Abstract
Isolevuglandins (isoLGs) are stereo and structurally isomeric γ-ketoaldehydes produced through free radical-induced oxidation of arachidonates. Some isoLG isomers are also generated through enzymatic cyclooxygenation. Post-translational modification of proteins by isoLG is associated with loss-of-function, cross-linking and aggregation. We now report that a low level of modification by one or two molecules of isoLG has a profound effect on the activity of a multi subunit protease, calpain-1. Modification of one or two key lysyl residues apparently suffices to abolish catalytic activity. Covalent modification of calpain-1 led to intersubunit cross-linking. Hetero- and homo-oligomers of the catalytic and regulatory subunits of calpain-1 were detected by SDS PAGE with Western blotting. N-Acetyl-glycyl-lysine methyl ester and β-amyloid(11-17) peptide EVHHQKL were used as models for characterizing the cross-linking of protein lysyl residues resulting from adduction of iso[4]LGE2. Aminal, bispyrrole and trispyrrole cross-links of these two peptides were identified and fully characterized by mass spectrometry. Aminal and bispyrrole dimers were both detected. Furthermore, a complex mixture of derivatives of the bispyrrole cross-link containing one or more additional atoms of oxygen was found. Interesting differences are evident in the predominant crosslink type generated in the reaction of iso[4]LGE2 with these peptides. More aminal cross-link versus bispyrrole is formed during reaction of the dipeptide with iso[4]LGE2. In contrast, more bispyrrole versus aminal cross-link is formed during the reaction of EVHHQKL with iso[4]LGE2. It is tempting to speculate that the EVHHQKL peptide-pyrrole modification forms noncovalent aggregates that favor the production of covalent bispyrrole cross-links because β-amyloid(11-17) tends to spontaneously oligomerize.
Graphical Abstract
INTRODUCTION
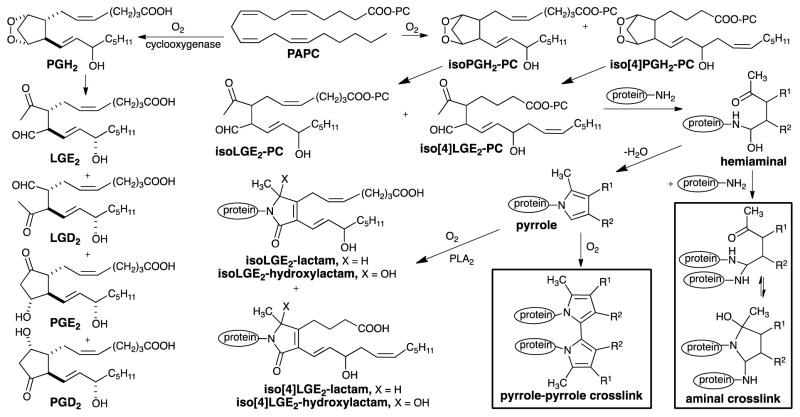
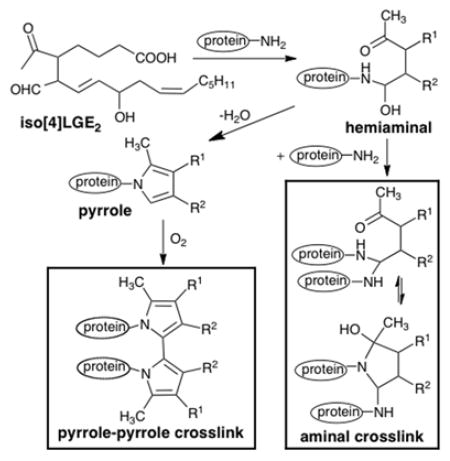
Isolevuglandins (isoLGs) are a family of γ-keto aldehydes1 generated as phospholipid esters by free radical-induced cyclooxygenation of arachidonyl phospholipids, e.g. PAPC (Scheme 1). Endoperoxide intermediates, e.g., isoPGH2-PC or iso[4]PGH2-PC, spontaneously rearrange to isoLGs, e.g., isoLGE2-PC, with prostanoid side chains or structural isomers, e.g., iso[4]LGE2-PC, with non-prostanoid side chains. Two stereoisomers of isoLGs, LGE2 and LGD2, with prostanoid side chains and stereochemistry, are also generated through cyclooxygenase-promoted oxygenation of arachidonate followed by spontaneous rearrangements2 that compete with enzymatic rearrangements of the endoperoxide PGH2 to prostaglandins (Scheme 1), prostacyclins and thromboxanes.3 Like other reactive intermediates,4 free isoLGs have never been detected in vivo presumably because they rapidly react, in seconds,5 with primary amines such as the ε-amino group of protein lysyl residues and ethanolamine phospholipids.6 They form covalent adducts with orders of magnitude greater avidity than other lipid oxidation products such as 4-hydroxynonenal and malondialdehyde (MDA).7–9 These adducts incorporate the primary amino group in a pyrrole ring that is readily oxidized to lactam and hydroxylactam derivatives.10 IsoLG-protein adduction has potential biological consequences. For example, preferential adduction of isoLG to a specific Lys in the mitochondrial enzyme Cyp27A1 abolishes its ability to metabolize cholesterol leading to cholesterol accumulation and retinal pathology.11, 12 Besides producing monomeric adducts, exposure of ovalbumin to relatively large concentrations of isoLGs was shown to generate protein-protein crosslinks13 and similar treatment of cells in culture produced DNA-protein crosslinks14 orders of magnitude more readily than other lipid oxidation-derived electrophiles, such as MDA. In the human eye, isoLG adducted calpain-1 accumulates in the trabecular meshwork (TM), and isoLG adduction abolishes the enzyme’s activity.15 Crosslinking of proteins by isoLGs may impede aqueous outflow through the TM and lead to elevated intraocular pressure in primary open angle glaucoma (POAG). Adduction and crosslinking of the subfamily of isoLGs produced through the cyclooxygenase pathway, i.e., LGE2 and LGD2 (Scheme 1), may foster aggregation of the β-amyloid peptide contributing to Alzheimer’s disease (AD).16–18 Aminal cross-link19 and pyrrole-pyrrole cross-link20 structures depicted in Scheme 1 have been postulated, but direct evidence supporting either alternative is lacking. Understanding the nature of isoLG-mediated cross-linking may provide insights into the involvement of lipid oxidation in the etiology of age-related diseases such as POAG and AD and suggest new therapeutic countermeasures.
Scheme 1.
Generation of isoLGs and postulated structures of their protein adducts and cross-links.
To characterize protein-isoLG cross-links, reactions between iso[4]LGE2 and a heterodimeric protein calpain-1 were investigated, as well as the reactions between iso[4]LGE2 and two peptide model systems, N-acetylglycyl-lysine methyl ester and the β-amyloid(11-17) peptide EVHHQKL. We now present evidence for the generation of cross-links between the catalytic and regulatory subunits of calpain-1 by iso[4]LGE2. Mass spectroscopic evidence demonstrates the generation of both pyrrole-pyrrole and aminal cross-links between lysyl residues resulting from adduction of iso[4]LGE2 with a dipeptide, N-acetyl-glycyl-lysine methyl ester, and with the β-amyloid(11-17) peptide EVHHQKL. Additional complexity was discovered to arise through further oxygenation of bispyrrole cross-links resulting in a complex mixture of derivatives containing one or more additional oxygen atoms.
EXPERIMENTAL PROCEDURES
General methods
All chemicals were high purity analytical or HPLC grade. The following commercially available materials were used as received: calpain-1 (from human erythrocytes) was from Calbiochem (La Jolla, CA). Calpain-1 (from human erythrocytes) was from Calbiochem (La Jolla, CA). N-Acetylglycyllysine methyl ester was from Bachem (Torrance, CA), β-amyloid(11-17) peptide EVHHQKL (β-amyloid residues 11-17) was from Peptide 2.0 Inc (Chantilly, VA), N-ethylmorpholine (NEM) was from Aldrich (Milwaukee, WI). Sodium acetate, ammonium bicarbonate, disodium hydrogen phosphate and monosodium phosphate were from Fisher Scientific Co. Chromatography was performed with ACS HPLC grade solvents.
Preparation of iso[4]LGE2 -modified calpain-1
Calpain-1 was modified with 10 equivalents of iso[4]LGE2. Calpain-1 (100 μg, 8.9 nmol) in 190 μl of a solution containing 20 mM imidazole pH6.8, 5 mM β–mercaptoethanol, 1 mM EDTA, 1 mM EGTA, 30 % v/v glycerol was mixed with 310 μL of sodium acetate solution (10 mM, pH 6.8). Iso[4]LGE2 stock (11 μL of 1 μg/μL in methanol, 30 nmol total) was added to calpain-1 and placed on a shaker (IKA MTS 2/4 digital microtiter shaker, 300 rpm) for 40 min at room temperature. This procedure was repeated a total of 3 times. The final reaction mixture was quenched by addition of glycine (2 μL, 100 mM, 178 nmol, 2 molar equivalent to iso[4]LGE2) with mixing then flash-frozen in liquid nitrogen and stored at −80 °C until analysis.
Preparation of Iso[4]LGE2 -modified model peptides
Reactant peptide stocks were prepared as follows: N-acetylglycyllysine methyl ester (25 mM) and β-amyloid(11-17) peptide EVHHQKL (1 mg/mL) were each dissolved in 50 mM N-ethylmorpholine acetate, pH 8.6. Iso[4]LGE2 in methanol (4.4 μL, 12.5 nmol, 4.4 μg) was added to 100 μl N-acetylglycyllysine methyl ester (2.5 μmol, 647.5 μg) (molar ratio of iso[4]LGE2: dipeptide = 1:200) and reacted under air on a digital microtiter shaker at 25 °C. After incubation for various times (1, 4, 7, 15, 21 and 50 days), aliquots (~1.2 nmol, 10 μL) were withdrawn from the reaction mixture, dried in a speed vac (Savant) and stored at −20 °C for further analysis. Two different molar ratios (iso4: peptide = 1:2000 and 1:200) were studied for both the dipeptide and β-amyloid(11-17) peptide EVHHQKL. Modified peptides generated under each of these reaction conditions were then analyzed by MALDI-TOF MS and LC MS/MS.
