Abstract
Introduction
Autosomal dominant cortical tremor, myoclonus, and epilepsy (ADCME) syndrome is a genetically heterogeneous and under-recognized disease characterized by tremulous movements mimicking essential tremor, myoclonus, and rare generalized tonic-clonic seizures. Here we described the clinical and electrophysiological features of three siblings with ADCME syndrome mimicking juvenile myoclonic epilepsy (JME).
Methods
Three siblings (two females and one male) diagnosed with ADCME were analyzed by electroencephalogram (EEG), somatosensory evoked potentials, and accelerometric recordings. The results were compared with 14 JME patients without tremor and 14 with essential tremor (ET).
Results
The shared features of the siblings were cortical tremor, myoclonia, epilepsy, migraine, and psychiatric symptoms. In all siblings, tremor had started before myoclonic epilepsy associated with 4–6 Hz generalized spike and wave discharges. The N20-P25 and P25-N35 amplitudes were substantially higher in the three siblings with ADCME. Although tremor frequencies were similar to those of the ET group, the siblings had mild interrupting low-amplitude myoclonus, suggestive of cortical tremor, in the accelerometric analysis.
Conclusion
We presented a detailed clinical evaluation with electrophysiological confirmation of ADCME syndrome in a Turkish family. This rare clinical picture might be misdiagnosed as JME and should be kept in mind to ensure correct diagnosis and to provide a homogenous group for genetic studies.
Keywords: Cortical tremor, myoclonus, epilepsy
INTRODUCTION
Tremor is a predominant symptom in some epilepsy patients. The generators of this tremor are not clear and are mainly speculated to be a valproate (VPA), enhanced essential tremor (ET) or comorbidity (1). However, there are families with an autosomal dominant syndrome, described with various acronyms, comprising cortical tremor, myoclonus, and epilepsy (2,3,4,5,6,7).
Electrophysiological studies are needed for differential diagnosis (5,8). We think that this genetic syndrome is underdiagnosed because of the lack of awareness. Here, we described three siblings of a Turkish family with autosomal dominant cortical tremor, myoclonus, and epilepsy (ADCME) syndrome initially misdiagnosed as juvenile myoclonic epilepsy (JME).
METHODS
Three siblings were re-evaluated clinically when tremor was recognized as their common complaint. The Ethical Committee of Istanbul University, Istanbul School of Medicine approved the study and informed consent was obtained from the patients. The neurophysiological evaluations included the following:
1. Electroencephalography
Background activity, response to intermittent photic stimulation, hyperventilation, and other pathological findings were investigated by an experienced clinical neurophysiologist (BB).
2. Accelerometer
Recordings were made by using an electromyography/evoked potential (EMG/EP) device (Viasys Healtcare Neurocare, Nicolet Viking Select Neurodiagnostic System Version 11.1). Surface EMG was recorded by bar electrodes placed over the forearm flexor and extensor muscles. Accelerometric recordings were made using 3 axes movement sensors (Square Movement Sensor, 20X20 mm, TEMEC Instruments B.V. P.O. Box 3011 NL-6460, Kerkrade, Netherlands). Simultaneous recordings were made using the four channels in the following order:
Channel 1: Accelerometer sensor on the extensor side of the third metacarpophalangeal joint.
Channel 2: Surface EMG electrode on the extensor muscles of the forearm.
Channel 3: Surface EMG electrode on the flexor muscle of the forearm.
Channel 4: Accelerometer sensor on the lateral side of the second metacarpophalangeal joint.
The tests were performed in a silent and comfortable room maintained at a constant temperature. Recordings from right and left arms were performed in the following positions: a) In a resting position; b) When the arm was extended; c) When the arm was extended and holding a standard load of 500 g; d) When the arm was extended and holding 1000 g. Each recording lasted 30 s for each analysis. Data was evaluated visually and with a computer based analysis program (Viking Select Master Software V8.1, Viasys Healthcare, CareFusion Switzerland: 317 Sàrl A-One Business Centre Zone D’activités Vers-la-Pièce no 10 CH-1180 Rolle, Suisse). The sampling was done at 800 Hz with 30000 data points and a 90% confidence interval.
