Abstract
Sustained activation of JAK/STAT3 signaling pathway is classically described in Multiple Myeloma (MM). One explanation could be the silencing of the JAK/STAT suppressor genes, through the hypermethylation of SHP-1 and SOCS-1, previously demonstrated in MM cell lines or in whole bone marrow aspirates. The link between such suppressor gene silencing and the degree of bone marrow invasion or the treatment response has not been evaluated in depth. Using real-time RT-PCR, we studied the expression profile of three JAK/STAT suppressor genes: SHP-1, SHP-2 and SOCS-1 in plasma cells freshly isolated from the bone marrows of MM patients and healthy controls. Our data demonstrated an abnormal repression of such genes in malignant plasma cells and revealed a significant correlation between such defects and the sustained activation of the JAK/STAT3 pathway during MM. The repressed expression of SHP-1 and SHP-2 correlated significantly with a high initial degree of bone marrow infiltration but was, unexpectedly, associated with a better response to the induction therapy. Collectively, our data provide new evidences that substantiate the contribution of JAK/STAT suppressor genes in the pathogenesis of MM. They also highlight the possibility that the decreased gene expression of SHP-1 and SHP-2 could be of interest as a new predictive factor of a favorable treatment response, and suggest new potential mechanisms of action of the therapeutic molecules. Whether such defect helps the progression of the disease from monoclonal gammopathy of unknown significance to MM remains, however, to be determined.
Introduction
Multiple Myeloma (MM) is a hematological malignancy that accounts for about 10% of hematological tumors. It is characterized by plasma cell monoclonal proliferation within the bone marrow, associated with a production of a monoclonal protein [1–3]. Many advances have been made in the field of MM treatment [4]. However, despite the improvement of patient survival achieved by the available therapeutic strategies, MM remains a frequent and an incurable disease [5]. Thus, understanding the biological aberrancies that drive the development of MM is crucial to reduce relapses and to obtain durable remissions.
It is broadly accepted that the bone marrow microenvironment is critical for the development of MM, since it provides the malignant plasma cells with the required cytokines and growth factors [6–8]. Among these cytokines, IL-6 plays fundamental role in the growth and survival of malignant cells, in part through the activation of Janus tyrosine kinases/Signal Transducers and Activators of Transcription (JAK/STAT) signaling pathway [9–12]. In MM cells, the JAK/STAT signaling pathway seems to be constitutively activated [13–16]. This constitutive activation appears to confer a survival advantage to the MM cells through an anti-apoptotic effect. Besides, it is also involved in MM cell drug-resistance [13, 17].
The JAK/STAT pathway is stringently regulated at several steps by 3 main families of proteins: the PIAS (Protein Inhibitor of Activated STATs), the tyrosine phosphatases (SHP-1 and SHP-2), and the SOCS (Suppressor of cytokine signal transduction), particularly SOCS-1 (reviewed in [18]). Although the IL-6/JAK/STAT signaling pathway was largely explored in the field of hematological malignancies, particularly MM, the role played by the JAK/STAT suppressor genes was less extensively investigated. Actually, SHP-1 and SOCS-1 genes were reported to be highly methylated in MM cell lines or in whole bone marrow aspirates from MM patients [19–22]. Little is known about SHP-1, SHP-2 and SOCS-1 genes expression within the malignant plasma cells per se (freshly isolated from MM patients bone marrows). Moreover, the relationship between the silencing of JAK/STAT suppressor genes and the degree of bone marrow invasion or treatment response is not well documented. Herein, we examined the expression of the JAK/STAT regulating genes (SHP-1, SHP-2 and SOCS-1) in the malignant plasma cells isolated from bone marrows of MM patients. Our data demonstrated an abnormal repression of such genes in freshly isolated malignant plasma cells and revealed a significant correlation between gene silencing of such repressors and the sustained activation of the JAK/STAT3 pathway in MM. In addition, we demonstrated the presence of a negative correlation between the transcription of the studied genes and the bone marrow plasma cells infiltration degree. In an unexpected manner, repression of SHP-1, SHP-2 genes expression was associated to a better initial treatment response.
Materials and methods
Patients and controls
Forty-five patients with newly diagnosed and untreated MM were prospectively enrolled in this study. For each patient, laboratory investigations determined the isotype of the monoclonal immunoglobulin, Durie and Salmon stage as well as the percentage of medullary plasma cell infiltration. After enrollment, all patients received Dexamethasone (DXM) and Thalidomide induction therapy. The response to treatment was evaluated three months later based on the International Myeloma Working Group uniform response criteria for MM [23]. The main biological features of the enrolled patients are summarized in Table 1. The control group consisted of 16 age-and sex-matched bone marrow healthy donors (10 men, 6 women, mean age: 46.3 years). The study protocol was approved by the national medical ethics committee and all patients and healthy donors gave their written informed consent before the study in accordance with the Declaration of Helsinki.
Table 1. Demographic and main clinical and biological features of the MM patients at diagnosis.
| Patients | n = 45 |
|---|---|
| Gender | 27/18 |
| Mean age (years) | 51.7 |
| Durie and Salmon stage (n) | |
| stage IIA | 2 |
| stage IIIA | 36 |
| stage IIIB | 7 |
| Mean (range) bone marrow infiltration | 41% (10–100) |
| Treatment response subcategory (n) (only 40 patients with an available follow-up data) | |
| Complete response | 9 |
| Very good partial response | 5 |
| Partial response | 21 |
| Stable disease | 5 |
Medullar plasma cell isolation
Bone marrow mononuclear cells were isolated on Ficoll-Hypaque gradient and plasma cells were isolated using CD138 magnetic beads (Miltenyi Biotec, Bergisch-Gladbach, Germany) according to manufacturer’s instructions. Purity was assessed by flow cytometry (FACS Canto, Becton Dickinson) and the following labeled mAbs: FITC-conjugated anti-CD38, PE-Conjugated anti-CD138, PE-Conjugated anti-CD19, Cychrome-Conjugated anti-CD45 and APC-H7-conjougated CD56 (BD Biosciences, Le Pont de Claix, France). Purity of isolated plasma cells (CD38+ CD138+) ranged from 95% to 99%.
