Abstract
T‐lymphokine‐activated killer cell‐originated protein kinase (TOPK) plays critical roles in cancer cell proliferation as well as maintenance of cancer stem cells (CSC). Small cell lung cancer (SCLC) has highly aggressive phenotype, reveals early spread to distant sites, and results in dismal prognosis with little effective treatment. In this study, we demonstrate that TOPK expression was highly upregulated in both SCLC cell lines and primary tumors. Similar to siRNA‐mediated TOPK knockdown effects, treatment with a potent TOPK inhibitor, OTS514, effectively suppressed growth of SCLC cell lines (IC 50; 0.4–42.6 nM) and led to their apoptotic cell death. TOPK inhibition caused cell morphologic changes in SCLC cells, elongation of intercellular bridges caused by cytokinesis defects or neuronal protrusions induced by neuronal differentiation in a subset of CSC‐like SCLC cells. Treatment with OTS514 suppressed forkhead box protein M1 (FOXM1) activity, which was involved in stemness of CSC. Furthermore, OTS514 treatment reduced CD90‐positive SCLC cells and showed higher cytotoxic effect against lung sphere‐derived CSC‐like SCLC cells. Collectively, our results suggest that targeting TOPK is a promising approach for SCLC therapy.
Keywords: Cancer stem cell, kinase inhibitors, molecular target, small cell lung cancer, TOPK
T‐lymphokine‐activated killer cell–originated protein kinase (TOPK), also known as PBK or PDZ‐binding kinase, is a Ser/Thr protein kinase that is highly expressed in a wide range of human malignancies, including acute myeloid leukemia (AML), breast cancer, Ewing sarcoma, kidney cancer and colorectal cancer.1, 2, 3, 4, 5, 6, 7 However, in normal adult tissues, TOPK expression is limited to the testis where it plays a key role in spermatogenesis.8 We and others reported that TOPK is essential in the cancer cell mitosis,1, 9, 10 much highly expressed in cancer stem cells (CSC)‐enriched tumor cell population, and associated with poor prognosis of multiple types of cancer.11 Because CSCs are in general resistant to conventional as well as molecular‐targeted anti‐cancer therapies, new treatment strategies targeting critical signaling pathways, in the replication and survival of CSC, including the TOPK pathway, should be developed.12, 13 Our efforts in this line of drug development identified two small molecular compounds, OTS514 and its derivative OTS964, as potent TOPK inhibitors.14 Treatment with TOPK inhibitors inhibited cytokinesis and effectively suppressed growth of cancer cells,6, 7 and induced complete tumor regression in xenograft models of human lung cancer.14
Small cell lung cancer (SCLC) is a subtype of lung cancer that affects more than 200 000 people worldwide every year with a very high mortality rate, <15% of 2‐year survival.15, 16 In the last three decades, their survival rate has been improved very little.17 These clinically aggressive characteristics of SCLC might be related to predominant presence of CSC subpopulation in tumor,18 since SCLC cells are probably derived from self‐renewing pulmonary neuroendocrine progenitor cells.19, 20
In this study, we report that TOPK was overexpressed in the majority of SCLC cell lines and primary tumors, and that either knockdown of TOPK or treatment with a TOPK inhibitor (OTS514) exhibited growth inhibitory effects in all tested SCLC cell lines. Our results imply that TOPK could be a promising therapeutic target for SCLC treatment and the TOPK inhibitor OTS514 would be clinically investigated as a novel class of anti‐SCLC agent.
Materials and Methods
Cell lines
Three adherent SCLC cell lines (DMS114, H69AR and H446), five suspension SCLC cell lines (H69, H82, H146, H524 and H2171), and two normal fetal lung fibroblasts cell lines (IMR‐90 and WI‐38) were purchased from the American Type Culture Collection (ATCC) (Manassas, VA, USA). DMS273 cell line was purchased from European Collection of Authenticated Cell Cultures (ECACC, Salisbury, UK). SBC‐3 and SBC‐5 cell lines were purchased from Japanese Collection of Research Bioresources Cell Bank (JCRB) (Suita, Japan). IMR‐90, WI‐38, SBC3, and SBC5 cell lines were cultured in EMEM medium (Life Technologies, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic‐antimycotic solution (Sigma‐Aldrich, St. Louis, MO, USA). Other SCLC cell lines were cultured in RPMI medium (Life Technologies) supplemented with 10% FBS. All cells were maintained at 37°C in humidified air with 5% CO2. Cell authentication results for SBC3, SBC5, DMS114, H446, H82 and H524 cells were previously described.21
Knockdown experiments
To knockdown expression of TOPK, SCLC cell lines were transiently transfected with 20 pmol/mL of oligo siRNA using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA) reagent according to manufacturer's instructions. Oligo siRNAs of si‐TOPK (5′‐GUGUGGCUUGCGUAAAUAA‐3′) and si‐control (Mission siRNA Universal Negative Control, catalog #SIC001) were purchased from Sigma‐Aldrich.
Cell viability analyses
Cell viability was assessed by methyl thiazolyl tetrazolium (MTT) assay using the Cell Counting Kit‐8 (Dojindo Molecular Technologies Inc., Kumamoto, Japan). Briefly, 4 × 103 of adherent SCLC cells or 4 × 104 of suspension SCLC cells were seeded into 96‐well flat plates. The SCLC cells treated with OTS514 compound or those transfected with oligo siRNAs were cultured for 72 h before the MTT assay. In case of LS‐derived cells, the cells treated with OTS514 were cultured for 48 h. After one‐hour reaction with the Cell Counting Kit‐8, the 96‐well plate was read at 450 nm of wavelength in the iMark microplate absorbance reader (Bio‐Rad, Hercules, CA, USA). All of these experiments were done in triplicate.
