Abstract
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive human malignancies. The Yes‐associated protein‐1 (YAP) plays a critical role in cell proliferation, apoptosis and angiogenesis. Verteporfin is a photosensitizer used in photodynamic therapy and also a small molecular inhibitor of the Hippo‐YAP pathway. However, little is known about whether verteporfin could inhibit YAP activity in PDAC cells. Our present results showed that verteporfin suppressed the proliferation of human PDAC PANC‐1 and SW1990 cells by arresting cells at the G1 phase, and inducing apoptosis in dose‐ and time‐dependent manners. Verteporfin also inhibited the tumor growth on the PDAC xenograft model. Treatment with verteporfin led to downregulation of cyclinD1 and cyclinE1, modulation of Bcl‐2 family proteins and activation of PARP. In addition, verteporfin exhibited an inhibitory effect on angiogenesis and vasculogenic mimicry via suppressing Ang2, MMP2, VE‐cadherin, and α‐SMA expression in vitro and in vivo. Mechanism studies demonstrated that verteporfin impaired YAP and TEAD interaction to suppress the expression of targeted genes. Our results provide a foundation for repurposing verteporfin as a promising anti‐tumor drug in the treatment of pancreatic cancer by targeting the Hippo pathway.
Keywords: Angiogenesis, apoptosis, pancreatic cancer, vasculogenic mimicry, verteporfin
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive human malignancies, and the overall 5‐year survival rate of is <5%.1 The incidence rate of PDAC is annually increasing because of the obesity, high‐fat diets, diabetes, smoking, alcoholic dependence, and chronic pancreatitis.2 The standard chemotherapeutic regimen for advanced PDAC only prolongs the survival for 3 months.3, 4 Therefore, it is urgently required to seek novel therapeutic strategies.
The Yes‐associated protein 1 (YAP) is the most critical downstream effector in the Hippo signaling cascade, and plays an essential role in physiological and pathological process.5 YAP serves as a transcriptional co‐activator and regulates the activity of various transcription factors, such as TEADs and CTGF, which are implicated in cell proliferation, apoptosis, chemoresistance and angiogenesis.6 YAP is highly expressed in several types of malignant tumors including pancreatic cancer,7, 8, 9 and is shown to participate in the development, progression and recurrence of PDAC.10, 11 Hyperactivated YAP could enhance the secretion of microfibrillar associated protein 5 (MFAP5) to promote neoangiogenesis in vitro and in vivo.12 Therefore, YAP may be a potential target for cancer therapies.
As one of the hallmarks of cancer, angiogenesis is characterized by the formation of new and irregular blood vessels to supply nutrients and oxygen in tumors.13 Tumor cells secret pro‐angiogenic factors to facilitate tumor growth and metastasis. Consequently, angiogenesis inhibition has become an important therapeutic strategy to prevent tumor progression and metastasis. Vasculogenic mimicry (VM), another form of tumor vascularization, has drawn an attention, since it is independent of angiogenesis and formed by highly invasive and genetically dysregulated cancer cells.14, 15 VM is detected in various solid tumors including pancreatic cancer.16, 17, 18 Tumors with high VM abilities are often highly aggressive and associated with poor prognosis.19 Pancreatic cancer is one of the most vascularized and angiogenic solid tumors.20 Therefore, inhibition of angiogenesis and VM could suppress the progression of pancreatic cancer.
Verteporfin is a photosensitizer clinically used for photodynamic therapy to treat neovascularization caused by age‐related macular degeneration.21 Recently, studies have shown that verteporfin could inhibit YAP activation by disrupting YAP‐TEAD interactions and preventing YAP induced oncogenic growth.22 Verteporfin was shown to inhibit angiogenesis and the growth and migration of human retinoblastoma cells via disrupting the YAP‐TEAD‐associated downstream proto‐oncogenes.23 Furthermore, verteporfin effectively inhibited the expression of YAP and endothelial growth factor receptor (EGFR) and enhanced the effects of cytotoxic drugs in killing cells from esophageal cancer24 and lung cancer.25 The results from a phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer indicated that verteporfin was feasible and safe, and might represent a useful supplement for the treatment of pancreatic cancer.26
In this study, we have shown that verteporfin inhibited the proliferation of PDAC cells by arresting cells at G1 phase via reducing the expression of cyclinD1 and cyclinE1, and inducing apoptosis by activating the intrinsic apoptotic signaling pathway. Verteporfin also inhibited tumor angiogenesis by downregulating angiopoietin‐2 (Ang2) through inhibiting YAP activity, and suppressed VM by downregulating MMP2, VE‐cadherin and α‐SMA expression. The above mechanism studies on YAP regulation by verteporfin provide insights into potential approaches with verteporfin in PDAC therapy.
Materials and Methods
Cell culture
Human umbilical vein endothelial cells (HUVECs) and human pancreatic ductal cells (HPDE6) were purchased from Fuheng biology (Shanghai, China). Human PDAC PANC‐1 and SW1990 cells were obtained from CTCC (Shanghai, China). Cells were cultured in Dulbecco's modified Eagle's medium (Hyclone, Logan, Utah, USA) containing 10% fetal bovine serum (Gibco, Carlsbad, CA, USA) in a humidified incubator containing 5% CO2 at 37°C.
Transmission electron microscopy
Conventional electron microscopy was used to visualize PANC‐1 and SW1990 cells morphology at the ultrastructural level. The cells after verteporfin treated were fixed with 2.5% glutaraldehyde, and post‐fixed in 1% phosphate‐buffered osmium tetroxide. After being embedded, sectioned, and double‐stained with uranyl acetate and lead citrate, images were captured with a transmission electron microscope (JEM‐1200EX, JEOL Ltd, Tokyo, Japan).
Flow cytometry for cell cycle and apoptosis assay
For cell cycle assay, 1 × 106 cells were harvested, washed, and fixed with ice‐cold 70% ethanol overnight at 4°C. After washing again, cells were incubated with DAPI and RNaseA for 30 min at room temperature, and then analyzed cytometrically. For cell apoptosis assay, cells were harvested and incubated with 1 μL of Zombie violet stain and 5 μL of FITC‐conjugated Annexin V for 30 min (Biolegend, San Diego, CA, USA), and then analyzed cytometrically. Three independent repeated experiments were performed.
