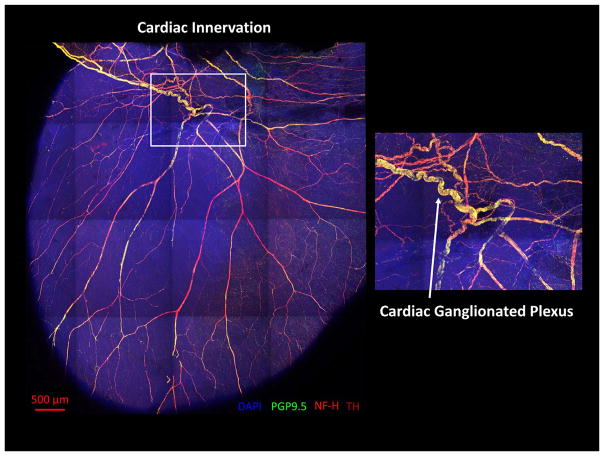
The intrinsic cardiac nervous system (ICNS) is a distributed network of ganglia and interconnecting nerves on the surface of the heart located in the epicardial fat pads.1 These ganglia contain an entire repertoire of neurons that constitute the “little brain” on the heart (Figure 1).2 They consist of efferent motor neurons, afferent sensory neurons, and local circuit neurons/interneurons, which allows for local reflex control independent of higher centers of the autonomic nervous system. These ganglia also have projections to atrial and ventricular tissue. The afferent neurons are mechanosensory, chemosensory, and multimodal in nature, transducing a variety of chemicals such as substance P, bradykinin, and calcitonin gene–related peptide. Changes in neural activity in the ICNS result in altered electrical activation patterns in both atria and ventricles.3
Figure 1.
The “little brain” on the heart. Neural innervation of the murine heart: Confocal microscopic image of a mouse heart stained with antibodies for nuclear marker 4′,6-diamidino-2-phenylindole (DAPI), pan-neuronal marker protein gene product 9.5 (PGP9.5), neurofilament heavy (NF-H), and tyrosine hydroxylase (TH). Mouse heart was cleared using the passive CLARITY technique. Image courtesy of Shivkumar Lab at UCLA and Gradinaru Lab at the California Institute of Technology.
It is clear that these ganglionated plexi (GPs) have a role to play in the initiation and maintenance of atrial fibrillation.4 During pulmonary vein isolation, with wide circumferential ablation, slowing of sinus or atrioventricular conduction occurs likely as a result of interrupting axons of passage between the ganglia. Controversially, one of the many possible treatments for atrial fibrillation includes targeting the GPs with catheter ablation.5,6 I-123 metaiodobenzylguanidine (MIBG) is a radiolabeled adrenergic blocking agent, which accumulates at sympathetic nerve endings and provides information on the distribution of sympathetic nerve fibers and function. MIBG imaging after pulmonary vein isolation for atrial fibrillation shows regional sympathetic denervation.7,8 Interestingly, there is evidence that reinnervation occurs after some time in areas of sympathetic denervation identified with early imaging.9 MIBG imaging is being used to quantitate autonomic “denervation” and potentially predicting recurrences.10
In the present study by Lemery et al,10 MIBG imaging was performed before and early (1 week) and late (3–4 months) after atrial fibrillation and GP ablation. They showed that regional innervation immediately after ablation was decreased in 3 patients and increased in 1 and showed a less prominent change in another. But with repeat imaging at a later stage (3–4 months), the denervation increased. Their report shows that regional denervation postablation persists for at least 3–4 months.