Western blot analysis and antibodies
After washing with phosphate‐buffered saline (PBS) twice, cultured cells were harvested and kept at −80°C until use. For western blot analysis, total proteins were extracted from cells using the IP lysis buffer (Thermo Scientific, Waltham, MA, USA) supplemented with the protease inhibitor cocktail III (EMD Millipore, Billerica, MA, USA). Then, the proteins were separated by electrophoresis using 4–20% SDS‐PAGE gel (Bio‐Rad) and transferred onto PVDF membranes. After blocking with skim milk (EMD Millipore) overnight, the membranes were incubated with the first antibody, respectively: anti‐TOPK antibody (BD Biosciences, Franklin Lakes, NJ, USA), anti‐FOXM1 antibody (Santa Cruz Biotechnology, Dallas, TX, USA), anti‐phospho‐FOXM1 antibody (Cell Signaling Technology, Danvers, MA, USA). We generated a mouse anti‐MELK (maternal embryonic leucine‐zipper kinase) monoclonal antibody using partial recombinant MELK protein of 264–601 amino acids.7 An anti‐β‐actin antibody (Sigma‐Aldrich) was used as a loading control.
Quantitative RT‐PCR
From cultured cancer cells, total RNA was extracted using RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and 1 μg of total RNA was reversely transcribed by SuperScript III First‐Strand Synthesis System (Invitrogen) according to the manufacturer's instructions. Aliquots of the reversely‐transcribed cDNA products were quantified by real‐time RT‐PCR using the ViiA 7 system (Life Technologies). The PCR primer sequences are 5′‐AGACCCTAAAGATCGTCCTTCTG‐3′ and 5′‐GTGTTTTAAGTCAGCATGAGCAG‐3′ for TOPK, 5′‐CAACCGCTACTTGACATTGGA‐3′ and 5′‐TCACCGGGAACTGGATAGG‐3′ for FOXM1, and 5′‐CGACCACTTTGTCAAGCTCA‐3′ and 5′‐GGTTGAGCACAGGGTACTTTATT‐3′ for GAPDH. Expression level of each gene was normalized with that of GAPDH.
Flow cytometry analyses
For Annexin‐V/PI staining analysis, DMSO‐ or OTS514‐treated SCLC cells were collected, spun down, washed with PBS, and then resuspended in 50 μL of binding buffer containing 2 μL of APC‐conjugated Annexin‐V antibody (eBioscience, San Diego, CA, USA). After 20 min incubation on ice, the cells were further stained with 100 μL of binding buffer containing 1 μL of propidium iodide (PI) (eBioscience). For cleaved caspase‐3 analysis, SCLC cells were prepared as aforementioned and resuspended in 500 μL of Cytofix/Cytoperm solution (eBioscience). After 20 min incubation on ice, the cells were resuspended with 100 μL of buffer containing 20 μL of PE‐conjugated anti‐cleaved caspase‐3 antibody (eBioscience). For neuronal differentiation detection, prepared SCLC cells were stained with FITC‐conjugated anti‐human CD56 antibody (eBioscience) for 20 min at room temperature. To measure expression levels of CD90 surface protein, cells were stained with APC‐conjugated anti‐human CD90 antibody (5E10) (BD Pharmingen) for 15 min at room temperature. After washing with PBS, samples were subjected to flow cytometry instruments (FACS Calibur or FACS LSRII; Becton Dickinson, San Jose, CA, USA) and analyzed using Flow Jo software (Treestar, Ashland, OR, USA).
Sphere formation assays
1 × 104 of adherent SCLC cells (SBC3, H446, and H69AR) were initially seeded onto the ultra‐low attachment 96‐well plate (Corning, Lowell, MA, USA) and cultured for 8 days to examine lung sphere (LS) formation under treatment with TOPK inhibitor OTS514. Subsequently, through gentle pipetting, the detached SCLC cells were transferred into another ultra‐low attachment 96‐well plate for additional 7‐day culture. Then LS formation was examined by an inverted microscope Axio Vert.A1 TL (Carl Zeiss Microscopy, Thornwood, NY, USA).
Statistical analyses
Data were expressed as mean ± one standard deviation. Differences between two groups were examined for the statistical significance by the student's t‐test, using GraphPad Prism 6 software (GraphPad, San Diego, CA, USA). Differences at P < 0.05 were considered to be significant.
Results
High levels of TOPK expression in SCLC cell lines and primary SCLC tissues
To examine TOPK expression levels in SCLC, we performed immunoblot analyses using 11 human SCLC cell lines (six adherent cells and five suspension cells) and two normal fetal lung fibroblast (NFLF) cell lines. Our results showed that TOPK protein was highly expressed in the majority of the SCLC cell lines examined, whereas it was hardly detectable in two NFLF normal cell lines (Fig. 1a,b). In addition, the Oncomine database22 revealed that TOPK expression in primary SCLC tissues was significantly higher than in normal lung tissues (P = 0.0008) (Fig. 1c).
Figure 1.
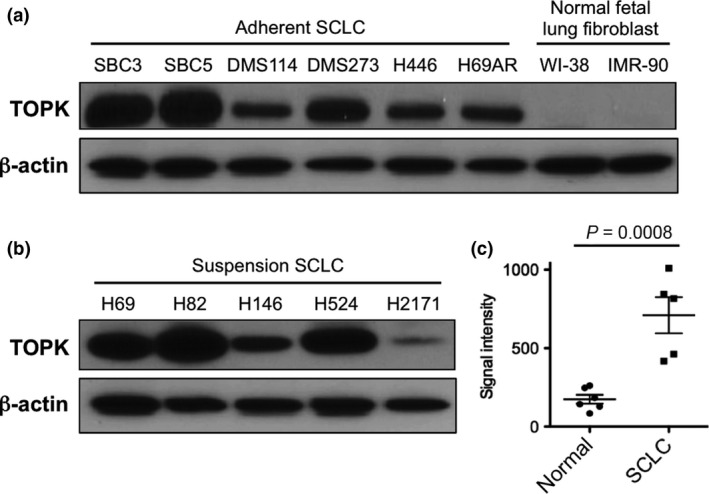
T‐lymphokine‐activated killer cell‐originated protein kinase (TOPK) is highly expressed in small cell lung cancer (SCLC) cell lines and primary SCLC tumors. Endogenous TOPK protein expression levels were examined by western blot analysis of six adherent SCLC cell lines, two NFLF (normal fetal lung fibroblast) cells (a), and five suspension SCLC cell lines (b). (c) The expression of TOPK mRNA is significantly upregulated in primary SCLC tumors (n = 5) compared with that of normal lung tissues (n = 6). Horizontal lines represent the means ± standard deviations.
