Abstract
Proton beam therapy (PBT) is a potential new alternative to treatment with photon radiotherapy that may reduce the risk of late toxicity and secondary cancer, especially for pediatric tumors. The goal of this study was to evaluate the long‐term benefits of PBT in cancer survivors. A retrospective observational study of pediatric patients who received PBT was performed at four institutions in Japan. Of 343 patients, 62 were followed up for 5 or more years. These patients included 40 males and 22 females, and had a median age of 10 years (range: 0–19 years) at the time of treatment. The irradiation dose ranged from 10.8 to 81.2 GyE (median: 50.4 GyE). The median follow‐up period was 8.1 years (5.0–31.2 years). The 5‐, 10‐ and 20‐year rates for grade 2 or higher late toxicities were 18%, 35% and 45%, respectively, and those for grade 3 or higher late toxicities were 6%, 17% and 17% respectively. Univariate analysis showed that the irradiated site (head and neck, brain) was significantly associated with late toxicities. No malignant secondary tumors occurred within the irradiated field. The 10‐ and 20‐year cumulative rates for all secondary tumors, malignant secondary tumors, and malignant nonhematologic secondary tumors were 8% and 16%, 5% and 13%, and 3% and 11%, respectively. Our data indicate that PBT has the potential to reduce the risk of late mortality and secondary malignancy. Longer follow‐up is needed to confirm the benefits of PBT for pediatric tumors.
Keywords: Late toxicity, pediatrics, proton, radiotherapy, secondary cancer
Recent progress in treatment of pediatric tumors has improved survival and almost 70% of patients can now be cured.1 However, late toxicities of radiotherapy may occur in long‐term survivors, and reduction of quality of life due to growth and development retardation and secondary cancer is a significant problem for pediatric patients.2, 3 Armstrong et al.4 showed that mortality due to the original malignancy begins to plateau beyond 20 years, while death from non‐recurrence causes increases. Between 15 and 30 years, the cumulative mortality attributable to the primary disease only increases from 6.3% to 7.8%, while that due to non‐recurrence causes increases from 2.0% to 7.0%. Schoot et al.5 found that 77% of long term survivors (median follow‐up 10.5 years, n = 31) after radiotherapy for pediatric head and neck tumor experience late toxicities of grade 3 or 4; Ducassou et al.6 showed that 23% of survivors for 5 or more years (n = 22) after radiotherapy for neuroblastoma experienced late toxicities of grade 3 or more; and Perwein et al.7 found that 50% of long‐term survivors (median follow‐up 7.4 years, n = 16) after radiotherapy for neuroblastoma experienced late toxicity of grade 3 or more.
Proton beam therapy (PBT) is likely to have reduced risks of late toxicity and secondary cancer, and Sethi et al.8 found a 10‐year cumulative incidence of 0% for all in‐field secondary malignancies after PBT. However, long‐term follow‐up is difficult because of tumor recurrence or cases lost in follow‐up, and this results in limited information on late toxicity after radiotherapy. This is particularly true for PBT because of the relatively short history of the technique. Here, we evaluated late toxicity in long‐term follow‐up after PBT in a multicenter study in Japan.
All data were published in a previous report.9 The current manuscript examines late toxicity in long‐term survivors.
Materials and Methods
A retrospective observational study in pediatric patients who received PBT was performed at four institutions in Japan. Institutional Review Boards approved the study at all institutes. All 343 patients aged <20 years old at the time of receiving PBT at these sites from January 1983 to August 2014 were initially enrolled. Previously, we report the overview of this retrospective observational study.9 In the report we mainly analyzed overall survival and late toxicities of all patients. The study showed that main purpose of PBT was to reduce the risk of late toxicity and secondary cancer. We consider that long‐term follow‐up is an essential condition to evaluate the risk of late toxicity and secondary cancer. However, the study included a number of short follow‐up patients. So here we perform the secondary analysis focused on long‐term survivors.
Of these patients, 62 were followed‐up for 5 or more years. There were 40 males and 22 females, and the median age at treatment was 10 years old (range: 0–19). The irradiated site were head and neck (n = 24), brain (n = 22), body trunk (n = 9) and others (n = 7). The irradiation dose ranged from 10.8 to 81.2 GyE (median: 50.4 GyE). The median follow‐up period in the 62 patients was 8.1 years (range: 5.0–31.2 years).