SOCS-1, SHP-1 and SHP-2 mRNAs expression by Real-Time (RT)-PCR
Total RNA was extracted using RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s procedure. The quality and purity of the extracted RNA was assessed by a direct visualization on agarose gel electrophoresis and a calculation of the ratio of 260/280 nm absorbance. Extracted RNA was reverse transcribed using the Murine-Moloney Leukemia Virus (MMLV) reverse transcriptase (Invitrogen, Cergy Pontoise, France) and random hexamers (Promega, Madison, WI, USA) according to standard procedures. SOCS-1, SHP-1 and SHP-2 mRNAs were quantified by real-time RT-PCR using SYBR Green PCR Master Mix (Applied Biosystems, Branchburg, NJ, USA) and 300 nM of the chosen couples of primers. Forward and reverse primers for SHP-1, SHP-2 and SOCS1 were, respectively, 5’-GGTCACCCACATCAAGGTCAT-3’ and 5’-TGTCGAAGGTCTCCAAACCAC-3’, 5’-GAGAGCAATGACGGCAAGTCT-3’ and 5’-CCTCCACCAACGTCGTATTTC-3’, 5’CCCTTCTTGTAGGATGGTAGCACAC3’ and 5’-GGCTCTGCTGCTGTGGAGAC-3’.
The optimal primers and PCR conditions were previously determined. Accordingly, for each gene, different combinations of primers were tested at three different concentrations using a serial dilution of a positive control. The best couple of primers was chosen based on its sensitivity (the lowest cycle threshold), specificity (a cycle threshold equal to 40 in the non-template controls) and efficiency (slope = -3.3). For each couple of the chosen primers, the PCR product was visualized on agarose gel electrophoresis and a direct sequencing of the PCR product confirmed its specificity. All the samples were tested in duplicate and run on the same plate for the same gene. Amplification was performed using 40 cycles of denaturation at 95°C for 15 s and annealing and extension at 60°C for 1 min using an ABI PRISM 7700 sequence detection system (Applied Biosystems). Data were normalized referring to the expression of the endogenous gene RPLP0 (Ribosomal Protein, Large, P0) using an available gene expression assay (with a tested efficiency equal to one) and TaqMan PCR Master Mix (Applied Biosystems) by calculating 2-ΔCT, with ΔCT the difference in threshold cycles for target and reference.
Immunochemistry
Immunohistochemistry was performed on paraffin-embedded bone marrow biopsies from 21 patients. Following reduction of endogenous peroxidase activity, deparaffinized slides were incubated at 4°C overnight with a rabbit anti-human phosphorylated STAT3 (p-STAT3 Tyr705) antibody diluted at 1:50 (clone D3A7; Cell Signaling Technology). Labeling was revealed by the R.T.U. VectaStain universal Elite ABC kit (Vector Laboratories). Briefly, after two washes, slides were incubated with a horse serum blocking solution included in the kit for 20 min. After washing, slides were incubated with a secondary biotinylated antibody, then stained with diaminobenzidine substrate (Novocastra) during 3 min. Slides were then washed twice with distilled water, counterstained with 1% hematoxylin and analyzed under a light microscope at a 40x magnification by two independent investigators in a blinded fashion. Positively stained plasma cells were evaluated for each sample.
Statistical analysis
Statistical analyses were performed using StatView, SPSS and/or GraphPad Prism software. The levels of mRNA expression for SHP-1, SHP-2 and SOCS-1 in plasma cells were compared between the different study groups using the non-parametric Mann-Whitney U test. Correlations between the gene expression of SHP-1, SHP-2 and SOCS-1 and either the bone marrow infiltration, the response to treatment or STAT3 signaling activation were analyzed using Spearman’s rank correlation. The χ2 test was used to compare the frequency of patients exhibiting a hypoexpression of SOCS-1, SHP-1 and SHP-2 in the different sub-groups identified. The statistical significance was assigned to p values < 0.05.
Results
Expression of SHP-1, SHP-2 and SOCS-1 genes is repressed in malignant plasma cells
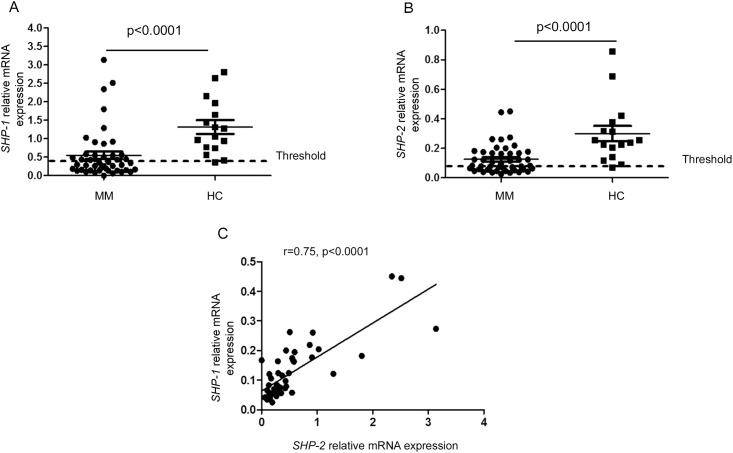
The transcription of SHP-1, SHP-2 and SOCS-1 genes was studied using real-time RT- PCR in the purified bone marrow plasma cells isolated from patients and healthy donors. Within the group of healthy donors, SHP-1 gene expression in plasma cells ranged from 0.36 to 2.8 with a median of 1.2. Comparatively, SHP-1 gene expression was significantly reduced within the MM group (p < 0.0001) (Fig 1A). When the threshold of normal transcription of SHP-1 was calculated as the value of the 5th percentile obtained in the healthy control group, we showed that 27 out of 45 MM patients (60%) displayed a significant reduction in SHP-1 gene expression (Fig 1A).
Fig 1. SHP-1 and SHP-2 gene expression in bone marrow plasma cells isolated from MM patients and healthy donors.
(A, B) Statistical dot plots showing the mRNA levels of SHP-1 and SHP-2 relative to the endogenous gene RPLP0 in sorted bone marrow plasma cells of healthy controls (HC, n = 16) and multiple myeloma patients (MM, n = 45). (C) Correlation between SHP-1 and SHP-2 relative gene expression within MM bone marrow plasma cells. Spearman rank correlation (r) and p values are indicated.
Similar results were obtained with SHP-2. In the control group, SHP-2 gene expression ranged from 0.07 to 0.8 with a median of 0.08. Its expression was significantly reduced in the malignant plasma cells from MM patients (p < 0.0001). When the threshold of normal expression of SHP-2 was determined as above, 23 out of 45 MM patients (51%) had a reduced SHP-2 gene expression (Fig 1B). Interestingly, in the malignant plasma cells, we found a significant correlation between SHP-1 and SHP-2 transcript expression (r = 0.75, p < 0.0001) (Fig 1C).
