Abstract
Objective
To determine the efficacy of anakinra (recombinant interleukin-1 receptor antagonist) in improving 28-day survival in sepsis patients with features of macrophage activation syndrome (MAS). Despite equivocal results in sepsis trials, anakinra is effective in treating MAS, a similar entity with fever, disseminated intravascular coagulation (DIC), hepatobiliary dysfunction (HBD), cytopenias, and hyperferritinemia. Hence, sepsis patients with MAS features may benefit from IL-1 receptor blockade.
Design
Re-analysis of de-identified data from the phase III randomized interleukin-1 receptor antagonist trial in severe sepsis (Opal, et. al. Crit Care Med. 1997 Jul;25(7):1115–24).
Setting
Multi-center study recruiting through 91 centers from 11 countries in Europe and North America.
Participants
Sepsis patients with MODS and/or shock (original study) were re-grouped based on presence or absence of concurrent HBD and DIC as features of MAS (HBD/DIC group). The “non-HBD/DIC” group included patients with only HBD, only DIC or neither.
Intervention
Treatment with anakinra or placebo.
Main Outcome(s) and Measure(s)
28-day mortality.
Statistical analysis
descriptive statistics, chi-square, ANOVA, logistic and Cox regression.
Results
Data were available for 763 adults from the original study cohort, randomized to receive either anakinra or placebo. Concurrent HBD/DIC was noted in 43 patients (5.6% of total, ages 18–75; 47% women). The 28-day survival was similar in both anakinra and placebo-treated non-HBD/DIC patients (71.4% vs. 70.8%, p=.88). Treatment with anakinra was associated with significant improvement in the 28-day survival rate in HBD/DIC patients (65.4% anakinra vs. 35.3% placebo), with HR for death 0.28 (0.11–0.71, p = 0.0071) for the treatment group in Cox regression.
Conclusions and Relevance
In this subgroup analysis, IL-1 receptor blockade was associated with significant improvement in survival of patients with sepsis and concurrent HBD/DIC. A prospective randomized trial using features of MAS for mortality risk stratification should be undertaken to confirm the role of IL-1 blockage.
Keywords: “Macrophage Activation Syndrome”, “multi-organ dysfunction syndrome”, “Sepsis mortality”, “hepatobiliary dysfunction”, “disseminated intravascular coagulation”, anakinra, “IL-1 receptor antagonist”
INTRODUCTION
“Sepsis” constitutes various degrees of systemic inflammation in the context of microbial infection (1), with production of pro-inflammatory cytokines including interleukin-1 (IL-1), IL-6, IL-18, and tumor necrosis factor (TNF) (2,3). These cytokines may contribute to development of sepsis induced “multi-organ dysfunction syndrome (MODS)” and/or “hypo-perfusion”, identified as “severe sepsis” and “septic shock”, respectively, based on “SCCM/ESIM/ACCP/ATS/SIS International Sepsis Definitions Conference” guidelines (1).
Following the discovery of IL-1 as “human leukocytic pyrogen” in1977 (4), IL-1 receptor antagonist (IL-1Ra) was purified in 1981 (5) and was demonstrated to have an important role in regulating systemic inflammatory responses (3–6). IL-1Ra (3,7) is a 25 kilo-Dalton (KD) naturally occurring plasma protein that binds to the type 1 IL-1 receptor, inhibiting both IL-1α and IL-1β signal transduction (7). Anakinra is a 17 KD recombinant, non-glycosylated human IL-1Ra. Prior to its approval to treat other inflammatory diseases (3), anakinra was investigated in a series of clinical trials for treatment of patients with sepsis and severe sepsis (9–12). An initial phase I study was conducted with intravenous doses as high as 10 mg/kg/hr for 72 hours (9), which established the safety and tolerability of anakinra at high doses. A subsequent phase II open label clinical trial demonstrated dose related efficacy in sepsis syndrome and septic shock without treatment related adverse outcomes (10). In an ensuing phase III randomized double blind trial comparing 1 or 2 mg/kg/hr with placebo, anakinra failed to meet the primary endpoint of improved 28-day survival (11), but did improve survival in patients with MODS, a predicted mortality probability of >24% at study entry, or septic shock (11). Based upon these results, a subsequent confirmatory Phase III trial was undertaken in patients with severe sepsis or septic shock (12). This latter study was discontinued following an interim analysis, in which no survival benefits could be demonstrated (12). This, and the other clinical trials of anakinra in sepsis, did not reveal either increased mortality rate or serious/non-serious adverse reactions in anakinra over placebo groups (9–12).