Statistical analysis
Rates for late toxicities and secondary tumors were calculated using the Kaplan–Meier method. A log‐rank test was used to examine differences for age, gender, irradiated site, irradiation dose, and irradiation volume. All analyses were performed with SPSS v. 11.0 (SPSS Inc., Chicago, IL, USA). Toxicities were graded using the Common Terminology Criteria for Adverse Events ver. 3.0.
Results
At the final follow‐up, 22 of 62 patients had late toxicities of grade 2 or more, with a median time from the first day of PBT to the day of diagnosis of the late toxicity of 4.5 years (range: 0.8–28.2 years). Four of the 22 patients had multiple late toxicities of grade 2 or more, and 6 had deformities of grade 2 or more, with all deformities occurring in patients irradiated in the head and neck or brain. The 5‐, 10‐ and 20‐year rates of late toxicities of grade 2 or more were 18% (95% CI 8–27%), 35% (95% CI 22–49%) and 45% (95% CI 24–65%) respectively, and those for late toxicities of grade 3 or more were 6% (95% CI 0–13%), 17% (95% CI 5–28%) and 17% (95% CI 5–28%), respectively (Fig. 1).
Figure 1.
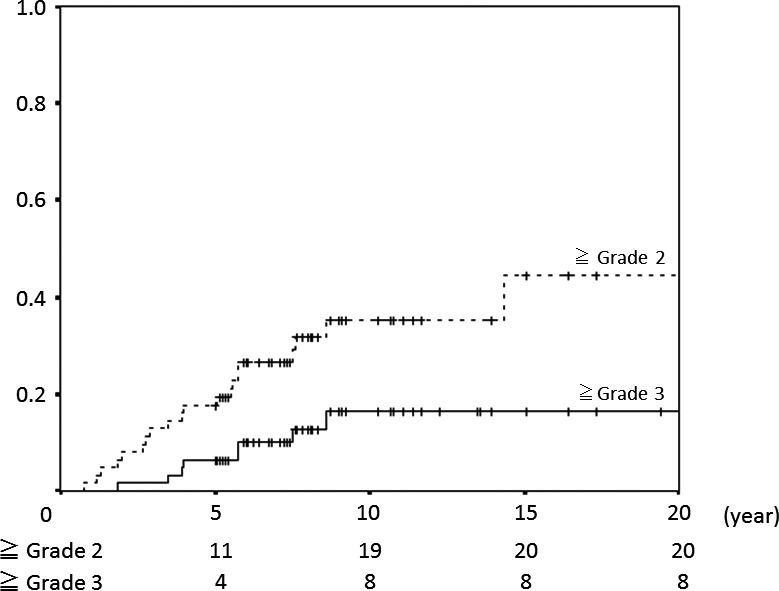
Incidence of late adverse events in all patients.
Univariate analysis showed that the irradiated site (head and neck, brain) was significantly associated with late toxicity (Fig. 2). The 5‐ and 10‐year late toxicity rates after head and neck or brain irradiation were 22% (95% CI 11–33%) and 42% (95% CI 27–57%) at grade 2 or more and 8% (95% CI 0–16%) and 20% (95% CI 7–32%) at grade 3 or more. For patients who did not have a head and neck or brain tumor, no late toxicity of grade 2 or more occurred within 20 years. Age (>10 or ≤10 years old), gender, irradiation dose (>40 or ≤40 GyE) and irradiation volumes (<100 or ≤100 mL) were not significantly associated with late toxicity. Multivariate analysis showed no significant difference in late toxicities for any factors. Six patients had late toxicity after the age of 20. And 11 of 30 late toxicities occurred after the age of 20. Figure 3 presents the relationship between onset age and Grade of late toxicity.
Figure 2.