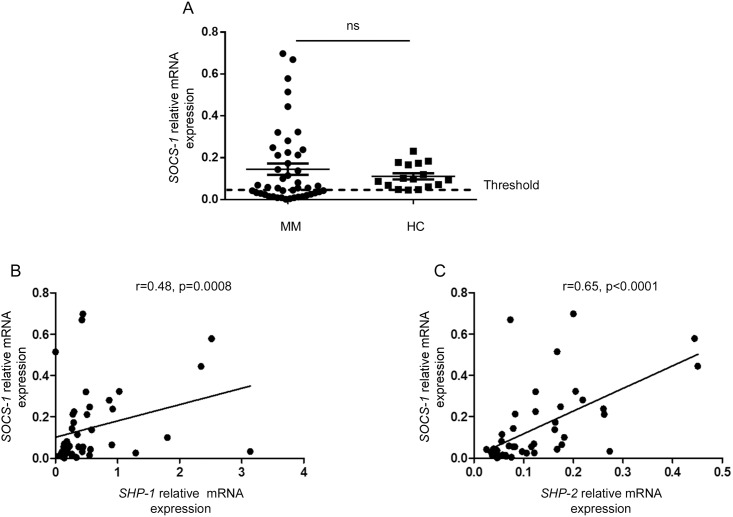
Regarding SOCS-1 gene expression, no significant difference was found between the healthy donor and the patient group (Fig 2A). Moreover, within the patient group, SOCS-1 gene expression profiles were very heterogeneous. Indeed, SOCS-1 gene expression was increased in 13 MM patients (exceeding the 95th percentile of the healthy control group) and decreased in 20 other cases (below the 5th percentile of the healthy control group). Thus, within the MM group, SOCS-1 gene expression displayed a different pattern compared to SHP-1 and SHP-2. Nevertheless, SOCS-1 gene expression was significantly correlated to that of either SHP-1 or SHP-2 (Fig 2B and 2C).
Fig 2. SOCS-1 gene expression in bone marrow plasma cells isolated from MM patients and healthy donors.
(A) Statistical dot plots showing the mRNA levels of SOCS-1 relative to the endogenous gene RPLP0 in sorted bone marrow plasma cells of healthy controls (HC, n = 16) and MM patients (MM, n = 45). (B, C) Correlation between SOCS-1 and SHP-1, SOCS-1 and SHP-2 relative gene expression in MM bone marrow plasma cells. Spearman rank correlation (r) and p values are indicated.
Analysis of SHP-1, SHP-2 and SOCS-1 gene expression according to the clinicopathological features of MM
Bone marrow infiltration
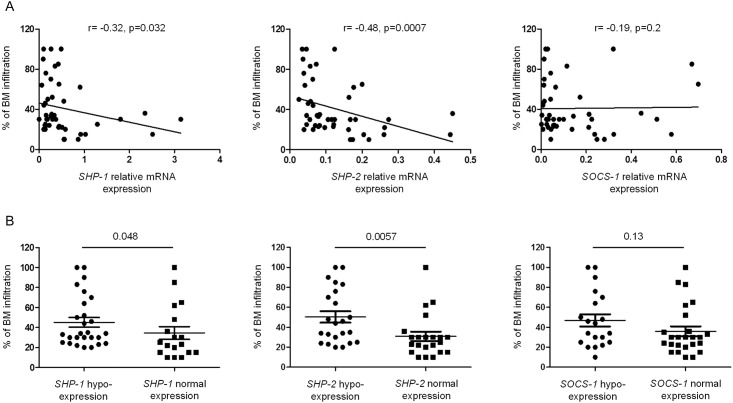
The percentage of bone marrow infiltration in the patient group varied from 10% to 100%, with a median of 30%. To evaluate SHP-1, SHP-2 and SOCS-1 gene expression according to the degree of bone marrow infiltration, several statistical analyses were performed. Using Spearman correlation test, we observed the presence of a negative correlation between SHP-1 and SHP-2 gene expression and the level of bone marrow infiltration (r = -32, p = 0.032 and r = -48, p = 0.0007, respectively) (Fig 3A). The less SHP-1 and SHP-2 were expressed in malignant plasma cells, the more the bone marrow was infiltrated. When the patient group was subdivided into 2 subgroups according to normal or decreased expression of the regulating genes, the level of bone marrow infiltration was significantly increased in the subgroups of patients with lower expression of SHP-1 or SHP-2 (p = 0.048 and p = 0.0057, respectively) (Fig 3B).
Fig 3. SHP-1 and SHP-2 gene expression inversely correlate with the level of bone marrow infiltration.
(A) Correlation between bone marrow infiltration and the relative gene expression of SHP-1, SHP-2 and SOCS-1 in the sorted bone marrow plasma cells. Spearman rank correlation (r) and p values are indicated. (B) The degree of bone marrow infiltration is compared in patients exhibiting normal or decreased expression of SHP-1, SHP-2 and SOCS-1 genes. P values are indicated.
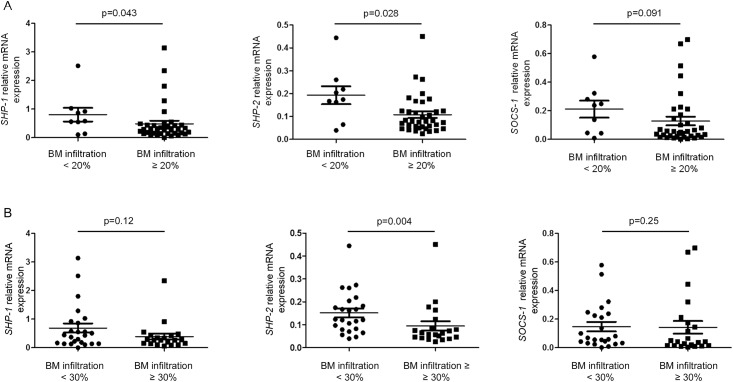
The relationship between the expression of regulating genes and the bone marrow infiltration was further analyzed by stratifying patients according to 2 thresholds of infiltration: 20% and 30%. An infiltration ≥ 20% was significantly associated with a reduced expression of SHP-1 and SHP-2 genes (p = 0.043 and p = 0.028, respectively) (Fig 4A). Interestingly, an infiltration degree ≥ 30% was associated only with a reduced expression of SHP-2 (p = 0.004) (Fig 4B).
Fig 4. Gene expression of SHP-1, SHP-2 and SOCS-1 according to the degree of bone marrow infiltration.
(A, B) The relative gene expression of SHP-1, SHP-2 and SOCS-1 was compared in patients subdivided according to two different thresholds (20% and 30%) of bone marrow infiltration. P values are indicated.
