Abstract
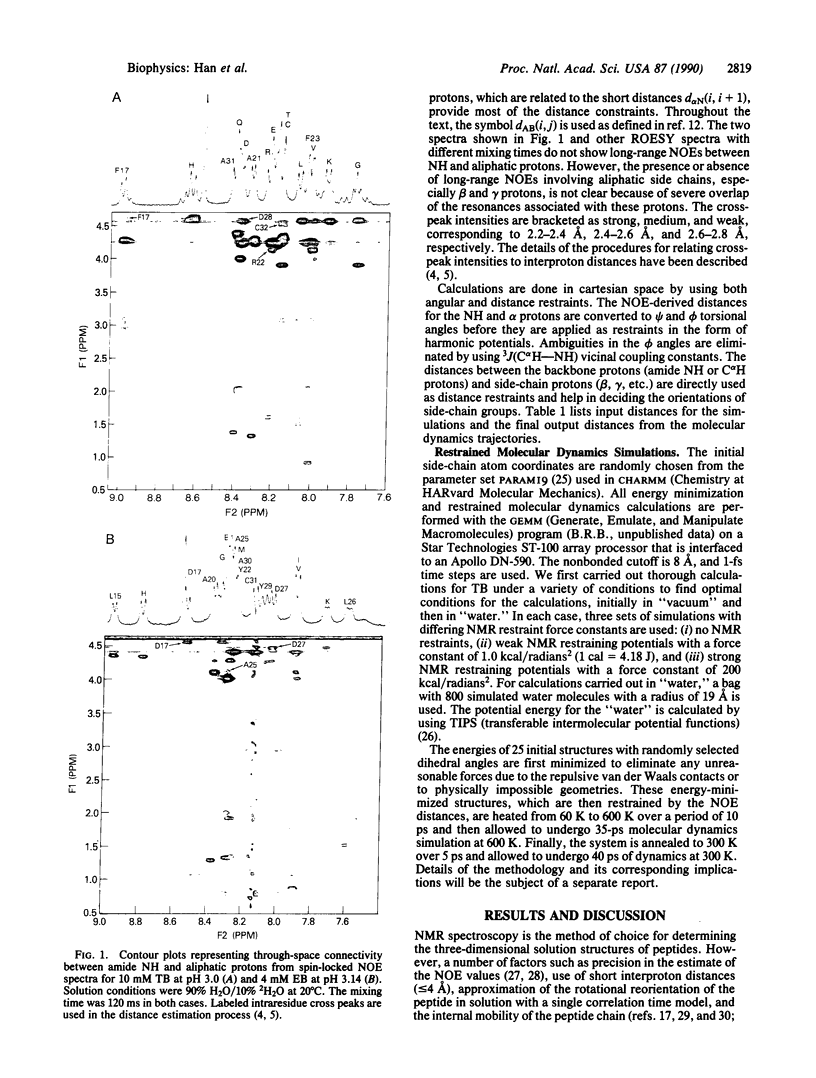
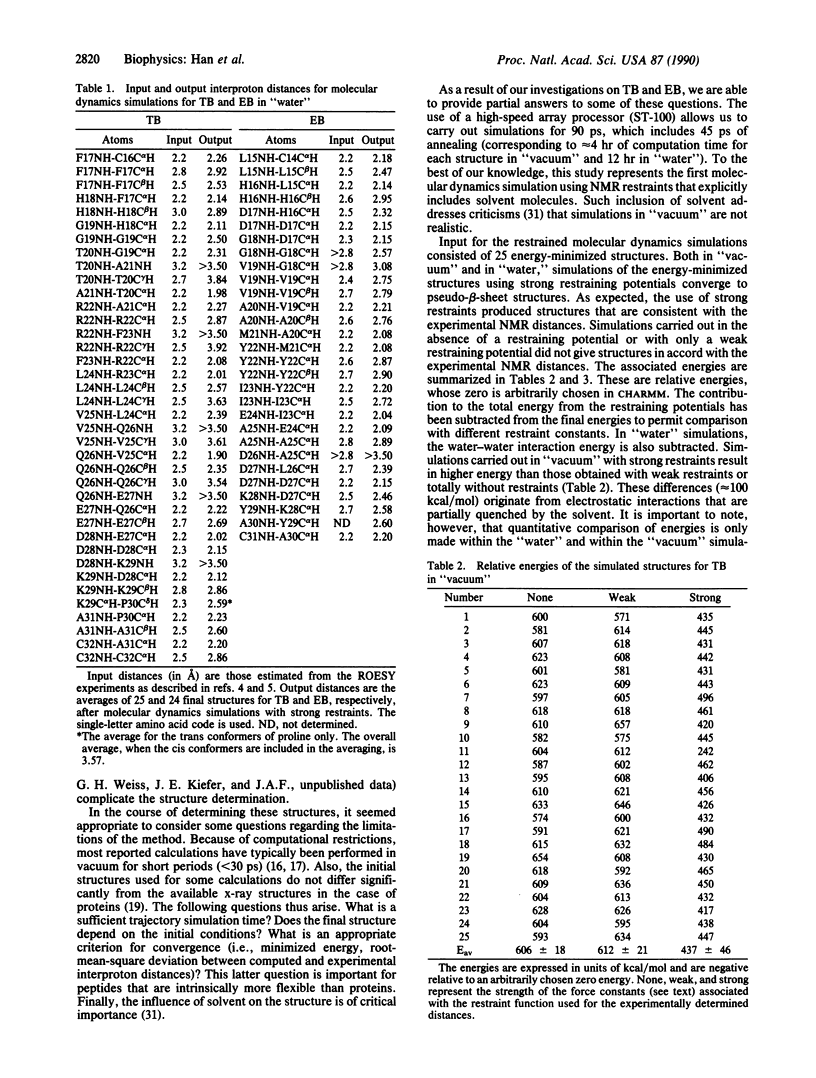
A restrained molecular dynamics simulation approach that explicitly includes the effect of the surrounding solvent molecules is applied to the NMR determination of the conformations of the B-loop fragments of human transforming growth factor alpha and epidermal growth factor. Backbone interproton distance restraints are obtained by using two-dimensional rotating frame nuclear Overhauser effect spectroscopy (ROESY). The simulations are carried out both in "vacuum" and in "water." The results are discussed in terms of the energetics, agreement with the NMR distances, and the flexibility of the peptides.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appella E., Robinson E. A., Ullrich S. J., Stoppelli M. P., Corti A., Cassani G., Blasi F. The receptor-binding sequence of urokinase. A biological function for the growth-factor module of proteases. J Biol Chem. 1987 Apr 5;262(10):4437–4440. [PubMed] [Google Scholar]
- Bost K. L., Smith E. M., Blalock J. E. Regions of complementarity between the messenger RNAs for epidermal growth factor, transferrin, interleukin-2 and their respective receptors. Biochem Biophys Res Commun. 1985 May 16;128(3):1373–1380. doi: 10.1016/0006-291x(85)91092-7. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Clore G. M., Gronenborn A. M., Karplus M. Solution conformations of human growth hormone releasing factor: comparison of the restrained molecular dynamics and distance geometry methods for a system without long-range distance data. Protein Eng. 1987 Oct-Nov;1(5):399–406. doi: 10.1093/protein/1.5.399. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Clore G. M., Gronenborn A. M., Karplus M. Three-dimensional structure of proteins determined by molecular dynamics with interproton distance restraints: application to crambin. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3801–3805. doi: 10.1073/pnas.83.11.