Abstract
Background Plants require nitrogen (N) for growth, development and defence against abiotic and biotic stresses. The extensive use of artificial N fertilizers has played an important role in the Green Revolution. N assimilation can involve a reductase series ( → → ) followed by transamination to form amino acids. Given its widespread use, the agricultural impact of N nutrition on disease development has been extensively examined.
Scope When a pathogen first comes into contact with a host, it is usually nutrient starved such that rapid assimilation of host nutrients is essential for successful pathogenesis. Equally, the host may reallocate its nutrients to defence responses or away from the site of attempted infection. Exogenous application of N fertilizer can, therefore, shift the balance in favour of the host or pathogen. In line with this, increasing N has been reported either to increase or to decrease plant resistance to pathogens, which reflects differences in the infection strategies of discrete pathogens. Beyond considering only N content, the use of or fertilizers affects the outcome of plant–pathogen interactions. feeding augments hypersensitive response- (HR) mediated resistance, while ammonium nutrition can compromise defence. Metabolically, enhances production of polyamines such as spermine and spermidine, which are established defence signals, with nutrition leading to increased γ-aminobutyric acid (GABA) levels which may be a nutrient source for the pathogen. Within the defensive N economy, the roles of nitric oxide must also be considered. This is mostly generated from by nitrate reductase and is elicited by both pathogen-associated microbial patterns and gene-for-gene-mediated defences. Nitric oxide (NO) production and associated defences are therefore dependent and are compromised by .
Conclusion This review demonstrates how N content and form plays an essential role in defensive primary and secondary metabolism and NO-mediated events.
Keywords: Nitric oxide, nitrate, ammonium, Pseudomonas, nitrate reductase, polyamines, plant defence
INTRODUCTION
Plant and crop biologists considering the role of nitrogen (N) have, quite correctly, concentrated on its key role in driving growth, development and yield. Whilst mechanisms of N uptake and assimilation have been the focus of many studies (for example, Liu et al., 2015), away from NO the importance of N in plant defence against pathogens has received limited attention. While not ignored by plant scientists, the importance of N in plant defence against pathogens has not, in our opinion, achieved the prominence that it deserves. In this review, we outline how N uptake and metabolism provide the building blocks for plant defence against pathogens, or mobilizing N away from the invader to influence virulence or symptom development. Additionally, N drives the generation of nitric oxide (NO), an important defence signal. Given the importance of N fertilizers in agriculture, the effects of N arising from interplay between soil, plant genotype and pathogen require investigation.
NITROGEN ASSIMILATION
Nitrogen is taken-up in two different ways depending on when and whether it exists in or forms. Three families of transporters (NRT1, NRT2 and CLC) have been linked to uptake and translocation of nitrate in plants. Long-distance transport is regulated by transporters such as AtNRT1.5 and AtNRT1.8 which, in Arabidopsis, are involved in loading and unloading into the root stele or from the shoot vasculature (Dechorgnat et al., 2011). uptake is carried out by plasma membrane-located AMT/MEP/Rh transporters (Khademi et al., 2004).
After take-up by roots, is first reduced to by cytosolic nitrate reductase (cNR) where NAD(P)H is used as electron donor and further plastidal nitrite reductase reduces nitrite to ammonium. In contrast, is taken up by roots and transported into plastids, then further assimilated to glutamate by glutamine and glutamate synthase (Crawford and Forde, 2002). assimilation is a more energetically demanding process compared with , as reduction to requires one NADH and a further three NADPH equivalents for further reduction to in the plastid (Noctor and Foyer, 1998).
is assimilated into amino acids via the glutamate synthase/glutamine-2-oxoglutarate aminotransferase (GOGAT) cycle. A range of aminotransferases will transfer the amino group from glutamate to catalyse the formation of amino acids. A notable example is asparagine synthetase (AS) which forms asparagine and glutamate and which, with glutamine synthetase (GS), plays an important role in N assimilation (Lam et al., 1996). The link with 2-oxoglutarate (2-OG) integrates N assimilation with the bioenergetic tricarboxylic acid (TCA) cycle. Within amino acid biosynthetic pathways, 2-OG plays an important role in providing the required carbon skeletons.
NITROGEN FERTILIZATION AND PATHOGENICITY
The interplay for N between host and pathogen may be dramatically affected by agricultural practices which depend on extensive application of synthetic N fertilizer. The increase in the application of N fertilizers over the past few decades has been a major factor in improving crop productivity (Kant et al., 2011). However, such fertilizer use can have conflicting effects on plant–pathogen interactions. Although N input increases plant defence, it also increases the availability of N compounds for exploitation by pathogens (Tavernier et al., 2007), and overapplication of nitrogen fertilizers has been shown to enhance disease development (Solomon et al., 2003). It has been suggested that increased N supply causes greater disease susceptibility through changes in canopy structure that can provide an environment favourable for pathogen growth. However, in yellow rust (caused by Puccinia striiformis), leaf N content rather than canopy structure was important for sustaining epidemics on winter wheat (Neumann et al., 2004). Increased supply of N to the plant led to higher spore production by the powdery mildew fungus Oidium lycopersicum, and increased leaf colonization by the bacterium Pseudomonas syringae pv tomato suggested that increased leaf N caused greater susceptibility to these pathogens. N fertilization has been shown to increase levels of powdery mildew attack on cereals in the field, and experiments carried out on seedlings of six different barley cultivars showed a positive correlation between N application and powdery mildew disease severity (Jensen and Munk, 1997). A further link between host and pathogen N was noted by Robert et al. (2002) who correlated spore production by the rust fungus Puccinia triticina in wheat seedlings. The number of spores was 70 % less in the low N plants but the percentage of N in the spores was higher than in the leaves, suggesting that the pathogen is highly efficient at taking up N from the host and indeed the effectiveness of this mechanism(s) would be important for virulence.