In contrast to its effects in sepsis, anakinra, either alone or in combination with other treatments, has been reported to be effective in the management of macrophage activation syndrome (MAS), previously termed secondary or reactive hemophagocytic lymphohistiocytosis (HLH) (13). Clinically, MAS presents as a fulminant cytokine storm and often fatal cause of MODS (13–17), associated with pancytopenia, tissue hemophagocytosis, liver dysfunction, coagulopathy, and/or central nervous system dysfunction (18–22), occurring as a complication of infection, malignancies, and autoimmune disorders (13,15). The excessive inflammatory response in MAS is reflected in the amplified transcription of ferritin, and tissue-bound and soluble CD163 (23–25), both of which have also been demonstrated in severe sepsis (26–31).
Given the clinical and cytokine similarities of MAS and severe sepsis, together with the observed favorable response of MAS to anakinra, the possibility that severe sepsis patients with features of MAS may respond to IL-1 receptor blockade merits consideration. This study revisits the data from the confirmatory phase III randomized clinical trial of IL-1Ra (anakinra) in severe sepsis (12), re-analyzing the data to investigate the response to anakinra in patients with features of MAS [hepatobiliary dysfunction (HBD) and disseminated intravascular coagulation (DIC)] (18–22).
PATIENTS AND METHODS
Data Source
The data from the previously published original confirmatory phase III placebo-controlled trial of interleukin-1 receptor antagonist in severe sepsis (12) was reanalyzed. In this trial 906 patients with severe sepsis and septic shock were recruited from 91 centers in 11 countries across Europe and North America, randomly assigned to either treatment or placebo (1:1 ratio). The primary end point was defined as 28-day survival; end organ dysfunction and/or failure were determined by consensus of a steering committee according to the guidelines in the antecedent phase III clinical trial (11) with additional modifications (12). The original study was stopped at the second interim analysis for futility. At the time of study termination, 58 patients had completed the three-day infusion (drug or placebo) and were in various stages of 28-day follow up. A group of 143 patients were in various stages of screening and infusion, without completing the full infusion course. This group was dropped from the study and their data were not available for the current analysis.
Regulatory Process
Each participating center obtained Institutional Review Board (IRB) approval, and all subjects gave written informed consent to participate in the original trial. The data set included demographic, clinical, and outcome data for subjects in the study, each identified by a study specific number. None of the personal identifiers were disclosed in the data set that was used for this study.
Variables
The available data for each subject used for this study included: age, gender, presence or absence of shock, DIC, HBD, or acute kidney injury (AKI), survival at day 28, predicted mortality, and treatment. Definitions of DIC and organ dysfunction [HBD, shock, acute respiratory distress syndrome (ARDS), AKI] were as described previously in the original open-label trial (11) (Table 1). The definitions of shock and DIC were amended in the confirmatory phase III clinical trial (12). Twenty-eight-day survival was the primary end point of this study. The Acute Physiology and Chronic Health Evaluation (APACHE) II score was used to calculate predicted death, which strongly correlated with APACHE II(11,12). The APACHE II score and the specifics of infecting organisms were not available as part of the dataset. All of the subjects with missing data for treatment, 28-day outcome or both DIC and HBD domains, were excluded, as these were the main variables of interest in this study.
Table 1.
Variable definitions used in the original study by Opal et al (12)
| Variable | Definition |
|---|---|
| Shock | Either of the following:
|
| DIC |
Both criteria: No anti-coagulation:
|
| AKI | Either of the following
|
| HBD | At least two of the following:
|
| ARDS | According to criteria of Murray, et al (47) |
DIC: disseminated intravascular coagulation
PT: prothrombin time
PTT: partial thromboplastin time
HBD: hepatobiliary dysfunction
ARDS: acute respiratory distress syndrome
AKI: acute kidney injury
AST: aspartate aminotransferase
ALT: alanine aminotransferase
“Disease” and “non-Disease” Groups
In keeping with the objective of the study, features of MAS were defined as the presence of both HBD and DIC in this severe sepsis cohort. This choice of definition was guided by the clinical characteristics of MAS as determined in major studies (18–22), and each patient was classified into one of the two main groups of interest:
HBD/DIC group: patients whose clinical presentation met the definitions of both HBD and DIC and
Non-HBD/DIC group: patients who did not have both HBD and DIC, and included patients with only HBD, only DIC, or neither.
Treatment vs. Placebo
The original study was a randomized, placebo controlled study. Patients in each group had therefore been randomly assigned at a 1:1 ratio to receive either placebo or anakinra, administered intravenously at 2.0 mg/kg/hr for 72 hours continuously.
