Abstract
Background Plants, though sessile, employ various strategies to defend themselves against herbivorous insects and convey signals of an impending herbivore attack to other plant(s). Strategies include the production of volatiles that include terpenoids and the formation of symbiotic associations with fungi, such as arbuscular mycorrhiza (AM). This constitutes a two-pronged above-ground/below-ground attack–defence strategy against insect herbivores.
Scope Terpenoids represent an important constituent of herbivore-induced plant volatiles that deter herbivores and/or attract their predators. Terpenoids serve as airborne signals that can induce defence responses in systemic undamaged parts of the plant and also prime defence responses in neighbouring plants. Colonization of roots by AM fungi is known to influence secondary metabolism in plants; this includes alteration of the concentration and composition of terpenoids, which can boost both direct and indirect plant defence against herbivorous insects. Enhanced nutrient uptake facilitated by AM, changes in plant morphology and physiology and increased transcription levels of certain genes involved in the terpenoid biosynthesis pathway result in alterations in plant terpenoid profiles. The common mycorrhizal networks of external hyphae have added a dimension to the two-pronged plant defence strategy. These act as conduits to transfer defence signals and terpenoids.
Conclusion Improved understanding of the roles of terpenoids in plant and AM defences against herbivory and of interplant signalling in natural communities has significant implications for sustainable management of pests in agricultural ecosystems.
Keywords: Terpenoids, herbivorous insects, indirect defence, induced defence, priming, arbuscular mycorrhiza, common mycorrhizal networks
INTRODUCTION
Terpenoids represent the largest and structurally the most diverse group of volatiles released by plants. Biologically, a wide array of terpenoids can enable plants to interact with other organisms, such as insects, pathogens and neighbouring plants (Kant et al., 2004; Mercke et al., 2004; Kappers et al., 2005; Cheng et al., 2007a, b). Terpenoids are emitted either constitutively or induced in response to biotic (Dudareva et al., 2006, 2013; Unsicker et al., 2009; Rasmann et al., 2012) and abiotic (Gouinguené and Turlings, 2002; Loreto and Schnitzler, 2010) stresses.
Attack by insects induces plants to emit a blend of volatile organic compounds (VOCs). Terpenoids are important members of the class of herbivore-induced plant volatiles (HIPVs) (Gershenzon and Dudareva, 2007; Mumm et al., 2008). Some terpenoids serve as repellents (Laothawornkitkul et al., 2008; Unsicker et al., 2009; Maffei, 2010), while others function in indirect plant defence by attracting arthropods that prey upon or parasitize herbivores (Kessler and Baldwin, 2001; Rasmann et al., 2005; Schnee et al., 2006). Additionally, terpenoids are produced in response to oviposition and are involved in the attraction of egg-parasitizing insects (Conti et al., 2008; Büchel et al., 2011; Tholl et al., 2011; Hilker and Fatouros, 2015).
In addition to their roles in direct and indirect defences, plant terpenoids, along with other HIPVs, such as green leaf volatiles (GLVs), serve as airborne signals that can be perceived by undamaged systemic parts of the same plant (Frost et al., 2007; Heil and Silva Bueno, 2007) and by neighbours (Karban et al., 2000). In response to perceived volatile signals, plants express defence genes and synthesize secondary metabolites (Shulaev et al., 1997; Arimura et al., 2000b; Sugimoto et al., 2014) or prime their defences against pests (Engelberth et al., 2004; Heil and Kost, 2006; Ton et al., 2006). Although primed plants do not show any trait of resistance, they become prepared to respond more rapidly and more intensely when attacked (Conrath et al., 2006; Heil and Ton, 2008).
The synthesis of terpenoids can be altered by numerous biotic and abiotic factors (Owen and Peñuelas, 2005; Peñuelas and Munné-Bosch, 2005; Brunetti et al., 2013). Among such influencing factors is the formation of arbuscular mycorrhiza (AM), defined as a symbiotic association of plant roots with soil fungi belonging to the phylum Glomeromycota. Arbuscular mycorrhizal fungi are heterokaryotic, obligate symbionts that confer on plants multifarious benefits, like improved access to nutrients and water and enhanced resistance to biotic and abiotic stresses (Finlay, 2008; Smith and Read, 2008; Miransari, 2010; Ruiz-Lozano et al., 2012; Evelin et al., 2013). In return for such colossal benefits, the fungus obtains carbon from the plants (Smith and Gianinazzi-Pearson, 1988; Smith and Read, 2008). Arbuscular mycorrhiza interconnects plants by means of an extensive subterranean hyphal network. This network is specialized for nutrient (primarily phosphate) and water uptake (Miller et al., 1995). The bidirectional exchange of nutrients between the symbionts takes place at highly branched intracellular structures called arbuscules, which are formed in the inner cortex of the plant root by the mycobiont (Harrison, 2005; Parniske, 2008). This interaction plays a crucial role in plant ecosystem functioning, as more than 80 % of the terrestrial plant species rely on AM fungi for their mineral nutrition (Smith and Read, 2008).
The formation of AM changes the physiology and ecology of the plant. Arbuscular mycorrhiza potentially strengthens both direct and indirect plant defence systems (Pozo and Azcón-Aguilar, 2007; Jung et al., 2012; Borowicz, 2013) by altering the secondary metabolism of the plant (Hohnjec et al., 2005; Walker et al., 2012). Formation of AM has been demonstrated to change the concentration and composition of terpenoids (Copetta et al., 2006; Khaosaad et al., 2006; Kapoor et al., 2007; Rapparini et al., 2008). This alters the plant’s attractiveness and also the insect’s behaviour (Schausberger et al., 2012; Babikova et al., 2014a; Shrivastava et al., 2015). Cascading effects on higher trophic levels have also been reported (Gange et al., 2003), as have indirect effect on predators and parasitoids of herbivores (Gange et al., 2003; Guerrieri et al., 2004; Laird and Addicott, 2007). Consequently, increased knowledge of the mechanisms that influence production of terpenoids in AM plants will make important contributions to the biocontrol and integrated management of pests.
In this review, readers are first introduced to the terpenoids that contribute to HIPVs, and their synthesis in the plant cell. The review emphasizes the role of terpenoids in plant defence against herbivorous insects (Fig. 1) and discusses their probable role in airborne signalling within the plant and to nearby plants. It then focuses on the significance of terpenoids in AM-mediated reinforcement of direct and indirect defences against herbivory (Fig. 2), further discussing various mechanisms underlying changes in the concentration and composition of terpenoids in mycorrhizal plants. Finally, it outlines the prospects for bioengineered terpenoid-producing plants and AM symbiosis in the sustainable management of pests in agricultural systems.
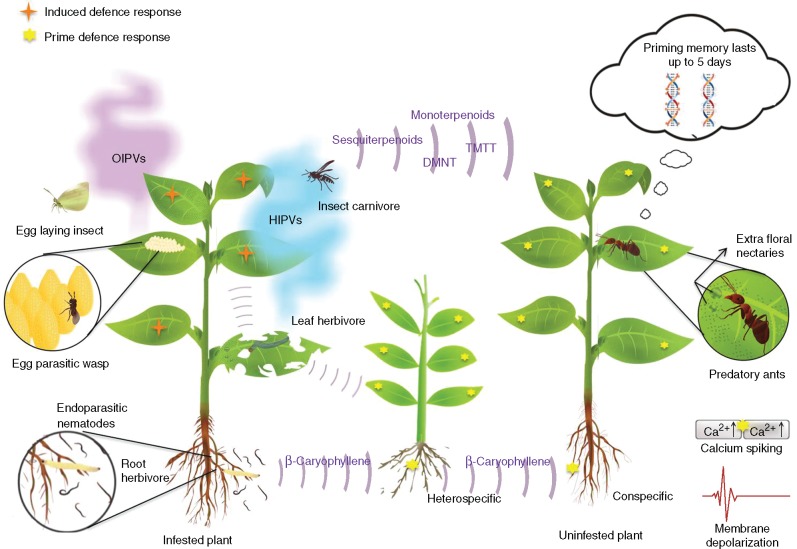
Fig. 1.
Overview of terpenoids in plant defence against herbivorous insects. Volatile terpenoids that belong to the HIPVs (herbivore-induced plant volatiles) and OIPVs (oviposition-induced plant volatiles) are released in response to herbivore attack and oviposition, respectively. Terpenoids induce defence responses in the systemic parts of the same plant. These volatiles attract insect carnivores that feed on the herbivores, thereby inducing indirect defence in plants, and prime neighbouring conspecific and heterospecific plants. The perception of terpenoids by neighbouring plants results in influx of calcium ions and membrane depolarization. Epigenetic regulation of this priming response is reported to evoke the priming memory for up to 5 d. Terpenoids also affect tritrophic interactions in soil.
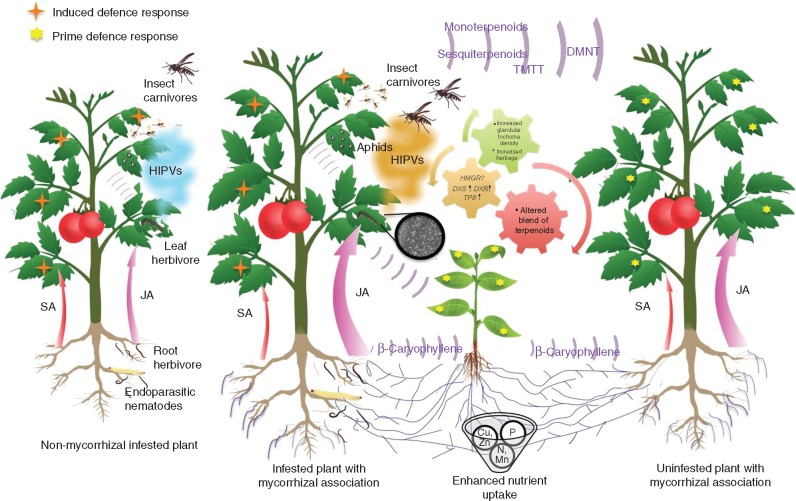
Fig. 2.