Decrease of cell viability by siRNA‐mediated knockdown of TOPK
To examine the growth promoting effect of TOPK in SCLC cells, we performed a loss of function approach with specific siRNA (si‐TOPK) using six adherent SCLC cell lines. Our results from quantitative RT‐PCR showed that knockdown of TOPK by si‐TOPK significantly decreased the TOPK expression in all of six adherent SCLC cell lines, compared with si‐control (**P < 0.01, ***P < 0.001) (Fig. 2a). Immunoblot analyses confirmed reduction of TOPK protein levels in the SCLC cell lines tested at 48 h after si‐TOPK transfection (Fig. 2b). MTT assay concordantly revealed significant decrease of the cell viability in the SCLC cell lines transfected with si‐TOPK, compared with those transfected with si‐control (*P < 0.05, **P < 0.01 or ***P < 0.001) (Fig. 2c). These results suggested that TOPK is likely to play critical roles in the proliferation and/or survival of SCLC cells.
Figure 2.
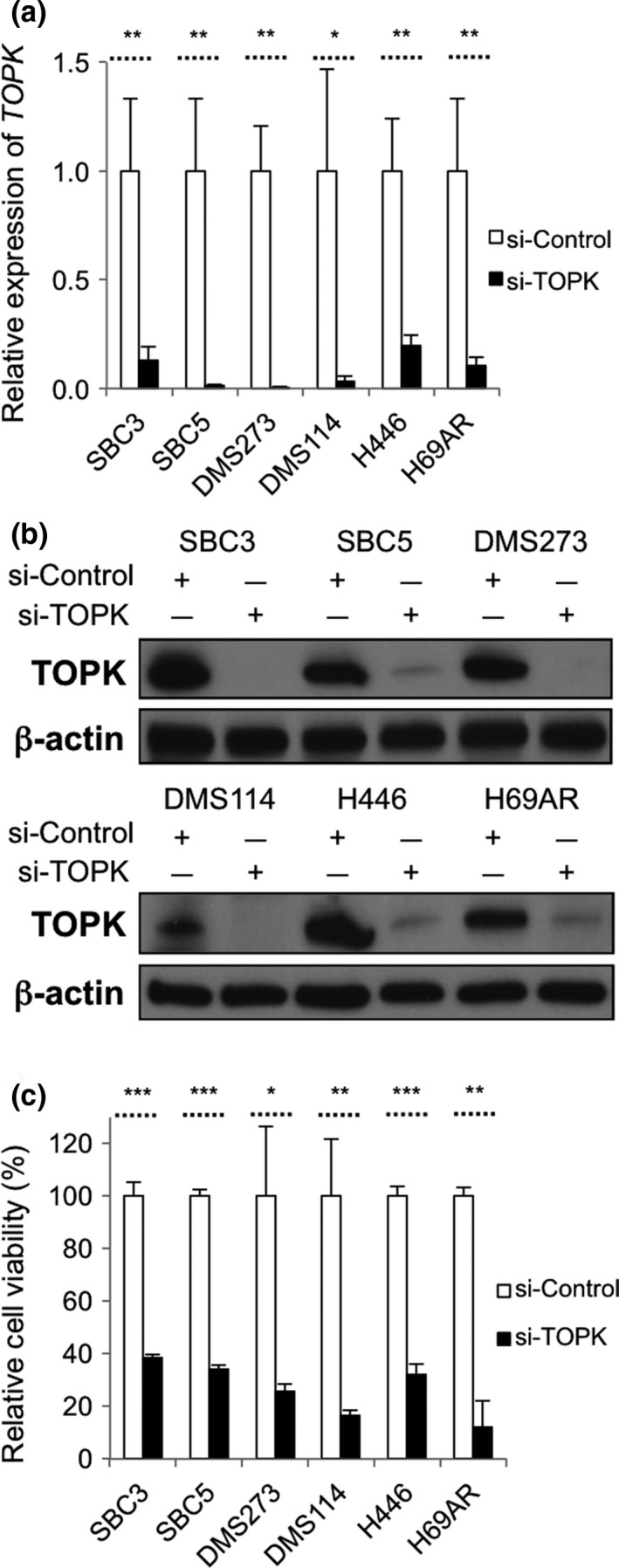
siRNA‐mediated TOPK knockdown results in decrease of cell viability in SCLC cells. Real‐time RT‐PCR (a) and western blot (b) analyses were conducted to examine expression levels of TOPK and TOPK protein levels in six adherent SCLC cells at 48 h after transfection with control siRNA or TOPK siRNA (*P < 0.05; **P < 0.01). (c) MTT assays were performed 6 days after transfection with siRNAs (*P < 0.05; **P < 0.01; ***P < 0.001).
Growth‐suppressive activity of TOPK inhibitor in SCLC cell lines
We then assessed growth‐suppressive effects of a potent TOPK inhibitor, OTS514.14 Since TOPK is known to be auto‐phosphorylated,1 we first examined effect of OTS514 on TOPK protein itself in the SCLC cells. We treated four SCLC cell lines with 10 or 20 nM of OTS514 for 48 h, and found that OTS514 treatment reduced the TOPK protein level in a dose‐dependent manner (Fig. 3a), indicating that the autophosphorylation might be required to maintain the stability of TOPK protein. Similar to the TOPK knockdown effect, OTS514 treatment exhibited strong growth‐suppressive effects on all of the six adherent SCLC cell lines with the half‐maximum inhibitory concentration (IC50) of 1.3–8.4 nM (Fig. 3b). We also examined five suspension SCLC cell lines and found that OTS514 treatment revealed stronger growth‐suppressive effects on three cell lines (H69, H82, and H524 with IC50 of 0.4–7.2 nM) that have very high levels of TOPK expression, but revealed relatively weaker growth‐suppressive effects on two cell lines (H146 and H2171 with IC50 of 39.3 nM and 42.6 nM, respectively), which showed relatively lower TOPK expression levels (Fig. 3c). It is notable that H446 cells bearing high CSC properties,23 and H69AR cells which are resistant to multi‐cytotoxic agents,24 were also very sensitive to this compound with IC50 values of 8.4 nM and 7.3 nM, respectively. Microscopic observation apparently exhibited cytotoxic effects of OTS514 in adherent SCLC cells in a dose‐dependent manner, while two NFLF cells remained undamaged with the treatment at the same concentration (Fig. 3d).