Statistical Analysis
Statistical analysis was performed to compare demographic, clinical, and outcome variables between 4 subgroups comprised of HBD/DIC and non-HBD/DIC patients who received anakinra or placebo. Descriptive statistics was performed for the variables, using mean and standard deviation for continuous variables (age, predicted death), or proportion and percent for binomial variables. Comparative statistics included t tests for continuous variables, as well as Fisher Exact test and Chi Square test for binomial variables. Kaplan-Meier survival estimates were also compared between the drug and control groups who were HBD/DIC-positive, negative, or equivocal. The possibility of post-randomization imbalances was investigated by comparing pre-treatment variables in drug vs. control groups, using between-group t test or chi-square. Drug effects were examined while controlling for potential confounders using logistic regression, as well as Cox proportional hazards models. A logistic regression model predicting 28-day survival was examined in the HBD/DIC subgroup to determine the association of drug with outcome, after controlling for potentially confounding covariates. In order to test whether the drug effect differed significantly across patients who were HBD/DIC-positive, -negative, or –equivocal (defined as having either HBD+ or DIC+ but not both), we also tested a logistic regression model in the full sample using a drug × diagnosis interaction term in the model. To rule out the possibility that multi-colinearity might have affected our multivariate models, we tested for this by examining variance inflation (VIF) in weighted regression. SAS version 9.3 (Cary, NC) was used for all analyses. A nominal p value of <0.05 between groups was considered statistically significant.
RESULTS
Subject Group
De-identified data was available on 763 study patients who had completed the study. All of these study patients were included in the analysis. The necessary variables for the current subgroup investigation were available in all of the patients. Of these, 43 patients (46.6% women) demonstrated both HBD and DIC; 26 (60.4%) were treated with anakinra (17 placebo). The remaining 720 subjects (40.7% women) were similarly distributed in anakinra or placebo groups: 484 subjects (67.2%) on anakinra, 236 subjects receiving placebo (Table 2). Despite the planned 1:1 randomization of the patients in the original trial, the observed ratio of anakinra to placebo group in the studied patient cohort was 2:1. A similar distribution is observed in the HBD/DIC subgroup. This has been confirmed with the investigators of the study to be merely a chance finding, likely related to early termination of the study.
Table 2.
Characteristics and associations of patients dichotomized based on presence or absence of HBD/DIC
| All N = 763 | HBD/DIC N= 43 | Non- HBD/DIC N= 720 | p Value | |
|---|---|---|---|---|
| Mean Age (range) | 57.9 (17–95) | 52.3 (18–75) | 58.3 (17–95) | 0.025 |
| Female Sex (%) | 313 (41) | 20 (46.5) | 293 (40.7) | 0.523 |
| rIL-1Ra treatment (%) | 510 (66.8) | 26 (60.4) | 484 (67.2) | NA |
| Mean Survival Day | 21.4 | 16.5 | 21.7 | 0.0004 |
| Predicated Death (range) | 0.346 (0.015–0.986) | 0.553 (0.143–0.986) | 0.345 (0.015–0.95) | 0.0001 |
| Alive in Day 28 | 527 (69.1) | 23 (53.5) | 504 (70.0) | 0.0273 |
| Concurrent Dysfunctions (%) | ||||
| ARDS | 194 (25.4) | 9 (20.9) | 185 (25.7) | 0.590 |
| AKI | 232 (30.4) | 26 (60.5) | 206 (28.6) | 0.0001 |
| Shock | 613 (80.3) | 41 (95.3) | 572 (79.4) | 0.0089 |
HBD: hepatobiliary dysfunction
DIC: disseminated intravascular coagulation
APACHE: Acute Physiology and Chronic Health Evaluation
ARDS: acute respiratory distress syndrome
AKI: acute kidney injury
rIL-1Ra: anakinra (recombinant interleukin-1 receptor antagonist)
Statistically significant p value is set at 0.05.
Patient Characteristics in HBD/DIC and Non-HBD/DIC Groups
Clinical and outcomes characteristics of the patients are reported in the original study publication (12). The descriptive details of the subgroups reported herein are outlined in Table 2. In comparative analysis, the HBD/DIC group subjects were significantly younger than their non-HBD/DIC counterparts (p = 0.025) with significantly higher predicted mortality scores at study entry (p = 0.0001). The HBD/DIC patients were less likely to be alive at 28 days (53.5% vs. 70.0%, p = 0.027). Average survival duration in HBD/DIC patients was 16.5 days, significantly shorter than the 21.7 days in non-HBD/DIC patients (p = 0.0004). ARDS was noted in similar proportions in both groups, but AKI and shock were both more frequent among the HBD/DIC patients (p = 0.0001 for AKI, p = 0.0089 for shock).
