Abstract
The neuroepithelial stem cell marker nestin is a cytoskeletal protein that regulates cell proliferation, invasion, and stemness in various tumors, including pancreatic tumors. In the present study, we examined the expression and roles of phosphorylated nestin in pancreatic cancer cells. Nestin phosphorylation at threonines 315 (Thr315) and 1299 (Thr1299) was observed during mitosis in human pancreatic cancer cells. Nestin phosphorylation was positively correlated with a cell proliferation marker, MIB‐1 expression in human pancreatic cancer samples. Transfection of MIA PaCa‐2 cells with nestin mutated at Thr315 and/or Thr1299 (to suppress phosphorylation) resulted in lower proliferation rates than those in control groups. Transfecting MIA PaCa‐2 cells with wild‐type nestin or with nestin mutated at Thr315 increased migration and invasion. In contrast, transfection with nestin mutated at both phosphorylation sites (Thr315 and Thr1299) did not enhance cell migration or invasion. In an intra‐splenic xenograft experiment using MIA PaCa‐2 cells, tumors expressing the nestin double mutant formed fewer liver metastases than tumors expressing wild‐type nestin. Nestin phosphorylation at these two sites was decreased upon treatment with inhibitors for cyclin dependent kinases, AKT, and Aurora in PANC‐1 cells, which express a high baseline level of phosphorylated nestin. These findings suggest that phosphorylation of nestin at Thr315 and/or Thr1299 affects cell proliferation, and inhibition of both phosphorylation sites suppresses invasion and metastasis of human pancreatic cancer. Inhibiting nestin phosphorylation at these two sites may represent a novel therapeutic strategy for pancreatic cancer.
Keywords: Metastasis, nestin, pancreatic cancer, phosphorylation, proliferation
Pancreatic cancer is associated with extremely poor prognosis due to high incidence of metastases.1 Cancer cell migration is an important prerequisite for cancer cell invasion and metastasis. Cell migration and structure are modulated by cytoskeletal components. The neuroepithelial stem cell marker nestin is a class VI intermediate filament (IF) protein2 that interacts with microfilaments and IF proteins including vimentin, desmin, α‐internexin, and synemin to form heterodimers.3 Nestin contributes to vimentin disassembly during mitosis4 and to the inactivation of proapoptotic cyclin‐dependent kinase 5 (CDK5).5 Nestin has 28 possible phosphorylation sites (P48681, UniProtKB/Swiss‐Prot). Phosphorylation of nestin at two different sites has been reported in myocytes and central nervous system progenitor cells.5 CDK5 and Cdc2 induce phosphorylation at threonines 316 (Thr316) and 1495 (Thr1495) of rat nestin,5, 6 modulating mitosis‐associated cytoplasmic reorganization.7 Furthermore, we have recently found that nestin regulates cell growth, invasion, and stemness in glioblastoma cells through alteration of cyclin D1 and heat shock cognate 71‐kDa protein.8 These findings suggest that nestin is not merely a structural protein serving as a progenitor cell marker, but that it also plays important roles in cell proliferation and migration.
Exocrine cells in the pancreas are derived from nestin‐expressing progenitor cells,9 and activation of oncogenic Kras in a nestin‐positive cell lineage is sufficient to induce premalignant pancreatic intraepithelial neoplasia (PanIN) lesions in mice.10 We previously reported that nestin is highly expressed in 30% of human pancreatic cancer samples,11 and that nestin inhibition reduces cell migration and invasion abilities in vitro and liver metastasis in vivo.12 Furthermore, the metastatic potential of human pancreatic cancer cells correlates with stemness, and the epithelial mesenchymal transition is dependent on nestin expression.13, 14 Together, these results indicate that nestin is a possible therapeutic target for pancreatic cancer treatment. In the present study, to develop options for nestin‐targeted therapy, we focused on nestin phosphorylation levels and roles in pancreatic cancer cells.
Here, we report that Thr315 and Thr1299 of human nestin, corresponding to Thr316 and Thr1495 of rat nestin, were phosphorylated mainly during mitosis in pancreatic cancer cells, and suppressing phosphorylation at these nestin residues inhibited the proliferation, migration, invasion, and metastasis of human pancreatic cancer cells.
Materials and methods
Materials
Reagents were purchased from the following companies: mouse monoclonal anti‐nestin antibody from R&D Systems, Inc. (Minneapolis, MN, USA); rabbit polyclonal anti‐Thr315 and anti‐Thr1299 phosphorylated nestin antibodies from Santa Cruz Biotechnology (p‐nestin, sc‐33879 for phosphorylated‐Thr315 of human nestin, sc‐33880 for phosphorylated‐Thr1299 of human nestin; Santa Cruz, CA, USA); rat monoclonal anti‐phosphorylated Histone H3 (phospho S28, ab10543) from Abcam (Cambridge, UK); mouse monoclonal anti‐MIB‐1 antibody from Dako Denmark A/S (Glostrup, Denmark); Histofine Simple Stain kit from Nichirei (Tokyo, Japan); Zenon rabbit IgG labeling kit (Z‐25351), Hoechst 33342, and Click‐iT EdU Pacific Blue Flow Cytometry Assay Kit from Invitrogen (Carlsbad, CA, USA); DSRed‐Express2‐N1 vector from Clontech (Mountain View, CA, USA); FuGene HD transfection reagent from Roche Diagnostics (Mannheim, Germany); the WST‐8 Cell Counting Kit from Wako Pure Chemical Industries (Osaka, Japan); Matrigel invasion chambers from BD Bioscience (Franklin Lakes, NJ, USA); Diff‐Quick staining kit from Sysmex Corp. (Kobe, Japan).