3. Median Nerve Somatosensorial Evoked Potentials (SEPs) and C Reflex Recordings
A five channel EMG/EP machine (CareFusion, Synergy, Middletown, WI, 53562 USA) was used. Right and left median nerves were stimulated submaximally with superficial electrodes. C reflex was recorded with superficial electrodes placed on the thenar muscles. Recordings were made simultaneously with four channels: C3′-Fz, Cz′-Fz, C4′-Fz, and the thenar muscles at rest. Responses were averaged 250 times and recorded at least 2 times. We measured the latency of N20 and P25 components (ms), N20-P25 and P25-N35 amplitudes (microvolt), and observed the C reflex when present. The SEPs were considered as “giant” if the amplitudes of N20-P25 or P25-N35 exceeded normal control mean values by +3 standard deviation (SD).
Statistical Analysis
The results of the siblings were compared with JME patients without tremor (n:14) and patients with ET (n:14). Statistical Package for the Social Sciences (IBM SPSS Statistics, New York, USA) version 20.0 software was used for statistical evaluations and p<0.05 was accepted as the limit of significance. Comparison of SEP results of the groups was conducted using the non-parametric Kruskal Wallis test.
RESULTS
The parents, without any neurological complaints, were from the same village but there was no consanguinity. The deceased grandmother, one living uncle, and one living aunt, all from the maternal side, had a history of epilepsy, but they could not be evaluated in our institution. Clinical and neurophysiologic findings of the siblings are summarized in Table 1. All described a feeling of sudden weakness in the legs under stressful conditions causing them to drop, but their formal neurological examinations were normal without any ataxia or sensory motor deficits. They showed a similar clinical picture characterized by tremor, migraine, and psychiatric disorders, which had begun before epilepsy. Improvement of epileptic seizures with treatment was observed in all siblings but without one-year remission. Tremor was not responsive to beta-blockers and primidone could not be used due to drowsiness in two cases. The siblings did not show any progression during the follow-up period of 5 years.
Table 1.
Clinical and laboratory features of the siblings
| Patient/Current Age/Sex | S1/31 y/M | S2/27 y/F | S3/26 y/F |
|---|---|---|---|
| Age at seizure onset | 17 y | 16 y | 14 y |
| Seizure type, age at onset | MYO 17y, GTC 26y, FS 3y | MYO 16y, ABS 16y | MYO 14y, ABS 15y, GTC 14y |
| Tremor; age at onset, dominant side | 16, Left >Right | 16, Left >Right | 13–14, Left >Right |
| Migraine without aura | + | + | + |
| Psychiatric features | + D | + A | + D, A |
| Medications | VPA | VPA, LTG | VPA, LTG, LEV |
| EEG | Asymmetric 4–5 GSW and PSW Increase in HV Background activity: 9–10 Hz | 4–5 Hz GSW Induced by IPS Background activity: 9–10 Hz | 5–6 Hz GSW and PSW Induced by HV and IPS Background activity: 8–9 Hz |
| MRI | Normal | Pituitary microadenoma (subclinical) | Normal |
| Last Medication | VPA 1250 mg/day | VPA 1000 mg/day | VPA 500 mg/day + LEV1500 mg/day |
| Frequency of seizures (at presentation) | 1 MYO/Week | 1 MYO/Day 1–2 ABS/Month | 1–2 MYO/Day |
| Frequency of seizures (last medication) | 1 MYO/Month | 1 MYO/Month | 3–4 MYO/Month |
M: male; F: female; Y: years; MYO: myoclonus; ABS: absence; GTC: Generalized Tonic-Clonic; FS: Febrile Seizures; D: depression; A: anxiety; VPA: Na-Valproate; LTG: lamotrigine; LEV: levetiracetam; GSW: generalized spike and waves; PSW: polyspike and waves; HV: hyperventilation; IPS: intermittent photic stimulation; MRI: magnetic resonance imaging
1. EEG (Table 1)
EEG revealed normal background activity with 4–6 Hz generalized spike and wave (GSW) and polyspike and wave (PSW) discharges (Figure 1).