Figure 3.
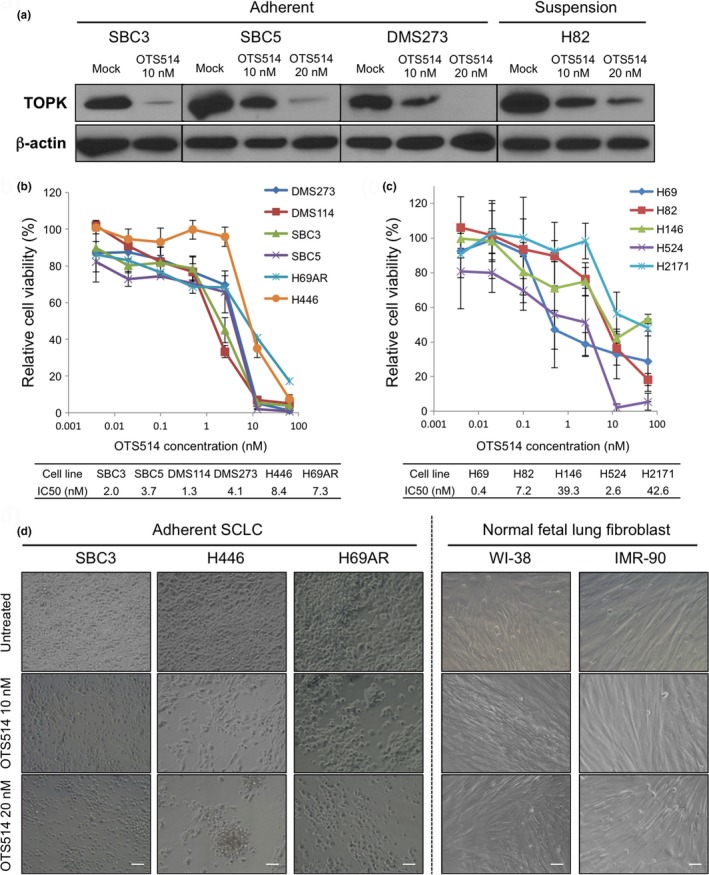
Treatment with TOPK inhibitor shows marked growth‐suppressive activity against SCLC cells. (a) Western blot analyses were performed to measure TOPK protein levels in adherent and suspension SCLC cells, 48 h after treatment with OTS514 (0, 10, and 20 nM). MTT assay was performed in six adherent SCLC cells (b) or in five suspension SCLC cells (c). All cells were measured their viability after treatment with OTS514 at different concentrations (0–50 nM) for 72 h. Graphs indicate relative cell viability at each OTS514 concentration, compared to a negative control (untreated). (d) The cellular effects induced by OTS514 treatment (10 nM or 20 nM) in three adherent SCLC cells and two NFLF cells were evaluated at 72 h after treatment by microscopic observation (×200 magnification). Scale bar indicates 50 μm.
Treatment of TOPK inhibitor induces apoptosis in SCLC cells
To address the molecular mechanism of cytotoxic effects by OTS514, we evaluated its effects on apoptosis during the treatment. Flow cytometry analyses after 48 h of OTS514 treatment revealed increased proportion of apoptotic cells in a dose‐dependent manner (Fig. 4a). We then explored the use of an antibody against an active (cleaved) form of caspase‐3 for the detection of the execution phase of apoptotic events. As shown in Fig. 4b, OTS514 treatment induced activation of caspase‐3 in a dose‐dependent manner in SCLC cells.
Figure 4.
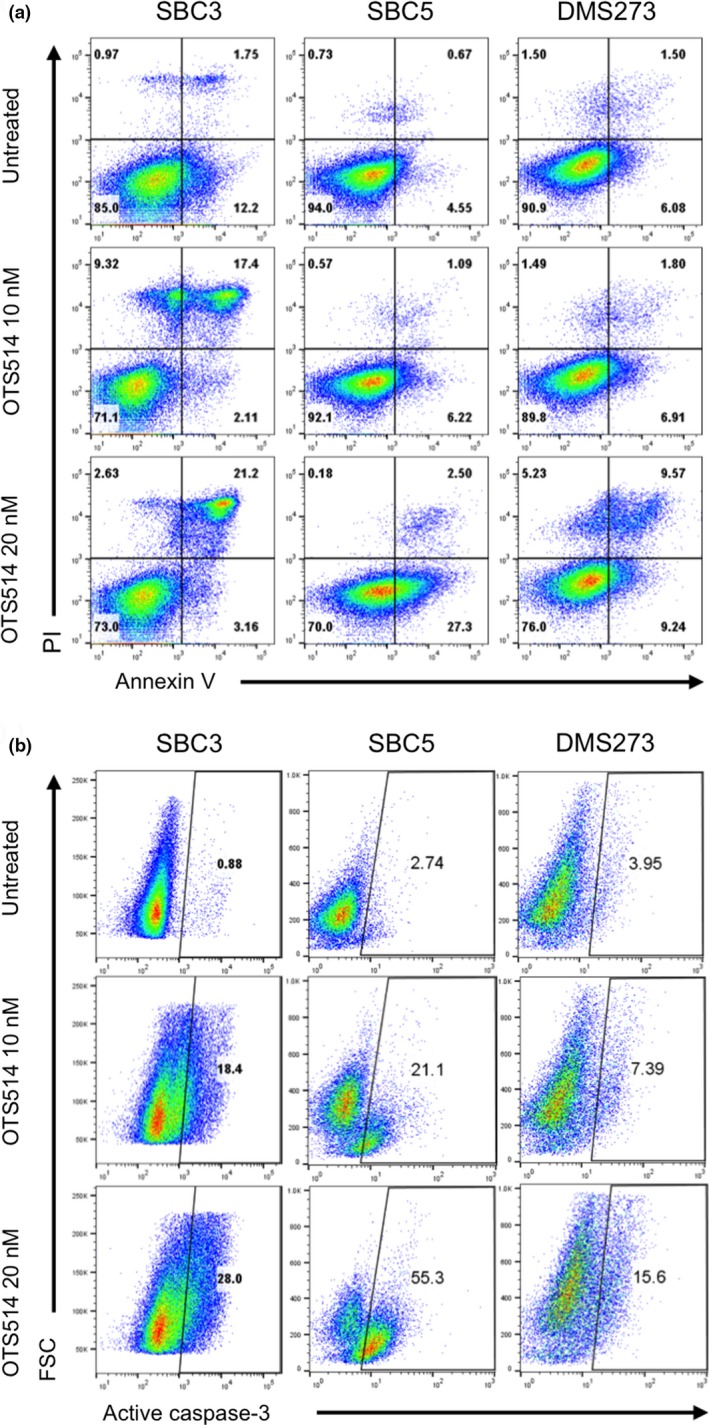
Treatment with TOPK inhibitor causes apoptosis in SCLC cells. (a) Three adherent SCLC cell lines were treated with 10 or 20 nM of OTS514. At 48 h of the treatment, Annexin‐V and PI staining assay was performed to detect apoptosis. The numbers represent the percentage of cells in each quarter. (b) At 72 h of treatment with 10 or 20 nM of OTS514, flow cytometry analysis was conducted to comparatively quantify levels of cleaved caspase‐3 activity in SCLC cells. The numbers depict the percentage of cells in each gate.
Cell morphological changes by TOPK inhibition
We previously reported that knockdown of TOPK resulted in significant suppression of cancer cell growth with a unique cell phenotype of elongated intercellular bridges, probably due to failure in the process of cytokinesis.3 We therefore examined morphological changes induced by TOPK knockdown in two adherent SCLC cells and found that TOPK‐depleted cells showed elongated intercellular bridges (white arrows) as well as dendrite‐like neuronal protrusions (yellow arrows), which were not observed in control cells (Fig. 5a). Since