Abstract
Background Plant–parasitic nematode interactions occur within a vast molecular plant immunity network. Following initial contact with the host plant roots, plant-parasitic nematodes (PPNs) activate basal immune responses. Defence priming involves the release in the apoplast of toxic molecules derived from reactive species or secondary metabolism. In turn, PPNs must overcome the poisonous and stressful environment at the plant–nematode interface. The ability of PPNs to escape this first line of plant immunity is crucial and will determine its virulence.
Scope Nematodes trigger crucial regulatory cytoprotective mechanisms, including antioxidant and detoxification pathways. Knowledge of the upstream regulatory components that contribute to both of these pathways in PPNs remains elusive. In this review, we discuss how PPNs probably orchestrate cytoprotection to resist plant immune responses, postulating that it may be derived from ancient molecular mechanisms. The review focuses on two transcription factors, DAF-16 and SKN-1, which are conserved in the animal kingdom and are central regulators of cell homeostasis and immune function. Both regulate the unfolding protein response and the antioxidant and detoxification pathways. DAF-16 and SKN-1 target a broad spectrum of Caenorhabditis elegans genes coding for numerous protein families present in the secretome of PPNs. Moreover, some regulatory elements of DAF-16 and SKN-1 from C. elegans have already been identified as important genes for PPN infection.
Conclusion DAF-16 and SKN-1 genes may play a pivotal role in PPNs during parasitism. In the context of their hub status and mode of regulation, we suggest alternative strategies for control of PPNs through RNAi approaches.
Keywords: MAMP- and PAMP-triggered immunity, oxidative burst, reactive species, phytoalexins, plant parasitic nematodes, DAF-16/FoxO, SKN-1/Nrf2, cytoprotective mechanisms, insulin/IGF-1, DAF pathway, dauer
INTRODUCTION
Plants have evolved immune defence mechanisms against pathogens that employ two different detection systems. As summarized in the classic zig-zag model, defence is based on (1) perception of conserved microbial-associated molecular patterns (MAMPs) or pathogen-associated molecular patterns (PAMPs) by cell surface-localized pattern recognition receptors (PRRs) that initiates basal immunity known as MTI/PTI (MAMP- or PAMP-triggered immunity) and (2) recognition of pathogenic effectors by intracellular nucleotide-binding domain leucine-rich repeat proteins (NB-LRRs) leading to effector-triggered immunity (ETI) (Jones and Dangl, 2006; Dodds and Rathjen, 2010). Although our knowledge of immune signalling components is currently poor with regard to plant/nematode interactions, molecular mechanisms of plant defence imply a certain degree of conservation among a broad range of studied pathosystems (Goverse and Smant, 2014; Zipfel, 2014; Holbein et al., 2016). During the early stages of infection, plant recognition of plant-parasitic nematodes (PPNs) leads rapidly to the production of reactive species [reactive oxygen (ROS) and reactive nitrogen (RNS)] and toxic molecules derived from secondary metabolism (Melillo et al., 2011). This toxic environment induced by the host plant results in oxidative stress in the animal. Through comparison with the Caenorhabditis elegans model system, we postulate that PPNs orchestrate an adapted response against the stressful conditions imposed by plant immunity.
In free-living nematodes, the dauer stage refers to an arrested developmental variant that circumvents harsh environmental conditions (Hu, 2007; Perry et al., 2009; Crook, 2014). Interestingly, dauer larvae share similarities with infective juvenile formation or pre-parasitic stage parasitic nematodes (Hu, 2007; Dieterich and Sommer, 2009; Davies and Curtis, 2011; Crook, 2014; Sommer and Mayer, 2015). For instance, pre-parasitic plant nematodes show certain identical morphological and metabolic features, such as a strong cuticle and fat storage (Cassada and Russell, 1975; Robinson et al., 1987; Davies and Curtis, 2011). Another relevant similarity is the high resistance of PPNs to oxidative stress (Larsen, 1993; Dubreuil et al., 2011; Vicente et al., 2015). With regard to the evolutionary history of nematodes, Blaxter and Koutsovoulos (2014) proposed that the transition from a free-living habit to parasitism defined three origins of plant parasitism in the phylum Nematoda. Interestingly, Sommer and Mayer (2015) have postulated that dauer formation might be a pre-adaptation that drove nematodes to phoresy, necromeny and then parasitism (Dieterich and Sommer, 2009; Crook, 2014). Morphological and physiological characteristics of dauer larvae have been seen as evolutionary catalysts that give rise to the predisposition of nematodes to withstand harsh conditions. Such an environment is met by the parasitic nematode in direct contact with the arsenal of toxic molecules produced by the host immune system. Comparative genomics using C. elegans and PPNs have enabled the identification of C. elegans DAF (dauer abnormal formation) orthologues in different clades of PPNs (McCarter et al., 2003; Abad et al., 2008; Opperman et al., 2008; Ogawa et al., 2009; Dieterich and Sommer, 2009; Crook, 2014; Cotton et al., 2014; Burke et al., 2015). Moreover, the characterization of some essential DAF genes has been published for other free-living, necromenic and animal-parasitic nematodes (APNs), supporting the central role of these genes in any nematode lifestyle (Birnby et al., 2000; Ogawa et al., 2009; Bento et al., 2010; Sommer and Mayer, 2015; Albarqi et al., 2016).
In this review, we provide an overview of the molecular interplay between plants and nematodes, with particular attention given to the early stages of infection. In the first section, we describe one component of plant immunity that allows the production of reactive species and toxic metabolites in response to nematode intrusion. In the second section, we report on whether certain genes play pivotal and conserved roles in PPN stress responses during plant parasitism. Finally, the review describes the potential function of C. elegans DAF-16 and SKN-1 transcription factor genes involved in adaptative responses to environmental stresses through three essential pathways, namely the antioxidant pathway, the detoxification pathway and the unfolding protein response (UPR). Conserved regulatory components of DAF-16 and SKN-1 in PPNs and modes of action are discussed.
SECTION I: PLANT BASAL DEFENCE, THE APOPLAST AND THE REDOX BALANCE
Early perception of nematode intrusion
PPNs have evolved sophisticated strategies to overcome plant innate immunity, to mitigate host cell damage, and to promote feeding site development and reproduction (Gheysen and Mitchum, 2011). The recent review by Goverse and Smant (2014) highlighted that PPNs must address critical developmental transitions in plant parasitism, including (1) host synchronization, (2) host attraction, (3) host invasion, (4) migration inside the host, (5) initiation of a permanent feeding structure, (6) expansion of a permanent feeding structure and (7) maintenance of a transfer cell-like function. These authors note that several sequential ‘go/no-go checkpoints’ during the plant–nematode interaction underpin a complex and dynamic interplay. Interestingly, fine-tuned and coordinated response strategies occur throughout plant–pathogen interactions, and the interactions are constantly evolving within specific spatio-temporal and environmental phenomena (Thomma et al., 2011; Pritchard and Birch, 2014; Andolfo and Ercolano, 2015). To date, the later events of PPN invasion in host root tissues have been extensively studied, i.e. when the nematode becomes sedentary, and plant defence is widely suppressed (reviewed by Goverse and Smant, 2014). Nevertheless, it would be noteworthy to provide investigations of oxidative stress responses from the very moment that PPNs invade the host, to elucidate the molecular interaction between the nematode and host plant at early parasitism. More recently, Manosalva et al. (2015) showed that PPNs secrete conserved molecules, so-called ascarosides, eliciting MAMP responses at nanomolar levels in various plants. The ascarosides represent a class of pheromones exclusively identified in the phylum Nematoda (Choe et al., 2012a). Similar to other known MAMPs such as flagellin (flg22) and chitin, ascaroside perception triggers an enhanced microbial-associated molecular-patterns-triggered immunity (MTI) response against a broad spectrum of pathogens (Manosalva et al., 2015). Even if ascarosides are proposed to be MAMPs from PPNs, their cognate PRRs have not yet been identified. It was shown that some ascarosides are continuously secreted by nematodes (Noguez et al., 2012; Schroeder, 2015), suggesting that ascarosides might be diffused in the rhizosphere and can trigger plant defence responses before physical contact. In addition, PPN entry into root tissues is facilitated by mechanical force or the release of enzymes that alter cell-wall integrity (by cell-wall-degrading enzymes (CWDEs) (Bellincampi et al., 2014; Bohlmann and Sobczak, 2014). Comparing genomic and transcriptomic approaches across distant PPN species, Rai et al. (2015) provided a comprehensive view of the CWDEs produced by nematodes from initial to late infectious stages. Nematode intrusion into a host cell may also activate the production of damage-associated molecular patterns (DAMPs) (Haegeman et al., 2011; Mitchum et al., 2013) that induce several downstream signalling events in plant immunity (Boller and Felix, 2009; Heil and Land, 2014). However, DAMPs have not yet been identified in plant–nematode interactions. Although the activation of basal defence responses remains underexplored, numerous reviews have shed light on their relevance to identify key molecular players that are involved at early stages of infection (Feng and Shan, 2014; Holbein et al., 2016).
The toxic cocktail of plant immunity
Plant defence responses trigger secondary metabolism towards the synthesis of toxic molecules, i.e. phytoalexins that belong to a class of low-molecular-mass secondary metabolites (Chitwood, 2002; Ahuja et al., 2012). The secretion of phytoalexins is also correlated with the formation of root border cells when the root tips are exposed to a plant pathogen (Cannesan et al., 2011; Baetz and Martinoia, 2014). Investigation of root infection in banana (Musa spp.) by the burrowing nematode Radopholus similis, for example, revealed local induction and accumulation of phenalenone-type phytoalexins, which are derived from the phenylpropanoid pathway (Hölscher et al., 2014). Similarly, production and exudation of phytoalexins in soybean root tissues infected with the cyst nematode (CN) Heterodera glycines are also restricted to resistant cultivars (Huang and Barker, 1991). Overexpression of the Arabidopsis phytoalexin-deficient 4 gene (AtPAD4), a lipase-like protein involved in plant defence, promoted by salicylic acid (SA) and phytoalexins, enhances resistance in soybean roots in response to PPN species Meloidogyne incognita (root-knot nematode, RKN) and Heterodera glycines (CN) (Youssef et al., 2013). An increased level of phytoalexins thus helps to induce the plant defence machinery following PPN attack. Increased understanding of their toxic activity and species-specific responses will benefit the development of disease control strategies.
Although plants produce a combination of toxic molecules that comprise a large variety of secondary metabolites, they also release reactive species. During nematode invasion, the plant generates an unfavourable oxidative environment to the parasite that plays a pivotal role in the MTI and ETI defence signalling pathways, triggering ROS accumulation through host immunity (Torres et al., 2006). The host plant senses and finely adapts its cellular redox status by activating the antioxidant pathway, which also occurs in an NPR1 (non-expressor of pathogenesis-related 1)-dependent manner (Després et al., 2003; Mou et al., 2003). Regulation of the antioxidant pathway enables the expression of genes encoding ROS-scavenging enzymes (Apel and Hirt, 2004; Foyer and Noctor, 2005), preventing intracellular oxidative damage to the host while inducing oxidative stress in the pathogen inside the host apoplast (Delaunois et al., 2014). Oxidative stress is also sensed and orchestrated by NPR1, which acts as a key regulator in plant defence responses, including the SA pathway (Pieterse and Van Loon, 2004; Siddique et al., 2014). NPR1 overexpression increases MTI and ETI responses, and is associated with a decrease in the number of galls and egg masses in response to M. incognita infection (Priya et al., 2011).
During the early stage of infection, the perception of PPNs triggers an oxidative burst in root tissues. At the plant cell surface, two major oxidant enzyme families govern the oxidative burst responses, corresponding to the plasma membrane-localized nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOXs, which are also called respiratory burst oxidases, or Rbohs) and/or the cell-wall-localized (or apoplastic) peroxidases (Lambeth, 2004; Mittler et al., 2011; Siddique et al., 2014). In addition, other oxidative enzymes are also implicated in the production of reactive species, such as xanthine oxidases, oxalate oxidases and amine oxidases (Wojtaszek, 1997; Hewezi et al., 2010; Moschou and Roubelakis-Angelakis, 2014; Iberkleid et al., 2015). Together, these enzymes contribute to the generation of reactive species, with distinct roles in MTI- and ETI-signalling responses (Daudi et al., 2012; Considine et al., 2015). Moreover, both the spatio-temporal aspect and the intensity of ROS released are relevant in the immune response, with the plant immune system generally governed by biphasic ROS production. At a first level, ROS are produced at low concentration in plant cells, which can then be followed by a second level of synthesis with much higher concentrations. The latter level also leads to the local self-sacrifice of a few plant cells, known as the hypersensitive response (HR) or cell death (Levine et al., 1994; Foyer and Noctor, 2005). The HR is particularly toxic for microbial invaders, in incompatible interactions, as H2O2 reaches an estimated concentration of 5–10 mm (Levine et al., 1994; Desikan et al., 1998).