Durie-Salmon staging system
According to the classic Durie-Salmon staging system, the majority of the studied patients (36/45 cases; 80%) exhibited a stage IIIA. Seven patients (15.6%) were classified stage IIIB and only 2 patients (4.4%) displayed a stage IIA. Despite a trend toward decreased expression of the three genes within the stage IIIB subgroup, we could not find any significant difference of expression when compared with stage IIIA and IIIB subgroups (data not shown). Similarly, there was no association between the reduced expression of the studied genes and disease stage (χ2 test, p > 0.05) (data not shown).
Treatment response
Three months after the beginning of induction therapy, the treatment response was evaluated in 40 out of 45 patients according to the International Myeloma Working Group uniform response criteria [23]. Several analyses were carried out to study the relationship between the expression of the regulating genes and the response to the treatment.
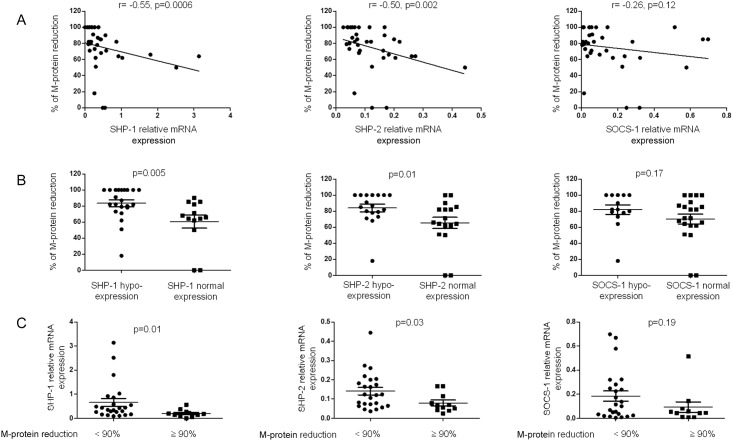
Unexpectedly, Spearman correlation analysis showed that the transcription of SHP-1 and SHP-2 negatively correlated with the percentage of reduction of the serum monoclonal protein (5 patients with light chain MM were excluded from this analysis) (r = -0.55, p = 0.0006 and r = -0.50, p = 0.002, respectively) (Fig 5A). The less SHP-1 and SHP-2 mRNAs were expressed, the stronger the response to the treatment. Additional analyses confirmed this finding. Accordingly, the percent reduction of the serum monoclonal protein was significantly higher within the group of patients exhibiting reduced expression of SHP-1 or SHP-2 (p = 0.005 and p = 0.01, respectively) (Fig 5B). When the patients were stratified according to their degree of response to treatment (as assessed by the percent reduction in serum monoclonal protein), SHP-1 and SHP-2 gene expression were significantly decreased within the subgroup of patient with a better response (≥ 90% reduction; p = 0.01 and p = 0.03, respectively) (Fig 5B).
Fig 5. Gene expression of SHP-1, SHP-2 and SOCS-1 according to the percent reduction of the monoclonal component.
(A) Correlation between the reduction of the serum monoclonal component (M-component) and the relative expression of SHP-1, SHP-2 and SOCS-1 in sorted bone marrow plasma cells. Spearman rank correlation (r) and p values are indicated. (B) Levels of reduction in the serum M-component are compared between patients exhibiting normal or reduced expression of SHP-1, SHP-2 and SOCS-1. P values are indicated. (C) The relative gene expression of SHP-1, SHP-2 and SOCS-1 was compared between the two patient subgroups with a decrease in the M-component <90% or ≥90%. P values are indicated.
Based on the International Myeloma Working Group uniform response criteria [23], we could grossly separate all the 40 patients into “responders” (including patients with either complete or very good partial response), and “non-responders” (including those with partial or no response). Using the χ2 test, there was a significant relationship between reduced SHP-1 expression and the “responder” status (p = 0.026, Table 2).
Table 2. Numbers of “non-responders” (patients with partial or no response) and “responders” (patients with either complete or very good partial response) in “reduced expression” or “normal expression” groups for each of the studied gene.
P values are indicated.
| Non-responders | Responders | Chi-square test | |
|---|---|---|---|
| SHP-1 | |||
| Reduced expression | 13 | 12 | P = 0.026 |
| Normal expression | 13 | 2 | |
| SHP-2 | |||
| Reduced expression | 12 | 10 | P = 0.12 |
| Normal expression | 14 | 4 | |
| SOCS-1 | |||
| Reduced expression | 9 | 8 | P = 0.17 |
| Normal expression | 17 | 6 |
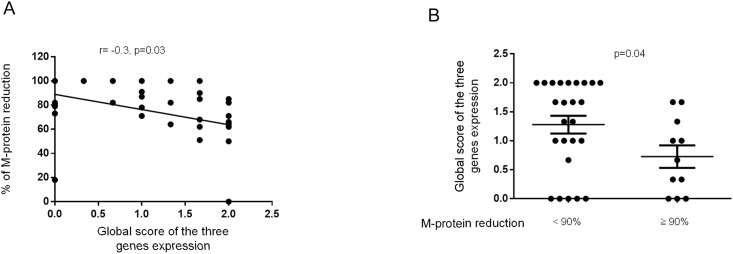
Finally, we attributed a score for gene expression ranging from 0 to 2. The score 2 was given to patients presenting a normal expression, 1 for those having a slightly decreased expression but close to the fixed threshold, 0 for an expression below the threshold. A global expression score for the three genes (calculated as the average of the 3 scores attributed to SHP-1, SHP-2 and SOCS-1 gene expression) was given to each patient. In line with the previous results, this scoring allowed us to show that the global gene expression score negatively correlated with the percent reduction in serum monoclonal protein (r = -0.3, p = 0.03) (Fig 6A). Moreover, this global score was significantly decreased in the subgroup of patients with a better response (≥ 90%; p = 0.04) (Fig 6B).
Fig 6. Relationship between treatment response and the global gene expression score.
(A) The graph recapitulates the correlation between the global score for SHP-1, SHP-2 and SOCS-1 gene expression and the percent reduction in the serum monoclonal component (M-component). Spearman rank correlation (r) and p values are indicated. (B) The global expression score of SHP-1, SHP-2 and SOCS-1 genes was compared between the two patient subgroups with a M-component reduction level <90% or ≥90%. P values are indicated.