SCLC is thought to derive from self‐renewing pulmonary neuroendocrine progenitor cells,19, 20 we hypothesized that TOPK inhibition might promote neuronal differentiation in a subpopulation of SCLC cells that retained undifferentiated stem cell phenotype. To address this question, we investigated expression of a neural differentiation maker, NCAM (neural cell adhesion molecule, also called CD56) in SCLC cells treated with OTS514 for 48 h. Flow cytometry analyses showed increased subpopulation of CD56‐postivie SCLC cells (Fig. 5b).
Figure 5.
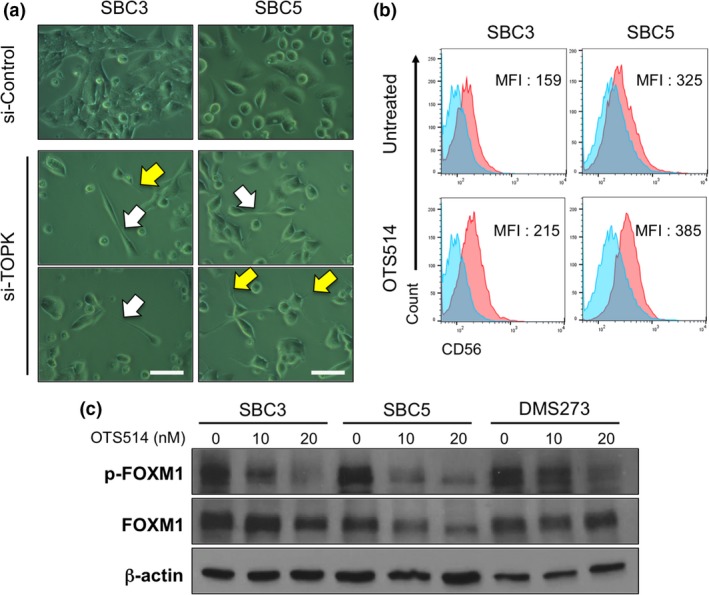
TOPK inhibition induces cytokinetic defects or differentiation into neuron‐like cells. (a) Two adherent SCLC cells were transfected with si‐Control or si‐TOPK, and microscopic observation was conducted 48 h later (×400 magnification). A scale bar indicates 50 μm. Yellow arrows and white arrows depict neuronal protrusions and intercellular bridge formation, respectively. (b) Two adherent SCLC cells were treated with 10 nM of OTS514 and flow cytometry analysis was performed to detect CD56 protein expression levels after 48‐h treatment. Numbers in histogram indicate the mean fluorescence intensity (MFI) corresponding to surface CD56 expression in SCLC cells. (c) Western blot analyses were performed to measure protein levels of total FOXM1 and phosphorylated FOXM1 in adherent SCLC cells untreated or treated with OTS514 for 48 h.
TOPK inhibitor downregulates FOXM1 activity
To further understand the mechanism of action of OTS514, we examined possible TOPK‐signaling pathways in SCLC cells. Since forkhead box protein M1 (FOXM1) was reported to function as an oncogenic transcriptional factor25, 26 and a master regulator of mitosis and stemness in CSC,27, 28, 29, 30 we investigated FOXM1 activity at protein level in the OTS514‐treated SCLC cells. We found that an active form of FOXM1, phosphorylated FOXM1 protein, was reduced (although the amounts of total FOXM1 protein were different in different cell lines) in adherent SCLC cells treated with OTS514 (Fig. 5c). Accordingly, OTS514 treatment reduced protein level of MELK, which is a downstream of FOXM1 and involved in the cancer stemness,7 as shown in Fig. S1a. It was also interesting that OTS514 treatment downregulated FOXM1 transcriptional level in two out of three SCLC cell lines (Fig. S1b), likely as we previously observed in kidney cancer cells after TOPK knockdown.7 Collectively, these results suggested that OTS514 treatment suppressed FOXM1 and MELK activity that play important roles in the proliferation/stemness of CSC.