Overview of arbuscular mycorrhiza (AM)-reinforced defence against herbivorous insects. Plants colonized by AM fungi are more tolerant by virtue of superior growth and nutrient uptake. Formation of AM may result in increased glandular trichome density, availability of substrates, induction of MEP (higher expression of DXS and DXR) and MVA (higher expression of HMGR) pathways, and induction of terpene synthases (TPSs). These factors in various combinations result in changes in the terpenoid profile in mycorrhizal (M) plants, inducing both direct and indirect defence responses against herbivore attack in the plant. Mycorrhizal colonization results in amplification of a wound signal, leading to priming of neighbouring plants. Common mycelial networks (CMNs) serve as signalling conduits between interconnected plants under herbivore attack. JA, jasmonic acid; SA, salicylic acid.
TERPENOIDS IN HIPVS
Terpenoids are one of the important constituents of volatiles that are released by plants in response to herbivore attack (Gershenzon and Dudareva, 2007; Mumm et al., 2008). They are low molecular weight compounds derived from the basic five-carbon building blocks of isopentenyl diphosphate (IPP). The key players among the terpenoid volatiles that significantly contribute to HIPVs are monoterpenes (C10), sesquiterpenes (C15) and homoterpenes such as 4,8-dimethylnona-l,3,7-triene (DMNT) and 4,8,12-trimethyltrideca-1,3,7,11-tetraene (TMTT) (Leitner et al., 2005; Arimura et al., 2008; Mithöfer and Boland, 2012). Isoprene (2-methyl-1,3-butadiene), although not produced by many plants, has also been demonstrated to play an important role in defence against insect herbivory (Laothawornkitkul et al., 2008).
There are two pathways for the production of terpenoids: the cytoplasmic mevalonate (MVA) pathway and the plastidial 2-C-methyl-d-erythritol 4-phosphate (MEP) pathway (Aharoni et al., 2005; Rodríguez-Concepcion, 2006; Cheng et al., 2007a). Both pathways generate universal precursors for terpenoid synthesis from IPP and its isomer dimethylallyl diphosphate (DMAPP). While monoterpenes are synthesized via the MEP pathway, sesquiterpenes are produced by the MVA pathway. In contrast to the conventional allocation, which suggests the MVA and MEP pathways are strictly independent, there is emerging evidence that the two pathways cross-talk by allowing IPP to shuttle between different compartments (Piel et al., 1998; Bick and Lange, 2003; Bartram et al., 2006; Rodríguez-Concepcion, 2006). However, it has been found that ∼80 % of the IPP derived from the MEP pathway contributes to sesquiterpene biosynthesis following herbivory (Bartram et al., 2006; Arimura et al., 2008).
Condensation of C5 units gives rise to all-trans or all-cis prenyl diphosphate precursors that are converted by the terpene synthase (TPS) enzymes of different subfamilies into acyclic, mono-, bi- or tricyclic monoterpenes, sesquiterpenes or semivolatile diterpenes (Chen et al., 2011). Terpene synthases are generally multiproduct enzymes, and thus even a single TPS can significantly enhance the diversity of terpenoids (Gershenzon, 1994; Tholl, 2006; Arimura et al., 2008). The primary terpene skeletons may be further modified through secondary enzymatic reactions, such as dehydrogenations, hydroxylations, methylations and acylations (Dudareva et al., 2006).
Some terpenoids, such as β-ionone, are produced not directly from IPP, but instead from tetraterpenes such as carotenoids, by carotenoid cleavage dioxygenases (Dudareva et al., 2013). Homoterpenes such as DMNT and TMTT are synthesized by oxidative degradation of the sesquiterpene (3S)-(E)-nerolidol and the diterpene geranyl linalool by cytochrome P450 enzymes (Arimura et al., 2009; Maffei, 2010).
TERPENOIDS IN DEFENCE AGAINST HERBIVORY
Direct interaction
Terpenoids can serve as repellents and reduce larval feeding and oviposition by herbivores (De Boer et al., 2004; Laothawornkitkul et al., 2008; Unsicker et al., 2009; Maffei, 2010). For example, linalool (a monoterpenoid) and (E)-β-farnesene (a sesquiterpene) produced by plants repel herbivores and aphids, respectively (Aharoni et al., 2003; Unsicker et al., 2009; Maffei, 2010). Although the exact mechanisms by which terpenoids affect insect pests are not known, probable processes include the inhibition of ATP synthase, alkylation of nucleophiles and interference with moulting (Langenheim, 1994). Terpenoids such as α-pinene and β-pinene have been shown to disturb the nervous system in insects by inhibition of acetylcholinesterase (Yeom et al., 2012).
Indirect above-ground interactions
Terpenoids emitted as a result of herbivore attack have an important role in a plant’s indirect defences, attracting predators or parasites of herbivores and facilitating location of the attacked plants (Heil, 2008). For example, infestation of lima bean leaves by spider mites (Tetranychus urticae) triggers the de novo production of terpenoids such as (E)-β-ocimene, linalool, DMNT and TMTT (Dicke et al., 1990, 1999; De Boer et al., 2004; Shimoda et al., 2005), which lure the predacious mites (Phytoseiulus persimilis) that prey on spider mites (Takabayashi and Dicke, 1996). The volatiles from spider mite-infested lima beans, treated with fosmidomycin (an inhibitor of the MEP pathway) were less attractive to the predatory mites than those from infested control plants, indicating the significance of terpenoids in indirect defence (Mumm et al., 2008).
The high chemical diversity within HIPV mixtures complicates identification of the compound(s) actually responsible for signalling herbivore enemies. However, it has been demonstrated by investigation of individual compounds that terpenoids such as the homoterpene TMTT can attract predatory mites (De Boer et al., 2004). Genetic engineering for enhanced expression of genes encoding enzymes for the formation of terpenoids has ascertained the role of individual compounds in tritrophic interactions. Transgenic Arabidopsis thaliana overexpressing strawberry nerolidol synthase, a TPS, attracted more predatory P. persimilis mites (Kappers et al., 2005). Similarly, overexpression of a corn TPS gene (TPS10) in A. thaliana augmented the attractiveness of these transgenic plants to the parasitic wasp Cotesia marginiventris (Schnee et al., 2006). Interestingly, changes in HIPV blends emitted at different times can impact the interactions among a plant, its herbivores and their parasitoids, and stimulate different preferences for herbivores and their parasitoids (Mathur et al., 2013; Pashalidou et al., 2015). The generalist Spodoptera littoralis preferred undamaged Brassica juncea plants, whereas its parasitoid (C. marginiventris) preferred 48-h damaged plants (Mathur et al., 2013). In Brassica nigra, parasitoid wasps (Cotesiaglomerata) were attracted to plants infested with eggs just before and shortly after larval hatching of Pieris brassicae (Pashalidou et al., 2015). The authors have correlated this preference to temporal changes in the blend of HIPVs (terpenoids).
Response to oviposition
Plants may respond to herbivore egg deposition and activate defences before actual feeding injury is initiated, which might be a successful tactic to reduce impending herbivory (Hilker et al., 2002; Mumm and Hilker, 2006; Pashalidou et al., 2015). Analogous to HIPVs, plant volatiles induced specifically by insect oviposition are termed oviposition-induced plant volatiles (OIPVs) (Hilker and Fatouros, 2015). Terpenoids are important members of the class of OIPVs (Conti et al., 2008; Tholl et al., 2011). The OIPV-specific terpenoids attract egg parasitoids (Wegener and Schulz, 2002; Mumm and Hilker, 2005; Büchel et al., 2011). Intriguingly, the attractiveness of egg-laden foliage to the egg parasitoid has been related to an increase in transcription levels of sesquiterpene synthase (Köpke et al., 2010; Beyaert et al., 2012). Oviposition on Pinus sylvestris needles by the sawfly Diprion pini induced both local and systemic emission of terpenoid volatiles (Hilker et al., 2002; Mumm and Hilker, 2006). This response was specific to oviposition, and could not be induced by artificial wounding (Hilker and Fatouros, 2015). However, volatile cues to attract egg parasitoids have not yet been identified.
Response to below-ground infestation
Below-ground VOC patterns are generally distinct from volatiles released from above-ground plant tissues (Peñuelas et al., 2014). Terpenes are the most prominent VOCs emitted from below-ground tissues (Rasmann et al., 2005; Ali et al., 2011; Palma et al., 2012; Peñuelas et al., 2014), and among these sesquiterpenes are the compounds that show the greatest diffusion in the soil (Hiltpold and Turlings, 2008). Terpenoids have been shown to play a crucial role in the specificity of below-ground tritrophic interactions (Rasmann and Turlings, 2008). The most well-studied example is the induction of (E)-β-caryophyllene by maize roots infested by larvae of the leaf beetle Diabrotica virgifera virgifera, which attracted the entomopathogenic nematode Heterorhabditis megidis (Rasmann et al., 2005). On the other hand, (E)-β-caryophyllene also served as an attractant aiding D. virgifera larvae to identify a susceptible host (Robert et al., 2012). One possible explanation for the contradictory observations in the above studies is that as maize roots only emit (E)-β-caryophyllene (Hiltpold and Turlings, 2008), it can be presumed that the entomopathogenic nematode H. megidis has developed an adaptation to take cues from the (E)-β-caryophyllene emitted by maize roots for efficient prey-searching.
Multiple herbivore infestations
In nature, plants are generally infested by two or more herbivore species, either concurrently or serially. However, most of the studies in this area have been conducted on single-herbivore attack under controlled conditions. Infestation by two or more insect species causes complex variations in volatile profile, and cannot be predicted on the basis of observations on single herbivores. When two or more herbivores co-infest, the effects may be negative or additive, or one type of herbivore takes priority. For example, concurrent occurrence of herbivory above- as well as below-ground by S.littoralis and D. virgifera, respectively, negatively influenced tritrophic signalling due to decreased (E)-β-caryophyllene production by maize roots (Rasmann and Turlings, 2007). This may be explained by reduced availability of a C source required for the synthesis of the terpenoid precursors. On the other hand, HIPVs emitted by lima beans and pepper plants infested by two herbivore species attracted more predatory mites and predatory mirid bugs, respectively, compared with volatiles emitted by plants infested by either herbivore separately (Dicke et al., 2009). Furthermore, most studies are performed under highly controlled conditions, which impedes application of the results in natural environments. Thus, a major challenge is the development of experimental designs that consider the ecological reality of infestations.