NITROGEN USE BY PATHOGENS
Any consideration of the role of N in plant demand must also assess the requirements of the pathogen. The N required by fungal pathogens for growth comes entirely from plant sources such as , and amino acids, the exact combination of which will vary depending on the part of the plant being infected. Some reports suggest that N sources in the plant are limiting, and that N starvation controls pathogenicity genes and may be a cue for disease development (Thomma et al., 2006; López-Berges et al., 2010). However, other studies suggest that there is a plentiful supply of N for fungal pathogen growth (Solomon et al., 2003; Pageau et al., 2006; Tavernier et al., 2007; Walters and Bingham, 2007). A large number of amino acids have been shown to be present in the apoplast of tomato leaves, with some of them, such as glutamine, glutamate, alanine and γ-aminobutyric acid (GABA) at millimolar concentrations, sufficient to support pathogen growth during the early stages of infection (Solomon and Oliver, 2001). Interestingly, these authors observed an increase in the amino acid concentration between 7 and 14 d after infection which was correlated with increased fungal biomass in the leaf. This may suggest a long-term requirement for N nutrition from the host. This could be extracted through increased protease activity, possibly due to the induction of an extracellular serine protease P69B (Solomon and Oliver, 2001). An additional source of host N nutrition is GABA produced by the irreversible conversion of glutamate by glutamate decarboxylase. It is usually found in plants at low concentrations but levels rise in response to a number of stresses, such as low temperature, heat shock and drought (Forde and Lea, 2007). Solomon and Oliver (2001) showed that Cladosporium fulvum was able to utilize GABA as an N source, with fungal growth on GABA similar to that when grown on aspartate or glutamate, suggesting its potential as an efficient N source for pathogen growth.
ROLE OF NITROGEN NUTRITION IN PLANT DEFENCE
Direct contributions by N to defensive metabolites
Against the trends noted above, other studies have noted that increased N resulted in increased host resistance; for example, in the interactions of Fusarium oxysporum f. sp. lycopersici and Botrytis cinerea with tomato plants which showed increased resistance with greater N application. Such results suggest specific and complex pathogen–N interactions (Hoffland et al., 2000). Increased N can contribute to every level of plant defence; constitutive or induced. Plants reconfigure their primary and secondary metabolism in response to pathogen infection, and these are also influenced by N (Ward et al., 2010). The nutrition regime will impact on the patterns of amino acid biosynthesis to affect gene expression, including those of defence genes. N levels have been shown to affect the production of constitutive defences based on alkaloids (Stout et al., 1998) and (poly) phenolics under different N regimes (Johnson et al., 1987).
Other studies have focused on establishing how ‘expensive’ the production of constitutive defences is or how N could be distributed against the competing demands of growth and defence (Herms and Mattson, 1992; Huot et al., 2014). Metabolite ‘hot spots’ for competition include phenylalanine-derived phenolics and N-containing cyanogenic glycosides (Goodger et al., 2007). Models suggesting how interacting plant defence hormones act to establish the relative balance between the plant growth vs. defence requirements have been recently proposed (Huot et al., 2014). In the context of such considerations, the role of the soil should not be ignored. Biological mineralization of organic material can influence dynamics of N availability. For instance, ammonification of organic forms of N and further nitrification alter the availability of different N forms to the plant during its growth. These dynamic changes in N status can become extreme with changes in pH (Fu et al., 1987), soil humidity and oxygen levels (Smith et al., 1998).
Beyond altered availability of N, the form of N can affect disease development and plant resistance. For instance, symptoms of black root rot (Rhizoctonia solani) of sugar beets (Afanasiev and Carlson, 1942) or Fusarium wilt on tomato (Borrero et al., 2012) were reported to increase following nutrition. In a recent metabolomic study, we have found that nutrition enhances the content of apoplastic sugar and amino acids as well as GABA, thereby increasing the availability of nutrients to the invading pathogen; in this case P. syringae (Gupta et al., 2013). Conversely, under nutrition, increased resistance to P. syringae was observed. Leaving aside -mediated impacts on NO generation (considered below), other defence-associated features were also augmented. One was the increase in polyamine levels, i.e. putrescine, spermidine and spermine production (Gupta et al., 2013). Polyamines are known to increase plant resistance; for example, increasing in barley (Hordeum vulgare) during the hypersensitive response (HR)-associated resistance response against the powdery mildew fungus B. graminis f. sp. hordei (Cowley and Walters, 2002a, b). Polyamines are now well established as signal molecules influencing the cell cycle, DNA and protein synthesis, as well as programmed cell death (Tiburcio et al., 2014). Polyamines are oxidatively deaminated by a series of amine oxidases to produce hydrogen peroxide (H2O2), and this has been suggested to contribute to oxidative cross-linking of cell walls to reduce pathogen ingress. H2O2 has been shown to be required for polysaccharide–protein cross-linking and lignification for penetration defence. Furthermore, aminoaldehydes and 1,3-diaminopropane from polyamine oxidation are involved in secondary metabolite synthesis and abiotic stress tolerance (Cona et al., 2006). Polyamines are also involved in systemic resistance. For instance, methyl jasmonate- (MJ) induced systemic resistance in powdery mildew infection in barley was accompanied by increased production of putrescine and spermidine along with other defence-related metabolites (Walters et al., 2002).
Nitrogen mobilization as a defence strategy
The efficient remobilization of N from leaves during grain filling is important to enable the plant to meet the high demands of the growing seed, and in cereals such as barley and wheat, flag leaf senescence correlates with grain N content. However, N remobilization can also occur prematurely in response to many environmental factors including pathogen attack (Masclaux-Daubresse et al., 2010). In this context, it is relevant that Olea and colleagues (2004) showed that during infection of tomato by the bacterial speck pathogen P. syringae pv. tomato, aspartate synthetase (AS) expression increased in the phloem of the main and secondary veins of the leaf. As the product of the AS-dependent catalysis of glutamine and aspartate is aspargine, which is preferred for energy storage and transport in some species due to the high C/N ratio, such vascular AS expression would reduce the availability of important nutrients to the pathogen.