TOPK inhibitor preferentially suppresses the lung sphere formation
To further evaluate the therapeutic potential of OTS514 on CSC subpopulation, we examined the protein expression level of CD90, one of the putative SCLC CSC markers,31, 32 in OTS514‐treated and ‐untreated SCLC cells. Flow cytometry analysis showed that OTS514 treatment clearly decreased proportion of CD90‐positive cells (Fig. 6a) as well as the intensity of CD90 (Fig. 6b) in all SCLC cells examined. We also conducted lung sphere (LS) formation assay because adherent SCLC cells can grow as spheres that are enriched with CSC subpopulation harboring higher in vitro clonogenic and in vivo tumorigenic potentials.33 The LS formation was developed through serial passage of cancer cells under low attachment culture condition as described previously.21 After microscopic confirmation of LS development after 15 days of culture, we mechanistically dissociated LS into single cell suspension and treated these LS‐derived SCLC cells with or without OTS514. Subsequently, we compared the sensitivity to OTS514 treatment between the LS‐derived SCLC cells and parental adherent SCLC cells by MTT assay, and found that OTS514 treatment more significantly suppressed the cell viability of LS‐derived SCLC cells than that of parental adherent SCLC cells in a dose‐dependent manner (Fig. 6c), indicating a possibility that OTS514 treatment might more effectively suppress the CSC subpopulation of SCLC cells.
Figure 6.
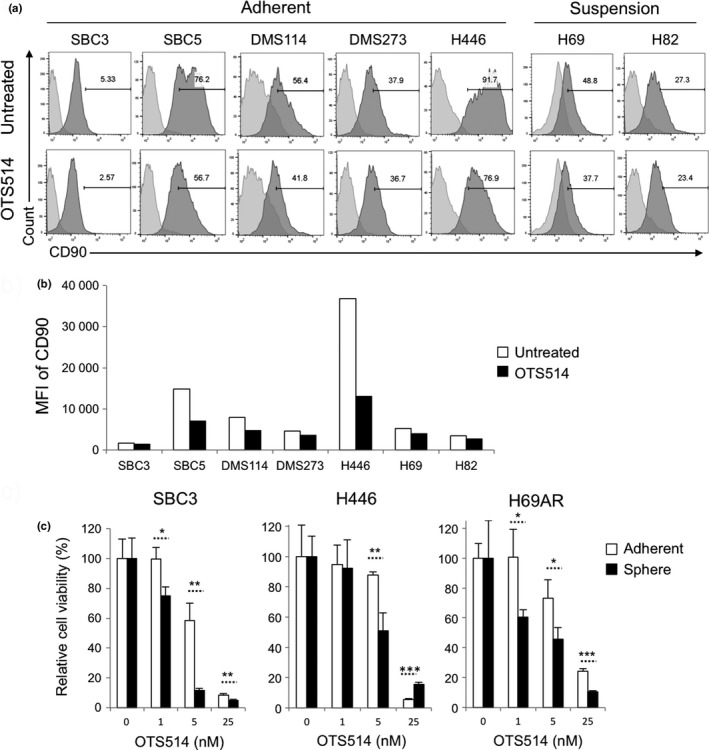
Treatment with TOPK inhibitor preferentially suppresses CSCs of SCLC. (a, b) Five adherent and two suspension SCLC cells were treated with 10 nM of OTS514 and flow cytometry analysis was performed to detect CD90 protein expression levels after 48‐h treatment. Numbers in histogram indicate the frequency (%) (a) or mean fluorescence intensity (MFI) (b) corresponding to surface CD90 expression in SCLC cells. (c) Three adherent SCLC cells were cultured in the ultra‐low attachment plate for LS formation. Then, these LS‐derived SCLC cells and corresponding parental cells were cultured with or without OTS514 (1 nM, 5 nM or 25 nM) in normal culture plates for 48 h followed by MTT assays. Graphs indicate relative cell viability at each OTS514 concentration, compared to untreated control cells (*P < 0.05, **P < 0.01, ***P < 0.001).
Discussion
T‐LAK cell‐originated protein kinase has been shown to be highly upregulated in various types of human cancer and its overexpression is correlated with poor prognosis of cancer patients,34, 35 but its biological or clinicopathological roles in SCLC, an aggressive phenotype of tumor enriched with CSC subpopulation, have not been addressed. Since it was suggested as one of CSC consensus markers,11 TOPK may play pivotal roles in development/progression of SCLC. Indeed, this study demonstrated that TOPK was highly expressed in the great majority of SCLC cell lines as well as primary SCLC tumors, compared with normal control cells or normal lung tissues.
