Abstract
Aims
A pilot randomized controlled trial (RCT) to assess the feasibility and potential efficacy of assertive community treatment (ACT) in adults with alcohol dependence.
Methods
Single blind, individually randomized, pilot RCT of 12 months of ACT plus treatment as usual (TAU) versus TAU alone in adults (age 18+ years) with alcohol dependence and a history of previous unsuccessful alcohol treatment attending specialist community alcohol treatment services. ACT aimed to actively engage participants for 12 months with assertive, regular, minimum weekly contact. ACT was combined with TAU. TAU comprised access to the full range of services provided by the community teams. Primary outcome is mean drinks per drinking day and percent days abstinent at 12 months follow up. Analysis of covariance was conducted using 80% confidence intervals, appropriate in the context of a pilot trial.
Results
A total of 94 participants were randomized, 45 in ACT and 49 in TAU. Follow-up was achieved with 98 and 88%, respectively at 12 months. Those in ACT had better treatment engagement, and were more often seen in their homes or local community than TAU participants. At 12 months the ACT group had more problems related to drinking and lower quality of life than TAU but no differences in drinking measures. The ACT group had a higher percentage of days abstinent but lower quality of life at 6 months. The ACT group had less unplanned healthcare use than TAU.
Conclusions
An trial of ACT was feasible to implement in an alcohol dependent treatment population.
Trial registration
ISRCTN22775534
Introduction
Excessive alcohol use places a considerable burden on society, and is the fifth leading cause of disability globally (Lim et al., 2012). In England, ~4% of the adult population are alcohol dependent (McManus et al., 2009) and the government has identified provision of effective treatment as a priority in reducing these costs (HM Government, 2012). Current specialist alcohol service provision in the UK focuses on discrete, time-limited episodes of intervention, typically up to 12 weeks duration and emphasizes personal choice and motivation. However, there are high rates of non-attendance in the treatment population (Mitchell and Selmes, 2007), <40% complete a treatment programme (Passetti et al., 2008), and after successful completion approximately one-third continue to drink heavily and have poor long-term outcomes (Marshall et al., 1994). For this group of patients alcohol dependence is a chronic relapsing disorder and in typical practice, treatment episodes extending over many years is typical. Current alcohol care pathways require significant levels of motivation and self-efficacy to navigate, that few patients possess (Gilburt et al., 2015).
Assertive community treatment (ACT) is a model of intensive case management which has been shown to be effective in improving engagement and retaining those with serious mental illness and those with alcohol and drug use comorbidities (Dieterich et al., 2010). A number of studies have applied individual components of ACT to the treatment of alcohol dependence with positive outcomes (Gilbert, 1998; Stout et al., 1999; Hilton et al., 2001). A non-randomized cohort study of assertive engagement methods resulted in a significantly greater number of patients completing treatment and entering aftercare (Passetti et al., 2008). However, the potential benefits of individual elements of ACT in patients with alcohol dependence without severe mental illness remain unclear, and there have been no published randomized controlled trials investigating the impact of ACT in this population to date. We therefore conducted a pilot randomized controlled trial of ACT in people with alcohol dependence and a history of previous unsuccessful treatment to investigate (a) the feasibility of recruiting and retaining people seeking treatment for alcohol dependence in a clinical trial of ACT and (b) the potential efficacy of ACT on the drinking behaviours and quality of life of people with alcohol dependence and a history of disengagement from specialist alcohol treatment services.
Method
This study (ACTAD trial) was reviewed and approved by the National Research Ethics Service Committee London—Chelsea (REC number: 08/H0801/113) and the trial was registered with the International Standard Randomized Controlled Trial registry (ISRCTN22775534) prior to the commencement of data collection. Progress of the trial, adherence to protocol and participant safety were overseen by an independent trial steering committee. A detailed protocol for the study has already been published (Gilburt et al., 2012).
Trial design
We undertook a single blind, pilot randomized controlled trial of ACT plus treatment as usual (TAU) compared with a TAU alone for people with alcohol dependence. The trial was conducted across two NHS trusts in South London: participants were recruited at the point of referral to one of three specialist community drug and alcohol services for a new episode of treatment. Participants were recruited between 23 March 2010 and 23 January 2012.
An equal number was randomized to each treatment condition. Randomization was conducted using a secure independent service at the level of the individual and was stratified by alcohol dependence (≤30 and >30) as measured by the Severity of Alcohol Dependence Questionnaire (Stockwell et al., 1979) and by site.
As a pilot study, the sample size was selected to determine feasibility and to allow for an analysis of potential effect prior to designing a full scale definitive trial. In order to ascertain these parameters, we considered a clinically meaningful difference in alcohol consumption between the groups at 12 months to be of the order of 30% and estimated the numbers required with 80% power and an alpha of 0.2, appropriate for a pilot study, using two-sided test. In addition, we considered an acceptable rate of follow-up at 12 months to be not less than 75%. Our required sample size was estimated as 45 in each group at baseline, with the expectation that at least 34 would be followed-up at 12 months.
