Abstract
The lymph node (LN) is an important immune system in which a number of antigen‐presenting cells are present that induce rapid immune responses to foreign antigens. While a great number of macrophages exist in lymph nodes, recent studies using animal models have shown that lymph node sinus macrophages are associated with the induction of anti‐tumor immunity, playing a significant role in host immune responses against tumor cells. In colorectal tumor, malignant melanoma, and endometrial tumor, it was shown that a high density of CD169‐positive macrophages in the LN sinus was a predictive factor for better clinical prognosis. The observations that the density of CD169‐positive macrophages in the LN sinus was positively associated with the density of infiltrating T or NK cells in tumor tissues, indicates the significance of CD169‐positive macrophages in anti‐tumor immune reactions of tumor patients. Moreover, antigen delivery targeting LN macrophages is also considered to be promising approach for vaccination. In this article, we have summarized the significance of CD169‐positive LN macrophages in anti‐tumor immunity.
Keywords: Cancer immunology, CD169, lymph node, sinus macrophage, tumor‐associated macrophage
Distribution of Macrophages in Lymph Nodes
Spleen and lymph nodes (LNs) are respectively involved in the filtration of blood and lymph flow, and immune responses are induced by the activation of lymphocytes and natural killer (NK) cells, which is dependent on antigen presenting cells (APCs) including dendritic cells and macrophages. Lymph nodes are bean‐like nodules that are usually <1 cm in size, but they are spread throughout the entire body, and their total weight corresponds to that of a single organ. LNs are also located around the tumor nodule in many kinds of malignant tumors (Fig. 1a). Lymph nodes are composed of an outer cortex and a central medullary part with a space referred to as the LN sinus that is filled with lymphatic fluid in which a number of macrophages are distributed.1, 2 Of these LN regions, antigens in the lymphatic fluid first reach the LN sinus, where LN sinus macrophages phagocytize pathogens, foreign substances, and abnormal antigens derived from tumor cells that flow into the lymphatic fluid. These substances are also captured in DCs localized in follicular and paracortex area. The LN sinus is proactively associated with inducing antigen‐specific immune responses.
Figure 1.
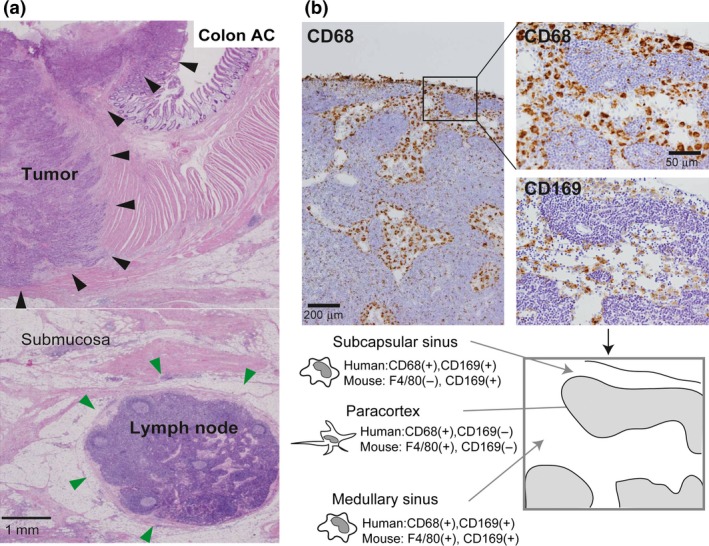
Histological distribution of macrophages in a lymph node (LN). (a) The hematoxylin and eosin section of human colon adenocarcinoma is presented. Large tumor nodules (indicated by black arrowhead) are located in mucosa and muscularis propria, and lymph node (indicated by green arrowhead) is seen in subserosa. (b) Serial sections of a human LN were immunostained for CD68 (pan‐macrophage marker) and CD169. Subcapsular sinus macrophages and medullary sinus macrophages are positive for CD68 and CD169 in humans (Top). Comparison of mouse LN macrophage F4/80 and CD169 staining with human LN CD68 and CD169 macrophage staining is shown at the bottom. CD169 is expressed on both subcapsular sinus macrophages and medullary sinus macrophages in the mouse; however, the staining pattern of F4/80 (pan‐macrophage marker) in mouse subcapsular sinus macrophages differs from that of CD68 staining of these macrophages in humans. Paracortex macrophages are negative for CD169 in both humans and mice.
