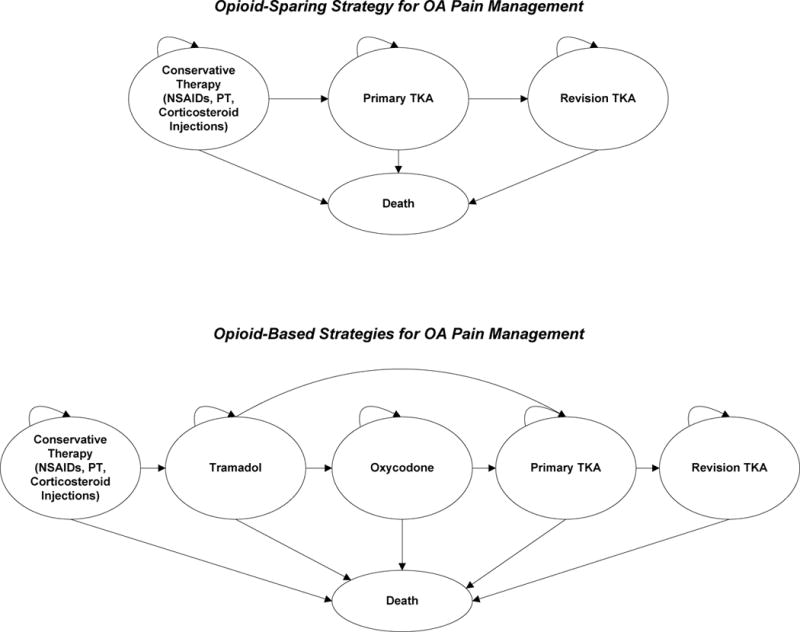
The figure depicts the treatment sequences through which subjects can progress. Each year while on a regimen, subjects are evaluated for sufficient pain relief, discontinuation, or major toxicity. If the regimen fails to provide pain relief or the subject experiences a major toxicity or discontinuation, the subject is removed from the regimen and progresses to the next regimen in the sequence. Conservative therapy includes non-steroidal anti-inflammatory drugs, physical therapy, and corticosteroid injections. Following conservative therapy, subjects on the opioid-sparing strategy may either undergo TKA immediately or enter a waiting period (not depicted), taking acetaminophen as needed for pain relief, until deemed eligible and accept surgical intervention. Subjects on opioid-based strategies progress to tramadol following conservative therapy; those on the tramadol + oxycodone sequence may advance to oxycodone if tramadol fails to provide sufficient pain relief in the first or any subsequent year of tramadol treatment. Following treatment with opioids, subjects on opioid-based strategies may progress to total knee arthroplasty or take acetaminophen as needed (not depicted) until eligible and willing to undergo surgical intervention. Death may occur at any stage throughout the treatment sequence. Abbreviations: NSAIDs, non-steroidal anti-inflammatory drugs; PT, physical therapy; TKA, total knee arthroplasty; PRN, as needed.
Subjects were removed from analgesic regimens due to major toxicity, lack of efficacy, or voluntary discontinuation. Major toxicities were associated with distinct costs, QoL decrements, and probabilities of death and were categorized as cardiovascular (CV) events (myocardial infarction, stroke, heart failure) and fractures (hip, upper or lower extremities). Lack of efficacy included failure to provide sufficient pain relief any year of treatment. We further incorporated voluntary discontinuation, defined as removal from the regimen for reasons other than major adverse events and lack of analgesic efficacy, to account for subjects’ intolerance of opioid medication. Subjects could become eligible for TKA upon removal from pharmacologic regimens. TKA eligibility criteria were stratified by OA severity, age, race, and sex. TKA recipients were further eligible for revision TKA if the primary TKA failed.
Model Inputs
Cohort Characteristics
We constructed a patient cohort to emulate the socio-demographic and clinical attributes of a population of Americans with knee OA, defined as Kellgren-Lawrence Grade 2 or 3, who had previously undergone treatment with NSAIDs, PT, and corticosteroid injections.2,17 We derived race/ethnicity, sex, and obesity distributions from the 2012 National Health Interview Survey.18 Table 1 presents cohort characteristics, non-OA medical costs, and QoL utilities.
Table 1.
