Abstract
Epithelial–mesenchymal transition (EMT) plays an important role in the progression of lung carcinoma. Podocalyxin (PODXL), which belongs to the CD34 family and regulates cell morphology, has been linked to EMT in lung cancer, and PODXL overexpression is associated with poor prognosis in several different classes of cancers. The aim of this study was to clarify the role of PODXL overexpression in EMT in lung cancer, and to determine the prognostic value of PODXL overexpression in tumors from lung cancer patients. The morphology, EMT marker expression, and migration and invasion abilities of engineered A549 PODXL‐knockdown (KD) or PODXL‐overexpression (OE) lung adenocarcinoma cells were examined. PODXL expression levels were assessed by immunohistochemistry in 114 human clinical lung adenocarcinoma specimens and correlated with clinical outcomes. PODXL‐KD cells were epithelial in shape, whereas PODXL‐OE cells displayed mesenchymal morphology. Epithelial markers were upregulated in PODXL‐KD cells and downregulated in PODXL‐OE cells, whereas mesenchymal markers were downregulated in the former and upregulated in the latter. A highly selective inhibitor of phosphatidylinositol 3‐kinase‐Akt signaling attenuated EMT of PODXL‐OE cells, while a transforming growth factor inhibitor did not, suggesting that PODXL induces EMT of lung adenocarcinoma cells via the phosphatidylinositol 3‐kinase pathway. In lung adenocarcinoma clinical specimens, PODXL expression was detected in minimally invasive and invasive adenocarcinoma, but not in non‐invasive adenocarcinoma. Disease free survival and cancer‐specific survival were significantly worse for patients whose tumors overexpressed PODXL. PODXL overexpression induces EMT in lung adenocarcinoma and contributes to tumor progression.
Keywords: Akt, epithelial mesenchymal transition, lung adenocarcinoma, phosphatidylinositol 3‐kinase, podocalyxin
Lung cancer is a leading cause of disease related mortality all over the world.1 Although several chemotherapies and molecular targeted therapies have been developed, the prognosis for lung cancer remains poor due to metastasis. One promising approach to combat distant metastasis in patients with lung cancer is to inhibit the process of epithelial to mesenchymal transition (EMT), which involves the loss of cell polarity and change to mesenchymal phenotype.2 EMT is known to drive lung cancer progression by promoting both the invasion of adjacent tissues and the development of resistance to anti‐cancer agents.3 Although several cytokines, including transforming growth‐factor β (TGF‐β), have received significant attention as major inducers of EMT during cancer progression, the roles of these factors in controlling EMT in the context of lung cancer have not been studied extensively.4
Podocalyxin (PODXL) is a transmembrane glycoprotein and member of the CD34 family, which is widely expressed on vascular endothelial cells, as well as hematopoietic stem and progenitor cells.5 PODXL is essential for maintaining the elaborate structure of kidney podocytes,6 and has a broader role in modifying cell shape.7 However, abnormal expression of PODXL occurs in several malignancies, including leukemia, breast and prostate cancer,5 and PODXL overexpression has been shown to be a poor prognostic factor in colorectal cancer,8 breast cancer,9 urothelial bladder cancer,10 pancreatic ductal adenocarcinoma11 and gastric cancer.12 In the lung cancer context, Meng et al.13 report that PODXL is upregulated during TGF‐β‐induced EMT, and silencing of PODXL reduced morphologic change and expression of E‐cadherin and vimentin during TGF‐β‐induced EMT in the A549 lung adenocarcinoma cell line. To date, the clinicopathological significance of PODXL expression in lung adenocarcinoma has not been investigated. The purpose of this study was to clarify the role of PODXL in EMT using lung adenocarcinoma cells, and address the prognostic significance of PODXL overexpression in clinical samples of lung adenocarcinoma.
Materials and Methods
Cell lines and culture conditions
The human lung adenocarcinoma cell lines, A549 and NCI‐H358, human lung squamous cell carcinoma cell line, NCI‐H520, and human large cell lung carcinoma cell line, NCI‐H460, were purchased from ATCC and maintained in D‐MEM (Wako, Osaka, Japan) supplemented with 10% FBS (Nippon Bio‐supp. Center, Tokyo, Japan). Platinum‐GP (Plat‐GP), a retroviral packaging cell line, was purchased from Cell Biolabs (San Diego, CA, USA) and also maintained in D‐MEM with 10% FBS. Cell lines were cultured in a humidified 5% CO2 incubator at 37°C.
Lentivirus‐mediated knockdown of podocalyxin expression
Lentiviral vectors expressing shRNA specific for the human PODXL gene and pLKO.1‐puro non‐mammalian shRNA control were purchased from Sigma (Mission shRNA; St. Louis, MO, USA). For transduction, A549 cells were seeded and incubated at 60% confluence. After replacement of the medium with D‐MEM containing 8 μg/mL hexadimethrine bromide (Sigma), lentiviral particles were added. The culture medium with virus particles was removed after 8.5 h incubation; D‐MEM with 10% FBS was added, and the transduced cells were incubated at 37°C for 48 h. Cells were then selected for drug resistance in the presence of 5 μg/mL puromycin (Sigma). Expression of PODXL was assessed by RT‐PCR and immunoblotting.
Lentivirus‐mediated overexpression of podocalyxin
A PODXL open reading frame fragment was amplified by polymerase chain reaction (PCR) with KOD FX Neo (TOYOBO, Osaka, Japan), and the sequence of the PCR product was verified as wild‐type by sequencer. The purified DNA PCR product and the pMSCV plasmid (Riken Bioresource Center, Tsukuba, Japan) were ligated using an In‐Fusion HD Cloning Kit (Clontech Laboratories, Mountain View, CA, USA). Plat‐GP packaging cells were seeded at 70% confluence. Lipofectamine 3000 (Thermo Fisher Scientific, Waltham, MA, USA) was used to transfect the plasmid containing PODXL‐pMSCV and the packaging plasmid pVZV (Clontech Laboratories) into the Plat‐GP cells. After 24 h of incubation at 37°C, the complete medium was replaced. After a further 24 h incubation, cell culture supernatants were collected through a 0.45‐μm filter and stored at −80°C. Prior to transduction, A549 cells were seeded and incubated at 30% confluence. After replacement of the medium with D‐MEM containing 5 μg/mL polybrene (Nacalai Tesque, Kyoto, Japan), the purified virus in D‐MEM containing 5 μg/mL polybrene was added to the medium. The virus‐containing medium was discarded after 24 h incubation and the transduced cells were incubated with D‐MEM containing 10% FBS at 37°C for 24 h. Cells were selected for drug resistance in the presence of 5 μg/mL puromycin.