AIRBORNE SIGNALLING TO NEIGHBOURING PLANTS AND SYSTEMIC PARTS OF THE SAME PLANT
The airborne volatile signals from herbivore-damaged plants (emitters) enable nearby conspecific and heterospecific undamaged plants (receivers) to foresee the impending arrival of herbivores and tailor their defence accordingly (Baldwin and Schultz, 1983; Arimura et al., 2000a; Engelberth et al., 2004; Karban et al., 2006; Heil and Silva Bueno, 2007; Ramadan et al., 2011). Herbivore-induced plant volatiles serve as external signals for within-plant communication, and elicit a defence response in systemic parts of the affected plant (Karban et al., 2006; Frost et al., 2007; Heil and Silva Bueno, 2007; Park et al., 2007; Das et al., 2013). Damaged leaves immediately release VOCs and communicate more quickly with leaves located nearby that are not directly connected by vasculature (Heil and Ton, 2008). Plants may react to the signals connected with the presence of herbivores by upregulating defence genes (Arimura et al., 2000b), leading to increased production of defence-related metabolites such as phytohormones, proteinase inhibitors, terpenoids and/or extrafloral nectar (Tscharntke et al., 2001; Engelberth et al., 2004; Kost and Heil, 2006; Frost et al., 2008a; Blande et al., 2010). These changes are ultimately translated into reduced herbivory and improved fitness of receiver (Karban and Maron, 2002; Kost and Heil, 2006; Muroi et al., 2011). The responses include a combination of priming and induced defences, according to the allocation cost of different classes of defence, with plants priming more expensive responses and inducing less costly metabolites, such as extrafloral nectar or HIPVs, to attract natural enemies of the herbivore (Kost and Heil, 2006; Frost et al., 2008b). Participation of volatiles in interplant below-ground interactions is not well elucidated (Schenkel et al., 2015; Delory et al., 2016). Whether VOCs emitted by roots in the rhizosphere can diffuse into the phyllosphere and convey signals to prime above-ground parts of the same plant is also not effectively documented (Erb et al., 2008).
ROLE OF TERPENOIDS IN AIRBORNE SIGNALLING
An important step in understanding the mechanistic foundations of airborne priming is the elucidation of the actual messengers. Green-leaf volatiles and terpenoids are two important components of HIPVs. Green-leaf volatiles, which are aldehydes, alcohols and esters resulting from lipoxygenase cleavage of fatty acids, account for the distinctive odour of damaged leaves (Paré and Tumlinson, 1999). Although evidence for GLVs as priming signals has been observed in several plant species (Farag and Paré, 2002; Ruther and Fürstenau, 2005; Ruther and Kleier, 2005; Kost and Heil, 2006; Sugimoto et al., 2014), reports on terpenoids have been variable. The role of volatile terpenes in plant–plant interactions was initially reported in lima bean, where terpenoids such as β-ocimene, DMNT, TMTT and linalool, released upon feeding of T.urticae, induced the expression of defence genes encoding lipoxygenase (synthesis of jasmonic acid) and the pathogenesis-related protein PR-2 (β-1,3-glucanase) (Arimura et al., 2000b). In maize, however, terpenoids were not associated with priming defence responses in the receiver plants (Ruther and Fürstenau, 2005).
Early events in the perception of volatile signals comprise an alteration of the plasma membrane potential (Vm) and an increase in cytosolic calcium ([Ca2+]cyt) (Zebelo et al., 2012). It was observed that GLVs such as (E)-2-hexenal, (Z)-3-hexenal and (Z)-3-hexenyl acetate induced stronger Vm depolarization and a greater increase in cytosolic calcium flux compared with terpenoids such as α-pinene and β-caryophyllene. These terpenoids induced a significant Vm depolarization with respect to controls, but did not exert any significant effect on [Ca2+]cyt homeostasis (Zebelo et al., 2012). Moreover, Vm depolarization was found to increase with increasing GLV concentration. Green-leaf volatiles are immediately released after damage and their release ceases within a few minutes of damage (Arimura et al., 2009), while the release of monoterpenes typically starts 24 h after attack (Dudareva et al., 2006; Pichersky et al., 2006). The emission of terpenoids is often systemic and extended (Paré and Tumlinson, 1999). These observations indicate that GLVs are better candidates than terpenoids for conveying airborne signals of herbivore attack. Further studies are required to identify the messengers (volatiles) involved in transmitting signals within and to nearby plants. The complementary approach of using plant mutants deficient in various components of HIPVs (GLVs or terpenoids) has enabled the role of individual compounds in plant–plant signalling to be deciphered (Baldwin et al., 2006). However, using this technique, Paschold et al. (2006) observed that neither GLVs nor terpenoids prime the expression of defence genes in Nicotiana attenuata. The role of various HIPVs as volatile priming signals has continued to be uncertain because in most studies healthy plants were treated with synthetic volatiles, a procedure that does not satisfactorily mimic the exact timing and concentrations of HIPV emissions in nature. Furthermore, genetic manipulation of plants for enhanced synthesis of HIPVs may result in several undesirable effects (Erb et al., 2015). As individual volatile compounds do not participate in plant–insect interactions in isolation, another key issue for exploration is the interactive effects of different VOCs in these interactions. Furthermore, techniques based on the limit of detection of terpenoids do not take into consideration the sensitivity of perception by biological systems (insects), and hence do not necessarily provide biologically useful information.
After herbivore departure, plants likely cease to release HIPVs that attract parasitoids (Puente et al., 2008). If emission were to continue, signals would deliver unreliable information to parasitoids, which would then be incapable of tracking their hosts. Receiver plants are not aware of how much later the herbivores will arrive, and therefore have no clues regarding how long the primed state should be maintained. However, very little is known about how receiver plants control the duration of the primed state, which is of importance in terms of the arrival time of herbivores. The molecular mechanisms involved in sustaining the primed state are also unresolved. Ali et al. (2013) demonstrated that the priming effect of HIPVs on resistance against herbivores is memorized and stored by plants through epigenetic regulation of DNA, with plants able to evoke this memory when attacked by herbivores. Treatment with HIPV was shown to result in demethylation of cytosine sites in the promoter region of a herbivore-responsive gene for Bowman–Birk-type trypsin inhibitor (TI). Further experiments are required to substantiate understanding of the epigenetic control of airborne signalling between plants.
ARBUSCULAR MYCORRHIZA AND HERBIVOROUS INSECT RESISTANCE
Arbuscular mycorrhizal fungi are reported to affect the performance of herbivores (Gange and West, 1994; Vicari et al., 2002; Pozo and Azcón-Aguilar, 2007; Gehring and Bennett, 2009; Borowicz, 2013). Arbuscular mycorrhiza symbioses adversely affect root-feeding insects, while their effects on leaf-feeding insects are variable (Pozo and Azcón-Aguilar, 2007; Gehring and Bennett, 2009; Borowicz, 2013). The extent of protection also changes with the feeding style of the attacking herbivore. Arbuscular mycorrhiza symbiosis seems to benefit phloem-sucking insects (aphids) (Gange et al., 1999; Koricheva et al., 2009), while effects on chewing and leaf-mining insects are largely adverse (Gange and West, 1994; Vicari et al., 2002; Hoffmann et al., 2009); counter-examples, however, also exist (Babikova et al., 2014b; Shrivastava et al., 2015). This considerable variation can be ascribed to some extent to the species (plant, fungus and herbivore) involved in the tripartite interactions (Bennett and Bever, 2007; Gange, 2007; Leitner et al., 2010; Pineda et al., 2010). Akin to the complexity of plant–herbivore–natural enemy tripartite interactions, AM also affects predators and parasitoids of herbivores (Gange et al., 2003; Guerrieri et al., 2004; Laird and Addicott, 2007).
Participating AM fungi may induce resistance to neighbouring plants, via hyphal networks functioning as plant–plant underground communication systems (Song et al., 2010; Babikova et al., 2013). Common mycorrhizal network serve as conduits facilitating the transfer of defence signals and also terpenoids between neighbouring plants under herbivore attack (Song et al., 2014).
ROLE OF TERPENOIDS IN AM-REINFORCED RESISTANCE AGAINST HERBIVOROUS INSECTS
The significance of below-ground interactions between plant and AM fungi for assessing VOC emission rates and their consequent ecological role in the deployment of indirect defences by plants has been emphasized (Rapparini et al., 2008). The indirect effect of AM on herbivore defence has been correlated to changes in the blend of terpenoids that alter plant attractiveness and insect behaviour (Babikova et al., 2014a). In Phaseolus challenged by spider mites, for example, AM symbiosis with Funneliformis mosseae increased the emission of β-ocimene and β-caryophyllene, resulting in increased attraction of predators of spider mites (Schausberger et al., 2012). Similarly, Shrivastava et al. (2015) observed a greater defence response against beet armyworm (Spodoptera exigua) in AM than in non-mycorrhizal plants, partly attributable to the difference in levels and blends of terpenoids. Arbuscular mycorrhiza formation led to enhanced levels of monoterpenes and sesquiterpenes, including monoterpenes such as myrcene, which were not detected in non-mycorrhizal plants. Myrcene is a semiochemical utilized by insects for communication, e.g. to deter thrips (Broughton and Harrison, 2012) or to attract aphidophagous hoverflies in a terrestrial orchid (Stökl et al., 2011).