Furthermore, the intensity and spatio-temporal nature of ROS release are also strongly dependent on the host genotype, nematode pathotype (virulent or avirulent), and environmental conditions which can determine the compatibility of the interaction (Bhattacharjee, 2012; Goverse and Smant, 2014; Feng and Shan, 2014). For example, strong oxidative bursts have been identified in early responses of wheat cultivars in incompatible interactions with Heterodera avenae, which are correlated with the up-regulation of several apoplastic peroxidases (Simonetti et al., 2009; Kong et al., 2015). The penetration of tomato root tissues by virulent or avirulent RKN populations generates the production of reactive species in a local and rapid manner. Indeed, the capacity of the plant to induce the HR can also be pathotype-dependent. For example, whilst the HR has been observed in Mi-resistant plants challenged with avirulent PPN (incompatible interaction), virulent pathotypes can overcome MTI, without triggering the HR, and develop feeding sites (compatible interaction) (Waetzig et al., 1999; Melillo et al., 2006, 2011). Interestingly, Siddique et al. (2014) showed a positive role for H2O2 in Arabidopsis susceptibility to cyst nematodes, indicating that nematodes can also induce ROS to promote infection. Correlations between nematode virulence and resistance to oxidative stress have been observed in the pinewood nematode, Bursaphelenchus xylophilus (Vicente et al., 2015). Together, these findings support the idea that the establishment of a cytoprotective mechanism against environmental oxidative stresses is a crucial prerequisite for successful PPN infection at both early and later time points after contact with the plant cell.
In general, reactive species primarily trigger two biological effects. First, they may create a cytotoxic environment for PPNs. For example, the superoxide anion () is highly damaging and diffuses over a short distance, whereas hydrogen peroxide (H2O2) is less reactive but can cross cell membranes and diffuses into the cell (Winterbourn, 2008). Secondly, ROS, such as hydrogen peroxide, and RNS, such as nitric oxide (NO), also act as signalling molecules at local and systemic levels, thereby influencing many cellular processes (Mittler et al., 2011; Suzuki et al., 2011; Considine et al., 2015; Sandalio and Romero-Puertas, 2015). Interestingly, RKN infection is not sufficient to trigger ROS in Rk-resistant cowpea plants and leads to a delayed defence response without an obvious HR (Das et al., 2008). These authors speculate that PPNs may alleviate or neutralize ROS release from the host plant to avoid localized cell death, through the manipulation of plant ROS-scavenging enzymes (Das et al., 2010). In fact, it was shown that Heterodera schachtii infection activates plant NOXs in plants to stimulate a local ROS production; subsequently, the release triggers antioxidant pathways (Siddique et al., 2014). Another study reported the discovery of a secretory effector (10A06) from Heterodera glycines to support this hypothesis (Hewezi et al., 2010). The Heterodera schachtii 10A06-homologue effector also interacts with Arabidopsis spermidine synthase 2 (SPDS2), which increases the activity of polyamine oxidase (PAO) and consequently enhances the antioxidant pathway in host plants (Hewezi et al., 2010). In contrast, PAO overexpression in Nicotiana tabacum plants leads to a greater H2O2 accumulation that is associated with disease tolerance to bacteria and oomycetes (Moschou et al., 2009; Moschou and Roubelakis-Angelakis, 2014). It seems that PAO enzymatic activity plays a pivotal role in cellular redox homeostasis in response to pathogen infection. Following these findings, it was proposed that PPNs might modulate the production of reactive species, not as toxic compounds, but as signalling molecules to activate antioxidant pathways (Goverse and Smant, 2014; Holbein et al., 2016).
The role of reactive species as signalling molecules is also crucial in the animal cell, particularly in those in which an oxidative stress response is implicated (Lambeth, 2004; Nathan and Cunningham-Bussel, 2013). Given the proximity of the PPN epidermis to the cell wall, H2O2 can diffuse across the nematode cell membrane and thus influences the redox cellular homeostasis of the animal. By extension, the plant cellular redox homeostasis can influence the PPN redox homeostasis and PPN behaviour during parasitism. The plant redox homeostasis perception by PPNs could be considered as a parameter integrated to the ‘go/no-go checkpoints’ model (Goverse and Smant, 2014). In the second section, we will discuss the potential signalling pathway involved in ROS perception by PPNs.
Extensive evidence has shown that ectoparasitic and endoparasitic PPNs employ sophisticated adaptation mechanisms related to their mode of parasitism (Evans and Perry, 2009; Dieterich and Sommer, 2009; Crook, 2014). We previously stated that PPNs are confronted with a hostile environment from the first contact with the host cell wall. Indeed, the oxidative burst in plant occurs at early stages of PPN infection. Most proteome and transcriptome analyses have reported that PPNs evade reactive species in the apoplast through the release of ROS scavengers, glutathione peroxidase (GPx), peroxidase (PER), peroxiredoxin (PRXs) and catalases (CTLs) (Bellafiore et al., 2008; Dubreuil et al., 2011; Shinya et al., 2013). We will provide an overview on this point in the second section. PPNs also secrete effectors to enable the control of cellular signalling pathways and thus to stabilize long-term relations with their host plants (Bellafiore and Briggs, 2010; Kyndt et al., 2013; Mantelin et al., 2015). In addition to Hs10A06 protein, two other nematode effectors have been shown to fine-tune oxidative stress responses in the host plant. The first example implicates nematode effectors Hs4F01 and Hg4F01 identified from two CNs, Heterodera schachtii and Heterodera glycines. These 4F01 effectors are secreted proteins similar to annexins in plants (Gao et al., 2003; Patel et al., 2010). Clark et al. (2010) have proposed that Arabidopsis annexins participate in the oxidative stress response. Hs4F01 overexpression in Arabidopsis plants is beneficial to PPN parasitism, unlike the RNAi-mediated knockdown effect. Consequently, the interaction of 4F01 with an Arabidopsis oxidoreductase suggests that this effector may be implicated in the regulation of oxidative stress responses in the host plant. The second example concerns the transthyretin-like (TTL) protein that is secreted by Meloidogyne javanica, which is likely to occur during early stages of infection. The Mj-TTL5 effector interacts with a ferredoxin: thioredoxin reductase catalytic subunit (AtFTRc) from Arabidopsis. This interaction is correlated with ROS scavenging and plant susceptibility against PPNs. Because ROS, particularly H2O2, are signalling molecules in plant immunity, their suppression is associated with the attenuation of host resistance to nematode infection (Lin et al., 2016). Notably, both PPN effectors (annexin-like and transthyretin-like effectors) are derived from ancient protein families that are conserved in the plant and animal kingdoms, with their functions often associated with oxidative stress (Richardson and Cody, 2009; Clark et al., 2010; Lauritzen et al., 2015).
Whilst ROS-scavenging enzymes act as cytoprotective determinants, effectors may also play a key regulatory role in plant signalling pathways. Most investigations to date on the plant–nematode interaction have focused on how PPN effectors modulate these pathways. Although rarely discussed, understanding that PPNs may also struggle against the plant oxidative stress responses appears essential. To face this stressful environment, PPNs may imply a complex orchestration of several cellular signalling pathways in different organs within these nematodes. In the following section, we raise questions about how PPNs manage the oxidative stress response and which molecular players may be determinants of this process.
SECTION II: POTENTIAL KEY REGULATORS OF OXIDATIVE STRESS RESPONSES IN PPNs
DAF-16 and SKN-1: from the C. elegans model system to PPNs
DAF-16 and SKN-1 in the C. elegans oxidative stress response.
The generation of daf mutants in C. elegans in conjunction with epistasis analysis and genetic population studies revealed various key genes in the DAF pathway (Fielenbach and Antebi, 2008). Consequently, numerous daf orthologue genes have been identified in the phylum Nematoda. For example, the DAF-12 gene encodes a nuclear receptor acting at the crossroads of important pathways, such as entry/exit regulation of the dauer formation. These pathways have also been implicated in development, metabolism and behaviour in other phylogenetically distant free-living nematodes, APNs (Ogawa et al., 2009; Bento et al., 2010; Albarqi et al., 2016) and in the PPN B. xylophilus (Zhao et al., 2013). Other genes from the DAF pathway have been reported to be necessary for parasitism, such as DAF-21/HSP90 (Birnby et al., 2000; Gillan et al., 2009; Lourenço-Tessutti et al., 2015). This finding raises the question of whether other DAF genes are determinants at different steps during the parasitic nematode life cycle.
Hence, an intimate connection between the C. elegans dauer stage and DAF pathway and oxidative stress responses has been described, supported by a remarkable level of resistance to hydrogen peroxide in the dauer stage, which is 20-fold higher than observed in the nematode adult stage (Larsen, 1993). Similarly, nematode J2s that have not yet entered the root (pre-parasitic plant nematodes) also provide pronounced resistance to oxidative stress, correlating with their degree of virulence (Dubreuil et al., 2011; Vicente et al., 2015; Li et al., 2016). In fact, previous findings have reported the insulin/IGF-1 signalling (IIS) pathway as a mediator between development and stress responses following the model ‘should I stay or should I go’ (Schindler and Sherwood, 2014). Specifically, the IIS pathway modulates oxidative stress responses in nematodes at several levels and acts more generally in animals (McElwee et al., 2004; Fielenbach and Antebi, 2008; Kenyon, 2010; Murphy and Hu, 2013). Activation of the dauer formation relies on the removal of the DAF pathway repression that is mediated by IIS (Matyash et al., 2004) (Fig. 1). In C. elegans, null mutations in daf-2, a membrane receptor for insulin, led to a constitutive dauer formation and enhanced resistance to oxidative stress in animals (Larsen, 1993; Honda and Honda, 1999). Two key regulators of the oxidative stress response, SKN-1 and DAF-16, are negatively regulated by DAF-2 and, thus, by the IIS pathway. Remarkably, the IIS pathway is distinct from the DAF pathway. Moreover, even if both SKN-1 and DAF-16 are regulated by DAF-2 and respond to environmental stresses, only DAF-16 is implicated in dauer entry (Tullet et al., 2008; Ewald et al., 2015). The remaining question to be considered is whether SKN-1 and DAF-16 are conserved functional genes in plant nematode parasitism.
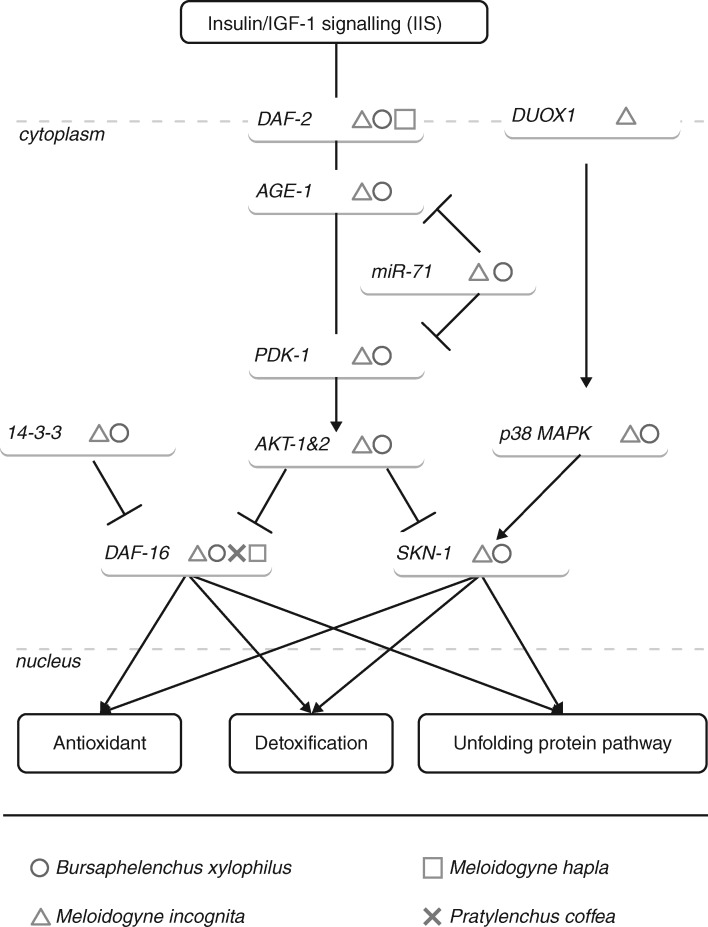
Fig. 1.