Model Inputs
| Parameter | Estimate | Data Source Used in Derivations | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Cohort Characteristics | ||||||
| Demographics | Mean (SD) | Assumption US Census Bureau 201244 NHIS 201218 |
||||
| Mean Age | 60 (0) | |||||
| Percent Female | 55% | |||||
| WOMAC Pain | 60 (10) | |||||
| Male | Female | |||||
| Race/Ethnicity | Percent | Mean (SD) BMI | Percent | Mean (SD) BMI | US Census Bureau 201244 NHANES 2009–201045 |
|
|
|
||||||
| White Non-Hispanic | 83% | 30.2 (6.0) | 79% | 30.4 (7.1) | ||
| White Hispanic | 6% | 29.5 (6.3) | 7% | 30.6 (6.6) | ||
| African American | 11% | 30.2 (6.1) | 13% | 33.1 (8.0) | ||
| Nonobese/Obese | ||||||
| Quality of Life Utilities | Age Group | |||||
| WOMAC Pain | 50 – 54 | 55 – 64 | 65 – 74 | OAI Brazier et al. 200446 |
||
|
|
||||||
| 16 – 40 | 0.780/ 0.769 | 0.786/ 0.775 | 0.810/ 0.799 | |||
| 41 – 70 | 0.714/ 0.703 | 0.720/ 0.709 | 0.744/ 0.733 | |||
| 71 – 100 | 0.656/ 0.645 | 0.662/0.651 | 0.685/ 0.675 | |||
| Age Group | MCBS 200947 NHANES 2009–201045 Red Book Online®7 CPI |
|||||
| Underlying Medical Costs* | 50–54 | 55–59 | 60–64 | 65–69 | 70–74 | |
|
|
||||||
| $2,700 | $3,500 | $4,300 | $4,600 | $5,300 | ||
| Treatment Characteristics
| |||
|---|---|---|---|
| Tramadol | Oxycodone | Data Source Used in Derivations | |
| Cost* | Red Book Online®7 CPI Levinson et al. 20058 IMS 20119 Medicare Physician Fee Schedule 2012B23 Volkow 201424 Wright et al. 20146 Bhala et al. 199933 Solomon et al. 201034 Miller et al. 201129 FRAX28 Moore et al. 200520 |
||
| First Year | $600 | $2,300 | |
| Subsequent Years | $800 | $2,800 | |
| Efficacy | |||
| Average WOMAC Pain Reduction in First Year | 15 | 16 | |
| Probability of Late Failure | 24.0% | 12.8% | |
| Adverse Effects | |||
| Minor Toxicity | 78% | 78% | |
| Major Toxicity | 0.04% | 0.33% | |
| Cardiovascular | – | 0.28% | |
| Fracture | 0.04% | 0.05% | |
| Discontinuation due to minor toxicity | 22% | 22% | |
| Total Knee Replacement | Opioid-Sparing Strategy | Opioid-Based Strategies | |
| Cost* | Losina et al. 200927 Buntin et al. 200548 Teeny et al. 200349 Katz et al. 200750 Zywiel et al. 20115 |
||
| First Year | $20,500 | $20,500 | |
| Efficacy | |||
| Average WOMAC Pain Reduction in First Year | 41 | 37 | |
| Probability of Early Revision | 1.2% | 1.3% | |
Costs in 2014 US Dollars.
Abbreviations: OA, osteoarthritis; K-L, Kellgren-Lawrence grade; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; NHIS, National Health Interview Survey; NHANES, National Health and Nutrition Examination Survey; MCBS, Medicare Current Beneficiary Survey; CPI, Consumer Price Index; FRAX, Fracture Risk Assessment Tool
Treatment Characteristics
Efficacy
Treatment strategies were associated with absolute decreases in pain severity, evaluated by the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Pain Subscale;19 pain decrements for each regimen were stratified by pain severity upon entering the regimen. The absolute pain reductions achieved in the first year on opioid regimens were adjusted to account for discontinuation; we assumed that 90% of subjects would continue treatment at 3 months, and 78% at 12 months.20 Final pain reductions were derived by multiplying the average WOMAC Pain decrease for persons remaining on treatment for an entire year by the proportion of the cohort remaining on the regimen. Tramadol was associated with a mean change in WOMAC Pain in the first year of 15 points, and oxycodone had a first year pain decrease of 16 points.21 In all subsequent years of treatment, each regimen carried a probability of late pain failure, defined as the likelihood of failing to maintain pain relief. Due to the scarcity of long-term data, we assumed the late pain failure rate of tramadol was equivalent to that of NSAIDs, resulting in a 24% likelihood of pain failure in subsequent years of treatment.22 Based on clinician expert opinion, we assigned oxycodone a late pain failure rate half that of tramadol.
Costs
Annual costs of tramadol and oxycodone were derived by converting Average Wholesale Prices (AWPs) from Red Book Online to Average Sales Prices (ASPs) by discounting brand name and generic drug costs by 26% and 68% respectively.7,8 We then weighted ASPs of brand name drugs as 7% and generic drugs as 93%, reflecting the ratio of prescriptions filled with brand and generic drugs respectively.9 Similarly to pain reduction, final drug cost in the first year was adjusted to account for discontinuation throughout the year.20 First-year costs for tramadol and oxycodone were $600 and $2,300 respectively.6–9,20,23,24 Annual costs of pharmacologic regimens included the cost of the analgesic, physician visits, and, for oxycodone, diversion of prescribed medication to illicit use. We incorporated 2 annual office visits for subjects on tramadol and 6 for oxycodone. To derive a cost of diversion, we first multiplied the proportion of opioid abusers obtaining the analgesic from friends or relatives (68%)25 by the aggregate healthcare costs of abuse26 in order to obtain a healthcare burden of opioid abuse attributable to legitimate prescriptions of $19.6 billion per year. The final cost of diversion per prescription, $95, was derived by dividing the healthcare cost of abuse attributable to legitimate prescriptions by the total number of opioid prescriptions in the US (207 million24). We assumed 12 prescriptions of oxycodone annually, resulting in $1,100 per person per year in attributable illicit use. We used sensitivity analysis, as described below, to examine the effect of uncertainty in the estimate of illicit use cost.