Pancreatic cancer cell lines and pancreatic cancer tissues
Pancreatic cancer cell lines PANC‐1 and MIA PaCa‐2 were obtained from the Cell Resource Center for Biomedical Research at the Institute of Development, Aging, and Cancer of Tohoku University (Sendai, Japan). Cells were grown in RPMI 1640 medium containing 10% fetal bovine serum (FBS). All cells were cultured in a humidified atmosphere containing 5% CO2 in an incubator at 37°C. Formalin‐fixed paraffin embedded tissues (n = 30) were obtained from patients with pancreatic ductal adenocarcinoma (PDAC) who underwent surgery at Tokyo Metropolitan Geriatric Hospital. This study was conducted in accordance with the principles outlined in the Declaration of Helsinki, 2008, and informed consent for the usage of tissues was obtained from each patient. Experiments were approved by the Research Ethics Committee of Tokyo Metropolitan Geriatric Hospital (permit #R16‐03).
Fluorescent staining and immunohistochemistry
Pancreatic cancer cells were fixed in 4% paraformaldehyde (PFA), and were incubated with anti‐nestin (1:50 dilution), anti‐phosphorylated nestin (Thr315 or Thr1299, 1:100 dilution), or phosphorylated Histone H3 (1:100 dilution) overnight at 4°C, followed by incubation with an Alexa 488‐ or 568‐labeled anti‐mouse IgG antibody (1:1000 dilution), an anti‐rabbit IgG antibody (1:1000 dilution), or an anti‐rat IgG antibody (1:1000 dilution). Anti‐phosphorylated nestin antibodies were raised against a short amino acid sequence containing phosphorylated Thr316 or Thr1495 of rat nestin. The rat‐raised phospho‐antibodies react with the corresponding phosphorylation sites of human nestin (Thr315 and Thr1299). To determine cells' DNA synthetic period (S‐phase), a Click‐iT EdU Imaging Kit was used according to the manufacturer's protocol. Stained cells were observed under a Digital Eclipse C1 TE2000‐E microscope (Nikon Instech Co., Ltd., Kanagawa, Japan) as previously described.15 All assays were performed in triplicate.
Paraffin‐embedded PDAC tissue sections (3.5 μm) were immunostained using a Histofine Simple Stain kit as previously reported.16 Tissue sections were incubated overnight at 4°C in the absence (negative controls) or presence of an anti‐nestin antibody (1:100 dilution), anti‐phosphorylated nestin antibodies (1:100 dilution), or an anti‐MIB‐1 antibody (1:200 dilution). Then, the bound antibodies were detected with Simple Stain MAX PO (M) or (R) reagent, using diaminobenzidine‐tetrahydrochloride chromogen as the substrate. Nuclear expression of MIB‐1 and nestin was observed under 200× magnification. Average expression values from five fields were used for statistical analysis.
Flow cytometry
Rabbit polyclonal anti‐Thr315 and anti‐Thr1299 phosphorylated nestin antibodies were labeled with Alexa Fluor 488 using a Zenon antibody labeling kit Life Technologies (Carlsbad, CA, USA). Cells were incubated for 20 min at 4°C in 10% human serum, and then incubated (5 × 105 cells/50 μL) with each antibody for 30 min at room temperature. Dead cells were labeled with the addition of 1 μg propidium iodide. We prepared rabbit IgG isotype control‐treated cells as negative controls.
Cell cycle was analyzed using a Click‐iT EdU Pacific Blue Flow Cytometry Assay Kit according to the manufacturer's protocol. Briefly, 10 μM EdU was added into the culture medium, and cells were incubated for 60 min at 37°C. Cells were fixed with 4% PFA for 60 min, and then EdU was labeled with Pacific Blue. Seven‐aminoactinomycin D was added to measure DNA content and cell cycle distribution, and cell cycle analysis was performed using a BD FACSAria II flow cytometer. All assays were performed in triplicate.
Wild‐type or mutant nestin‐expressing MIA PaCa‐2 cells
Wild‐type nestin was transfected into MIA PaCa‐2 cells or PANC‐1 cells as previously described12 using DSRed‐Express2‐N1 vector (wild‐type cells). We also prepared expression constructs harboring single mutations at individual phosphorylation sites of nestin (Thr315 and Thr1299) to inhibit phosphorylation, and also a double mutant construct unable to be phosphorylated at either site (mut‐nes1, 2, and 3, respectively). To accomplish these mutations we changed adenines (A) at nucleotides (nts) 1076 and 4028 of the nestin gene (NM_006617.1) to guanines (G), thereby changing the phosphorylation‐site threonines into alanines. MIA PaCa‐2 cells (1 × 105 cells/mL) were transfected with 3 μg of DSRed‐Express2‐N1 vector containing mutated nestin cDNA using FuGene HD, and the cells were passaged and cultured with 600 μg/mL Geneticin. Independent colonies were isolated by ring cloning. As a negative control, empty vector was transfected into MIA PaCa‐2 cells or PANC‐1 cells (EV cells).
Western blot analysis
Lysates from MIA PaCa‐2 cells were subjected to SDS‐PAGE. Membranes were incubated with an anti‐nestin antibody (1:1000 dilution) or anti‐phosphorylated nestin antibodies (anti‐Thr315 or anti‐Thr1299, 1:8000 dilution), and then with the appropriate secondary antibody (1:4000 dilution). Membranes were reblotted with an anti‐glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) antibody (1:5000 dilution) to confirm equal loading. All assays were performed in triplicate.
Anchorage‐dependent cell growth assays
MIA PaCa‐2 cells or PANC‐1 cells (5 × 103 cells/well) were seeded in 96‐well plates, and a WST‐8 cell proliferation assay was performed after 72 h using an ELISA plate reader (Bio‐Rad Lab., Hercules, CA, USA) at 450 nm.17 All assays were performed in triplicate.