Regulatory components of DAF-16 and SKN-1 identified in plant-parasitic nematodes. DAF-2 is the insulin receptor during IIS signaling. In C. elegans, IIS signalling activates the phosphorylation cascade of PDK-1/AKT, leading to the sequestration of DAF-16 and SKN-1 in the cytoplasm. miR-71 expression inhibits the phosphorylation cascade, allowing the translocation of DAF-16 and SKN-1 to the nucleus. The genes identified for B. xylophilus, M. incognita, M. hapla or P. coffea are tagged with a dedicated symbol (see key). The most relevant regulatory components of DAF-16 and SKN-1 are highlighted here according to the identification of the orthologous genes between plant-parasitic nematodes and C. elegans.
Search for DAF-16 and SKN-1 orthologues in PPNs.
The free radical theory initially postulated that ageing is associated with an increased intracellular accumulation of ROS (Beckman and Ames, 1998; Shore and Ruvkun, 2013). Likewise, the oxidative resistance of a C. elegans mutant in the IIS was associated with lifespan extension (two- to three-fold), generating a rising interest in this pathway. DAF-16 was identified as a core component in the management of oxidative stress (Murakami and Jonhson, 2001). In relation to parasitic nematodes, combined in silico and transcriptional analyses have shown the presence of C. elegans DAF-16 orthologous genes in APNs from the genera Strongyloides (Massey et al., 2003; Hu et al., 2010; Hunt et al., 2015) and Ancylostoma (Gao et al., 2009, 2015). Whole genome sequence data for PPNs has also revealed that the DAF-16 orthologue gene from C. elegans is extended to Meloidogyne hapla (Opperman et al., 2008), Pratylenchus coffea (Burke et al., 2015), B. xylophilus (Kikuchi et al., 2011) and M. incognita (Abad et al., 2008).
DAF-16 is a forkhead class transcription factor, homologous to the human FoxO (Forkhead box O) protein family and also conserved across animal species (Obsil and Obsilova, 2011; Nakagawa et al., 2013, Webb et al., 2016). In silico protein sequence analysis based on the winged DNA-binding domain (DBD) allowed potential orthologues to be identified in PPNs (Table 1). The high degree of conservation for its DBD may be explained by its high connectivity within several signalling pathways (Shimeld et al., 2010). The decoding of SKN-1 function with regard to stress responses and ageing has also been studied (Blackwell et al., 2015). Futhermore, SKN-1 is also well conserved in animals and represents a functional orthologue of the human nuclear transactivator Nfr2 (NF-E2-related factor), a major regulator of oxidative stress responses (Kensler et al., 2007) and other signalling pathways, including developmental pathways (Hayes and Dinkova-Kostova, 2014). Conservation of the SKN-1 regulatory pathway was confirmed following the sequencing of the genome for the PPN B. xylophilus (Kikuchi et al., 2011). Notably, Choe et al. (2012b) revealed that SKN-1 is generally well conserved among PPNs and APNs. We extended the search for SKN-1 in PPNs through BLAST searches by using C. elegans SKN-1 as the query protein sequence (Table 2). Like DAF-16, SKN-1 is conserved at its DNA-binding motif, which is composed of a CNC (Cap-n-Collar) and BR (Basic Region) (Rupert et al., 1998; Choe et al., 2012b). From the available PPN genomic resources we outlined that DAF-16 and SKN-1 are conserved upon the presence of predicted DBD in PPNs.
Table 1.
Homology searches of DAF-16 in the phylum Nematoda for its respective core DNA-binding regions
| Species | Nematode clade | Current annotation | Core DNA binding region | Website/Reference |
|---|---|---|---|---|
| Caenorhabditis elegans | V | O16850 | 222AGWKNSIRHNLSLHSRF238 | Massey et al. (2003) |
| Pristionchus pacificus | V | L0CML2 | AGWKNSIRHNLSLHSRF | Ogawa et al. (2011) |
| Strongyloides stercoralis | IVa | Q6WKW2 | AGWKNSIRHNLSLHNRF | Massey et al. (2003) |
| Meloidogyne hapla | IVb | Contig353.frz3.gene4 | QGWKNSIRHNLSLHSRF | Nematode.net; Opperman et al. (2008) |
| Meloidogyne incognita | IVb | Minc17526 (UPI00060F5D60) | WGWQNSIRHNLSLHDCF | Meloidogyne genomic resources (INRA); Abad et al. (2008) |
| Globodera pallida | IVb | GPLIN_001276900 | SGWKNSVRHNLSLNKCF | Gene DB |
| Heterodera schachtii | IVb | HS00253 | QGWKNSIRHNLSLHSRF | Nematode.net |
| Bursaphelenchus xylophilus | IVb | H2DMI5 | AGWKNSIRHNLSLHSRF | Kikuchi et al. (2011) |
| **:**:******.. * |
The predicted residues involved in direct contact with DNA are highlighted in bold according to a previous analysis of the forkhead DNA-binding domain in DAF-16 (Obsil and Obsilova, 2011; Nakagawa et al., 2013). Protein BLAST searches were performed using C. elegans DAF-16 proteins as query sequences. Multiple amino acid alignments were performed with Clustal Omega (Sievers et al., 2011). The consensus symbols refer to fully conserved (*), strongly similar (:) and weakly similar (.) sequences. ‘Nematode clade’ refers to the five major phylogenetic groups within nematodes according to Blaxter (1998).
Table 2.
Homology searches of SKN-1 in the phylum Nematoda for its core DNA-binding regions
| Species | Nematode clade | Current annotation | Adjacent basic region | Core DNA binding region | Website/reference(s) |
|---|---|---|---|---|---|
| Caenorhabditis elegans | V | P34707 | 452QRKRGRQSKDEQL464 | 506RKIRRRGKNKVAARTCRQRR525 | Rupert et al. (1998), Choe et al. (2012b) |
| Pristionchus pacificus | V | H3EVC1 | PRRRGRQSKDEQL | RKIRRRGKNKVAARTCRQRR | Choe et al. (2012b) |
| Strongyloides stercoralis | IVa | SS01750 | KKKAGRVSKDNEL | RNIRRRGRNKIAAKKVRINR | Nematode.net; Choe et al. (2012b) |
| Meloidogyne hapla | IVb | Contig1686.frz3.gene3 | KGKRGRRSKDDSL | KKIRRRGRNKLAARKCRDRR | Nematode.net |
| Meloidogyne incognita | IVb | Minc09034 (MI04199) | KGKRGRRSKDDSL | KKIRRRGRNKLAARKCRDRR | Nematode.net; Choe et al. (2012b) |
| Globodera pallida | IVb | GPLIN_000599400 | KCKRGRKSKDNSL | KKIRRRGRNKFAAQKCRERR | Gene DB |
| Heterodera schachtii | IVb | HS01483 | KSKRGRKSKDNSL | KKIRRRGRNKLAAQKCRERR | Nematode.net; Choe et al., 2012b |
| Bursaphelenchus xylophilus | IVb | BUX.s01653·203 | QRKRGRQSKDEQL | KKIRRRGRNKVAARKCRERR | Nematode net; Kikuchi et al. (2011) |
| : ** ***:.* | ::*****:**.**:. * .* |
The predicted residues involved in direct contact with DNA are highlighted in bold according to a previous analysis of the Cap’n’collar (CNC) DNA-binding domain and adjacent basic region (BR) in SKN-1 (Rupert et al., 1998, Choe et al., 2012b; Blackwell et al., 2015). Protein BLAST searches were performed using C. elegans SKN-1 proteins as query sequences. Multiple amino acid alignments were performed with Clustal Omega (Sievers et al., 2011). The consensus symbols refer to fully conserved (*), strongly similar (:) and weakly similar (.) sequences. ‘Nematode clade’ refers to the five major phylogenetic groups within nematodes according to Blaxter (1998).
Targets of SKN-1 and DAF-16 during stress response in nematodes
As mentioned previously, SKN-1 and DAF-16, together with their respective orthologous genes in other animals, are the subject of numerous investigations focusing on development, ageing, lifespan extension and immunity (Shivers et al., 2008; Kenyon, 2010; Martins et al., 2016). As the gene function of DAF-16 and SKN-1 has been extensively examined, we have paid greater attention to their central role during the management of cellular stress responses and cytoprotection (Shore and Ruvkun, 2013) and their potential link to PPNs. Several studies have generated data on global gene expression of daf-16 and skn-1. For instance, Murphy et al. (2003) published transcriptome profiles of daf-2, age-1 and daf-16/daf-2 mutants in comparison with those of wild-type animals and by combining the mutants with RNAi screens targeting the DAF-2/IGF-1 and DAF-16/IGF-1 pathways. In addition, transcriptome analyses have been performed in wild-type animals that were treated with skn-1 dsRNA under normal or oxidative conditions (Park et al., 2009). Other studies based on promoter analysis, epistasis, chromatin immuno-precipitation (ChIP) assays and/or proteomic analysis have supported these findings and enriched the targets of these genes (Oliveira et al., 2009; Niu et al., 2011; Glover-Cutter et al., 2013; Tepper et al., 2013; Tullet, 2015). Moreover, their orthologous genes in mammals and invertebrates have been fully elucidated (Hayes and Dinkova-Kostova, 2014; Webb and Brunet, 2014; Martins et al., 2016; Webb et al., 2016). DAF-16 and SKN-1 are responsible for gene activation of up to 500 and 846 genes, respectively, with some overlapping targets. Several functional gene groups from these interactions are implicated in the antioxidant pathway, the detoxification pathway and the unfolding response pathway (Espinosa-Diez et al., 2015; Klotz et al., 2015; Pajares et al., 2015).
The nematode antioxidant pathway.
In C. elegans, DAF-16 and SKN-1 act in concert to up-regulate the expression of two catalases (CTL-1 and CTL-2) and two superoxide dismutases (SOD-1 and SOD-3). Consistently, SOD-3 and CTL genes are highly expressed in dauer larvae and daf-2 mutants (Vanfleteren and de Vreese, 1995; Honda and Honda, 1999). SKN-1 also up-regulates expression of two genes that encode glutathione peroxidases. DAF-16 and SKN-1 are also associated with the up-regulation of the PRDX-2 gene that encodes peroxiredoxin in C. elegans (Oláhová and Veal, 2015). Comparative genomics studies have enabled ROS-scavenging enzymes to be identified in PPNs (Abad et al., 2008; Opperman et al., 2008; Bird et al., 2009). Their role in parasitism has been experimentally validated using proteomic and molecular approaches (Shinya et al., 2013). Additionally, secretomes from M. incognita and B. xylophilus have revealed several proteins linked to the antioxidant pathway (Bellafiore et al., 2008; Rosso et al., 2009; Shinya et al., 2013; Mitchum et al., 2013). These studies have found mitochondrial and cytoplasmic SODs and CTLs in M. incognita and B. xylophilus (Bellafiore et al., 2008; Shinya et al., 2013). Moreover, the virulence of B. xylophilus is correlated with the degree of catalase expression and, by extension, its resistance to oxidative stress (Vicente et al., 2015). The PPN secretome also comprises two other relevant ROS-scavengers, GPX and PRX enzymes that are implicated in the regulation of a defensive oxidative burst from the plant. Seven PRX genes have been found in the genome of M. incognita (Abad et al., 2008). PRX expression occurs in the exophytic phase and reaches a higher expression level during RKN parasitism (Dubreuil et al., 2011). Here, PRX gene silencing affects nematode parasitism, suggesting that PRX promotes oxidative stress resistance in PPNs. Li et al. (2016) have also recently reported a cytoprotective role of PRX in B. xylophilus during the infestation of whitebark pine (Pinus bungean). These results showed that PRXs from M. incognita and B. xylophilus exhibit strong antioxidant activities in parasitic stages. The role of these antioxidant enzymes is similar to that observed in C. elegans during biotic and abiotic stress and ageing (Ewbank, 2006; Golden and Melov, 2007).
The nematode detoxification pathway.