Gene expression vectors and transfection
Full‐length human YAP ectopic expression lentivirus vector and the negative control (GeneChem, Shanghai, China) were transfected into HUVECs according to the manufacturer's instruction. YAP shRNA (5′‐CCCAGTTAAATGTTCACCAAT‐3′) and scrambled control shRNA (GenePharma, Shanghai, China) were transfected into HUVECs with the PolyJet DNA in vitro transfection reagent (SignaGen, Ijamsville, MD, USA).
Transwell assay
The assay was performed as previously described.27 Briefly, cells were added to the upper compartment of the chamber. After incubation for 24 h, cells in the lower compartment were fixed with methanol, and stained with 0.1% crystal violet. The number of migrated cells in five randomly selected fields (magnification, 100×) was counted under microscopy. The experiments were repeated at least in triplicate.
In vitro tube formation assay
The assay was performed as previously described.27 Briefly, 24‐well culture plates were coated with 200 μL of Matrigel matrix (Corning, Bedford, MA, USA). Cells (2.5 × 105/well) were seeded in plates. Twenty‐four hours after treatments, images were captured with an Olympus microscope. The number of tubular structures in five randomly chosen fields (magnification, 40×) was quantified with the Image‐Pro Plus 6.0 software (Media Cybernetics, Rockville, MD, USA).
Chicken chorioallantoic membrane (CAM) assay
Chorioallantoic membrane assay was performed as described previously.28 Groups of 10 fertilized chicken eggs were incubated at 37.8°C and 60–70% relative humidity for 8 days. A small hole was then drilled on the broad end of each egg and a window was carefully created on the eggshell. The chorioallantoic membranes were treated with either vehicle or verteporfin. The windows were then sealed with cellophane tape. After incubation for further 48 h, the CAMs were photographed. Angiogenesis was quantified by counting the number of branching blood vessels.
Animal experiments
The animal experiments were approved by the Animal Care and Use Committee of Shandong University. Six‐week‐old male BALB/c nude (nu/nu) mice were purchased from Beijing HFK Bioscience (Beijing, China). The experimental protocol has been described previously.29, 30 Briefly, the PANC‐1 or SW1990 cells (107 cells in 100 μL PBS) were subcutaneously inoculated into the right axilla of mice after a week of acclimatization. Tumor growth was measured daily with calipers. The tumor volume was calculated as a 2 × b × π/6, where b is the length in millimeters and a is the width in millimeters. When tumors reached a mean volume of 70–150 mm3, the animals were randomized to two groups. The mice in the verteporfin group were intraperitoneally injected with verteporfin (100 mg/kg body weight) every 2 days for 3 weeks. The control mice received the intraperitoneal injection with an equal volume of PBS. The tumors were harvested at the end of the experiments.
Statistical analysis
The data were expressed as the mean ± standard deviation. Comparisons were made with one‐way anova followed by Dunnet's t‐test. The GraphPad Prism 5 software (La Jolla, CA, USA) was used to evaluate statistical significance. A value of P < 0.05 was considered to indicate a statistically significant difference.
Antibody and reagents, Cell viability assay, Colony formation assay, TUNEL assay, Quantitative real‐time PCR, Western blot, immunohistochemistry and periodic acid‐schiff staining. Please see details in Supplemental additional materials and methods.
Results
Verteporfin inhibits cell proliferation and cell cycle progression
Incubation of verteporfin reduced the viability of PANC‐1 and SW1990 cells in dose‐ and time‐dependent manners. The IC50 of verteporfin was 6.8, 2.6, and 1.4 μM in PANC‐1 cells after incubation for 24, 48 and 72 h, respectively (Fig. 1a). Similarly, the IC50 in SW1990 cells was 7.7, 3.6 and 1.7 μM after incubation for 24, 48, and 72 h, respectively (Fig. 1b). Flow cytometry assays revealed that verteporfin increased the percentage of cells in G1 phase and decreased the percentage of cells in S phase (Fig. 1c,d). Additionally, the number of colonies formed by verteporfin‐pretreated PDAC cells was significantly less than those by control cells (Fig. 1d,e).
Figure 1.
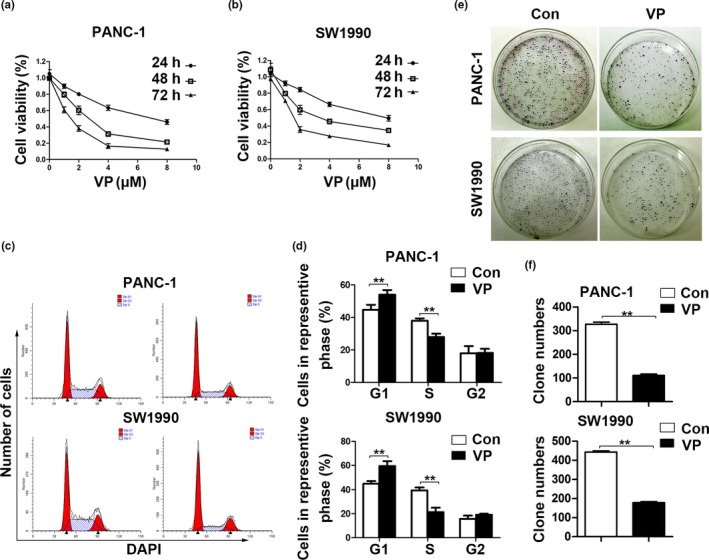
Verteporfin inhibits cell proliferation and cell cycle progression in vitro. (a, b) PANC‐1 and SW1990 cells were incubated with vehicle or verteporfin at various concentrations for 24, 48, and 72 h. Cell viability was assessed (%). (c) Proportion of PANC‐1 and SW1990 cells at different phases of cell cycle was measured by flow cytometry. (d) Representative histograms depicting cell cycle profiles of PANC‐1 and SW1990 cells were shown. (e, f) Representative colonies formed by PANC‐1 or SW1990 cells treated with vehicle or verteporfin were shown in the left panel, and the quantified results in the right panel. The experiments were performed in triplicate. Data were presented as mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001.