Cell migration and invasion assays
Migration and invasion assays were performed using a modified Boyden chamber technique as described previously.12 Briefly, cells were suspended in serum‐free medium at a density of 1 × 105 cells/500 μL. The lower compartment was filled with 750 μL of medium containing 10% FBS. After 20 h of incubation, the cells that migrated through the membrane to the lower surface of the filter were fixed, stained with a Diff‐Quick staining kit, and counted under a light microscope. The cell numbers on each membrane were counted in five high‐power fields (200×). Cell invasion assays were performed in the same manner using Matrigel‐coated inserts. All assays were performed in triplicate.
Liver metastatic models using intra‐splenic injection
MIA PaCa‐2 cells (1 × 105 cells) were injected into the spleens of 6‐week‐old male NOD/Shi‐scid, IL‐2Rγnull (NOG) mice (n = 6 for each experimental group, Central Institute for Experimental Animals, Kanagawa Japan) as previously reported.13 After 8 weeks, animals were euthanized and the livers were excised. All animal experiments were carried out according to the institutional animal care guidelines of the Nippon Medical School Animal Ethical Committee (#23‐133).
Screening for inhibitors of nestin phosphorylation in PANC‐1 cells
We screened for molecules that regulate nestin phosphorylation using the SCADS inhibitor kit III (the Screening Committee of Anticancer Drugs), which contains a total of 95 compounds, in two 96‐well plates.18 PANC‐1 cells, which express a high baseline level of phosphorylated nestin, were seeded in 96‐well plates on day 1 (1 × 103 cells/well), and inhibitors were added at a concentration of 5 μM on day 3. On day 5, we examined alterations in nestin phosphorylation by performing fluorescent staining with anti‐phosphorylated nestin antibodies (anti‐Thr315 or anti‐Thr1299) as described above. Nestin phosphorylation levels were analyzed using an In Cell Analyzer 6000 (GE Healthcare UK Ltd, Amersham Place, UK).
Statistical analysis
All quantitative data are presented as mean ± standard error of the mean (SEM). Data were compared using a one‐way ANOVA, and P‐values <0.05 were considered statistically significant. Analyses were performed using StatView J version 5.0 (SAS Institute, Inc., Cary, NC, USA).
Results
Immunofluorescent analysis showed that total nestin was diffusely localized in all PANC‐1 cells (Fig. 1, left upper and lower panels, red). In contrast, nestin phosphorylated at Thr315 and Thr1299 (p‐Thr315 and p‐Thr1299, respectively) was strongly expressed in a few small round to oval‐shaped PANC‐1 cells (Fig. 1, middle upper and lower panel, green, arrows). Merged images show that these phosphorylated nestin‐positive PANC‐1 cells do not have nuclear membranes, but they have blue‐stained chromosomes (Fig. 1, right upper and lower panels). To characterize the phosphorylated nestin‐positive cells, we performed immunofluorescent staining using an anti‐histone H3 antibody, a marker of mitosis (M phase), and ErdU staining to detect S‐phase cells. Phosphorylated nestin‐positive PANC‐1 cells (p‐Thr315 and p‐Thr1299) were positive for histone H3, but negative for ErdU (Fig. 2a, arrows), indicating nestin phosphorylation at Thr315 and Thr1299 mainly occurs during the mitotic phase of PANC‐1 cells (Fig. 2b). Flow cytometry using an anti‐p‐Thr315 or ‐p‐Thr1299 antibody also showed that most phosphorylated nestin‐positive PANC‐1 cells were in the G2/M phase of the cell cycle (Fig. 3).
Figure 1.
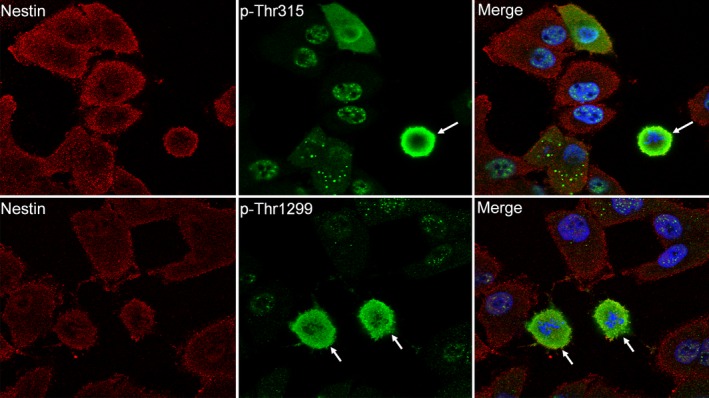
Expression of nestin and phosphorylated nestin in PANC‐1 cells. Nestin was expressed in most PANC‐1 cells, but phosphorylated nestin was only observed in a small number of round‐ to oval‐shaped cells (arrows). Red, nestin; green, phosphorylated nestin; blue, DAPI; p‐Thr315, phosphorylated at Thr315 of nestin; p‐Thr1299, phosphorylated at Thr1299 of nestin. 1000× magnification.
Figure 2.