Macrophages are professional phagocytes, and have long been considered to be involved in innate immune responses. It is well established that many macrophages are distributed in lympho‐reticular organs including spleen and LNs.3, 4 Macrophages are detected in the subcapsular sinus, the medullary sinus, and the medullary cord, and, according to animal studies, their functions and phenotypes are somewhat different in each location.5 LN sinus macrophages are known to express the surface antigen CD169. CD169 is a Type I lectin with a molecular weight of 185 kDa, which specifically recognizes sialic acid‐containing sugar chains.6 While CD169‐positive macrophages are mainly found in the LNs and spleen, CD169‐positive macrophages are also found in the intestinal tract, liver, and bone marrow, although only in small numbers.7 Macrophages detected in the subcapsular sinus and the medullary sinus of LNs express CD169 (Figs 1b,2a); however, macrophages in the medullary cord of both rodent and human LNs are negative for CD169. In addition, there tends to be more macrophages in the medullary sinus than in the subcapsular sinus in human LNs compared to rodent LNs (Fig. 1b). Human sinus macrophages also express programmed cell death 1 ligand 1 (PD‐L1), CD163, CD204, and Fascin, and are negative for CD11c which is a marker for dendritic cells (Fig. 2a,b). CD11c‐positive cells are preferentially detected in medullary area. In our preliminary studies, LN sinus macrophages expressed PD‐L1, CD163, and CD204 in almost all cases, although the expression of CD169 showed individual variation. Since there are no research studies focused on the functions of these antigens expressed on sinus macrophages, the functional mechanisms of these antigens in sinus macrophages have been uncovered. PD‐L1 is known to be expressed not only on tumor cells but also on macrophages, and PD‐L2 is also expressed on macrophages.8, 9 Blocking PD‐1‐PD‐L1/2 signal might enhance antigen presentation of sinus macrophages to lymphocytes in LN, although further studies are necessary to elucidate the functional significance of PD‐L1/2 on macrophages in anti‐tumor immune response.
Figure 2.
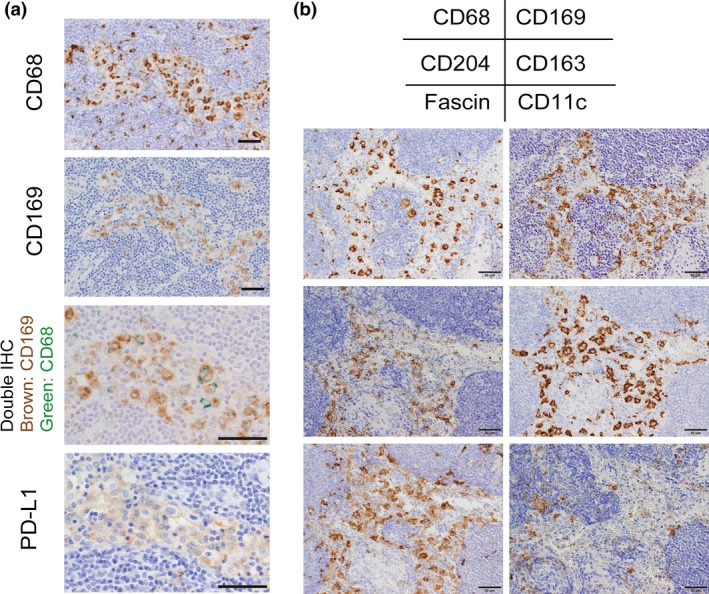
Molecules expressed on macrophages in a lymph node (LN). (a) Serial sections of a human LN were immunostained for CD68, CD169, and PD‐L1. Double immunohistochemistry (IHC) of CD68 and CD169 was also added. Sinus CD169‐positive macrophages express CD68 and PD‐L1. (b) Serial sections of a human LN were immunostained for CD68, CD169, CD204, CD163, Fascin, and CD11c. Sinus macrophages express CD163, CD204, and Fascin. CD11c was expressed on dendritic cells but not on sinus macrophages. Scale bar; 50 μm.