Mortality and Treatment with Anakinra
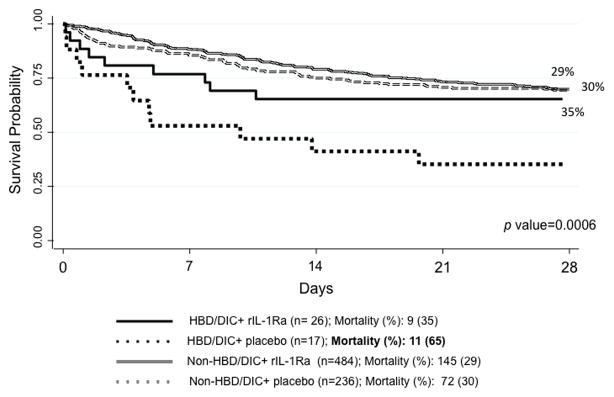
The outcome of death related to anakinra versus placebo treatment was determined over the course of the study observation period for HBD/DIC and non-HBD/DIC patients. As shown in Figure 1, 28-day mortality was equivalent for the non-HBD/DIC patients who received anakinra (29.7%) or placebo (30.5%, p = 0.999). However, the 28-day mortality in HBD/DIC patients who received anakinra was significantly lower (34.6%) than was noted for HBD/DIC patients who received placebo (64.7%, p = 0.0006), corresponding to a 47% reduction in mortality associated with anakinra.
Figure 1.
Treatment with IL-1Ra significantly improves 28-day survival in HBD/DIC sepsis, but does not change the outcome in non-HBD/DIC sepsis.
HBD: hepatobiliary dysfunction
DIC: disseminated intravascular coagulation
rIL-1Ra: anakinra (abbreviation for recombinant interleukin-1 receptor antagonist)
HBD/DIC group: patients with severe sepsis who demonstrate BOTH HBD and DIC features
Non-HBD/DIC group: patients with either HBD alone (no DIC), DIC alone (no HBD, or neither.
Statistically significant pP value is set at 0.05.
Confirmatory Analysis
Given the post hoc nature of this investigation, confirmatory statistical evaluations were performed to identify any post-randomization imbalances or confounders. We confirmed that the “predicted death probability score” was normally distributed with a mean 0.36 ± 0.21 and a median = 0.32, in the full sample. Probability of death was well balanced between treatment and placebo groups [mean “predicted death” of 0.358 ± 0.21 in the treated group (n = 510) and 0.354 ± 0.21 in the placebo group (n = 253; p value = 0.81)]. The risk of death between HBD/DIC patients who received anakinra treatment (means 0.57 ± 0.22) versus placebo group (0.53 ± 0.25) was also similar in t test comparison (p = 0.54). Other covariate values are shown for HBD/DIC-positive patients stratified by treatment in Table 3. None of these were statistically significant. Taken together, these findings indicate the treatment groups are balanced on risk of death and other potential confounding variables.
Table 3.
Values of pre-treatment variables in treatment vs. control groups in patients with HBD/DIC
| Variable | Treatment (n=26) | Control (n=17) | p value |
|---|---|---|---|
| Age | 49.6 ± 12.7 | 56.3 ± 19.4 | 0.18 |
| Female Sex | 12 (46.2%) | 8 (47.1%) | 0.95 |
| AKI | 17 (65.4%) | 9 (52.9%) | 0.41 |
| ARDS | 6 (23.1%) | 3 (17.7%) | 0.28 |
| Risk of death | 0.57 ± 0.22 | 0.53 ± 0.25 | 0.54 |
To further confirm the results, the patients were divided into three groups including HBD/DIC-positive (n = 43), HBD/DIC-negative (n = 470), who have neither HBD nor DIC, as well as 217 who have either HBD or DIC positive but not both (HBD/DIC-equivocal). There is a large significant difference in 28-day survival between these 3 groups, with HBD/DIC-positive patients having the lowest survival rate (54%), followed by HBD/DIC-equivocal patients (58%), and HBD/DIC-negative patients having the highest survival rate (77%; Chi-square = 32.1, p < 0.0001, phi = 0.21).
In univariate analysis (using Chi-square for categorical variables and t-test for age), age (p < 0.0001), AKI (p < 0.0001), and ARDS (p = 0.0066) are associated with 28-day mortality, while gender (p= 0.73), infectious-cause (p= 0.66), shock (p=.22), and sepsis-cause (p= 0.21) are not. Therefore, age, AKI, and ARDS were used as covariates in examining the association of drug with 28-day mortality in HBD/DIC patients.
In the logistic regression model in HBD/DIC-positive patients, the drug was associated with a reduced rate of death in HBD/DIC patients, after controlling for covariates, with an adjusted odds of 28-day mortality 87% lower than HBD/DIC patients on placebo [OR for death 0.13 (0.03–0.71), p = 0.018].
Also in the logistic regression model using all patients, the diagnosis × treatment interaction was significant (p = 0.025), indicating different drug effects on mortality, depending on HBD/DIC status. In HBD/DIC-equivocal patients, treatment with anakinra was associated with increased incidence of 28-day mortality compared to controls, but anakinra therapy was associated with reduced incidence of 28-mortality in HBD/DIC-positive patients, compared with “non-HBD/DIC” controls.