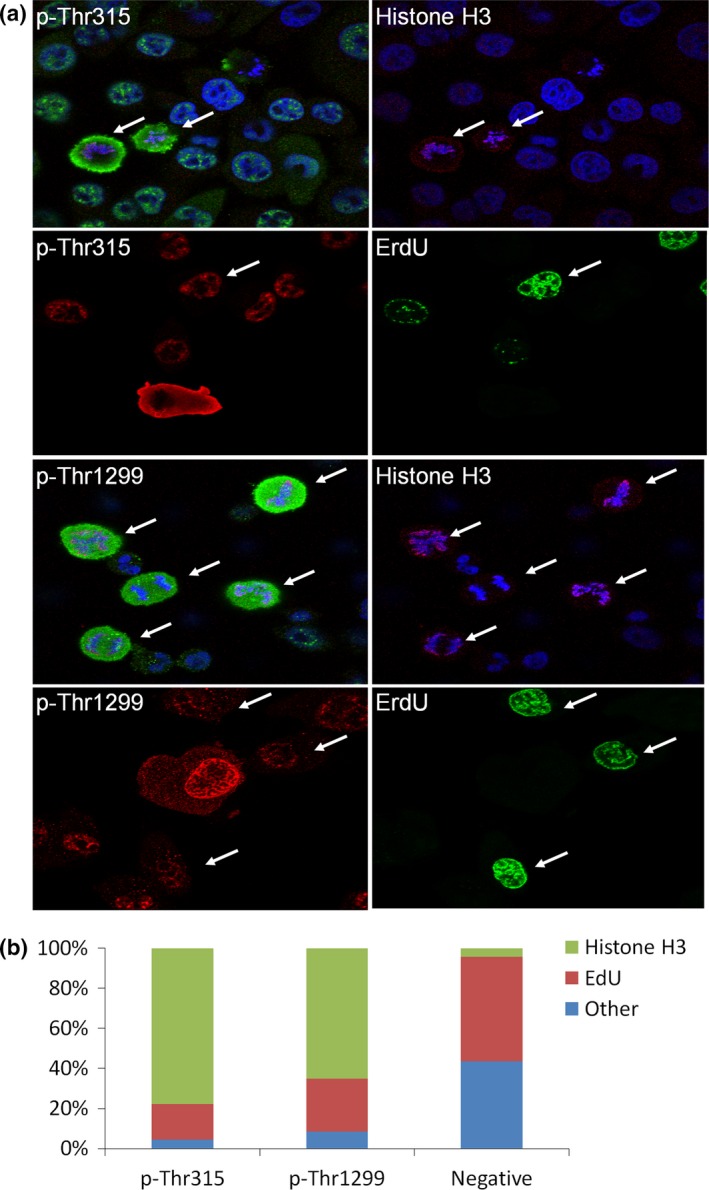
Relationship between mitosis and phosphorylated nestin in PANC‐1 cells. (a) Phosphorylated nestin (p‐Thr315 and p‐Thr1299) expressed in M phase cells that were positive for phosphorylated histone H3 staining (arrows), but negative for ErdU staining (S‐phase, arrows). Green or red, phosphorylated nestin; red, histone H3; green, ErdU; blue, DAPI; 1000× magnification for p‐Thr315 and 600× for p‐Thr1299. (b) Percentage of Histon H3 and EdU‐positive cells in p‐Thr315 or p‐Thr1299‐positive cells.
Figure 3.
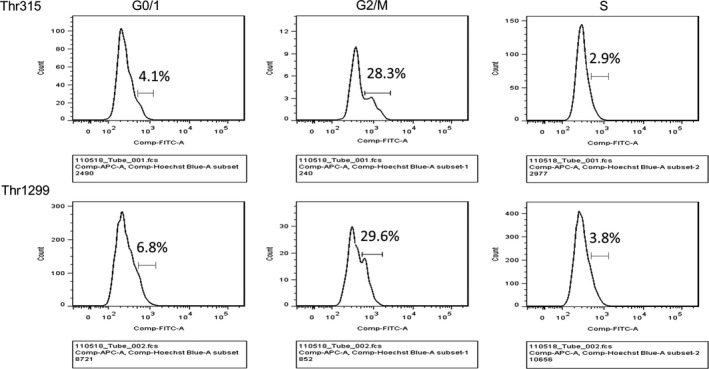
Flow cytometry analysis of phosphorylated nestin in PANC‐1 cells. X‐axis indicates fluorescent intensity of phosphorylated nestin. The cells in G2/M phase of the cell cycle showed higher intensity of phosphorylated nestin as compared with the cells in G0/1 and S phase.
Next, we performed immunohistochemical analysis using anti‐phosphorylated nestin (p‐Thr315 and p‐Thr1299) and anti‐MIB‐1 antibodies to clarify the relationship between nestin phosphorylation and mitotic ability in human pancreatic tissues. In human pancreatic cancer samples, p‐Thr315 and p‐Thr1299 was observed in the nuclei (Fig. 4a, left upper and lower panels and insets), while total nestin was mainly expressed in the cytoplasm of pancreatic cancer cells (right upper panel). Most cells of normal pancreatic tissue were negative for phosphorylated nestin, while mitotic acinar cell was positive for phosphorylated nestin (Fig. 4a, right lower panel and inset). MIB‐1, which is a proliferative cell marker was localized in the nuclei of cancer cells, and phosphorylation at Thr1299 was positively correlated with MIB‐1 expression in human pancreatic cancer tissues (Fig. 4b, P < 0.0001, R2 = 0.588).
Figure 4.
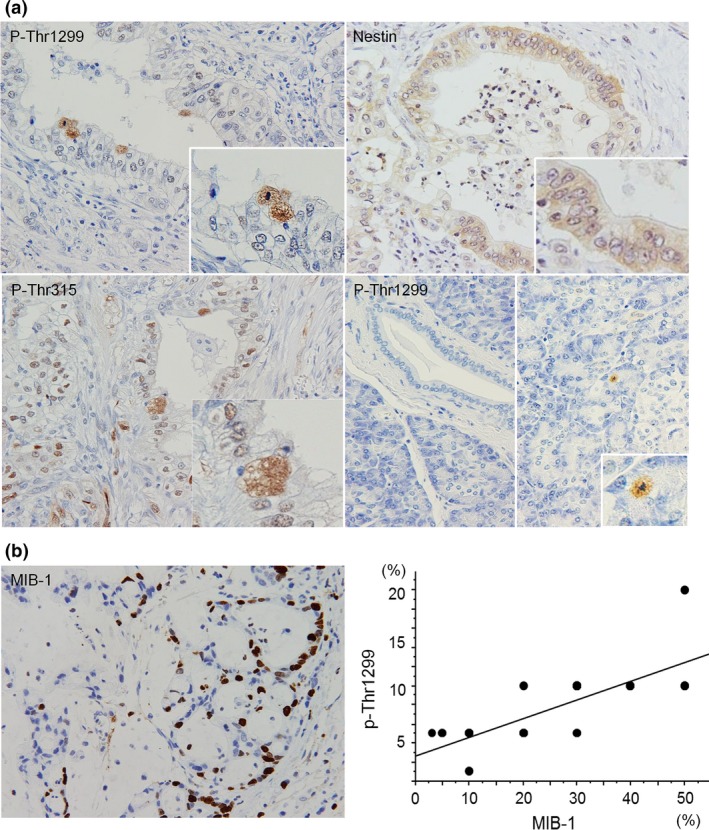
Immunohistochemical analysis of MIB‐1 and phosphorylated nestin. (a) Phosphorylated nestin (p‐Thr315 andp‐Thr1299) localized in the nuclei of pancreatic cancer cells, while total nestin mainly localized in the cytoplasm of cancer cells. Most cells in normal pancreatic tissue did not show positive for phosphorylated nestin, but mitotic cells were positive for phosphorylated nestin. (b) MIB‐1 was localized in the nuclei of cancer cells. A positive correlation between p‐Thr1299 and MIB‐1 was observed in pancreatic cancer cases.