We applied an initial cost of $20,500 for TKA, which included the cost of surgery and rehabilitation. Yearly TKA follow-up, consisting of a physician office visit and knee radiograph, contributed an annual cost of $100.27
Toxicity
Tramadol and oxycodone were associated with major CV events and fractures. Due to lack of long-term data, we assumed the rate of major toxicity in subsequent years was one-half the rate in the first year. Using FRAX® WHO Fracture Risk Assessment Tool28 and relative risks derived from Miller et al.,29 we applied a 0.04% and 0.05% likelihood of fractures in the first year of tramadol and oxycodone treatment, respectively. Fractures were associated with a mortality of 8.9%30,31 and cost of $14,600.32 Major CV events also accompanied oxycodone treatment with likelihoods of 0.28% and 0.14% in the first year and all subsequent years of treatment respectively.33,34 We applied a 24.9% probability of death due to major CV toxicity.33 Minor toxicity included nausea, vomiting, constipation, and somnolence. Tramadol and oxycodone were associated with a 78% likelihood of minor toxicity in all years of treatment35,36 and 22% probability of discontinuation during the first year of treatment.20
TKA carried risks of myocardial infarction, pulmonary embolism, pneumonia, and prosthetic joint infection. Each complication was associated with a unique cost, QoL decrement, and mortality. We further included a one year post-surgical recovery period for all subjects undergoing TKA, reflecting the decreased QoL associated with surgical recuperation. Details of these derivations have been previously published.2
Impact of chronic opioid use on TKA outcomes
Published literature suggests that chronic opioid use prior to TKA may result in higher rates of early revision and persistent pain.5 Data published by Zywiel and colleagues suggest that there is a significantly higher prevalence of revision TKAs and exploratory arthroscopies due to persistent pain in persons utilizing opioids prior to TKA compared to persons without pre-surgical opioid use.5 On the basis of these studies, we applied a 10% relative increase in the probability of early revision and a 10% relative reduction in pain relief achieved in the first year following TKA for opioid-treated subjects.
Sensitivity Analyses
We performed sensitivity analyses to assess the impact of variation in key parameters on our cost-effectiveness estimates. We simultaneously varied the age of the cohort, duration of pain relief, likelihood of minor toxicity, and the impact of opioid use on TKA outcomes. In the base case, we increased the probability of early revision and decreased the amount of pain relief achieved from TKA by 10% for opioid-treated subjects; we additionally evaluated 5% and 0% decrement in TKA outcomes compared to the opioid-sparing strategy. We further assessed each strategy without the option of TKA, to reflect the clinical course of patients averse to surgical intervention.
We conducted probabilistic sensitivity analyses37 to evaluate the effects of simultaneously varying three key parameters: discontinuation, the effect of opioids on TKA outcomes, and the cost of diversion to illicit use for the T+O strategy. The discontinuation rate was assumed to be normally distributed with mean (SD) of 22% (2%);20 opioid effect on TKA efficacy was assumed to have a uniform distribution, producing a reduction in TKA effectiveness (compared to the opioid-sparing strategy) ranging from 0% to 14%;5 the cost of diversion to illicit use for oxycodone was assumed to be uniformly distributed, ranging from $0 to the base case annual cost of $1,100.
RESULTS
Base Case Analysis
In the base case, tramadol-treated patients remained on tramadol for 2.4 years and oxycodone-treated patients remained on oxycodone for 2.9 years, on average. As long as opioids effectively control pain, individuals may not be sufficiently symptomatic to consider TKA. This period of additional pain control delays the decision to undergo TKA, thereby reducing its utilization. Compared to the OS strategy, the T and T+O strategies delayed TKA by 7 and 9 years, respectively, reducing primary TKA utilization by 4% and 10% and revision TKA use by 23% and 39%. Quality-adjusted life expectancy (QALE), cost, proportion of patients undergoing TKA, and time to TKA for each strategy are presented in Table 2. The opioid-sparing strategy led to a discounted QALE of 11.49 QALYs and lifetime cost of $130,300. Incorporating tramadol into the treatment sequence decreased QALE by 0.01 QALYs compared to OS strategy while increasing cost to $131,000. The T+O strategy was associated with a discounted QALE of 11.49 and cost of $134,900. Because the T and T+O strategies reduced QALE while increasing cost compared to the OS strategy, they were termed Dominated.
Table 2.
Cost-effectiveness of tramadol and tramadol + oxycodone under base case assumptions
| Sequence | QALE | Cost | ICER | % TKA Utilization | Time to TKA (years) |
|---|---|---|---|---|---|
| Opioid-Sparing | 11.49 | $130,300 | 66% | 6 | |
| Tramadol | 11.47 | $131,000 | Dominated | 63% | 13 |
| Tramadol + Oxycodone | 11.49 | $134,900 | Dominated | 59% | 16 |
Time to TKA is reported as the number of years between ending conservative therapies and receiving primary TKA. A strategy that leads to greater costs without clinical benefit is termed Dominated.