The xenobiotic/endobiotic detoxification pathway is one line of defence mediated by SKN-1 and/or DAF-16 in nematodes (Depuydt et al., 2013; Blackwell et al., 2015). Xeno- and endobiotics refer to toxic molecules produced exogenously or endogenously. The detoxification pathway is conserved across the animal, plant and fungal kingdoms (Blokhina et al., 2003; Xu et al., 2005; Lindholm et al., 2006). In a general manner, the host plant can produce a wide diversity of secondary metabolites with potential effects on PPNs (Ohri and Pannu, 2010; Caretto et al., 2015). The toxic properties of phytoalexins that are secreted in response to nematode infections are specifically seen as xenobiotics (Chitwood, 2002). A large group of these metabolites are derived from phenolic compounds. For example, we previously mentioned that in the R. similis–Musa spp. interaction, the phytoalexin nematicide (phenalenone) is detected at high concentrations in the lesions induced by the parasite (Wuyts et al., 2007; Hölscher et al., 2014). The best detoxification mechanism characterized in nematodes is in C. elegans, with studies regarding ageing and immune responses (Gems and Doonan, 2008; Shivers et al., 2008; Engelmann and Pujol, 2010). The SKN-1-dependent detoxification pathway has previously been proposed to play an important role in PPNs to disarm the plant’s xenobiotic metabolism that belongs to its defensive arsenal (Kikuchi et al., 2011; Choe et al., 2012b). This pathway involves three detoxification enzyme systems that are essentially hosted in the endoplasmic reticulum (ER) (Xu et al., 2005; Lindholm et al., 2006; Blackwell et al., 2015). The phase 1 detoxification system involves enzymes from the cytochrome P450 (CYP) and short chain dehydrogenase (SDR) families, whereas phase 2 concerns enzymes from two other families, i.e. glutathione S-transferases (GSTs) and UDP-glucuronosyl transferases (UGTs). These enzymes orchestrate the inactivation of xeno- and endobiotics by the suppression or addition of functional groups. During phase 3, conjugated toxins are exported from cells by dedicated transporters, such as the ATP-binding cassette (ABC) transporter family. A transcriptome analysis revealed a high gene expression level of GST-1 during plant parasitism by M. incognita (Dubreuil et al., 2007; Bellafiore et al., 2008), with GST-1 being secreted from oesophageal secretory glands, suggesting a functional role in nematode feeding sites. Additionally, Shinya et al. (2013) also detected a total of 12 antioxidant proteins, including two GSTs (GST-1 and GST-3), based on a secretome analysis of B. xylophilus. Interestingly, aurovertin D, a secreted metabolite isolated from the nematophagous fungus Pochonia clamydosporia, exhibits strong nematicidal properties against C. elegans and M. incognita (Wang et al., 2015a). Epistasis analyses of daf-2 and daf-16 mutants in C. elegans have shown that these mutants are resistant and hypersensitive, respectively, to aurovertin D treatment. Probably, the daf-16 mutant is unable to detoxify this molecule. As well as exobiotics, the formation of endobiotics should also be taken into account. During host infection, cellular components of the nematode are exposed to reactive species, generating protein carbonylation, lipid peroxidation or oxidative by-products of nucleic acids. For example, reactive species produced in the plant cell wall can cause lipid peroxidation of membranes in PPNs. Peroxidized lipids are also targets for glutathione lipid hydroperoxidases and GSTs from the detoxification pathway (Gems and Doonan, 2008). Finally, one role of the detoxification pathway in nematode parasitism is to protect the animal from nematicidal compounds produced by the host immunity. For this reason, the generation of xenobiotic inhibitors has been proposed as a strategy for suppressing parasitic nematodes (Choe et al., 2012b).
The nematode unfolding protein pathway.
Other genes are up-regulated by SKN-1 and DAF-16 to support the maintenance of proteostasis, and these include heat shock proteins (HSPs) that stabilize protein folding by direct interactions (Murphy et al., 2003). For example, DAF-16 enhances HSP gene expression (HSP-16, HSP-12·6 and SIP-1) (Hsu et al., 2003). Besides the role in the antioxidant pathway, GST enzymes are capable of preventing the cysteine oxidation in proteins. In addition, thioredoxins (THXs) and protein disulfide isomerases (PDIs) also participate in protein maintenance by reducing or rearranging disulfide bond formation. SKN-1 up-regulates the expression of one gene that encodes THX and two that encode PDI (Glover-Cutter et al., 2013). Proteomic analysis also found these protein families in the secretome of PPNs (Bellafiore et al., 2008; Rosso et al., 2009; Shinya et al., 2013; Mitchum et al., 2013). Another aspect of protein maintenance concerns a link between the detoxification pathway and the ER, which is associated with the oxidative stress response. Inhibition of the detoxification pathway increases oxidative damage and thus amplifies oxidative stress. DAF-16 and SKN-1 orchestrate the redox balance between the ER and cytoplasmic redox status, allowing the adaptation of cellular homeostasis during stress (Ron and Walter, 2007; Safra et al., 2014; Cominacini et al., 2015). Moreover, the implication of SKN-1 in modulation of the unfolding protein response (UPR) has been reported. A ChIP assay on SKN-1 revealed several important genes related to the UPR signalling pathway that are positively regulated by SKN-1 under oxidative stress (Niu et al., 2011). The UPR signalling pathway prevents misfolded proteins by triggering protein maintenance and slowing global transcriptional activity. This molecular mechanism affects the traffic jam of proteins in the ER (Glover-Cutter et al., 2013). Moreover, UPR maintenance homoeostasis and DAF-16-regulated genes have been associated with the secretion of proteins that were implicated in detoxification and cytoprotection (Shore and Ruvkun, 2013; Safra et al., 2014).
SKN-1 and DAF-16 regulate the antioxidant, the UPR and the detoxification pathways during cellular events. They are important components at the crossroads of several cellular pathways, more specifically involved in oxidative stress responses. Numerous enzymes under the control of DAF-16 and SKN-1 are often detected in the secretome of PPNs in the early stages of parasitism, as cited previously. Regardless of whether C.elegans DAF-16 and SKN-1 orthologues in PPNs may be crucial in the early stages, they represent a potential defensive strategy against host immunity (Fig. 2). However, activation of the antioxidant, detoxification and UPR pathways strongly consumes NADPH and GSH and represents a huge cost in terms of energy and reducing power. Over time, this cost is critical for the cellular redox balance towards oxidative stress and, consequently, generates a poisonous environment for the nematode leading to intoxication. To defend itself, the small animal may deploy an offensive strategy by curtailing locally the production of plant defensive compounds. This requires the secretion of effectors capable of controlling plant immune responses. We mentioned previously that genes encoding TTL proteins have been identified as effector-modulating host immunity factors in APNs and PPNs (Goverse and Smant, 2014; Mantelin et al., 2015). Curiously, numerous transthyretin-like proteins in C. elegans are significantly up-regulated in DAF-16- and IIS-dependent manners (Depuydt et al., 2013) and are thus associated with innate immunity against microbial nematicides (Treitz et al., 2015). The TTL gene family may be an example of potential nematode effectors that are secreted in response to the oxidative stress response induced by its host plant.
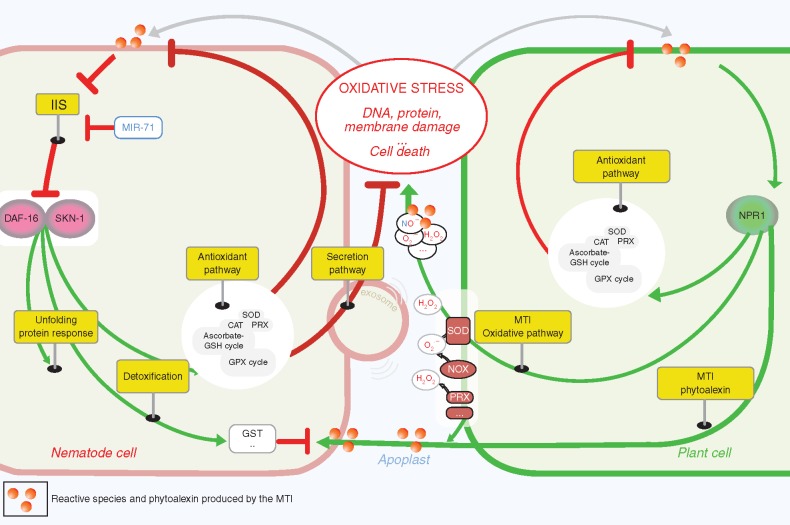
Fig. 2.
How do plant-parasitic nematodes alleviate the stress of an apoplast ‘on fire’? A model is proposed to explain the DAF-16 and SKN-1 functions that were orchestrated within different cellular pathways to resist the release of toxic compounds (reactive species and phytoalexins) by the plant cell early in infection. In this model, the nematodes sense the physiological state of the plant cell by detecting reactive species, and they generate an adapted response. The findings illustrated in this figure are based primarily on multi-omic resources from plant-parasitic nematodes and functional genomics data from the nematode model system C. elegans. The plant defence response activates the oxidative pathway, leading to the release of reactive species in the apoplast. At the same time, the plant activates its antioxidant pathway to protect the plant cell from oxidative damage. Secondary metabolism is modulated to produce phytoalexins, which represent xenobiotics to the nematode cell. Additionally, the perception of reactive species leads the nematode cell to activate its oxidative stress response. In C. elegans, this pathway is orchestrated by DAF-16 and SKN-1, two transcription factors that are conserved in the animal kingdom. DAF-16 and SKN-1 are negatively regulated by the insulin/IGF-1 signalling (IIS) pathway and positively regulated by miR-71. As in the plant cell, the activation of the antioxidant pathways has a cytoprotective function. In parallel, ROS-scavenging enzymes can be secreted in the apoplast to mitigate the plant’s oxidative burst. The unfolding protein response (UPR) adapts cellular homeostasis and protects proteins directly from oxidative damage induced by oxidative stress. The detoxification pathway covers the phytoalexins produced by the plant cell to suppress their toxicity. GSH, glutathione; CAT, catalase; PER, peroxidase; PRX, peroxiredoxin; SOD, superoxide dismutase; GPX, glutathione peroxidase; SKN-1, skinhead transcription factor-1; DAF-16, dauer formation 16; DAF-12, dauer formation-12; NPR1, non-pathogenic related protein-1; NOX, NADPH oxidase; MTI, MAMP-triggered immunity; MIR-71, micro RNA-71; IIS, insulin IGF1 signalling.
Regulatory elements of DAF-16 and SKN-1 in nematodes
DAF-16 and SKN-1 contribute to the orchestration of several adaptive cellular pathways in response to various environmental stimuli (Schindler and Sherwood, 2014; Blackwell et al., 2015). In C. elegans, the effects of aspirin and SA on stress responses have been assessed (Ayyadevara et al., 2013; Wan et al., 2013). In a survival assay, aspirin (0·5–1 mm) and SA (1 mm) were administered through the nematode medium, leading to an increase of 1·5- to 3·5-fold in the expression of genes encoding SOD, CTL and GST proteins (Ayyadevara et al., 2013). In contrast, daf-16 mutant fails to up-regulate SOD and CTL genes under SA application, implicating DAF-16 in the SA-mediated activation of these genes. The nematode lifespan then increases by 12–30 % when small animals are treated with aspirin or SA upon exposure to 5 mm H2O2. This finding correlates activation of the antioxidant pathway with the nematode’s capacity to resist harsh oxidative stress in a DAF-16-dependent manner. Moreover, protein aggregation decreases under oxidative stress upon SA treatment underlying the role of this signalling molecule within the UPR pathway in C. elegans. In fact, SA, Me-SA and its derivatives (called salicylates) are ancient signalling molecules that are found across taxa to have physiological impacts in the animal kingdom (Klessig et al., 2016). In the pine–B. xylophilus interaction, SA elevation is significantly detected at early stages of infection (Li et al., 2016). Moreover, ascaroside pheromones from diverse PPNs were found to activate MTI and especially genes from the SA pathway in host plants (Manosalva et al., 2015). Interestingly, a screen of plant exudates revealed that SA is an attractant for M. incognita, suggesting an impact of the signalling molecule on nematode physiology and behaviour (Wuyts et al., 2006). It is tempting to speculate that DAF-16 or SKN-1 pathways in PPNs could be triggered by SA perception. In such cases, sensing SA to activate an oxidative stress response might represent an advantage for PPNs to resist the plant immune system in the early stages of infection.