Calpain-1 activity assay
Calpain-1 was assayed with a calpain activity assay kit (Biovision Inc., Mountainview, CA) following the protocol recommended by the manufacturer. The fluorometric assay was based on detecting the cleavage of a calpain substrate, Ac-LLY-AFC, that fluoresces at 505 nm, whereas the cleaved substrate fluoresces at 400 nm. Thus, calpain-1 (26 μL of 2.286 μM, 6.6 μg, 0.0595 nmol) was added to iso[4]LGE2 (0.21 μg 0.595 nmol) in 74 μL of aqueous sodium acetate (10 mM), mixed well and reacted under air on shaker (IKA MTS 2/4 digital microtiter shaker, 300 rpm) at 25 °C. At each time point (3, 10, 30 and 90 min), one aliquot (25 μL) was taken and quenched with 1.2 μL 1 mM glycine (1.2 nmol) before activity assay. The aliquot (26.2 μL) was added to extraction buffer (58.8 μL), 10x reaction buffer (Ready-to-Use reagent from Biovision calpain activity assay kit) (10 μl) and calpain substrate (5 μL). Then the mixture was incubated at 37 °C for 1 hour in the dark and fluorescence was detected using Spectra Max Gemini spectrofluorometer (excitation 400 nm, emission 505 nm) (Molecular Devices, Sunnyvale, CA). A negative control was performed by using the same method with calpain inhibitor Z-LLY-FMK (Biovision calpain activity assay kit).
MALDI-TOF MS analysis
Initial diagnostic analysis of peptide samples was performed by MALDI-TOF mass spectrometry on an AB Sciex4800 Plus MALDI TOF/TOF instrument. Reaction aliquots were resuspended in 10% v/v aqueous acetonitrile, 0.1% v/v formic acid, and Ziptip purified (Millipore, ZipTip with 0.6 μL C18 resin). The Ziptip was washed 3x with 0.1% v/v formic acid/water (wash solution) and eluted with 5 μL of 80% v/v acetonitrile/0.1% v/v formic acid (elution solution). Two microliters of eluant was mixed with 1 μL of matrix [α-cyano-4-hydroxycinnamic acid (CHCA), 10 mg/ml in 50% v/v acetonitrile, 0.1% v/v formic acid], and 1.5 μL applied to the MALDI target plate. MS spectra were generated with 1000 laser shots per spectrum at a laser intensity of 3000. A 6 peptide mass standards kit (AB Sciex) was used to calibrate the instrument with minimum signal-to-noise ratio of 20 and maximum error of 100 ppm. Data was processed with Data Explorer 4.0 (AB Sciex) software.
Electrospray LC-MS/MS analysis
Iso[4]LGE2-modified peptides were analyzed by LC MS/MS on an API 3000 triple qudarupole electrospray mass spectrometer (AB Sciex) equipped with a Waters Acquity UPLC system.21 Modified β-amyloid(11-17) peptide was chromatographed on a C18 DIONEX Acclaim column (300 μm x 150 mm, 5μm particle size) with aqueous trifluoroacetic acid (TFA)/acetonitrile solvents, a flow rate of 20 μl/min, and the following gradient: 2–12% v/v B over 5 min, to 60 % v/v over 48 min, to 98% v/v B over 2 min, and hold for 10 min (solvent A, 0.1% v/v TFA; solvent B, 0.1% v/v TFA in acetonitrile). Modified acetyl-gly-lys-O-methyl ester peptide was chromatographed on C18 Waters HSS column (2.1 x 150 mm, 1.8 μm particle size) with aqueous formic acid (FA)/acetonitrile solvents, at a flow rate of 70 μl/min with the following gradient: 2–10% v/v B over 2 min, to 98 % v/v within 25 min, and hold for 18 min (solvent A, 0.1% v/v FA, solvent B, 0.1% v/v FA in acetonitrile). Chromatography effluents were monitored with the mass spectrometer operated in the positive mode, nitrogen as source gas and scanning from m/z 220–2000. The ion spray voltage was set at 4000 V and source temperature was set at 200 °C.
QTOF MS/MS analysis
Following ZipTip purification, iso[4]LGE2-modified N-acetylglycyllysine methyl ester was analyzed on a quadrupole-time-of-flight (Q-TOF-2) mass spectrometer (Waters). The modified peptide (133 fmol/μl) was infused onto a capillary column (PicoFrit™ 75μm x 70 mm, 15 μ tip ID; New Objective Inc., Woburn, MA) at 0.5 μl/min using a syringe pump (Harvard apparatus, Pump 11) with a 100 μl Hamilton syringe, and ionized using an electrospray source designed in-house. The mass spectrometer was operated in MS and MS/MS modes. MS data was collected over the mass range of 50–1500 m/z and MS/MS data was analyzed with MassLynx v4.1 software (Waters). The instrument was calibrated using a solution of [Glu1]-Fibrinopeptide B (Sigma) in 50% v/v aqueous acetonitrile containing 0.1% v/v formic acid. The intensity of the peak from the MS/MS spectrum at m/z 785.84 m/z was used as a reference for calibration with mass measurement error of ≤ 10 ppm.
Electrophoresis and Western analysis
Sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Western analysis were performed according to published protocols.22 Proteins (~ 5–10 μg) were suspended in Laemmli sample buffer,23 and fractionated on a 4–12% acylamide gradient gel. Gels were either stained with Coomassie Blue and staining intensity quantified using a GS-710 scanner and QuantityOne® software (Bio-Rrad (Hercules, CA). or electroblotted to polynivyl difluoride (PVDF) membrane (Millipore, Bedford, MA) for Westrern analysis. PVDF membranes were treated with Odyssey blocking buffer (Li-Cor Cat# 927-40000) and probed with anti-calpain-1 small subunit (Fisher Cat#01674303) mAb or anti-calpain-1 catalytic subunit (Fisher Cat#01674304) mAb. Goat anti-mouse IRDye® 680 (Odyssey Cat#926-32220) was used as the secondary antibody. The immunoreactive bands were detected and quantified using a Li-Cor infrared imaging system.
RESULTS
Iso[4]LGE2 abolishes calpain-1 protease activity
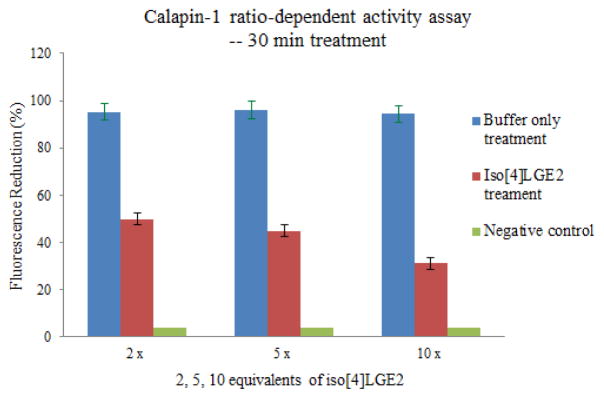
Calpains are ubiquitous calcium-dependent cysteine proteases that exist in two forms in all tissues, μ-calpain and m-calpain. Both forms comprise a large subunit of about 80 kDa and a small subunit of about 28 kDa. The activity of calpain-1 extracted from glaucomatous trabecular meshwork (TM) tissue is significantly less than calpain-1 extracted from normal TM in spite of the fact that the levels of calpain-1 in glaucotomous TM are greatly elevated compared with those in normal TM.15 Because isoLG-protein adducts accumulate in TM tissue in individuals with primary open angle glaucoma, we speculated that the paradoxical accumulation of inactive calpain-1 in TM might be explicable if modification by isoLGs renders calpain-1 inactive. We previously confirmed that treatment of calpain-1 with isoLGs in vitro abolishes its catalytic activity.15 To further characterize the relationship between calpain-1 enzymatic activity and protein modification by isoLGs, we monitored the time and isoLG/protein ratio dependence of activity loss upon treatment with iso[4]LGE2 in vitro. The reaction was quenched by addition of glycine and the resulting mixture was assayed for enzymatic activity and further characterized by Western analysis and mass spectrometry (vide infra). Our data show that exposure of calpain-1 to iso[4]LGE2 in vitro diminished enzyme activity in a time- (Figure 1) and ratio-dependent (Figure 2) manner.
Figure 1.
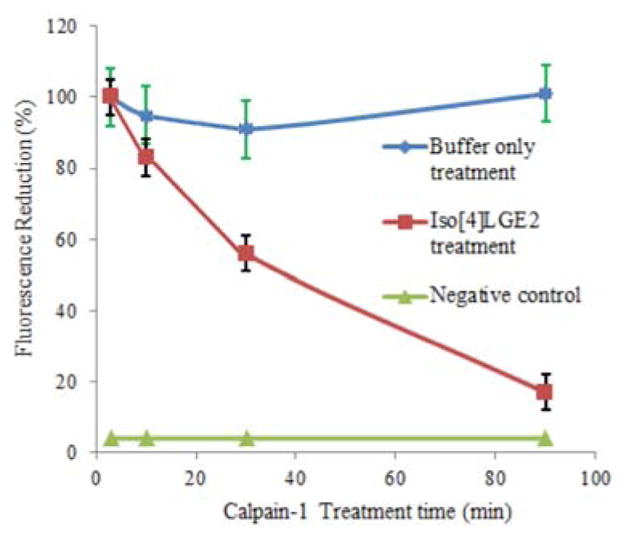
Time-course for the decrease of calpain-1 activity after a treatment with 10 equivalents of iso[4]LGE2 at 37 °C.