Neuromodulation is a potentially effective way at reducing afferent, efferent, or local circuit neuronal activity and may also affect remodeling in intrathoracic extracardiac ganglia.11 This can be done in a reversible or a permanent way. In this article, Lemery et al and other groups5,10,12 have shown that targeting the ICNS endocardially with catheter ablation alters cardiac autonomic activity and can influence the recurrence of atrial arrhythmias. Other methods of ICNS neuromodulation include low-level vagal nerve stimulation and botulinum toxin injection, both of which have shown some success at reducing recurrences of atrial arrhythmias.13–15
It is not yet clear which is the best way to modulate the ICNS, how many GPs should be targeted, what are the long-term consequences of these therapies, and whether they should be targeted at all. There is experimental evidence to show that disconnecting the myocyte from any neural control can have major adverse consequences in the setting of acute ischemia.16 There are also data suggesting that ablation of GPs may actually be proarrhythmic, leading to both atrial and ventricular arrhythmias.17,18 Further, the recent Atrial Fibrillation Ablation and Autonomic Modulation via Thorascopic Surgery (AFACT) study showed that GP ablation in AF patients had no effect on AF recurrence and resulted in major adverse events such as pacemaker implantation likely due to interruption of axonal fields projecting to the sinus node and conduction system.19 It is likely that more precise therapies derived from a better scientific understanding of these structures could potentially improve therapeutic outcomes in atrial fibrillation with less off-target consequences. The ability to directly record an “electrocardioneurogram” on the heart gives a new technique to study these structures from an interventional electrophysiologist’s perspective.20 Until we get more translational scientific data, it is wise to proceed with caution in ablating these structures.
Acknowledgments
Mr Rajendran was supported by National Institutes of Health (NIH) National Institute of General Medical Sciences Grant 2T32GM065823, American Heart Association Grant 15PRE22230011 and NIH National Heart, Lung and Blood Institute (NHLBI) Grant F31HL127974.
References
- 1.Armour JA, Murphy DA, Yuan BX, Macdonald S, Hopkins DA. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec. 1997;247:289–298. doi: 10.1002/(SICI)1097-0185(199702)247:2<289::AID-AR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 2.Armour JA. Potential clinical relevance of the “little brain” on the mammalian heart. Exp Physiol. 2008;93:165–176. doi: 10.1113/expphysiol.2007.041178. [DOI] [PubMed] [Google Scholar]
- 3.Cardinal R, Page P, Vermeulen M, Ardell JL, Armour JA. Spatially divergent cardiac responses to nicotinic stimulation of ganglionated plexus neurons in the canine heart. Auton Neurosci. 2009;145:55–62. doi: 10.1016/j.autneu.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Scherlag BJ, Nakagawa H, Jackman WM, Yamanashi WS, Patterson E, Po S, Lazzara R. Electrical stimulation to identify neural elements on the heart: their role in atrial fibrillation. J Interv Card Electrophysiol. 2005;13:37–42. doi: 10.1007/s10840-005-2492-2. [DOI] [PubMed] [Google Scholar]
- 5.Katritsis DG, Giazitzoglou E, Zografos T, Pokushalov E, Po SS, Camm AJ. Rapid pulmonary vein isolation combined with autonomic ganglia modification: a randomized study. Heart Rhythm. 2011;8:672–678. doi: 10.1016/j.hrthm.2010.12.047. [DOI] [PubMed] [Google Scholar]
- 6.Nakagawa H, Scherlag BJ, Patterson E, Ikeda A, Lockwood D, Jackman WM. Pathophysiologic basis of autonomic ganglionated plexus ablation in patients with atrial fibrillation. Heart Rhythm. 2009;6:S26–S34. doi: 10.1016/j.hrthm.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 7.Arimoto T, Tada H, Igarashi M, Sekiguchi Y, Sato A, Koyama T, Yamasaki H, Machino T, Kuroki K, Kuga K, Aonuma K. High washout rate of iodine-123-metaiodobenzylguanidine imaging predicts the outcome of catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2011;22:1297–1304. doi: 10.1111/j.1540-8167.2011.02123.x. [DOI] [PubMed] [Google Scholar]