Downstream of the stimulus perception, oxidative stress response is modulated by evolutionary conserved signalling pathways in the animal kingdom (Shivers et al., 2008; van der Hoeven et al., 2011; Shore and Ruvkun, 2013; Blackwell et al., 2015; Klotz et al., 2015; Penkov et al., 2015). In a general manner, the regulation of DAF-16 and SKN-1 involves an intricate network of post-translational modifications and protein–protein interactions. Interestingly, some important regulatory elements cited below have been identified in PPNs and a few have been validated for their implication in plant parasitism by an RNAi approach (Fig. 1). Moreover, C. elegans mutants affected in these genes have shown a contrasting response to oxidative stress. As discussed previously, the IIS pathway plays a pivotal role in stress responses. Insulin perception by the DAF-2 receptor activates the phosphoinositide-dependent kinase-1/serine-threonine kinase (PDK-1/AKT) phosphorylation cascade that prevents nuclear trans-localization of DAF-16 and SKN-1 (Lin et al., 2001; Henderson and Johnson, 2001). Reciprocally, SKN-1 negatively regulates IIS through a positive feedback loop (Oliveira et al., 2009). Futhermore, the Akt phosphorylation sites in DAF-16 mediate binding of the 14-3-3 scaffolding proteins and contribute to its sequestration within the cytoplasm (Li et al., 2007). At a post-transcriptional level, miR-71 silences the gene expression of AGE-1 and AKT, positioning this miRNA as an inhibitor of the IIS pathway (de Lencastre et al., 2010; Boulias and Horvitz, 2012). In addition, the p38 mitogen-activated protein kinase (MAPK) pathway also plays an important function in orchestration of the oxidative stress response. Exogenous ROS exposure or an endogenous production by dual oxidase 1 (DUOX1) is associated with SKN-1 translocation in the nucleus via the p38 MAPK pathway (Inoue et al., 2005; van der Hoeven et al., 2011). To our knowledge, some of these genes from the IIS and p38 MAPK pathways have been explored for their physiological and virulence roles in PPNs. Genome sequencing of M. incognita and B. xylophilus revealed C. elegans genes orthologous to 14-3-3, PDK-1, AGE-1 and AKT-1&2 (Abad et al., 2008; Shinya et al., 2010; Kikuchi et al., 2011; Liu et al., 2011). Silencing of DUOX1 and MPK-1 genes in M. incognita compromised nematode parasitism in tomato plants (Charlton et al., 2010; Dong et al., 2016). Interestingly, deep sequencing analysis from M. incognita has identified miR-71 as one of the most frequently produced miRNAs at the pre-infective J2 stage and in eggs (Wang et al., 2015b; Zhang et al., 2016). Beyond the central role of the IIS and p38 MAPK pathways for the oxidative stress response orchestration, other post-translational modifications and protein interactions of DAF-16 and SKN-1 might potentially be of interest in the understanding of plant–nematode parasitism (Yen et al., 2011; Riedel et al., 2013; Benayoun et al., 2015; Blackwell et al., 2015).
Furthermore, the gene expression profile of DAF-16 and SKN-1 in C. elegans has been essentially reported in the intestinal cells but also in hypodermis and neural cells (Libina et al., 2003; Chávez et al., 2007; Murphy et al., 2007; Paek et al., 2012). Nevertheless, their expression can vary according to the physiological context. For example, nematode infection by a fungal pathogen is associated with the expression and activation of DAF-16 in the epidermal tissues in contact with the pathogen (Zou et al., 2013). Similarly, DAF-16 and SKN-1 activation can drastically increase in intestinal cells during exposure to xenobiotics or pathogens (Chávez et al., 2007; van der Hoeven et al., 2011; Papp et al., 2012). More in depth, DAF-16 is subjected to a positive feedback regulation in intestinal cells following the model known as ‘FOXO-to-FOXO’ (Murphy et al., 2007). In other words, a systemic signal amplifying DAF-16 gene expression and its activation is propagated within intestinal cells and throughout other tissues in the animal. A similar mechanism has also been mentioned for SKN-1 (Staab et al., 2013). The cell-to-cell communication comprises miR-71 expression in the neuronal system and is linked to DAF-16 activation in the intestinal cells (Boulias and Horvitz, 2012). The DAF-16 activity in intestinal cells influences the systemic signalling by coordinating downstream gene expression in a tissue-specific way, such as SOD-3 expression in the epidermis and muscles. (Libina et al., 2003). In PPNs, epidermis and intestine are two organs exposed to plant xenobiotics and ROS released in the extracellular matrix. PRX immunolocalization in M. incognita has been observed in the hypodermis and the pseudocoelom surrounding the stylet and amphidial pouches, which suggests a protective role when locally in contact with the feeding cells (Dubreuil et al., 2011). By in situ hybridization, Vicente et al. (2015) have shown that CTL-1 and CTL-2 genes are expressed in the intestinal cells of B. xylophilus. The orchestration of antioxidant responses probably involves a complex signalling network between various organs within the nematode. Following Murphy’s model (Murphy et al., 2007) in C. elegans, the nematode intestine is an organ with a fundamental role in the DAF-16 and SKN-1 pathways. The systemic signalling that is propagated from the intestinal cells seems to resonate with other organs, orchestrating a coherent oxidative stress response.
CONCLUDING REMARKS
Given the considerable economic impact of PPN pathogens on global crop yield (Chitwood, 2003), the development of management strategies for disease control requires particular attention. Recent research has focused on either plant breeding for engineering durable disease resistance to PPNs or on identification of effectors and their role in successful parasitism. With advances in whole genome sequencing in both the model species C. elegans and parasitic nematode species, significant progress has been made in identifying molecular players that modulate important biological processes. The present review investigates events prior to PPN establishment of successful infection and how these nematodes are able to manage the stressful environment imposed by the host plant throughout the parasitic life cycle. We portray the putative role of PPN orthologues of C.elegans DAF-16 and SKN-1 in sensing and tackling the first line of host defence. DAF-16 and SKN-1 are ancient transcription factors conserved across the animal kingdom, which play essential roles in nematode development and environmental adaptation. We propose that they might also play an important role in PPNs in counteracting oxidative stress conditions produced by the host plant throughout invasion and migration.
Hub proteins have been defined as conserved proteins that are highly connected within cellular pathways and act as strong regulators of organism development and environmental adaptation (Dietz et al., 2010; Grants et al., 2015). DAF-16 and SKN-1 share these features and can be considered as hub proteins (Schindler and Sherwood, 2014; Webb and Brunet, 2014). Such proteins are often identified as cellular targets by pathogens (Mukhtar et al., 2011; Schleker and Trilling, 2013). In C. elegans, as in plants, the immune system also involves a first line of protection against pathogens, resulting from the activation of an oxidative burst (Engelmann and Pujol, 2010; Papp et al., 2012) and simultaneous activation of cellular preservation mechanisms regulated by DAF-16 and SKN-1 (Shore and Ruvkun, 2013; Espinosa-Diez et al., 2015; Klotz et al., 2015). Finally, in plant–nematode interactions, the host triggers an oxidative burst and the PPN has to fight against it. If SKN-1 and DAF-16 orchestrate oxidative stress responses in C. elegans, probably PPN orthologues could do the same following a partially similar mechanism.
More generally, it was shown that DAF-16 and the IIS pathway are at the crossroads of redox and metabolism in C. elegans (Penkov et al., 2015). This finding supports the hypothesis that there are developmental checkpoints in C. elegans in accordance with the model ‘should I stay/should I go’ (Murphy and Hu, 2013; Schindler and Sherwood, 2014). A hypothesis would be that the IIS pathway comprising C. elegans DAF-16 orthologues in PPNs takes part in the ‘go/no-go checkpoints’ model during nematode parasitism and its exophytic stage (Goverse and Smant, 2014; Schindler and Sherwood, 2014). Therefore, it would be interesting to establish comparisons between the ‘go/no-go checkpoint’ model from plant–nematode interactions and the ‘should I stay or should I go’ model derived from the IIS established in C. elegans.
Many current nematode control measures are based on nematicides. These agrochemicals can have toxic effects on human health and also be limited to only reduced nematicidal activity at increased soil depths, where nematode populations can occur. A common alternative biotechnological approach for nematode control, which is based on engineering plant resistance, relies on the induction of RNAi silencing to target essential nematode genes for nematode development, reproduction, metabolism or stress response (Rosso et al., 2009; Li et al., 2011; Dutta et al., 2015; Rehman et al., 2016). The high connectivity and important functions of DAF-16 and SKN-1 in the nematode response against oxidative stress and other cellular pathways make them attractive upstream transcription factor targets for RNAi, and represent an alternative to RNAi pyramiding strategies, as several essential genes may be silenced simultaneously. Down-regulation of DAF-16 and SKN-1 probably affect not only the expression of genes encoding ROS scavengers and detoxification enzymes, but also PPN cell functions during their exophytic stage, particularly during abiotic and biotic stress.
In conclusion, genomics data for the C. elegans model system, together with those for PPNs, are enabling advances in understanding adaptation mechanisms involved in nematode parasitism, in addition to how PPN effectors modulate plant cellular pathways. Unravelling of the molecular mechanisms involved in PPNs for managing plant defence responses is furthering our general understanding of the pathosystem. From a biotechnological point of view, this may offer the possibility for identification of new targets in the development of durable resistance to PPNs in plants.
ACKNOWLEDGMENTS
We are grateful to EMBRAPA, CAPES, CNPQ and FAPDF for financial support for scientific research. We also thank Susan Casement and Gilbert Engler for helping in editing the written English.