The levels of enzymes involved in nitrogen assimilation such as NR and glutamine synthetase 2 (GS2) decrease with leaf age, whereas levels of glutamate dehydrogenase (GDH) and glutamine synthetase 1 (GS1) increase. The expression and activity of these latter two enzymes can therefore be used as senescence markers. Interestingly, following infection of tobacco leaves with viruses, and virulent and avirulent strains of the bacterial pathogen P. syringae, the use of fungal elicitors and application of phytohormones such as salicylic acid (SA) showed alterations in GS1 and GDH activity similar to senescence. Most notably there was an overall decrease in GS activity and an increase in GS1 and GDH expression, the exact response varying and depending on the individual interaction (Pageau et al., 2006). Similar effects on senescence markers were also observed by Tavernier et al., (2007) when looking at the compatible interaction of the hemibiotrophic pathogen Colletotrichum lindemuthianum with Phaseolus vulgaris. It was noted that GS1 expression was greater with infection by the avirulent strains of P. syringae whereas GHD expression was more associated with cell death occurring as a result of both resistance responses and disease development. Taking all of these observations together, it is possible to hypothesize that GS1 acts like a metabolic defence gene, remobilizing N away from the infection site in a scorched-earth defence mechanism that has been referred to as ‘slash and burn’ (Pageau et al., 2006; Tavernier et al., 2007).
Nitric oxide in plant defence
The production of NO is a feature of microbially catalysed oxidoreductive reactions occurring between and during soil N cycling. Plants possess a number of distinct pathways for production of NO, located in different cellular compartments and with activation dependent on physiological, developmental and stress conditions (Gupta, 2011). Cytosolic NR, mitochondrial nitrite NO reductase, plasma membrane nitrite NO reductase and xanthine oxido-reductase are reductive pathways. Nitric oxide synthase-like enzyme (NOS-like)-, polyamine- and hydroxylamine-mediated pathways are oxidative in nature. Central to all of these mechanisms is a dependence on the relative availability of N assimilation products (see below). NO has a specific role in each compartment, possibly interacting with local signal events. For example, NO has recently been shown to modulate mitochondrial alternative oxidase and aconitase activities, thereby helping the plant in shift from primary metabolism towards amino acid biosynthesis (Cvetkovska and Vanlerberghe, 2012a, b; Gupta et al., 2012a, b). In the case of peroxisomal NO production, this has been shown to inhibit catalase and glyoxylate oxidase activity; enzymes which play roles in β-oxidation and in the detoxification of reactive oxygen species (ROS) (Ortega-Galisteo et al., 2012). Although a number of such enzyme activities have been linked to disease resistance, further investigation is necessary (Chern et al., 2013).
Nitric oxide appears to play important roles in many facets of plant defence. The first report came from Noritake et al. (1996), who reported that NO plays a role in accumulation of phytoalexin biosynthesis. Later, the role of NO in driving cell death during the HR was defined in plants (Durner et al., 1998; Delledonne et al., 2001, 1998). These authors and many others, including ourselves (Mur et al., 2005), have shown that the rate of NO production influences the kinetics of HR formation. Mechanistically, this is likely to involve an interaction with ROS which is also central to forming the HR (Delledonne et al., 1998; Torres et al., 2002; Yun et al., 2011). Due to its high diffusibility and lipophilic nature, NO can cross plant membranes and react with superoxide , leading to the generation of peroxynitrite (ONOO–). Excess NO can also react with , leading to accumulation of less toxic NO2, N2O3 and N2O4.
The NO–ROS interactions are also important in downstream signalling pathways. Nitrosylation is another mechanism where reversible reaction of NO with the thiol groups of reduced cysteine residues plays a role in activation and inactivation of specific protein functions (Gupta, 2011). Due to the high content of iron and thiols, mitochondrial NO can nitrosylate many proteins in mitochondria. For example, the P. syringae-derived elicitor harpin induced mitochondrial NO production and nitrosylation of the photorespiratory mitochondrial glycine decarboxylase complex (GDC). Since NADH is needed for redox control, nitrosylation leads to inhibition of GDC, which can affect overall redox balance and promote cell death (Palmieri et al., 2010).
Nitric oxide also plays a role in defence responses induced by pathogen-associated molecular patterns (PAMPs) (Zeidler et al., 2004). As a signalling component of PAMP-triggered immunity (PTI) and effector-triggered immunity (ETI) (Delledonne et al., 1998), NO can be considered part of the Zig–Zag model of plant defence (Jones and Dangle, 2006). Additionally, recognized PAMPs located on P. syringae pv. tomato were demonstrated to elicit NO-mediated stomatal closure and thereby reduce the penetration efficiency of the pathogen. The bacterial virulence factor coronatine has been shown to block this effect, thus allowing entry into the sub-stomatal chamber (Melotto et al., 2006). NO has also been shown to influence the formation of papillae, which are cell wall appositions produced in response to fungal pathogens and central to many non-host defence mechanisms (Prats et al., 2005). Taking all of these points together, N availability, together with subsequent NO generation, will have a wide-ranging impact on the ability of plants to withstand microbial attack. In terms of both NO production elicited via PTI and ETI, one of the most important downstream effects is the initiation of SA biosynthesis (Durner et al., 1998). SA plays a central role in both localized and systemic resistance to infection, and NO is now known to be an integral component of the signalling pathway (Mur et al., 2013). Extensive bioinformatic analysis of NO-responsive promoters in arabidopsis in response to infection found that cis-elements linked to SA responsiveness were prominent (Palmieri et al., 2008). Further, NO is known to nitrosylate key cysteines on TGA-class transcription factors to aid in the initiation of SA-dependent gene expression (Lindermayr and Durner, 2009; Lindermayr et al., 2010; Gupta, 2011; Mur et al., 2013; Yu et al., 2014). This can be countered through nitrosylation of A NONEXPRESSOR OF PATHOGENESIS-RELATED PROTEIN1 (NPR1) leading to oligomerization of NPR1 within the cytoplasm to reduce TGA activation (Tada et al., 2008; Lindermayr et al., 2010). On the other hand, Després and colleagues (2003) reported that TGA1 relies on the oxidation state of cysteine residues to mediate the interaction with NPR. Another key SA protein that is nitrosylated is the SA-binding protein 3 (SABP3) which has carbonic anhydrase activity (Wang et al., 2009). Silencing SABP3 gene expression suppressed a HR elicited by P. syringae pv. tomato (Slaymaker et al., 2002).