Antibodies and reagents
Anti‐PODXL rabbit polyclonal antibody (HPA002110), anti‐GAPDH mouse monoclonal antibody (WH0002597M1) and anti‐vimentin mouse monoclonal antibody (V6389) were purchased from Sigma. Anti‐Human‐E‐Cadherin mouse monoclonal antibody (M106) was purchased from Takara Bio (Shiga, Japan). Anti‐N‐cadherin mouse monoclonal antibody (sc‐59987), anti‐SNAI 1 rabbit polyclonal antibody (sc‐28199) and anti‐twist mouse monoclonal antibody (sc‐81417) were from Santa Cruz (Dallas, TX, USA). Anti‐Fibronectin rabbit polyclonal antibody (ab23750) and anti‐αSMA rabbit polyclonal antibody (ab5694) were purchased from Abcam (Cambridge, UK). Anti‐Smad2 mouse monoclonal antibody (#3103), anti‐phosoho‐Smad2 rabbit polyclonal antibody (#3101), anti‐Akt rabbit polyclonal antibody (#9272) and anti‐phospho‐Akt (Ser473) rabbit polyclonal antibody (#9271) were from Cell Signaling Technology (Danvers, MA, USA). Recombinant Human TGF‐β1 Protein was purchased from R&D Systems (Minneapolis, MN, USA). LY294002, as a selective inhibitor of phosphatidylinositol 3‐kinase (PI3K), was purchased from Cell Signaling Technology. SB431542, as a potent and selective inhibitor of TGF‐β type 1 receptor kinases, was purchased from Sigma.
Immunoblotting
Cells were washed with PBS, and proteins were extracted using radioimmunoprecipitation assay (RIPA) buffer (Cell Signaling Technology) containing 1 mM phenylmethylsulfonyl fluoride (PMSF) (Sigma) and phosphatase inhibitor cocktail 2 (Sigma). The concentration of extracted protein was measured by Bradford protein assay (Bio‐Rad, Hercules, CA, USA). Electrophoresis was performed on 7.5% acrylamide gels, and proteins were transferred onto polyvinylidenefluoride (PVDF) membranes (Bio‐Rad). Primary antibodies against PODXL, GAPDH, vimentin, E‐cadherin, N‐cadherin, Fibronectin, αSMA, Smad2, phosphor‐Smad2, Akt, phospho‐Akt, SnaI and twist were diluted at 1:250, 1:2000, 1:500, 1:500, 1:200, 1:500, 1:100 1:1000, 1:1000, 1:1000, 1:1000, 1:200 and 1:200, respectively. Secondary antibodies (anti‐mouse IgG HRP‐linked antibody and anti‐rabbit IgG, HRP‐linked antibody [Cell Signaling Technology]) were diluted at 1:2000. Cell extracts were detected using the Western Lighting Plus‐ECL (PerkinElmer, Boston, MA, USA). The figures were photographed using Chemi Doc Touch (Bio‐Rad). These results were attained from more than three independently performed experiments.
RNA extraction and quantitative real‐time RT‐PCR
Total RNA was extracted with an RNeasy Mini Kit (Qiagen, Hilden, Germany). Reverse transcription was done using Superscript III Reverse Transcriptase (Thermo Fisher Scientific), according to the manufacturer's protocol. Quantitative RT‐PCR was performed using a StepOnePlus system (Applied Biosystems, Foster City, CA, USA). The relative expression levels were calculated using the standard curve method.
ELISA
The expression of TGF‐β1 in condition medium was assessed in duplicate by ELISA. Human TGF‐β1 Quantikine ELISA Kit (R&D Systems) was used, following the manufacturer's instructions. The condition medium of A549 and the PODXL‐OE cells were collected and activated to immunoreactive TGF‐β1 detectable by the Quantikine TGF‐β1 immunoassay; an assay diluent was added, and standard, control or activated samples were added to each well. After incubation, the cells were washed, TGF‐β1 Conjugate was added, followed by washing and the addition of Substrate Solution. Next, Stop Solution was added. Absorbance was then assessed with a plate reader at 450 and 540 nm.
Cell migration assay
Cells (2 × 106 in 2 mL of serum free medium) were plated in the upper chambers of the wells of tissue culture plates containing polyethylene terephthalate membranes (pore size 8 μm, 6 cm in diameter; Corning Glass Work, Corning, NY, USA). The lower chamber contained 3 mL standard medium with 10% FBS. The cells on the lower side of the filter were stained using a Diff‐quik staining kit (Siemens, Munich, Germany) after incubation for 6 h at 37°C, and the number of cells was counted in four microscopic fields per well at a magnification of ×10. Data were collected from three independently performed experiments.
Cell invasion assay
Cells (2.5 × 105 in 200 μL of serum free medium) were plated in the upper chamber of a Matrigel Invasion well (pore size 8 μm, 24‐well; CORNING). The lower chamber contained 750 μL standard medium with 10% FBS. The cells on the lower side of the filter were stained after incubation for 20 h at 37°C, and the number of cells were counted as above.
Immunohistochemistry and analysis of prognosis
Podocalyxin expression was immunohistochemically examined in 114 primary lung adenocarcinoma specimens resected from patients who underwent surgery between 2010 and 2011 at Osaka University Hospital. The specimens were diagnosed as 16 non‐invasive adenocarcinomas, 43 minimally invasive adenocarcinomas and 55 invasive adenocarcinomas, according to standard lung adenocarcinoma classification guidelines.14 Histological specimens were fixed in 10% formalin and routinely processed for paraffin embedding. Paraffin‐embedded samples were stored in a dark room at room temperature. Sections were cut at 4‐μm thickness. Staining was performed using an automated VENTANA BenchMark GX (Roche, Basel, Switzerland) system with anti‐PODXL antibody (1:500; Sigma). The results were evaluated independently by two pathologists (H.K. and E.M.). Cases showing positive staining in more than 5% of tumor cells were considered PODXL‐positive. The114 patients were assessed for overall survival, disease free survival (DFS), and cancer related survival. The study was approved by the Osaka University ethical review board (No. 15234).
Statistical analysis
The χ2‐test, t‐test and Mann–Whitney U‐test were used to compare the results. Survival curves were calculated using the Kaplan–Meier method and tests for significance were based on log‐rank test results. The DFS was defined as the time between the date of pulmonary resection and the date of recurrent disease. All patient characteristics were tested against DFS using a Cox regression analysis based on the tested variable. All statistical analyses were performed using JMP version 10.2 for Windows (SAS Institute, Cary, NC, USA). A P‐value of ≤0.05 was considered to be statistically significant.
Results
Change of morphology and epithelial–mesenchymal transition marker expression in knockdown of podocalyxin expression and podocalyxin overexpression cells
When PODXL expression was knocked down, cell shape became more epithelial with the formation of tight cell clusters, and a reduction in the number of spindle cells (Fig. 1a). Immunoblotting (Fig. 1b) and quantitative RT‐PCR (Fig. 1c) showed that E‐cadherin was upregulated, and N‐cadherin, vimentin, fibronectin and αSMA were downregulated in podocalyxin knockdown (PODXL‐KD) cells (Fig. 1b).
Figure 1.