LITERATURE CITED
- Abad P, Gouzy J, Aury JM, et al. 2008. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nature Biotechnology 26: 909–915. [DOI] [PubMed] [Google Scholar]
- Ahuja I, Kissen R, Bones AM.. 2012. Phytoalexins in defense against pathogens. Trends in Plant Science 17: 73–90. [DOI] [PubMed] [Google Scholar]
- Albarqi MMY, Stoltzfus JD, Pilgrim AA, et al. 2016. Regulation of life cycle checkpoints and developmental activation of infective larvae in Strongyloides stercoralis by dafachronic acid. PloS Pathogens 12: e1005358. doi.org/10.1371/journal.ppat.1005358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andolfo G, Ercolano MR.. 2015. Plant innate immunity multicomponent model. Frontiers in Plant Science 6: 987. doi:10.3389/fpls.2015.00987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel K, Hirt H.. 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology 55: 373–399. [DOI] [PubMed] [Google Scholar]
- Ayyadevara S, Bharill P, Dandapat A, et al. 2013. Aspirin inhibits oxidant stress, reduces age-associated functional declines, and extends lifespan of Caenorhabditis elegans. Antioxidants & Redox Signaling 18: 481–90. [DOI] [PubMed] [Google Scholar]
- Baetz U, Martinoia E.. 2014. Root exudates: the hidden part of plant defense. Trends in Plant Science 19: 90–98. [DOI] [PubMed] [Google Scholar]
- Beckman KB, Ames BN.. 1998. The free radical theory of aging matures. Physiological Reviews 78: 547–581. [DOI] [PubMed] [Google Scholar]
- Bellafiore S, Briggs SP.. 2010. Nematode effectors and plant responses to infection. Current Opinion in Plant Biology 13: 442–448. [DOI] [PubMed] [Google Scholar]
- Bellafiore S, Shen Z, Rosso MN, Abad P, Shih P, Briggs SP.. 2008. Direct identification of the Meloidogyne incognita secretome reveals proteins with host cell reprogramming potential. PloS Pathogens 4: e1000192. doi:10.1371/journal.ppat.1000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellincampi D, Cervone F, Lionetti V.. 2014. Plant cell wall dynamics and wall-related susceptibility in plant-pathogen interactions. Frontiers in Plant Science 5: 228. doi:10.3389/fpls.2014.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benayoun BA, Pollina EA, Brunet A.. 2015. Epigenetic regulation of ageing: linking environmental inputs to genomic stability. Nature Reviews. Molecular Cell Biology 16: 593–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bento G, Ogawa A, Sommer RJ.. 2010. Co-option of the hormone-signalling module dafachronic acid-DAF-12 in nematode evolution. Nature 466: 494–497. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee S. 2012. The language of reactive oxygen species signaling in plants. Journal of Botany 332: 1–22. [Google Scholar]
- Bird DM, Williamson VM, Abad P, et al. 2009. The genomes of root-knot nematodes. Annual Review of Phytopathology 47: 333–351. [DOI] [PubMed] [Google Scholar]
- Birnby D, Link EM, Vowels JJ, Tian H, Colacurcio P, Thomas H.. 2000. A transmembrane guanylyl cyclase (DAF-11) and Hsp90 (DAF-21) regulate a common set of chemosensory behaviors in C. elegans. Genetics 155: 85–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell TK, Steinbaugh MJ, Hourihan JM, Ewald CY, Isik M.. 2015. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radical Biology and Medicine 88: 290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaxter M. 1998. Caenorhabditis elegans is a nematode. Science 282: 2041–2046. [DOI] [PubMed] [Google Scholar]
- Blaxter M, Koutsovoulos G.. 2014. The evolution of parasitism in Nematoda. Parasitology 142: 826–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokhina O, Virolainen E, Fagerstedt K V.. 2003. Antioxidants, oxidative damage and oxygen deprivation stress. Annals of Botany 91: 179–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlmann H, Sobczak M.. 2014. The plant cell wall in the feeding sites of cyst nematodes. Frontiers in Plant Science 5: 89. doi:10.3389/fpls.2014.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T, Felix G.. 2009. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annual Review of Plant Biology 60: 379–406. [DOI] [PubMed] [Google Scholar]
- Boulias K, Horvitz HR.. 2012. The C. elegans MicroRNA mir-71 acts in neurons to promote germline-mediated longevity through regulation of DAF-16/FOXO. Cell Metabolism 15: 439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke M, Scholl EH, Bird DMK, et al. 2015. The plant parasite Pratylenchus coffeae carries a minimal nematode genome. Nematology 17: 621–637. [Google Scholar]
- Cannesan MA, Gangneux C, Lanoue A, et al. 2011. Association between border cell responses and localized root infection by pathogenic Aphanomyces euteiches. Annals of Botany 108: 459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caretto S, Linsalata V, Colella G, Mita G, Lattanzio V.. 2015. Carbon fluxes between primary metabolism and phenolic pathway in plant tissues under stress. International Journal of Molecular Sciences 16: 26378–26394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassada RC, Russell RL.. 1975. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Developmental Biology 46: 326–342. [DOI] [PubMed] [Google Scholar]
- Chávez V, Mohri-Shiomi A, Maadani A, Vega LA, Garsin DA.. 2007. Oxidative stress enzymes are required for DAF-16-mediated immunity due to generation of reactive oxygen species by Caenorhabditis elegans. Genetics 176: 1567–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton WL, Harel HYM, Bakhetia M, Hibbard JK, Atkinson HJ, McPherson MJ.. 2010. Additive effects of plant expressed double-stranded RNAs on root-knot nematode development. International Journal for Parasitology 40: 855–864. [DOI] [PubMed] [Google Scholar]
- Chitwood DJ. 2002. Phytochemical based strategies for nematode control. Annual Review of Phytopathology 40: 221–249. [DOI] [PubMed] [Google Scholar]
- Chitwood DJ. 2003. Research on plant-parasitic nematode biology conducted by the United States Department of Agriculture-Agricultural Research Service. Pest Management Science 59: 748–753. [DOI] [PubMed] [Google Scholar]
- Choe A, Von Reuss SH, Kogan D, et al. 2012a. Ascaroside signaling is widely conserved among nematodes. Current Biology 22: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe KP, Leung CK, Miyamoto MM.. 2012b. Unique structure and regulation of the nematode detoxification gene regulator, SKN-1: implications to understanding and controlling drug resistance. Drug Metabolism Reviews 44: 209–223. doi:10.3109/03602532.2012.684799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark G, Konopka-Postupolska D, Hennig J, Roux S.. 2010. Is annexin 1 a multifunctional protein during stress responses? Plant Signaling & Behavior 5: 303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cominacini L, Mozzini C, Garbin U, et al. 2015. Endoplasmic reticulum stress and Nrf2 signaling in cardiovascular diseases. Free Radical Biology & Medicine 88: 233–242. [DOI] [PubMed] [Google Scholar]
- Considine MJ, Sandalio M L, Helen Foyer C.. 2015. Unravelling how plants benefit from ROS and NO reactions, while resisting oxidative stress. Annals of Botany 116: 469–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton JA, Lilley CJ, Jones LM, et al.2014. The genome and life-stage specific transcriptomes of Globodera pallida elucidate key aspects of plant parasitism by a cyst nematode. Genome Biology 15: R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook M. 2014. The dauer hypothesis and the evolution of parasitism: 20 years on and still going strong. International Journal of Parasitology 44: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, DeMason DA, Ehlers JD, Close TJ, Roberts PA.. 2008. Histological characterization of root-knot nematode resistance in cowpea and its relation to reactive oxygen species modulation. Journal of Experimental Botany 59: 1305–1313. [DOI] [PubMed] [Google Scholar]
- Das S, Ehlers JD, Close TJ, Roberts PA.. 2010. Transcriptional profiling of root-knot nematode induced feeding sites in cowpea (Vigna unguiculata L. Walp.) using a soybean genome array. BMC Genomics 11: 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daudi A, Cheng Z, O’Brien JA, et al. 2012. The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. The Plant Cell 24: 275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KG, Curtis RHC.. 2011. Cuticle surface coat of plant-parasitic nematodes. Annual Review of Phytopathology 49: 135–156. [DOI] [PubMed] [Google Scholar]
- Delaunois B, Jeandet P, Clément C, Baillieul F, Dorey S, Cordelier S.. 2014. Uncovering plant-pathogen crosstalk through apoplastic proteomic studies. Frontiers in Plant Science 5: 1–18. doi:10.3389/fpls.2014.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depuydt G, Xie F, Petyuk VA, et al. 2013. Reduced insulin/insulin-like growth factor-1 signaling and dietary restriction inhibit translation but preserve muscle mass in Caenorhabditis elegans. Molecular & Cellular Proteomics 12: 3624–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R, Reynolds A, Hancock JT, Neill SJ.. 1998. Harpin and hydrogen peroxide both initiate programmed cell death but have differential effects on defence gene expression in Arabidopsis suspension cultures. Biochemical Journal. 330: 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Després C, Chubak C, Rochon A, et al. 2003. The arabidopsis NPR1 disease resistance protein is a novel cofactor that confers redox regulation of DNA binding activity to the basic domain/leucine zipper transcription factor TGA1. The Plant Cell 15: 2181–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich C, Sommer RJ.. 2009. How to become a parasite – lessons from the genomes of nematodes. Trends in Genetics 25: 203–209. [DOI] [PubMed] [Google Scholar]
- Dietz KJ, Jacquot JP, Harris G.. 2010. Hubs and bottlenecks in plant molecular signalling networks. New Phytologist 188: 919–938. [DOI] [PubMed] [Google Scholar]
- Dodds PN, Rathjen JP.. 2010. Plant immunity: towards an integrated view of plant-pathogen interactions. Nature Reviews. Genetics 11: 539–548. [DOI] [PubMed] [Google Scholar]
- Dong L, Xu J, Chen S, Li X, Zuo Y.. 2016. Mi-flp-18 and Mi-mpk-1 genes are potential targets for Meloidogyne incognita control. Journal of Parasitology 102: 208–213. [DOI] [PubMed] [Google Scholar]
- Dubreuil G, Magliano M, Abad P, Rosso MN.. 2007. Transcriptome analysis of root-knot nematode functions induced in the early stages of parasitism. New Phytologist 176: 426–436. [DOI] [PubMed] [Google Scholar]
- Dubreuil G, Deleury E, Magliano M, Jaouannet M, Abad P, Rosso MN.. 2011. Peroxiredoxins from the plant parasitic root-knot nematode, Meloidogyne incognita, are required for successful development within the host. International Journal for Parasitology 41: 385–396. [DOI] [PubMed] [Google Scholar]
- Dutta TK, Banakar P, Rao U.. 2015. The status of RNAi-based transgenic research in plant nematology. Frontiers in Microbiology 5: 760. doi:10.3389/fmicb.2014.00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann I, Pujol N.. 2010. Innate immunity in C. elegans. Advances in Experimental Medecine and Biology 708: 105–121. [DOI] [PubMed] [Google Scholar]
- Espinosa-Diez C, Miguel V, Mennerich D, et al. 2015. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biology 6: 183–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AAF, Perry RN.. 2009. Survival mechanisms In: Perry RN, Moens M, Starr JL, eds. Root-knot nematodes. Wallingford, UK: CABI Publishing, 201–222. [Google Scholar]
- Ewald CY, Landis JN, Abate JP, Murphy CT, Blackwell TK.. 2015. Dauer-independent insulin/IGF-1-signalling implicates collagen remodelling in longevity. Nature 519: 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewbank JJ. 2006 Signaling in the immune response. In: Monica D, Patterson G, eds. The C. elegans Research Community, WormBook. doi/10.1895/wormbook.1.83.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Shan L.. 2014. ROS open roads to roundworm infection. Science Signaling 7(320): pe10. doi:10.1126/scisignal.2005273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielenbach N, Antebi A.. 2008. C. elegans dauer formation and the molecular basis of plasticity. Genes and Development 22: 2149–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Noctor G.. 2005. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. The Plant Cell 17: 1866–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Allen R, Maier T, Davis EL, Baum TJ, Hussey RS.. 2003. The parasitome of the phytonematode Heterodera glycines. Molecular Plant-Microbe Interactions 16: 720–726. [DOI] [PubMed] [Google Scholar]
- Gao X, Frank D, Hawdon JM.. 2009. Molecular cloning and DNA binding characterization of DAF-16 orthologs from Ancylostoma hookworms. International Journal for Parasitology 39: 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Goggin K, Dowling C, Qian J, Hawdon JM.. 2015. Two potential hookworm DAF-16 target genes, SNR-3 and LPP-1: gene structure, expression profile, and implications of a cis-regulatory element in the regulation of gene expression. Parasites & Vectors 8: 14. doi:10.1186/s13071-014-0609-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gems D, Doonan R.. 2008. The nematode Caenorhabditiselegans : oxidative stress and aging in the nematode Caenorhabditis elegans In: Miwa S, Beckman KB, Muller FL, eds. Oxidative Stress in Aging: From Model Systems to Human Diseases. Totowa, NJ: Humana Press, 81–108. [Google Scholar]
- Gheysen G, Mitchum MG.. 2011. How nematodes manipulate plant development pathways for infection. Current Opinion in Plant Biology 14: 415–421. [DOI] [PubMed] [Google Scholar]