EFFECTS OF ARBUSCULAR MYCORRHIZA ON TERPENOIDS
Arbuscular mycorrhiza symbiosis can affect a number of volatile organic compounds, including terpenes. Arbuscular mycorrhiza fungal colonization has been shown to enhance the production of triterpenoids (Akiyama and Hayashi, 2002), apocarotenoids (Klingner et al., 1995; Fester et al., 2002; Strack and Fester, 2006; Akiyama, 2007; Walter and Strack, 2011) and abscisic acid (Meixner et al., 2005) in roots of various plants. Systemic effects of AM on the quantity and quality of terpenoids in above-ground parts of plants have also been mooted (Kapoor et al., 2002a, b; Copetta et al., 2006; Khaosaad et al., 2006; Kapoor et al., 2007; Zubek et al., 2010; Weisany et al., 2015; Rydlová et al., 2016). These studies have so far been largely confined to the effects of AM on individual components of terpenoids or a suite of terpenoids (essential oil composition) that have pharmaceutical value. Arbuscular mycorrhiza may enhance the biosynthesis of an individual terpenoid either by increase in isoprene precursors through the induction of biosynthesis pathways and/or by induction of terpene synthase enzymes (Shrivastava et al., 2015). Increases in the level of substrates by enhanced P uptake and increased photosynthetic efficiency have been described (Wright et al., 1998a, b; Kapoor et al., 2002a, b; Rasouli-Sadaghiani et al., 2010). The role of P is perceptible in the synthesis of terpenoids both via the MVA pathway, which requires acetyl-CoA, ATP and NADPH, and via the MEP pathway, requiring glyceraldehyde phosphate and pyruvate, of which P is a constituent. Photosynthesis provides ATP and carbon substrate (glyceraldehyde-3-phosphate or pyruvate) for isoprene synthesis. Increased foliar biomass in AM plants results in greater photosynthetic capacity and thus increased production of total photosynthates required for terpenoid biosynthesis (Niinemets et al., 2002; Cao et al., 2008; Hofmeyer et al., 2010). However, P nutrition alone fails to explain terpenoid accumulation in AM fungus-colonized plants (Copetta et al., 2006; Khaosaad et al., 2006; Rydlová et al., 2016). This is not unanticipated, assuming that the biosynthesis of isoprene precursors is regulated by complex mechanisms, some of them independent of P nutrition (Kirby and Keasling, 2009; Vranova et al., 2012; Kumari et al., 2013).
The enzyme 1-deoxy-d-xylulose 5-phosphate synthase (DXS) catalyses the rate-limiting step of the MEP pathway. Walter et al. (2000) first demonstrated fungal-induced upregulation of DXS and DXR (1-deoxy-D-xylulose 5-phosphate reductoisomerase) transcript levels in AM-colonized roots of various cereals. This was followed by a series of reports on the upregulation of DXS transcripts in mycorrhizal roots of various plants (Hans et al., 2004; Strack and Fester, 2006; Floß et al., 2008). Transcription of genes encoding DXS and DXR enzymes is upregulated by AM symbiosis and correlated with quantitative terpenoid concentration in leaves (Mandal et al., 2015a). This increase in transcription and terpenoid content has been ascribed to an increased concentration of the phytohormone jasmonic acid (Mandal et al., 2015a; Nair et al., 2015) and/or improved mineral nutrient availability (Mandal et al., 2015a), and may therefore be influenced by both nutritional and non-nutritional mechanisms (Mandal et al., 2013). Results obtained so far suggest that the AM fungal-mediated increase in concentrations of terpenoids is due to enhanced production of IPP/DMAPP derived from the MEP pathway (Mandal et al., 2015a). There were have been no reports of AM-mediated changes in the MVA pathway in the literature until recently, when Venkateshwaran et al. (2015) reported that mevalonic acid is crucial for the transduction of symbiotic signals produced by AM fungi to induce symbiotic gene expression in plants.
Arbuscular mycorrhiza influences the concentration of specific terpenoids and their derivatives in plants by upregulating the transcription of downstream genes of the dedicated biosynthesis pathway (Mandal et al., 2015a, b). Induction of TPS family genes TPS31, TPS32 and TPS33 in mycorrhizal tomato (Zouari et al., 2014) further suggests a probable mechanism underlying the change in terpenoid profile observed in AM plants.
Glandular trichomes are one of the most common secretory structures that produce and accumulate terpenoids in plants (Karban and Baldwin, 1997; Van Schie et al., 2007; Kang et al., 2010; Schilmiller et al., 2010). A direct relation between augmented concentration of terpenoids and glandular trichome density has been observed in a number of plants (Ringer et al., 2005; Bartram et al., 2006; Behnam et al., 2006; Muñoz-Bertomeu et al., 2006). Correspondingly, an increase in trichome density upon colonization by AM fungi has often been proposed to augment concentration of terpenoids (Copetta et al., 2006; Kapoor et al., 2007; Morone-Fortunato and Avato, 2008). It was demonstrated in Artemisia annua that AM enhances glandular trichomes by inducing the transcription of TTG1 (transparent testa glabra 1), a transcription factor that acts at the top of the regulatory hierarchy of trichome development (Mandal et al., 2015a). However, continued studies are required to elucidate the mechanisms of enhanced production of glandular trichomes and further ascertain the role of phytohormones in AM plants.
CONCLUSIONS AND FUTURE PROSPECTS
The volatile nature of terpenoids confers the ability to act as efficient signalling molecules. Potential deployment in pest management practices in agriculture depends upon the efficient control of emission (augmenting or repressing) in plants (Vickers et al., 2014). Genetic manipulation of plants for terpenoid emission is a promising method to alter tritrophic interactions. In recent years, transgenic plants producing terpenoids have been used to repel herbivores (Aharoni et al., 2003), deter oviposition (McCallum et al., 2011) and attract predators (Bouwmeester et al., 2003; Kappers et al., 2005; Beale et al., 2006) and parasitoids (Schnee et al., 2006). The physiological cost of terpenoid production has been assumed to be minor, given their low molecular weight and the relatively low concentrations emitted (Dicke and Sabelis, 1990; Halitschke et al., 2000). On the other hand, a number of studies have demonstrated that constitutive transgenic production of terpenes can result in negative physiological effects on the plant (Aharoni et al., 2003; Robert et al., 2013). These effects may be manifested as stunted growth, reduced reproductive yield and also enhanced conspicuousness and attractiveness of plants to pests (Robert et al., 2013). Furthermore, constitutive emission of HIPVs by transgenic plants would render these emissions unreliable as cues for natural enemies that might waste hunting time in prey-free environments (Gish et al., 2015). Therefore, synchronized engineering strategies that consider herbivore-induced emissions are required to circumvent these cost effects. Further studies are required to evaluate the physiological and ecological costs of terpenoid manipulation in the field to determine the future of this approach for environmental pest management strategies (Robert et al., 2013).
Engineering of tritrophic interactions to successfully protect crop species requires consideration of a number of aspects (Bouwmeester et al., 2003; Degenhardt et al., 2003). For example, identification of an appropriate carnivore species for effective control of herbivore populations is required – one that is naturally present in the cultivation area and attracted by manipulating a known terpenoid (Vickers et al., 2014). Engineered emissions, however, should not attract other herbivores. The overall benefit of manipulated terpenoid emissions can be significantly enhanced by making the release inducible, by inserting a herbivore-inducible tissue-specific promoter with the terpene synthase gene (Degenhardt et al., 2009). Such controlled release would prevent the attraction of herbivores by healthy plants and would lead to recruitment of natural enemies only when the plant is attacked by herbivores (Robert et al., 2013). The lack of understanding of mechanisms by which plants recognize and respond to olfactory cues restricts the prospects for the utilization of terpenoids in crop plants. The highly simplified community structure of large-scale agricultural plantings is another challenge for the effective application of HIPVs, as natural enemy attraction may be ineffective in controlling pests in the core regions of large agricultural fields (Gish et al., 2015).
Alteration of the terpenoid profile in AM plants appears to be one of the important mechanisms for augmented defence against herbivorous insects. Different AM fungal species have variable effects on terpenoid blends (Kapoor et al., 2002b; Sailo and Bagyaraj, 2005; Arpana et al., 2008), and consequently likely differentially influence plant–herbivore and higher trophic level interactions. In this context, comparative studies using different AM fungal species are warranted, to enable differentiation of universal from species-specific responses, and also to identify those AM fungal species efficient in defence against specific herbivores. As the efficiency of AM symbiosis may be limited by nutrient availability in agricultural fields, comprehensive studies are also required to evaluate the relevance of AM symbiosis to herbivore defence under different nutrient regimes.
ACKNOWLEDGEMENTS
We are grateful to Professor R. Geeta, Professor Rajesh Tandon and Professor Sudheshna Mazumdar-Leighton for critically reviewing the manuscript and for their valuable comments. We thank the Research Council of the University of Delhi, Delhi, India, for financial assistance.