Recruitment
All potential participants were in the first instance identified and approached by a member of the community drug and alcohol team who informed them about the trial and obtained verbal consent to being contacted by a researcher. A member of the research team met with the participant to explain the trial further and check eligibility for the trial. Participants who met all the criteria were invited to provide written consent for the trial.
Inclusion and exclusion criteria
All participants met the following criteria: (a) age 18 years or over; (b) able to understand English sufficiently well to obtain informed consent and complete the assessment instruments; (c) attended an NHS community addiction service in either of the participating trusts for alcohol dependence on at least one previous occasion in the last 5 years; and (d) an ICD-10 diagnosis of alcohol dependence as determined using the Composite International Diagnostic Interview (Robins et al., 1988). We excluded patients who were: (a) unable to provide written informed consent; (b) street homeless; (c) diagnosed with a psychotic disorder; (d) in receipt of assertive outreach services or had community mental health team input once a month or more; (e) had severe cognitive impairment as determined by the Mini Mental State Examination score of ≤10 (Folstein et al., 1975); and (f) who had a history of violence to staff or were registered under the UK Multi-Agency Public Protection Arrangement.
Interventions
Assertive community treatment
The intervention was informed by the original ACT model used for people with psychosis and findings from research identifying effective elements of assertive outreach in UK studies (Burns et al., 2000). The ACT intervention comprised:
A maximum caseload of 15 ACT patients per ACT practitioner.
Input from a multidisciplinary team (including psychiatrists and substance misuse specialists).
Regular contact (minimum of once a week), with 50% of contacts occurring outside of the service settings either in the patients’ home or neighbourhood, and in which short frequent contacts rather than long complex contacts were encouraged.
Assertive engagement where there were persistent and repeated attempts to contact, and an emphasis on maintaining contact and building relationships.
A focus on both health and social care needs, including accommodation, leisure, occupation and physical and mental health.
A flexible approach, focusing on the patient's goals even when these were peripheral to the alcohol dependence.
Practitioners were explicit about their role both in care planning and in visits.
An ethos of ‘going out of your way’, where practitioners are encouraged to step outside of professional roles and ‘go the extra mile’ for patients.
Extended care provided for a prolonged period of 1 year.
An intervention manual was developed in collaboration with experts in the provision of ACT. The manual outlined the core components of the ACT intervention and the principles of delivery in line with components of the alcohol treatment pathway. This included hours of operation, caseload, target client group, source of referrals, assessment, interventions and care plan and the use of interventions for alcohol dependence. Policies on training and development for ACT practitioners, home visiting, lone working and risk assessment and the withdrawal from treatment were also incorporated. Finally the manual provided an overview of the research processes involved in the trial.
Treatment as usual
Participants randomized to the intervention arm received TAU plus ACT, while those randomized to the control group received TAU alone. TAU included the allocation of a keyworker when available with contact as required as per local team policies. Contact was primarily conducted within a service setting (community addictions service or general practice) via an appointment-based system. These NHS services specialize in treatment of patients with drug and/or alcohol dependence. They are located in community-based treatment centres and staffed by multidisciplinary teams comprising specialist addiction psychiatrists, nurses, clinical psychologists, social workers, counsellors and community support workers. They provide a wide range of interventions in accordance with national clinical guidelines (Department of Health, 2007; National Institute for Health and Care Excellence, 2011). Participants received a full assessment of alcohol, social and physical health needs and a risk assessment. The focus of treatment was primarily on alcohol dependence, promoting abstinence and relapse prevention. This included access to medical detoxification, psychological interventions focused on drinking behaviour and aftercare as required. TAU also included input from specialists in addiction psychiatry, clinical psychology and social work where available. When these services were not directly provided by the community drug and alcohol treatment service, participants were most often referred or signposted to other relevant agencies as required. The majority of participants were discharged to primary care from the specialist service within 12 weeks of being allocated a keyworker unless significant risks were identified. Failure to attend several appointments resulted in discharge from the service. These approaches are broadly in line with national service delivery at the time of the study. In addition we collected data on the actual care received in order to understand the content of TAU.
Training and support
A training programme for ACT staff was developed by the team in collaboration with experts in ACT and addictions treatment. The training comprised workshops providing information on ACT, its history, implementation in mental health and trial processes; a 1-day clinical placement with a mental health assertive outreach team shadowing a member of staff; and a final workshop day focused on the application of ACT in addictions services. A total of seven practitioners from the trial sites attended the training (four from one service, three from the second) including substance misuse, nursing and addiction psychiatry professionals. Additional training was delivered on a one-to-one basis to a further three practitioners at a third service setting following late incorporation into the trial, and in response to staff turnover.
In addition to existing team-based clinical supervision, practitioners delivering ACT were encouraged to attend monthly ACT group supervision meetings throughout the course of the study. These meetings were facilitated by the Chief Investigator, an experienced addictions psychiatrist, and the trial manager. Meetings focused on enabling practitioners to share experience and practice in order to support delivery of ACT, address clinical issues arising as a result of the intervention, and reinforce fidelity of the ACT intervention.