Verteporfin induces cell apoptosis
Under electron microscopy, control cells displayed regular oval shape with prominent nucleus, distinct nucleolus and maintained the integrity of cellular membranes; while verteporfin‐treated cells featured significant cytoplasmic vacuolization, large vacuoles, autophagosome, and swollen mitochondria (Fig. 2a). Verteporfin significantly induced apoptosis in PANC‐1 and SW1990 cells as detected by TUNEL assay (Fig. 2b). Annexin V–FITC/Zombie violet apoptosis assay showed that the apoptosis rates of PANC‐1 and SW1990 cells treated with verteporfin were approximately 30% and 24%, respectively (Fig. 2c,d). Similarly, verteporfin also inhibited the viability and induced the apoptosis of HPDE6 and HUVECs in relatively more doses (Fig. S1).
Figure 2.
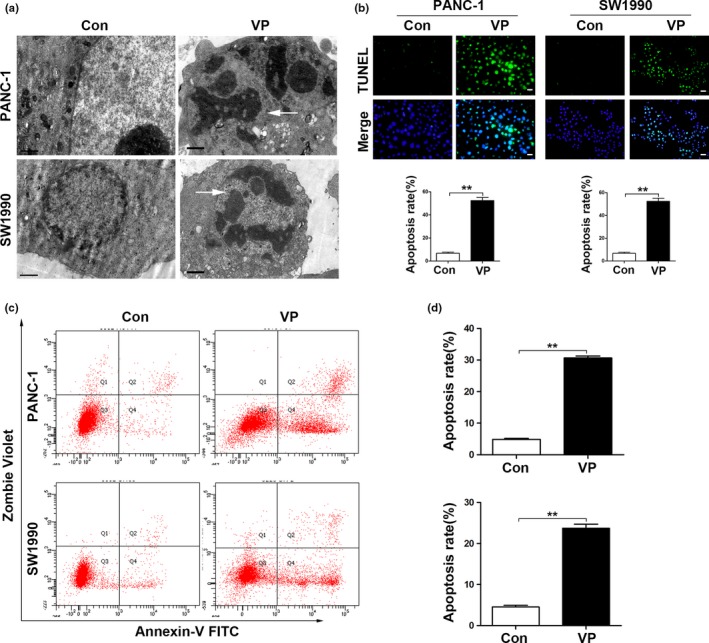
Verteporfin induces cell apoptosis in pancreatic cancer cells. (a) Ultrastructure of PDAC cells treated with vehicle control or verteporfin (Magnification ×10 000, bar = 1 μM) was examined by electron microscopy. (b) PDAC cells were incubated with vehicle control or verteporfin and then stained by TUNEL (Magnification ×200, bar = 50 μM). TUNEL positive cells were quantified. (c) Representative dot plots were from cytometrically analyzed apoptotic cells. (d) Percentages of apoptotic cells (%) were quantified. The experiments were performed in triplicate. Data were presented as mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001.
Verteporfin regulates the expression of proteins involved in cell cycle progression and apoptosis via disrupting YAP‐TEAD interaction
Mizuno et al. found that YAP1 knockdown led to an accumulation of cells in G0 and G1 phase as well as a significant loss of S phase cells in mesothelioma cell lines. Mechanism studies demonstrated that YAP directly regulated the expressions of cyclinD1 and foxhead box m1 (FOXM1).31 Here, verteporfin inhibited the expression of cyclinD1 and cyclinE1 in a dose‐dependent manner (Fig. 3a). Verteporfin also increased the expression of Bax, decreased the expression of Bcl‐2, and activated PARP (Fig. 3b). Verteporfin also suppressed necrosis‐associated protein expression in PDAC cells (Fig. S2). It has been shown that verteporfin abrogated the interaction of YAP and TEAD.22 As expected, verteporfin inhibited the expression of total YAP, phosphoYAP and TEAD in a dose‐dependent manner (Fig. 3c). The results indicate that verteporfin may induce cell G1 phase arrest and apoptosis via disrupting the YAP‐TEAD interaction.
Figure 3.
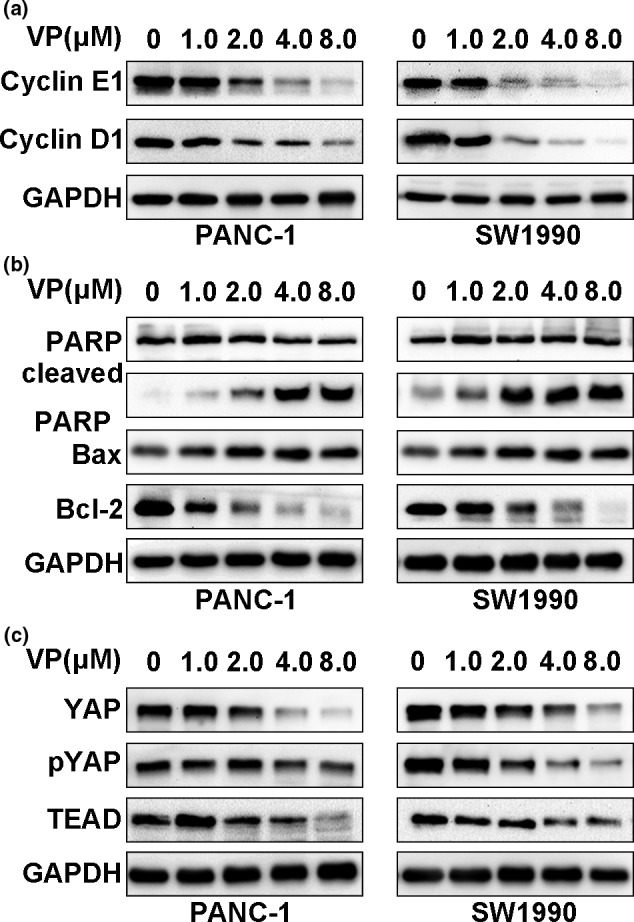
Verteporfin inhibits cell cycle progression and induces apoptosis via disrupting YAP‐TEAD interaction. PDAC cells were treated with verteporfin at various concentrations for 24 h, and then subjected to Western blotting for detecting the expression of cyclinD1 and cyclinE1 (a), the expression of PARP, cleaved PARP, Bax and Bcl‐2 (b), and the expression of proteins in the Hippo pathway, YAP, pYAP and TEAD (c).