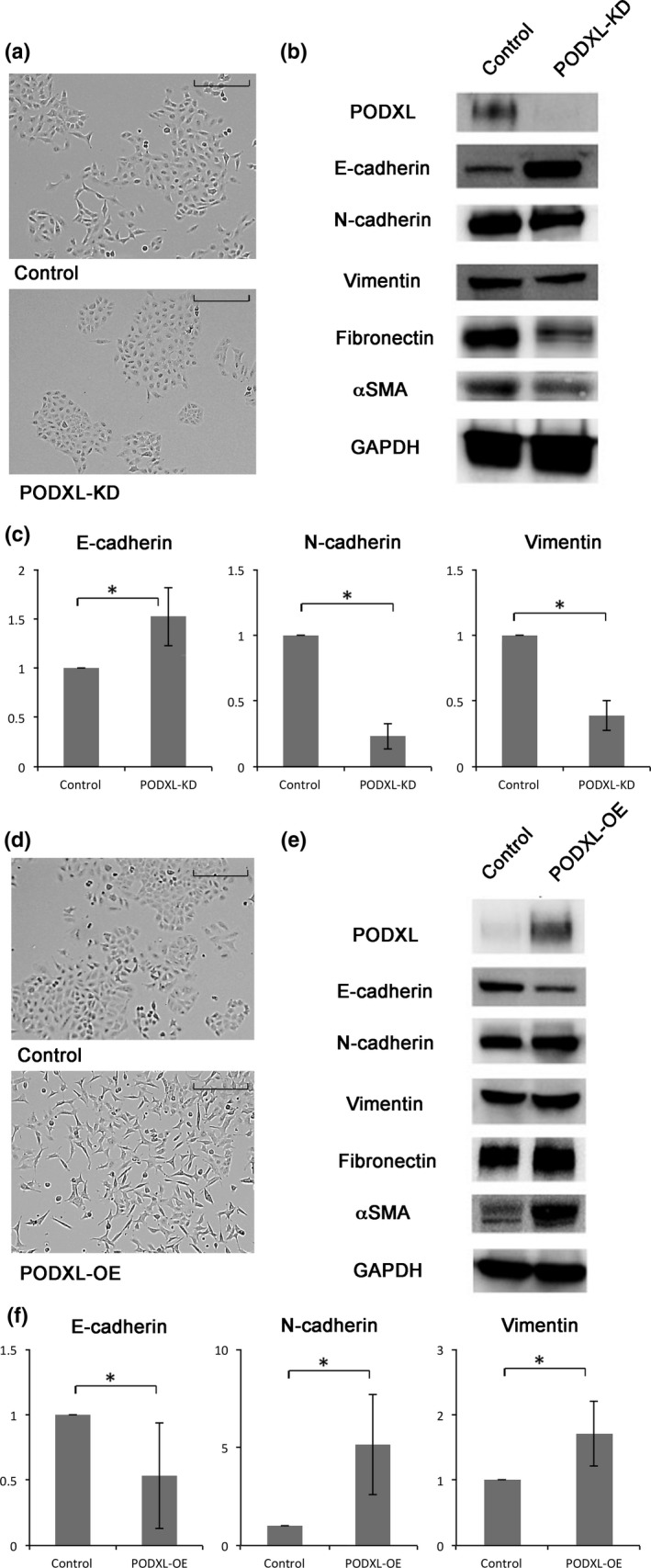
Cell morphology and epithelial–mesenchymal transition (EMT) marker expression of podocalyxin (PODXL)‐knockdown (KD) and overexpression (OE) cells. (a) The shape of control A549 cells and PODXL‐KD cells was observed under phase contrast. Scale bar = 100 μm. (b) Expression of PODXL, E‐cadherin, N‐cadherin, vimentin, fibronectin and αSMA were measured by immunoblotting in PODXL‐KD cells. (c) Expression of E‐cadherin, N‐cadherin and vimentin was measured by quantitative RT‐PCR in PODXL‐KD cells. The values are the means ± SD of three experiments. *P ≤ 0.05 (Mann–Whitney U‐test). (d) The shape of PODXL‐OE A549 cells was compared under phase contrast microscope. Scale bar = 100 μm. (e) Expression of PODXL, E‐cadherin, N‐cadherin, vimentin, fibronectin and αSMA were measured by immunoblotting in PODXL‐OE cells. (f) Expression of E‐cadherin, N‐cadherin and vimentin were measured by quantitative RT‐PCR in PODXL‐OE cells. The values are shown as above.
When PODXL was overexpressed, the morphology of cells became more spindle‐shaped, with loose cell connections (Fig. 1d). Immunoblotting revealed that E‐cadherin was downregulated, and N‐cadherin, vimentin, fibronectin, and αSMA were upregulated (Fig. 1e). Quantitative RT‐PCR showed that the mRNA level of E‐cadherin was reduced by approximately half, and N‐cadherin and vimentin expression increased 5‐fold and 1.7‐fold, respectively (Fig. 1f). Similar phenotypic changes were observed when PODXL was overexpressed in NCI‐H358 cells (Fig. S1). Because PODXL is expressed at a low level in NCI‐H358 cells, PODXL knockdown experiments were not conducted with this cell line.
TGF‐β1 induced podocalyxin expression and epithelial–mesenchymal transition
To investigate the relationship between PODXL expression and EMT induced by TGF‐β1, A549 cells were treated with 2 ng/mL of TGF‐β1 for 48 h, after which PODXL and EMT markers were assessed by immunoblotting. We found that E‐cadherin was downregulated, while N‐cadherin and vimentin as well as PODXL were upregulated in A549 cells treated with TGF‐β (Fig. 2a).
Figure 2.