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clore G. M., Gronenborn A. M., Brünger A. T., Karplus M. Solution conformation of a heptadecapeptide comprising the DNA binding helix F of the cyclic AMP receptor protein of Escherichia coli. Combined use of 1H nuclear magnetic resonance and restrained molecular dynamics. J Mol Biol. 1985 Nov 20;186(2):435–455. doi: 10.1016/0022-2836(85)90116-0. [DOI] [PubMed] [Google Scholar]
- Compton L. A., Johnson W. C., Jr Analysis of protein circular dichroism spectra for secondary structure using a simple matrix multiplication. Anal Biochem. 1986 May 15;155(1):155–167. doi: 10.1016/0003-2697(86)90241-1. [DOI] [PubMed] [Google Scholar]
- Cooke R. M., Wilkinson A. J., Baron M., Pastore A., Tappin M. J., Campbell I. D., Gregory H., Sheard B. The solution structure of human epidermal growth factor. 1987 May 28-Jun 3Nature. 327(6120):339–341. doi: 10.1038/327339a0. [DOI] [PubMed] [Google Scholar]
- Han K. H., Ferretti J. A., Niu C. H., Lokeshwar V., Clarke R., Katz D. Conformational and receptor binding properties of human EGF and TGF-alpha second loop fragments. J Mol Recognit. 1988 Jun;1(3):116–123. doi: 10.1002/jmr.300010304. [DOI] [PubMed] [Google Scholar]
- Han K. H., Niu C. H., Roller P. P., Ferretti J. A. Conformation of the second disulfide loop in human transforming growth factor-alpha studied by two-dimensional NMR spectroscopy. Biopolymers. 1988 Jun;27(6):923–937. doi: 10.1002/bip.360270604. [DOI] [PubMed] [Google Scholar]
- Heath W. F., Merrifield R. B. A synthetic approach to structure-function relationships in the murine epidermal growth factor molecule. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6367–6371. doi: 10.1073/pnas.83.17.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard A. E., Kollman P. A. An analysis of current methodologies for conformational searching of complex molecules. J Med Chem. 1988 Sep;31(9):1669–1675. doi: 10.1021/jm00117a001. [DOI] [PubMed] [Google Scholar]
- Jelicks L. A., Naider F. R., Shenbagamurthi P., Becker J. M., Broido M. S. A type II beta-turn in a flexible peptide: proton assignment and conformational analysis of the alpha-factor from Saccharomyces cerevisiae in solution. Biopolymers. 1988 Mar;27(3):431–449. doi: 10.1002/bip.360270307. [DOI] [PubMed] [Google Scholar]