Verteporfin suppresses tumor growth in PDAC xenograft model
In subsequent study, we further observed the effect of verteporfin on the PDAC xenograft model. Tumors from verteporfin‐treated mice were significantly smaller than those from vehicle‐treated controls (Fig. 4a,b). The weights of tumors harvested from mice at the end of experiments showed a similar trend to the volume of tumors (Fig. 4c). Immunohistochemical analysis of tumor sections showed that verteporfin decreased the expression of YAP, cyclinD1 and cyclinE1 (Fig. 4d). Fewer Ki‐67 positive‐cells were observed in tumors treated with verteporfin compared with control tumors. TUNEL assay showed increased apoptosis in verteporfin‐treated tumors (Fig. 4e).
Figure 4.
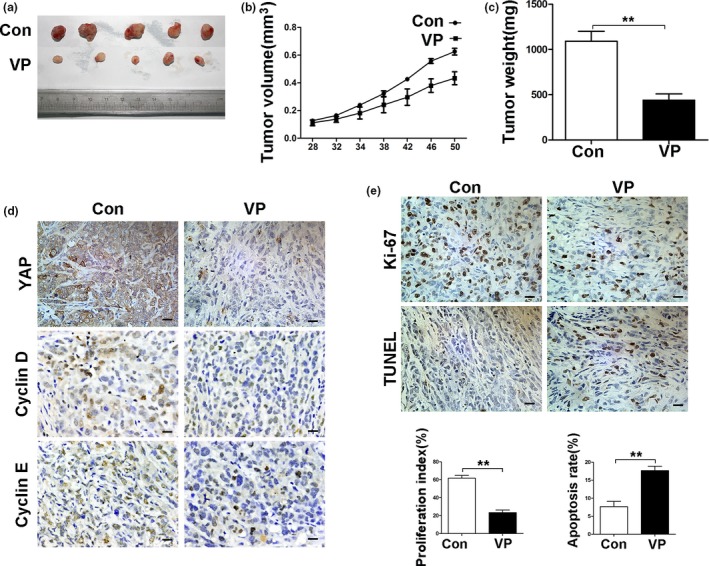
Verteporfin suppresses tumor growth in the PDAC xenograft model. The treatment schedule was shown in Materials and Methods. (a) Tumors were measured and tumor volume calculated at indicated time points. At the end of the experiment, tumors were excised and photographed (b), and weighed (c). (d) Tumoral expression of YAP, cyclinD1 and cyclinE1 was detected by IHC. (e) Tumor sections were stained with an anti‐Ki67 antibody and TUNEL (Magnification× 400, bar = 25 μM). Proliferation and apoptosis indexes were quantified. Data were presented as mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001.
YAP transcriptionally regulates Ang2 expression to enhance angiogenesis
Ang2 is the critical marker for vasculogenic events, and we have reported that Ang2 contributed to neo‐angiogenesis.27 In addition, YAP plays an important role in angiogenic sprouting and remodeling of HUVECs, and the expression of Ang2 correlates to YAP expression.32 Here, we showed that depletion of YAP inhibited, while overexpression of YAP increased, Ang2 expression (Fig. 5a,b). Accordingly, depletion of YAP inhibited, while overexpression of YAP promoted, the migration of HUVECs (Fig. 5c,d). Depletion of YAP impaired, while overexpression of YAP, promote the capillary tube formation ability of HUVECs (Fig. 5e,f).
Figure 5.
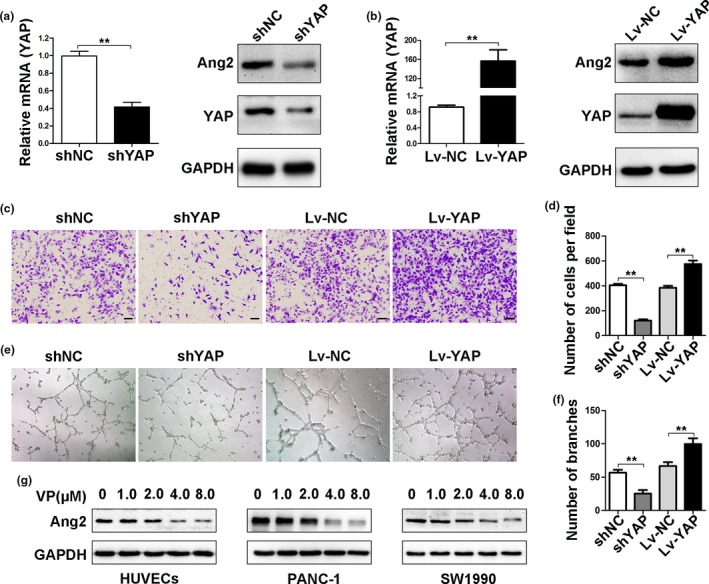
YAP regulates Ang2 expression to enhance angiogenesis. (a) Human umbilical vein endothelial cells (HUVECs) were transfected with control or YAPshRNAs. YAP mRNA was detected by qRT‐PCR, and YAP and Ang2 proteins by Western blot analysis. (b) HUVECs were transfected with control or YAP ectopic expression vectors. YAP mRNA was determined by qRT‐PCR, and YAP and Ang2 proteins by Western blot analysis. (c–f) HUVECs were transfected with control or YAPshRNAs, or control or YAP ectopic expression vectors. (c) cell migration was analyzed by transwell assays(Magnification ×100, bar = 100 μM), and (e, angiogenesis by tube formation assays (Magnification ×40). Cell migration (d) and tube formation (f) were quantified. (g) The expression of Ang2 was detected by Western blot analysis in HUVECs and PDAC cells treated with verteporfin at various concentrations. Data were presented as mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001.