SHP-1, SHP-2 and SOCS-1 gene expression negatively correlates with the activation of the JAK/STAT3 pathway
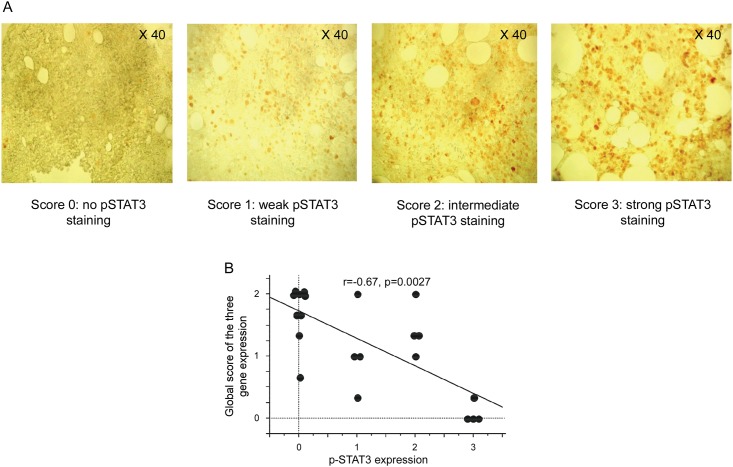
The JAK/STAT3 activation pathway was analyzed by studying the expression of the phosphorylated form of STAT3 (p-STAT3). Immunohistochemistry was performed on paraffin embedded bone marrow sections from 21 out of 45 enrolled MM patients. A score of STAT3 phosphorylation, ranging from 0 to 3, was attributed according to the intensity of staining (Fig 7A). Activation of the JAK/STAT3 pathway was observed in 12 out of 21 patients (57%) (Table 3). Moreover, in 18 of the 21 patients (85.7%), the p-STAT3 score was concordant with the relative gene expression level SHP-1, SHP-2 and SOCS-1 (Table 3). Interestingly, a significant correlation was found between the decreased expression of the three studied genes and the activation of the JAK/STAT3 pathway (p = 0.004 for SHP-1, p = 0.007 for SHP-2 and p = 0.02 for SOCS-1; data not shown). Finally, the correlation between the activation of the JAK/STAT3 pathway and the global score of SHP-1, SHP-2 and SOCS-1 gene expression was tested. The global level of expression of these regulating genes was negatively correlated to the activation of the JAK/STAT3 pathway (r = -67, p = 0.0027) (Fig 7B).
Fig 7. Relationship between the activation of the JAK/STAT pathway and the gene expression of SHP-1, SHP-2 and SOCS-1.
(A) Consecutive sections of paraffin-embedded MM bone marrow biopsies (40x magnification) were stained with rabbit anti-human p-STAT3 or isotype control. Representative samples are shown from 4 MM patients displaying a score of 0, 1, 2 and 3 (no p-STAT3 staining, weak p-STAT3 staining, intermediate p-STAT3 staining and strong pSTAT3 staining, respectively). (B) The graph recapitulates the correlation between the global score of SHP-1, SHP-2 and SOCS-1 gene expression and the score for p-STAT3 staining intensity. Spearman rank correlation (r) and p values are indicated.
Table 3. SHP-1, SHP-2 and SOCS-1 gene expression profiles and the corresponding Immunohistochemistry (IHC) scores for p-STAT3 staining in the indicated patients.
| Patient number | SHP-1 relative expression | SHP-2 relative expression | SOCS-1 relative expression | IHC SCORE | Concordance |
|---|---|---|---|---|---|
| 1 | normal | normal | normal | 0 | concordant |
| 2 | normal | normal | normal | 0 | concordant |
| 3 | normal | normal | decreased | 0 | concordant |
| 4 | normal | normal | normal | 0 | concordant |
| 5 | Slightly decreased | normal | normal | 0 | concordant |
| 6 | decreased | decreased | normal | 0 | discordant |
| 7 | normal | normal | normal | 0 | concordant |
| 8 | normal | normal | normal | 0 | concordant |
| 9 | Slightly decreased | normal | normal | 0 | concordant |
| 10 | Slightly decreased | Slightly decreased | Slightly decreased | 1 | concordant |
| 11 | decreased | decreased | Slightly decreased | 1 | concordant |
| 12 | decreased | Slightly decreased | normal | 1 | concordant |
| 13 | normal | normal | normal | 1 | discordant |
| 14 | decreased | normal | normal | 2 | concordant |
| 15 | normal | normal | normal | 2 | discordant |
| 16 | decreased | normal | normal | 2 | concordant |
| 17 | Slightly decreased | decreased | normal | 2 | concordant |
| 18 | decreased | decreased | Slightly decreased | 3 | concordant |
| 19 | decreased | decreased | decreased | 3 | concordant |
| 20 | decreased | decreased | decreased | 3 | concordant |
| 21 | decreased | decreased | decreased | 3 | concordant |
Discussion
JAK/STAT is a common signaling pathway shared by many cytokines and growth factors [24–28]. This notion supports the important and complex role that this pathway plays in the biology of hematopoietic cells, oncogenesis, and therapy applied to some blood malignancies [28–32]. Particularly, the IL-6R/JAK/STAT3 pathway largely contributes to the MM multistep transformation process and pathogenesis, making its therapeutic targeting an attractive strategy that could favor durable remissions in MM patients [33–36]. The JAK/STAT signaling pathway is regulated at various levels by different molecules such as SOCS-1, SHP-1 and SHP-2, all of which are known to suppress the JAK/STAT signaling pathway. The aberrant and constitutive activation of the JAK/STAT pathway during MM suggests that epigenetic silencing of its regulating genes might be involved in this abnormality. In fact, previous data demonstrated that hypermethylation of SHP-1 and SOCS-1 genes is a frequent event in MM. These results were mainly demonstrated in MM cell lines or in whole bone marrow aspirates from MM patients [20–22]. Thus, our first goal was to confirm that the reported hypermethylation resulted in the silencing or at least repression in the expression of these genes within the malignant plasma cells per se. Using RT-PCR, we could study the gene expression profile of SHP-1, SHP-2 and SOCS-1 in plasma cells freshly isolated from the bone marrow of MM patients. Compared with healthy donors, we were able to demonstrate the presence of a global and significant decrease in SHP-1 and SHP-2 gene expression in patient plasma cells. Thus, these finding are in line with the reported hypermethylation of SHP-1 in MM [21]. Moreover, we found a very close and significant correlation between the inactivation of SHP-1 and SHP-2 genes, suggesting the involvement of a common mechanism underlying their inactivation during MM. Even though the SOCS-1 gene expression pattern differs from those of SHP-1 and SHP-2, we could demonstrate that SOCS-1 gene expression was inactivated in about 44% of patients. This result further corroborates one previous study showing a hypermethylation of SOCS-1 in 40% of MM patients [22]. The heterogeneous profile of SOCS-1 gene expression leads us to hypothesize that the mechanisms involved in the inactivation of SOCS-1 differ from those supporting the inhibition of SHP-1 and SHP-2. Surprisingly, some of our patients (13 out of 45; 29%) exhibited a significantly enhanced expression of the SOCS-1 gene. SOCS-1 is classically described as a tumor suppressor in many cancers, including hematopoietic malignancies. However, its ambivalent role was previously reported, since its overexpression seems to be associated to an oncogenic effect in other settings (reviewed in [37]). Moreover, this hyperexpression could also be beneficial by inhibiting the oncogenic events through the degradation of oncoproteins [38]. The limited number of patients with hyper-expression of SOCS-1 did not allow us to characterize in depth this unexpected feature.