It is important not to forget the interacting pathogen, where NO can also play a role in host invasion. NO has been shown to be generated in the appressoria of Blumeria graminis and Magnaporthe oryzae to aid penetration of the host (Prats et al., 2008; Samalova et al., 2013). In the case of Phytophthora cryptogea, the virulence factor/elicitor cryptogein aids pathogenesis by promoting host cell death via NO generation (Lamotte et al., 2004). Thus, not only N nutrition, but the relative availability of N to drive either host or pathogen NO generation is a relevant consideration.
Role of N nutrition in NO generation during defence against pathogens
The NR pathway catalyses the reduction of nitrate to nitrite using NADH as electron donor [NAD(P)H + 3H2O+ + 2 → NAD+ + 2NO + 5H2O]. It is now well established that and play a role in increased NR activity and NO emissions (Gupta et al., 2005; Planchet et al., 2005). In an important recent development, it was shown that S-nitrosothiol (SNO) signalling regulates both nitrate uptake and reduction, to fine-tune nitrate homeostasis (Frungillo et al., 2014). It is therefore possible that defence-associated NO could influence this homeostatic mechanism to favour N diversion either away from the infection site or towards defensive metabolism.
nutrition is also of importance, as this leads to reduced levels of NO via suppression of NR activity (Planchet et al., 2005; Gupta et al., 2013). The NR NO-generating mechanism has been shown to be central to the HR in response to avirulent Pseudomonas interactions with the host (Melotto et al., 2006; Gupta et al., 2013; Vitor et al., 2013). Gupta and colleagues (2013) also reported that during compatible interaction between tobacco and P. syringie pv. tabaci the NR NO-generated mechanism increased plant resistance. A similar trend of response was observed in Verticillium dahliae infection in arabidopsis (Shi and Li, 2008). Yamamoto-Katou et al. (2006) showed that silencing NR leads to reduced levels of NO in response to elicitin. These authors concluded that mitochondrial NO plays a role in defence. Mitochondria produce NO from complex III and IV of mitochondrial electron transport. This process requires relatively low oxygen levels (Gupta et al., 2005), a situation that could arise due to defence responses depleting local oxygen levels within tissues. The implication of all of these studies is that the strength of NO generation and, thus, the efficacy of the defence response, is strongly dependent on the relative availability of / .
A parallel consideration must be NO generation during plant defence via a NOS-like pathway (Delledonne et al., 1998; Wendehenne et al., 2001) Despite having no homologue in higher plants, it was shown that NOS-like activity is responsible for NO during plant response to various pathogen infection and resistance responses (Chaki et al., 2009; Yoshioka et al., 2009). However, evidence for this is mainly based on the use of pharmacological NOS inhibitors such as arginine analogues; PBITU [S,S′-1,3-phenylene-bis(1,2-ethanediyl)-bis-isothiourea], l-NAME (NG-nitro-l-arginine methyl ester), l-NMMA (NG-monomethyl-l-arginine), l-NIL [N6-(1-iminoethyl)-l-lysine)] or AET [2-(2-aminoethyl) isothiourea] (Gaupels et al., 2011). This stated, direct assays of NOS-like activity have suggested an involvement in disease resistance to the necrotrophic pathogen Botrytis cinerea in Nicotiana benthamiana (Asai and Yoshioka, 2009), and cryptogein-induced cell death (Besson-Bard et al., 2008). Whatever the exact nature of this NOS-like activity, its dependence on arginine retains the link with N assimilation, NO and defence.
A similar indirect link with N is implicit in the polyamine-mediated NO pathway (Tun et al., 2006). As in the NOS-mediated NO pathway, in the polyamine NO pathway arginine acts as a substrate for production of spermine and spermidine to produce NO. Polyamine generation requires arginine to act as a substrate for the enzyme arginine decarboxylase. Arginine levels can be determined by modulation of arginase, leading to altered NO production (Flores et al., 2008). Normal levels of NO were returned by providing spermine to the plants, suggesting that the polyamine synthesis from arginine is involved in the production of NO. The mechanisms through which polyamines mediate NO generation are not completely known. However, polyamines clearly play a role in plant defence (Walters, 2003), with increased spermine in tobacco leading to increased resistance against Pseudomonas tabaci and also the hemibiotrophic oomycete Phytophthora parasitica var. nicotiana (Moschou et al., 2009) and potentiating defence against Pseudomonas viridiflava in arabidopsis (Gonzalez et al., 2011). Defence mechanisms involving polyamines may include ROS production and cell death as well as cell wall reinforcement mechanisms (Walters, 2003).
CONCLUDING REMARKS
Far from considering N assimilation as only providing the building blocks of primary metabolism, this review has suggested that its appropriate concentration and form is essential for effective plant defence. N influences both constitutive and induced defences, as an element in key signal molecules such as NO and polyamines. We have highlighted how resistance based upon PTI, ETI or the mobilization of nutrients away from the site of infection (‘slash and burn’) could all be compromised under different N conditions. The potential importance of these observations cannot be overstated, and breeding programmes focusing on resistance need also to consider interactions under different N regimes. Continued advances in the development of disease-resistant crop plants requires further understanding of how N, NO and polyamines contribute to PTI and ETI, together with elucidation of mechanisms involved in movement of N resources away from the site of infection. Understanding of how different pathogens, with different infection strategies, respond to N levels to influence nutrition and pathogenicity is also of fundamental importance in this context.