- Kaiser E. T., Kézdy F. J. Amphiphilic secondary structure: design of peptide hormones. Science. 1984 Jan 20;223(4633):249–255. doi: 10.1126/science.6322295. [DOI] [PubMed] [Google Scholar]
- Krüger P., Strassburger W., Wollmer A., van Gunsteren W. F. A comparison of the structure and dynamics of avian pancreatic polypeptide hormone in solution and in the crystal. Eur Biophys J. 1985;13(2):77–88. doi: 10.1007/BF00256528. [DOI] [PubMed] [Google Scholar]
- Montelione G. T., Wüthrich K., Nice E. C., Burgess A. W., Scheraga H. A. Identification of two anti-parallel beta-sheet conformations in the solution structure of murine epidermal growth factor by proton magnetic resonance. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8594–8598. doi: 10.1073/pnas.83.22.8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilges M., Gronenborn A. M., Brünger A. T., Clore G. M. Determination of three-dimensional structures of proteins by simulated annealing with interproton distance restraints. Application to crambin, potato carboxypeptidase inhibitor and barley serine proteinase inhibitor 2. Protein Eng. 1988 Apr;2(1):27–38. doi: 10.1093/protein/2.1.27. [DOI] [PubMed] [Google Scholar]
- Sippl M. J., Scheraga H. A. Cayley-Menger coordinates. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2283–2287. doi: 10.1073/pnas.83.8.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gunsteren W. F., Berendsen H. J., Geurtsen R. G., Zwinderman H. R. A molecular dynamics computer simulation of an eight-base-pair DNA fragment in aqueous solution: comparison with experimental two-dimensional NMR data. Ann N Y Acad Sci. 1986;482:287–303. doi: 10.1111/j.1749-6632.1986.tb20962.x. [DOI] [PubMed] [Google Scholar]
- Wright P. E., Dyson H. J., Lerner R. A. Conformation of peptide fragments of proteins in aqueous solution: implications for initiation of protein folding. Biochemistry. 1988 Sep 20;27(19):7167–7175. doi: 10.1021/bi00419a001. [DOI] [PubMed] [Google Scholar]