Verteporfin inhibits angiogenesis by downregulating Ang2 expression
Western blot analysis showed that verteporfin suppressed Ang2 expression in a dose‐dependent manner (Fig. 5g). Verteporfin inhibited the migration and tube formation of HUVECs (Fig. 6a,b). In a CAM angiogenesis model, verteporfin exhibited an anti‐angiogenic activity as well (Fig. 6c). In the xenograft model, verteporfin‐treated tumors expressed less Ang2 (Fig. 6d) and CD31 (Fig. 6e) than control tumors as detected by IHC (Fig. 6d).
Figure 6.
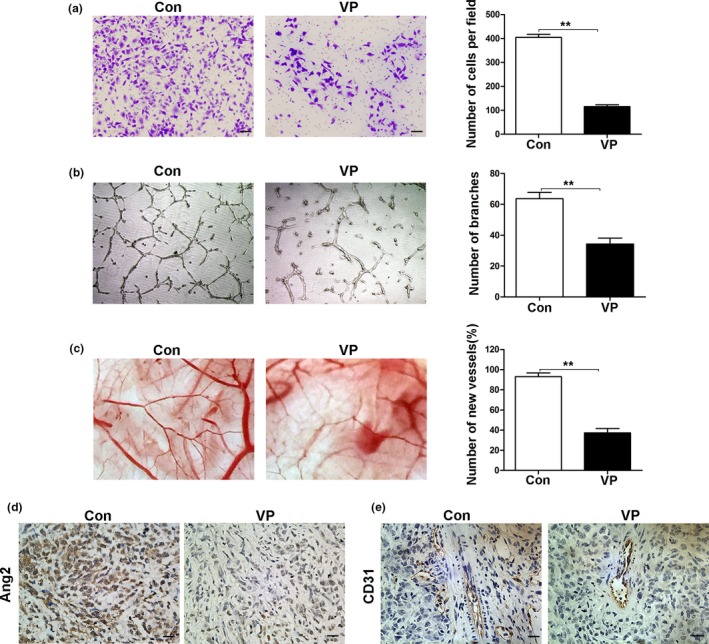
Verteporfin inhibits angiogenesis by downregulating Ang2 expression. (a–c) Human umbilical vein endothelial cells (HUVECs) were pre‐treated with verteporfin at 4 μM concentration for 24 h. Cell migration was analyzed by transwell assays (Magnification ×100, bar = 100 μM) (a), and angiogenesis by tube formation assays (Magnification ×40) (b) and by chorioallantoic membrane assays (c). (d, e) The expression of Ang2 and CD31 was detected by IHC (Magnification ×400, bar = 25 μM). Data were presented as mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001.
Verteporfin suppresses the migration of PDAC cells and formation of VM via downregulating MMP2,VE‐cadherin and α‐SMA expression
The formation of VM by tumor cells contributes to tumor rapid progression, drug resistance and metastasis.33 Verteporfin treatment inhibited the migration ability of PDAC cells as examined by transwell assays (Fig. 7a). PANC‐1 and SW1990 cells were able to form tube‐like structures, which was disrupted by verteporfin treatment (Fig. 7b). Verteporfin treatment inhibited the expression of MMP2, VE‐cadherin and α‐SMA in a dose‐dependent manner (Fig. 7c), three markers for VM.34 To quantify VM, we double stained tumor sections with antibodies against CD31 and PAS. CD31 is a well‐known marker for endothelial cells, while PAS is used to detect basement membrane structures and VM networks in tumors.17 Verteporfin treatment resulted in less detectable VM (branching networks of PAS+ structures and lacking CD31 staining) compared to the controls (Fig. 7d). Verteporfin‐treated tumors had lower expression levels of MMP2, VE‐cadherin and α‐SMA than control tumors as detected by IHC (Fig. 7e). Similar results were observed in the SW1990 xenograft model (Fig. S3).
Figure 7.
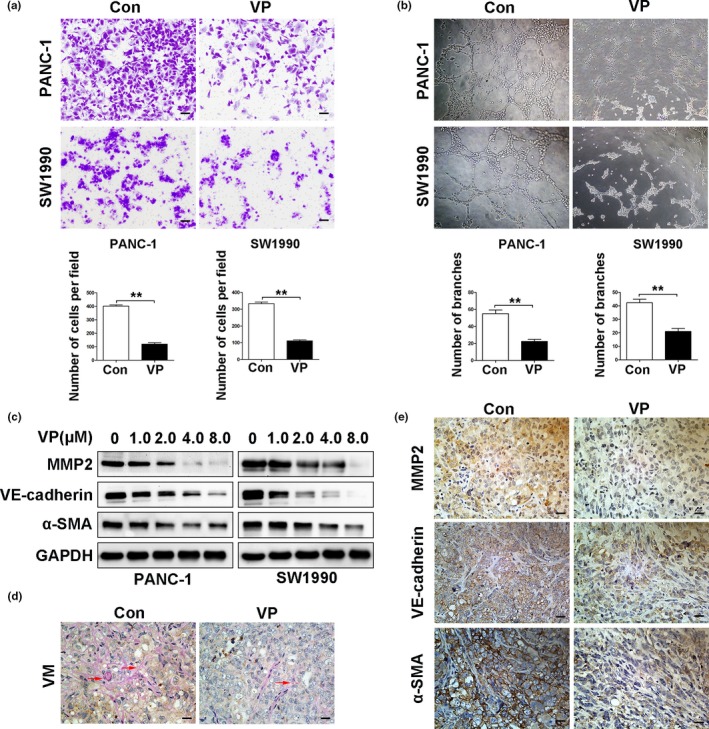
Verteporfin suppresses vasculogenic mimicry in vitro and in vivo. PANC‐1 and SW1990cells were pre‐treated with verteporfin at 4 μM concentration for 24 h. (a) Cell migration was analyzed by transwell assays (Magnification ×100, bar = 100 μM), and (b) VM by tube formation assays (Magnification ×40). The above results were quantified. (c) The expression of MMP2, VE‐cadherin and α‐SMA was detected by Western blot analysis in PANC‐1 and SW1990 cells treated with verteporfin at various concentrations. (d) VM formed in tumors harvested from Figure 4 was detected by staining tumor sections with anti‐CD31 and anti‐PAS antibodies (red arrow, CD31‐negative and PAS positive). (e) The expression of MMP2, VE‐cadherin and α‐SMAin tumors harvested from Figure 4 was detected by IHC (Magnification ×400, bar = 25 μM). Data were presented as mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
Yes‐associated protein‐1 is highly expressed in various malignant tumors including pancreatic cancer, and its activation is an independent predictor of the survival of cancer patients.8, 35, 36 Although initially regarded as a photo‐sensitizer in photodynamic therapy, verteporfin has recently been reported to exhibit anti‐tumor activities by disrupting YAP‐TEAD interaction.22 In accordance with previous reports that verteporfin inhibited the growth of cells from esophageal cancer24 and lung cancer,25 our present results demonstrated that verteporfin also suppressed the growth of pancreatic cancer cells and induced apoptosis in vitro and in vivo. Verteporfin treatment led to downregulation of cyclinD1 and cyclinE1, modulation of Bcl‐2 family proteins, and activation of PARP. Verteporfin had also displayed a profound effect on angiogenesis through suppressing Ang2 expression. Verteporfin suppressed the migration of PDAC cells and the formation of VM by downregulating MMP2, VE‐cadherin and α‐SMA (Fig. 8). The encouraging results warrant a further investigation of verteporfin as an anti‐tumor agent in PDAC.