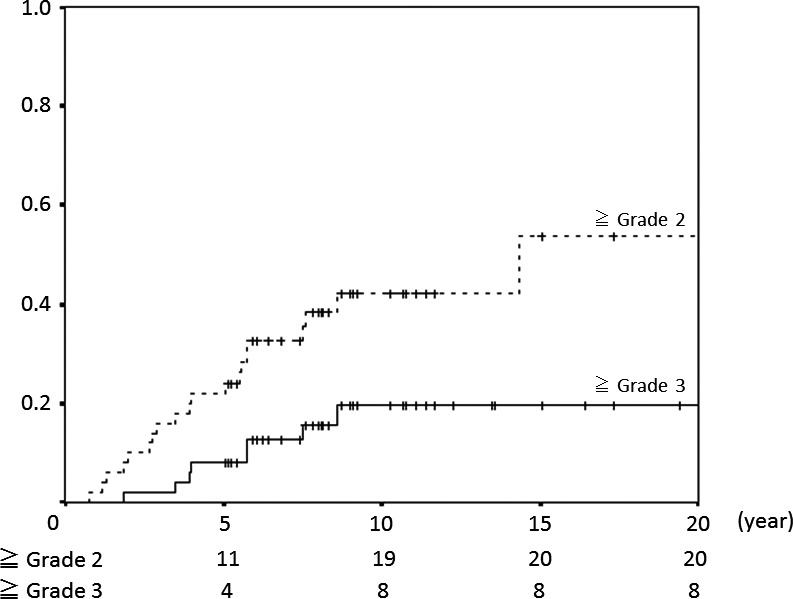
Incidence of late adverse events in cases of head and neck or brain tumor.
Figure 3.
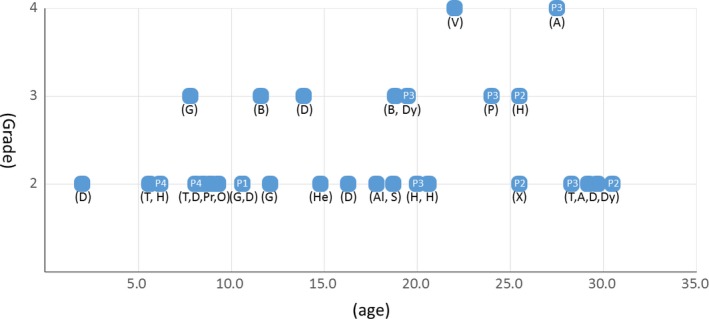
Figure between brackets indicates kind of late toxicity. A, Angiostenosis; Al, Alopecia; B, Brain injury; D, Deformity; Dy, Dysphagia; G, Growth hormone deficiency; H, Hearing impairment; He, Headache; O, Otitis media; P, Pneumonitis; Pr, Precocious puberty; S, Seizure; T, Thyroid dysfunction; V, Visual impairment; X, Xerostomia. Four patients (P1, P2, P3, P4) had multiple late toxicities.
As previously reported several patients had a secondary tumor.9 In this report, four of 62 patients had a secondary tumor after PBT, including three malignant tumors (osteosarcoma, thyroid cancer and acute myelocytic leukemia (AML)) and one case of pituitary adenoma. All malignant secondary tumors occurred outside the irradiated field, with only pituitary adenoma occurring within the irradiated field. The 10‐ and 20‐year cumulative incidences for all secondary tumors (osteosarcoma, thyroid cancer, AML and pituitary adenoma), malignant secondary tumors (osteosarcoma, thyroid cancer and AML) and malignant solid secondary tumors (osteosarcoma and thyroid cancer) were 8% (95% CI 0–18%) and 16% (95% CI 0–33%), 5% (95% CI 0–11%) and 13% (95% CI 0–29%), and 3% (95% CI 0–9%) and 11%(95% CI 0–27%), respectively. The incidences of all malignant secondary cancers and in‐field malignant secondary cancers are shown in Figure 4.
Figure 4.
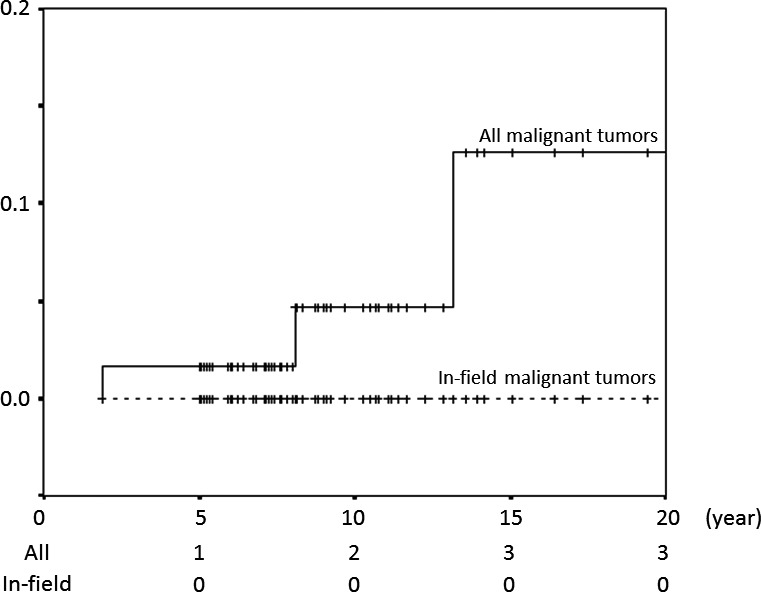
Incidences of all malignant secondary cancers and in‐field malignant secondary cancers.