Intervention fidelity
Staff providing care to participants in both arms of the trial completed a contact log detailing the care they provided for each patient following each contact. The log, developed from a previous study of assertive outreach, included details about the mode of contact, (i.e. face to face, telephone), setting, focus of contact, and the member of staff involved (Burns et al., 2000). To enhance accuracy, data were additionally collected from the electronic clinical records of each participating service following completion of the trial. Data highlighting additional contacts with a participant and information absent from contacts recorded in the log was used to supplement existing information.
Outcome measures
Feasibility and acceptability of delivering ACT to people with alcohol dependence was measured by levels of recruitment into the trial and retention of participants in both arms of the study at 12 months follow-up.
The primary outcome measures were mean drinks per drinking day and percent days abstinent at 12 months measured using the Time Line Follow Back form 90 (TLFB; Miller, 1995). Secondary outcomes included total alcohol consumed, other consumption measures at 6 months and other drug use measured using TLFB, alcohol-related problems (Alcohol Problems Questionnaire; APQ; Drummond, 1990), severity of alcohol dependence (Severity of Alcohol Dependence Questionnaire; SADQ; Stockwell et al., 1979), health utility (EQ-5D; EuroQol Group, 1990), health-related quality of life (SF-12; Ware et al., 1996), motivation to change (Readiness to Change Treatment version; Heather et al., 1999), social network involvement (Important People and Activities Inventory; Zywiak et al., 2002), health service utilization (York Service Use Questionnaire; Drummond et al., 2009). All measured at baseline and then 6 and 12 months after randomization. Data were collected through face-to-face interviews conducted by trained researchers working on this trial who were blinded to the treatment allocation. All participants were reminded by the researcher at the beginning of each follow-up interview not to reveal the treatment allocation they received. For each time point (including the baseline) assessment was made was of the previous 6 months. However, in the case of TLFB form 90 for alcohol and drugs the time period covered at each time point was the previous 90 days.
Statistical analysis
The main hypothesis, stated as a null hypothesis, is that TAU augmented with ACT is no more effective than TAU in reducing alcohol consumption 12 months after randomization. The primary outcome measure was mean drinks per drinking day and percent days abstinent at 12 months follow up. Our primary analysis was by intention-to-treat in which participants are analysed as part of their allocated group irrespective of the treatment received. This provides the most rigorous estimate of effectiveness. The primary outcome measure was analysed using an analysis of covariance approach adjusting for known confounding variables such as baseline mean drinks per drinking day, age and gender. If the assumptions underlying ANCOVA were not met transformations were undertaken and if transformations were not viable alternative non-parametric approaches were used. Continuous secondary measures were analysed in a similar manner. Categorical variables were analysed using chi-squared statistics and binary outcomes analysed using logistic regression controlling for known confounding variables. Estimates and the 80% confidence intervals are presented.
Results
Feasibility of recruitment
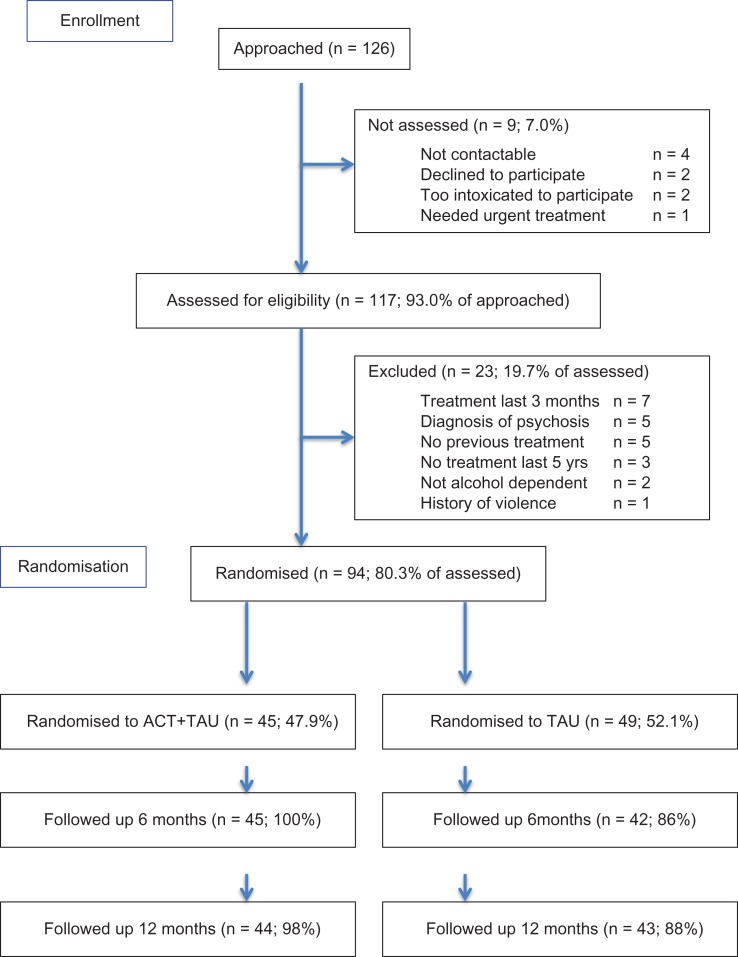
Feasibility outcomes for this study were assessed by recruitment and retention rates in both arms of the study. Participant flow is indicated in the Consort diagram (Fig. 1). Of 126 individuals assessed for eligibility, four could not be contacted after referral by the team, two declined to participate, two were assessed as too intoxicated to participate and one needed to be seen immediately by the clinical team so could not be seen by a researcher. A total of 117 (93.0%) patients were assessed for eligibility to take part. Of all, 23 did not meet the inclusion criteria (seven had been seen for treatment in the last 3 months, five had been diagnosed with a psychotic disorder, five had no history of treatment with the service, three had not been seen by the service within the last 5 years, two were not alcohol dependent and one had a significant history of violence).