LITERATURE CITED
- Aharoni A, Giri AP, Deuerlein S, et al. 2003. Terpenoid metabolism in wild-type and transgenic Arabidopsis plants. The Plant Cell 15: 2866–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni A, Jongsma MA, Bouwmeester HJ.. 2005. Volatile science? Metabolic engineering of terpenoids in plants. Trends in Plant Science 10: 594–602. [DOI] [PubMed] [Google Scholar]
- Akiyama K. 2007. Chemical identification and functional analysis of apocarotenoids involved in the development of arbuscular mycorrhizal symbiosis. Bioscience, Biotechnology, and Biochemistry 71: 1405–1414. [DOI] [PubMed] [Google Scholar]
- Akiyama K, Hayashi H.. 2002. Arbuscular mycorrhizal fungus promoted accumulation of two new triterpenoids in cucumber roots. Bioscience, Biotechnology, and Biochemistry 66: 762–769. [DOI] [PubMed] [Google Scholar]
- Ali JG, Alborn HT, Campos-Herrera R, et al. 2011. Subterranean, herbivore induced plant volatile increases biological control activity of multiple beneficial nematode species in distinct habitats. PLoS One 7: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M, Sugimoto K, Ramadan A, Arimura G.. 2013. Memory of plant communications for priming anti-herbivore responses. Scientific Reports 3: 1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura G, Ozawa R, Shimoda T, Nishioka T, Boland W, Takabayashi J.. 2000a. Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature 406: 512–515. [DOI] [PubMed] [Google Scholar]
- Arimura G, Tashiro K, Kuhara S, Nishioka T, Ozawa R, Takabayashi J. 2000b. Gene responses in bean leaves induced by herbivory and by herbivore-induced volatiles. Biochemical and Biophysical Research Communications 277: 305–310. [DOI] [PubMed] [Google Scholar]
- Arimura G, Garms S, MaVei M, et al. 2008. Herbivore-induced terpenoid emission in Medicago truncatula: concerted action of jasmonate, ethylene and calcium signalling. Planta 227: 453–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura G, Matsui K, Takabayashi J.. 2009. Chemical and molecular ecology of herbivore-induced plant volatiles: proximate factors and their ultimate functions. Plant Cell Physiology 50: 911–923. [DOI] [PubMed] [Google Scholar]
- Arpana J, Bagyaraj DJ, Prakasa Rao EVS, Parameswaran TN, Abdul Rahiman BA.. 2008. Symbiotic response of patchouli [Pogostemon cablin (Blanco) Benth.] to different arbuscular mycorrhizal fungi. Advances in Environmental Biology 2: 20–24. [Google Scholar]
- Babikova Z, Gilbert L, Bruce TJA, et al.2013. Underground signals carried through common mycelial networks warn neighbouring plants of aphid attack. Ecology Letters 16: 835–843. [DOI] [PubMed] [Google Scholar]
- Babikova Z, Gilbert L, Randall KC, Bruce TJA, Pickett JA, Johnson D.. 2014a. Increasing phosphorus supply is not the mechanism by which arbuscular mycorrhiza increase attractiveness of bean (Vicia faba) to aphids. Journal of Experimental Botany 65: 5231–5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babikova Z, Gilbert L, Bruce TJA, Dewhirst S, Pickett JA, Johnson D.. 2014b. Arbuscular mycorrhizal fungi and aphids interact by changing host plant quality and volatile emission. Functional Ecology 28: 375–385. [Google Scholar]
- Baldwin IT, Schultz JC.. 1983. Rapid changes in tree leaf chemistry induced by damage: evidence for communication between plants. Science 221: 277–279. [DOI] [PubMed] [Google Scholar]
- Baldwin IT, Halitschke R, Paschold A, von Dahl CC, Preston CA.. 2006. Volatile signaling in plant-plant interactions: “talking trees” in the genomics era. Science 311: 812–815. [DOI] [PubMed] [Google Scholar]
- Bartram S, Jux A, Gleixner G, Boland W.. 2006. Dynamic pathway allocation in early terpenoid biosynthesis of stress-induced lima bean leaves. Phytochemistry 67: 1661–1672. [DOI] [PubMed] [Google Scholar]
- Beale MH, Birkett MA, Bruce TJ, et al.2006. Aphid alarm pheromone produced by transgenic plants affects aphid and parasitoid behavior. Proceedings of the National Academy of Sciences of the USA 103: 10509–10513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnam S, Farzaneh M, Ahmadzadeh M, Tehrani AS.. 2006. Composition and antifungal activity of essential oils of Mentha piperita and Lavendula angustifolia on post-harvest phytopathogens. Communications in Agriculture and Applied Biological Sciences 71: 1321–1326. [PubMed] [Google Scholar]
- Bennett AE, Bever JD.. 2007. Mycorrhizal species differentially alter plant growth and response to herbivory. Ecology 88: 210–218. [DOI] [PubMed] [Google Scholar]
- Beyaert I, Köpke D, Stiller J, Hammerbacher A, et al.2012. Can insect egg deposition ‘warn’ a plant of future feeding damage by herbivorous larvae? Proceedings of the Royal Society, B 279: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick JA, Lange BM.. 2003. Metabolic cross talk between cytosolic and plastidial pathways of isoprenoid biosynthesis: unidirectional transport of intermediates across the chloroplast envelope membrane. Archives of Biochemistry and Biophysics 415: 146–154. [DOI] [PubMed] [Google Scholar]
- Blande JD, Holopainen JK, Li T.. 2010. Air pollution impedes plant-to-plant communication by volatiles. Ecology Letters 13: 1172–1181. [DOI] [PubMed] [Google Scholar]
- De Boer JG, Posthumus MA, Dicke M.. 2004. Identification of volatiles that are used in discrimination between plants infested with prey or non-prey herbivores by a predatory mite. Journal of Chemical Ecology 30: 2215–2230. [DOI] [PubMed] [Google Scholar]
- Borowicz VA. 2013. The impact of arbuscular mycorrhizal fungi on plant growth following herbivory: a search for pattern. Acta Oecologica 52: 1–9. [Google Scholar]
- Bouwmeester HJ, Kappers IF, Verstappen FW, et al. 2003. Exploring multi-trophic plant–herbivore interactions for new crop protection methods In: Pickett J, ed. Proceedings of the BCPC International Congress: Crop Science and Technology. Alton: British Crop Protection Council, 1123–1134. [Google Scholar]
- Broughton S, Harrison J.. 2012. Evaluation of monitoring methods for thrips and the effect of trap colour and semiochemicals on sticky trap capture of thrips (Thysanoptera) and beneficial insects (Syrphidae, Hemerobiidae) in deciduous fruit trees in Western Australia. Crop Protection 42: 156–163. [Google Scholar]
- Brunetti C, George RM, Tattini M, Field K, Davely MP.. 2013. Metabolics in plant environmental physiology. Journal of Experimental Botany 64: 4011–4020. [DOI] [PubMed] [Google Scholar]
- Büchel KK, Malskies S, Mayer M, et al. 2011. How plants give early herbivore alert: volatile terpenoids emitted from elm attract egg parasitoids to plants laden with eggs of the elm leaf beetle. Basic and Applied Ecology 12: 403–412. [Google Scholar]
- Cao B, Dang QL, Yü X, Zhang S.. 2008. Effects of [CO2] and nitrogen on morphological and biomass traits of white birch (Betula papyrifera) seedlings. Forest Ecology Management 254: 217–224. [Google Scholar]
- Chen F, Tholl D, Bohlmann J, Pichersky E.. 2011. The family of terpene synthases in plants: a mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. The Plant Journal 66: 212–229. [DOI] [PubMed] [Google Scholar]
- Cheng AX, Lou YG, Mao YB, Lu S, Wang LJ, Chen XY.. 2007a. Plant terpenoids: biosynthesis and ecological functions. Journal of Integrative Plant Biology 49: 179−186. [Google Scholar]
- Cheng AX, Xiang CY, Li JX, et al. 2007b. The rice (E)-β-caryophyllene synthase (OsTPS3) accounts for the major inducible volatile sesquiterpenes. Phytochemistry 68: 1632–1641. [DOI] [PubMed] [Google Scholar]
- Conrath U, Beckers GJM, Flors V, et al. 2006. Priming: getting ready for battle. Molecular Plant-Microbe Interactions 19: 1062–1071. [DOI] [PubMed] [Google Scholar]
- Conti E, Zadra C, Salerno G, et al. 2008. Changes in the volatile profile of Brassica oleracea due to feeding and oviposition by Murgantia histrionica (Heteroptera: Pentatomidae). European Journal of Entomology 105: 839–847. [Google Scholar]
- Copetta A, Lingua G, Berta G.. 2006. Effects of three AM fungi on growth, distribution of glandular hairs, and essential oil production in Ocimum basilicum L. Var. Genovese. Mycorrhiza 16: 485–494. [DOI] [PubMed] [Google Scholar]
- Das A, Lee SH, Hyun TK, Kim SW, Kim JY.. 2013. Plant volatiles as method of communication. Plant Biotechnology Reports 7: 9–26. [Google Scholar]
- Degenhardt J, Gershenzon J, Baldwin IT, Kessler A.. 2003. Attracting friends to feast on foes: engineering terpene emission to make crop plants more attractive to herbivore enemies. Current Opinion in Biotechnology 14: 169–176. [DOI] [PubMed] [Google Scholar]
- Degenhardt J, Hiltpold I, Köllner TG, et al. 2009. Restoring a maize root signal that attracts insect-killing nematodes to control a major pest. Proceedings of the National Academy of Sciences of the USA 106: 13213–13218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delory BM, Delaplace P, Fauconnier ML, du Jardin P.. 2016. Root-emitted volatile organic compounds: can they mediate belowground plant-plant interactions? Plant and Soil 402: 1–26, in press. doi:10.1007/s11104-016-2823-3. [Google Scholar]
- Dicke M, Sabelis MW.. 1990. Does it pay plants to advertize for bodyguards? Towards a cost-benefit analysis of induced synomone production In: Lambers H, Cambridge ML, Konings H, Pons TL, eds. Causes and consequences of variation in growth rate and productivity of higher plants. The Hague: SPB Academic Publishing, 341–358. [Google Scholar]
- Dicke M, Vanbeek TA, Posthumus MA, et al.1990. Isolation and identification of volatile kairomone that affects acarine predator-prey interactions – involvement of host plant in its production. Journal of Chemical Ecology 16: 381–396. [DOI] [PubMed] [Google Scholar]
- Dicke M, Gols R, Ludeking D, Posthumus MA.. 1999. Jasmonic acid and herbivory differentially induce carnivore-attracting plant volatiles in lima bean plants. Journal of Chemical Ecology 25: 1907–1922. [Google Scholar]
- Dicke M, van Loon JJA, Soler R.. 2009. Chemical complexity of volatiles from plants induced by multiple attack. Nature 5: 317–324. [DOI] [PubMed] [Google Scholar]