- Gillan V, Maitland K, McCormack G, Him NA, Devaney E.. 2009. Functional genomics of hsp-90 in parasitic and free-living nematodes. International Journal for Parasitology 39: 1071–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover-Cutter KM, Lin S, Blackwell TK.. 2013. Integration of the unfolded protein and oxidative stress responses through SKN-1/Nrf. PloS Genetics 9: e1003701. doi:10.1371/journal.pgen.1003701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden TR, Melov S.. 2007. Gene expression changes associated with aging in C. elegans In: Monica D, Patterson G, eds. The C. elegans Research Community, WormBook. doi/10.1895/wormbook.1.127.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goverse A, Smant G.. 2014. The activation and suppression of plant innate immunity by parasitic nematodes. Annual Review of Phytopathology 52: 243–265. [DOI] [PubMed] [Google Scholar]
- Grants JM, Goh GYS, Taubert S.. 2015. The mediator complex of Caenorhabditis elegans: insights into the developmental and physiological roles of a conserved transcriptional coregulator. Nucleic Acids Research 43: 2442–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegeman A, Jones JT, Danchin EGJ.. 2011. Horizontal gene transfer in nematodes: a catalyst for plant parasitism? Molecular Plant-Microbe Interactions 24: 879–887. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Dinkova-Kostova AT.. 2014. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends in Biochemical Sciences 39: 199–218. [DOI] [PubMed] [Google Scholar]
- Heil M, Land WG.. 2014. Danger signals - damaged-self recognition across the tree of life. Frontiers in Plant Science 5: 578. doi:10.3389/fpls.2014.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson ST, Johnson TE.. 2001. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Current Biology 11: 1975–1980. [DOI] [PubMed] [Google Scholar]
- Hewezi T, Howe PJ, Maier TR, et al. 2010. Arabidopsis spermidine synthase is targeted by an effector protein of the cyst nematode Heterodera schachtii. Plant Physiology 152: 968–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoeven R, McCallum KC, Cruz MR, Garsin DA.. 2011. Ce-Duox1/BLI-3 generated reactive oxygen species trigger protective SKN-1 activity via p38 MAPK signaling during infection in C. elegans. PloS Pathogens 7: e1002453. doi:10.1371/journal.ppat.1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbein J, Grundler FMW, Siddique S.. 2016. Plant basal resistance to nematodes: an update. Journal of Experimental Botany 67: 2049–2061. [DOI] [PubMed] [Google Scholar]
- Hölscher D, Dhakshinamoorthy S, Alexandrov T, et al. 2014. Phenalenone-type phytoalexins mediate resistance of banana plants (Musa spp.) to the burrowing nematode Radopholus similis. Proceedings of the National Academy of Sciences of the United States of America 111: 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Y, Honda S.. 1999. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. The FASEB Journal 13: 1385–1393. [PubMed] [Google Scholar]
- Hu PJ. 2007. Dauer In: Riddle D, ed. The C. elegans Research Community, WormBook doi/10.1895/wormbook.1.127.1, http://www.wormbook.org. [Google Scholar]
- Hu M, Lok JB, Ranjit N, Massey HC, Sternberg PW, Gasser RB.. 2010. Structural and functional characterisation of the fork head transcription factor-encoding gene, Hc-daf-16, from the parasitic nematode Haemonchus contortus (Strongylida). International Journal for Parasitology 40: 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JS, Barker KR.. 1991. Glyceollin I in soybean–cyst nematode interactions : spatial and temporal distribution in roots of resistant and susceptible soybeans. Plant Physiology 96: 1302–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt VL, Tsai IJ, Coghlan A, et al. 2015. The genomic basis of parasitism in the Strongyloides clade of nematodes. Nature Genetics 48:299-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu AL, Murphy CT, Kenyon C.. 2003. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 300: 1142–1145. [DOI] [PubMed] [Google Scholar]
- Iberkleid I, Sela N, Brown Miyara S.. 2015. Meloidogyne javanica fatty acid- and retinol-binding protein (Mj-FAR-1) regulates expression of lipid-, cell wall-, stress- and phenylpropanoid-related genes during nematode infection of tomato. BMC Genomics 16: 272. doi:10.1186/s12864-015-1426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Hisamoto N, An JH, et al. 2005. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes & Development 19: 2278–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JDG, Dangl JL.. 2006. The plant immune system. Nature 444: 323–329. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N, Biswal S.. 2007. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annual Review of Ppharmacology and Toxicology 47: 89–116. [DOI] [PubMed] [Google Scholar]
- Kenyon CJ. 2010. The genetics of ageing. Nature 464: 504–512. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Cotton JA, Dalzell JJ, et al. 2011. Genomic insights into the origin of parasitism in the emerging plant pathogen Bursaphelenchus xylophilus. PloS Pathogens 7: e1002219. doi:10.1371/journal.ppat.1002219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klessig DF, Tian M, Choi HW.. 2016. Multiple targets of salicylic acid and its derivatives in plants and animals. Frontiers in Immunology 7: 206. doi:10.3389/ mmu.2016.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz LO, Sanchez-Ramos C, Prieto-Arroyo I, Urbanek P, Steinbrenner H, Monsalve M.. 2015. Redox regulation of FoxO transcription factors. Redox Biology 6: 51–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong LA, Wu DQ, Huang WK, et al. 2015. Large-scale identification of wheat genes resistant to cereal cyst nematode Heterodera avenae using comparative transcriptomic analysis. BMC Genomics 16: 801. doi:10.1186/s12864-015-2037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyndt T, Vieira P, Gheysen G, de Almeida-Engler J.. 2013. Nematode feeding sites: unique organs in plant roots. Planta 238: 807–818. [DOI] [PubMed] [Google Scholar]
- Lambeth JD. 2004. NOX enzymes and the biology of reactive oxygen. Nature Reviews. Immunology 4: 181–189. [DOI] [PubMed] [Google Scholar]
- Larsen PL. 1993. Aging and resistance to oxidative damage in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America 90: 8905–8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen SP, Boye TL, Nylandsted J.. 2015. Annexins are instrumental for efficient plasma membrane repair in cancer cells. Seminars in Cell and Developmental Biology 45: 32–38. [DOI] [PubMed] [Google Scholar]
- de Lencastre A, Pincus Z, Zhou K, Kato M, Lee SS, Slack FJ.. 2010. MicroRNAs both promote and antagonize longevity in C. elegans. Current Biology 20: 2159–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C.. 1994. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79: 583–593. [DOI] [PubMed] [Google Scholar]
- Li J, Tewari M, Vidal M, Sylvia Lee S.. 2007. The 14-3-3 protein FTT-2 regulates DAF-16 in Caenorhabditis elegans. Developmental Biology 301: 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Todd TC, Lee J, Trick HN.. 2011. Biotechnological application of functional genomics towards plant-parasitic nematode control. Plant Biotechnology Journal 9: 936–944. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhang Q, Zhou X.. 2016. A 2-Cys peroxiredoxin in response to oxidative stress in the pine wood nematode, Bursaphelenchus xylophilus. Scientific Reports 6: 27438. doi:10.1038/srep27438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libina N, Berman JR, Kenyon C.. 2003. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell 115: 489–502. [DOI] [PubMed] [Google Scholar]
- Lin K, Hsin H, Libina N, Kenyon C.. 2001. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nature Genetics 28: 139–145. [DOI] [PubMed] [Google Scholar]
- Lin B, Zhuo K, Chen S, et al. 2016. A novel nematode effector suppresses plant immunity by activating host reactive oxygen species-scavenging system. New Phytologist 209: 1159–1173. doi:10.1111/nph.13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm D, Wootz H, Korhonen L.. 2006. ER stress and neurodegenerative diseases. Cell Death and Differentiation 13: 385–392. [DOI] [PubMed] [Google Scholar]
- Liu D, Chen L, Duan Y.. 2011. Molecular cloning and phylogenetic analysis of two plant-parasitic nematode 14-3-3 genes. Journal of Agricultural Science 3: 86–95. [Google Scholar]
- Lourenço-Tessutti IT, Souza Junior JDA, Martins-de-Sa D, et al. 2015. Knock-down of heat-shock protein 90 and isocitrate lyase gene expression reduced root-knot nematode reproduction. Phytopathology 105: 628–637. [DOI] [PubMed] [Google Scholar]
- Manosalva P, Manohar M, von Reuss SH, et al. 2015. Conserved nematode signalling molecules elicit plant defenses and pathogen resistance. Nature Communications 6: 7795. doi:10.1038/ncomms8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantelin S, Thorpe P, Jones JT.. 2015. Suppression of plant defences by plant-parasitic nematodes In: Carolina E, Carmen F, eds. Advances in botanical research. San Diego: Academic Press, 325–337. [Google Scholar]
- Martins R, Lithgow GJ, Link W.. 2016. Long live FOXO: unraveling the role of FOXO proteins in aging and longevity. Aging Cell 15: 196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey HC, Nishi M, Chaudhary K, Pakpour N, Lok JB.. 2003. Structure and developmental expression of Strongyloides stercoralis fktf-1, a proposed ortholog of daf-16 in Caenorhabditis elegans. International Journal for Parasitology 33: 1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyash V, Entchev E V., Mende F, et al. 2004. Sterol-derived hormone(s) controls entry into diapause in Caenorhabditis elegans by consecutive activation of DAF-12 and DAF-16. PloS Biology 2: e280. doi:10.1371/journal.pbio.0020280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter JP, Mitreva MD, Martin J, et al. 2003. Analysis and functional classification of transcripts from the nematode Meloidogyne incognita. Genome Biology 4: R26. doi:10.1186/gb-2003-4-4-r26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwee JJ, Schuster E, Blanc E, Thomas JH, Gems D.. 2004. Shared transcriptional signature in Caenorhabditis elegans dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. Journal of Biological Chemistry 279: 44533–44543. [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Suzuki N, et al. 2011. ROS signaling: the new wave? Trends in Plant Science 16: 300–309. [DOI] [PubMed] [Google Scholar]
- Melillo MT, Leonetti P, Bongiovanni M, Castagnone-Sereno P, Bleve-Zacheo T.. 2006. Modulation of reactive oxygen species activities and H2O2 accumulation during compatible and incompatible tomato – root-knot nematode interactions. New Phytologist 170: 501–512. [DOI] [PubMed] [Google Scholar]
- Melillo MT, Leonetti P, Leone A, Veronico P, Bleve-Zacheo T.. 2011. ROS and NO production in compatible and incompatible tomato–Meloidogyne incognita interactions. European Journal of Plant Pathology 130: 489–502. [Google Scholar]
- Mitchum MG, Hussey RS, Baum TJ, et al. 2013. Nematode effector proteins: an emerging paradigm of parasitism. New Phytologist 199: 879–894. [DOI] [PubMed] [Google Scholar]
- Moschou PN, Roubelakis-Angelakis KA.. 2014. Polyamines and programmed cell death. Journal of Experimental Botany 65: 1285–1296. [DOI] [PubMed] [Google Scholar]
- Moschou PN, Sarris PF, Skandalis N, et al. 2009. Engineered polyamine catabolism preinduces tolerance of tobacco to bacteria and oomycetes. Plant Physiology 149: 1970–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou Z, Fan W, Dong X.. 2003. Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 113: 935–944. [DOI] [PubMed] [Google Scholar]
- Mukhtar MS, Carvunis AR, Dreze M, et al. 2011. Independently evolved virulence effectors converge onto hubs in a plant immune system network. Science 333: 596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S, Johnson TE.. 2001. The OLD-1 positive regulator of longevity and stress resistance is under DAF-16 regulation in Caenorhabditis elegans. Current Biology 11: 1517–1523. [DOI] [PubMed] [Google Scholar]
- Murphy CT, Hu PJ.. 2013. Insulin/insulin-like growth factor signaling in C. elegans In: The C. elegans Research Community, WormBook: 1–30. doi/10.1895/wormbook.1.127.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, et al. 2003. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424: 277–283. [DOI] [PubMed] [Google Scholar]
- Murphy CT, Lee SJ, Kenyon C.. 2007. Tissue entrainment by feedback regulation of insulin gene expression in the endoderm of Caenorhabditis elegans. Proceedings of the National Academy of Sciences USA 104: 19046–19050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Gisselbrecht SS, Rogers JM, Hartl DL, Bulyk ML.. 2013. DNA-binding specificity changes in the evolution of forkhead transcription factors. Proceedings of the National Academy of Sciences of the United States of America 110: 12349–12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C, Cunningham-Bussel A.. 2013. Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nature Reviews. Immunology 13: 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu W, Lu ZJ, Zhong M, et al.2011. Diverse transcription factor binding features revealed by genome-wide ChIP-seq in C. elegans. Genome Research 21: 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguez JH, Conner ES, Zhou Y, Ciche TA, Ragains JR, Butcher RA.. 2012. A novel ascaroside controls the parasitic life cycle of the entomopathogenic nematode Heterorhabditis bacteriophora. ACS Chemical Biology 7: 961–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obsil T, Obsilova V.. 2011. Structural basis for DNA recognition by FOXO proteins. Biochimica et Biophysica Acta – Molecular Cell Research 1813: 1946–1953. [DOI] [PubMed] [Google Scholar]