The multi-colinearity test found all variance inflation factor (VIF) less than 1.2 for all predictors, indicating no issue with multi-collinearity.
In the Cox regression analysis of HBD/DIC-positive patients only, treatment was significantly associated with time to death (p = 0.0071) with a HR of0.28 (0.11–0.71) for anakinra vs. placebo groups, after adjusting for age, AKI, ARDS, and death risk score. In Cox regression in the full sample, using 6 groups of HBD/DIC × Treatment [HBD/DIC-positive, -negative, or -equivocal by treatment (anakinra versus placebo)] with HBD/DIC-positive placebo treated as the reference group, the hazard ratio (HR) for the HBD/DIC-positive treatment group was 0.28 (0.12–0.68; p=0.0051) after adjusting for age, AKI, ARDS, and risk score. This indicates that the HBD/DIC-positive patients who received the drug had a significantly reduced likelihood of 28-day mortality, compared with HBD/DIC patients in the placebo group. KM Survival estimates for the 6 combinations of HBD/DIC and anakinra demonstrates the survival advantage for the HBD-positive/anakinra group over the HBD-positive/placebo group begins on Day 1 and becomes more apparent on Day 5.
DISCUSSION
The benefits of IL-1 receptor blockade in modifying the detrimental effects of the inflammatory process have been demonstrated since 1984 (3); yet, the appropriate target population among sepsis/MODS patients who would benefit from treatment with anakinra has remained elusive. The recent recognition of and development of treatments for MAS may be informative, as MAS and severe sepsis share many clinical, laboratory, and pathologic features, including elevated serum ferritin (28,29), cytopenias, hepatic dysfunction, coagulopathy (28,29), CNS dysfunction (30), tissue hemophagocytosis (31), and elevated macrophage expression of CD163+ cells throughout the reticuloendothelial system (27,32). These shared clinical/laboratory features of MAS and severe sepsis occur in the context of a “cytokine storm” in which IL-1 plays a major role (2,3,6,15).
Anakinra is a recombinant human IL-1 receptor antagonist with a short half-life of about 3–4 hours (7), wide therapeutic range (7), and high safety profile (7). The use of anakinra as a treatment option for MAS was initially reported in 2006 (33) with subsequent case reports/series reporting the benefits of IL-1 receptor blockade, mainly in children with MAS complicating systemic juvenile idiopathic arthritis and adults with Still disease (34–44). It is therefore plausible that a subset of severe sepsis patients develop MAS, and that the associated severe organ dysfunction is responsive to IL-1 blockade with anakinra.
In recent studies to develop validated case definitions for MAS, attributes of HBD (liver enzyme elevation) and DIC (hypofibrinogenemia, thrombocytopenia) are consistently among the most prevalent clinical features encountered in patients with presumed MAS (18,20). Both HBD and DIC were readily assessed in the original anakinra/sepsis phase III study data, permitting the use of the presence of HBD and DIC as a marker for possible MAS in the current analysis.
Mortality rates of adult MAS have been reported as up to 60% in various studies (21,22), comparable to the observed mortality rate noted in the HBD/DIC patients randomized to placebo. Treatment with anakinra reduced mortality in HBD/DIC patients to the mortality rate of non-HBD/DIC patients, but did not lead to a meaningful difference in survival in non-HBD/DIC patients compared to placebo. These findings suggest severe sepsis patients with HBD/DIC may represent a state of cytokine storm similar to MAS and high mortality risk, who may be the most appropriate patients to receive immune modulating and cytokine-targeted therapies. The identification of the appropriate sub-group of sepsis patients to target for potentially life-saving anti-cytokine targeted therapy provides a rationale for future prospective investigations to verify the utility of IL-1 blockade in sepsis associated with features of MAS.
Compared to activated protein C (45) as an intervention for sepsis, anakinra has no significant reported safety issues since introduction in the market in 2001 (46), with higher survival benefits in the selected population in this study and a lower NNT. While the subset analysis undertaken herein offers suboptimal power, these favorable findings merit future randomized studies to establish the benefits of anakinra in sepsis patients with features of MAS as well as MAS patients who have complicating sepsis.
CONCLUSION
The present findings support the possibility that anakinra treatment provides a survival benefit in septic patients with features of MAS. The safety and wide therapeutic margin of anakinra and the central role of IL-1 in the cytokine storm in severe sepsis patients merit reconsideration of this therapeutic as a potential treatment option in carefully selected groups of sepsis patients with attributes of MAS.
Footnotes
Reprints may be ordered.