Figure 2.
Change in calpain-1 enzymatic activity after a treatment with 2, 5 or 10 equivalents of iso[4]LGE2 for 30 min at 37 °C (n = 3, ± SD).
Notably, the inhibitory effect was not linearly related to isoLG:protein ratio. Five equivalents of isoLG had little additional inhibitory effect over a 2:1 molar ratio and the inhibition caused by a 10 equivalents of isoLG to protein was less than twice that of 2 equivalents. This behavior suggests that a low level of modification by one or two molecules of isoLG has a profound effect on the enzymatic activity, and that additional modifications only produced minor additional inhibitory effects. The time dependence of the effect of exposure of the protein to 10 equivalents of iso[4]LGE2 was also examined. Enzymatic activity decreased by 50% within 30 min and gradually decreased to 10% by 90 min while the activity of the control, treated with buffer alone, was unchanged.
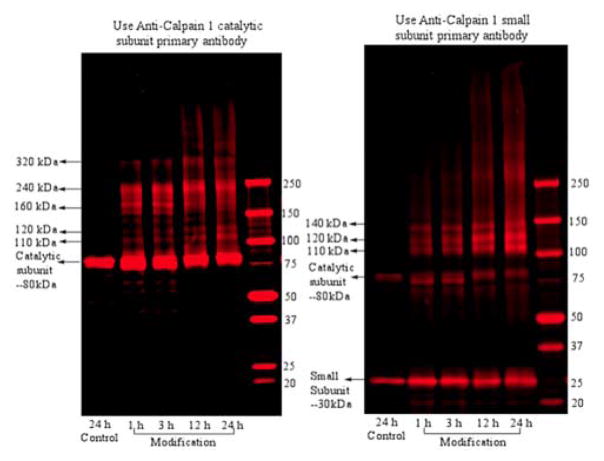
Iso[4]LGE2 Generates Inter-subunit Cross-links in Calpain-1
The time course of iso[4]LGE2 modification of calpain-1 was monitored. The oligomerizations were terminated by the addition of excess glycine after various incubation times, and the reaction mixtures were fractionated by SDS-PAGE and detected by Western blot analysis (Figure 3). Apparent dimers and higher oligomers were prominent after only 1 h of incubation with 50 equivalents of iso[4]LGE2. Notably, intense bands for the calpain-1 catalytic subunit, its dimer and trimer at 80, 160, 240 and 320 kDa were detected with the catalytic subunit antibody but not with the regulatory subunit antibody (Figure 3). By contrast, the control, calpain-1 in the absence of iso[4]LGE2, exhibited no oligomerization after incubation for 24 h. The anti-calpain-1 catalytic subunit antibody detected a band at 110 kDa that likely corresponds to a hetero dimer of one catalytic subunit (80 kDa) with one regulatory subunit (28 kDa). It also detected bands at 160 kDa, 240 kDa and 320 kDa that likely correspond to a dimer, trimer and tetramer of the catalytic subunit. The anti-calpain-1 regulatory subunit antibody also detected a band at 110 kDa, again suggesting a hetero dimer of one catalytic subunit and one regulatory subunit. Bands detected by the calpain-1 regulatory subunit antibody at 120 kDa could correspond to a tetramer of the regulatory subunit, and at 140 kDa could correspond to a heterotrimer of two regulatory subunits cross-linked with one catalytic subunit. Both the anti-catalytic and anti-regulatory antibodies exhibited some low level cross-reactivity in the Western analyses in Figure 3. Although the molecular structure of the protein cross-link remains unknown,13, 24 the oligomers are resistant to dissociation by SDS and heat under reducing conditions, indicating a high probability that the cross-link involves covalent bonding.
Figure 3.
Western blot analysis of iso[4]LGE2 (50 equivalents) modified calpain-1 after reaction for 1 h, 3 h, 12 h and 24 h. Left panel: developed with anti-calpain-1 catalytic subunit. Right panel: developed with anti-calpain-1 small regulatory subunit.
Mass spectrometric analysis of the iso[4]LGE2-peptide adducts
N-Acetyl-glycyl-lysine methyl ester (N-acetyl-gly-lys-OMe) and β-amyloid(11-17) peptide EVHHQKL were used as models for characterizing the modification of protein lysyl residues resulting from adduction of iso[4]LGE2. Iso[4]LGE2 was incubated with N-acetyl-glycyl-lysine methyl ester or β-amyloid(11-17) peptide EVHHQKL individually at pH 8.6 (50 mM N-ethylmorpholine acetate) and 25 °C for several days. The resulting mixtures were dried, resuspended in 10% v/v acetonitrile containing 0.1% v/v formic acid and analyzed by mass spectrometry. Modification reactions conducted in acidic (50 mM phosphate buffer, pH 5.8) or neutral (50 mM phosphate buffer, pH 7.0) solutions resulted in similar outcomes as in basic buffer. The reaction product profile exhibited no relationship with buffer pH (vide infra).
(i) Modification of N-acetyl-gly-lys methyl ester by iso[4]LGE2
MALDI-TOF analysis of the reaction product mixture from iso[4]LGE2 with a large excess of N-acetyl-glycyl-lysine methyl ester showed major ions at m/z 576, 592, 608, 853, and 1149 corresponding to protonated pyrrole, lactam, hydroxylactam, aminal cross-link and bispyrrole in addition to ions resulting from the loss of one or two molecules of water from those adducts. Figure 4 shows a representative spectrum revealing the presence of various adducts in the reaction product mixture (see supplementary Information Table 1S for the molecular weights of various iso[4]LGE2-(N-acetyl-gly-lys-OMe) adducts).
Figure 4.
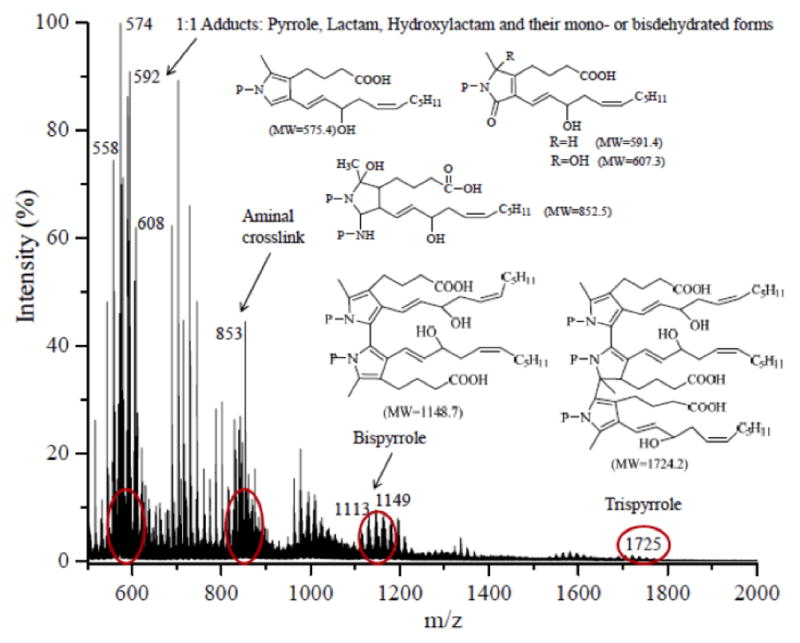
MALDI-TOF analysis of the reaction mixture from N-acetyl-glycyl-lysine methyl ester and iso[4]LGE2 (molar ratio = 200:1) (50 mM NEM·OAc aqueous solution with 4% methanol, v/v, pH 8.6) at 25 °C for 4 days.
The reaction products for various reactant molar ratios were recorded (see Supplementary Information Figure S1 (1) for N-acetyl-glycyl-lysine methyl ester: iso[4]LGE2 = 2000:1, (2) for 1000:1 and (3) for 200:1 in 1, 4, 7, 15, 21 and 50 day reactions). The initial concentration of N-acetyl-glycyl-lysine methyl ester was constant while various ratios of iso[4]LGE2 were added. At various incubation times, identical aliquots of reaction mixture were purified by Ziptip and then analyzed. One-to-one adducts, i.e., pyrrole, lactam and hydroxylactam and their dehydration products were generated very quickly in the reaction of iso[4]LGE2 with N-acetyl-glycyl-lysine methyl ester (see Supplementary Information Figure S1A).