Fig. 1.
Trial consort diagram.
A total of 94 (80.3%) provided consent and were randomized. Overall, 45 participants were randomized to ACT plus TAU arm (47.9%) and 49 participants were randomized to the TAU arm (52.1%). The follow-up rates at 6 months were 100% (n = 45) in the ACT plus TAU arm and 86% (n = 42) in the TAU arm. The follow-up rates at 12 months were 98% (n = 44) in the ACT plus TAU arm and 88% (n = 43) in the TAU arm.
Baseline characteristics
The baseline demographics of the sample are shown in Table 1. The majority of the sample was male (61%), white (87%), not currently in a marital relationship (68%), and living in rented or temporary accommodation (76%), were parents (68%), and had a basic level of educational qualifications (67%). The mean age was 43 years.
Table 1.
Demographics of the sample
| ACT + TAU (n = 45) | TAU (n = 49) | Overall (n = 94) | |
|---|---|---|---|
| Mean age (SD) | 42.6 (9.6) | 43.2 (9.3) | 43.0 (9.6) |
| Male, n (%) | 26 (58) | 31 (63) | 57 (61) |
| Ethnicity, n (%) | |||
| White | 38 (84) | 44 (90) | 82 (87) |
| Black | 2 (4) | 1 (2) | 3 (3) |
| Asian | 3 (6) | 2 (4) | 5 (4) |
| Other | 4 (2) | 2 (4) | 6 (6) |
| Marital status, n (%) | |||
| Never married | 18 (40) | 20 (41) | 38 (40) |
| With partner | 6 (13) | 4 (8) | 10 (11) |
| Married | 10 (22) | 10 (20) | 20 (21) |
| Separated | 6 (13) | 5 (10) | 11 (12) |
| Divorced | 5 (12) | 8 (16) | 13 (14) |
| Widowed | 0 | 2 (5) | 2 (2) |
| Accommodation, n (%) | |||
| Owner/ occupier | 11 (24) | 12 (24) | 23 (24) |
| Rented/ temporary | 34 (76) | 37 (76) | 71 (76) |
| Educational qualifications, n (%) | |||
| None | 17 (38) | 14 (28) | 31 (33) |
| GCSE or equivalent or higher | 28 (62) | 35 (86) | 63 (67) |
| Children, n (%) | 30 (67) | 34 (69) | 64 (68) |
At baseline no significant differences were observed between the groups in terms of study outcome measures (Table 2). All participants met the criteria for alcohol dependence with lower than population average mental and physical health-related quality of life. The majority were in the pre-contemplative stage of change and had high levels of alcohol dependence.
Table 2.