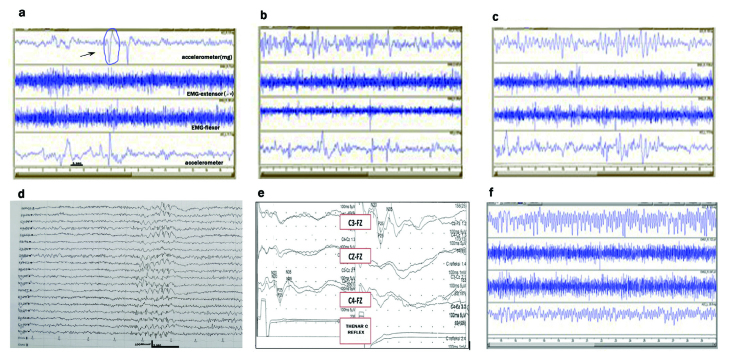
Figure 1.
a–f. The polygraphic recordings of accelerometric analysis of the S2 patient with posture (a), with a 500 g weight, arrow shows myoclonus (b), and a 1000 g weight (c) showing increase of tremulous movements with intervening brief myoclonic jerks. (d) EEG showing fast generalized spike and waves. (e) Giant SEP- absence of C reflex. (f) Example of a typical control patient with essential tremor by accelerometric analysis
2. Accelerometric analysis results
In the siblings, tremor did not show a consistent pattern in the computerized analysis. Its mean peak frequency and amplitude were 7.04±0.94 Hz and 65.23±65.22 milligravity, respectively. In the ET group with a typical consistent tremor pattern, the mean peak frequency was 7.06±1.57 Hz and the amplitude was 83.33±80.61 milligravity. There was no statistical difference between the two groups. Under 500 and 1000 g of weight load, the frequency and amplitude of tremor were increased in all patients, as shown in Figure 1 a–c. Besides this, intervening low-amplitude myoclonic movements of the fingers were typically observed in the siblings.
3. SEP Results (Table 2)
Table 2.
Somatosensory evoked potentials results and comparison
| GROUP | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Siblings | JME without tremor | Essential tremor | Statistics | |||||||
| Mean± Standard deviation | Minimum | Maximum | Mean± Standard deviation | Minimum | Maximum | Mean ±Standard deviation | Minimum | Maximum | Significance* | |
| Right N20 latency (ms) | 19.28±2.03 | 17.10 | 21.10 | 19.55±1.41 | 17.50 | 21.51 | 20.63±1.99 | 18.00 | 23.20 | n.s. |
| Left N20 latency (ms) | 18.80±2.09 | 17.40 | 21.20 | 19.35±1.29 | 17.50 | 21.67 | 20.53±1.81 | 18.17 | 23.50 | n.s. |
| Right P25 latency (ms) | 24.73±1.58 | 23.15 | 26.30 | 25.62±5.07 | 20.67 | 38.20 | 26.76±2.96 | 20.85 | 30.80 | n.s. |
| Left P25 latency (ms) | 24.43±1.68 | 23.25 | 26.35 | 25.16±4.44 | 20.20 | 35.80 | 26.89±2.80 | 21.20 | 30.30 | n.s. |
| Right N35 latency (ms) | 34.92±2.90 | 32.00 | 37.80 | 35.55±7.42 | 26.90 | 54.60 | 33.60±4.28 | 23.10 | 39.75 | n.s. |
| Left N35 latency (ms) | 33.93±3.23 | 31.35 | 37.55 | 35.19±6.87 | 26.20 | 51.90 | 35.28±5.73 | 23.10 | 44.55 | n.s. |
| Right N20-P25 amplitude (μV) | 7.53±2.17 | 5.90 | 10.00 | 4.25±1.16 | 3.10 | 6.60 | 2.93±0.90 | 0.92 | 4.20 | p=0.001 |
| Left N20-P25 amplitude (μV) | 6.57±2.00 | 4.50 | 8.50 | 3.95±0.98 | 2.30 | 5.80 | 3.07±1.11 | 1.30 | 4.60 | p=0.008 |
| Right P25-N35 amplitude (μV) | 6.73±1.74 | 5.40 | 8.70 | 2.31±1.23 | 0.94 | 4.90 | 1.60±0.71 | 0.35 | 2.80 | p=0.01 |
| Left P25-N35 amplitude (μV) | 5.80±1.73 | 3.80 | 6.90 | 1.91±0.96 | 0.76 | 3.69 | 1.41±0.90 | 0.29 | 3.10 | p=0.012 |
Kruskal Wallis test,
n.s: Not significant 274
The mean N20-P25 and P25-N35 amplitudes of the siblings were statistically higher than those found in both control groups. SEP responses were categorized to be ‘giant’ in all three siblings bilaterally, in 5 patients (35%) in the JME without tremor group (but only unilaterally), and in none of ET group. None of the siblings had C reflex.