The direct link between the hypermethylation of SHP-1, SHP-2 and SOCS-1 genes and the hyperactivation of the JAK/STAT signaling pathway in MM is not well established. Indeed, such correlation was only studied in MM cell lines [22]. Notably, using immunohistochemistry to study p-STAT3 on patients’ bone marrow specimens, we observed the presence of a global negative correlation between the sustained activation of the JAK/STAT3 pathway and the gene expression of SHP-1, SHP-2 and SOCS-1 in patient plasma cells.
Data evaluating the relation between genes regulating the JAK/STAT3 pathway and the bone marrow infiltration status at MM diagnosis is lacking. Thus, in a second step, we studied the relationship between the gene expression of SHP-1, SHP-2 and SOCS-1 and the degree of patient bone marrow infiltration at baseline. Our analyses highlighted that the inactivation of SHP-1 and SHP-2 was significantly associated with a high degree of initial bone marrow invasion. Thus, we consolidate the idea that the inactivation of genes regulating the JAK/STAT3 pathway can support tumor expansion through the repression of JAK/STAT signaling. In addition to its anti-apoptotic effect, the JAK/STAT pathway can also enhance IL-6 production [39, 40]. This can lead to the establishment of a positive feedback loop that amplifies the IL-6R/JAK/STAT3 pathway. It is also likely that the uncontrolled JAK/STAT signaling prompts plasma cells to be more responsive to the survival signals delivered by the microenvironment (e.g. IL-6). Moreover, the significant correlation between bone marrow infiltration and the inhibition of SHP-1 and SHP-2 gene expression raises the possibility that their degree of inactivation may represent a biomarker that indirectly reflects tumor mass and the level of bone marrow invasion at diagnosis. Finally, it was suggested that aberrant methylation of some genes, such as SHP-1 and SOCS-1, helps the progression from monoclonal gammopathy of undetermined significance (MGUS) to MM [19, 41]. In line with this, since our results show that the decreased gene expression of SHP-1, SHP-2 was associated with a more extensive bone marrow invasion at diagnosis, we can hypothesize that such patients may be those who progressed more rapidly from MGUS to MM.
The JAK-STAT pathway was incriminated in MM chemo-resistance to multiple agents, including dexamethasone. Indeed, JAK-STAT inhibitors can revert dexamethasone-resistance in MM cells [13, 15, 17, 42–44]. The involvement of JAK-STAT signaling in drug resistance to thalidomide or its analogs is not described. Unexpectedly, our results rather suggest that the inactivation of SHP-1 and SHP-2 genes is associated with a strong response to combined dexamethasone/thalidomide induction therapy. Indeed, all the analyses performed showed that the expression of SHP-1 and SHP-2 genes inversely correlates with the degree of treatment response. The less SHP-1 and SHP-2 genes were expressed, the better the response to treatment. A favorable response was globally more associated to the inactivation of SHP-1 than to that of SHP-2. Accordingly, when patients were subdivided according to the International Myeloma Working Group uniform response criteria into “responders” (complete or very good partial response), and “non-responders” (partial or no response), only SHP-1 inactivation was significantly associated to the “responder” status. One explanation for the association of a favorable response with the inactivation of SHP gene expression could be that therapeutic agents, particularly thalidomide, act through the inhibition of cytokines such as IL-6 that sustain tumor growth and survival [45]. Patients exhibiting a decreased expression of the regulating genes could be therefore more susceptible to the growth factor starvation occurring during treatment. Hence, the level of SHP gene expression could be a predictive factor of a favorable response to induction therapy. Another hypothesis could be that thalidomide and\or dexamethasone may act similarly to other molecules by inducing the transcription of SHP-1/2 in the malignant plasma cells [46–49]. Subsequently, this could efficiently inhibit the JAK/STAT pathway and induce apoptosis of tumor cells. In this setting, thalidomide and\or dexamethasone would be more effective in patients showing a significant inactivation of SHP gene expression. This could be of interest to adapt and personalize the available therapies according to patient profiles.
Overall, our data provide evidence that repression of SHP-1, SHP-2 and SOCS-1 gene expression in patient plasma cells supports the constitutive activation of the JAK/STAT3 pathway and probably leads to higher degrees of bone marrow invasion. Interestingly, this aberrancy is associated to a better response to thalidomide and dexamethasone induction therapy. The identification of such subgroups of patients showing greater or lesser responsiveness to particular anti-cancer therapies could be helpful in the development of more adapted treatment strategy. Whether the gene silencing of SHP-1/2 and SOCS-1 favors the progression from MGUS to MM remains to be determined. Finally, our work further emphasizes the implication of SHP-1, SHP-2 and SOCS-1 genes as suppressors of tumor growth in different cancers [37, 50–52].