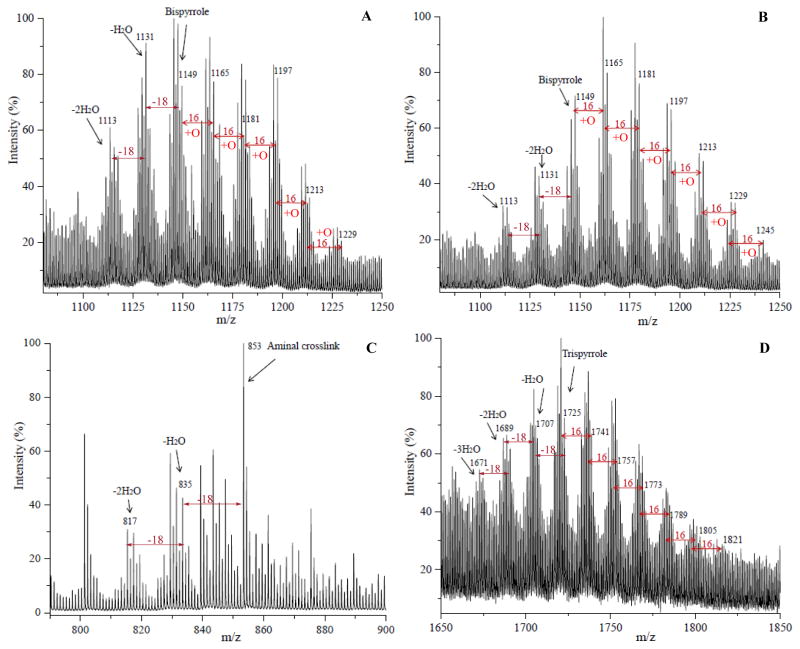
Cross-link adducts appear to include aminals, bispyrroles, trispyrroles and their dehydrated forms. With higher iso[4]LGE2 ratios and longer incubation times, a higher percentage (comparison of cross-link peak intensity with one-to-one adducts) of apparent bispyrrole adducts was generated. Specifically, bispyrrole peak intensity was higher when the molar ratio of N-acetyl-glycyl-lysine methyl ester to iso[4]LGE2 was 200:1 than 2000:1 (compare Supplementary Information Figures 3.1S 3A–F). This suggests that excess dipeptide interferes with pyrrole-pyrrole cross-link formation. Bispyrrole peak intensity was also higher when N-acetyl-glycyl-lysine methyl ester reaction time with iso[4]LGE2 was 15 days versus 1 day. Derivatives of the polypyrroles, i.e., bispyrrole and trispyrrole, showed a sequence of ions corresponding to further addition of oxygen atoms (+16). Each polypyrrole parent ion is accompanied by a series of ions corresponding to the addition of one or more atoms of oxygen to the polypyrrole. The details will be discussed below.
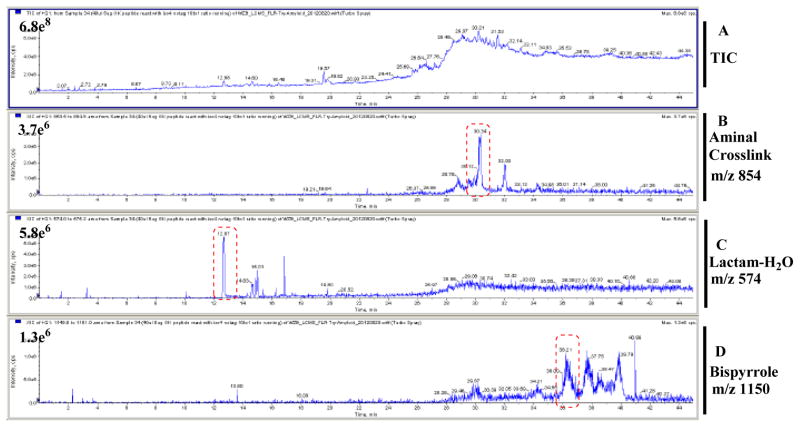
The product mixture from reaction of iso[4]LGE2 with N-acetyl-gly-lys-OMe was separated by reverse phase HPLC using an acetonitrile/water gradient and monitored by LC-ESI extracted ion channels (Figure 5). The major products corresponding to lactam (trace 5C), aminal cross-link (trace 5B) and bispyrrole (trace 5D) eluted in order of decreasing polarity at about 12, 30, and 36 minutes, respectively.
Figure 5.
LC-ESI for the product mixture from the reaction of N-acetyl-glycyl-lysine methyl ester with iso[4]LGE2 (molar ratio = 200:1 at 25 °C for 4 days) with selected ion monitoring for aminal cross-link, lactam-H2O and bispyrrole. A) TIC, B) aminal cross-link m/z 854.1 (+ H+), C) lactam-H2O m/z 574.7 (+ H+), D) bispyrrole m/z 1150.5 (+ H+).
MALDI-TOF MS analysis of the fractions eluting at about 12, 30, 36 and 41 minutes confirmed the presence of the lactam, as well as aminal, bispyrrole and trispyrrole cross-links. Thus, MALDI-TOF MS analysis (Figure 6 panels A and B) of the product eluting at 36 minutes showed major ions at m/z 1149.7, 1131.7 and 1113.7 corresponding to protonated bispyrrole in addition to ions resulting from the loss of one and two molecules of water. MALDI-TOF MS analysis (Figure 6 panel C) of the product eluting at 30 minutes showed major ions at m/z 853.5, 835.5 and 817.5 corresponding to protonated aminal cross-link in addition to ions resulting from the loss of one and two molecules of water. MALDI-TOF MS analysis (Figure 6 panel D) of the product eluting at 41 minutes showed major ions at 1725.1, 1707.1, and 1689.0 corresponding to protonated bispyrrole in addition to ions resulting from the loss of one, two or three molecules of water.
Figure 6.
MALDI-TOF MS spectra: panel A (4 days reaction) and B (15 days reaction) for 36 min HPLC fractions, panel C (15 days reaction) for 30 min HPLC fraction, panel D (15 days reaction) for 41 min HPLC fraction of products from the reaction of N-acetyl-glycyl-lysine methyl ester with iso[4]LGE2 (molar ratio = 200:1 at 25 °C for 4 and 15 days).
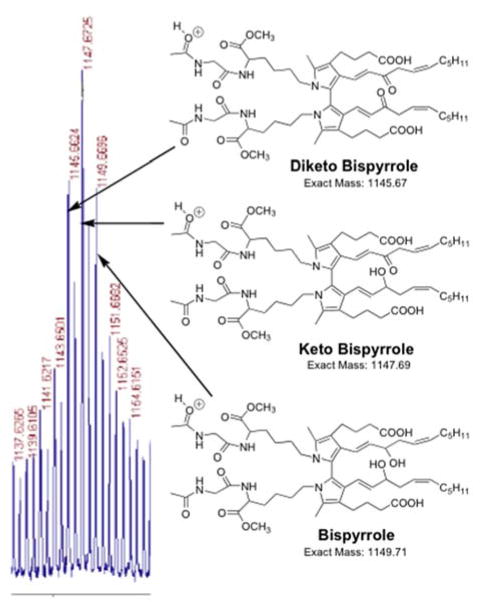
Additional complexity of the reaction product mixture is clearly evident in the MALDI-TOF MS spectrum (Figure 7) of an HPLC fraction containing bispyrrole from the reaction for 21 days of N-acetyl-glycyl-lysine methyl ester with iso[4]LGE2 (molar ratio = 1000:1 at 25 °C). Prominent peaks corresponding to the loss of 2H and 4H presumably correspond to keto bispyrrole and diketobispyrrole generated by oxidative allylic hydroxyl to keto conversions. As for the bispyrrole, further addition of oxygen atoms to the keto bispyrrole and diketo bispyrrole likely contributes to the envelope of peaks associated with the corresponding polyoxygenated bispyrrole derivatives.
Figure 7.
MALDI-TOF MS spectrum of HPLC fraction containing bispyrrole from the reaction of N-acetyl-glycyl-lysine methyl ester with iso[4]LGE2 (molar ratio = 1000:1 at 25 °C for 21 days).
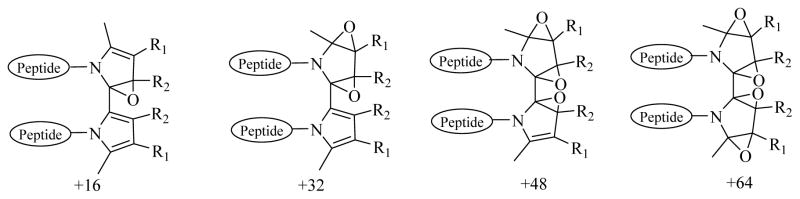
A series of ions are present in the mass spectrum of the isoLG-N-acetyl-gly-lys-OMe bispyrrole cross-link chromatographic fraction corresponding to the addition of one or more atoms of oxygen to the bispyrrole, and the relative amount of these products of further oxidation is greater after incubation for 15 days compared to 4 days (Figure 6 panels A and B, respectively). The peak intensity of products corresponding to addition of oxygen atoms to the bispyrrole was higher compared with bispyrrole and its dehydrated forms after 15 days modification than after 4 days. The pattern of further addition of oxygen atoms (+16) was also observed for the trispyrrole products. This formation of a family of polyoxygenated derivatives is reminiscent of the oxidation of the pyridinium bisretinoid A2E25, 26 (see Discussion below). Thus, pyrrole oligomerizes to form polypyrroles, and all of these products undergo further oxidation. Possible structures for oxidized bispyrrole products of (+16, +32, +48, +64) are shown in Scheme 2.
Scheme 2.
Possible structures for products of oxidized bispyrrole (+16, +32, +48, +64).