Figure 8.
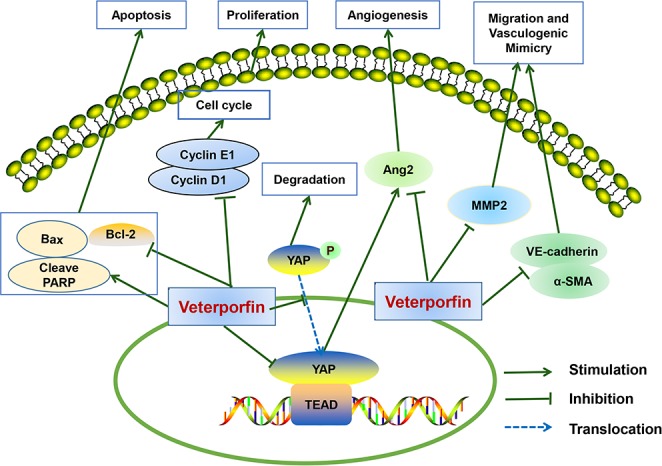
Schematic of a hypothetical mechanism for the anti‐tumor effects of verteporfin in pancreatic ductal adenocarcinoma (PDAC) cells. Verteporfin suppresses cell survival, migration, angiogenesis and vasculogenic mimicry of pancreatic cancer via disrupting the YAP‐TEAD interaction. Verteporfin inhibits cell cycle progression by suppressing cyclinD1 and cyclinE1 expression. Verteporfin induces cell apoptosis by activating the intrinsic apoptosis pathway. Verteporfin also inhibits Ang2, MMP2, VE‐cadherin and α‐SMA expression to suppress angiogenesis and vasculogenic mimicry.
Recent studies suggest that the Hippo‐YAP pathway is implicated in cell proliferation, apoptosis, chemo‐resistance and angiogenesis.6, 37 YAP regulates the transcription of cyclinD1 and FOXM1, which are important factors for G1 progression and G1/S transition.31 Hyperactivated YAP also regulates the insensitivity to apoptosis of cancer cells.37 Activated YAP resulted in gemcitabine resistance in pancreatic cancer.38 Thus, YAP has become a promising target for seeking novel cancer therapeutics. It has been reported that verteporfin induced the sequestration of YAP in the cytoplasm by increasing 14‐3‐3σ expression, thereby inhibiting YAP activation.39 Verteporfin interfered with the YAP‐TEAD pathway, resulting in downregulation of the downstream proto‐oncogenes, such as c‐myc and Axl, thus inducing growth inhibition, apoptosis, and G0/G1‐phase cell cycle arrest of human retinoblastoma cells.23 In the present study, we showed that verteporfin inhibited the proliferation and induced apoptosis of PDAC cells by regulating cell cycle‐ and apoptosis‐related proteins via suppressing YAP‐TEAD interaction.
Hippo signaling is also involved in tumor progression, metastasis, and angiogenesis.6 In cholangiocarcinoma, hyperactivated YAP enhanced the expression of pro‐angiogenic MFAP5.10 The YAP‐TEAD complex regulates the expression of genes participating in angiogenesis and cell migration, such as CTGF, CYR61and VEGF‐A, which could be inhibited by verteporfin.23 Ang2 is one member of the angiopoietin family which induces destabilization, disassociation from the endothelial cell basement membrane, migration and sprouting of endothelium cells, thus regulating tumor angiogenesis and progression.40 High expression of Ang2 is associated with poor prognosis of patients with breast cancer,41 colorectal carcinoma42 and melanoma.43 The role of Ang2 in pancreatic cancer progression has also been reported, and silencing Ang2 exerted an anti‐angiogenesis effect.44 Ang2 stimulated the migration and invasion, and induced metastasis of pancreatic cancer cells through MMP2‐mediated pathway.45 Our previous study has shown that inhibition of Ang2 by T7 peptide, an active fragment of full‐length Tumstatin, inhibited angiogenesis and exerted its antitumor effects.27 It has been recently reported that YAP could regulate vascular remodeling and angiogenesis through transcriptionally targeting Ang2.32 Our present results have shown that verteporfin could decrease Ang2 expression to regulate angiogenesis through disrupting YAP and TEAD interaction.