LN Macrophages and Anti‐Tumor Immunity in Animal Studies
Anti‐tumor immunity is observed to a greater or lesser degree in patients with malignant diseases, and the induction of anti‐tumor immune responses is considered to be one of the factors related to the effectiveness of conventional anti‐tumor chemotherapy.10, 11 In the cases of malignant diseases other than hematological malignancies, tumor‐antigen‐loaded APCs are considered to induce antigen‐specific T‐cell responses and generate cytotoxic T lymphocytes (CTLs) in LNs, therefore the LNs are thought to be important for the establishment and the maintenance of anti‐tumor immune responses.12, 13 There are many macrophages in the subcapsular sinus of the LN, which, as mentioned above, is located in the first line for incoming lymph. In contrast, dendritic cells are preferentially distributed in the lymph follicle. Sinus macrophages in the LN engulf antigens via several receptors including scavenger receptors (Fig. 2),14, 15 and are considered to present antigen‐derived peptide to T‐ and B‐lymphocytes. The critical role of LN macrophages in anti‐tumor immunity of LNs, is evidenced by recent studies using animal models. It is well known that subcutaneous injection of dead tumor cells induces tumor‐antigen specific CTLs. By exploiting this phenomenon, and by using genetically modified mice in which CD169‐positive macrophages were specifically depleted by diphtheria toxin, Asano et al.13 demonstrated that CD169‐positive subcapsular sinus macrophages in LNs are preferentially involved in antigen‐presentation and the induction of CTLs. Benhard et al. additionally demonstrated that not only dendritic cells but also CD169‐positive subcapsular sinus macrophages induced CTL responses. Notably, by using an adenoviral particle selectively trapped in marginal zone CD169‐positive macrophage and CD11c‐DTR mice, they found that dendritic cells induced CTLs that react to strongly binding epitopes whereas macrophages generated CTLs that react to a broader range of epitopes.16 The potential role of CD169 in these events was investigated, and CD169 has been considered to contribute to the uptake of sialylated antigen and to be useful as a target of antigen delivery for vaccine.17, 18 However, the result that CTL responses to tumor cells were not different between wild type mice and CD169‐deficient mice indicated that CD169 function was not of great importance for immune reactions to tumor cells.13 Muraoka et al.19 demonstrated that CHP nanogel‐conjugated tumor antigen was specifically engulfed by medullary sinus macrophages, induced antigen‐specific CTLs and suppressed tumor development. These findings indicated that medullary sinus macrophages, in addition to subcapsular sinus macrophages and dendritic cells, also functioned in antigen presentation.
Thus emerging data related to the significance of CD169‐positive subcapsular and medullary sinus macrophages in the generation of CTLs has been reported. On the other hand, Pucci et al.20 demonstrated that subcapsular sinus macrophages engulfed melanoma‐derived extracellular vesicles and subsequently suppressed the induction of protumor B‐cells. They suggested that subcapsular sinus macrophages act as a physical barrier to B cell activation under specific circumstances. With regard to B‐cell function in tumor development, Coussens's group reported that B lymphocytes and humoral immunity are involved in tumor development through the activation of M2 or protumor myeloid cells,21, 22 and they also showed that a B cell‐depleting αCD20 monoclonal antibody improved response to platinum‐ and Taxol‐based chemotherapy in squamous cell carcinoma.23 These findings indicate the existence of complex mechanisms in cell–cell interaction between protumor B lymphocytes and LN macrophages; however, LN macrophage‐mediated B lymphocyte activation might be a novel target for anti‐tumor immunotherapy.
Significance of LN Macrophages in Anti‐Tumor Immunity in Human Colorectal Tumor
Although several studies using murine models have indicated the significance of LN macrophages in anti‐tumor immune responses and tumor progression, there have not been many studies describing correlations between LN macrophages and anti‐tumor immunity or tumor progression. A high number of infiltrating lymphocytes (especially CD8‐positive cells) into tumor tissues or of circulating lymphocytes in peripheral blood have been reported to be related to favorable clinical prognosis in several malignant tumors.24, 25, 26, 27 Recent studies also demonstrated that higher infiltration of CD8‐positive lymphocytes into the tumor microenvironment is correlated with a higher rate of pathological complete response in breast tumor treated with neoadjuvant chemotherapy.28, 29 These findings indicate that CD8‐positive cytotoxic lymphocytes (CTLs) are the critical mediators related to anti‐tumor immune responses in human tumors; however, it has not yet been discovered how CTLs are generated in tumor patients.