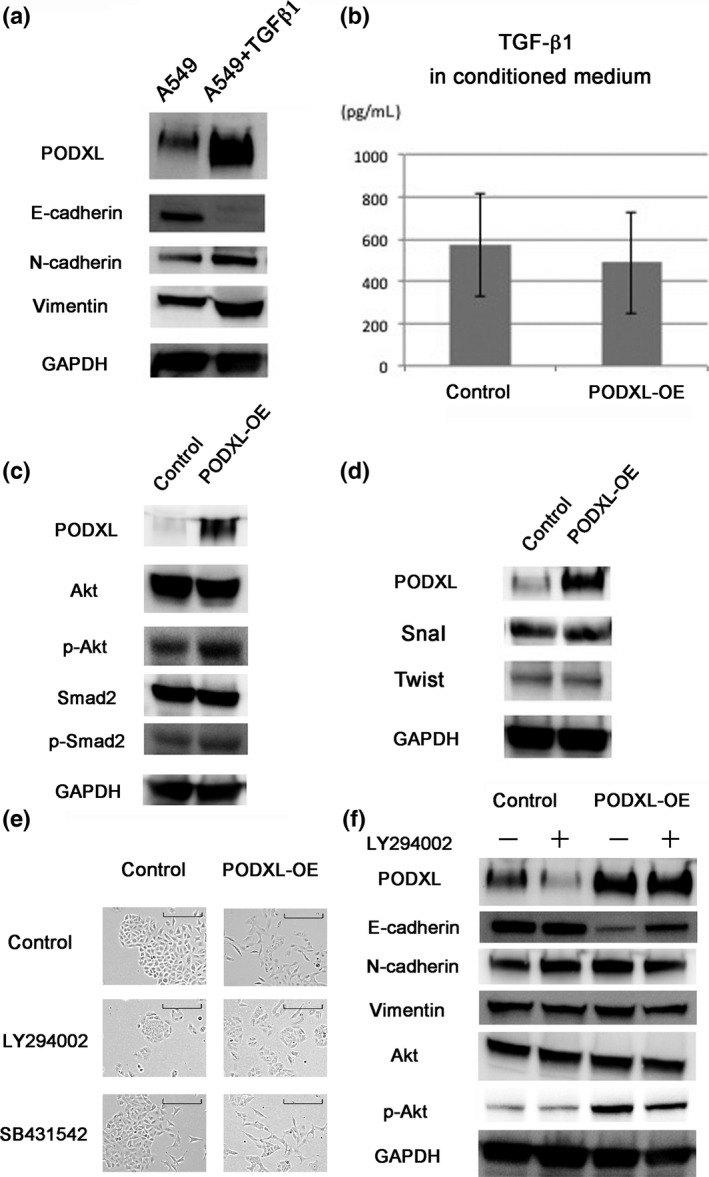
Role of PI3K‐Akt signaling in podocalyxin (PODXL)‐induced epithelial–mesenchymal transition (EMT). (a) Immunoblotting showed that TGF‐β induced EMT, and also induced PODXL expression in A549 cells. (b) The concentration of TGF‐β1 in conditioned medium was determined using a Human TGF‐beta 1 Quantikine ELISA Kit. There was no significant difference in the expression of TGF‐β1 in conditioned medium between A549 and PODXL overexpression (OE) cells (P = 0.60). (c) Phosphorylation of Akt and Smad2 was examined by immunoblotting in PODXL‐OE cells. (d) Immunoblotting did not reveal upregulation of Snail or Twist in PODXL‐OE cells. (e) PODXL‐OE cells were treated with LY294002 (10 μM) or SB431542 (10 μM) for 48 h. The change of cell shape was observed under phase contrast. Scale bar = 100 μm. (f) EMT markers and Akt signaling components were analyzed by immunoblotting in PODXL‐OE cells treated with or without LY294002 (10 μM) for 48 h.
Concentration of TGF‐β1 in conditioned medium
To examine the effect of TGF‐β1 on EMT, its concentration in conditioned medium was determined using ELISA. The mean TGF‐β1 concentration in five samples of medium from A549 cells was 573 pg/mL, while that in five samples of medium from PODXL‐OE cells was 488 pg/mL, which was not a significant difference (P = 0.60) (Fig. 2b). This result suggested that PODXL‐induced EMT was not influenced by TGF‐β1 secreted from the tumor cells.
Role of PI3K‐Akt signaling in podocalyxin‐induced EMT
To clarify the mechanisms driving PODXL‐induced EMT, the phosphorylation levels of the PI3K‐Akt pathway components, Smad2 and Akt, in PODXL‐OE cells were examined by immunoblotting. When PODXL was overexpressed, the levels of phospho‐Akt and phospho‐Smad2 were upregulated, consistent with activation of the pathway (Fig. 2a). To clarify the relationship between PODXL expression and EMT, Snail and Twist, transcription factors known to regulate EMT, were investigated. Immunoblotting did not reveal upregulation of Snail or Twist in PODXL‐OE cells (Fig. 2d). Next, to confirm the role of PI3K‐Akt signaling in PODXL‐induced EMT, a highly selective inhibitor of PI3K, LY294002, was added to the PODXL‐OE cells. Although PODXL‐OE cells normally display mesenchymal morphology, the addition of LY294002 induced changes in cell shape to a more epithelial form with tight cell connections (Fig. 2b). In contrast, the addition of the potent and selective TGF‐β signaling inhibitor, SB431542, did not dramatically attenuate morphological changes induced in PODXL‐OE cells (Fig. 2b). Immunoblotting revealed that the addition of LY294002 upregulated the expression of epithelial markers and downregulated the expression of mesenchymal markers (Fig. 2c). These data suggested that PODXL induced EMT of lung adenocarcinoma cells by activating PI3K‐Akt signaling.
Effect of podocalyxin on cell migration and the invasive phenotype
Cell migration and invasion assays revealed that knockdown of PODXL expression significantly reduced the number of cells migrating through the membrane or Matrigel, respectively (Fig. 3a,b). In the reciprocal experiment, PODXL overexpression significantly increased the number of cells migrating through the membrane or Matrigel, respectively (Fig. 3c,d). These findings indicated that PODXL regulated cell motility, and overexpression was related to the aggressive phenotype of lung adenocarcinoma cells. To investigate the role of PI3K‐Akt signaling in upregulated migration and invasion induced by PODXL overexpression, cell migration and invasion of PODXL‐OE cells was assessed after treatment with the PI3K inhibitor LY294002. We found that the number of migrating PODXL‐OE cells was significantly reduced as compared to untreated PODXL‐OE cells (Fig. 3e,f). These findings indicate that PODXL regulates cell motility under PI3K‐Akt signaling and its overexpression is related to the aggressive phenotype of lung adenocarcinoma cells.
Figure 3.