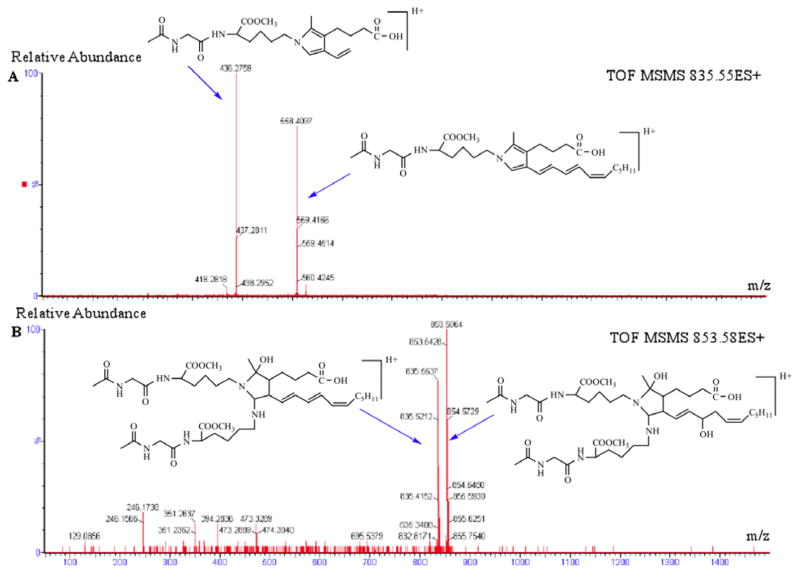
Tandem MS/MS analysis of the aminal cross-link ion was performed by Q-TOF mass spectrometry. ESI-MS/MS analysis of Zip-Tip purified reaction mixture from N-acetyl-glycyl-lysine methyl ester and iso[4]LGE2 (molar ratio = 200:1 at 25 °C for 4 days) provided additional support for the structure postulated for the aminal cross-link. Previously, it was postulated that an initial iso[4]LGE2 Schiff base adduct with the lysine residue of N-acetyl-gly-lys-OMe is an electrophilic intermediate that undergoes aminal cross-linking with another nucleophilic side chain residue of a second molecule of N-acetyl-glycyl-lysine methyl ester.8 The MS/MS spectrum of the parent ion (m/z 853.5) from the putative aminal cross-link shows peaks at m/z 853.5 and 835.5 corresponding to the [M+H]+ ion from iso[4]LGE2-(N-acetyl-gly-lys-OMe) aminal cross-link and its dehydrated form (Figure 8B). The MS/MS spectrum of m/z 835.5 (Figure 8A) exhibits two diagnostic daughter ions at m/z 558.4 and 436.3. The ion at m/z 558.4, which is isobaric with protonated dehydrated pyrrole, corresponds to loss of one molecule of N-acetyl-gly-lys-OMe (−259) and one molecule of H2O (−18). The ion at m/z 436.3 corresponds to loss of non-3-en-1-yne (C9H14) (−122). The proposed structure of the parent and each daughter ion is shown in Figure 8 and Scheme 3. See Supplementary Information Figure S2 B–D and Scheme S1 for ESI-MS/MS spectra and fragmentation structures of the pyrrole, lactam and hydroxylactam one-to-one adducts.
Figure 8.
ESI-MSMS spectra A) for fragmentation of the dehydrated aminal crosslink (+ H+) ion at m/z 835, and B) the molecular ions (+ H+) from the iso[4]LGE2(N-acetyl-gly-lys-oMe aminal crosslink at m/z 853 and its dehydrated form (+ H+) at m/z 835.
Scheme 3.
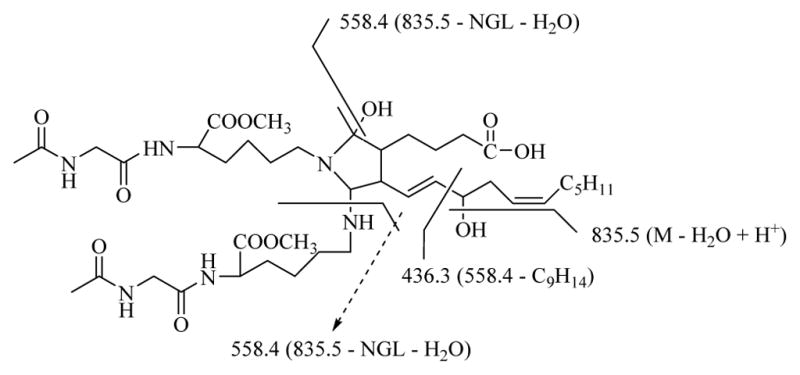
Possible fragmentation of an iso[4]LGE2-(N-acetyl-gly-lys-OMe) aminal cross-link at m/z 853.5 (M + H+).
(ii) Modification of the β-amyloid(11-17) peptide EVHHQKL by iso[4]LGE2
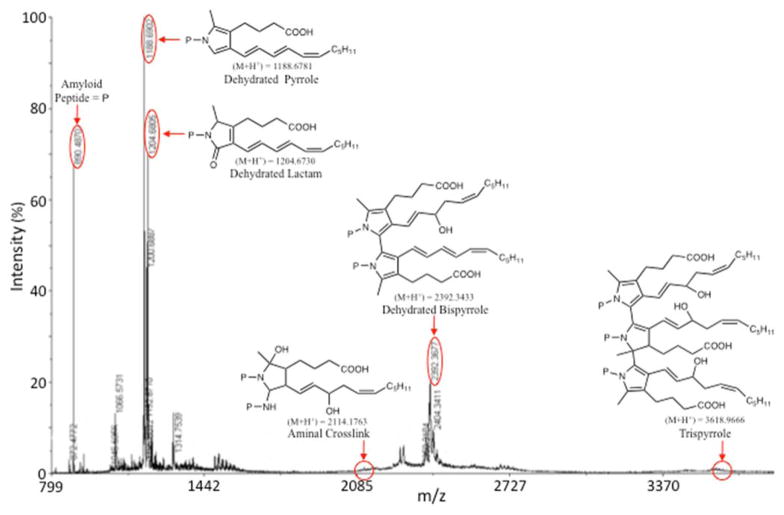
MALDI-TOF MS analysis of the reaction product mixture from iso[4]LGE2 with β-amyloid(11-17) peptide EVHHQKL (Figure 9) showed major ions at m/z 1188, 1204 and 2392 corresponding to dehydrated protonated pyrrole, lactam and bispyrrole, respectively. Other crosslinks, i.e., aminal and trispyrrole, subsequently identified after HPLC fractionation of the reaction product mixture (vide infra) are not prominent. The regions where they are expected to appear are circled. See appendix Table S1 for the molecular weights of various iso[4]LGE2-β-amyloid(11-17) EVHHQKL peptide adducts.
Figure 9.
MALDI-TOF MS analysis of the product mixture from the reaction of β-amyloid(11-17) peptide EVHHQKL with iso[4]LGE2 (molar ratio = 200:1) (50 mM NEM·OAc aqueous solution with 4% v/v methanol, v/v, pH 8.6) 25 °C for 7 days.
Reaction products were recorded for two reactant molar ratios (see Supplementary Information Figure S3 (1) for β-amyloid(11-17) EVHHQKL peptide: iso[4]LGE2 = 2000:1 and (2) 200:1 measured after incubation for 1, 4, 7, 15, 21 and 50 days). The initial concentration of β-amyloid(11-17) EVHHQKL peptide was the same for both reactions while various ratios of iso[4]LGE2 were added. At various incubation times, identical aliquots of the reaction mixture were purified by Ziptip and then analyzed. The 1:1 adducts, i.e., pyrrole, lactam and hydroxylactam and their dehydrated forms, were generated very quickly in the reaction of iso[4]LGE2 with β-amyloid(11-17) EVHHQKL peptide (see Supplementary Information Figure S3 1A). Cross-link adducts included aminals, bispyrroles, trispyrroles and their dehydrated forms. With higher iso[4]LGE2 ratio and longer incubation times, a higher percentage (comparison of cross-link peak intensity with one-to-one adducts) of bispyrrole adducts was generated. Specifically, bispyrrole peak intensity was higher when the molar ratio of β-amyloid(11-17) EVHHQKL peptide to iso[4]LGE2 was 200:1 than 2000:1 (see Supplementary Information Figure S3 2A–F). Bispyrrole peak intensity was also higher (5 % more) when the reaction time for the β-amyloid(11-17) EVHHQKL peptide with iso[4]LGE2 was 15 days compared to just 1 day. A similar trend was observed with the dipeptide. It agrees with the conclusion the dipeptide or EVHHQKL peptide interferes with bispyrrole formation. Bispyrrole formation is an oxidative coupling process that presumably involves the formation of a pyrrole cation radical that reacts with a second pyrrole. Apparently, these peptides react with the putative cation radical intermediate in competition with a second molecule of pyrrole.
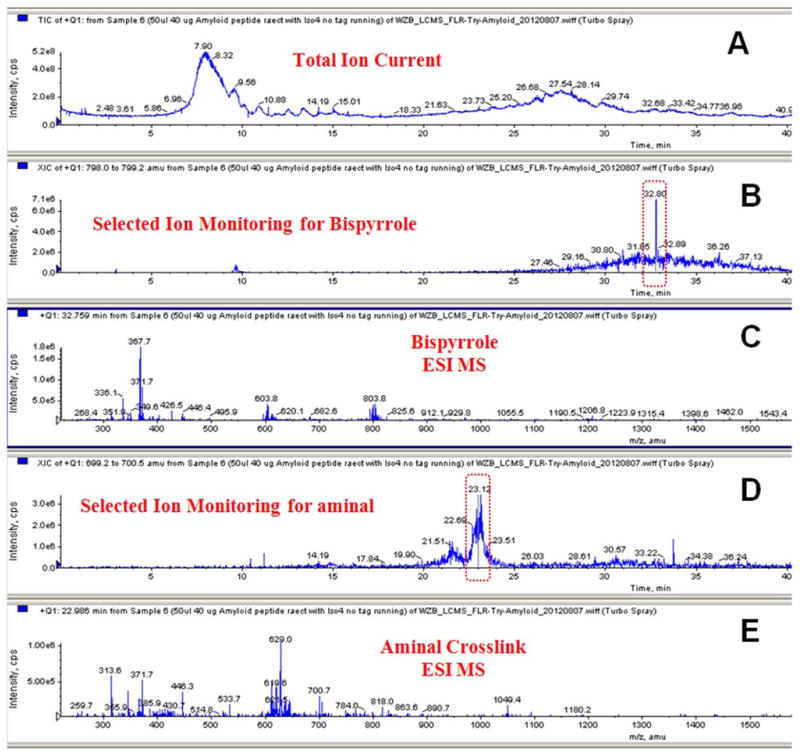
The product mixture from reaction of iso[4]LGE2 with β-amyloid(11-17) peptide EVHHQKL was separated by reverse phase HPLC using an acetonitrile/water gradient and monitored by LC-ESI extracted ion channels (Figure 10). The major products corresponding to aminal cross-link (trace 10C) and bispyrrole (trace 10B) eluted in order of decreasing polarity at about 22 and 32 minutes.