Based on the above findings, it was suggested that the number and proportion of CD169‐positive macrophages in LN sinuses reflect the tumor immunocompetence of tumor patients; however, the association between human CD169 and anti‐tumor immunity has not been analyzed. Therefore, in order to verify whether or not an anti‐tumor immune mechanism is linked to CD169‐positive macrophages in humans, we analyzed the correlation of CD169‐positive macrophages of regional LNs to anti‐tumor immune reactions and clinical prognoses in human colorectal tumors.30 CD169 expression in human LN sinus macrophages was immunohistochemically analyzed; however, interestingly, it was found that the CD169‐positive rate in lymph node sinus macrophages differed widely from case to case. In other words, in contrast to mouse cases, CD169 expression in LN sinus macrophages was nearly negative in some human cases. A statistical analysis of the post‐operative survival rate and clinicopathological factors of the CD169 high and CD169 low groups, which were based on the CD169 expression level in the lymph nodes (Fig. 3), was performed. The results showed that an increased density of CD169‐positive macrophages in the sinus area and higher percentages of CD169‐positive cells in CD68‐positive sinus macrophages were significantly correlated with higher T stages, non‐LN metastasis status, and, notably, high CD8‐positive lymphocyte infiltration into primary tumor tissues. Patients with a higher density or percentage of CD169‐positive macrophages showed significantly better overall survival. The density of CD68‐positive macrophages in LN sinus was associated to neither any clinicopathological factors and the density of CTLs. These observations suggested that CD169 expression in sinus macrophages was closely involved in the induction of anti‐tumor immune responses and exerted a beneficial effect on the clinical course.
Figure 3.
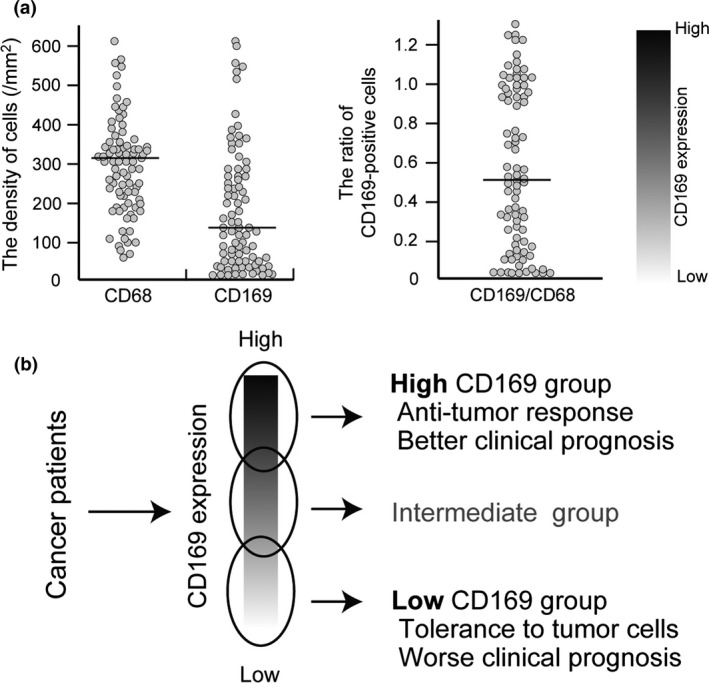
Summary of the data of the study of colorectal cancer. (a) Scatter diagrams of the number of CD68‐ and CD169‐positive macrophages (left) and the ratio of CD169+:CD68+ macrophages (right) in the LN sinus. The value of the CD169+:CD68+ macrophage ratio in the LN sinus indicated the variation of CD169 expression in individuals. (b) Cases with high CD169 expression in LN sinus macrophages showed high infiltration of CD8‐positive CTLs and better clinical course, and vice versa. These phenomena were observed when the cases were divided into high or low CD169 expression groups based on median cell numbers or based on the CD169+:CD68+ ratio. However, cases with intermediate CD169 expression are also seen and it might be difficult to strictly classify these cases into either a high or low CD169 expression group.
Significance of LN Macrophages in Anti‐Tumor Immunity in Human Melanoma and Endometrial Tumor
We subsequently carried out similar research using resected samples from patients with melanoma and endometrial tumors.31, 32 Similar to the study of colorectal tumors, higher expression of CD169 in LN macrophages was significantly associated with better clinical course in patients with melanoma and endometrial tumors. The density of CD68‐positive macrophages in LN sinus was associated to neither any clinicopathological factors and infiltration of immune cells in melanoma and endometrial tumors as well as that in colorectal tumors. In endometrial tumors, increased density and percentage of CD169‐positive LN macrophages correlated well with higher density of infiltrating CD8‐positive lymphocytes and CD57‐positive natural killer cells in primary tumor tissues, and with lower clinical stage and non‐LN metastasis. In melanoma, although there was no significant association between CD169 expression and clinical stages, increased percentage of CD169‐positive LN macrophages showed a strong link with a higher density of infiltrating CD8‐positive lymphocytes in primary tumor tissues and non‐LN metastasis. In our studies in three kinds of tumors, neither gender nor age was associated with either the density or the percentage of CD169‐positive LN macrophages. At the present time, similar studies are underway to examine the correlations between CD169 expression in sinus macrophages and clinicopatholocal factors including the number of infiltrating effector cells.