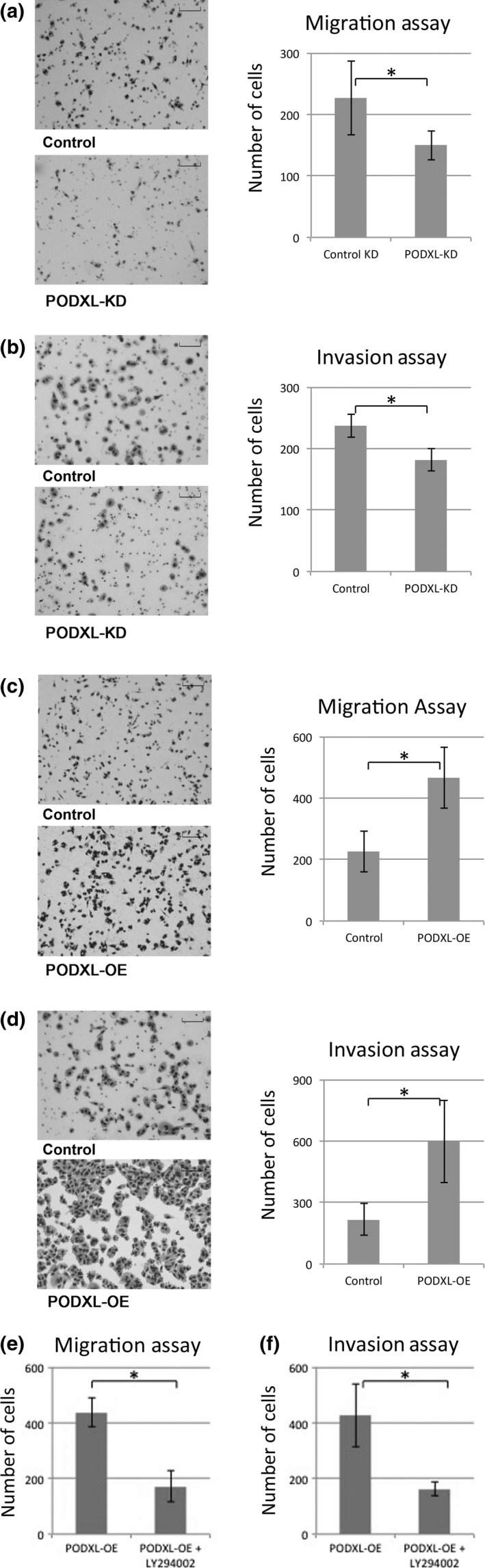
Migration and invasive phenotype affected by podocalyxin (PODXL). (a) Motility assays were performed, and cells traversing the filter were counted. Representative findings of A549 cells traversing the filter are shown. The number of stained cells were counted in four microscopic fields per well at a magnification of ×10. Data were collected from three independently performed experiments. The values are the means ± SD of three experiments. *P ≤ 0.05 (Mann–Whitney U‐test). Scale bar = 100 μm. (b) Invasion assays were performed with podocalyxin knockdown (PODXL‐KD) cells. (c) Motility assays were performed using PODXL‐overexpression (OE) cells. (d) Invasion assays were performed with PODXL‐OE cells. (e, f) Cell migration and invasion of PODXL‐OE cells was assessed after treatment with the PI3K inhibitor LY294002.
Effect of podocalyxin on epithelial–mesenchymal transition markers in various lung carcinoma cell lines
The effect of PODXL on EMT marker expression was examined in other lung carcinoma cell lines (Fig. S2). PODXL expression level was correlated to EMT characters in adenocarcinoma cell lines A549 and NCI‐H358, and squamous cell carcinoma cell line NCI‐H520. As for large cell carcinoma cell line NCI‐H460, the expression of PODXL, E‐cadherin and N‐cadherin was hardly detected. We found that the expression of PODXL was proportional to mesenchymal character in some of the cell lines.
Podocalyxin expression in clinical samples of lung adenocarcinoma
To examine the clinicopathological significance of PODXL expression, immunohistochemistry was performed on 114 cases of lung adenocarcinoma, and the relationships between PODXL positivity and clinicopathological characteristics were analyzed. Because heterogeneous staining for PODXL was noted in the same samples, cases with positive staining in >5% of the tumor cells were considered to be PODXL‐positive regardless of histological features. Fifty‐three cases (46.5%) were positive for PODXL expression, and 61 cases (53.5%) were negative. All PODXL‐positive tumors were diagnosed as invasive or minimally invasive adenocarcinomas. In contrast, all cases of non‐invasive adenocarcinoma (16 cases) were PODXL‐negative (Fig. 4). In the immunohistochemistry findings, PODXL‐positive tumor cells showed weak staining for E‐cadherin and strong staining for vimentin as compared to PODXL‐negative tumor cells in the same sample (Fig. 4d–h). As compared to the lepidic predominant subtype, non‐lepidic predominant samples tended to be positive for PODXL (Fig. S3).
Figure 4.
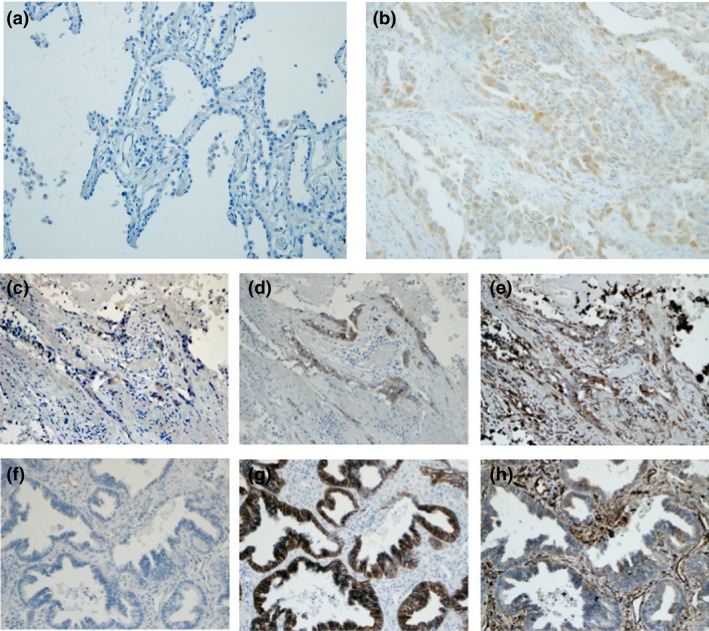
Immunohistochemistry of lung adenocarcinoma. Panels show representative findings of podocalyxin (PODXL) staining in a non‐invasive adenocarcinoma (a) and in an invasive adenocarcinoma (b). Representative images of PODXL‐positive area (c), E‐cadherin (d) and vimentin (e) in serial sections of lung adenocarcinoma. Representative images of PODXL‐negative area (f), E‐cadherin (g) and vimentin (h) in serial sections of the same sample. Original magnification was ×200.
When age, gender, histology, pathological node status, pathological stage, operation type and postoperative therapy were considered, only age and histology were significantly different between PODXL‐positive and PODXL‐negative cases (Table 1). Seven variables, including age, gender, pathologic nodal metastasis, pathologic stage, operating procedure, adjuvant therapy and PODXL expression, were analyzed using a Cox proportional hazards regression model to assess the variables affecting DFS in lung adenocarcinoma patients. The univariate analyses revealed that pathologic nodal metastasis, pathologic stage and PODXL expression were related to DFS (Table 2). Although no significant difference in overall survival was detected, DFS and cancer‐specific survival were significantly worse for PODXL‐positive lung adenocarcinoma patients compared with those that were PODXL‐negative (Fig. 5). Multivariate analysis revealed that PODXL expression was an independent variable predicting DFS (Table 3). These data indicated that PODXL expression was an unfavorable prognostic factor in lung adenocarcinoma.
Table 1.
Clinicopathological features of podocalyxin (PODXL) positive and negative cases
| PODXL (−) | PODXL (+) | P‐value | |
|---|---|---|---|
| N | 61 (53.5%) | 53 (46.5%) | |
| Age | 63.5 (32–86) | 69.6 (40–84) | 0.022 |
| Gender (M/F) | 30/31 | 24/29 | 0.68 |
| Histology (AIS/MIA/IA) | 16/24/21 | 0/19/34 | <0.0001 |
| Pathologic N status (0/1/2) | 56/4/1 | 48/3/2 | 0.76 |
| Pathologic stage (I/II/III/IV) | 52/5/1/3 | 46/4/2/1 | 0.73 |
| Operation (lobectomy/segmentectomy/partial resection) | 40/13/8 | 39/8/6 | 0.63 |
| Adjuvant therapy (no/yes) | 49/9 | 48/4 | 0.20 |
AIS, adenocarcinoma in situ; F, female; IA, invasive adenocarcinoma; M, male; MIA, minimally invasive adenocarcinoma.