Data Availability
All relevant data are within the paper.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351(18):1860–73. 10.1056/NEJMra041875 [DOI] [PubMed] [Google Scholar]
- 2.Blade J, Cibeira MT, Fernandez de Larrea C, Rosinol L. Multiple myeloma. Ann Oncol. 2010;21 Suppl 7:vii313–9. [DOI] [PubMed] [Google Scholar]
- 3.Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111(6):2962–72. 10.1182/blood-2007-10-078022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung C. Role of Immunotherapy in Targeting the Bone Marrow Microenvironment in Multiple Myeloma: An Evolving Therapeutic Strategy. Pharmacotherapy. 2016. [DOI] [PubMed] [Google Scholar]
- 5.Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516–20. 10.1182/blood-2007-10-116129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitsiades CS, Mitsiades NS, Munshi NC, Richardson PG, Anderson KC. The role of the bone microenvironment in the pathophysiology and therapeutic management of multiple myeloma: interplay of growth factors, their receptors and stromal interactions. Eur J Cancer. 2006;42(11):1564–73. 10.1016/j.ejca.2005.12.025 [DOI] [PubMed] [Google Scholar]
- 7.Seidl S, Kaufmann H, Drach J. New insights into the pathophysiology of multiple myeloma. Lancet Oncol. 2003;4(9):557–64. [DOI] [PubMed] [Google Scholar]
- 8.Rajkumar SV, Mesa RA, Fonseca R, Schroeder G, Plevak MF, Dispenzieri A, et al. Bone marrow angiogenesis in 400 patients with monoclonal gammopathy of undetermined significance, multiple myeloma, and primary amyloidosis. Clin Cancer Res. 2002;8(7):2210–6. [PubMed] [Google Scholar]
- 9.Klein B, Zhang XG, Lu ZY, Bataille R. Interleukin-6 in human multiple myeloma. Blood. 1995;85(4):863–72. [PubMed] [Google Scholar]
- 10.Klein B, Tarte K, Jourdan M, Mathouk K, Moreaux J, Jourdan E, et al. Survival and proliferation factors of normal and malignant plasma cells. Int J Hematol. 2003;78(2):106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hilbert DM, Kopf M, Mock BA, Kohler G, Rudikoff S. Interleukin 6 is essential for in vivo development of B lineage neoplasms. J Exp Med. 1995;182(1):243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shain KH, Yarde DN, Meads MB, Huang M, Jove R, Hazlehurst LA, et al. Beta1 integrin adhesion enhances IL-6-mediated STAT3 signaling in myeloma cells: implications for microenvironment influence on tumor survival and proliferation. Cancer Res. 2009;69(3):1009–15. 10.1158/0008-5472.CAN-08-2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10(1):105–15. [DOI] [PubMed] [Google Scholar]
- 14.Morgan GJ, Walker BA, Davies FE. The genetic architecture of multiple myeloma. Nat Rev Cancer. 2012;12(5):335–48. 10.1038/nrc3257 [DOI] [PubMed] [Google Scholar]
- 15.Bharti AC, Shishodia S, Reuben JM, Weber D, Alexanian R, Raj-Vadhan S, et al. Nuclear factor-kappaB and STAT3 are constitutively active in CD138+ cells derived from multiple myeloma patients, and suppression of these transcription factors leads to apoptosis. Blood. 2004;103(8):3175–84. 10.1182/blood-2003-06-2151 [DOI] [PubMed] [Google Scholar]
- 16.Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol. 2003;171(7):3863–71. [DOI] [PubMed] [Google Scholar]
- 17.Shi L, Wang S, Zangari M, Xu H, Cao TM, Xu C, et al. Over-expression of CKS1B activates both MEK/ERK and JAK/STAT3 signaling pathways and promotes myeloma cell drug-resistance. Oncotarget. 2010;1(1):22–33. 10.18632/oncotarget.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shuai K, Liu B. Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol. 2003;3(11):900–11. 10.1038/nri1226 [DOI] [PubMed] [Google Scholar]
- 19.Chim CS, Liang R, Leung MH, Kwong YL. Aberrant gene methylation implicated in the progression of monoclonal gammopathy of undetermined significance to multiple myeloma. J Clin Pathol. 2007;60(1):104–6. 10.1136/jcp.2006.036715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galm O, Yoshikawa H, Esteller M, Osieka R, Herman JG. SOCS-1, a negative regulator of cytokine signaling, is frequently silenced by methylation in multiple myeloma. Blood. 2003;101(7):2784–8. 10.1182/blood-2002-06-1735 [DOI] [PubMed] [Google Scholar]
- 21.Chim CS, Fung TK, Cheung WC, Liang R, Kwong YL. SOCS1 and SHP1 hypermethylation in multiple myeloma: implications for epigenetic activation of the Jak/STAT pathway. Blood. 2004;103(12):4630–5. 10.1182/blood-2003-06-2007 [DOI] [PubMed] [Google Scholar]
- 22.Reddy J, Shivapurkar N, Takahashi T, Parikh G, Stastny V, Echebiri C, et al. Differential methylation of genes that regulate cytokine signaling in lymphoid and hematopoietic tumors. Oncogene. 2005;24(4):732–6. 10.1038/sj.onc.1208032 [DOI] [PubMed] [Google Scholar]
- 23.Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467–73. 10.1038/sj.leu.2404284 [DOI] [PubMed] [Google Scholar]
- 24.Valentino L, Pierre J. JAK/STAT signal transduction: regulators and implication in hematological malignancies. Biochem Pharmacol. 2006;71(6):713–21. 10.1016/j.bcp.2005.12.017 [DOI] [PubMed] [Google Scholar]
- 25.Moriggl R, Topham DJ, Teglund S, Sexl V, McKay C, Wang D, et al. Stat5 is required for IL-2-induced cell cycle progression of peripheral T cells. Immunity. 1999;10(2):249–59. [DOI] [PubMed] [Google Scholar]
- 26.Moriggl R, Sexl V, Piekorz R, Topham D, Ihle JN. Stat5 activation is uniquely associated with cytokine signaling in peripheral T cells. Immunity. 1999;11(2):225–30. [DOI] [PubMed] [Google Scholar]
- 27.Friedrich K, Kammer W, Erhardt I, Brandlein S, Sebald W, Moriggl R. Activation of STAT5 by IL-4 relies on Janus kinase function but not on receptor tyrosine phosphorylation, and can contribute to both cell proliferation and gene regulation. Int Immunol. 1999;11(8):1283–94. [DOI] [PubMed] [Google Scholar]
- 28.Ferrajoli A, Faderl S, Ravandi F, Estrov Z. The JAK-STAT pathway: a therapeutic target in hematological malignancies. Curr Cancer Drug Targets. 2006;6(8):671–9. [DOI] [PubMed] [Google Scholar]