Vasculogenic mimicry is an alternative type of blood supplement independent of endothelial vessels, formed by the aggressive metastatic tumor cells.15, 33 VM is shown to enhance tumor growth, chemoresistance and metastasis. Vascular signaling pathway, embryonic/stem cell pathways, and hypoxia/hypoxia‐reoxygenation signaling pathways are implicated in tumor cell VM, which enhances a high degree of plasticity phenotype and promotes embryonic stem cells markers expression.15, 46 It has been reported that YAP plays an important role in regulating stem cells in epithelial tissues,47 but it is unknown whether YAP participates in VM. MMP2, VE‐cadherin and α‐SMA are key markers for VM.34 In the present study, we found that verteporfin suppressed VM via downregulating MMP2, VE‐cadherin and α‐SMA expression. The dual effects of verteporfin on tumor angiogenesis and VM may synergize to improve the efficacy of its anti‐tumor activities against PDAC.
In summary, verteporfin is an FDA‐approved photosensitizer for treating age‐related macular degeneration and exhibits limited toxicity,21 and could inhibit tumorigenesis via disrupting YAP‐TEAD complexes.48 Our present results have demonstrated that verteporfin suppressed the growth of pancreatic cancer cells by inducing G1 phase arrest and apoptosis in vitro and in vivo. The mechanisms accounting for its activities include downregulation of cyclinD1 and cyclinE1, modulation of Bcl‐2 family proteins and activation of PARP. Verteporfin has also displayed an inhibitory effect on angiogenesis by suppressing Ang2 expression, and the migration of PDAC cells and VM via downregulating MMP2, VE‐cadherin and α‐SMA expression. The encouraging results warrant a future investigation of verteporfin as a novel therapeutic in the treatment of pancreatic cancer.
Conflict of Interest
We have no conflict of interest.
Supporting information
Fig. S1. Verteporfin inhibits the viability and induces the apoptosis of HPDE6 and HUVECs.
Fig. S2. Verteporfin suppresses necrosis‐associated protein expression in PDAC cells.
Fig. S3. Verteporfin suppresses vasculogenic mimicry in SW1990 xenografts.
Appendix S1. Materials and methods.
Acknowledgments
This work was supported by the National Major Research and Development Program of China (2016YFC0106004), Shandong Provincial Science and Technology Development Planning, China (2015GSF121040) and Shandong Provincial Natural Science Foundation, China (ZR2012HL05 and ZR2015HL080).
Cancer Sci 108 (2017) 478–487
Funding Information
National Major Research and Development Program of China (2016YFC0106004), Shandong Provincial Science and Technology Development Planning, China (2015GSF121040) and Shandong Provincial Natural Science Foundation, China (ZR2012HL05 and ZR2015HL080).
References
- 1. Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014; 371: 1039–49. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66: 7–30. [DOI] [PubMed] [Google Scholar]
- 3. Faris JE, Blaszkowsky LS, McDermott S et al FOLFIRINOX in locally advanced pancreatic cancer: the Massachusetts General Hospital Cancer Center experience. Oncologist 2013; 18: 543–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Von Hoff DD, Ervin T, Arena FP et al Increased survival in pancreatic cancer with nab‐paclitaxel plus gemcitabine. N Engl J Med 2013; 369: 1691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self‐renewal. Nat Cell Biol 2011; 13: 877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sebio A, Lenz HJ. Molecular pathways: Hippo signaling, a critical tumor suppressor. Clin Cancer Res 2015; 21: 5002–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kang W, Tong JH, Chan AW et al Yes‐associated protein 1 exhibits oncogenic property in gastric cancer and its nuclear accumulation associates with poor prognosis. Clin Cancer Res 2011; 17: 2130–9. [DOI] [PubMed] [Google Scholar]
- 8. Sohn BH, Shim JJ, Kim SB et al Inactivation of Hippo pathway is significantly associated with poor prognosis in hepatocellular carcinoma. Clin Cancer Res 2016; 22: 1256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diep CH, Zucker KM, Hostetter G et al Down‐regulation of yes associated protein 1 expression reduces cell proliferation and clonogenicity of pancreatic cancer cells. PLoS One 2012; 7: e32783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang S, Zhang L, Purohit V et al Active YAP promotes pancreatic cancer cell motility, invasion and tumorigenesis in a mitotic phosphorylation‐dependent manner through LPAR3. Oncotarget 2015; 6: 36019–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morvaridi S, Dhall D, Greene MI et al Role of YAP and TAZ in pancreatic ductal adenocarcinoma and in stellate cells associated with cancer and chronic pancreatitis. Sci Rep 2015; 5: 16759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marti P, Stein C, Blumer T et al YAP promotes proliferation, chemoresistance, and angiogenesis in human cholangiocarcinoma through TEAD transcription factors. Hepatology 2015; 62: 1497–510. [DOI] [PubMed] [Google Scholar]
- 13. Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell 2011; 146: 873–87. [DOI] [PubMed] [Google Scholar]
- 14. Maniotis AJ, Folberg R, Hess A et al Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol 1999; 155: 739–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kirschmann DA, Seftor EA, Hardy KM et al Molecular pathways: vasculogenic mimicry in tumor cells: diagnostic and therapeutic implications. Clin Cancer Res 2012; 18: 2726–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu WB, Xu GL, Jia WD et al Prognostic significance and mechanisms of patterned matrix vasculogenic mimicry in hepatocellular carcinoma. Med Oncol 2011; 28(Suppl 1): S228–38. [DOI] [PubMed] [Google Scholar]
- 17. Tan LY, Mintoff C, Johan MZ et al Desmoglein 2 promotes vasculogenic mimicry in melanoma and is associated with poor clinical outcome. Oncotarget 2016; 7: 46492–508. doi:10.18632/oncotarget.10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guo JQ, Zheng QH, Chen H et al Ginsenoside Rg3 inhibition of vasculogenic mimicry in pancreatic cancer through downregulation of VE‐cadherin/EphA2/MMP9/MMP2 expression. Int J Oncol 2014; 45: 1065–72. [DOI] [PubMed] [Google Scholar]