DISCUSSION
We present three siblings with ADCME syndrome from Turkey. Their common complaints were tremor like movements, myoclonic jerks, migraine, and psychiatric disorders into addition to rare generalized tonic-clonic and absence seizures. The cardinal features of ADCME were tremulous movements that actually represented brief cortical distal myoclonus. These mild involuntary movements were usually unnoticed even though they could be visually observed with detailed examination of the fingers or hands. An accelerometric study may help to verify this different type of tremulous movement (8). The recordings of our siblings showed arrhythmic, or semi-rhythmic, low-amplitude tremors with a mean frequency of 7 Hz and superimposing mild myoclonus of the fingers, which were seen at rest but increased with posture and action. Rare polygraphic studies in ADCME revealed similar findings (8).
Giant SEPs and long latency reflexes (C reflex) were reported in some patients with ADCME to support the cortical origin of myoclonus (5,7). On the other hand, giant SEPs were also described in some cases of JME (9). Remarkably, comparison of our SEP results showed that N20-P25 and P25-N35 amplitudes of the 3 siblings were statistically higher than both control groups with ET and JME without tremor, which was not reported before. Different from some other studies, we did not observe C reflex in any of our patients (5).
EEGs of our siblings demonstrated normal background activity with regular GSW-PSWs. The initial frequency of GSWs was 4 to 6 Hz, which was clearly faster than the classical 3–4 Hz GSWs. This peculiar EEG pattern with high frequency GSWs was not reported before in ADCME. In 2 of 3 siblings, discharges were sensitive to intermittent photic stimulation, consistent with previous studies, but these changes are not specific for this syndrome (5).
All our patients had concomitant psychiatric diseases including depression and anxiety as well as migraine without aura. Psychiatric comorbidity was reported to be high in ADCME patients (10,11). Including the first Turkish family reported before, migraine seemed to be higher in European pedigrees in comparison to those from Japan (4,10,11).
Little is known about the pathophysiology of ADCME. We observed electrophysiological changes supporting the presence of cortical hyperexcitability. There are two hypotheses: cerebellar and intracortical dysfunction could result from a channelopathy (migraine comorbidity supported this theory) or decreased cortical inhibition may be caused by dysfunction of the cerebello-thalamo-cortical loop as a result of primary cerebellar pathology (12). Remarkably, some authors have shown pathologic abnormalities in the cerebellum of ADCME patients supporting this theory (12). Although extensive genetic investigations of numerous pedigrees have been performed and three different loci have been described, no definite gene for ADCME has been found thus far.
Despite these well-defined cardinal features, ADCME syndrome has not been accepted by the International League Against Epilepsy classification. This fact might limit its increased recognition and investigations crucial to identifying the responsible genes.
In conclusion, we reported ADCME in a Turkish family to highlight clinical and electrophysiological details. We want to emphasize that these patients were misdiagnosed with JME and that their recognition and delineation from other syndromes are essential to create homogenous groups for planning new genetic and other pathophysiological studies.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of İstanbul University İstanbul School of Medicine.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – Z.A.Ö., B.B.; Design – Z.A.Ö., B.B.; Supervision – B.B., A.E.Ö; Resources – Z.M., Z.A.Ö.; Materials – Z.A.Ö.; Data Collection and/or Processing – Z.A.Ö., Z.M.; Analysis and/or Interpretation – Z.A.Ö., B.B., Z.M., A.E.Ö, E.O.A.; Literature Search – E.O.A., Z.A.O., B.B.; Writing Manuscript – E.O.A., Z.A.Ö.; Critical Review – Z.M., A.E.O, B.B.; Other – Z.A.Ö.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Morgan JC, Sethi KD. Drug-induced tremors. Lancet Neurol. 2005;4:866–876. doi: 10.1016/S1474-4422(05)70250-7. http://dx.doi.org/10.1016/S1474-4422(05)70250-7. [DOI] [PubMed] [Google Scholar]