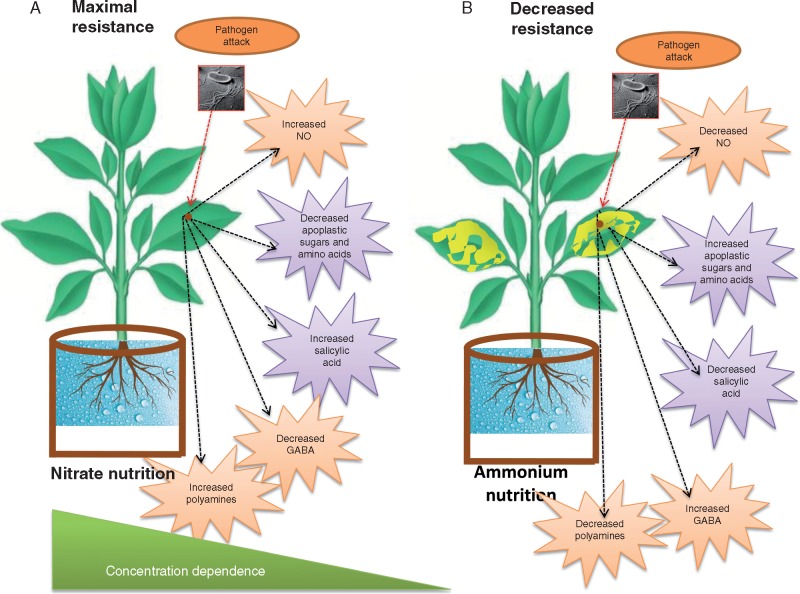
Fig. 1.
Effect of vs. on plant resistance to pathogen infection. Growth on nutrition leads to increased levels of NO, SA, PR gene expression, induction of the polyamine pathway, a decrease in apoplastic sugars and amino acids, and an overall increase in plant resistance in a concentration-dependent manner. Growth on nutrition leads to increased levels of apoplastic sugars and amino acids, reduced levels of SA and PR gene expression, induction of GABA biosynthesis and reduced plant defence response.
ACKNOWLEDGEMENTS
This work is currently being supported by a DST-UKIERI thematic partnership award to K.J.G. and L.A.J.M. K.J.G. is currently supported by a Ramalingaswami Fellowship and Innovate Young Biotechnology Award (IYBA) by the Department of Biotechnology, Government of India. We thank Aakanksha Wany for critical reading of the manuscript, and also thank the handling editor for providing valuable suggestions for improvement of this manuscript.
REFERENCES
- Afanasiev MM, Carlson WE.. 1942. The relation of phosphorus and nitrogen ratio to the amount of seedling diseases of sugar beets. American Society of Sugar Beet Technologists 407–411. [Google Scholar]
- Asai S, Yoshioka H.. 2009. Nitric oxide as a partner of reactive oxygen species participates in disease resistance to nectrotophic pathogen Botryis cinerea in Nicotiana benthamiana. Molecular Plant-Microbe Interactions 22: 619–629. [DOI] [PubMed] [Google Scholar]
- Besson-Bard A, Courtois C, Gauthier A, et al. 2008. Nitric oxide in plants: production and cross-talk with Ca2+ signaling. Molecular Plant 1: 218–228. [DOI] [PubMed] [Google Scholar]
- Borrero C, Trillas MI, Delgado A, Aviles M.. 2012. Effect of ammonium/nitrate ratio in nutrient solution on control of Fusarium wilt of tomato by Trichoderma asperellum T34. Plant Pathology 61: 132–139. [Google Scholar]
- Chaki M, Fernandez-Ocana AM, Valderrama R, et al. 2009. Involvement of reactive nitrogen and oxygen species (RNS and ROS) in sunflower–mildew interaction. Plant and Cell Physiology 50: 265–279. [DOI] [PubMed] [Google Scholar]
- Chern M, Bai W, Chen X, Canlas PE, Ronald PC.. 2013. Reduced expression of glycolate oxidase leads to enhanced disease resistance in rice. Peer Journal 1: e28. doi:10.7717/peerj.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cona A, Rea G, Angelini R, Federico R, Tavladoraki P.. 2006. Functions of amine oxidases in plant development and defence. Trends in Plant Science 11: 80–88. [DOI] [PubMed] [Google Scholar]
- Cowley T, Walters DR.. 2002a. . Polyamine metabolism in an incompatible interaction between barley and the powdery mildew fungus, Blumeria graminis f. sp hordei. Journal of Phytopathology-Phytopathologische Zeitschrift 150: 581–586. [Google Scholar]
- Cowley T, Walters DR.. 2002b. . Polyamine metabolism in barley reacting hypersensitively to the powdery mildew fungus Blumeria graminis f. sp hordei. Plant, Cell & Environment 25: 461–468. [Google Scholar]
- Crawford NM, Forde BG.. 2002. Molecular and developmental biology of inorganic nitrogen nutrition. Arabidopsis Book 1: e0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvetkovska M, Vanlerberghe GC.. 2012a. Alternative oxidase modulates leaf mitochondrial concentrations of superoxide and nitric oxide. New Phytologist 195: 32–39. [DOI] [PubMed] [Google Scholar]
- Cvetkovska M, Vanlerberghe GC.. 2012b. Coordination of a mitochondrial superoxide burst during the hypersensitive response to bacterial pathogen in Nicotiana tabacum. Plant, Cell & Environment 35: 1121–1136. [DOI] [PubMed] [Google Scholar]
- Dechorgnat J, Nguyen CT, Armengaud P, et al. 2011. From the soil to the seeds: the long journey of nitrate in plants. Journal of Experimental Botany 62: 1349–1359. [DOI] [PubMed] [Google Scholar]
- Delledonne M, Xia Y, Dixon RA, Lamb C.. 1998. Nitric oxide functions as a signal in plant disease resistance. Nature 394: 585–588. [DOI] [PubMed] [Google Scholar]
- Delledonne M, Zeier J, Marocco A, Lamb C.. 2001. Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proceedings of the National Academy of Sciences, USA 98: 13454–13459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Després C, Chubak C, Rochon A, et al. 2003. The Arabidopsis NPR1 disease resistance protein is a novel cofactor that confers redox regulation of DNA binding activity to the basic domain/leucine zipper transcription factor TGA1. The Plant Cell 15(9): 2181–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durner J, Wendehenne D, Klessig DF.. 1998. Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proceedings of the National Academy of Sciences, USA 95: 10328–10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores T, Todd CD, Tovar-Mendez A, et al. 2008. Arginase-negative mutants of Arabidopsis exhibit increased nitric oxide signaling in root development. Plant Physiology 147: 1936–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frungillo L, Skelly MJ, Loake GJ, Spoel SH, Salgado I.. 2014. S-Nitrosothiols regulate nitric oxide production and storage in plants through the nitrogen assimilation pathway. Nature Communications 5: 5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde BG, Lea PJ.. 2007. Glutamate in plants: metabolism, regulation, and signalling. Journal of Experimental Botany 58: 2339–2358. [DOI] [PubMed] [Google Scholar]