Discussion
Comprehensive data from this study have been reported elsewhere, but this previous analysis included cases with a variety of tumors and a short follow‐up time.9 The advantages of PBT for pediatric patients are likely to be fewer late adverse events and a reduced risk secondary malignancy, since comparisons of dose distribution between PBT and photon radiotherapy indicate a decreased dose to normal tissue in PBT.10, 11 Late toxicity has been found in long‐term follow‐up of pediatric patients after photon radiotherapy. In an analysis of 17 patients who survived for at least 5 years (median 20 years) after radiotherapy for head and neck pediatric rhabdomyosarcoma, Paulino et al. found late effects of treatment in 11 cases with facial growth retardation, nine with neuroendocrine dysfunction, nine with visual/orbital problems, seven with dental abnormalities, and six with hearing loss.12 The severity was unclear, but these data show a high rate of late toxicity and similar late toxicities to those found in our patients (deformation, neuroendocrine dysfunction, and hearing impairment).
Our data showed 10‐year late toxicity rates after PBT for head and neck cancer of 42% at grade 2 or more and 20% at grade 3 or more, respectively. Based on a simple comparison with the above rates for photon radiotherapy, PBT appears to reduce the risk of late toxicity. In a preliminary study of PBT for pediatric rhabdomyosarcoma, Ladra et al. found rates of late toxicity of 28% for grade 2 and 7% for grade 3, but it should be noted that the cases included truncal site tumors and the follow‐up period was relatively short.13 In patients with parameningeal rhabdomyosarcoma, Childs et al.14 found that PBT in a relatively small group of 10 patients reduced long‐term toxicities compared with a historical control group treated with photon radiotherapy. At this time, more than a third of late toxicities occurred age 20 or older. This result indicates that a routine follow‐up is needed in adult life to evaluate the precise late effect of PBT.
There is also limited information on secondary malignancy after PBT.8 Some reports have shown that PBT can reduce the risk of secondary malignancy,15, 16 and recently Sethi et al. showed that the 10‐year cumulative incidences of secondary tumors in survivors of retinoblastoma were significantly lower in those treated with PBT compared to photon therapy for both in‐field (0% vs 14%) and all (5% vs 14%) secondary malignancies. In our analysis, the 10‐year cumulative rates of in‐field and all secondary malignancies were 0% and 5%, respectively, which are exactly the same as those in Sethi et al. Stringent comparison with photon radiotherapy is difficult because of variation in patient backgrounds, but these data suggest that PBT has a lower risk of radiotherapy‐induced malignancy.
The risk of subsequent malignancy continues to increase after 40 years old,17 and at age 55 the cumulative incidence of new malignancy reaches 16.3%. This shows that radiotherapy is a risk factor for late mortality and subsequent malignancy. Improved outcomes of pediatric tumors requires increased long‐term survival after radiotherapy, and our data indicate that PBT has the potential to reduce the risk of late mortality and subsequent malignancy. However, the follow‐up period was still short and the number of patients was insufficient for comparison with photon historical data, in particular. Longer follow‐up is needed to confirm these advantages of PBT for pediatric tumors.
Disclosure Statement
Dr. Hiroki Shirato received donations from Hitachi Ltd., Shimadzu Corp., and Jokoh. All other authors had no financial support or relationship to this manuscript.
Acknowledgments
Masashi Mizumoto wrote the first draft of the manuscript. This research was supported by a grant for Practical Research for Innovative Cancer Control (15ck0106186h0001) from the Japan Agency for Medical Research and Development (AMED); in part by Grants‐in‐Aid for Scientific Research (B) (15H04901) and Young Scientists (B) (25861064) from the Ministry of Education, Science, Sports and Culture of Japan; and in part by the Translational Research Network Program of the Ministry of Education, Science, Sports and Culture of Japan.