Figure 10.
LC-ESI for the reaction mixture from β-amyloid(11-17) peptide and iso[4]LGE2 (molar ratio = 200:1 at 25 °C for 7 days) with selected ion recording for bispyrrole and aminal cross-links. A) TIC, B) SIM for bispyrrole, C) ESI MS showing peaks corresponding to bispyrrole (m/z 804.1; z = 3) and (m/z 603.3; z = 4) ions, D) SIM for aminal cross-link, E) ESI MS showing peaks corresponding to dehydrated aminal (m/z = 1048.6; z = 2) and (m/z = 699.4; z = 3) ions.
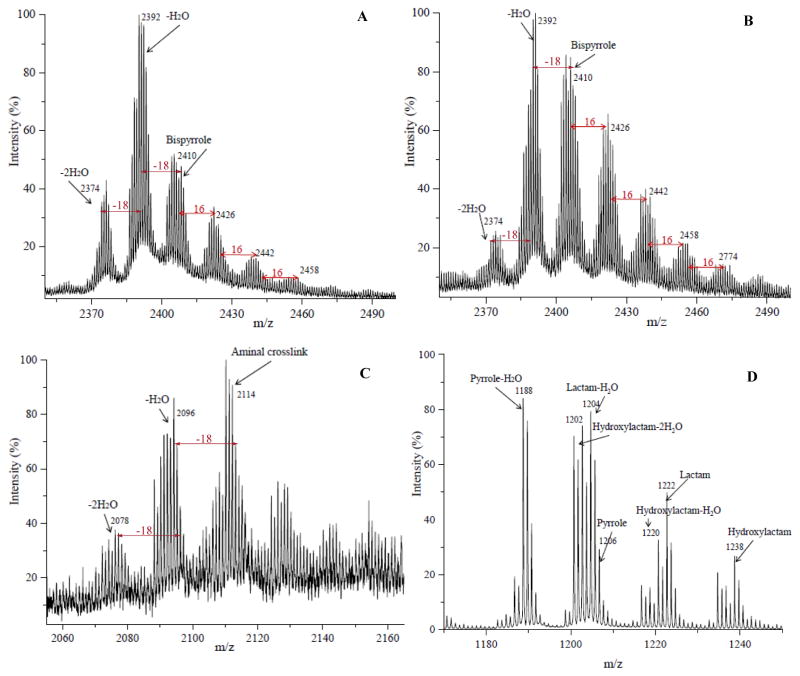
MALDI-TOF MS analysis of the fractions eluted at 22 and 32 minutes confirmed the presence of the aminal and bispyrrole cross-links. MALDI-TOF MS analysis of the aminal fraction (Figure 11C) showed major ions at m/z 2114.2, 2096.2 and 2078.2 corresponding to protonated aminal cross-link in addition to ions resulting from the loss of one and two molecules of water. MALDI-TOF MS analysis of the bispyrrole fraction (Figures 11A and 11B) showed major ions at m/z 2410.3, 2392.3 and 2374.3 corresponding to protonated bispyrrole in addition to ions resulting from the loss of one and two molecules of water. A series of peaks are also present in the mass spectrum of the isoLG-β-amyloid(11-17) peptide chromatographic fraction corresponding to the addition of one or more atoms of oxygen to the bispyrrole, and the relative amount of these products of further oxidation as indicated by the relative peak intensity of further oxidized bispyrrole (products with addition of oxygen atoms) compared with bispyrrole and its dehydrated forms was greater after 15 days incubation than after 7 days (Figure 11 panels A and B, respectively). Monomeric pyrrole, lactam and hydroxylactam derivatives are evident in the MALDI-TOF MS of the HPLC fraction eluting at 15 minutes (Figure 11D).
Figure 11.
MALDI-TOF MS spectra: panels A (7 days reaction) and B (15 days reaction) for 32 min HPLC fractions containing bispyrrole, panels C and D (7 days reaction) for 22 min, 15 min HPLC fractions of products from reaction of the β-amyloid peptide EVHHQKL with iso[4]LGE2 reaction (200:1 molar ratio at 25 °C) containing aminal crosslink and monomeric adducts respectively.
Similar to the iso[4]LGE2-(N-acetyl-gly-lys-OMe) bispyrrole cross-link and that discussed above, a series of products of further addition of oxygen (multiple +16 m/z increments) was also observed for the trispyrrole adduct. Thus, pyrrole derivatives incorporating protein lysyl residue ε-amino groups oligomerize to form polypyrroles that can undergo further oxidation.
DISCUSSION
The biological chemistry of isoLGs is complex and difficult to study owing to their proclivity for covalent adduction with biomolecules. Our efforts to understand this chemistry have spanned nearly four decades. Enabled by fundamental research on the chemistry of the bicyclic peroxide nucleus of the prostaglandin endoperoxide PGH2, progress in understanding isoLG chemistry began in 1977 with our discovery that this bicyclic peroxide undergoes a novel rearrangement that produces a γ-ketoaldehyde, levulinaldehyde.27 That insight guided the demonstration in 1984 that PGH2 itself similarly rearranges to two isomeric γ-ketoaldehydes,28 levuglandin (LG)E2 and LGD2, and the discoveries that LGs avidly react with proteins to form covalent adducts,5 and that this adduction can lead to protein-protein13 and DNA-protein cross-links.14 In 1994 we postulated that stereo and structural LG isomers would be produced by free radical-induced oxidation of arachidonyl phospholipids.9 We then demonstrated that such isomers, referred to collectively as isoLGs, are formed upon free radical-induced oxidation of low-density lipoprotein.29 Thus, although free, unadducted isoLGs have never been isolated from biological samples owing to their proclivity for covalent adduction with proteins and ethanolamine phospholipids,30 our previous studies established the production of isoLG-protein and isoLG-ethanolamine phospholipid derivatives in vivo12, 15, 29, 31–33 and some biological consequences of these lipid oxidation-derived protein modifications.11, 34–36
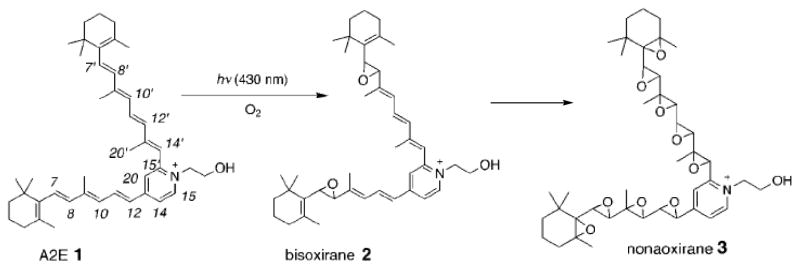
The present study revealed new complexities by showing that isoLGs form both pyrrole-pyrrole and aminal crosslinks, and showed that isoLG-derived pyrrole-pyrrole dimers can undergo further oxygenation to produce complex mixtures of polyoxygenated cross-linked species (see Figures 6 and 11 above). Another example of the generation of complex mixtures of polyoxygenated lipids was reported previously for the pyridinium bisretinoid A2E that undergoes addition of multiple atoms of oxygen to form a family of polyoxygenated derivatives (Scheme 4 and Figure 12).25, 26
Scheme 4.
Structures of A2E (1), the bisoxirane (2) and nonaoxirane (3). A collage of images from Figures 1 and 3 in Angew Chem Int Ed Engl (2002) 41, 814–817.
Figure 12.
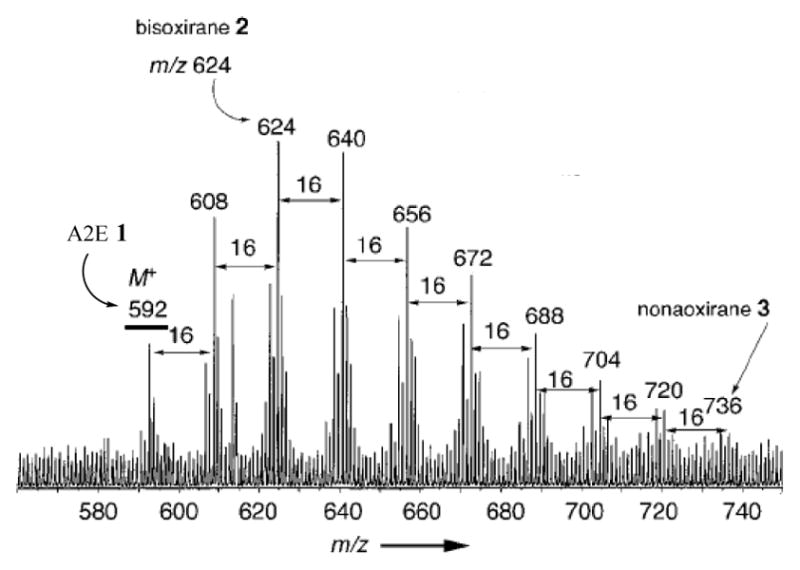
ESI-Q-TOF spectrum showing a series of ions that differ in m/z by 16 owing to the addition of oxygen atoms to A2E. Image from Figure 3 in Angew Chem Int Ed Engl (2002) 41, 814–817.