To determine the roles of phosphorylated nestin, we prepared expression vectors containing nestin cDNA mutated at either Thr315, Thr1299, or both Thr315 and Thr1299. These nestin mutations led to amino acid changes from threonine to alanine at the phosphorylation sites (Fig. 5a, mut‐nes1, 2, and 3 respectively). MIA PaCa‐2 cells express low baseline levels of nestin, thus we transfected these cells with wild‐type or mutant nestin constructs11. Nestin phosphorylation at Thr315 and Thr1299 was decreased in mutant nestin‐transfected cells (mut‐nes3) compared to wild‐type nestin‐transfected (Wild‐type) cells (Fig. 5b, second and third panels). Multinuclear cells were observed in the mut‐nes3 group as seen by phase contrast microscopy (Fig. 5c, arrows). To examine the number of multinuclear cells in the mut‐nes groups, we counted the number of spindle type cells and multinuclear cells in each group. The mut‐nes1, 2, and 3 group cells showed a significant increase in number of multinuclear cells, but the number of spindle cells was not different from wild‐type nestin‐ or EV‐transfected cells (Fig. 5d, *P < 0.05).
Figure 5.
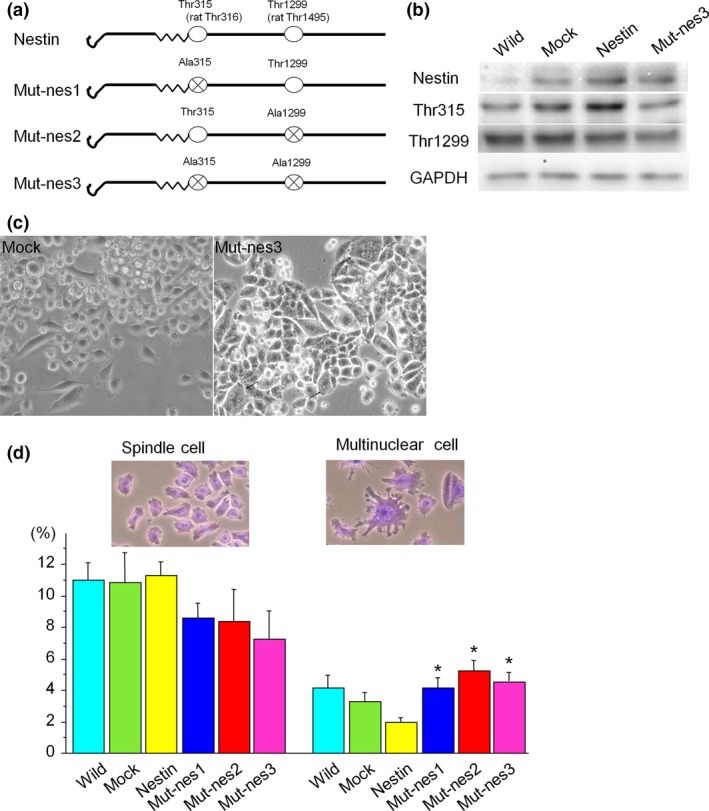
Transfection of MIA PaCa‐2 cells with wild‐type or mutant nestin expression vectors (a) Wild‐type nestin possesses threonines at residues 315 and 1299, and we performed mutations of nestin cDNA that changed these amino acids to alanines. (b) Western blot analysis of phosphorylated nestin (Thr315 and 1299) in non‐treated cells (Non‐transfected), empty vector‐transfected cells (EV), wild‐type nestin‐transfected cells (Wild‐type), and cells transfected with nestin mutated at both phosphorylation sites (Mut‐nes3). (c) Phase contrast images of EV and Mut‐nes3 cells. Arrows indicate multinuclear cells. (d) Cells transfected with Mut‐nes1, 2, or 3 constructs show an increase in number of multinuclear cells (*P < 0.05 vs. non‐transfected, EV, and Wild‐type cells).
Next, we examined the biological role(s) of nestin phosphorylation at Thr315 and Thr1299. Mut‐nes1, 2, and 3 cells showed a significant decrease in cell growth rate compared to cells in control groups (Fig. 6a, *P < 0.05 vs. wild‐type and EV). Wild‐type nestin‐transfected cells as well as mut‐nes1 and 2 cells showed an increase in cell migration and invasion (Fig. 6b,c *P < 0.05 vs. EV cells12), but mut‐nes 3 cells did not show any differences in cell migration or invasion (Fig. 6b,c, #P < 0.05 mut‐nes3 vs. wild‐type, mut‐nes1 and 2). PANC‐1 cells transfected with mut‐nes1, 2, or 3 showed a decrease in cell proliferation (Fig. 6d), and cell migration and invasion was enhanced in nestin transfected and mut‐nes1 cells, but not in mut‐nes2 and three cells (Fig. 6e,f). These findings suggest that phosphorylation of Thr315 or Thr1299 correlates with cell proliferation, but does not affect cancer cell migration and invasion. Inhibiting phosphorylation at both sites synergistically reduced pancreatic cancer cell migration and invasion (Table 1).