Table 2.
Univariate analysis of disease free survival
| Factors | Hazard ratio | 95% CI | P‐value |
|---|---|---|---|
| Age | |||
| Over 70 versus under 70 | 1.14 | 0.46–2.75 | 0.78 |
| Gender | |||
| Male versus female | 0.93 | 0.37–2.25 | 0.87 |
| Pathologic N status | |||
| pN1 versus pN0 | 10.37 | 3.16–30.31 | 0.0004 |
| pN2 versus pN0 | 12.30 | 1.84–49.82 | 0.015 |
| Pathologic stage | |||
| II versus I | 3.45 | 0.98–9.67 | 0.054 |
| III versus I | 9.73 | 1.48–37.47 | 0.023 |
| Operation | |||
| Segmentectomy versus lobectomy | 0.67 | 0.16–2.01 | 0.51 |
| Partial resection versus lobectomy | 0.34 | 0.019–1.66 | 0.22 |
| Adjuvant therapy | |||
| No versus yes | 0.35 | 0.14–1.09 | 0.068 |
| PODXL expression | |||
| Positive versus negative | 2.53 | 1.04–6.74 | 0.042 |
CI, confidence interval.
Figure 5.
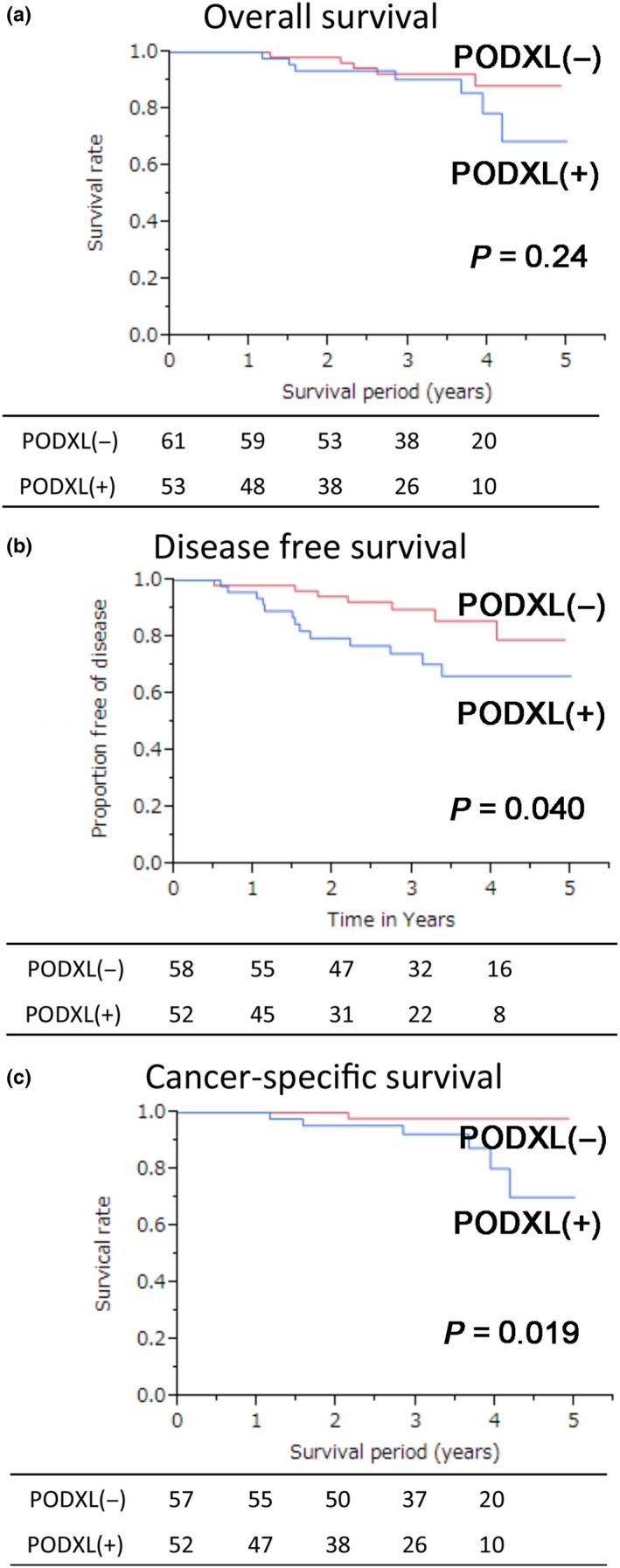
Kaplan–Meier curves of lung adenocarcinoma patients. Overall survival (a), disease free survival (b), and cancer‐specific survival (c) were calculated using Kaplan–Meier curves comparing podocalyxin (PODXL)‐positive and PODXL‐negative patients.
Table 3.
Multivariate analysis of disease free survival
| Factors | Hazard ratio | 95% CI | P‐value |
|---|---|---|---|
| Pathologic stage | |||
| II versus I | 3.73 | 1.05–10.50 | 0.043 |
| III versus I | 9.84 | 1.48–38.92 | 0.023 |
| PODXL expression | |||
| Positive versus negative | 2.65 | 1.08–7.10 | 0.034 |
CI, confidence interval; PODXL, podocalyxin.
Discussion
The present study is the first to address the relationship between PODXL expression and EMT in lung adenocarcinoma. PODXL is known to inhibit adhesion between neighboring foot processes in kidney cells.6 Chen et al.15 report that the interaction between ezrin and PODXL in A549 cells is important for TGF‐β induced EMT through actin filament reorganization. Thus, PODXL has a crucial role in regulating cell shape, as well as cell movement. Our study showed that EMT was induced in PODXL‐OE cells, and, conversely, PODXL knockdown inhibited PODXL‐induced EMT, similar to the results reported in Meng et al.13 In addition, our data revealed that PODXL‐mediated induction of EMT of lung adenocarcinoma cells was regulated by PI3K‐Akt signaling, and PODXL influenced cell motility in the A549 lung carcinoma cell line. These findings are broadly consistent with a suite of other PODXL studies. PODXL was reported to activate several signaling pathways, including PI3K, Rac and Rho, in a range of different cancers.16, 17, 18 The PI3K‐Akt pathway is known to trigger a cascade of responses, including cell growth, proliferation and activation of pro‐survival pathways, motility, EMT and angiogenesis.19 PODXL overexpression increases cell migration and invasion via MAPK and PI3K pathways in breast and prostate cancer,16 and promotes cell invasion and resistance to temozolomide‐induced apoptotic stress by enhancing the activation of the PI3K‐Akt signaling pathway in brain tumors.17 PODXL also influences cell migration by disrupting cell–cell junctions and cell invasion by increasing expression of matrix metalloprotease 9.20, 21 In contrast to inhibition of the PI3K‐Akt pathway, inhibition of TGF‐β signaling did not affect PODXL‐induced EMT. Consistent with the results of Meng et al.,13 we observed that the knockdown of PODXL expression inhibited TGF‐β‐induced EMT of A549 cells (data not shown). Although many transcript factors, including Snail and Twist, are known to regulate EMT,22, 23 immunoblotting did not reveal upregulation of Snail or Twist in PODXL‐OE cells. However, EMT was induced by PODXL expression, as shown by changes in EMT markers, including E‐cadherin, N‐cadherin, vimentin, fibronectin and αSMA. These findings suggest that PODXL is essential for TGF‐β‐induced EMT, but classical TGF‐β signaling is not required for PODXL‐induced EMT.