Abbreviations: QALE, quality-adjusted life expectancy; ICER, incremental cost-effectiveness ratio; TKA, total knee arthroplasty
Sensitivity Analyses
One-way sensitivity analyses
Impact of age and use of TKA
For all ages of opioid initiation, we evaluated each strategy without the option of progressing to surgical intervention, representing patients unable or unwilling to undergo TKA (Table 3). When TKA was not a treatment option, the opioid-sparing strategy was associated with a QALE of 11.11 QALYs and cost of $118,000. Incorporating tramadol into the treatment sequence increased QALE by 0.05 QALYs and cost by $1,800 compared to the OS strategy, resulting in an ICER of $39,600/QALY. Adding oxycodone further increased QALE to 11.20 QALYs and cost to $125,000, leading to an ICER of $116,800/QALY compared to tramadol alone. When the age of opioid initiation was increased to 70 years and TKA was not a treatment option, the ICER of tramadol decreased to $36,900/QALY and the ICER for the T+O strategy was reduced to $98,300/QALY.
Table 3.
Cost-effectiveness of tramadol and tramadol + oxycodone in strategies without surgical intervention
| Sequence | QALE | Cost | ICER |
|---|---|---|---|
| Age 50 | |||
|
| |||
| Opioid-Sparing | 13.72 | $116,500 | |
| Tramadol | 13.77 | $118,200 | $40,800 |
| Tramadol + Oxycodone | 13.81 | $123,600 | $127,900 |
|
| |||
| Age 60 | |||
|
| |||
| Opioid-Sparing | 11.11 | $118,000 | |
| Tramadol | 11.15 | $119,800 | $39,600 |
| Tramadol + Oxycodone | 11.20 | $125,000 | $116,800 |
|
| |||
| Age 70 | |||
|
| |||
| Opioid-Sparing | 8.18 | $108,300 | |
| Tramadol | 8.23 | $110,000 | $36,900 |
| Tramadol + Oxycodone | 8.28 | $114,600 | $98,300 |
ICERs reported as incremental costs in 2014 USD per QALY gained compared to the alternative treatment.
Abbreviations: QALE, quality adjusted life expectancy; ICER, incremental cost-effectiveness ratio
Multi-way sensitivity analyses
The T and T+O strategies were assessed under a range of values for duration of pain relief, toxicity, impact on TKA outcomes, and ages of treatment initiation, shown in Figure 2. The T+O strategy had ICERs above $150,000/QALY under all variations in toxicity, late pain failure, and effect on TKA outcomes.
Figure 2. Incremental Cost-Effectiveness Ratios of Tramadol with Varying Effects on TKA, Late Failure, Toxicity, and Age.
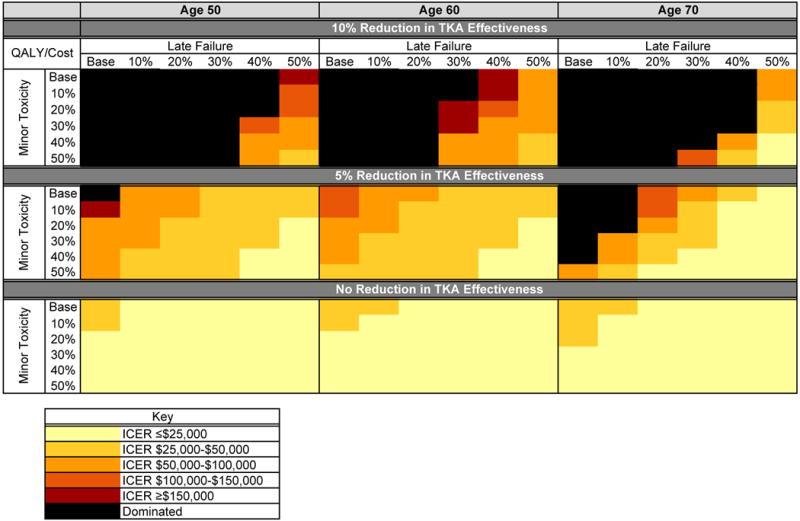
This figure illustrates the ICERs estimated for the tramadol strategy under a variety of conditions. The relative reduction in late failure and minor toxicity are taken from the base case assumptions. Decrements in total knee arthroplasty (TKA) effectiveness after opioid use were taken relative to the opioid-sparing strategy. TKA effectiveness includes pain relief after surgery and rate of early revision. Base case decrement in TKA outcomes following tramadol or tramadol followed by oxycodone was 10%. Late failure is defined as the probability of the analgesic failing to maintain pain relief in any subsequent year of treatment. Scenarios where tramadol increases cost and decreases quality-adjusted life years (QALYs) compared to an alternate strategy were termed Dominated.
Duration of benefit and minor toxicity
Increasing the duration of tramadol effectiveness by 50% resulted in a discounted QALE of 11.50 and cost of $131,100 for the T strategy, leading to an ICER of $85,300/QALY. Variations in minor toxicity had minimal effects on the cost-effectiveness of the T and T+O strategies when the probability of late pain failure was within 20% of the base case value.