Outcomes by allocated group at baseline, 6 and 12 months
| Baseline | Month 6 | Month 12 | ||||
|---|---|---|---|---|---|---|
| ACT + TAU (n = 45) | TAU (n = 49) | ACT + TAU (n = 43) | TAU (n = 44) | ACT+TAU (n = 42) | TAU (n = 45) | |
| Alcohol use in past 90 days | ||||||
| Mean drinksa consumed (SE) | 2074 (232.9) | 1926 (175.1) | 722.8 (167.9) | 816.3 (160.6) | 728.9 (152.3) | 774.1 (148.7) |
| Mean drinks per day (SE) | 23.05 (2.6) | 21.41 (1.9) | 8.032 (1.9) | 9.070 (1.8) | 8.099 (1.7) | 8.601 (1.6) |
| Mean percent days abstinent (SE) | 13.80 (2.9) | 14.24 (2.8) | 67.83 (5.8) | 57.40 (6.2) | 65.45 (5.6) | 57.41 (6.0) |
| Alcohol problems (APQ scored 1 to 23, 23 most severe) | 11.48 (0.6) | 11.42 (0.5) | 6.37 (0.9) | 6.02 (0.7) | 6.83 (0.9) | 5.29 (0.7) |
| EQ5D (scored 0–1, 0 worse health utility) | 0.62 (0.1) | 0.50 (0.1) | 0.61 (0.1) | 0.68 (0.1) | 0.61 (0.1) | 0.71 (0.1) |
| Health-related quality of life (SF12 scored 0–100, 0 most severe) | ||||||
| Mental component | 30.61 (1.82) | 30.66 (1.93) | 37.08 (2.61) | 39.15 (2.16) | 39.28 (0.06) | 40.84 (2.23) |
| Physical component | 42.30 (1.93) | 31.50 (1.87) | 44.09 (2.07) | 44.22 (1.96) | 43.93 (1.99) | 45.58 (1.99) |
| Readiness to change, n (%)b | ||||||
| Pre-contemplation | 0 | 0 | 1 (3.3) | 1 (2.9) | 1 (4.8) | 0 |
| Contemplation | 35 (81.4) | 37 (75.5) | 15 (50) | 16 (48.4) | 14 (66.7) | 21 (77.8) |
| Action | 8 (18.6) | 12 (24.5) | 14 (46.7) | 17 (50.0) | 6 (28.6) | 6 (22.2) |
| Severity of dependence (SADQ scored 0–60, 60 most dependent) | 32.38 (2.102) | 31.86 (2.043) | 18.19 (2.233) | 20.41 (2.581) | 18.50 (2.273) | 15.13 (1.937) |
aOne drink = 1 UK unit of alcohol = 8 g ethanol.
bData on Readiness to Change was not obtained from two participants at baseline and 23 at 6 months, and 39 at 12 months. This was because these Readiness to Change questions were not applicable to participants who were already abstinent at these time points.
Intervention delivery
Compared with participants randomized to the TAU arm of the trial, participants receiving ACT + TAU were in contact with services for a significantly longer period of time, (t(76.77) = 15.62, P < 0.001), received a greater mean number of contacts during treatment (t(57.75) = 10.52, P < 0.001), and received a significantly greater percentage of contacts in a venue other than the addictions treatment service (t(66.34) = 7.47, P < 0.001).
Primary outcomes
Tables 3 and 4 contain outcomes at 6 and 12 months. Data presented has been adjusted by baseline covariates; baseline value, age and gender. There were reductions in the total alcohol consumed and increases in the percent days abstinent and number of participants abstinent at 12 months. There were reductions in drinks per drinking day in both groups at 12 months compared to baseline but the difference between allocated groups was small and not statistically significant (Table 5).
Table 3.
Contact with staff during treatment by allocated group
| ACT + TAU (n = 45) | TAU (n = 46) | Mean difference (80% CI) | P-value | |
|---|---|---|---|---|
| Length of contact, mean days (SD) | 335.09 (55.87) | 95.54 (87.35) | 239.55 (219.72; 259.37) | 0.000 |
| Number of contacts with participant, mean (SD) | ||||
| Face to face | 22.04 (12.79) | 5.09 (4.52) | 16.96 (14.34; 19.58) | 0.000 |
| Telephone | 22.47 (12.14) | 3.17 (4.29) | 19.29 (16.81; 21.78) | 0.000 |
| Group | 1.98 (4.59) | 1.20 (2.78) | 0.78 (−0.25; 1.81) | 0.330 |
| Number of contacts with carer, mean (SD) | ||||
| Face to face | 0.16 (0.52) | 0 | 0.16 (0.06; 0.26) | 0.051 |
| Telephone | 2.50 (4.53) | 0.57 (1.38) | 1.94 (1.01; 2.86) | 0.009 |
| Frequency of contact per week, mean (SD) | ||||
| All contacts | 1.0 (0.41) | 1.90 (2.28) | −0.89 (−1.34; −0.45) | 0.012 |
| All contacts with participant | 0.95 (0.39) | 1.83 (2.30) | −0.88 (−1.33; −0.43) | 0.014 |
| Face to face and group contacts | 0.48 (0.28) | 0.99 (1.71) | −0.51 (−0.84; −0.18) | 0.051 |
| Place of contact, mean % (SD) | ||||
| Addictions service | 20.67 (15.01) | 59.49 (31.95) | −38.83 (−45.65; −32.00) | 0.000 |
| Home/community | 17.84 (17.91) | 0.58 (2.67) | 17.25 (13.75; 20.76) | 0.000 |
| Telephone | 54.09 (17.89) | 37.49 (32.28) | 16.59 (9.54; 23.65) | 0.003 |
| Other | 7.34 (8.05) | 1.86 (7.70) | 5.49 (3.35; 7.62) | 0.001 |
Table 4.