IsoLG modification of calpain-1
Previously, we found that isoLG modification of proteins is associated with loss-of-function, cross-linking and aggregation. We showed that isoLG inhibits mitosis of sea urchin eggs, and one molecule of isoLG per tubulin monomer abolished its ability to assemble into microtubules.34 Covalent isoLG adduction abolishes the catalytic activity of Cyp27A1, a cytochrome P 450 enzyme. Selective modification of a single lysyl residue, Lys358, in vitro resulted in loss of function. Modification of this key lysyl residue in vivo by isoLG was also detected in human retina.11, 12, 36
There are elevated levels of the protease calpain-1 in glaucomatous TM based on Western analysis.15 In addition, isoLG-modification of calpain-1 in vivo may promote its ubiquitination and the formation of aggregates that resist proteasomal processing as we have observed in vitro15, 37 and presumably contribute to the impeded aqueous humor outflow through the TM that results in elevated intraocular pressure. It appears that calpain-1 is upregulated to promote proteolysis of damaged and post-translationally modified proteins. However, this enzyme function is often diminished by isoLG-modification that renders it inactive. In addition, isoLG modification makes proteins resistant to proteolysis, e.g., by the 20S proteasome.38 Thus, isoLG adduction presumably contributes to accumulation and inactivation of calpain in the TM owing to aggregation and/or resistance to proteolysis.
In the present study, we showed that calpain-1 lost its proteolytic activity with t1/2 = ~40 min upon incubation with 10 equivalents of iso[4]LGE2, and as little as 2 equivalents was almost as effective. This suggested that modification of one or two lysyl residues suffices to abolish enzymatic activity. The types and extent of cross-linking between subunits of the heterodimeric calpain-1 by iso[4]LGE2 were characterized. Using SDS-PAGE with Western blotting with antibodies against the catalytic and regulatory subunits, we detected covalently cross-linked hetero and homo oligomers of calpain-1 catalytic and regulatory subunits.
IsoLG modification of the β-amyloid(11-17) peptide EVHHQKL
We used N-acetyl-glycyl-lysine methyl ester (N-acetyl-Gly-Lys-OMe) and the β-amyloid(11-17) peptide EVHHQKL as models for characterizing the cross-linking of protein lysyl residues resulting from adduction of iso[4]LGE2. Aminal, bispyrrole and trispyrrole cross-links of two model peptides were identified and fully characterized by mass spectrometry, and a large variety of derivatives of the bispyrrole cross-link containing one or more additional atoms of oxygen were discovered. An interesting difference is evident in the predominant crosslink type generated in the reaction of iso[4]LGE2 with N-acetyl-Gly-Lys-OMe compared to the β-amyloid(11-17) peptide EVHHQKL. More aminal cross-link versus bispyrrole is formed during reaction of the dipeptide with iso[4]LGE2. In contrast, more bispyrrole versus aminal cross-link is formed during the reaction of EVHHQKL with iso[4]LGE2. Since β-amyloid tends to spontaneously oligomerize and a dodecanoyl amide of the N-terminal amino group of β-amyloid(11-17) self-assembles into nanofibrils,39 it is tempting to speculate that non-covalent EVHHQKL oligomers containing peptide-derived pyrrole modifications favor pyrrole-pyrrole coupling leading to irreversible aggregation. Previously, the fact that PGH2 accelerates the formation of amyloid β (Aβ)1-42 oligomers in conjunction with lipid modification suggested that LG adduction to Aβ1-42 provides a mechanism that links cyclooxygenase activity to the accumulation of oligomers of Aβ.17 It was suggested that the formation of neurotoxic oligomers of the Aβ1-42 peptide may be a consequence of the covalent cross-linking that was observed upon treatment of Aβ1-42 with LGE2 in vitro.16 We now hypothesize that covalent cross-linking may be especially favorable for Aβ1-42 because its proclivity for noncovalent oligomerization may facilitate the irreversible formation of covalent pyrrole-pyrrole cross-links. Future studies might seek evidence for noncovalent assembly of an isoLG-pyrrole derivative of the EVHHQKL peptide under oxygen free conditions where oxidative coupling, i.e., covalent pyrrole-pyrrole dimerization, is precluded. Our observations on the crosslinking of EVHHQKL by iso[4]LGE2 will facilitate investigation of the influence of such covalent crosslinking on the proclivity of N-dodecanoyl β-amyloid(11-17) toward self assembly, e.g., to determine if crosslinking promotes nanofibril formation.
As suggested in Scheme 1, cyclic and acyclic forms of the aminal crosslink are likely to be in equilibrium. It is important to note that the molecular structures suggested for iso[4]LGE2 crosslinked species shown in the figures and schemes cannot be distinguished from alternative isobaric structures with the data available, and the crosslinks are likely to consist of a complex mixture of structural isomers including 16 different stereoisomeric forms owing to the 4 stereocenters in the pyrrolidine ring of the cyclic aminal crosslink.
Supplementary Material
Acknowledgments
FUNDING SOURCES
This work was supported by NIH Grants EY011373, EY016813 and GM021249.
ABBREVIATIONS
- Aβ
amyloid β
- kDa
kilodalton
- EDTA
ethylenediaminetetraacetic acid
- EGTA
ethylene glycol tetraacetic acid
- ESI
electrospray ionization
- 1H-NMR
proton magnetic resonance
- HPLC
high performance liquid chromatography
- iso[4]LGE2
iso[4]levuglandin E2
- isoLGE2
isolevuglandin E2
- isoLGD2
isolevuglandin D2
- LC
liquid chromatography
- LG
levuglandin
- LGD2
levuglandin D2
- LGE2
levuglandin E2
- MALDI-TOF
matrix assisted laser desorption ionization-time of flight
- MeOH
methanol
- MS/MS
tandem mass spectroscopy
- NEM
N-ethyl morpholine
- PAGE
polyacrylamide gel electrophoresis
- PE
phosphatidylethanolamine
- PGs
prostaglandins
- PGD2
prostaglandin D2
- PGE2
prostaglandin E2
- PGH2
prostaglandin endoperoxide H2
- ppm
parts per million
- PVDF
polyvinylidene fluoride
- Rf
retention factor
- SDS
sodium dodecyl sulfate
- TFA
trifluoroacetic acid
- TLC
thin layer chromatography
- TM
trabecular meshwork
- TMS
trimethyl silylane
- UV
ultraviolet
Footnotes
Table S1. Molecular weight summary iso[4]LGE2 peptide derivatives. Figure S1. N-Acetyl-Gly-Lys-O-Methyl ester reactions with iso[4]LGE2 in different molar ratio and time points. Figure S2. ESI-MSMS of molecular ions at A) aminal cross-link, B) pyrrole, C) lactam and D) hydroxylactam corresponding to an iso[4]LGE2–(N-acetyl-Gly-Lys-OMe) adduct. Scheme S1. Possible fragmentation of (N-acetyl-Gly-Lys-OMe)-iso[4]LGE2 adducts. Figure S3. Amyloid(11-17) EVHHQKL peptide reactions with iso[4]LGE2 in different molar ratio and time points. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Salomon RG. Levuglandins and isolevuglandins: Stealthy toxins of oxidative injury. Antioxid Redox Signal. 2005;7:185–201. doi: 10.1089/ars.2005.7.185. [DOI] [PubMed] [Google Scholar]
- 2.Zagorski MG, Salomon RG. Prostaglandin endoperoxides. 11 Mechanism of amine-catalyzed fragmentation of 2,3-dioxabicyclo[2.2.1]heptane. J Am Chem Soc. 1980;102:2501–2503. [Google Scholar]
- 3.Boutaud O, Andreasson KI, Zagol-Ikapitte I, Oates JA. Cyclooxygenase-dependent lipid-modification of brain proteins. Brain Pathol. 2005;15:139–142. doi: 10.1111/j.1750-3639.2005.tb00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiteller G, Afzal M. The action of peroxyl radicals, powerful deleterious reagents, explains why neither cholesterol nor saturated fatty acids cause atherogenesis and age-related diseases. Chemistry. 2014;20:14928–14945. doi: 10.1002/chem.201404383. [DOI] [PubMed] [Google Scholar]
- 5.Salomon RG, Jirousek MR, Ghosh S, Sharma RB. Prostaglandin endoperoxides 21. Covalent binding of levuglandin E2 with proteins. Prostaglandins. 1987;34:643–656. doi: 10.1016/0090-6980(87)90289-9. [DOI] [PubMed] [Google Scholar]
- 6.Guo L, Gragg SD, Chen Z, Zhang Y, Amarnath V, Davies SS. Isolevuglandin-modified phosphatidylethanolamine is metabolized by NAPE-hydrolyzing phospholipase D. J Lipid Res. 2013;54:3151–3157. doi: 10.1194/jlr.M042556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salomon RG, Sha W, Brame C, Kaur K, Subbanagounder G, O’Neil J, Hoff HF, Roberts LJ. Protein adducts of iso 4 levuglandin E-2, a product of the isoprostane pathway, in oxidized low density lipoprotein. J Biol Chem. 1999;274:20271–20280. doi: 10.1074/jbc.274.29.20271. [DOI] [PubMed] [Google Scholar]