- 8.Wenning C, Lange PS, Schulke C, Vrachimis A, Monnig G, Schober O, Eckardt L, Schafers M. Pulmonary vein isolation in patients with paroxysmal atrial fibrillation is associated with regional cardiac sympathetic denervation. EJNMMI Res. 2013;3:81. doi: 10.1186/2191-219X-3-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mabuchi M, Imamura M, Kubo N, Morita K, Noriyasu K, Tsukamoto T, Yasuda K, Tamaki N. Sympathetic denervation and reinnervation after the maze procedure. J Nucl Med. 2005;46:1089–1094. [PubMed] [Google Scholar]
- 10.Lemery R, Ben-Haim S, Wells G, Ruddy T. I-1-2-3-Metaiodobenzylguanidine (MIBG) imaging in patients with atrial fibrillation undergoing mapping and ablation of autonomic ganglia. Heart Rhythm. 2017;14:128–132. doi: 10.1016/j.hrthm.2016.08.038. [DOI] [PubMed] [Google Scholar]
- 11.Shivkumar K, Ajijola OA, Anand I, et al. Clinical neurocardiology defining the value of neuroscience-based cardiovascular therapeutics. J Physiol. 2016;594:3911–3954. doi: 10.1113/JP271870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katritsis DG, Pokushalov E, Romanov A, Giazitzoglou E, Siontis GC, Po SS, Camm AJ, Ioannidis JP. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. J Am Coll Cardiol. 2013;62:2318–2325. doi: 10.1016/j.jacc.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 13.Lo LW, Chang HY, Scherlag BJ, Lin YJ, Chou YH, Lin WL, Chen SA, Po SS. Temporary suppression of cardiac ganglionated plexi leads to long-term suppression of atrial fibrillation: evidence of early autonomic intervention to break the vicious cycle of “AF begets AF. J Am Heart Assoc. 2016;5(7):e003309. doi: 10.1161/JAHA.116.003309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pokushalov E, Kozlov B, Romanov A, et al. Long-term suppression of atrial fibrillation by botulinum toxin injection into epicardial fat pads in patients undergoing cardiac surgery: one-year follow-up of a randomized pilot study. Circ Arrhythm Electrophysiol. 2015;8:1334–1341. doi: 10.1161/CIRCEP.115.003199. [DOI] [PubMed] [Google Scholar]
- 15.Shen MJ, Shinohara T, Park HW, et al. Continuous low-level vagus nerve stimulation reduces stellate ganglion nerve activity and paroxysmal atrial tachyarrhythmias in ambulatory canines. Circulation. 2011;123:2204–2212. doi: 10.1161/CIRCULATIONAHA.111.018028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He B, Lu Z, He W, Wu L, Cui B, Hu X, Yu L, Huang C, Jiang H. Effects of ganglionated plexi ablation on ventricular electrophysiological properties in normal hearts and after acute myocardial ischemia. Int J Cardiol. 2013;168:86–93. doi: 10.1016/j.ijcard.2012.09.067. [DOI] [PubMed] [Google Scholar]
- 17.Lo LW, Scherlag BJ, Chang HY, Lin YJ, Chen SA, Po SS. Paradoxical long-term proarrhythmic effects after ablating the “head station” ganglionated plexi of the vagal innervation to the heart. Heart Rhythm. 2013;10:751–757. doi: 10.1016/j.hrthm.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 18.Osman F, Kundu S, Tuan J, Jeilan M, Stafford PJ, Ng GA. Ganglionic plexus ablation during pulmonary vein isolation—predisposing to ventricular arrhythmias? Indian Pacing Electrophysiol J. 2010;10:104–107. [PMC free article] [PubMed] [Google Scholar]
- 19.Driessen AH, Berger WR, Krul SP, van den Berg NW, Neefs J, Piersma FR, Chan Pin Yin DR, de Jong JS, van Boven WP, de Groot JR. Ganglion Plexus Ablation in Advanced Atrial Fibrillation: The AFACT Study. J Am Coll Cardiol. 2016;68:1155–1165. doi: 10.1016/j.jacc.2016.06.036. [DOI] [PubMed] [Google Scholar]
- 20.Rajendran PS, Nakamura K, Ajijola OA, Vaseghi M, Armour JA, Ardell JL, Shivkumar K. Myocardial infarction induces structural and functional remodelling of the intrinsic cardiac nervous system. J Physiol. 2016;594:321–341. doi: 10.1113/JP271165. [DOI] [PMC free article] [PubMed] [Google Scholar]