The Microenvironment and CD169 Expression in LNs
In our in vitro studies using human monocyte‐derived macrophages, CD169 expression was found to be induced by type 1 interferon (IFN),30 consistent with a previous report.33 However, this observation was inconsistent with data of rodents in which CD169 expression was not affected by IFN.34 Therefore, we next examined which cells in LNs express type 1 IFN. Since an antibody suitable for immunohistochemistry of IFN‐α is commercially available, we immunostained IFN‐α in LN samples. Although positive IFN‐α reactions were observed in some of the LN samples, positive signals were detected on CD68‐positive and CD169‐negative macrophages that are not morphologically similar to sinus macrophages. Positive IFN‐α signals were also detected on CD303‐positive plasmacytoid dendritic cells. The detailed mechanisms of IFN‐α production by these cells have never been clarified. It has been suggested that the immunological condition related to IFN‐α production by these cells affects CD169 expression and the capacity of antigen presentation to effector cells. IFN‐α signaling is known to be closely related to anti‐tumor responses, and single nucleotide polymorphisms in type 1 IFN‐α receptor genes were reported to be associated with altered overall survival of patients with glioma.35 Therefore, genetic background also potentially influences IFN‐α signaling in LNs.
Targeting Macrophages; Novel Methods for Promising Vaccination
A number of methods have been investigated for targeting antigen to LN macrophages as part of the development of future lymphatic‐targeted vaccines. Shiku H. and his colleagues reported that vaccines of a tumor antigen (NY‐ESO‐1, HER2, and MAGE‐A4) complexed with cholesteryl pullulan (CHP) nanogels induced CD8‐ and CD4‐positive lymphocytes and antibody production that specifically reacted to antigen in tumor patients, and thereafter vaccination using CHP nanogels induced tumor regression in some patients with malignant tumors.36, 37, 38, 39, 40, 41, 42 In these studies, increased antibody reactions to vaccinated antigens were showed to predict the efficacy of anti‐tumor immune responses. In addition, antigen spreading following vaccination was also observed in around half patients treated with CHP nanogels.43 As described above, CHP nanogels were found to be preferentially engulfed by the medullary sinus macrophages of the LN. Thus, these observations indicated the significance of LN sinus macrophages in the development of anti‐tumor immune responses. Chen et al.,17 using newly developed sialic acid‐conjugated liposomes that successfully deliver antigen to CD169‐positive macrophages, stimulated the production of antigen‐specific T cells. CD169‐targeting liposomes with lipid antigen induced activation of iNKT cells in a CD1d dependent manner.44 Reddy et al.45 demonstrated that nanoparticles that were smaller than 25 nm in size flowed in lymph vessels and reached the LN sinus in which nanoparticles are phagocytosed by CD11c‐positive dendritic cells, whereas the targeting efficacy of nanoparticles that were 100 nm in size was very low. Although they had never evaluated the macrophages in the article, small‐size nanoparticles seemed to be trapped by sinus macrophages in the picture figure.45 Nanoparticles with cyclic dinucleotides (which are an agonist of the stimulator of IFN genes) and peptides were successfully delivered to macrophages and dendritic cells in LNs and induced a greater expansion of peptide‐specific CD4‐positive T cells; moreover, tumor development was significantly reduced by vaccination.46 Thus a lymphatic‐targeted vaccine is considered to be a promising approach to improving vaccine efficacy, and CD169‐positive macrophages are now of interest as APC to which antigens might be efficiently delivered. Since the efficacy of antigen delivery using biomaterials is dependent on the size and the nature of the surface molecules/ligands, it is necessary that methods to deliver antigens and adjuvants to APCs in LNs are developed in further studies.