- Fu MH, Xu XC, Tabatabai MA.. 1987. Effect of pH on nitrogen mineralization in crop-residue-treated soils. Biology and Fertility of Soils 5: 115–119. [Google Scholar]
- Gaupels F, Kuruthukulangarakoola GT, Durner J.. 2011. Upstream and downstream signals of nitric oxide in pathogen defence. Current Opinion in Plant Biology 14: 707–714. [DOI] [PubMed] [Google Scholar]
- Gonzalez ME, Marco F, Minguet EG, et al. 2011. Perturbation of spermine synthase gene expression and transcript profiling provide new insights on the role of the tetraamine spermine in Arabidopsis defense against Pseudomonas viridiflava. Plant Physiology 156: 2266–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodger JQ, Choo TY, Woodrow IE.. 2007. Ontogenetic and temporal trajectories of chemical defence in a cyanogenic eucalypt. Oecologia 153: 799–808. [DOI] [PubMed] [Google Scholar]
- Gupta KJ. 2011. Protein S-nitrosylation in plants: photorespiratory metabolism and NO signaling. Science Signaling 4: jc1. [DOI] [PubMed] [Google Scholar]
- Gupta KJ, Stoimenova M, Kaiser WM.. 2005. In higher plants, only root mitochondria, but not leaf mitochondria reduce nitrite to NO, in vitro and in situ. Journal of Experimental Botany 56: 2601–2609. [DOI] [PubMed] [Google Scholar]
- Gupta KJ, Igamberdiev AU, Mur LA.. 2012a. NO and ROS homeostasis in mitochondria: a central role for alternative oxidase. New Phytologist 195: 1–3. [DOI] [PubMed] [Google Scholar]
- Gupta KJ, Shah JK, Brotman Y, et al. 2012b. Inhibition of aconitase by nitric oxide leads to induction of the alternative oxidase and to a shift of metabolism towards biosynthesis of amino acids. Journal of Experimental Botany 63: 1773–1784. [DOI] [PubMed] [Google Scholar]
- Gupta KJ, Brotman Y, Segu S, et al. 2013. The form of nitrogen nutrition affects resistance against Pseudomonas syringae pv. phaseolicola in tobacco. Journal of Experimental Botany 64: 553–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herms DA, Mattson WJ.. 1992. The dilemma of plants – to grow or defend. Quarterly Review of Biology 67: 283–335. [Google Scholar]
- Hoffland E, Jeger MJ, van Beusichem ML.. 2000. Effect of nitrogen supply rate on disease resistance in tomato depends on the pathogen. Plant and Soil 218: 239–247. [Google Scholar]
- Huot B, Yao J, Montgomery BL, He SY.. 2014. Growth–defense tradeoffs in plants: a balancing act to optimize fitness. Molecular Plant 7: 1267–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen B, Munk L.. 1997. Nitrogen-induced changes in colony density and spore production of Erysiphe graminis f sp hordei on seedlings of six spring barley cultivars. Plant Pathology 46: 191–202. [Google Scholar]
- Johnson ND, Liu B, Bentley BL.. 1987. The effects of nitrogen-fixation, soil nitrate, and defoliation on the growth, alkaloids, and nitrogen levels of Lupinus succulentus (Fabaceae). Oecologia 74: 425–431. [DOI] [PubMed] [Google Scholar]
- Jones JD, Dangl JL.. 2006. The plant immune system. Nature 444: 323–329. [DOI] [PubMed] [Google Scholar]
- Kant S, Bi YM, Rothstein SJ.. 2011. Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency. Journal of Experimental Botany 62: 1499–1509. [DOI] [PubMed] [Google Scholar]
- Khademi S, O’Connell J 3rd, Remis J, Robles-Colmenares Y, Miercke LJ, Stroud RM.. 2004. Mechanism of ammonia transport by Amt/MEP/Rh: structure of AmtB at 1·35 A. Science 305: 1587–1594. [DOI] [PubMed] [Google Scholar]
- Lam HM, Coschigano KT, Oliveira IC, MeloOliveira R, Coruzzi GM.. 1996. The molecular-genetics of nitrogen assimilation into amino acids in higher plants. Annual Review of Plant Physiology and Plant Molecular Biology 47: 569–593. [DOI] [PubMed] [Google Scholar]
- Lamotte O, Gould K, Lecourieux D, et al. 2004. Analysis of nitric oxide signaling functions in tobacco cells challenged by the elicitor cryptogein. Plant Physiology 135: 516–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindermayr C, Durner J.. 2009. S-Nitrosylation in plants: pattern and function. Journal of Proteomics 2: 1–9. [DOI] [PubMed] [Google Scholar]
- Lindermayr C, Sell S, Muller B, Leister D, Durner J.. 2010. Redox regulation of the NPR1–TGA1 system of Arabidopsis thaliana by nitric oxide. The Plant Cell 22: 2894–2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Chen X, Wu K, Fu X.. 2015. Nitrogen signaling and use efficiency in plants: what’s new? Current Opinion in Plant Biology 27: 192–198. [DOI] [PubMed] [Google Scholar]