We attempted to address the biological role of TOPK in growth of SCLC cells by two loss‐of‐function approaches, depletion of TOPK expression by siRNA and inhibition of TOPK kinase activity by a small molecular compound OTS514. Both approaches led to strong growth‐suppressive effects on the majority of SCLC cell lines with rapid induction of apoptosis, clearly indicating that TOPK is indispensable for the proliferation and/or survival of SCLC cells. Interestingly, OTS514 treatment could induce apoptosis even in the CSC‐like H446 cells, suggesting that OTS514 is a promising treatment modality for refractory SCLC where CSCs are enriched and anti‐apoptotic proteins are overexpressed.36 Except two SCLC cell lines (H146 and H2171) having low levels of TOPK expression, nine SCLC cell lines with high TOPK expression were very sensitive to OTS514 treatment with IC50 values ranging from 0.4–8.4 nM. It was also important that high concentration (20 nM) of OTS514 treatment did not exhibited cytotoxicity against two normal (NFLF) cells where TOPK expression was hardly detectable. These findings supported high efficacy and selectivity of TOPK inhibitor for cancer treatment, as we reported in xenograft models of human cancer.14 In addition, TOPK inhibition induced morphological changes in SCLC cells, such as intercellular bridges and neuronal protrusions. Considering TOPK's roles in cancer cell mitosis and stemness, it was likely that most proliferating cancer cells might undergo cytokinetic failure that resulted in cell death, meanwhile a subpopulation of CSC was provoked to differentiate into neuron‐like cell morphology. Thus, TOPK inhibitor might also have the function as a differentiation inducer, which aims to force some subpopulation of SCLC cells to resume the neuronal differentiation from progenitor phenotype. Related with a possible TOPK pathway, our analyses indicated that OTS514 treatment decreased the activity of FOXM1, which was accompanied by reduction of MELK protein. MELK is a downstream of FOXM1 and also plays important roles in SCLC CSCs.21 It is likely that autophosphorylation of TOPK is critical for maintenance of TOPK protein stability, thus TOPK inhibition by OTS514 treatment is a very effective way to suppress TOPK's downstream molecules, FOXM1 and MELK. Therefore, targeting these correlative molecules may provide a new modality to attack the vulnerabilities of CSC, as we previously showed preferential effects of MELK inhibitor on SCLC CSCs.21
There is a possibility of complex cross‐talk mechanism between TOPK and FOXM1. For example, our results showed that OTS514 treatment could downregulate transcriptional level of the FOXM1 gene in SBC5 and DMS273 cells. Indeed, our recent work using kidney cancer cell lines showed significant reduction of FOXM1 transcriptional level after knockdown of TOPK.7 It was still unclear why total FOXM1 protein level was drastically reduced in the OTS514‐treated SBC5 cells, but considering higher FOXM1 protein level and more CD90+ cell population in SBC5 cells than those in SBC3 and DMS273 cells, the TOPK‐mediated upregulation of FOXM1 might be critical in the SBC5 cells which harbored more CSC‐like subpopulation.
Finally, we investigated therapeutic potential of OTS514 on CSC and found that OTS514 treatment was effective to eliminate CD90+ CSC subpopulation in all seven SCLC cell lines examined. We also used a LS formation approach and demonstrated stronger growth‐suppressive effects of OTS514 on the CSC‐like SCLC cells. Our results suggested that OTS514 could target CSC and thus might be applied to a subset of human cancers where CSCs play dominant roles, such as glioblastoma, neuroblastoma, and malignant peritoneal mesothelioma.
In summary, we have clarified that TOPK plays pivotal roles in cancer progression and/or stem cell maintenance in SCLC cells. Our findings collectively suggest that the TOPK inhibitor OTS514 may be a promising molecular‐targeted therapy to treat patients with SCLC.
Disclosure Statement
Y.N. is a stock holder and a scientific advisor of OncoTherapy Science, Inc. J.P. is a scientific advisor of OncoTherapy Science, Inc. T.M. and Y.M. are employees of OncoTherapy Science, Inc. The other authors declare no competing interests.
Supporting information
Fig. S1. Effects of OTS514 treatment in SCLC cells. (a) Western blot analyses were performed to measure protein levels of phosphorylated FOXM1, total FOXM1, and MELK in 3 adherent SCLC cells untreated or treated with 10 nM of OTS514 for 48 h. (b) Real‐time RT‐PCR showed relative expression levels of FOXM1 in three adherent SCLC cells untreated or treated with 10 nM of OTS514 for 48 h.
Acknowledgments
This work was supported in part by a Team Science Award of UCCCC (The University of Chicago Medicine Comprehensive Cancer Center), that from Cancer Research Foundation, and by a grant from OncoTherapy Science Inc. We appreciate Mr. Masao Mizuno for his gift to support this work.
Cancer Sci 108 (2017) 488–496
Funding Information
The University of Chicago Medicine Comprehensive Cancer Center, OncoTherapy Science Inc., Cancer Research Foundation
References
- 1. Park JH, Lin ML, Nishidate T, Nakamura Y, Katagiri T. PDZ‐binding kinase/T‐LAK cell‐originated protein kinase, a putative cancer/testis antigen with an oncogenic activity in breast cancer. Cancer Res 2006; 66: 9186–95. [DOI] [PubMed] [Google Scholar]
- 2. Komatsu M, Yoshimaru T, Matsuo T et al Molecular features of triple negative breast cancer cells by genome‐wide gene expression profiling analysis. Int J Oncol 2013; 42: 478–506. [DOI] [PubMed] [Google Scholar]
- 3. Park JH, Nishidate T, Nakamura Y, Katagiri T. Critical roles of T‐LAK cell‐originated protein kinase in cytokinesis. Cancer Sci 2010; 101: 403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu F, Zykova TA, Kang BS et al Bidirectional signals transduced by TOPK‐ERK interaction increase tumorigenesis of HCT116 colorectal cancer cells. Gastroenterology 2007; 133: 219–31. [DOI] [PubMed] [Google Scholar]