Impact of chronic opioid use on TKA outcomes
Lessening the impact of opioid use on TKA outcomes from a 10% relative reduction in the base case to a 5% reduction resulted in an ICER of $110,600/QALY for the T strategy. Decreasing the probability of late pain failure or minor toxicity by only 10% while the impact of opioid use on TKA outcomes was lessened led to ICERs below $100,000/QALY for tramadol. When we assumed opioid use prior to TKA had no influence on TKA outcomes, tramadol maintained ICERs below $50,000/QALY under all scenarios evaluated. As the age of opioid initiation increased, tramadol appeared less cost-effective; however, this trend was not maintained when we assumed opioid use prior to TKA had no effect on TKA outcomes.
Probabilistic sensitivity analysis
We also evaluated the effects of simultaneously varying the rate of discontinuation of opioid regimens, the effect of opioid use on TKA outcomes, and cost of diversion for oxycodone through probabilistic sensitivity analyses. At a willingness to pay (WTP) threshold of $100,000/QALY, tramadol had a 32% likelihood of being the cost-effective treatment option when the age of opioid initiation was 60 years; that probability decreased to 9% when the age of treatment initiation was increased to 70 years. Increasing the WTP threshold to $150,000/QALY increased the likelihood of cost-effectiveness of tramadol to 36% (Table 4) for age 60. The T+O strategy was not the cost-effective option under any of the conditions assessed.
Table 4.
Results of probabilistic sensitivity analysis: probability of cost-effectiveness of treatment strategies at each WTP threshold
| Sequence | Willingness to Pay Threshold | |||
|---|---|---|---|---|
| $50,000 | $100,000 | $150,000 | ||
| Age 50 | ||||
|
| ||||
| Opioid-Sparing | 83% | 76% | 72% | |
| Tramadol | 17% | 24% | 27% | |
|
| ||||
| Age 60 | ||||
|
| ||||
| Opioid-Sparing | 81% | 68% | 63% | |
| Tramadol | 19% | 32% | 36% | |
|
| ||||
| Age 70 | ||||
|
| ||||
| Opioid-Sparing | 97% | 91% | 88% | |
| Tramadol | 3% | 9% | 12% | |
The probabilities of cost-effectiveness of the opioid-sparing and tramadol sequences are presented.37 The probability of cost-effectiveness of the tramadol + oxycodone strategy did not exceed 1%, and is, thus, not depicted. These results are based on 100 iterations, varying the discontinuation rate of opioid strategies in the first year of treatment, the effect of opioid use on TKA outcomes, and annual cost of illicit use for the oxycodone regimen. The discontinuation rate was assumed to be normally distributed with mean (SD) of 22% (2%);20 opioid effect on TKA efficacy was assumed to have a uniform distribution, producing a reduction in TKA effectiveness (compared to the opioid-sparing strategy) ranging from 0% to 14%;5 the cost of diversion to illicit use for oxycodone was assumed to be uniformly distributed, ranging from $0 to the base case annual cost of $1,100.
Abbreviations: WTP, willingness to pay
DISCUSSION
We used the OAPol Model to evaluate the cost-effectiveness of incorporating opioids into the treatment of knee OA patients without prevalent comorbidities. Under the base case assumptions, we found that neither tramadol nor tramadol followed by oxycodone is cost-effective in these patients. These results are highly dependent on the effect of opioid use on TKA outcomes; when we assumed opioid use prior to TKA had no effect on early revision rates or pain outcomes, tramadol emerged as cost-effective, with ICERs below $50,000/QALY. Decreasing the cost of illicit use and diversion for oxycodone did not affect the cost-effectiveness of the T+O strategy. We further found that incorporating tramadol, but not tramadol followed by oxycodone, into knee OA management may be a cost-effective option in patients unwilling or unable to undergo surgical interventions.
To our knowledge, this is the first cost-effectiveness evaluation of opioids in knee OA patients. A previous cost-utility analysis evaluated the incremental costs and benefits of less potent and potent opioids and duloxetine as compared to NSAIDs in knee OA treatment, and found that tramadol and oxycodone increased QALYs compared to COX-2 selective and non-selective NSAIDs; however both opioid strategies were dominated by duloxetine. These analyses were conducted from a private payer perspective, used NSAIDs as a comparator, and did not model progression of knee OA or surgical intervention.38 An additional economic analysis of tramadol in OA patients in Spain concluded that tramadol is cost-saving compared to NSAIDs with proton pump inhibitors. These results cannot be compared to those we present, as the analysis did not include opioid-induced fractures and evaluated costs and outcomes over a six month period, thereby failing to consider long-term effects.39
A key parameter in our analysis was the effect of opioid use on TKA outcomes. Diminished TKA efficacy following opioid use has been documented in small cohorts. A matched-control study evaluating the effects of opioid utilization at least six weeks prior to TKA found a substantially higher prevalence of subsequent surgical intervention in the patients requiring opioid therapy prior to TKA, with eight revisions for uncontrolled pain or stiffness and five exploratory arthroscopies of 49 patients in the opioid group compared to none in their non-opioid counterparts.5 Further, patients prescribed opioids prior to TKA experienced more pain after TKA and required significantly larger doses for post-surgical pain management.40 These conclusions, however, are based on limited sample sizes and do not address the influence of potentially confounding factors – including BMI, preoperative pain and function levels, and comorbidities – on TKA outcomes. Currently published studies do not isolate the degree to which the drug itself influences TKA outcomes as compared with the personal traits of individuals using opioids. The effects of opioids on joint replacement outcomes are of concern among clinicians. This is emphasized by the Centers for Medicare and Medicaid Innovation (CMMI) Comprehensive Care for Joint Replacement (CJR) final rule, which, per orthopedists’ suggestions, includes narcotic use among the risk variables to be documented in the patient-reported outcomes data collection.41