- 29.Yamada O, Kawauchi K. The role of the JAK-STAT pathway and related signal cascades in telomerase activation during the development of hematologic malignancies. JAKSTAT. 2013;2(4):e25256 10.4161/jkst.25256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mughal TI, Girnius S, Rosen ST, Kumar S, Wiestner A, Abdel-Wahab O, et al. Emerging therapeutic paradigms to target the dysregulated Janus kinase/signal transducer and activator of transcription pathway in hematological malignancies. Leuk Lymphoma. 2014;55(9):1968–79. 10.3109/10428194.2013.863307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vainchenker W, Constantinescu SN. JAK/STAT signaling in hematological malignancies. Oncogene. 2013;32(21):2601–13. 10.1038/onc.2012.347 [DOI] [PubMed] [Google Scholar]
- 32.Sansone P, Bromberg J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol. 2012;30(9):1005–14. 10.1200/JCO.2010.31.8907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chatterjee M, Stuhmer T, Herrmann P, Bommert K, Dorken B, Bargou RC. Combined disruption of both the MEK/ERK and the IL-6R/STAT3 pathways is required to induce apoptosis of multiple myeloma cells in the presence of bone marrow stromal cells. Blood. 2004;104(12):3712–21. 10.1182/blood-2004-04-1670 [DOI] [PubMed] [Google Scholar]
- 34.Bommert K, Bargou RC, Stuhmer T. Signalling and survival pathways in multiple myeloma. Eur J Cancer. 2006;42(11):1574–80. 10.1016/j.ejca.2005.12.026 [DOI] [PubMed] [Google Scholar]
- 35.Chatterjee M, Jain S, Stuhmer T, Andrulis M, Ungethum U, Kuban RJ, et al. STAT3 and MAPK signaling maintain overexpression of heat shock proteins 90alpha and beta in multiple myeloma cells, which critically contribute to tumor-cell survival. Blood. 2007;109(2):720–8. 10.1182/blood-2006-05-024372 [DOI] [PubMed] [Google Scholar]
- 36.Lin H, Kolosenko I, Bjorklund AC, Protsyuk D, Osterborg A, Grander D, et al. An activated JAK/STAT3 pathway and CD45 expression are associated with sensitivity to Hsp90 inhibitors in multiple myeloma. Exp Cell Res. 2013;319(5):600–11. 10.1016/j.yexcr.2012.12.006 [DOI] [PubMed] [Google Scholar]
- 37.Beaurivage C, Champagne A, Tobelaim WS, Pomerleau V, Menendez A, Saucier C. SOCS1 in cancer: An oncogene and a tumor suppressor. Cytokine. 2016. [DOI] [PubMed] [Google Scholar]
- 38.Kamio M, Yoshida T, Ogata H, Douchi T, Nagata Y, Inoue M, et al. SOCS1 [corrected] inhibits HPV-E7-mediated transformation by inducing degradation of E7 protein. Oncogene. 2004;23(17):3107–15. 10.1038/sj.onc.1207453 [DOI] [PubMed] [Google Scholar]
- 39.Huang WL, Yeh HH, Lin CC, Lai WW, Chang JY, Chang WT, et al. Signal transducer and activator of transcription 3 activation up-regulates interleukin-6 autocrine production: a biochemical and genetic study of established cancer cell lines and clinical isolated human cancer cells. Mol Cancer. 2010;9:309 10.1186/1476-4598-9-309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee C, Oh JI, Park J, Choi JH, Bae EK, Lee HJ, et al. TNF alpha mediated IL-6 secretion is regulated by JAK/STAT pathway but not by MEK phosphorylation and AKT phosphorylation in U266 multiple myeloma cells. Biomed Res Int. 2013;2013:580135 10.1155/2013/580135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stanganelli C, Arbelbide J, Fantl DB, Corrado C, Slavutsky I. DNA methylation analysis of tumor suppressor genes in monoclonal gammopathy of undetermined significance. Ann Hematol. 2010;89(2):191–9. 10.1007/s00277-009-0818-3 [DOI] [PubMed] [Google Scholar]
- 42.Alas S, Bonavida B. Inhibition of constitutive STAT3 activity sensitizes resistant non-Hodgkin's lymphoma and multiple myeloma to chemotherapeutic drug-mediated apoptosis. Clin Cancer Res. 2003;9(1):316–26. [PubMed] [Google Scholar]
- 43.Zhu S, Wang Z, Li Z, Peng H, Luo Y, Deng M, et al. Icaritin suppresses multiple myeloma, by inhibiting IL-6/JAK2/STAT3. Oncotarget. 2015;6(12):10460–72. 10.18632/oncotarget.3399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J, Favata M, Kelley JA, Caulder E, Thomas B, Wen X, et al. INCB16562, a JAK1/2 selective inhibitor, is efficacious against multiple myeloma cells and reverses the protective effects of cytokine and stromal cell support. Neoplasia. 2010;12(1):28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hideshima T, Anderson KC. Molecular mechanisms of novel therapeutic approaches for multiple myeloma. Nat Rev Cancer. 2002;2(12):927–37. 10.1038/nrc952 [DOI] [PubMed] [Google Scholar]
- 46.Kim SM, Lee JH, Sethi G, Kim C, Baek SH, Nam D, et al. Bergamottin, a natural furanocoumarin obtained from grapefruit juice induces chemosensitization and apoptosis through the inhibition of STAT3 signaling pathway in tumor cells. Cancer Lett. 2014;354(1):153–63. 10.1016/j.canlet.2014.08.002 [DOI] [PubMed] [Google Scholar]
- 47.Lee JH, Chiang SY, Nam D, Chung WS, Lee J, Na YS, et al. Capillarisin inhibits constitutive and inducible STAT3 activation through induction of SHP-1 and SHP-2 tyrosine phosphatases. Cancer Lett. 2014;345(1):140–8. 10.1016/j.canlet.2013.12.008 [DOI] [PubMed] [Google Scholar]
- 48.Kim C, Cho SK, Kapoor S, Kumar A, Vali S, Abbasi T, et al. beta-Caryophyllene oxide inhibits constitutive and inducible STAT3 signaling pathway through induction of the SHP-1 protein tyrosine phosphatase. Mol Carcinog. 2014;53(10):793–806. 10.1002/mc.22035 [DOI] [PubMed] [Google Scholar]
- 49.Phromnoi K, Prasad S, Gupta SC, Kannappan R, Reuter S, Limtrakul P, et al. Dihydroxypentamethoxyflavone down-regulates constitutive and inducible signal transducers and activators of transcription-3 through the induction of tyrosine phosphatase SHP-1. Mol Pharmacol. 2011;80(5):889–99. 10.1124/mol.111.073676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu C, Guan Q, Wang Y, Zhao ZJ, Zhou GW. SHP-1 suppresses cancer cell growth by promoting degradation of JAK kinases. J Cell Biochem. 2003;90(5):1026–37. 10.1002/jcb.10727 [DOI] [PubMed] [Google Scholar]
- 51.Bard-Chapeau EA, Li S, Ding J, Zhang SS, Zhu HH, Princen F, et al. Ptpn11/Shp2 acts as a tumor suppressor in hepatocellular carcinogenesis. Cancer Cell. 2011;19(5):629–39. 10.1016/j.ccr.2011.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang T, Guo W, Yang Y, Liu W, Guo L, Gu Y, et al. Loss of SHP-2 activity in CD4+ T cells promotes melanoma progression and metastasis. Sci Rep. 2013;3:2845 10.1038/srep02845 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.