Copyright form disclosures: Dr. Carcillo received support for article research from the National Institutes of Health (NIH). His institution received grant support (support from NIGMS for Dr. Carcillo’s research related to MODS and from NIGMS supports his research in MODS/MAS). Dr. Chatham’s institution received grant support from SOBI (pending grant for clinical trial re: anakinra in macrophage activation syndrome). Dr. Cron provided expert testimony for Wicker Smith O’Hara McCoy & Ford P.A. (peds rheum case expert evaluation). His institution received grant support from SOBI (clinical trial of anakinra for MAS). Dr. Dinarello served as a board member for Bio-Techne. Dr. Opal consulted for BioAegis (advice on phase 2 study in severe pneumonia), Battelle (advice on preclinical development on hemoperfusion columns for sepsis), Biocardis (advice on rapid blood steam genomic diagnostic methodologies), ImmuneXpress (advice on rapid diagnosis of sepsis based on host response gene response), Grifolds (Advice on protein C use in sepsis studies), Octopharma (advice on plasma component therapy in sepsis), and Becton Dickinson (advice on new therapies for multi-drug resistant bacterial pathogens). He received royalties from Elsevier Publishers (Royalties for a textbook in Infectious dieases) and received support from Archeogen and Ketotek (data and safety monitoring boards). His institution received grant support from Asahi Kasei (Clinical coordinating center for their phase 3 trial in sepsis) and Cardeas (Clinical coordinating center for their phase 2 trial in severe pneumonia), Arsanis (preclincal study of monclonal antibodies for blood stream infection). The remaining authors have disclosed that they do not have any potential conflicts of interest.
Contributor Information
B. Shakoory, George Washington University, Washington, DC
J.A. Carcillo, University of Pittsburgh Medical Center, Pittsburgh, PA
W. W. Chatham, University of Alabama at Birmingham, Birmingham, AL
R. L. Amdur, George Washington University, Washington, DC
H. Zhao, Temple University, Philadelphia, PA
C.A. Dinarello, University of Colorado Denver, Aurora, CO
R.Q. Cron, University of Alabama at Birmingham, Birmingham, AL
S.M. Opal, Brown University, Providence, RI
References
- 1.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 2.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 3.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2010;117(14):3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinarello CA, Renfer L, Wolff SM. Human leukocytic pyrogen: purification and development of a radioimmunoassay. Proc Natl Acad Sci USA. 1977;74:4624–4627. doi: 10.1073/pnas.74.10.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinarello CA, Rosenwasser LJ, Wolff SM. Demonstration of a circulating suppressor factor of thymocyte proliferation during endotoxin fever in humans. J Immunol. 1984;127:2517–2519. [PubMed] [Google Scholar]
- 6.Dinarello CA. Interleukin-1 and the pathogenesis of the acute-phase response. New England Journal Of Medicine. 1984;311(22):1413–1418. doi: 10.1056/NEJM198411293112205. [DOI] [PubMed] [Google Scholar]
- 7.Granowitz EV, Clark BD, Mancilla J, Dinarello CA. Interleukin-1 receptor antagonist competitively inhibits the binding of interleukin-1 to the type II interleukin-1 receptor. J Biol Chem. 1991;266(22):14147–50. [PubMed] [Google Scholar]
- 8.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11(8):633–52. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Granowitz EV, Porat R, Mier JW, et al. Pharmacokinetics, safety and immunomodulatory effects of human recombinant interleukin-1 receptor antagonist in healthy humans. Cytokine. 1992;4(5):353–60. doi: 10.1016/1043-4666(92)90078-6. [DOI] [PubMed] [Google Scholar]
- 10.Fisher CJ, Slotman GJ, Opal SM, et al. Initial evaluation of human recombinant interleukin-1 receptor antagonist in the treatment of sepsis syndrome: a randomized, open-label, placebo-controlled multicenter trial. Crit Care Med. 1994;22(1):12–21. doi: 10.1097/00003246-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Fisher CJ, Dhainaut JF, Opal SM, et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA. 1994;271(23):1836–43. [PubMed] [Google Scholar]