Figure 6.
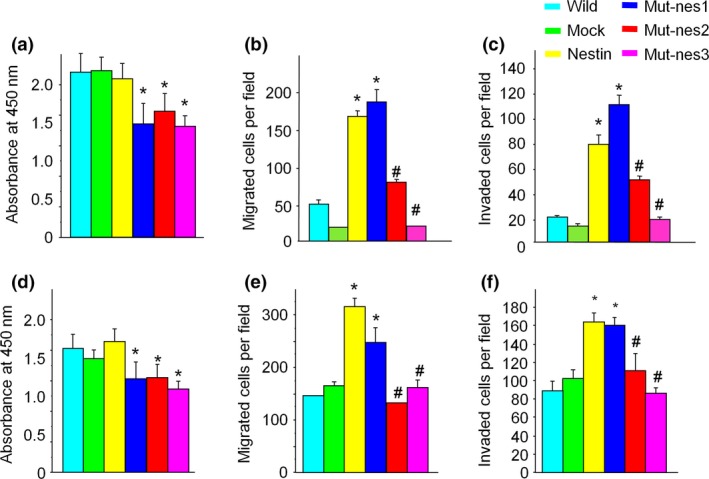
Changes in cell proliferation, migration, and invasion in cells expressing mutant nestin (a) MIA PaCa‐2 cells transfected with mut‐nes1, 2, or 3 showed decreases in cell proliferation (*P < 0.05 vs. non‐transfected and EV‐transfected cells). (b, c) MIA PaCa‐2 cells transfected with wild‐type nestin or mut‐nes1 show an increase in cell migration and invasion, but cells expressing mut‐nes2 and 3 showed a decrease in migration and invasion (#P < 0.05 vs. non‐transfected, EV, and mut‐nes‐2, 3 cells). (d) PANC‐1 cells transfected with mut‐1,2, or 3 showed a decrease of cell proliferation (*P < 0.05 vs. non‐transfected and EV cells). (e,f) PANC‐1 cells transfected with wild‐type nestin or mut‐nes1 show an increase in cell migration and invasion, but cells expressing mut‐nes2 and 3 showed a decrease in migration and invasion (#P < 0.05 vs. non‐transfected, EV, and mut‐nes‐2, 3 cells).
Table 1.
Effects of inhibition of nestin phosphorylation in pancreatic cancer
| Wild‐type nestin | Thr315 | Thr1299 | Thr315+1299 | |
|---|---|---|---|---|
| Growth | → | ↓ | ↓ | ↓ |
| Migration | ↑ | ↑ | ↑ | → |
| Invasion | ↑ | ↑ | ↑ | → |
Thr315: nestin expression vector mutated at threonine‐315 to suppress phosphorylation; Thr1299: nestin expression vector mutated at threonine‐1299 to suppress phosphorylation; Thr315+1299: nestin expression vector mutated at both threonine‐315 and ‐1299.
We examined whether inhibition of nestin phosphorylation can suppress metastasis in vivo. Since cell growth, migration, and invasion were most strongly inhibited by suppressing phosphorylation at both Thr315 and Thr1299 in vitro studies, we selected mut‐nes3 cells for animal experiments. Eight weeks after splenic injection of mut‐nes3 cells, liver metastasis in NOG mice was strongly decreased compared to mice injected with control cells (Fig. 7a). Increases in liver weight due to metastatic tumors were lower in mice injected with mut‐nes3 cells compared to mice injected with control cells (Fig. 7b, *P < 0.05 vs. non‐transfected, EV, and Wild‐type expressing cells).
Figure 7.
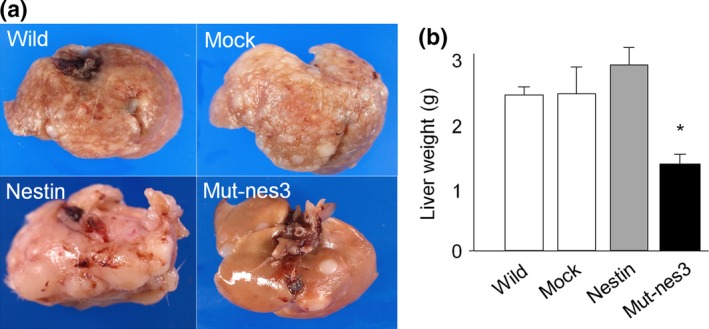
Liver metastasis in mice bearing xenografts with mutant nestin‐transfected cells (a) Liver metastasis in mice bearing xenografts expressing mut‐nes3 compared with controls (xenografts with non‐transfected cells or cells expressing EV or wild‐type nestin) in NOG mice (n = 6 in each group). (b) Liver weight was decreased in mice bearing Mut‐nes3‐expressing tumors than in mice with tumors expressing other constructs (*P < 0.05 vs. non‐transfected, EV, and wild‐type nestin cells).
Next, regulatory mechanisms of nestin phosphorylation were analyzed using an inhibitor kit. Nestin phosphorylation at residues Thr315 and Thr1299 was decreased upon addition of inhibitors for cyclin dependent kinases, AKT, and Aurora, as shown in Tables 2 and 3. Nine of the top 10 inhibitors of nestin phosphorylation at Thr315 were also inhibitors of phosphorylation at Thr1299, and a Cdk2/9 inhibitor resulted in the strongest inhibition at these two phosphorylation sites.
Table 2.