- 8.Difranco E, Subbanagounder G, Kim S, Murthi K, Taneda S, Monnier VM, Salomon RG. Formation and stability of pyrrole adducts in the reaction of levuglandin E(2) with proteins. Chem Res Toxicol. 1995;8:61–67. doi: 10.1021/tx00043a008. [DOI] [PubMed] [Google Scholar]
- 9.Kobierski ME, Kim SC, Murthi KK, Iyer RS, Salomon RG. Synthesis of a pyrazole isostere of pyrroles formed by the reaction of the epsilon-amino groups of protein lysyl residues with levuglandin E(2) J Org Chem. 1994;59:6044–6050. [Google Scholar]
- 10.Brame CJ, Salomon RG, Morrow JD, Roberts LJ. Identification of extremely reactive gamma-ketoaldehydes (isolevuglandins) as products of the isoprostane pathway and characterization of their lysyl protein adducts. J Biol Chem. 1999;274:13139–13146. doi: 10.1074/jbc.274.19.13139. [DOI] [PubMed] [Google Scholar]
- 11.Charvet CD, Laird J, Xu Y, Salomon RG, Pikuleva IA. Posttranslational modification by an isolevuglandin diminishes activity of the mitochondrial cytochrome P450 27A1. J Lipid Res. 2013;54:1421–1429. doi: 10.1194/jlr.M035790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charvet CD, Liao WL, Heo GY, Laird J, Salomon RG, Turko IV, Pikuleva IA. Isolevuglandins and Mitochondrial Enzymes in the Retina mass spectrometry detection of post-translational modification of sterol-metabolizing CYP27A1. J Biol Chem. 2011;286:20413–20422. doi: 10.1074/jbc.M111.232546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iyer RS, Ghosh S, Salomon RG. Levuglandin-E2 crosslinks proteins. Prostaglandins. 1989;37:471–480. doi: 10.1016/0090-6980(89)90096-8. [DOI] [PubMed] [Google Scholar]
- 14.Murthi KK, Friedman LR, Oleinick NL, Salomon RG. Formation of DNA-protein cross-links in mammalian cells by levuglandin E2. Biochemistry (Mosc) 1993;32:4090–4097. doi: 10.1021/bi00066a034. [DOI] [PubMed] [Google Scholar]
- 15.Govindarajan B, Laird J, Salomon RG, Bhattacharya SK. Isolevuglandin-modified proteins, including elevated levels of inactive calpain-1, accumulate in glaucomatous trabecular meshwork. Biochemistry (Mosc) 2008;47:817–825. doi: 10.1021/bi701517m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boutaud O, Montine TJ, Chang L, Klein WL, Oates JA. PGH2-derived levuglandin adducts increase the neurotoxicity of amyloid beta1-42. J Neurochem. 2006;96:917–923. doi: 10.1111/j.1471-4159.2005.03586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boutaud O, Ou JJ, Chaurand P, Caprioli RM, Montine TJ, Oates JA. Prostaglandin H2 (PGH2) accelerates formation of amyloid beta1-42 oligomers. J Neurochem. 2002;82:1003–1006. doi: 10.1046/j.1471-4159.2002.01064.x. [DOI] [PubMed] [Google Scholar]
- 18.Zagol-Ikapitte I, Masterson TS, Amarnath V, Montine TJ, Andreasson KI, Boutaud O, Oates JA. Prostaglandin H2-derived adducts of proteins correlate with Alzheimer’s disease severity. J Neurochem. 2005;94:1140–1145. doi: 10.1111/j.1471-4159.2005.03264.x. [DOI] [PubMed] [Google Scholar]
- 19.Jirousek MR, Murthi KK, Salomon RG. Electrophilic levuglandin E2-protein adducts bind glycine: A model for protein crosslinking. Prostaglandins. 1990;40:187–203. doi: 10.1016/0090-6980(90)90083-8. [DOI] [PubMed] [Google Scholar]
- 20.Boutaud O, Brame CJ, Chaurand P, Li JY, Rowlinson SW, Crews BC, Ji C, Marnett LJ, Caprioli RM, Roberts LJ, Oates JA. Characterization of the lysyl adducts of prostaglandin H-synthases that are derived from oxygenation of arachidonic acid. Biochemistry (Mosc) 2001;40:6948–6955. doi: 10.1021/bi002629k. [DOI] [PubMed] [Google Scholar]
- 21.Ni J, Yuan X, Gu J, Yue X, Gu X, Nagaraj RH, Crabb JW. Plasma protein pentosidine and carboxymethyllysine, biomarkers for age-related macular degeneration. Mol Cell Proteomics. 2009;8:1921–1933. doi: 10.1074/mcp.M900127-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crabb JW, Miyagi M, Gu XR, Shadrach K, West KA, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn ME, Salomon RG, Hollyfield JG. Drusen proteome analysis: An approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laemmli UK. Cleavage of structural proteins during assembly of head of bacteriophage-T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Iyer RS, Kobierski ME, Salomon RG. Generation of pyrroles in the reaction of levuglandin E(2) with proteins. J Org Chem. 1994;59:6038–6043. [Google Scholar]
- 25.Ben-Shabat S, Itagaki Y, Jockusch S, Sparrow JR, Turro NJ, Nakanishi K. Formation of a nonaoxirane from A2E, a lipofuscin fluorophore related to macular degeneration, and evidence of singlet oxygen involvement. Angew Chem Int Ed Engl. 2002;41:814–817. doi: 10.1002/1521-3773(20020301)41:5<814::aid-anie814>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 26.Sparrow JR, Vollmer-Snarr HR, Zhou J, Jang YP, Jockusch S, Itagaki Y, Nakanishi K. A2E-epoxides damage DNA in retinal pigment epithelial cells. Vitamin E and other antioxidants inhibit A2E-epoxide formation. J Biol Chem. 2003;278:18207–18213. doi: 10.1074/jbc.M300457200. [DOI] [PubMed] [Google Scholar]
- 27.Salomon RG, Salomon MF. 2,3-Dioxabicyclo [2.2.1] heptane. The strained bicyclic peroxide nucleus of prostaglandin endoperoxides. J Am Chem Soc. 1977;99:3501–3503. doi: 10.1021/ja00452a052. [DOI] [PubMed] [Google Scholar]
- 28.Salomon RG, Miller DB, Zagorski MG, Coughlin DJ. Solvent Induced Fragmentation of Prostaglandin Endoperoxides. New Aldehyde Products from PGH2 and a Novel Intramolecular 1,2-Hydride Shift During Endoperoxide Fragmentation in Aqueous Solution. J Am Chem Soc. 1984;106:6049–6060. [Google Scholar]
- 29.Salomon RG, Subbanagounder G, Singh U, O’Neil J, Hoff HF. Oxidation of low-density lipoproteins produces levuglandin-protein adducts. Chem Res Toxicol. 1997;10:750–759. doi: 10.1021/tx970016b. [DOI] [PubMed] [Google Scholar]
- 30.Wang H, Guo J, West XZ, Bid HK, Lu L, Hong L, Jang GF, Zhang L, Crabb JW, Linetsky M, Salomon RG. Detection and biological activities of carboxyethylpyrrole ethanolamine phospholipids (CEP-EPs) Chem Res Toxicol. 2014;27:2015–2022. doi: 10.1021/tx500216a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poliakov E, Meer SG, Roy SC, Mesaros C, Salomon RG. Iso 7 LGD(2) - Protein adducts are abundant in vivo and free radical-induced oxidation of an arachidonyl phospholipid generates this D series isolevuglandin in vitro. Chem Res Toxicol. 2004;17:613–622. doi: 10.1021/tx034185+. [DOI] [PubMed] [Google Scholar]
- 32.Li W, Laird JM, Lu L, Roychowdhury S, Nagy LE, Zhou R, Crabb JW, Salomon RG. Isolevuglandins covalently modify phosphatidylethanolamines in vivo: detection and quantitative analysis of hydroxylactam adducts. Free Radic Biol Med. 2009;47:1539–1552. doi: 10.1016/j.freeradbiomed.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Govindarajan B, Junk A, Algeciras M, Salomon RG, Bhattacharya SK. Increased isolevuglandin-modified proteins in glaucomatous astrocytes. Mol Vis. 2009;15:1079–1091. [PMC free article] [PubMed] [Google Scholar]
- 34.Murthi KK, Salomon RG, Sternlicht H. Levuglandin E2 inhibits mitosis and microtubule assembly. Prostaglandins. 1990;39:611–622. doi: 10.1016/0090-6980(90)90022-n. [DOI] [PubMed] [Google Scholar]
- 35.Schmidley JW, Dadson J, Iyer RS, Salomon RG. Brain Tissue Injury and Blood-Brain Barrier Opening Induced by Injection of LGE2 or PGE2. Prostaglan Leukotri Essent Fatty Acids. 1992;47:105–110. doi: 10.1016/0952-3278(92)90145-9. [DOI] [PubMed] [Google Scholar]
- 36.Charvet CD, Saadane A, Wang M, Salomon RG, Brunengraber H, Turko IV, Pikuleva IA. Pretreatment with pyridoxamine mitigates isolevuglandin-associated retinal effects in mice exposed to bright light. J Biol Chem. 2013;288:29267–29280. doi: 10.1074/jbc.M113.498832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Govindarajan B, Laird JM, Sherman R, Salomon RG, Bhattacharya SK. Neuroprotection in Glaucoma Using Calpain-1 Inhibitors: Regional Differences in Calpain-1 Activity in the Trabecular Meshwork, Optic Nerve and Implications for Therapeutics. CNS Neurol Disord Drug Targets. 2008;7:295–304. doi: 10.2174/187152708784936644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davies SS, Amarnath V, Montine KS, Bernoud-Hubac N, Boutaud O, Montine TJ, Roberts LJ. Effects of reactive gamma-ketoaldehydes formed by the isoprostane pathway (isoketals) and cyclooxygenase pathway (levuglandins) on proteasome function. FASEB J. 2002;16:715–717. doi: 10.1096/fj.01-0696fje. [DOI] [PubMed] [Google Scholar]
- 39.Deng M, Yu D, Hou Y, Wang Y. Self-assembly of peptide-amphiphile C12-Abeta(11-17) into nanofibrils. J Phys Chem B. 2009;113:8539–8544. doi: 10.1021/jp904289y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.