- 12.Opal SM, Fisher CJ, Jr, Dhainaut JF, et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. Crit Care Med. 1997;25(7):1115–24. doi: 10.1097/00003246-199707000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Schulert GS, Grom AA. Pathogenesis of macrophage activation syndrome and potential for cytokine- directed therapies. Annu Rev Med. 2015;66:145–59. doi: 10.1146/annurev-med-061813-012806. [DOI] [PMC free article] [PubMed] [Google Scholar]; Med. 2003;348(2):138–50. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 14.Mazodier K1, Marin V, Novick D, et al. Severe imbalance of IL-18/IL-18BP in patients with secondary hemophagocytic syndrome. Blood. 2005;106(10):3483–3489. doi: 10.1182/blood-2005-05-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emmenegger U, Schaer DJ, Larroche C, Neftel KA. Hemophagocytic syndromes in adults: current concepts and challenges ahead. Swiss Med Wkly. 2005;135(21–22):299–314. doi: 10.4414/smw.2005.10976. [DOI] [PubMed] [Google Scholar]
- 17.Ravelli A, Grom AA, Behrens EM, Cron RQ. Macrophage activation syndrome as part of systemic juvenile idiopathic arthritis: diagnosis, genetics, pathophysiology and treatment. Genes Immun. 2012;13(4):289–98. doi: 10.1038/gene.2012.3. [DOI] [PubMed] [Google Scholar]
- 18.Minoia F, Davì S, Horne A, et al. Clinical features, treatment, and outcome of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a multinational, multicenter study of 362 patients. Arthritis Rheumatol. 2014;66(11):3160–9. doi: 10.1002/art.38802. [DOI] [PubMed] [Google Scholar]
- 19.Ravelli A, Magni-Manzoni S, Pistorio A, et al. Preliminary diagnostic guidelines for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. J Pediatr. 2005;146(5):598–604. doi: 10.1016/j.jpeds.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66(9):2613–20. doi: 10.1002/art.38690. [DOI] [PubMed] [Google Scholar]
- 21.Rivière S, Galicier L, Coppo P, et al. Reactive hemophagocytic syndrome in adults: a retrospective analysis of 162 patients. Am J Med. 2014;127(11):1118–25. doi: 10.1016/j.amjmed.2014.04.034. [DOI] [PubMed] [Google Scholar]
- 22.Shabbir M, Lucas J, Lazarchick J, Shirai K. Secondary hemophagocytic syndrome in adults: a case series of 18 patients in a single institution and a review of literature. Hematol Oncol. 2011;29(2):100–6. doi: 10.1002/hon.960. [DOI] [PubMed] [Google Scholar]
- 23.Behrens EM, Beukelman T, Paessler M, Cron RQ. Occult macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis. J Rheumatol. 2007;34(5):1133–8. [PubMed] [Google Scholar]
- 24.Bleesing J, Prada A, Siegel DM, et al. The diagnostic significance of soluble CD163 and soluble interleukin-2 receptor alpha-chain in macrophage activation syndrome and untreated new-onset systemic juvenile idiopathic arthritis. Arthritis Rheum. 2007;56(3):965–71. doi: 10.1002/art.22416. [DOI] [PubMed] [Google Scholar]
- 25.Allen CE, Yu X, Kozinetz CA, McClain KL. Highly elevated ferritin levels and the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2008;50(6):1227–35. doi: 10.1002/pbc.21423. [DOI] [PubMed] [Google Scholar]
- 26.Møller HJ, Moestrup SK, Weis N, et al. Macrophage serum markers in pneumococcal bacteremia: Prediction of survival by soluble CD163. Crit Care Med. 2006;34(10):2561–6. doi: 10.1097/01.CCM.0000239120.32490.AB. [DOI] [PubMed] [Google Scholar]
- 27.Schaer DJ, Schleiffenbaum B, Kurrer M, et al. Soluble hemoglobin-haptoglobin scavenger receptor CD163 as a lineage-specific marker in the reactive hemophagocytic syndrome. Eur J Haematol. 2005;74(1):6–10. doi: 10.1111/j.1600-0609.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- 28.Castillo L, Carcillo J. Secondary hemophagocytic lymphohistiocytosis and severe sepsis/systemic inflammatory response syndrome/multiorgan dysfunction syndrome/macrophage activation syndrome share common intermediate phenotypes on a spectrum of inflammation. Pediatr Crit Care Med. 2009;10(3):387–92. doi: 10.1097/PCC.0b013e3181a1ae08. [DOI] [PubMed] [Google Scholar]
- 29.Castillo L. High elevated ferritin levels and the diagnosis of HLH/Sepsis/SIRS/MODS/MAS. Pediatr Blood Cancer. 2008 Nov;51(5):710. doi: 10.1002/pbc.21681. [DOI] [PubMed] [Google Scholar]
- 30.Hughes CG, Morandi A, Girard TD, et al. Association between endothelial dysfunction and acute brain dysfunction during critical illness. Anesthesiology. 2013;118(3):631–9. doi: 10.1097/ALN.0b013e31827bd193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strauss R, Neureiter D, Westenburger B, Wehler M, Kirchner T, Hahn EG. Multifactorial risk analysis of bone marrow histiocytic hyperplasia with hemophagocytosis in critically ill medical patients--a postmortem clinicopathologic analysis. Crit Care Med. 2004;32(6):1316–21. doi: 10.1097/01.ccm.0000127779.24232.15. [DOI] [PubMed] [Google Scholar]