Conclusion
Tumor‐associated macrophages (TAMs) infiltrating into tumor tissues are well known to promote the proliferation and metastasis of tumor cells, and a high density of TAMs in primary tumor tissues has been reported as a poor prognostic factor in many tumors.47, 48 Unlike TAMs, LN sinus macrophages have anti‐tumor activity via the induction of anti‐tumor CTLs and are important cells for tumor immunotherapy; thus the phenotype and functions of macrophages in patients with a malignant tumor differ in their distribution (Fig. 4). Antigen delivery targeting LN macrophages is also considered to be a promising approach for vaccination. Immunotherapy using immune checkpoint blockers is of interest for new anti‐tumor therapy; however, there are few biomarkers for evaluating anti‐tumor immune responses. The evaluation of CD169‐positive macrophages in LNs might be useful for evaluating the status of anti‐tumor immune responses and for predicting the effect of anti‐tumor therapy such as chemotherapy and immunotherapy.
Figure 4.
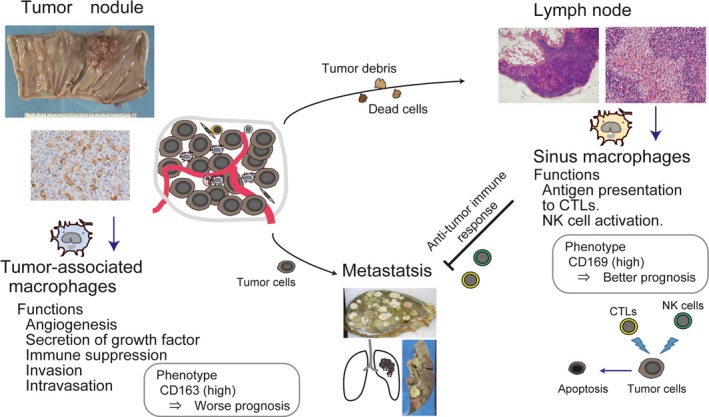
Scheme of the function of macrophages related to tumor development. (Left) TAMs have pro‐tumor functions and a high density of CD163 (a marker of pro‐tumor macrophages)‐positive TAMs has been reported as one of the predictive factors for worse clinical prognosis in many malignant tumors. (Right) CD169‐positive LN macrophages are considered to cross‐present tumor antigens to effector cells. A high density or percentage of CD169‐positive LN macrophages has been demonstrated to be one of the predictive factors for better clinical prognosis in colorectal cancer, melanoma, and endometrial cancer.
Conflict of Interest
The authors have no financial competing interests to declare.
Acknowledgments
This work was supported by JSPS KAKENHI (grant number JP25460497, JP25293089).
Cancer Sci 108 (2017) 290–295
Funding Information
JSPS KAKENHI (grant number JP25460497, JP25293089).
References
- 1. Martinez‐Pomares L, Gordon S. CD169+ macrophages at the crossroads of antigen presentation. Trends Immunol 2012; 33: 66–70. [DOI] [PubMed] [Google Scholar]
- 2. Gasteiger G, Ataide M, Kastenmüller W. Lymph node – an organ for T‐cell activation and pathogen defense. Immunol Rev 2016; 271: 200–20. [DOI] [PubMed] [Google Scholar]
- 3. Marmey B, Boix C, Barbaroux JB et al CD14 and CD169 expression in human lymph nodes and spleen: specific expansion of CD14+CD169‐ monocyte‐derived cells in diffuse large B‐cell lymphomas. Hum Pathol 2006; 37: 68–77. [DOI] [PubMed] [Google Scholar]
- 4. Junt T, Moseman EA, Iannacone M et al Subcapsular sinus macrophages in lymph nodes clear lymph‐borne viruses and present them to antiviral B cells. Nature 2007; 450: 110–4. [DOI] [PubMed] [Google Scholar]
- 5. Gray EE, Cyster JG. Lymph node macrophages. J Innate Immun 2012; 4: 424–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O'Neill AS, van den Berg TK, Mullen GE. Sialoadhesin – a macrophage‐restricted marker of immunoregulation and inflammation. Immunology 2013; 138: 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hartnell A, Steel J, Turley H, Jones M, Jackson DG, Crocker PR. Characterization of human sialoadhesin, a sialic acid binding receptor expressed by resident and inflammatory macrophage populations. Blood 2001; 97: 288–96. [DOI] [PubMed] [Google Scholar]
- 8. Horlad H, Ma C, Yano H et al An IL‐27/Stat3 axis induces expression of programmed cell death 1 ligands (PD‐L1/2) on infiltrating macrophages in lymphoma. Cancer Sci 2016; 107: 1696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miyoshi H, Kiyasu J, Kato T et al PD‐L1 expression on neoplastic or stromal cells is respectively a poor or good prognostic factor for adult T‐cell leukemia/lymphoma. Blood 2016; 128: 1374–81. [DOI] [PubMed] [Google Scholar]