Cancer Sci 108 (2017) 444–447
Clinical Trial Registration: Not relevant
Funding Information
Japan Agency for Medical Research and Development (AMED), (Grant/Award Number: ‘15ck0106186 h0001‘) Ministry of Education, Science, Sports and Culture of Japan, (Grant/Award Number: ‘15H04901‘, ‘25861064‘).
References
- 1. Jemal A, Clegg LX, Ward E et al Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer 2004; 101(1): 3–27. [DOI] [PubMed] [Google Scholar]
- 2. Oeffinger KC, Mertens AC, Sklar CA et al Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 2006; 355: 1572–82. [DOI] [PubMed] [Google Scholar]
- 3. Armstrong FD. Proton‐beam radiation therapy and health‐related quality of life in children with CNS tumors. J Clin Oncol 2012; 30: 2028–9. [DOI] [PubMed] [Google Scholar]
- 4. Armstrong GT, Liu Q, Yasui Y et al Late mortality among 5‐year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. J Clin Oncol 2009; 27: 2328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schoot RA, Slater O, Ronckers CM et al Adverse events of local treatment in long‐term head and neck rhabdomyosarcoma survivors after external beam radiotherapy or AMORE treatment. Eur J Cancer 2015; 51: 1424–34. [DOI] [PubMed] [Google Scholar]
- 6. Ducassou A, Gambart M, Munzer C et al Long‐term side effects of radiotherapy for pediatric localized neuroblastoma: results from clinical trials NB90 and NB94. Strahlenther Onkol 2015; 191: 604–12. [DOI] [PubMed] [Google Scholar]
- 7. Perwein T, Lackner H, Sovinz P et al Survival and late effects in children with stage 4 neuroblastoma. Pediatr Blood Cancer 2011; 57: 629–35. [DOI] [PubMed] [Google Scholar]
- 8. Sethi RV, Shih HA, Yeap BY et al Second nonocular tumors among survivors of retinoblastoma treated with contemporary photon and proton radiotherapy. Cancer 2014; 120(1): 126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mizumoto M, Murayama S, Akimoto T et al Proton beam therapy for pediatric malignancies: a retrospective observational multicenter study in Japan. Cancer Med 2016; 5: 1519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cotter SE, Herrup DA, Friedmann A et al Proton radiotherapy for pediatric bladder/prostate rhabdomyosarcoma: clinical outcomes and dosimetry compared to intensity‐modulated radiation therapy. Int J Radiat Oncol Biol Phys 2011; 81: 1367–73. [DOI] [PubMed] [Google Scholar]
- 11. Kozak KR, Adams J, Krejcarek SJ et al A dosimetric comparison of proton and intensity‐modulated photon radiotherapy for pediatric parameningeal rhabdomyosarcomas. Int J Radiat Oncol Biol Phys 2009; 74(1): 179–86. [DOI] [PubMed] [Google Scholar]
- 12. Paulino AC, Simon JH, Zhen W et al Long‐term effects in children treated with radiotherapy for head and neck rhabdomyosarcoma. Int J Radiat Oncol Biol Phys 2000; 48: 1489–95. [DOI] [PubMed] [Google Scholar]
- 13. Ladra MM, Szymonifka JD, Mahajan A et al Preliminary results of a phase II trial of proton radiotherapy for pediatric rhabdomyosarcoma. J Clin Oncol 2014; 32: 3762–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Childs SK, Kozak KR, Friedmann AM et al Proton radiotherapy for parameningeal rhabdomyosarcoma: clinical outcomes and late effects. Int J Radiat Oncol Biol Phys 2012; 82: 635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Athar BS, Paganetti H. Comparison of second cancer risk due to out‐of‐field doses from 6‐MV IMRT and proton therapy based on 6 pediatric patient treatment plans. Radiother Oncol 2011; 98(1): 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fontenot JD, Lee AK, Newhauser WD. Risk of secondary malignant neoplasms from proton therapy and intensity‐modulated X‐ray therapy for early‐stage prostate cancer. Int J Radiat Oncol Biol Phys 2009; 74: 616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Turcotte LM, Whitton JA, Friedman DL et al Risk of subsequent neoplasms during the fifth and sixth decades of life in the childhood cancer survivor study cohort. J Clin Oncol 2015; 33: 3568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]