- López-Berges MS, Rispail N, Prados-Rosales RC, Di Pietro A.. 2010. A nitrogen response pathway regulates virulence functions in Fusarium oxysporum via the protein kinase TOR and the bZIP protein MeaB. The Plant Cell 22: 2459–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masclaux-Daubresse C, Daniel-Vedele F, Dechorgnat J, Chardon F, Gaufichon L, Suzuki A.. 2010. Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Annals of Botany 105: 1141–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melotto M, Underwood W, Koczan J, Nomura K, He SY.. 2006. Plant stomata function in innate immunity against bacterial invasion. Cell 126: 969–980. [DOI] [PubMed] [Google Scholar]
- Moschou PN, Sarris PF, Skandalis N, et al. 2009. Engineered polyamine catabolism preinduces tolerance of tobacco to bacteria and oomycetes. Plant Physiology 149: 1970–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mur LA, Santosa IE, Laarhoven LJ, Holton NJ, Harren FJ, Smith AR.. 2005. Laser photoacoustic detection allows in planta detection of nitric oxide in tobacco following challenge with avirulent and virulent Pseudomonas syringae pathovars. Plant Physiology 138: 1247–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mur LA, Prats E, Pierre S, Hall MA, Hebelstrup KH.. 2013. Integrating nitric oxide into salicylic acid and jasmonic acid/ethylene plant defense pathways. Frontiers in Plant Science 4: 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Paveley ND, Beed FD, Sylvester-Bradley R.. 2004. Nitrogen per unit leaf area affects the upper asymptote of Puccinia striiformis f.sp tritici epidemics in winter wheat. Plant Pathology 53: 725–732. [Google Scholar]
- Noctor G, Foyer CH.. 1998. Ascorbate and glutathione: keeping active oxygen under control. Annual Review of Plant Physiology and Plant Molecular Biology 49: 249–279. [DOI] [PubMed] [Google Scholar]
- Noritoke T, Kawakita K, Doke N.. 1996. Nitric oxide induces phytoalexin accumulation in potato tuber tissues. Plant and Cell Physiology 37: 113–116. [Google Scholar]
- Olea F, Perez-Garcia A, Canton FR, et al. 2004. Up-regulation and localization of asparagine synthetase in tomato leaves infected by the bacterial pathogen Pseudomonas syringae. Plant and Cell Physiology, 45: 770–780. [DOI] [PubMed] [Google Scholar]
- Ortega-Galisteo AP, Rodriguez-Serrano M, Pazmino DM, Gupta DK, Sandalio LM, Romero-Puertas MC.. 2012. S-Nitrosylated proteins in pea (Pisum sativum L.) leaf peroxisomes: changes under abiotic stress. Journal of Experimental Botany 63: 2089–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pageau K, Reisdorf-Cren M, Morot-Gaudry JF, Masclaux-Daubresse C.. 2006. The two senescence-related markers, GS1 (cytosolic glutamine synthetase) and GDH (glutamate dehydrogenase), involved in nitrogen mobilization, are differentially regulated during pathogen attack and by stress hormones and reactive oxygen species in Nicotiana tabacum L. leaves. Journal of Experimental Botany 57: 547–557. [DOI] [PubMed] [Google Scholar]
- Palmieri MC, Lindermayr C, Bauwe H, Steinhauser C, Durner J.. 2010. Regulation of plant glycine decarboxylase by s-nitrosylation and glutathionylation. Plant Physiology 152: 1514–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri MC, Sell S, Huang X, et al. 2008. Nitric oxide-responsive genes and promoters in Arabidopsis thaliana: a bioinformatics approach. Journal of Experimental Botany 59: 177–186. [DOI] [PubMed] [Google Scholar]
- Planchet E, Jagadis Gupta K, Sonoda M, Kaiser WM.. 2005. Nitric oxide emission from tobacco leaves and cell suspensions: rate limiting factors and evidence for the involvement of mitochondrial electron transport. The Plant Journal 41: 732–743. [DOI] [PubMed] [Google Scholar]
- Prats E, Carver TL, Mur LA.. 2008. Pathogen-derived nitric oxide influences formation of the appressorium infection structure in the phytopathogenic fungus Blumeria graminis. Research in Microbiology 159: 476–480. [DOI] [PubMed] [Google Scholar]
- Prats E, Mur LA, Sanderson R, Carver TL.. 2005. Nitric oxide contributes both to papilla-based resistance and the hypersensitive response in barley attacked by Blumeria graminis f. sp. hordei. Molecular Plant Pathology 6: 65–78. [DOI] [PubMed] [Google Scholar]
- Robert C, Bancal MO, Lannou C.. 2002. Wheat leaf rust uredospore production and carbon and nitrogen export in relation to lesion size and density. Phytopathology 92: 762–768. [DOI] [PubMed] [Google Scholar]
- Samalova M, Johnson J, Illes M, Kelly S, Fricker M, Gurr S.. 2013. Nitric oxide generated by the rice blast fungus Magnaporthe oryzae drives plant infection. New Phytologist 197: 207–222. [DOI] [PubMed] [Google Scholar]
- Shi FM, Li YZ.. 2008. Verticillium dahliae toxins-induced nitric oxide production in Arabidopsis is major dependent on nitrate reductase. Biochemistry and Molecular Biology Reports 41: 79–85. [DOI] [PubMed] [Google Scholar]