- 5. Herrero‐Martin D, Osuna D, Ordonez JL et al Stable interference of EWS‐FLI1 in an Ewing sarcoma cell line impairs IGF‐1/IGF‐1R signalling and reveals TOPK as a new target. Br J Cancer 2009; 101: 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alachkar H, Mutonga M, Malnassy G et al T‐LAK cell‐originated protein kinase presents a novel therapeutic target in FLT3‐ITD mutated acute myeloid leukemia. Oncotarget 2015; 6: 33410–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kato T, Inoue H, Imoto S et al Oncogenic roles of TOPK and MELK, and effective growth suppression by small molecular inhibitors in kidney cancer cells. Oncotarget 2016; 7: 17652–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fujibuchi T, Abe Y, Takeuchi T et al Expression and phosphorylation of TOPK during spermatogenesis. Dev Growth Differ 2005; 47: 637–44. [DOI] [PubMed] [Google Scholar]
- 9. Ayllon V, O'Connor R. PBK/TOPK promotes tumour cell proliferation through p38 MAPK activity and regulation of the DNA damage response. Oncogene 2007; 26: 3451–61. [DOI] [PubMed] [Google Scholar]
- 10. Abe Y, Takeuchi T, Kagawa‐Miki L et al A mitotic kinase TOPK enhances Cdk1/cyclin B1‐dependent phosphorylation of PRC1 and promotes cytokinesis. J Mol Biol 2007; 370: 231–45. [DOI] [PubMed] [Google Scholar]
- 11. Shats I, Gatza ML, Chang JT et al Using a stem cell‐based signature to guide therapeutic selection in cancer. Cancer Res 2011; 71: 1772–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takebe N, Miele L, Harris PJ et al Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol 2015; 12: 445–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer 2005; 5: 275–84. [DOI] [PubMed] [Google Scholar]
- 14. Matsuo Y, Park JH, Miyamoto T et al TOPK inhibitor induces complete tumor regression in xenograft models of human cancer through inhibition of cytokinesis. Sci Transl Med 2014; 6: 259ra145. [DOI] [PubMed] [Google Scholar]
- 15. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62: 10–29. [DOI] [PubMed] [Google Scholar]
- 16. Sher T, Dy GK, Adjei AA. Small cell lung cancer. Mayo Clin Proc 2008; 83: 355–67. [DOI] [PubMed] [Google Scholar]
- 17. Oze I, Hotta K, Kiura K et al Twenty‐seven years of phase III trials for patients with extensive disease small‐cell lung cancer: disappointing results. PLoS ONE 2009; 4: e7835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Codony‐Servat J, Verlicchi A, Rosell R. Cancer stem cells in small cell lung cancer. Transl Lung Cancer Res 2016; 5: 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park KS, Liang MC, Raiser DM et al Characterization of the cell of origin for small cell lung cancer. Cell Cycle 2011; 10: 2806–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sutherland KD, Proost N, Brouns I, Adriaensen D, Song JY, Berns A. Cell of origin of small cell lung cancer: inactivation of Trp53 and Rb1 in distinct cell types of adult mouse lung. Cancer Cell 2011; 19: 754–64. [DOI] [PubMed] [Google Scholar]
- 21. Inoue H, Kato T, Olugbile S et al Effective growth‐suppressive activity of maternal embryonic leucine‐zipper kinase (MELK) inhibitor against small cell lung cancer. Oncotarget 2016; 7: 13621–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garber ME, Troyanskaya OG, Schluens K et al Diversity of gene expression in adenocarcinoma of the lung. Proc Natl Acad Sci U S A 2001; 98: 13784–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang Z, Zhou Y, Qian H et al Stemness and inducing differentiation of small cell lung cancer NCI‐H446 cells. Cell Death Dis 2013; 4: e633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mirski SE, Gerlach JH, Cole SP. Multidrug resistance in a human small cell lung cancer cell line selected in adriamycin. Cancer Res 1987; 47: 2594–8. [PubMed] [Google Scholar]
- 25. Alachkar H, Mutonga MB, Metzeler KH et al Preclinical efficacy of maternal embryonic leucine‐zipper kinase (MELK) inhibition in acute myeloid leukemia. Oncotarget 2014; 5: 12371–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Halasi M, Gartel AL. Targeting FOXM1 in cancer. Biochem Pharmacol 2013; 85: 644–52. [DOI] [PubMed] [Google Scholar]
- 27. Joshi K, Banasavadi‐Siddegowda Y, Mo X et al MELK‐dependent FOXM1 phosphorylation is essential for proliferation of glioma stem cells. Stem Cells 2013; 31: 1051–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee Y, Kim KH, Kim DG et al FoxM1 Promotes Stemness and Radio‐Resistance of Glioblastoma by Regulating the Master Stem Cell Regulator Sox2. PLoS ONE 2015; 10: e0137703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Song IS, Jeong YJ, Jeong SH et al FOXM1‐induced PRX3 regulates stemness and survival of colon cancer Cells via maintenance of mitochondrial function. Gastroenterology 2015; 149: 1006–16. [DOI] [PubMed] [Google Scholar]
- 30. Bao B, Wang Z, Ali S et al Over‐expression of FoxM1 leads to epithelial‐mesenchymal transition and cancer stem cell phenotype in pancreatic cancer cells. J Cell Biochem 2011; 112: 2296–306. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31. Salcido CD, Larochelle A, Taylor BJ, Dunbar CE, Varticovski L. Molecular characterisation of side population cells with cancer stem cell‐like characteristics in small‐cell lung cancer. Br J Cancer 2010; 102: 1636–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang P, Gao Q, Suo Z et al Identification and characterization of cells with cancer stem cell properties in human primary lung cancer cell lines. PLoS ONE 2013; 8: e57020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33. Qiu X, Wang Z, Li Y, Miao Y, Ren Y, Luan Y. Characterization of sphere‐forming cells with stem‐like properties from the small cell lung cancer cell line H446. Cancer Lett 2012; 323: 161–70. [DOI] [PubMed] [Google Scholar]
- 34. Zlobec I, Molinari F, Kovac M et al Prognostic and predictive value of TOPK stratified by KRAS and BRAF gene alterations in sporadic, hereditary and metastatic colorectal cancer patients. Br J Cancer 2010; 102: 151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wei DC, Yeh YC, Hung JJ et al Overexpression of T‐LAK cell‐originated protein kinase predicts poor prognosis in patients with stage I lung adenocarcinoma. Cancer Sci 2012; 103: 731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tagscherer KE, Fassl A, Campos B et al Apoptosis‐based treatment of glioblastomas with ABT‐737, a novel small molecule inhibitor of Bcl‐2 family proteins. Oncogene 2008; 27: 6646–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Effects of OTS514 treatment in SCLC cells. (a) Western blot analyses were performed to measure protein levels of phosphorylated FOXM1, total FOXM1, and MELK in 3 adherent SCLC cells untreated or treated with 10 nM of OTS514 for 48 h. (b) Real‐time RT‐PCR showed relative expression levels of FOXM1 in three adherent SCLC cells untreated or treated with 10 nM of OTS514 for 48 h.