- Ogawa A, Streit A, Antebi A, Sommer RJ.. 2009. A conserved endocrine mechanism controls the formation of dauer and infective larvae in nematodes. Current Biology 19: 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa A, Bento G, Bartelmes G, Dieterich C, Sommer RJ.. 2011. Pristionchus pacificus daf-16 is essential for dauer formation but dispensable for mouth form dimorphism. Development 138: 1281–1284. [DOI] [PubMed] [Google Scholar]
- Ohri P, Pannu SK.. 2010. Effect of phenolic compounds on nematodes – a review. Journal of Applied and Natural Science 2: 344–350. [Google Scholar]
- Oláhová M, Veal EA.. 2015. A peroxiredoxin, PRDX-2, is required for insulin secretion and insulin/IIS-dependent regulation of stress resistance and longevity. Aging Cell 14: 558–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira RP, Abate JP, Dilks K, et al. 2009. Condition-adapted stress and longevity gene regulation by Caenorhabditis elegans SKN-1/Nrf. Aging Cell 8: 524–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opperman CH, Bird DM, Williamson VM, et al. 2008. Sequence and genetic map of Meloidogyne hapla: a compact nematode genome for plant parasitism. Proceedings of the National Academy of Sciences 105: 14802–14807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paek J, Lo JY, Narasimhan SD, et al. 2012. Mitochondrial SKN-1/Nrf mediates a conserved starvation response. Cell Metabolism 16: 526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajares M, Jiménez-Moreno N, Dias IHK, Debelec B, Cuadrado A.. 2015. Redox control of protein degradation. Redox Biology 6: 409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp D, Csermely P, Sőti C.. 2012. A role for SKN-1/Nrf in pathogen resistance and immunosenescence in Caenorhabditis elegans. PloS Pathogens 8: e1002673. doi:10.1371/journal.ppat.1002673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Tedesco PM, Johnson TE.. 2009. Oxidative stress and longevity in Caenorhabditis elegans as mediated by SKN-1. Aging Cell 8: 258–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N, Hamamouch N, Li C, et al. 2010. A nematode effector protein similar to annexins in host plants. Journal of Experimental Botany 61: 235–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penkov S, Kaptan D, Erkut C, Sarov M, Mende F, Kurzchalia TV.. 2015. Integration of carbohydrate metabolism and redox state controls dauer larva formation in Caenorhabditis elegans. Nature Communications 6: 8060. doi.org/10.1038/ncomms9060. [DOI] [PubMed] [Google Scholar]
- Perry RN, Moens M, Starr JL (eds). 2009. Root-knot nematodes. Wallingford, UK: CABI Publishing. [Google Scholar]
- Pieterse CMJ, Van Loon LC.. 2004. NPR1: the spider in the web of induced resistance signaling pathways. Current Opinion in Plant Biology 7: 456–464. [DOI] [PubMed] [Google Scholar]
- Pritchard L, Birch PRJ.. 2014. The zigzag model of plant–microbe interactions: is it time to move on? Molecular Plant Pathology 15: 865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priya DB, Somasekhar N, Prasad J, Kirti P.. 2011. Transgenic tobacco plants constitutively expressing Arabidopsis NPR1 show enhanced resistance to root-knot nematode, Meloidogyne incognita. BMC Research Notes 4: 231. doi:10.1186/1756-0500-4-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai KM, Balasubramanian VK, Welker CM, Pang M, Hii MM, Mendu V.. 2015. Genome wide comprehensive analysis and web resource development on cell wall degrading enzymes from phyto-parasitic nematodes. BMC Plant Biology 15: 187. doi:10.1186/s12870-015-0576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman S, Gupta VK., Goyal AK.. 2016. Identification and functional analysis of secreted effectors from phytoparasitic nematodes. BMC Microbiology 16: 48. doi:10.1186/s12866-016-0632-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson SJ, Cody V (eds). 2009. Recent advances in transthyretin evolution, structure and biological functions. Heidelberg: Springer-Verlag. [Google Scholar]
- Riedel CG, Dowen RH, Lourenco GF, et al. 2013. DAF-16 employs the chromatin remodeller SWI/SNF to promote stress resistance and longevity. Nature Cell Biology 15: 491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MP, Atkinson HJ, Perry RN.. 1987. The influence of soil moisture and storage time on the motility, infectivity and lipid utilization of second stage juveniles of the potato cyst nematodes Globodera rostochiensis and G. pallida. Revue de Nematologie 10: 343–348. [Google Scholar]
- Ron D, Walter P.. 2007. Signal integration in the endoplasmic reticulum unfolded protein response. Nature Reviews. Molecular Cell Biology 8: 519–529. [DOI] [PubMed] [Google Scholar]
- Rosso MN, Jones JT, Abad P.. 2009. RNAi and functional genomics in plant parasitic nematodes. Annual Review of Phytopathology 47: 207–232. [DOI] [PubMed] [Google Scholar]
- Rupert PB, Daughdrill GW, Bowerman B, Matthews BW.. 1998. A new DNA-binding motif in the Skn-1 binding domain-DNA complex. Nature Structural Biology 5: 400–406. [DOI] [PubMed] [Google Scholar]
- Safra M, Fickentscher R, Levi-Ferber M, et al. 2014. The FOXO transcription factor DAF-16 bypasses ire-1 requirement to promote endoplasmic reticulum homeostasis. Cell Metabolism 20: 870–881. [DOI] [PubMed] [Google Scholar]
- Sandalio LM, Romero-Puertas MC.. 2015. Peroxisomes sense and respond to environmental cues by regulating ROS and RNS signalling networks. Annals of Botany 116: 475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler AJ, Sherwood DR.. 2014. Should I stay or should I go? Identification of novel nutritionally regulated developmental checkpoints in C. elegans. Worm 3: e979658. doi:10.4161/21624054.2014.979658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleker S, Trilling M.. 2013. Data-warehousing of protein-protein interactions indicates that pathogens preferentially target hub and bottleneck proteins. Frontiers in Microbiology 4: 51. doi:10.3389/fmicb.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimeld SM, Degnan B, Luke GN.. 2010. Evolutionary genomics of the Fox genes: origin of gene families and the ancestry of gene clusters. Genomics 95: 256–260. [DOI] [PubMed] [Google Scholar]
- Shinya R, Morisaka H, Takeuchi Y, Ueda M, Futai K.. 2010. Comparison of the surface coat proteins of the pine wood nematode appeared during host pine infection and in vitro culture by a proteomic aspproach. Phytopathology 100: 1289–1297. [DOI] [PubMed] [Google Scholar]
- Shinya R, Morisaka H, Kikuchi T, Takeuchi Y, Ueda M, Futai K.. 2013. Secretome analysis of the pine wood nematode Bursaphelenchus xylophilus reveals the tangled roots of parasitism and its potential for molecular mimicry. PloS ONE 8: e67377. doi:10.1371/journal.pone.0067377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivers RP, Youngman MJ, Kim DH.. 2008. Transcriptional responses to pathogens in Caenorhabditis elegans. Current Opinion in Microbiology 11: 251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore DE, Ruvkun G.. 2013. A cytoprotective perspective on longevity regulation. Trends in Cell Biology 23: 409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder FC. 2015. Modular assembly of primary metabolic building blocks: a chemical language in C. elegans. Chemistry and Biology 22: 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddique S, Matera C, Radakovic ZS, et al. 2014. Parasitic worms stimulate host NADPH oxidases to produce reactive oxygen species that limit plant cell death and promote infection. Science Signaling 7: ra33. [DOI] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen D, et al.2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Molecular Systems Biology 7: 539. doi:10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetti E, Veronico P, Melillo MT, Delibes A, Andrés MF, López-Braña I.. 2009. Analysis of class III peroxidase genes expressed in roots of resistant and susceptible wheat lines infected by Heterodera avenae. Molecular Plant-Microbe Interactions 22: 1081–1092. [DOI] [PubMed] [Google Scholar]
- Sommer RJ, Mayer MG.. 2015. Toward a synthesis of developmental biology with evolutionary theory and ecology. Annual Review of Cell and Developmental Biology 31: 453–471. [DOI] [PubMed] [Google Scholar]
- Staab TA, Griffen TC, Corcoran C, Evgrafov O, Knowles JA, Sieburth D.. 2013. The conserved SKN-1/Nrf2 stress response pathway regulates synaptic function in Caenorhabditis elegans. PLoS Genetics 9: e1003354. doi:10.1371/journal.pgen.1003354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R.. 2011. Respiratory burst oxidases: the engines of ROS signaling. Current Opinion in Plant Biology 14: 691–699. [DOI] [PubMed] [Google Scholar]
- Tepper RG, Ashraf J, Kaletsky R, Kleemann G, Murphy CT, Bussemaker HJ.. 2013. PQM-1 complements DAF-16 as a key transcriptional regulator of DAF-2-mediated development and longevity. Cell 154: 676–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BP, Nürnberger T, Joosten MH.. 2011. Of PAMPs and effectors: the blurred PTI-ETI dichotomy. The Plant Cell 23: 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Jones JDG, Dangl JL.. 2006. Reactive oxygen species signaling in response to pathogens. Plant Physiology 141: 373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treitz C, Cassidy L, Höckendorf A, Leippe M, Tholey A.. 2015. Quantitative proteome analysis of Caenorhabditis elegans upon exposure to nematicidal Bacillus thuringiensis. Journal of Proteomics 113: 337–350. doi:10.1016/j.jprot.2014.09.027. [DOI] [PubMed] [Google Scholar]
- Tullet JM. 2015. DAF-16 target identification in C. elegans: past, present and future. Biogerontology 16: 221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullet JM, Hertweck M, An JH, Baker J, et al. 2008. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell 132: 1025–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanfleteren R, de Vreese A.. 1995. The gerontogenes age-1 and daf-2 determine metabolic rate potential in aging Caenorhabditis elegans. The FASEB Journal 9: 1355–1361. [DOI] [PubMed] [Google Scholar]
- Vicente CSL, Ikuyo Y, Shinya R, Mota M, Hasegawa K.. 2015. Catalases induction in high virulence pinewood nematode Bursaphelenchus xylophilus under hydrogen peroxide-induced stress. PloS One 10: e0123839. doi:10.1371/journal. pone.0123839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waetzig G, Grundler FMW, Sobczak M.. 1999. Localization of hydrogen peroxide during the defence response of Arabidopsis thaliana against the plant-parasitic nematode Heterodera glycines. Nematology 1: 681–686. [Google Scholar]
- Wan QL, Zheng SQ, Wu GS, Luo HR.. 2013. Aspirin extends the lifespan of Caenorhabditis elegans via AMPK and DAF-16/FOXO in dietary restriction pathway. Experimental Gerontology 48: 499–506. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li L, Li D, Wang B, Zhang K, Niu X.. 2015a. Yellow pigment aurovertins mediate interactions between the pathogenic fungus Pochonia chlamydosporia and its nematode host. Journal of Agricultural and Food Chemistry 63: 6577–6587. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mao Z, Yan J, et al. 2015b. Identification of MicroRNAs in Meloidogyne incognita using deep sequencing. PloS ONE 10: e0133491. doi:10.1371/journal. pone.0133491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AE, Brunet A.. 2014. FOXO transcription factors: key regulators of cellular quality control. Trends in Biochemical Sciences 39: 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AE, Kundaje A, Brunet A.. 2016. Characterization of the direct targets of FOXO transcription factors throughout evolution. Aging Cell 15: 673–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. 2008. Reconciling the chemistry and biology of reactive oxygen species. Nature Chemical Review 4: 278–286. doi:10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- Wojtaszek P. 1997. Oxidative burst: an early plant response to pathogen infection. The Biochemical Journal 322: 681–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuyts N, Swennen R, de Waele D.. 2006. Effects of plant phenylpropanoid pathway products and selected terpenoids and alkaloids on the behaviour of the plant-parasitic nematodes Radopholus similis, Pratylenchs penetrans and Meloidogyne incognita. Nematology 8: 89–101. [Google Scholar]
- Wuyts N, Lognay G, Verscheure M, Marlier M, de Waele D, Swennen R.. 2007. Potential physical and chemical barriers to infection by the burrowing nematode Radopholus similis in roots of susceptible and resistant banana (Musa spp.). Plant Pathology 56: 878–890. [Google Scholar]
- Xu C, Li CY, Kong AN.. 2005. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Archives of Pharmacal Research 28: 249–268. [DOI] [PubMed] [Google Scholar]
- Yen K, Narasimhan SD, Tissenbaum HA.. 2011. DAF-16/Forkhead box O transcription factor: many paths to a single Fork(head) in the road. Antioxidants & Redox Signaling 14: 623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef RM, MacDonald MH, Brewer EP, Bauchan GR, Kim K-H, Matthews BF.. 2013. Ectopic expression of AtPAD4 broadens resistance of soybean to soybean cyst and root-knot nematodes. BMC Plant Biology 13: 67. doi:10.1186/1471-2229-13-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang Y, Xie F, et al. 2016. Identification and characterization of microRNAs in the plant parasitic root-knot nematode Meloidogyne incognita using deep sequencing. Functional & Integrative Genomics 16: 127–142. [DOI] [PubMed] [Google Scholar]
- Zhao L, Zhang S, Wei W, et al. 2013. Report chemical signals synchronize the life cycles of a plant-parasitic nematode and its vector beetle. Current Biology 23: 2038–2043. [DOI] [PubMed] [Google Scholar]
- Zipfel C. 2014. Plant pattern-recognition receptors. Trends in Immunology 35: 345–351. [DOI] [PubMed] [Google Scholar]
- Zou CG, Tu Q, Niu J, Ji XL, Zhang KQ.. 2013. The DAF-16/FOXO transcription factor functions as a regulator of epidermal inate immunity. PLoS Pathogens 9: e1003660. doi:10.1371/journal. ppat.1003660. [DOI] [PMC free article] [PubMed] [Google Scholar]