Mean and mean difference of ACT plus TAU versus TAU in outcomes at 6 months adjusted by baseline covariates
| ACT + TAU mean (80% CI) | TAU Mean (80% CI) | Mean difference (80% CI) | P-value | |
|---|---|---|---|---|
| Alcohol use in past 90 days | ||||
| Mean drinksa consumed | 719 (504; 934) | 820 (607; 1032) | −101 (−404; 202) | 0.67 |
| Mean drinks per day | 7.95 (5.57; 10.33) | 9.15 (6.79; 11.50) | −1.97 (−4.55; 2.16) | 0.64 |
| Mean percent days abstinent | 68.43 (60.63; 76.23) | 56.81 (49.09; 64.52) | 11.62 (0.64; 22.61) | 0.18 |
| Number abstinent (%) | 14 (32.6%) | 13 (29.5%) | – | 0.47 |
| Alcohol problems (APQ) | 6.37 (5.43; 7.30) | 6.03 (5.11; 6.95) | 0.34 (−0.97; 1.65) | 0.74 |
| EQ5D | 0.58 (0.53; 0.64) | 0.71 (0.65; 0.76) | −0.12 (−0.21; -0.04) | 0.05 |
| Health-related quality of life (SF12) | ||||
| Mental component | 36.84 (33.90; 39.77) | 38.89 (35.99; 41.78) | −2.05 (−6.17; 2.08) | 0.52 |
| Physical component | 43.73 (41.40; 46.06) | 44.33 (42.03; 46.23) | −0.60 (−3.87; 2.68) | 0.82 |
| Readiness to change n (%) | ||||
| Pre-contemplation | 1 (3.3) | 1 (2.9) | – | 0.96 |
| Contemplation | 15 (50.0) | 16 (47.1) | – | 0.37 |
| Action | 14 (46.7) | 17 (50.0) | – | |
| Severity of dependence (SADQ) | 17.94 (15.15; 20.74) | 20.64 (17.94; 23.34) | −2.70 (−6.59; 1.19) | |
aOne drink = 1 UK unit of alcohol = 8 g ethanol
Table 5.
Mean and mean difference of ACT plus TAU versus TAU in outcomes at 12 months adjusted by baseline covariates
| ACT+TAU mean (80% CI) | TAU mean (80% CI) | Mean difference (80% CI) | P-value | |
|---|---|---|---|---|
| Alcohol use in past 90 days | ||||
| Mean drinksa consumed | 722 (525; 918) | 780 (591; 969) | −58 (−330; 214) | 0.78 |
| Mean drinks per day | 8.02 (5.85; 10.19) | 8.67 (6.58; 10.77) | −0.65 (−3.68; 2.37) | 0.78 |
| Mean percent days abstinent | 65.56 (57.90; 73.22) | 57.30 (49.90; 64.70) | 8.26 (−2.39; 18.92) | 0.32 |
| Number abstinent (%) | 11 (26.2) | 13 (28.9) | – | 0.48 |
| Alcohol problems (APQ) | 6.85 (5.87; 7.83) | 5.28 (4.33; 6.22) | 1.57 (0.21; 2.93) | 0.14 |
| EQ5D | 0.59 (0.53; 0.65) | 0.72 (0.67; 0.78) | −0.14 (−0.22; -0.05) | 0.05 |
| Health-related quality of life (SF12) | ||||
| Mental component | 38.80 (36.08; 41.53) | 41.18 (38.52; 43.85) | −2.05 (−6.17; 2.08) | 0.42 |
| Physical component | 43.52 (41.10; 45.94) | 46.58 (44.21; 48.94) | −2.38 (−6.19; 1.43) | 0.25 |
| Readiness to change, n (%) | ||||
| Pre-contemplation | 1 (3.3) | 0 | – | 0.43 |
| Contemplation | 14 (66.7) | 21(77.8) | – | 0.26 |
| Action | 6 (28.6) | 6 (22.2) | – | |
| Severity of dependence (SADQ) | 18.42 (15.77; 21.07) | 15.21 (12.65; 17.78) | 3.21 (−0.48; 6.89) | |
aOne drink = 1 UK unit of alcohol = 8 g ethanol.
Secondary outcomes
Significant differences were observed between the groups at 6 months with the ACT plus TAU group having a higher percentage of days abstinent. At 6 months the TAU group had significantly fewer alcohol-related problems and health utility, measured using the EQ5D, was significantly better for the TAU group at 6 and 12 months. No other significant differences between groups on other secondary outcome measures, including APQ, SF12, Readiness to Change or SADQ, were found.
Service use
The use of services used in the previous 6 months at 6 and 12 month follow-up are presented in Table 6. At 6 and 12 months the ACT + TAU group used significantly more alcohol day care services than the TAU group and significantly more alcohol outpatient services at 12 months. At 6 months the ACT + TAU group had significantly fewer inpatient days, outpatient visits to non-alcohol-related services and significantly more GP visits. No significant differences were observed between the groups at 12 months.
Table 6.