- Dudareva N, Negre F, Nagegowda DA, Orlova I.. 2006. Plant volatiles: recent advances and future perspectives. Critical Reviews in Plant Sciences 25: 417–440. [Google Scholar]
- Dudareva N, Klempien A, Muhlemann K, Kaplan I.. 2013. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytologist 198: 16–32. [DOI] [PubMed] [Google Scholar]
- Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH.. 2004. Airborne signals prime plants against insect herbivore attack. Proceedings of the National Academy of Sciences of the USA 10: 1781–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb M, Ton J, Degenhardt J, Turlings TC.. 2008. Interactions between arthropod-induced aboveground and belowground defenses in plants. Plant Physiology 146: 867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb M, Veyrat N, Robert CAM, et al. 2015. Indole is an essential herbivore-induced volatile priming signal in maize. Nature Communications 6: 6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evelin H, Giri B, Kapoor R.. 2013. Ultrastructural evidence for AMF mediated salt stress mitigation in Trigonella foenum-graecum. Mycorrhiza 23: 71–86. [DOI] [PubMed] [Google Scholar]
- Farag MA, Paré PW.. 2002. C-6-green leaf volatiles trigger local and systemic VOC emissions in tomato. Phytochemistry 61: 545–554. [DOI] [PubMed] [Google Scholar]
- Fester T, Schmidt D, Lohse S, et al. 2002. Stimulation of carotenoid metabolism in arbuscular mycorrhizal roots. Planta 216: 148–154. [DOI] [PubMed] [Google Scholar]
- Finlay RD. 2008. Ecological aspects of mycorrhizal symbiosis: with special emphasis on the functional diversity of interactions involving the extraradical mycelium. Journal of Experimental Botany 59: 1115–1126. [DOI] [PubMed] [Google Scholar]
- Floβ DS, Hause B, Lange PR, Küster H, Strack D, Walter MH.. 2008. Knock-down of the MEP pathway isogene 1-deoxy-d-xylulose 5-phosphate synthase 2 inhibits formation of arbuscular mycorrhiza-induced apocarotenoids, and abolishes normal expression of mycorrhiza-specific plant marker genes. The Plant Journal 56: 86–100. [DOI] [PubMed] [Google Scholar]
- Frost CJ, Appel HM, Carlson JE, De Moraes CM, Mescher MC, Schultz JC.. 2007. Within-plant signalling by volatiles overcomes vascular constraints on systemic signalling and primes responses against herbivores. Ecology Letters 10: 490–498. [DOI] [PubMed] [Google Scholar]
- Frost CJ, Mescher MC, Dervinis C, Davis JM, Carlson JE, De Moraes CM.. 2008a. Priming defense genes and metabolites in hybrid poplar by the green leaf volatile cis‐3‐hexenylacetate. New Phytologist 180: 722–734. [DOI] [PubMed] [Google Scholar]
- Frost CJ, Mescher MC, Carlson JE, De Moraes CM.. 2008b. Plant defense priming against herbivores: getting ready for a different battle. Plant Physiology 146: 818–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gange AC. 2007. Insect-mycorrhizal interactions: patterns, processes and consequences In: Oghushi T, Craig TP, Price PW, eds. Ecological communities: plant mediation in indirect interactions. Cambridge: Cambridge University Press, 124–144. [Google Scholar]
- Gange AC, West HM.. 1994. Interactions between arbuscular mycorrhizal fungi and foliar-feeding insects in Plantago lanceolata L. New Phytologist 128: 79–87. [DOI] [PubMed] [Google Scholar]
- Gange AC, Bower E, Brown VK.. 1999. Positive effects of an arbuscular mycorrhizal fungus on aphid life history traits. Oecologia 120: 123–131. [DOI] [PubMed] [Google Scholar]
- Gange AC, Brown VK, Aplin MA.. 2003. Multitrophic links between arbuscular mycorrhizal fungi and insect parasitoids. Ecology Letters 6: 1051–1055. [Google Scholar]
- Gehring C, Bennett A.. 2009. Mycorrhizal fungal–plant–insect interactions: the importance of a community approach. Environmental Entomology 38: 93–102. [DOI] [PubMed] [Google Scholar]
- Gershenzon J. 1994. Metabolic costs of terpenoid accumulation in higher plants. Journal of Chemical Ecology 20: 1281–1328. [DOI] [PubMed] [Google Scholar]
- Gershenzon J, Dudareva N.. 2007. The function of terpene natural products in the natural world. Nature Chemical Biology 3: 408–414. [DOI] [PubMed] [Google Scholar]
- Gish M, De Moraes CM, Mescher MC.. 2015. Herbivore-induced plant volatiles in natural and agricultural ecosystems: open questions and future prospects. Current Opinion in Insect Science 9: 1–6. [DOI] [PubMed] [Google Scholar]
- Gouinguené SP, Turlings TCJ.. 2002. The effects of abiotic factors on induced volatile emissions in corn plants. Plant Physiology 129: 1296–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrieri E, Lingua G, Digilio MC, Massa N, Berta G.. 2004. Do interactions between plant roots and the rhizosphere affect parasitoid behaviour? Ecological Entomology 29: 753–756. [Google Scholar]
- Halitschke R, Keßler A, Kahl J, Lorenz A, Baldwin IT.. 2000. Ecophysiological comparison of direct and indirect defenses in Nicotiana attenuata. Oecologia 124: 408–417. [DOI] [PubMed] [Google Scholar]
- Hans J, Hause B, Strack D, Walter MH.. 2004. Cloning, characterization, and immunolocalization of a mycorrhiza-inducible 1-deoxy-d-xylulose 5-phosphate reductoisomerase in arbuscule-containing cells of maize. Plant Physiology 134: 614–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison MJ. 2005. Signaling in the arbuscular mycorrhizal symbiosis. Annual Review of Microbiology 59: 19–42. [DOI] [PubMed] [Google Scholar]
- Heil M. 2008. Indirect defence via tritrophic interactions. New Phytologist 178: 41–61. [DOI] [PubMed] [Google Scholar]
- Heil M, Kost C.. 2006. Priming of indirect defences. Ecology Letters 9: 813–817. [DOI] [PubMed] [Google Scholar]
- Heil M, Silva Bueno JC.. 2007. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proceedings of the National Academy of Sciences of the USA 104: 5467–5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M, Ton J.. 2008. Long-distance signalling in plant defence. Trends in Plant Science 13: 264–272. [DOI] [PubMed] [Google Scholar]
- Hilker M, Fatouros NE.. 2015. Plant responses to insect egg deposition. Annual Review of Entomology 60: 493–515. [DOI] [PubMed] [Google Scholar]
- Hilker M, Kobs C, Varama M, Schrank K.. 2002. Insect egg deposition induces Pinus sylvestris to attract egg parasitoids. Journal of Experimental Biology 205: 455–461. [DOI] [PubMed] [Google Scholar]
- Hiltpold I, Turlings TCJ.. 2008. Belowground chemical signaling in maize: when simplicity rhymes with efficiency. Journal of Chemical Ecology 34: 628–635. [DOI] [PubMed] [Google Scholar]
- Hoffmann D, Vierheilig H, Riegler P, Schausberger P.. 2009. Arbuscular mycorrhizal symbiosis increases host plant acceptance and population growth rates of the two-spotted spider mite Tetranychus urticae. Oecologia 158: 663–671.. [DOI] [PubMed] [Google Scholar]
- Hofmeyer PV, Seymour RS, Kenefic LS.. 2010. Production ecology of Thuja occidentalis. Canadian Journal of Forest Research 40: 1155–1164. [Google Scholar]
- Hohnjec N, Vieweg MF, Pühler A, Becker A, Küster H.. 2005. Overlaps in the transcriptional profiles of Medicago truncatula roots inoculated with two different glomus fungi provide insights into the genetic program activated during arbuscular mycorrhiza. Plant Physiology 137: 1283–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SC, Martinez-Medina A, Lopez-Raez JA, Pozo MJ.. 2012. Mycorrhiza-induced resistance and priming of plant defenses. Journal of Chemical Ecology 38: 651–664. [DOI] [PubMed] [Google Scholar]
- Kang JH, Shi F, Jones AD, Marks MD, Howe GA.. 2010. Distortion of trichome morphology by the hairless mutation of tomato affects leaf surface chemistry. Journal of Experimental Botany 61: 1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant MR, Ament K, Sabelis MW, Haring MA, Schuurink RC.. 2004. Differential timing of spider mite-induced direct and indirect defenses in tomato plants. Plant Physiology 135: 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappers IF, Aharoni A, Van Herpen TWJM, Luckerhoff LLP, Dicke M, Bouwmeester HJ.. 2005. Genetic engineering of terpenoid metabolism attracts, bodyguards to Arabidopsis. Science 309: 2070–2072. [DOI] [PubMed] [Google Scholar]
- Kapoor R, Giri B, Mukerji KG.. 2002a. Glomus macrocarpum: a potential bioinoculant to improve essential oil quality and concentration in dill (Anethum graveolens L.) and carum (Trachyspermum ammi (Linn.) Sprague). World Journal of Microbiology and Biotechnology 18: 459–463. [Google Scholar]
- Kapoor R, Giri B, Mukerji KG.. 2002b. Mycorrhization of coriander (Coriandrum sativum L.) to enhance the concentration and quality of essential oil. Journal of the Science Food and Agriculture 88: 1–4. [Google Scholar]
- Kapoor R, Chaudhary V, Bhatnagar AK.. 2007. Effects of arbuscular mycorrhiza and phosphorus application on artemisinin concentration in Artemisia annua L. Mycorrhiza 17: 581–587. [DOI] [PubMed] [Google Scholar]
- Karban R, Baldwin IT.. 1997. Induced responses to herbivory. Chicago: University of Chicago Press. [Google Scholar]
- Karban R, Maron J.. 2002. The fitness consequences of interspecific eavesdropping between plants. Ecology 83: 1209–1213. [Google Scholar]
- Karban R, Baldwin IT, Baxter KJ, Laue G, Felton GW.. 2000. Communication between plants: induced resistance in wild tobacco plants following clipping of neighboring sagebrush. Oecologia 125: 66–71. [DOI] [PubMed] [Google Scholar]
- Karban R, Shiojiri K, Huntzinger M, McCall AC.. 2006. Damage-induced resistance in sagebrush: volatiles are key to intra- and interplant communication. Ecology 87: 922–930. [DOI] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT.. 2001. Defensive function of herbivore induced plant volatile emissions in nature. Science 291: 2141–2144. [DOI] [PubMed] [Google Scholar]
- Khaosaad T, Vierheilig H, Nell M, Zitterl-Eglseer K, Novak J.. 2006. Arbuscular mycorrhiza alter the concentration of essential oils in oregano (Origanum sp., Lamiaceae). Mycorrhiza 6: 443–446. [DOI] [PubMed] [Google Scholar]
- Kirby J, Keasling JD.. 2009. Biosynthesis of plant isoprenoids: perspectives for microbial engineering. Annual Review of Plant Biology 60: 335–355. [DOI] [PubMed] [Google Scholar]