There are important limitations to our analyses. As trial durations for analgesics do not frequently exceed 12 weeks, our estimates of long-term efficacy and toxicity were largely informed by expert clinician opinion. Studies on analgesics’ efficacies and adverse effects do not always report previous treatment exposure of the study population; thus, estimates of pain relief and adverse effects for oxycodone we report are assumed to be independent of whether patients have received or failed tramadol or other opioid analgesics prior to oxycodone initiation. We evaluated patients without any prevalent comorbid conditions. Acetaminophen toxicity is substantially lower than that of opioids,42 particularly in a population without chronic comorbidities, thus we did not incorporate it into our analysis as it would have minimal impact on our results. Pharmacologic analgesics are generally more toxic in individuals of poorer health; thus, our results should not be generalized to the entire OA population. In a previous analysis, we examined the cost-effectiveness of using opioids in OA patients with comorbidities and found similar results, suggesting that tramadol leads to higher costs and worse quality-adjusted life expectancy.43 We acknowledge that our estimation of the cost of oxycodone diversion may exaggerate the healthcare burden of illicit opioid use attributable to the OA population; however, removing the cost of diversion had little effect on the cost-effectiveness of opioid-based strategies, thus the policy-relevant conclusions remained unchanged. Our assumption of decreased TKA effectiveness following opioid use was informed by few published studies of relatively small cohort sizes. We addressed this assumption in sensitivity analyses and found this to be an important factor in the cost-effectiveness of these agents.
Our findings have important implications for policy, research, and clinical care. The US spends over $1.5 billion1,6–9 on prescription opioids for knee OA patients without additional chronic conditions and over $28 billion on healthcare costs related to their illicit use.26 Our results suggest that opioids are not beneficial in this population if they are associated with even small (~10%) detriments in TKA outcomes. While patients without comorbidities may not be representative of the entire OA population, previous analyses similarly suggest that opioids are not cost-effective in a population with multiple comorbidities.43 However, at a willingness-to-pay threshold of $50,000/QALY, treatment of knee OA pain with tramadol (but not oxycodone) is an effective and cost-effective option in patients averse to surgical intervention. Given the risk of diversion and its associated cost for potent opioids, policy makers may consider limiting the use of potent opioids in knee OA patients. From a cost-effectiveness standpoint, both opioid-based strategies led to higher costs without providing additional benefits, unless patients were unwilling or unable to undergo TKA later. Lastly, from a research perspective, our work highlights the gap in knowledge regarding the effects of opioid use on TKA outcomes. The influence of opioid use on TKA should be considered a research priority in order to understand the role of opioids in knee OA treatment.
SIGNIFICANCE AND INNOVATION.
The finding that opioids do not appear to provide long-term clinical benefit if they diminish the effectiveness of total knee replacement suggests that, in general, clinicians should avoid prescribing opioids in patients with knee OA.
For OA patients who are averse to total knee replacement, tramadol appears to be an effective and cost-effective treatment.
This analysis shows for the first time that the long-term clinical benefit of opioids is highly dependent on their effects on TKA outcomes. This finding underscores the need for research on the influence of opioids on TKA outcomes.
Acknowledgments
Supported by: NIH/NIAMS R01AR064320, K24AR057827
Role of the Funding Source: The National Institutes Arthritis, Musculoskeletal and Skin Diseases funded the study but had no role in its design, conduct or reporting.
Footnotes
Author Contributions: Dr. Losina had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conception and design: Smith, Katz, Losina
Collection and assembly of data: Smith, Katz, Losina
Analysis and interpretation of the data: Smith, Katz, Collins, Solomon, Jordan, Suter, Yelin, Paltiel, Losina
Statistical expertise: Collins, Losina
Drafting of the article: Smith, Losina
Critical revision of the article for important intellectual content: Smith, Katz, Collins, Solomon, Jordan, Suter, Yelin, Paltiel, Losina
Final approval of the article: Smith, Katz, Collins, Solomon, Jordan, Suter, Yelin, Paltiel, Losina
Obtaining of funding: Losina
Competing interests: Dr. Katz is the President-Elect of the Osteoarthritis Research Society International. Drs. Katz and Losina are Deputy Editors for Biostatistics and Methodology for the Journal of Bone and Joint Surgery. Dr. Solomon receives salary support through a research contract to his institution from Pfizer. He also serves unpaid on the Executive Committee for the PRECISION trial, a trial testing the safety of NSAIDs.
Portions of this analysis were presented at the 2015 American College of Rheumatology Annual Meeting.