Mean service utilization (SE) over previous 6 months at 6 and 12 months by allocation
| Month 6 | Month 12 | |||||
|---|---|---|---|---|---|---|
| ACT + TAU | TAU | P-value | ACT+TAU | TAU | P-value | |
| Alcohol services | ||||||
| Day care | 26.5 (4.9) | 12.8 (7.2) | 0.12 | 29.0 (6.9) | 14.2 (5.3) | 0.09 |
| Outpatient | 7.2 (1.3) | 5.5 (1.2) | 0.39 | 6.9 (2.04) | 2.0 (0.7) | 0.02 |
| Inpatient nights | 33.2 (10.4) | 39.2 (10.9) | 0.7 | 61.1 (19.3) | 44.1 (13.0) | 0.5 |
| Other NHS services | ||||||
| Emergency department | 1.3 (0.4) | 2.0 (0.9) | 0.46 | 1.1 (0.3) | 1.4 (0.5) | 0.68 |
| Inpatient nights | 1.2 (9.1) | 26.8 (6.7) | 0.2 | 1.2 (0.8) | 4.2 (3.2) | 0.4 |
| Outpatient | 1.3 (0.2) | 1.9 (0.3) | 0.1 | 2.2 (0.9) | 1.6 (0.3) | 0.56 |
| Day case | 0.6 (0.5) | 0 | 0.4 | 0.4 (0.4) | 0.2 (0.2) | 0.63 |
| GP visit | 6.3 (0.6) | 4.2 (0.7) | 0.02 | 6.2 (0.9) | 5.6 (0.8) | 0.62 |
Discussion
This study demonstrates the feasibility of recruitment and retention of participants in a randomized controlled trial of ACT + TAU versus TAU. Of the 126 patients referred to the research team, 80.3% provided consent and were randomized. This compares favourably with previous RCTs in similar clinical populations where eligibility and consent rates were generally lower (UKATT Research Team, 2005). Further, the follow up rates were higher than in many previous RCTs with similar alcohol dependent clinical populations. We achieved a 92.6% follow-up rate at both 6 months and 12 months, exceeding our minimum target follow up rate of 75% at both follow up points, and may reflect the use of optimal methods to maximize follow-up (Woolard et al., 2004; Robinson et al., 2007; Zweben et al., 2009). The follow up rate was lower in the TAU group compared to the ACT + TAU group, which may have been related to the more intensive nature of the ACT intervention arm, and potentially a degree of disappointment in those randomized to TAU (Woolard et al., 2004). However, the difference in follow up rate was within acceptable limits and greater than the planned minimum follow up rate in both groups.
In terms of implementation of the ACT intervention, we found that the clinical teams were able to engage participants for a significantly longer period of time than in TAU, and close to the intended 365 days. There was no evidence of contamination between the two study arms. The ACT group also received a significantly greater number of contacts with specialist staff, and contacts were more often in participants’ homes or local communities, than in the TAU only group. Given that there are considerable differences in assertiveness, intensity and duration of treatment between ACT and treatment typically delivered by addiction services, this study demonstrates the feasibility of implementation of ACT in this population.
This pilot study was not designed to be statistically powered to provide a definitive test of the effectiveness of ACT + TAU versus TAU alone. Nevertheless there were some differences in outcome between the two groups principally at 6 months follow up, with the ACT + TAU reporting fewer drinking days but lower quality of life and greater alcohol-related problems than TAU. The ACT + TAU group also had greater engagement with alcohol services at both 6 and 12 months and significantly less unplanned inpatient care and outpatient hospital visits than the TAU group. The ACT + TAU group also had significantly more GP visits at 6 months. These differences may reflect improved facilitated access to planned health interventions supported by the ACT staff and a beneficial reduction in unplanned care. Overall this pilot RCT demonstrated the feasibility of conducting a trial of ACT + TAU versus TAU. A definitive trial is now warranted.
Acknowledgements
We would like to thank all the staff and patients in South West London and St George's NHS Trust, and South London and Maudsley NHS Foundation Trust for their support and participation in the research. In particular we would like to thank the ACT practitioners Georgia Bentley, Ann Nolan, Arlette L'Olive, and Andrew Fullagar, and clinicians, Gwen Jones, Megan Northcott, Sally Krumtally, Dr Irene Guerrini, Dr Emily Finch, Dr Emma Whicher, Dr Kishore Chalwa, and service user representatives, Georgina Villa and Paul Paterson.
Funding
This research was supported by a Medical Research Council project grant (reference no. G0701818) a grant from the Guy's and St Thomas’ Charity and the Department of Health. Colin Drummond was part funded by the NIHR Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London, and the NIHR Collaboration for Leadership in Applied Health Research and Care South London. The views expressed are those of the authors and not necessarily those of the NHS, NIHR or Department of Health.
CONFLICT OF INTEREST STATEMENT
The research team was in receipt of research grants from the Medical Research Council, Department of Health and the Guy's and St Thomas’ Charity administered by King's College London, to conduct this work. There are no other conflicts of interest to declare.