- Klingner A, Bothe H, Wray V, Marner FJ.. 1995. Identification of a yellow pigment formed in maize roots upon mycorrhizal colonization. Phytochemistry 38: 53–55. [Google Scholar]
- Köpke D, Beyaert I, Gershenzon J, Hilker M, Schmidt A.. 2010. Species-specific responses of pine sesquiterpene synthases to sawfly oviposition. Phytochemistry 71: 909–917. [DOI] [PubMed] [Google Scholar]
- Koricheva J, Gange AC, Jones T.. 2009. Effects of mycorrhizal fungi on insect herbivores: a meta-analysis. Ecology 90: 2088–2097. [DOI] [PubMed] [Google Scholar]
- Kost C, Heil M.. 2006. Herbivore-induced plant volatiles induce an indirect defence in neighbouring plants. Journal of Ecology 94: 619–628. [Google Scholar]
- Kumari S, Priya P, Misra G, Yadav G.. 2013. Structural and biochemical perspectives in plant isoprenoid biosynthesis. Phytochemistry Reviews 12: 255–291. [Google Scholar]
- Langenheim JH. 1994. Higher plant terpenoids: a phytocentric overview of their ecological roles. Journal of Chemical Ecology 20: 1223–1280. [DOI] [PubMed] [Google Scholar]
- Laird RA, Addicott JF.. 2007. Arbuscular mycorrhizal fungi reduce the construction of extrafloral nectaries in Vicia faba. Oecologia 152: 541–551. [DOI] [PubMed] [Google Scholar]
- Laothawornkitkul J, Paul ND, Vickers CE, et al. 2008. Isoprene emissions influence herbivore-feeding decisions. Plant, Cell & Environment 31: 1410–1415. [DOI] [PubMed] [Google Scholar]
- Leitner M, Boland W, Mithöfer A.. 2005. Direct and indirect defences induced by piercing-sucking and chewing herbivores in Medicago truncatula. New Phytologist 167: 597–606. [DOI] [PubMed] [Google Scholar]
- Leitner M, Kaiser R, Hause B, Boland W, Mithöfer A.. 2010. Does mycorrhization influence herbivore-induced volatile emission in Medicago truncatula? Mycorrhiza 20: 89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Schnitzler JP.. 2010. Abiotic stresses and induced BVOCs. Trends in Plant Science 15: 154–166. [DOI] [PubMed] [Google Scholar]
- Maffei ME. 2010. Sites of synthesis, biochemistry and functional role of plant volatiles. South African Journal of Botany 76: 612–631. [Google Scholar]
- Mandal S, Evelin H, Giri B, Singh VP, Kapoor R.. 2013. Arbuscular mycorrhiza enhances the production of stevioside and rebaudioside-A in Stevia rebaudiana via nutritional and non-nutritional mechanisms. Applied Soil Ecology 72: 187–194. [Google Scholar]
- Mandal S, Upadhyay S, Wajid S, et al. 2015a. Arbuscular mycorrhiza increase artemisinin accumulation in Artemisia annua by higher expression of key biosynthesis genes via enhanced jasmonic acid levels. Mycorrhiza 25: 345–357. [DOI] [PubMed] [Google Scholar]
- Mandal S, Upadhyay S, Singh VP, Kapoor R.. 2015b. Enhanced production of steviol glycosides in mycorrhizal plants: a concerted effect of arbuscular mycorrhizal symbiosis on transcription of biosynthetic genes. Plant Physiology and Biochemistry 89:100–106. [DOI] [PubMed] [Google Scholar]
- Mathur V, Tytgat TOG, Hordijk CA, et al. 2013. An ecogenomic analysis of herbivore-induced plant volatiles in Brassica juncea. Molecular Ecology 22: 6179–6196. [DOI] [PubMed] [Google Scholar]
- McCallum EJ, Cunningham JP, Lücker J, Zalucki MP, De Voss JJ, Botella JR.. 2011. Increased plant volatile production affects oviposition, but not larval development, in the moth Helicoverpa armigera. Journal of Experimental Biology 214: 3672–3677. [DOI] [PubMed] [Google Scholar]
- Meixner C, Ludwig-Muller J, Miersch O, Gresshoff P, Staehelin C, Vierheilig H.. 2005. Lack of mycorrhizal autoregulation and phytohormonal changes in the super nodulating soybean mutants nts1007. Planta 222: 709–715. [DOI] [PubMed] [Google Scholar]
- Mercke P, Kappers IF, Francel WAV, Oscar V, Marcel D, Harro JB.. 2004. Combined transcript and metabolite analysis reveals genes involved in spider mite induced volatile formation in cucumber plants. Plant Physiology 135: 2012–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MH, McGonigle TP, Addy HD.. 1995. Functional ecology of vesicular arbuscular mycorrhizas as influenced by phosphate fertilization and tillage in an agricultural ecosystem. Critical Reviews in Biotechnology 15: 241–255. [Google Scholar]
- Miransari M. 2010. Contribution of arbuscular mycorrhizal symbiosis to plant growth under different types of soil stress. Plant Biology 12: 563–569. [DOI] [PubMed] [Google Scholar]
- Mithöfer A, Boland W.. 2012. Plant defense against herbivores: chemical aspects. Annual Review of Plant Biology 63: 431–450. [DOI] [PubMed] [Google Scholar]
- Morone-Fortunato I, Avato P.. 2008. Plant development and synthesis of essential oils in micropropagated and mycorrhiza inoculated plants of Origanum vulgare L. ssp. hirtum (Link) Ietswaart. Plant Cell Tissue and Organ Culture 93: 139. [Google Scholar]
- Mumm R, Hilker M.. 2005. The significance of background odour for an egg parasitoid to detect plants with host eggs. Chemical Senses 30: 337–343. [DOI] [PubMed] [Google Scholar]
- Mumm R, Hilker M.. 2006. Direct and indirect chemical defence of pine against folivorous insects. Trends in Plant Science 11: 351–358. [DOI] [PubMed] [Google Scholar]
- Mumm R, Posthumus MA, Dicke M.. 2008. Significance of terpenoids in induced indirect plant defence against herbivorous arthropods. Plant, Cell & Environment 31: 575–585. [DOI] [PubMed] [Google Scholar]
- Muñoz-Bertomeu J, Arrillaga I, Ros R, Segura J.. 2006. Up-regulation of 1-deoxy-d-xylulose-5-phosphate synthase enhances production of essential oils in transgenic spike lavender. Plant Physiology 142: 890–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroi A, Ramadan A, Nishihara M.. 2011. The composite effect of transgenic plant volatiles for acquired immunity to herbivory caused by inter-plant communications. PLoS One 6: e24594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Kolet SP, Thulasiram HV, Bhargava S.. 2015. Systemic jasmonic acid modulation in mycorrhizal tomato plants and its role in induced resistance against Alternaria alternata. Plant Biology 17: 625–631. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Seufert G, Steinbrecher R, Tenhunen JD.. 2002. A model coupling foliar monoterpene emissions to leaf photosynthetic characteristics in Mediterranean Quercus species. New Phytologist 153: 257–275. [Google Scholar]
- Owen S, Peñuelas J.. 2005. Opportunistic emissions of volatile isoprenoids. Trends in Plant Science 10: 420–426. [DOI] [PubMed] [Google Scholar]
- Palma R, Mutis A, Manosalva L, et al.2012. Behavioral and electrophysiological responses of Hylastinus obscurus to volatiles released from the roots of Trifolium pratense L. Journal of Soil Science and Plant Nutrition 12: 183–193. [Google Scholar]
- Paré PW, Tumlinson JH.. 1999. Plant volatiles as a defense against insect herbivores. Plant Physiology 121: 325–332. [PMC free article] [PubMed] [Google Scholar]
- Park SW, Kaimoyo E, Kumar D, Mosher S, Klessig DF.. 2007. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 318: 113–116. [DOI] [PubMed] [Google Scholar]
- Parniske M. 2008. Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nature Reviews. Microbiology 6: 763–775. [DOI] [PubMed] [Google Scholar]
- Paschold A, Halitschke R, Baldwin IT.. 2006. Using ‘mute’ plants to translate volatile signals. The Plant Journal 45: 275–291. [DOI] [PubMed] [Google Scholar]
- Pashalidou FG, Gols R, Berkhout BW, et al.2015. To be in time: egg deposition enhances plant-mediated detection of young caterpillars by parasitoids. Oecologia 177: 477–486. [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Munné-Bosch S.. 2005. Isoprenoids: an evolutionary pool for photoprotection. Trends in Plant Science 10: 166–169. [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Asensio D, Tholl D, et al. 2014. Biogenic volatile emissions from the soil. Plant, Cell & Environment 37: 1866–1891. [DOI] [PubMed] [Google Scholar]
- Pichersky E, Noel JP, Dudareva N.. 2006. Biosynthesis of plant volatiles: nature's diversity and ingenuity. Science 311: 808–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel J, Donath J, Bandemer K, Boland W.. 1998. Mevalonate-independent biosynthesis of terpenoid volatiles in plants: induced and constitutive emission of volatiles. Angewandte Chemie, International Edition 37: 2478–2481. [DOI] [PubMed] [Google Scholar]
- Pineda A, Zheng SJ, van Loon JJA, Pieterse CMJ, Dicke M.. 2010. Helping plants to deal with insects: the role of beneficial soil-borne microbes. Trends in Plant Science 15: 507–514. [DOI] [PubMed] [Google Scholar]
- Pozo MJ, Azcón-Aguilar C.. 2007. Unraveling mycorrhiza-induced resistance. Current Opinion in Plant Biology 10: 393–398. [DOI] [PubMed] [Google Scholar]
- Puente ME, Kennedy GG, Gould F.. 2008. The impact of herbivore-induced plant volatiles on parasitoid foraging success: a general deterministic model. Journal of Chemical Ecology 34: 945–958. [DOI] [PubMed] [Google Scholar]
- Ramadan A, Muroi A, Arimura G.. 2011. Herbivore-induced maize volatiles serve as priming cues for resistance against post-attack by the specialist armyworm Mythimna separata. Journal of Plant Interactions 6: 155–158. [Google Scholar]
- Rapparini F, Llusia J, Peñuelas J.. 2008. Effect of arbuscular mycorrhizal (AM) colonization on terpene emission and content of Artemisia annua L. Plant Biology 10: 108–122. [DOI] [PubMed] [Google Scholar]
- Rasmann S, Turlings TC.. 2007. Simultaneous feeding by aboveground and belowground herbivores attenuates plant-mediated attraction of their respective natural enemies. Ecology Letters 10: 926–936. [DOI] [PubMed] [Google Scholar]
- Rasmann S, Turlings TC.. 2008. First insights into specificity of below ground tritrophic interactions. Oikos 117: 362–369. [Google Scholar]
- Rasmann S, Köllner TG, Degenhardt J, et al. 2005. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 434: 732–737. [DOI] [PubMed] [Google Scholar]
- Rasmann S, Hiltpold I, Ali J.. 2012. The role of root-produced volatile secondary metabolites in mediating soil interactions In: Montanaro G, Bartolomeo D, eds. Advances in selected plant physiology aspects. Rijeka: InTech, 269– 290. [Google Scholar]