- 10. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011; 480: 480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Palucka AK, Coussens LM. The basis of oncoimmunology. Cell 2016; 164: 1233–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lores B, García‐Estevez JM, Arias C. Lymph nodes and human tumors (review). Int J Mol Med 1998; 1: 729–33. [DOI] [PubMed] [Google Scholar]
- 13. Asano K, Nabeyama A, Miyake Y et al CD169‐positive macrophages dominate antitumor immunity by crosspresenting dead cell‐associated antigens. Immunity 2011; 34: 85–95. [DOI] [PubMed] [Google Scholar]
- 14. Martens JH, Kzhyshkowska J, Falkowski‐Hansen M et al Differential expression of a gene signature for scavenger/lectin receptors by endothelial cells and macrophages in human lymph node sinuses, the primary sites of regional metastasis. J Pathol 2006; 208: 574–89. [DOI] [PubMed] [Google Scholar]
- 15. Tanaka M, Asano K, Qiu CH. Immune regulation by apoptotic cell clearance. Ann N Y Acad Sci 2010; 1209: 37–42. [DOI] [PubMed] [Google Scholar]
- 16. Bernhard CA, Ried C, Kochanek S, Brocker T. CD169+ macrophages are sufficient for priming of CTLs with specificities left out by cross‐priming dendritic cells. Proc Natl Acad Sci USA 2015; 112: 5461–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen WC, Kawasaki N, Nycholat CM et al Antigen delivery to macrophages using liposomal nanoparticles targeting sialoadhesin/CD169. PLoS One 2012; 7: e39039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Poderoso T, Martínez P, Álvarez B et al Delivery of antigen to sialoadhesin or CD163 improves the specific immune response in pigs. Vaccine 2011; 29: 4813–20. [DOI] [PubMed] [Google Scholar]
- 19. Muraoka D, Harada N, Hayashi T et al Nanogel‐based immunologically stealth vaccine targets macrophages in the medulla of lymph node and induces potent antitumor immunity. ACS Nano 2014; 8: 9209–18. [DOI] [PubMed] [Google Scholar]
- 20. Pucci F, Garris C, Lai CP et al SCS macrophages suppress melanoma by restricting tumor‐derived vesicle‐B cell interactions. Science 2016; 352: 242–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell 2005; 7: 411–23. [DOI] [PubMed] [Google Scholar]
- 22. Andreu P, Johansson M, Affara NI et al FcRgamma activation regulates inflammation‐associated squamous carcinogenesis. Cancer Cell 2010; 17: 121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Affara NI, Ruffell B, Medler TR et al B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell 2014; 25: 809–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Naito Y, Saito K, Shiiba K et al CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 1998; 58: 3491–4. [PubMed] [Google Scholar]
- 25. Galon J, Costes A, Sanchez‐Cabo F et al Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313: 1960–4. [DOI] [PubMed] [Google Scholar]
- 26. Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour‐infiltrating lymphocytes in cancer: a systematic review with meta‐analysis. Br J Cancer 2011; 105: 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sasada T, Suekane S. Variation of tumor‐infiltrating lymphocytes in human cancers: controversy on clinical significance. Immunotherapy 2011; 3: 1235–51. [DOI] [PubMed] [Google Scholar]
- 28. Mao Y, Qu Q, Zhang Y, Liu J, Chen X, Shen K. The value of tumor infiltrating lymphocytes (TILs) for predicting response to neoadjuvant chemotherapy in breast cancer: a systematic review and meta‐analysis. PLoS One 2014; 9: e115103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Denkert C, von Minckwitz G, Brase JC et al Tumor‐infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2‐positive and triple‐negative primary breast cancers. J Clin Oncol 2015; 33: 983–91. [DOI] [PubMed] [Google Scholar]
- 30. Ohnishi K, Komohara Y, Saito Y et al CD169‐positive macrophages in regional lymph nodes are associated with a favorable prognosis in patients with colorectal carcinoma. Cancer Sci 2013; 104: 1237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saito Y, Ohnishi K, Miyashita A et al Prognostic significance of CD169+ lymph node sinus macrophages in patients with malignant melanoma. Cancer Immunol Res 2015; 3: 1356–63. [DOI] [PubMed] [Google Scholar]