In clinical specimens, immunohistochemical analyses showed that PODXL was only expressed in invasive or minimally invasive adenocarcinoma. Because EMT is known to play important roles in lung cancer invasion,24, 25 this result is consistent with our in vitro findings that PODXL was involved in EMT. Thus, we speculate that PODXL expression increases as malignancy progresses from non‐invasive adenocarcinoma to invasive adenocarcinoma. In recent years, overexpression of PODXL has been linked to the pathogenesis of several different forms of cancer, including breast20 and prostate cancer,26 malignant brain tumors,27 and hepatocellular28 and renal cell carcinoma.29 Consistent with these studies, DFS and cancer‐specific survival were significantly worse in PODXL‐positive lung adenocarcinoma patients. Thus, similar to other cancers, PODXL expression is an unfavorable prognostic factor in lung adenocarcinoma.
How the expression of PODXL is controlled remains unclear. Koch et al.30 report that high expression of PODXL in small cell lung cancer is linked to hypomethylation of the PODXL promoter. Consistent with the potential role of promoter dysfunction in the upregulation of PODXL expression, we found that the PODXL promoter was hypomethylated in the A549 cell line (data not shown). However, other mechanisms have been described, including TGF‐β‐mediated upregulation of PODXL expression,12 suggesting that the regulation of PODXL expression is complex, and additional pathways may contribute to PODXL overexpression in cancer cells.
The ERM proteins, including ezrin, radixin and moesin, are known to be general cross‐linkers between cortical actin filaments and plasma membrane.31 The PODXL–ezrin complex is associated with migration and invasion in breast and prostate cancer and hepatocellular carcinoma.16, 32 Thus, further investigation of the role of the PODXL–ezrin complex in the regulation of the lung cancer phenotype appears warranted.
Our results suggest that targeting PODXL might be a novel strategy for the treatment of lung adenocarcinoma. In this regard, there has been some recent progress towards the development of PODXL‐targeted therapies, including PODXL‐targeted monoclonal antibody therapy to inhibit primary tumor growth and systemic dissemination of breast cancer.33 In addition, Chijiiwa et al.34 suggest that miR‐5100, which directly binds to the 3′ untranslated region of the PODXL transcript and downregulates PODXL expression, could have therapeutic potential for the treatment of metastatic pancreatic cancer.
In conclusion, we demonstrated that PODXL overexpression induces EMT via the PI3K‐Akt signaling pathway in lung adenocarcinoma, and is involved in driving disease progression. Consistent with the in vitro findings, the analysis of clinical samples revealed that PODXL overexpression was significantly associated with more aggressive and invasive tumors, and was an unfavorable prognostic factor in lung adenocarcinoma.
Disclosure Statement
The authors have no conflict of interest to declare.
Supporting information
Fig. S1. Cell morphology and epithelial–mesenchymal transition (EMT) marker expression of podocalyxin (PODXL)‐overexpressing (OE) NCI‐H358 cells. (a) The shape of PODXL‐OE NCI‐H358 cells was compared to untransduced control cells under phase contrast. Scale bar = 100 μm. (b) Expression of E‐cadherin, N‐cadherin and vimentin was measured by immunoblotting.
Fig. S2. Podocalyxin (PODXL) expression level was correlated to epithelial–mesenchymal transition (EMT) characters in A5449, NCI‐H358 and NCI‐H520 cells.
Fig. S3. Number of cases with podocalyxin (PODXL)‐positive and PODXL‐negative features in lepidic or non‐lepidic predominant subtype of lung adenocarcinoma. Non‐lepidic predominant subtype tended to PODXL‐positive, as compared to lepidic predominant subtype.
Acknowledgment
This work was supported by Grants‐in‐Aid for Scientific Research (grant T264604700 to E.M.) and Grants‐in‐Aid for Scientific Research (grant 15H04943 to Y.S.).
Cancer Sci 108 (2017) 528–535
Funding Information
Grants‐in‐Aid for Scientific Research (grant T264604700 to E.M.) and Grants‐in‐Aid for Scientific Research (grant 15H04943 to Y.S.).
References
- 1. Spira A, Ettinger DS. Multidisciplinary management of lung cancer. N Engl J Med 2004; 350: 379–92. [DOI] [PubMed] [Google Scholar]
- 2. Thiery JP. Epithelial–mesenchymal transitions in development and pathologies. Curr Opin Cell Biol 2003; 15: 740–6. [DOI] [PubMed] [Google Scholar]
- 3. Abulaiti A, Shintani Y, Funaki S et al Interaction between non‐small‐cell lung cancer cells and fibroblasts via enhancement of TGF‐beta signaling by IL‐6. Lung Cancer 2013; 82: 204–13. [DOI] [PubMed] [Google Scholar]
- 4. Shintani Y, Maeda M, Chaika N, Johnson KR, Wheelock MJ. Collagen I promotes epithelial‐to‐mesenchymal transition in lung cancer cells via transforming growth factor‐beta signaling. Am J Respir Cell Mol Biol 2008; 38: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nielsen JS, McNagny KM. Novel functions of the CD34 family. J Cell Sci 2008; 121: 3683–92. [DOI] [PubMed] [Google Scholar]
- 6. Takeda T, Go WY, Orlando RA, Farquhar MG. Expression of podocalyxin inhibits cell–cell adhesion and modifies junctional properties in Madin‐Darby canine kidney cells. Mol Biol Cell 2000; 11: 3219–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burghardt T, Kastner J, Suleiman H et al LMX1B is essential for the maintenance of differentiated podocytes in adult kidneys. J Am Soc Nephrol 2013; 24: 1830–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Larsson A, Johansson ME, Wangefjord S et al Overexpression of podocalyxin‐like protein is an independent factor of poor prognosis in colorectal cancer. Br J Cancer 2011; 105: 666–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Forse CL, Yilmaz YE, Pinnaduwage D et al Elevated expression of podocalyxin is associated with lymphatic invasion, basal‐like phenotype, and clinical outcome in axillary lymph node‐negative breast cancer. Breast Cancer Res Treat 2013; 137: 709–19. [DOI] [PubMed] [Google Scholar]