- 2.Striano P, Chifari R, Striano S, de Fusco M, Elia M, Guerrini R, Casari G, Canevini MP. A new benign adult familial myoclonic epilepsy (BAFME) pedigree suggesting linkage to chromosome 2p11.1-q12.2. Epilepsia. 2004;45:190–192. doi: 10.1111/j.0013-9580.2004.39903.x. http://dx.doi.org/10.1111/j.0013-9580.2004.39903.x. [DOI] [PubMed] [Google Scholar]
- 3.Depienne C, Magnin E, Bouteiller D, Stevanin G, Saint-Martin C, Vidailhet M, Apartis E, Hirsch E, LeGuern E, Labauge P, Rumbach L. Familial cortical myoclonic tremor with epilepsy: the third locus (FCMTE3) maps to 5p. Neurology. 2010;74:2000–2003. doi: 10.1212/WNL.0b013e3181e396a8. http://dx.doi.org/10.1212/WNL.0b013e3181e396a8. [DOI] [PubMed] [Google Scholar]
- 4.Saka E, Saygi S. Familial adult onset myoclonic epilepsy associated with migraine. Seizure. 2000;9:344–346. doi: 10.1053/seiz.2000.0402. http://dx.doi.org/10.1053/seiz.2000.0402. [DOI] [PubMed] [Google Scholar]
- 5.van Rootselaar AF, van Schaik IN, van den Maagdenberg AM, Koelman JH, Callenbach PM, Tijssen MA. Familial cortical myoclonic tremor with epilepsy: a single syndromic classification for a group of pedigrees bearing common features. Mov Disord. 2005;20:665–673. doi: 10.1002/mds.20413. http://dx.doi.org/10.1002/mds.20413. [DOI] [PubMed] [Google Scholar]
- 6.Licchetta L, Pippucci T, Bisulli F, Cantalupo G, Magini P, Alvisi L, Baldassari S, Martinelli P, Naldi I, Vanni N, Liguori R, Seri M, Tinuper P. A novel pedigree with familial cortical myoclonic tremor and epilepsy (FCMTE): Clinical characterization, refinement of the FCMTE2 locus, and confirmation of a founder haplotype. Epilepsia. 2013;54:1298–1306. doi: 10.1111/epi.12216. http://dx.doi.org/10.1111/epi.12216. [DOI] [PubMed] [Google Scholar]
- 7.Striano P, Zara F, Striano S. Autosomal dominant cortical tremor, myoclonus and epilepsy: many syndromes, one phenotype. Acta Neurol Scand. 2005;111:211–217. doi: 10.1111/j.1600-0404.2005.00385.x. http://dx.doi.org/10.1111/j.1600-0404.2005.00385.x. [DOI] [PubMed] [Google Scholar]
- 8.Terada K, Ikeda A, Mima T, Kimura M, Nagahama Y, Kamioka Y, Murone I, Kimura J, Shibasaki H. Familial cortical myoclonic tremor as a unique form of cortical reflex myoclonus. Mov Disord. 1997;12:370–377. doi: 10.1002/mds.870120316. http://dx.doi.org/10.1002/mds.870120316. [DOI] [PubMed] [Google Scholar]
- 9.Salas-Puig J, Tunon A, Diaz M, Lahoz CH. Somatosensory evoked potentials in juvenile myoclonic epilepsy. Epilepsia. 1992;33:527–530. doi: 10.1111/j.1528-1157.1992.tb01704.x. http://dx.doi.org/10.1111/j.1528-1157.1992.tb01704.x. [DOI] [PubMed] [Google Scholar]
- 10.Crompton DE, Sadleir LG, Bromhead CJ, Bahlo M, Bellows ST, Arsov T, Harty R, Lawrence KM, Dunne JW, Berkovic SF, Scheffer IE. Familial adult myoclonic epilepsy: recognition of mild phenotypes and refinement of the 2q locus. Arch Neurol. 2012;69:474–481. doi: 10.1001/archneurol.2011.584. http://dx.doi.org/10.1001/archneurol.2011.584. [DOI] [PubMed] [Google Scholar]
- 11.Coppola A, Santulli L, Del Gaudio L, Minetti C, Striano S, Zara F, Striano P. Natural history and long-term evolution in families with autosomal dominant cortical tremor, myoclonus, and epilepsy. Epilepsia. 2011;52:1245–1250. doi: 10.1111/j.1528-1167.2011.03017.x. http://dx.doi.org/10.1111/j.1528-1167.2011.03017.x. [DOI] [PubMed] [Google Scholar]
- 12.Van Rootselaar AF, van der Salm SMA, Bour LJ, Edwards MJ, Brown P, Aronica E, Rozemuller-Kwakkel JM, Koehler PJ, Koelman JH, Rothwell JC, Tijssen MA. Decreased cortical inhibition and yet cerebellar pathology in familial cortical myoclonic tremor with epilepsy. Mov Disord. 2007;22:2378–2385. doi: 10.1002/mds.21738. http://dx.doi.org/10.1002/mds.21738. [DOI] [PubMed] [Google Scholar]