- 19. Cao Z, Bao M, Miele L et al Tumour vasculogenic mimicry is associated with poor prognosis of human cancer patients: a systemic review and meta‐analysis. Eur J Cancer 2013; 49: 3914–23. [DOI] [PubMed] [Google Scholar]
- 20. Lin SZ, Wei WT, Chen H et al Antitumor activity of emodin against pancreatic cancer depends on its dual role: promotion of apoptosis and suppression of angiogenesis. PLoS One 2012; 7: e42146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Henney JE. From the Food and Drug Administration. JAMA 2000; 283: 2779. [PubMed] [Google Scholar]
- 22. Liu‐Chittenden Y, Huang B, Shim JS et al Genetic and pharmacological disruption of the TEAD‐YAP complex suppresses the oncogenic activity of YAP. Genes Dev 2012; 26: 1300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brodowska K, Al‐Moujahed A, Marmalidou A et al The clinically used photosensitizer verteporfin (VP) inhibits YAP‐TEAD and human retinoblastoma cell growth in vitro without light activation. Exp Eye Res 2014; 124: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Song S, Honjo S, Jin J et al The Hippo coactivator YAP1 mediates EGFR overexpression and confers chemoresistance in esophageal cancer. Clin Cancer Res 2015; 21: 2580–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cheng H, Zhang Z, Rodriguez‐Barrueco R et al Functional genomics screen identifies YAP1 as a key determinant to enhance treatment sensitivity in lung cancer cells. Oncotarget 2016; 7: 28976–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huggett MT, Jermyn M, Gillams A et al Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. Br J Cancer 2014; 110: 1698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang F, Dong X, Xiu P et al T7 peptide inhibits angiogenesis via downregulation of angiopoietin‐2 and autophagy. Oncol Rep 2015; 33: 675–84. [DOI] [PubMed] [Google Scholar]
- 28. Li X, Kong X, Wang Y et al 53BP1 is a novel regulator of angiogenesis in breast cancer. Cancer Sci 2013; 104: 1420–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xiu P, Dong X, Dong X et al Secretory clusterin contributes to oxaliplatin resistance by activating Akt pathway in hepatocellular carcinoma. Cancer Sci 2013; 104: 375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. He C, Dong X, Zhai B et al MiR‐21 mediates sorafenib resistance of hepatocellular carcinoma cells by inhibiting autophagy via the PTEN/Akt pathway. Oncotarget 2015; 6: 28867–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mizuno T, Murakami H, Fujii M et al YAP induces malignant mesothelioma cell proliferation by upregulating transcription of cell cycle‐promoting genes. Oncogene 2012; 31: 5117–22. [DOI] [PubMed] [Google Scholar]
- 32. Choi HJ, Zhang H, Park H et al Yes‐associated protein regulates endothelial cell contact‐mediated expression of angiopoietin‐2. Nat Commun 2015; 6: 6943. [DOI] [PubMed] [Google Scholar]
- 33. Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med 2011; 17: 1359–70. [DOI] [PubMed] [Google Scholar]
- 34. Zhang C, Chen W, Zhang X et al Galunisertib inhibits glioma vasculogenic mimicry formation induced by astrocytes. Sci Rep 2016; 6: 23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang Y, Dong Q, Zhang Q et al Overexpression of yes‐associated protein contributes to progression and poor prognosis of non‐small‐cell lung cancer. Cancer Sci 2010; 101: 1279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pei T, Li Y, Wang J et al YAP is a critical oncogene in human cholangiocarcinoma. Oncotarget 2015; 6: 17206–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhao Y, Yang X. The Hippo pathway in chemotherapeutic drug resistance. Int J Cancer 2015; 137: 2767–73. [DOI] [PubMed] [Google Scholar]
- 38. Chen M, Wang M, Xu S et al Upregulation of miR‐181c contributes to chemoresistance in pancreatic cancer by inactivating the Hippo signaling pathway. Oncotarget 2015; 6: 44466–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang C, Zhu X, Feng W et al Verteporfin inhibits YAP function through up‐regulating 14‐3‐3Sigma sequestering YAP in the cytoplasm. Am J Cancer Res 2016; 6: 27–37. [PMC free article] [PubMed] [Google Scholar]
- 40. Vasudev NS, Reynolds AR. Anti‐angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis 2014; 17: 471–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Imanishi Y, Hu B, Jarzynka MJ et al Angiopoietin‐2 stimulates breast cancer metastasis through the alpha(5)beta(1) integrin‐mediated pathway. Cancer Res 2007; 67: 4254–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goede V, Coutelle O, Neuneier J et al Identification of serum angiopoietin‐2 as a biomarker for clinical outcome of colorectal cancer patients treated with bevacizumab‐containing therapy. Br J Cancer 2010; 103: 1407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Holopainen T, Saharinen P, D'Amico G et al Effects of angiopoietin‐2‐blocking antibody on endothelial cell‐cell junctions and lung metastasis. J Natl Cancer Inst 2012; 104: 461–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhou J, Zhang ZX, Zhao H et al Anti‐angiogenesis by lentivirus‐mediated small interfering RNA silencing of angiopoietin‐2 gene in pancreatic carcinoma. Technol Cancer Res Treat 2011; 10: 361–9. [DOI] [PubMed] [Google Scholar]
- 45. Zhang ZX, Zhou J, Zhang Y et al Knockdown of angiopoietin‐2 suppresses metastasis in human pancreatic carcinoma by reduced matrix metalloproteinase‐2. Mol Biotechnol 2013; 53: 336–44. [DOI] [PubMed] [Google Scholar]
- 46. Seftor RE, Hess AR, Seftor EA et al Tumor cell vasculogenic mimicry: from controversy to therapeutic promise. Am J Pathol 2012; 181: 1115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev 2016; 30: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Perra A, Kowalik MA, Ghiso E et al YAP activation is an early event and a potential therapeutic target in liver cancer development. J Hepatol 2014; 61: 1088–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Verteporfin inhibits the viability and induces the apoptosis of HPDE6 and HUVECs.
Fig. S2. Verteporfin suppresses necrosis‐associated protein expression in PDAC cells.
Fig. S3. Verteporfin suppresses vasculogenic mimicry in SW1990 xenografts.
Appendix S1. Materials and methods.