Top 10 inhibitors of nestin phosphorylation at Thr315
| Inhibitor | Pathway | Intensity |
|---|---|---|
| Cdk2/9 inhibitor | CDK | 160.185 |
| Cdk1/2 inhibitor III | CDK | 163.208 |
| Alsterpaullone, 2‐cyanoethyl | CDK | 163.591 |
| Akt Inhibitor IV | AKT | 164.482 |
| Aurora kinase/cdk inhibitor | Aurora | 179.331 |
| PKR inhibitor | PKR | 200.288 |
| AG1024 | IGF‐IR | 206.688 |
| ATM/ATR kinase inhibitor | ATM | 207.488 |
| JAK3 Inhibitor VI | Jak | 214.289 |
| ATM kinase inhibitor | ATM | 222.953 |
Table 3.
Top 10 inhibitors of nestin phosphorylation at Thr1299
| Inhibitor | Pathway | Intensity |
|---|---|---|
| Cdk2/9 inhibitor | CDK | 126.356 |
| Cdk1/2 inhibitor III | CDK | 128.243 |
| Alsterpaullone, 2‐cyanoethyl | CDK | 128.441 |
| JAK3 Inhibitor VI | Jak | 133.398 |
| Akt Inhibitor IV | AKT | 134.538 |
| Aurora kinase/cdk inhibitor | Aurora | 141.519 |
| ATM/ATR kinase inhibitor | ATM | 155.3 |
| PKR inhibitor | PKR | 135.471 |
| AG1024 | IGF‐IR | 130.167 |
| 1‐Azakenpaullone | GSK | 185.69 |
Discussion
The present study showed that phosphorylated nestin was detected in the mitotic phase of pancreatic cancer cells. Nestin phosphorylation affects several pancreatic cancer cell behaviors including cell growth, migration, invasion, and metastasis. Data from the present study and our previous studies8, 11, 12, 13, 14 indicate that nestin is a key factor in pancreatic cancer invasion and metastasis, and inhibition of nestin expression or phosphorylation may be a novel therapeutic strategy for treating pancreatic cancer.
Among several nestin phosphorylation sites, cell cycle‐related proteins induce phosphorylation of Thr316 and Thr1495 in rat nestin; the only commercially‐available phospho‐nestin antibodies are raised against Thr316‐ and Thr1495‐phosphorylated rat nestin.6 These antibodies cross‐react with phosphorylated Thr315 and Thr1299 of human nestin. Both Thr315‐ and Thr1299‐phosphorylated nestin were observed in the mitotic phase of cells. Previously, downstream molecules of nestin have been reported. Nestin can regulate cell proliferation via phosphorylation and reorganization of vimentin,4 and by modulating CDK5.19 In the early stages of central nervous system development, nestin is expressed in dividing cells.20 Nestin regulates stemness, cell growth, and invasion in glioblastoma cells through the alteration of HSC71.8 However, the effect of nestin on pancreatic cancer cell proliferation remains controversial.13, 14 We previously found that inhibiting nestin expression by short hairpin RNA (shRNA) suppressed growth of the glioblastoma cell line A172,15 the melanoma cell line A375,21 and the lung cancer cell line H1975.18 In the present study, inhibition of nestin phosphorylation suppressed cell growth and increased the number of multinuclear cells, suggesting that inhibiting nestin phosphorylation can disturb cancer cell division. We did not analyze downstream molecules that are involved in phosphorylated nestin. However, inhibition of phosphorylated nestin showed almost same effects as inhibition of total nestin.13 These results suggest that vimentin,4 CDK5,19 or HSC71,8 downstream molecules of nestin might be involved in cell proliferation, invasion and migration which were regulated by phosphorylated nestin.
In addition, we have shown that inhibiting nestin phosphorylation in cancer cells in vivo suppressed liver metastasis, likely via inhibiting cell migration, invasion, and growth in the liver. Wild type nestin induced cell migration and invasion in vitro, but did not enhance metastatic ability in vivo. These results indicate that interaction with stromal cells in vivo might affect nestin function. Our study has limitations. We performed animal study using wild type nestin‐transfected and both phosphorylated sites mutated nestin‐transfected cells; therefore, we did not clarify the roles of each phosphorylate site in vivo. Further studies are needed to clarify molecular mechanisms of nestin phosphorylation.
We previously reported that nestin expression level was higher in metastatic lesions compared to primary lesions.13 Nestin was continuously expressed in pancreatic cancer cells, while the phosphorylated form was only observed in the mitotic phase. In the present study, we found that inhibition of both phosphorylation sites suppressed human pancreatic cancer metastasis. These findings suggest that inhibiting nestin phosphorylation is more specific than inhibiting total nestin, and is more effective for inhibiting metastasis. Furthermore, most inhibitors of cyclin dependent kinases, Akt, or Aurora utilized in this study decreased nestin phosphorylation at both sites, suggesting that these molecules are upstream regulators of nestin phosphorylation. Molecular targeted therapies that inhibit nestin phosphorylation, such as inhibitors used in the present study, antibodies or small molecules, may be new candidates for pancreatic cancer treatment.
In conclusion, phosphorylated nestin regulates proliferation, invasion, and metastasis of pancreatic cancer cells. Inhibiting nestin phosphorylation may represent a novel therapeutic option for pancreatic cancer. Further studies are needed to clarify the mechanisms of nestin phosphorylation in pancreatic cancer, and to develop agents that inhibit nestin phosphorylation for the treatment of pancreatic cancer.
Disclosure Statement
The authors declare no conflict of interest.
Acknowledgments
We thank Drs. Tetsushi Yamamoto and Zenya Naito for helpful discussion, and Dr. Masahito Hagio for technical assistance (Department of Integrated Diagnostic Pathology, Nippon Medical School). This work was supported in part by a grant‐in‐aid from the Japan Society for the Promotion of Science (C, No. 25462127) and grants from the Cancer Research Institute of Kanazawa University and Mitsui Life Social Welfare Foundation to Y. Matsuda, and in part by a grant‐in‐aid from the Japan Society for the Promotion of Science (C, No. 25461027) to T. Ishiwata.