References
- 1.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Losina E, Paltiel AD, Weinstein AM, Yelin E, Hunter DJ, Chen SP, et al. Lifetime medical costs of knee osteoarthritis management in the United States: impact of extending indications for total knee arthroplasty. Arthritis Care Res (Hoboken) 2015;67:203–215. doi: 10.1002/acr.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64:465–474. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 4.Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570–587. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Zywiel MG, Stroh DA, Lee SY, Bonutti PM, Mont MA. Chronic opioid use prior to total knee arthroplasty. J Bone Joint Surg Am. 2011;93:1988–1993. doi: 10.2106/JBJS.J.01473. [DOI] [PubMed] [Google Scholar]
- 6.Wright EA, Katz JN, Abrams S, Solomon DH, Losina E. Trends in prescription of opioids from 2003–2009 in persons with knee osteoarthritis. Arthritis Care Res (Hoboken) 2014;66:1489–1495. doi: 10.1002/acr.22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Red Book Online®. Truven Health Analytics Inc.; 2013. [Google Scholar]
- 8.Levinson DR. Medicaid Drug Price Comparison: Average Sales Price to Average Wholsale Price. Office of the Inspector General: Department of Health and Human Serives. 2005 [Google Scholar]
- 9.IMS Institute for Healthcare Informatics. The Use of Medicines in the United States: Review of 2010. 2011 [Google Scholar]
- 10.Prescription Painkiller Overdoses in the US. CDC Vital Signs: Centers for Disease Control and Prevention. 2011 [Google Scholar]
- 11.Warner M, Chen LH, Makuc DM, Anderson RN, Minino AM. Drug Poisoning Deaths in the United States, 1980–2008. Hyattsville, MD: National Center for Health Statistics; 2011. (NCHS Data Brief, no 81). [PubMed] [Google Scholar]
- 12.Losina E, Daigle ME, Suter LG, Hunter DJ, Solomon DH, Walensky RP, et al. Disease-modifying drugs for knee osteoarthritis: can they be cost-effective? Osteoarthritis Cartilage. 2013;21:655–667. doi: 10.1016/j.joca.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siegel JE, Weinstein MC, Russell LB, Gold MR. Recommendations for reporting cost-effectiveness analyses. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1339–1341. doi: 10.1001/jama.276.16.1339. [DOI] [PubMed] [Google Scholar]
- 14.Ryen L, Svensson M. The Willingness to Pay for a Quality Adjusted Life Year: A Review of the Empirical Literature. Health Econ. 2014 doi: 10.1002/hec.3085. [DOI] [PubMed] [Google Scholar]
- 15.Losina E, Thornhill TS, Rome BN, Wright J, Katz JN. The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by growth in population size and the obesity epidemic. J Bone Joint Surg Am. 2012;94:201–207. doi: 10.2106/JBJS.J.01958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Losina E, Walensky RP, Reichmann WM, Holt HL, Gerlovin H, Solomon DH, et al. Impact of obesity and knee osteoarthritis on morbidity and mortality in older Americans. Ann Intern Med. 2011;154:217–226. doi: 10.1059/0003-4819-154-4-201102150-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Losina E, Weinstein AM, Reichmann WM, Burbine SA, Solomon DH, Daigle ME, et al. Lifetime risk and age at diagnosis of symptomatic knee osteoarthritis in the US. Arthritis Care Res (Hoboken) 2013;65:703–711. doi: 10.1002/acr.21898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Health Interview Survey (NHIS) Centers for Disease Control and Prevention, National Center for Health Statistics. 2012 [Google Scholar]
- 19.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 20.Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non-malignant pain: systematic review of randomised trials of oral opioids. Arthritis Res Ther. 2005;7:R1046–1051. doi: 10.1186/ar1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith SR, Deshpande BR, Collins JE, Katz JN, Losina E. Comparative pain reduction of oral non-steroidal anti-inflammatory drugs and opioids for knee osteoarthritis: systematic analytic review. Osteoarthritis Cartilage. 2016 doi: 10.1016/j.joca.2016.01.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott DL, Berry H, Capell H, Coppock J, Daymond T, Doyle DV, et al. The long-term effects of non-steroidal anti-inflammatory drugs in osteoarthritis of the knee: a randomized placebo-controlled trial. Rheumatology (Oxford) 2000;39:1095–1101. doi: 10.1093/rheumatology/39.10.1095. [DOI] [PubMed] [Google Scholar]
- 23.Medicare Fee Schedules. Centers for Medicare & Medicaid Services (CMS) 2012; 2014. [Google Scholar]
- 24.Volkow ND. In: America’s Addiction to Opioids: Herion and Prescription Drug Abuse. Control SCoIN, editor. National Institute on Drug Abuse; 2014. [Google Scholar]
- 25.Substance Abuse and Mental Health Services Administration. NSDUH Series H-48, HHS Publication No. (SMA) 14–4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. [Google Scholar]
- 26.Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, Roland CL. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med. 2011;12:657–667. doi: 10.1111/j.1526-4637.2011.01075.x. [DOI] [PubMed] [Google Scholar]