- Rasouli-Sadaghiani MH, Hassani A, Barin M, Danesh YR, Sefidkon F.. 2010. Effects of arbuscular mycorrhizal (AM) fungi on growth, essential oil production and nutrients uptake in basil. Journal of Medicinal Plants Research 4: 2222–2228. [Google Scholar]
- Ringer KL, Davis EM, Croteau R.. 2005. Monoterpene metabolism. Cloning, expression, and characterization of (−)-isopiperitenol/(−)-carveol dehydrogenase of peppermint and spearmint. Plant Physiology 137: 863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert CAM, Veyrat N, Glauser G, et al.2012. A specialist root herbivore exploits defensive metabolites to locate nutritious tissues. Ecology Letters 15: 55–64. [DOI] [PubMed] [Google Scholar]
- Robert CAM, Erb M, Hiltpoldt I, et al. 2013. Genetically engineered maize plants reveal distinct costs and benefits of constitutive volatile emissions in the field. Plant Biotechnology Journal 11: 628–639. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Concepcion M. 2006. Early steps in isoprenoid biosynthesis: multilevel regulation of the supply of common precursors in plant cells. Phytochemistry Reviews 5: 1–15. [Google Scholar]
- Ruiz-Lozano JM, Porcel R, Azcón C, Aroca R.. 2012. Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: new challenges in physiological and molecular studies. Journal of Experimental Botany 63: 4033–4044. [DOI] [PubMed] [Google Scholar]
- Ruther J, Fürstenau B.. 2005. Emission of herbivore-induced volatiles in absence of a herbivore – response of Zea mays to green leaf volatiles and terpenoids. Zeitschrift für Naturforschung C 60: 743–756. [DOI] [PubMed] [Google Scholar]
- Ruther J, Kleier S.. 2005. Plant-plant signaling: ethylene synergizes volatile emission in Zea mays induced by exposure to (Z)-3-hexen-1-ol. Journal of Chemical Ecology 31: 2217–2222. [DOI] [PubMed] [Google Scholar]
- Rydlová J, Jelínková M, Karel Dušek K, Dušková E, Miroslav Vosátka M, Püschel D.. 2016. Arbuscular mycorrhiza differentially affects synthesis of essential oils in coriander and dill. Mycorrhiza 26:123–131. [DOI] [PubMed] [Google Scholar]
- Sailo GL, Bagyaraj DJ.. 2005. Influence of different AM fungi on the growth, nutrition and forskolin content of Coleus forskohlii. Mycological Research 109: 795–798. [DOI] [PubMed] [Google Scholar]
- Schausberger P, Peneder S, Jürschik S, Hoffmann D.. 2012. Mycorrhiza changes plant volatiles to attract spider mite enemies. Functional Ecology 26: 441–449. [Google Scholar]
- Schenkel D, Lemfack MC, Piechulla B, Splivallo R.. 2015. A meta-analysis approach for assessing the diversity and specificity of belowground root and microbial volatiles. Frontiers in Plant Science 6: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilmiller A, Shi F, Kim J, et al. 2010. Mass spectrometry screening reveals widespread diversity in trichome specialized metabolites of tomato chromosomal substitution lines. The Plant Journal 62: 391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnee C, Kollner TG, Held M, Turlings TC, Gershenzon J, Degenhardt J.. 2006. The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proceedings of the National Academy of Sciences of the USA 103: 1129–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda T, Ozawa R, Sano K, Yano E, Takabayashi J.. 2005. The involvement of volatile infochemicals from spider mites and from food-plants in prey location of the generalist predatory mite Neoseiulus californicus. Journal of Chemical Ecology 31: 2019–2032. [DOI] [PubMed] [Google Scholar]
- Shrivastava G, Ownley BH, Augé RM, et al. 2015. Colonization by arbuscular mycorrhizal and endophytic fungi enhanced terpene production in tomato plants and their defense against a herbivorous insect. Symbiosis 65: 65–74. [Google Scholar]
- Shulaev V, Silverman P, Raskin I.. 1997. Airborne signalling by methyl salicylate in plant pathogen resistance. Nature 385: 718–721. [Google Scholar]
- Smith SE, Gianinazzi-Pearson V.. 1988. Physiological interactions between symbionts in vesicular-arbuscular mycorrhizal plants. Annual Review of Plant Physiology and Plant Molecular Biology 39: 221–244. [Google Scholar]
- Smith SE, Read DJ.. 2008. Mycorrhizal symbiosis, 4th edn.London: Academic Press. [Google Scholar]
- Song YY, Zeng SR, Xu JF.. 2010. Interplant communication of tomato plants through underground common mycorrhizal networks. PLoS One 5: e13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YY, Ye M, Li CY, et al. 2014. Hijacking common mycorrhizal networks for herbivore-induced defence signal transfer between tomato plants. Science Reports 4: 3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stökl J, Brodmann J, Dafni A, Ayasse M, Hansson BS.. 2011. Smells like aphids: orchid flowers mimic aphid alarm pheromones to attract hoverflies for pollination. Proceedings of the Royal Society, B 278: 1216–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack D, Fester T.. 2006. Isoprenoid metabolism and plastid reorganization in arbuscular mycorrhizal roots. New Phytologist 172: 22–34. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Matsui K, Iijima Y, et al. 2014. Intake and transformation to a glycoside of (Z)-3-hexenol from infested neighbors reveals a mode of plant odor reception and defense. Proceedings of the National Academy of Sciences of the USA 111: 7144–7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabayashi J, Dicke M.. 1996. Plant-carnivore mutualism through herbivore-induced carnivore attractants. Trends in Plant Science 1: 109–113. [Google Scholar]
- Tholl D. 2006. Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Current Opinion in Plant Biology 9: 297–304. [DOI] [PubMed] [Google Scholar]
- Tholl D, Sohrabi R, Huh JH, Lee S.. 2011. The biochemistry of homoterpenes – common constituents of floral and herbivore-induced plant volatile bouquets. Phytochemistry 72: 1635–1646. [DOI] [PubMed] [Google Scholar]
- Ton J, D'Alessandro M, Jourdie V. et al. 2006. Priming by airborne signals boosts direct and indirect resistance in maize. The Plant Journal 49: 16–26. [DOI] [PubMed] [Google Scholar]
- Tscharntke T, Thiessen S, Dolch R, Boland W.. 2001. Herbivory, induced resistance and interplant signal transfer in Alnus glutinosa. Biochemical Systematics and Ecology 29: 1025–1047. [Google Scholar]
- Unsicker SB, Kunert G, Gershenzon J.. 2009. Protective perfumes: the role of vegetative volatiles in plant defense against herbivores. Current Opinion in Plant Biology 12: 479–485. [DOI] [PubMed] [Google Scholar]
- Van Schie CCN, Haring MA, Schuurink RC.. 2007. Tomato linalool synthase is induced in trichomes by jasmonic acid. Plant Molecular Biology 64: 251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkateshwaran M. D, Chabaud M, et al. 2015. A role for the mevalonate pathway in early plant symbiotic signaling. Proceedings of the National Academy of Sciences of the USA 112: 9781–9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari M, Hatcher PE, Ayres PG.. 2002. Combined effect of foliar and mycorrhizal endophytes on an insect herbivore. Ecology 83: 2452–2464. [Google Scholar]
- Vickers CE, Bongers M, Liu Q, Delatte T, Bouwmeester H.. 2014. Metabolic engineering of volatile isoprenoids in plants and microbes. Plant Cell and Environment 37: 1753–1775. [DOI] [PubMed] [Google Scholar]
- Vranova E, Coman D, Gruissem W.. 2012. Structure and dynamics of the isoprenoid pathway network. Molecular Plant 5: 318–333. [DOI] [PubMed] [Google Scholar]
- Walker V, Couillerot O, Von Felten A, et al. 2012. Variation of secondary metabolite levels in maize seedling roots induced by inoculation with Azospirillum, Pseudomonas and Glomus consortium under field conditions. Plant Soil 356: 151–163. [Google Scholar]
- Walter MH, Strack D.. 2011. Carotenoids and their cleavage products: biosynthesis and functions. Natural Product Reports 28: 663–692. [DOI] [PubMed] [Google Scholar]
- Walter MH, Fester T, Strack D.. 2000. Arbuscular mycorrhizal fungi induce the non-mevalonate methylerythritol phosphate pathway of isoprenoid biosynthesis correlated with accumulation of the ‘yellow pigment’ and other apocarotenoids. The Plant Journal 21: 571–578. [DOI] [PubMed] [Google Scholar]
- Wegener R, Schulz S.. 2002. Identification and synthesis of homoterpenoids emitted from elm leaves after elicitation by beetle eggs. Tetrahedron 58: 315–319. [Google Scholar]
- Weisany W, Raei Y, Pertot I.. 2015. Changes in the essential oil yield and composition of dill (Anethum graveolens L.) as response to arbuscular mycorrhiza colonization and cropping system. Industrial Crops and Products 77: 295–306. [Google Scholar]
- Wright DP, Scholes JD, Read DJ.. 1998a. Effects of VA mycorrhizal colonization on photosynthesis and biomass production of Trifolium repens L. Plant, Cell & Environment 21: 209–216. [Google Scholar]
- Wright DP, Read DJ, Scholes JD.. 1998b. Mycorrhizal sink strength influences whole plant carbon balance of Trifolium repens L. Plant, Cell & Environment 21: 881–891. [Google Scholar]
- Yeom HJ, Kang JS, Kim GH, Park IK.. 2012. Insecticidal and acetylcholine esterase inhibition activity of Apiaceae plant essential oils and their constituents against adults of German cockroach (Blattella germanica). Journal of Agricultural and Food Chemistry 60: 7194–7203. [DOI] [PubMed] [Google Scholar]
- Zebelo SA, Matsui K, Ozawac R, Maffei ME.. 2012. Plasma membrane potential depolarization and cytosolic calcium flux are early events involved in tomato (Solanum lycopersicon) plant-to-plant communication. Plant Science 196: 93–100. [DOI] [PubMed] [Google Scholar]
- Zouari I, Salvioli A, Chialva M, et al. 2014. From root to fruit: RNA-Seq analysis shows that arbuscular mycorrhizal symbiosis may affect tomato fruit metabolism. BMC Genomics 15: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubek S, Stojakowska A, Anielska T, Turnau K.. 2010. Arbuscular mycorrhizal fungi alter thymol derivative contents of Inula ensifolia L. Mycorrhiza 20: 497–504. [DOI] [PubMed] [Google Scholar]