- 32.Schaer DJ, Schaer CA, Schoedon G, Imhof A, Kurrer MO. Hemophagocytic macrophages constitute a major compartment of heme oxygenase expression in sepsis. Eur J Haematol. 2006 Nov;77(5):432–6. doi: 10.1111/j.1600-0609.2006.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Behrens EM, Kreiger PA, Cherian S, Cron RQ. Interleukin 1 receptor antagonist to treat cytophagic histiocytic panniculitis with secondary hemophagocytic lymphohistiocytosis. J Rheumatol. 2006;33(10):2081–4. [PubMed] [Google Scholar]
- 34.Kelly A, Ramanan AV. A case of macrophage activation syndrome successfully treated with anakinra. Nat Clin Pract Rheumatol. 2008;4(11):615–20. doi: 10.1038/ncprheum0919. [DOI] [PubMed] [Google Scholar]
- 35.Kahn PJ, Cron RQ. Higher-dose Anakinra is effective in a case of medically refractory macrophage activation syndrome. J Rheumatol. 2013;40(5):743–4. doi: 10.3899/jrheum.121098. [DOI] [PubMed] [Google Scholar]
- 36.Loh NK, Lucas M, Fernandez S, Prentice D. Successful treatment of macrophage activation syndrome complicating adult Still disease with anakinra. Intern Med J. 2012;42(12):1358–62. doi: 10.1111/imj.12002. [DOI] [PubMed] [Google Scholar]
- 37.Durand M, Troyanov Y, Laflamme P, Gregoire G. Macrophage activation syndrome treated with anakinra. J Rheumatol. 2010;37(4):879–80. doi: 10.3899/jrheum.091046. [DOI] [PubMed] [Google Scholar]
- 38.Bruck N, Suttorp M, Kabus M, Heubner G, Gahr M, Pessler F. Rapid and sustained remission of systemic juvenile idiopathic arthritis-associated macrophage activation syndrome through treatment with anakinra and corticosteroids. J Clin Rheumatol. 2011;17(1):23–7. doi: 10.1097/RHU.0b013e318205092d. [DOI] [PubMed] [Google Scholar]
- 39.Rajasekaran S, Kruse K, Kovey K, et al. Therapeutic role of anakinra, an interleukin-1 receptor antagonist, in the management of secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction/macrophage activation syndrome in critically ill children. Pediatr Crit Care Med. 2014;15(5):401–8. doi: 10.1097/PCC.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 40.Simon DW, Aneja R, Carcillo JA, Halstead ES. Plasma exchange, methylprednisolone, IV immune globulin, and now anakinra support continued PICU equipoise in management of hyperferritinemia-associated sepsis/multiple organ dysfunction syndrome/macrophage activation syndrome/secondary hemophagocytic lymphohistiocytosis syndrome. Pediatr Crit Care Med. 2014;15(5):486–8. doi: 10.1097/PCC.0000000000000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tayer-Shifman OE, Ben-Chetrit E. Refractory macrophage activation syndrome in a patient with SLE and APLA syndrome - Successful use of PET- CT and Anakinra in its diagnosis and treatment. Mod Rheumatol. 2013 Oct 21; doi: 10.3109/14397595.2013.844403. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 42.Shafferman A, Birmingham JD, Cron RQ. High dose anakinra for treatment of severe neonatal Kawasaki disease: a case report. Pediatr Rheumatol Online J. 2014 Jul 11;12:26. doi: 10.1186/1546-0096-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar N, Goyal J, Goel A, Shakoory B, Chatham WW. Macrophage activation syndrome secondary to human monocytic ehrlichiosis. Indian J Hematol Blood Transfus. 2014 Sep;30(Suppl 1):145–7. doi: 10.1007/s12288-013-0299-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miettunen PM, Narendran A, Jayanthan A, Behrens EM, Cron RQ. Successful treatment of severe paediatric rheumatic disease-associated macrophage activation syndrome with interleukin-1 inhibition following conventional immunosuppressive therapy: case series with 12 patients. Rheumatology. 2011;50(2):417–9. doi: 10.1093/rheumatology/keq218. [DOI] [PubMed] [Google Scholar]
- 45.Cochrane Database Syst Rev. 2012 Dec 12;12:CD004388. doi: 10.1002/14651858.CD004388.pub6. Human recombinant protein C for severe sepsis and septic shock in adult and paediatric patients. Martí-Carvajal AJ1, Solà I, Gluud C, Lathyris D, Cardona AF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mertens M, Singh JA. Anakinra for rheumatoid arthritis: a systematic review. J Rheumatol. 2009;36(6):1118–25. doi: 10.3899/jrheum.090074. [DOI] [PubMed] [Google Scholar]
- 47.Murray JF, Matthay MA, Luce JM, et al. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720–723. doi: 10.1164/ajrccm/138.3.720. Erratum, Am Rev Respir Dis 1989; 139:1065. [DOI] [PubMed] [Google Scholar]