Cancer Sci 108 (2017) 354–361
Y. Matsuda and T. Ishiwata contributed equally to this study.
Funding Information
The Cancer Research Institute of Kanazawa University, the Japan Society for the Promotion of Science, (Grant / Award Number: ‘C, No. 25461027‘,’C, No. 25462127‘), Mitsui Life Social Welfare Foundation.
References
- 1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014; 64: 9–29. [DOI] [PubMed] [Google Scholar]
- 2. Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell 1990; 60: 585–95. [DOI] [PubMed] [Google Scholar]
- 3. Sjoberg G, Jiang WQ, Ringertz NR, Lendahl U, Sejersen T. Colocalization of nestin and vimentin/desmin in skeletal muscle cells demonstrated by three‐dimensional fluorescence digital imaging microscopy. Exp Cell Res 1994; 214: 447–58. [DOI] [PubMed] [Google Scholar]
- 4. Chou YH, Khuon S, Herrmann H, Goldman RD. Nestin promotes the phosphorylation‐dependent disassembly of vimentin intermediate filaments during mitosis. Mol Biol Cell 2003; 14: 1468–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sahlgren CM, Mikhailov A, Vaittinen S et al Cdk5 regulates the organization of Nestin and its association with p35. Mol Cell Biol 2003; 23: 5090–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matsuda Y, Suzuki G, Kusano T et al Phosphorylation of Thr(1495) of nestin in a mouse model of cerebral ischemia and reperfusion damage. Pathol Int 2013; 63: 448–56. [DOI] [PubMed] [Google Scholar]
- 7. Sahlgren CM, Mikhailov A, Hellman J et al Mitotic reorganization of the intermediate filament protein nestin involves phosphorylation by cdc2 kinase. J Biol Chem 2001; 276: 16456–63. [DOI] [PubMed] [Google Scholar]
- 8. Matsuda Y, Ishiwata T, Yoshimura H, Hagio M, Arai T. Inhibition of nestin suppresses stem cell phenotype of glioblastomas through the alteration of post‐translational modification of heat shock protein HSPA8/HSC71. Cancer Lett 2015; 357: 602–11. [DOI] [PubMed] [Google Scholar]
- 9. Esni F, Stoffers DA, Takeuchi T, Leach SD. Origin of exocrine pancreatic cells from nestin‐positive precursors in developing mouse pancreas. Mech Dev 2004; 121: 15–25. [DOI] [PubMed] [Google Scholar]
- 10. Carriere C, Seeley ES, Goetze T, Longnecker DS, Korc M. The Nestin progenitor lineage is the compartment of origin for pancreatic intraepithelial neoplasia. Proc Natl Acad Sci U S A 2007; 104: 4437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kawamoto M, Ishiwata T, Cho K et al Nestin expression correlates with nerve and retroperitoneal tissue invasion in pancreatic cancer. Hum Pathol 2009; 40: 189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matsuda Y, Naito Z, Kawahara K, Nakazawa N, Korc M, Ishiwata T. Nestin is a novel target for suppressing pancreatic cancer cell migration, invasion and metastasis. Cancer Biol Ther 2011; 11: 512–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matsuda Y, Yoshimura H, Ueda J, Naito Z, Korc M, Ishiwata T. Nestin delineates pancreatic cancer stem cells in metastatic foci of NOD/Shi‐scid IL2Rgamma(null) (NOG) mice. Am J Pathol 2014; 184: 674–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hagio M, Matsuda Y, Suzuki T, Ishiwata T. Nestin regulates epithelial‐mesenchymal transition marker expression in pancreatic ductal adenocarcinoma cell lines. Mol Clin Oncol 2013; 1: 83–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ishiwata T, Teduka K, Yamamoto T, Kawahara K, Matsuda Y, Naito Z. Neuroepithelial stem cell marker nestin regulates the migration, invasion and growth of human gliomas. Oncol Rep 2011; 26: 91–9. [DOI] [PubMed] [Google Scholar]
- 16. Matsuda Y, Fujii T, Suzuki T et al Comparison of fixation methods for preservation of morphology, RNAs, and proteins from paraffin‐embedded human cancer cell‐implanted mouse models. J Histochem Cytochem 2010; 59: 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ishiwata T, Matsuda Y, Yamamoto T, Uchida E, Korc M, Naito Z. Enhanced expression of fibroblast growth factor receptor 2 IIIc promotes human pancreatic cancer cell proliferation. Am J Pathol 2012; 180: 1928–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Narita K, Matsuda Y, Seike M, Naito Z, Gemma A, Ishiwata T. Nestin regulates proliferation, migration, invasion and stemness of lung adenocarcinoma. Int J Oncol 2014; 44: 1118–30. [DOI] [PubMed] [Google Scholar]
- 19. Sahlgren CM, Pallari HM, He T, Chou YH, Goldman RD, Eriksson JE. A nestin scaffold links Cdk5/p35 signaling to oxidant‐induced cell death. EMBO J 2006; 25: 4808–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tohyama T, Lee VM, Rorke LB, Marvin M, McKay RD, Trojanowski JQ. Nestin expression in embryonic human neuroepithelium and in human neuroepithelial tumor cells. Lab Invest 1992; 66: 303–13. [PubMed] [Google Scholar]
- 21. Akiyama M, Matsuda Y, Ishiwata T, Naito Z, Kawana S. Inhibition of the stem cell marker nestin reduces tumor growth and invasion of malignant melanoma. J Invest Dermatol 2013; 133: 1384–7. [DOI] [PubMed] [Google Scholar]