- 10. Boman K, Larsson AH, Segersten U et al Membranous expression of podocalyxin‐like protein is an independent factor of poor prognosis in urothelial bladder cancer. Br J Cancer 2013; 108: 2321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saukkonen K, Hagstrom J, Mustonen H et al Podocalyxin is a marker of poor prognosis in pancreatic ductal adenocarcinoma. PLoS One 2015; 10: e0129012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Laitinen A, Bockelman C, Hagstrom J et al Podocalyxin as a prognostic marker in gastric cancer. PLoS One 2015; 10: e0145079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meng X, Ezzati P, Wilkins JA. Requirement of podocalyxin in TGF‐beta induced epithelial mesenchymal transition. PLoS One 2011; 6: e18715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Travis WD, Brambilla E, Noguchi M et al Diagnosis of lung adenocarcinoma in resected specimens: implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification. Arch Pathol Lab Med 2013; 137: 685–705. [DOI] [PubMed] [Google Scholar]
- 15. Chen MJ, Gao XJ, Xu LN, Liu TF, Liu XH, Liu LX. Ezrin is required for epithelial–mesenchymal transition induced by TGF‐beta1 in A549 cells. Int J Oncol 2014; 45: 1515–22. [DOI] [PubMed] [Google Scholar]
- 16. Sizemore S, Cicek M, Sizemore N, Ng KP, Casey G. Podocalyxin increases the aggressive phenotype of breast and prostate cancer cells in vitro through its interaction with ezrin. Cancer Res 2007; 67: 6183–91. [DOI] [PubMed] [Google Scholar]
- 17. Wu H, Yang L, Liao D, Chen Y, Wang W, Fang J. Podocalyxin regulates astrocytoma cell invasion and survival against temozolomide. Exp Ther Med 2013; 5: 1025–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin CW, Sun MS, Liao MY et al Podocalyxin‐like 1 promotes invadopodia formation and metastasis through activation of Rac1/Cdc42/cortactin signaling in breast cancer cells. Carcinogenesis 2014; 35: 2425–35. [DOI] [PubMed] [Google Scholar]
- 19. Fukasawa H, Obayashi H, Schmieder S, Lee J, Ghosh P, Farquhar MG. Phosphorylation of podocalyxin (Ser415) prevents RhoA and ezrin activation and disrupts its interaction with the actin cytoskeleton. Am J Pathol 2011; 179: 2254–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Somasiri A, Nielsen JS, Makretsov N et al Overexpression of the anti‐adhesin podocalyxin is an independent predictor of breast cancer progression. Cancer Res 2004; 64: 5068–73. [DOI] [PubMed] [Google Scholar]
- 21. Ruhul Amin AR, Senga T, Oo ML, Thant AA, Hamaguchi M. Secretion of matrix metalloproteinase‐9 by the proinflammatory cytokine, IL‐1beta: a role for the dual signalling pathways, Akt and Erk. Genes Cells 2003; 8: 515–23. [DOI] [PubMed] [Google Scholar]
- 22. Fan Q, Qiu MT, Zhu Z et al Twist induces epithelial–mesenchymal transition in cervical carcinogenesis by regulating the TGF‐beta/Smad3 signaling pathway. Oncol Rep 2015; 34: 1787–94. [DOI] [PubMed] [Google Scholar]
- 23. Liu RY, Zeng Y, Lei Z et al JAK/STAT3 signaling is required for TGF‐beta‐induced epithelial–mesenchymal transition in lung cancer cells. Int J Oncol 2014; 44: 1643–51. [DOI] [PubMed] [Google Scholar]
- 24. Zhang J, Lu C, Kang J, Cao C, Li M. Involvement of ZEB1 and E‐cadherin in the invasion of lung squamous cell carcinoma. Mol Biol Rep 2013; 40: 949–56. [DOI] [PubMed] [Google Scholar]
- 25. Maeng YI, Kim KH, Kim JY et al Transcription factors related to epithelial mesenchymal transition in tumor center and margin in invasive lung adenocarcinoma. Int J Clin Exp Pathol 2014; 7: 4095–103. [PMC free article] [PubMed] [Google Scholar]
- 26. Casey G, Neville PJ, Liu X et al Podocalyxin variants and risk of prostate cancer and tumor aggressiveness. Hum Mol Genet 2006; 15: 735–41. [DOI] [PubMed] [Google Scholar]
- 27. Hayatsu N, Kaneko MK, Mishima K et al Podocalyxin expression in malignant astrocytic tumors. Biochem Biophys Res Commun 2008; 374: 394–8. [DOI] [PubMed] [Google Scholar]
- 28. Chen X, Higgins J, Cheung ST et al Novel endothelial cell markers in hepatocellular carcinoma. Mod Pathol 2004; 17: 1198–210. [DOI] [PubMed] [Google Scholar]
- 29. Hsu YH, Lin WL, Hou YT et al Podocalyxin EBP50 ezrin molecular complex enhances the metastatic potential of renal cell carcinoma through recruiting Rac1 guanine nucleotide exchange factor ARHGEF7. Am J Pathol 2010; 176: 3050–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koch LK, Zhou H, Ellinger J et al Stem cell marker expression in small cell lung carcinoma and developing lung tissue. Hum Pathol 2008; 39: 1597–605. [DOI] [PubMed] [Google Scholar]
- 31. Tsukita S, Yonemura S. ERM (ezrin/radixin/moesin) family: from cytoskeleton to signal transduction. Curr Opin Cell Biol 1997; 9: 70–5. [DOI] [PubMed] [Google Scholar]
- 32. Flores‐Tellez TN, Lopez TV, Vasquez Garzon VR, Villa‐Trevino S. Co‐expression of ezrin‐CLIC5‐podocalyxin is associated with migration and invasiveness in hepatocellular carcinoma. PLoS One 2015; 10: e0131605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Snyder KA, Hughes MR, Hedberg B et al Podocalyxin enhances breast tumor growth and metastasis and is a target for monoclonal antibody therapy. Breast Cancer Res 2015; 17: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chijiiwa Y, Moriyama T, Ohuchida K et al Overexpression of microRNA‐5100 decreases the aggressive phenotype of pancreatic cancer cells by targeting PODXL. Int J Oncol 2016; 48: 1688–700. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Cell morphology and epithelial–mesenchymal transition (EMT) marker expression of podocalyxin (PODXL)‐overexpressing (OE) NCI‐H358 cells. (a) The shape of PODXL‐OE NCI‐H358 cells was compared to untransduced control cells under phase contrast. Scale bar = 100 μm. (b) Expression of E‐cadherin, N‐cadherin and vimentin was measured by immunoblotting.
Fig. S2. Podocalyxin (PODXL) expression level was correlated to epithelial–mesenchymal transition (EMT) characters in A5449, NCI‐H358 and NCI‐H520 cells.
Fig. S3. Number of cases with podocalyxin (PODXL)‐positive and PODXL‐negative features in lepidic or non‐lepidic predominant subtype of lung adenocarcinoma. Non‐lepidic predominant subtype tended to PODXL‐positive, as compared to lepidic predominant subtype.