- 27.Losina E, Walensky RP, Kessler CL, Emrani PS, Reichmann WM, Wright EA, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169:1113–1121. doi: 10.1001/archinternmed.2009.136. discussion 1121–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.FRAX® WHO Fracture Risk Assessment Tool. University of Sheffield, UK: World Health Organization Collaborating Centre for Metabolic Bone Diseases; 2010. [Google Scholar]
- 29.Miller M, Sturmer T, Azrael D, Levin R, Solomon DH. Opioid analgesics and the risk of fractures in older adults with arthritis. J Am Geriatr Soc. 2011;59:430–438. doi: 10.1111/j.1532-5415.2011.03318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573–1579. doi: 10.1001/jama.2009.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li L, Setoguchi S, Cabral H, Jick S. Opioid use for noncancer pain and risk of fracture in adults: a nested case-control study using the general practice research database. Am J Epidemiol. 2013;178:559–569. doi: 10.1093/aje/kwt013. [DOI] [PubMed] [Google Scholar]
- 32.Healthcare Cost and Utilization Project (HCUP) Nationwide Inpatient Sample (INS), National Statistics on All Stays. HCUPnet: Agency for Healthcare Research and Quality; 2012. [Google Scholar]
- 33.Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382:769–779. doi: 10.1016/S0140-6736(13)60900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med. 2010;170:1968–1976. doi: 10.1001/archinternmed.2010.391. [DOI] [PubMed] [Google Scholar]
- 35.Ultram ER® (tramadol HCl) Extended-Release Tablets Prescribing Information. 2009 [Google Scholar]
- 36.Anastassopoulos KP, Chow W, Tapia CI, Baik R, Ackerman SJ, Biondi D, et al. Economic study on the impact of side effects in patients taking oxycodone controlled-release for noncancer pain. J Manag Care Pharm. 2012;18:615–626. doi: 10.18553/jmcp.2012.18.8.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006. [Google Scholar]
- 38.Wielage RC, Bansal M, Andrews JS, Klein RW, Happich M. Cost-utility analysis of duloxetine in osteoarthritis: a US private payer perspective. Appl Health Econ Health Policy. 2013;11:219–236. doi: 10.1007/s40258-013-0031-3. [DOI] [PubMed] [Google Scholar]
- 39.Vidal J, Benito P, Manresa A, Ly-Pen D, Batlle E, Blanco FJ, et al. Economic evaluation of tramadol/paracetamol in the management of pain in patients with osteoarthritis in Spain. Reumatol Clin. 2011;7:241–247. doi: 10.1016/j.reuma.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 40.Patanwala AE, Jarzyna DL, Miller MD, Erstad BL. Comparison of opioid requirements and analgesic response in opioid-tolerant versus opioid-naive patients after total knee arthroplasty. Pharmacotherapy. 2008;28:1453–1460. doi: 10.1592/phco.28.12.1453. [DOI] [PubMed] [Google Scholar]
- 41.Centers for M, Medicaid Services HHS. Medicare Program; Comprehensive Care for Joint Replacement Payment Model for Acute Care Hospitals Furnishing Lower Extremity Joint Replacement Services. Final rule Fed Regist. 2015;80:73273–73554. [PubMed] [Google Scholar]
- 42.McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22:363–388. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Katz JN, Smith SR, Collins JE, Solomon DH, Jordan JM, Hunter DJ, et al. Cost-effectiveness of nonsteroidal anti-inflammatory drugs and opioids in the treatment of knee osteoarthritis in older patients with multiple comorbidities. Osteoarthritis Cartilage. 2015 doi: 10.1016/j.joca.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Annual Estimates of the Resident Population for Selected Age Groups by Sex for the United States, States, Counties, and Puerto Rico Common Wealth and Municipios: April 1, 2010 to July 1, 2012. U.S Census Bureau Population Division; 2012. June 2012 ed. [Google Scholar]
- 45.Hyattsville MD, editor. Centers for Disease Control and Prevention (CDC) 2009–2010 National Health and Nutrition Examination Survey (NHANES) Data. National Center for Health Statistics (NCHS), U.S. Department of Health and Human Services; 2010. [Google Scholar]
- 46.Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care. 2004;42:851–859. doi: 10.1097/01.mlr.0000135827.18610.0d. [DOI] [PubMed] [Google Scholar]
- 47.Medicare Current Beneficiary Survey. Centers for Medicare & Medicaid Services; 2009. [Google Scholar]
- 48.Buntin MB, Deb P, Escarce J, Hoverman C, Paddock S, Sood N. Comparison of Medicare Spending and Outcomes for Beneficiaries with Lower Extremity Joint Replacements. Arlington, VA: RAND Health, Medicare Payment Advisory Commission; 2005. pp. 1–45. [Google Scholar]
- 49.Teeny SM, York SC, Mesko JW, Rea RE. Long-term follow-up care recommendations after total hip and knee arthroplasty: Results of the american association of hip and knee surgeons’ member survey. J Arthroplasty. 2003;18:954–962. doi: 10.1016/j.arth.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Katz JN, Mahomed NN, Baron JA, Barrett JA, Fossel AH, Creel AH, et al. Association of hospital and surgeon procedure volume with patient-centered outcomes of total knee replacement in a population-based cohort of patients age 65 years and older. Arthritis Rheum. 2007;56:568–574. doi: 10.1002/art.22333. [DOI] [PubMed] [Google Scholar]