- Slaymaker DH, Navarre DA, Clark D, del Pozo O, Martin GB, Klessig DF.. 2002. The tobacco salicylic acid-binding protein 3 (SABP3) is the.chloroplast carbonic anhydrase, which exhibits antioxidant activity and plays a role in the hypersensitive defense response. Proceedings of the National Academy of Sciences, USA 99: 11640–11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KA, Thomson PE, Clayton H, McTaggart IP, Conen F.. 1998. Effects of temperature, water content and nitrogen fertilisation on emissions of nitrous oxide by soils. Atmospheric Environment 32: 3301–3309. [Google Scholar]
- Solomon PS, Oliver RP.. 2001. The nitrogen content of the tomato leaf apoplast increases during infection by Cladosporium fulvum. Planta 213: 241–249. [DOI] [PubMed] [Google Scholar]
- Solomon PS, Tan KC, Oliver RP.. 2003. The nutrient supply of pathogenic fungi; a fertile field for study. Molecular Plant Pathology 4: 203–210. [DOI] [PubMed] [Google Scholar]
- Stout MJ, Brovont RA, Duffey SS.. 1998. Effect of nitrogen availability on expression of constitutive and inducible chemical defenses in tomato, Lycopersicon esculentum. Journal of Chemical Ecology 24: 945–963. [Google Scholar]
- Tada Y, Spoel SH, Pajerowska-Mukhtar K, Mou Z, Song J, Wang C.. 2008. Plant immunity requires conformational charges of NPR1 via S-nitrosylation and thioredoxins. Science 321: 952–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavernier V, Cadiou S, Pageau K, et al. 2007. The plant nitrogen mobilization promoted by Colletotrichum lindemuthianum in Phaseolus leaves depends on fungus pathogenicity. Journal of Experimental Botany 58: 3351–3360. [DOI] [PubMed] [Google Scholar]
- Thomma BP, Bolton MD, Clergeot PH, DE Wit PJ.. 2006. Nitrogen controls in planta expression of Cladosporium fulvum Avr9 but no other effector genes. Molecular Plant Pathology 7: 125–130. [DOI] [PubMed] [Google Scholar]
- Tiburcio AF, Altabella T, Bitrian M, Alcazar R.. 2014. The roles of polyamines during the lifespan of plants: from development to stress. Planta 240: 1–18. [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JD.. 2002. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proceedings of the National Academy of Sciences, USA 99: 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun NN, Santa-Catarina C, Begum T, et al. 2006. Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings. Plant and Cell Physiology 47: 346–354. [DOI] [PubMed] [Google Scholar]
- Vitor SC, Duarte GT, Saviani EE, Vincentz MGA, Oliveira HC, Salgado I.. 2013. Nitrate reductase is required for the transcriptional modulation and bactericidal activity of nitric oxide during the defense response of Arabidopsis thaliana against Pseudomonas syringae. Planta 238: 475–486. [DOI] [PubMed] [Google Scholar]
- Walters D, Cowley T, Mitchell A.. 2002. Methyl jasmonate alters polyamine metabolism and induces systemic protection against powdery mildew infection in barley seedlings. Journal of Experimental Botany 53: 7477–7456. [DOI] [PubMed] [Google Scholar]
- Walters DR. 2003. Polyamines and plant disease. Phytochemistry 64: 97–107. [DOI] [PubMed] [Google Scholar]
- Walters DR, Bingham IJ.. 2007. Influence of nutrition on disease development caused by fungal pathogens: implications for plant disease control. Annals of Applied Biology 151: 307–324. [Google Scholar]
- Wang YQ, Feechan A, Yun BW, et al. 2009. S-nitrosylation of AtSABP3 antagonizes the expression of plant immunity. Journal of Biological Chemistry 284: 2131–2137. [DOI] [PubMed] [Google Scholar]
- Ward JL, Forcat S, Beckmann M, et al. 2010. The metabolic transition during disease following infection of Arabidopsis thaliana by Pseudomonas syringae pv. tomato. The Plant Journal 63: 443–457. [DOI] [PubMed] [Google Scholar]
- Wendehenne D, Pugin A, Klessig DF, Durner J.. 2001. Nitric oxide: comparative synthesis and signaling in animal and plant cells. Trends in Plant Science 6: 177–183. [DOI] [PubMed] [Google Scholar]
- Yamamoto-Katou A, Katou S, Yoshioka H, Doke N, Kawakita K.. 2006. Nitrate reductase is responsible for elicitin-induced nitric oxide production in Nicotiana benthamiana. Plant and Cell Physiology 47: 726–735. [DOI] [PubMed] [Google Scholar]
- Yoshioka H, Asai S, Yoshioka M, Kobayashi M.. 2009. Molecular mechanisms of generation for nitric oxide and reactive oxygen species, and role of the radical burst in plant immunity. Molecular Cells 28: 321–329. [DOI] [PubMed] [Google Scholar]
- Yu M, Lamattina L, Spoel SH, Loake GJ.. 2014. Nitric oxide function in plant biology: a redox cue in deconvolution. New Phytologist 202: 1142–1156. [DOI] [PubMed] [Google Scholar]
- Yun BW, Feechan A, Yin M, et al. 2011. S-Nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 478: 264–268. [DOI] [PubMed] [Google Scholar]
- Zeidler D, Zahringer U, Gerber I, et al. 2004. Innate immunity in Arabidopsis thaliana: lipopolysaccharides activate nitric oxide synthase (NOS) and induce defense genes. Proceedings of the National Academy of Sciences, USA 101: 15811–15816. [DOI] [PMC free article] [PubMed] [Google Scholar]