References
- Burns T, Fiander M, Kent A, et al. (2000) Effects of case-load size on the process of care of patients with severe psychotic illness. Br J Psychiatry 177:427–433. [DOI] [PubMed] [Google Scholar]
- Dieterich M, Irving CB, Park B, et al. (2010) Intensive case management for severe mental illness. Cochrane Database Syst Rev doi:10.1002/14651858.CD007906.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health (2007) Drug Misuse and Dependence: UK Guidelines on Clinical Management. London: Department of Health (England), the Scottish Government, Welsh Assembly Government and Northern Ireland Executive. [Google Scholar]
- Drummond C. (1990) The relationship between alcohol dependence and alcohol related problems in a clinical population. Br J Addict 85:357–366. [DOI] [PubMed] [Google Scholar]
- Drummond C, Coulton S, James D, et al. (2009) Effectiveness and cost effectiveness of stepped care intervention for alcohol use disorders in primary care: a pilot study. B J Psychiatry 195:448–456. [DOI] [PubMed] [Google Scholar]
- EuroQol Group (1990) EuroQol: a new facility for the measurement of health-related quality of life. Health Policy 16:199–208. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR (1975) ‘Mini-mental state’: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198. [DOI] [PubMed] [Google Scholar]
- Gilbert FS. (1998) The effect of type of aftercare follow-up on treatment outcome among alcoholics. J Stud Alcohol 49:149–159. [DOI] [PubMed] [Google Scholar]
- Gilburt H, Burns T, Copello A, et al. (2012) Assertive community treatment for alcohol dependence (ACTAD): study protocol for a randomised controlled trial. Trials 13:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilburt H, Sinclair J, Drummond C (2015) Navigating the alcohol treatment pathway: a qualitative study from the service users’ perspective. Alcohol Alcohol 50:444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heather N, Luce A, Peck D, et al. (1999) The development of a treatment version of the Readiness to Change Questionnaire. Addict Res 7:63–68. [Google Scholar]
- Hilton ME, Maisto SA, Conigliaro J, et al. (2001) Improving alcoholism treatment across the spectrum of services. Alcohol Clin Exp Res 25:128–135. [PubMed] [Google Scholar]
- HM Government (2012) The UK Government's Alcohol Strategy. London: HMSO. [Google Scholar]
- Lim SS, Vos T, Flaxman AD, et al. (2012) A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall EJ, Edwards JG, Taylor C. (1994) Mortality in men with drinking problems: a 20-year follow-up. Addiction 89:1293–1298. [DOI] [PubMed] [Google Scholar]
- McManus S, Meltzer H, Brugha T, et al. (2009) Adult Psychiatric Morbidity in England, 2007: Results of a Household Survey. Leeds: The NHS Information Centre for Health and Social Care. [Google Scholar]
- Miller WR. (1995) Form 90: a structured assessment interview for drinking and related behaviours. Project MATCH Monograph 5. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; [Google Scholar]
- Mitchell AJ, Selmes T (2007) A comparative survey of missed initial and follow-up appointments to psychiatric specialties in the United Kingdom. Psychiatr Serv 8:868–871. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence (2011) Alcohol Use Disorders: Diagnosis, Assessment and Management of Harmful Drinking and Alcohol Dependence. NICE Clinical Guideline 115. London: National Institute for Health and Clinical Excellence. [PubMed] [Google Scholar]
- Passetti F, Jones G, Chawla K, et al. (2008) Pilot study of assertive community treatment methods to engage alcohol-dependent individuals. Alcohol Alcohol 43:451–455. [DOI] [PubMed] [Google Scholar]
- Robins LN, Wing J, Wittchen HU, et al. (1988) The Composite International Diagnostic Interview: an epidemiologic instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry 45:1069–1077. [DOI] [PubMed] [Google Scholar]
- Robinson KA, Dennison CR, Wayman DM, et al. (2007) Systematic review identifies number of strategies important for retaining study participants. J Clin Epidemiol 60:757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell TR, Hodgson RJ, Edwards G, et al. (1979) The development of a questionnaire to measure severity of alcohol dependence. Br J Addict 74:79–87. [DOI] [PubMed] [Google Scholar]
- Stout RL, Rubin A, Zwick W, et al. (1999) Optimizing the cost-effectiveness of alcohol treatment: a rationale for extended case monitoring. Addict Behav 24:17–35. [DOI] [PubMed] [Google Scholar]
- UKATT Research Team (2005) Effectiveness of treatment for alcohol problems: findings of the randomised UK alcohol treatment trial (UKATT). Br Med J 331:541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE Jr, Kosinski M, Keller SD (1996) A 12 Item Short Form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 34:220–233. [DOI] [PubMed] [Google Scholar]
- Woolard RH, Carty K, Wirtz P, et al. (2004) Follow-up of subjects in clinical trials: addressing subject attrition. Acad Emerg Med 11:859–866. [DOI] [PubMed] [Google Scholar]
- Zweben A, Fucito LM, O'Malley SS (2009) Effective strategies for maintaining research participation in clinical trials. Drug Inf J 43, doi:10.1177/009286150904300411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zywiak WH, Longabaugh R, Wirtz PW (2002) Decomposing the relationships between pre-treatment social network characteristics and alcohol treatment outcome. J Stud Alcohol 63:114–121. [PubMed] [Google Scholar]