- 32. Ohnishi K, Yamaguchi M, Erdenebaatar C et al Prognostic significance of CD169‐positive lymph node sinus macrophages in patients with endometrial carcinoma. Cancer Sci 2016; 107: 846–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Puryear WB, Akiyama H, Geer SD et al Interferon‐inducible mechanism of dendritic cell‐mediated HIV‐1 dissemination is dependent on Siglec‐1/CD169. PLoS Pathog 2013; 9: e1003291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van den Berg TK, van Die I, de Lavalette CR et al Regulation of sialoadhesin expression on rat macrophages. Induction by glucocorticoids and enhancement by IFN‐beta, IFN‐gamma, IL‐4, and lipopolysaccharide. J Immunol 1996; 157: 3130–8. [PubMed] [Google Scholar]
- 35. Fujita M, Scheurer ME, Decker SA et al Role of type 1 IFNs in antiglioma immunosurveillance–using mouse studies to guide examination of novel prognostic markers in humans. Clin Cancer Res 2010; 16: 3409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kitano S, Kageyama S, Nagata Y et al HER2‐specific T‐cell immune responses in patients vaccinated with truncated HER2 protein complexed with nanogels of cholesteryl pullulan. Clin Cancer Res 2006; 12: 7397–405. [DOI] [PubMed] [Google Scholar]
- 37. Kawabata R, Wada H, Isobe M et al Antibody response against NY‐ESO‐1 in CHP‐NY‐ESO‐1 vaccinated patients. Int J Cancer 2007; 120: 2178–84. [DOI] [PubMed] [Google Scholar]
- 38. Uenaka A, Wada H, Isobe M et al T cell immunomonitoring and tumor responses in patients immunized with a complex of cholesterol‐bearing hydrophobized pullulan (CHP) and NY‐ESO‐1 protein. Cancer Immun 2007; 7: 9. [PMC free article] [PubMed] [Google Scholar]
- 39. Kageyama S, Kitano S, Hirayama M et al Humoral immune responses in patients vaccinated with 1‐146 HER2 protein complexed with cholesteryl pullulan nanogel. Cancer Sci 2008; 99: 601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aoki M, Ueda S, Nishikawa H et al Antibody responses against NY‐ESO‐1 and HER2 antigens in patients vaccinated with combinations of cholesteryl pullulan (CHP)‐NY‐ESO‐1 and CHP‐HER2 with OK‐432. Vaccine 2009; 27: 6854–61. [DOI] [PubMed] [Google Scholar]
- 41. Kageyama S, Wada H, Muro K et al Dose‐dependent effects of NY‐ESO‐1 protein vaccine complexed with cholesteryl pullulan (CHP‐NY‐ESO‐1) on immune responses and survival benefits of esophageal cancer patients. J Transl Med 2013; 11: 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saito T, Wada H, Yamasaki M et al High expression of MAGE‐A4 and MHC class I antigens in tumor cells and induction of MAGE‐A4 immune responses are prognostic markers of CHP‐MAGE‐A4 cancer vaccine. Vaccine 2014; 32: 5901–7. [DOI] [PubMed] [Google Scholar]
- 43. Miyauchi K, Tsuchikawa T, Wada M et al Clinical relevance of antigen spreading pattern induced by CHP‐MAGE‐A4 cancer vaccination. Immunotherapy 2016; 8: 527–40. [DOI] [PubMed] [Google Scholar]
- 44. Barral P, Polzella P, Bruckbauer A et al CD169(+) macrophages present lipid antigens to mediate early activation of iNKT cells in lymph nodes. Nat Immunol 2010; 11: 303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reddy ST, van der Vlies AJ, Simeoni E et al Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol 2007; 25: 1159–64. [DOI] [PubMed] [Google Scholar]
- 46. Hanson MC, Crespo MP, Abraham W et al Nanoparticulate STING agonists are potent lymph node‐targeted vaccine adjuvants. J Clin Invest 2015; 125: 2532–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Takeya M, Komohara Y. Role of tumor‐associated macrophages in human malignancies: friend or foe? Pathol Int 2016; 66: 491–505. [DOI] [PubMed] [Google Scholar]
- 48. Komohara Y, Jinushi M, Takeya M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci 2014; 105: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


