Abstract
A major objective of the emerging field of exercise-oncology research is to determine the efficacy of, and biological mechanisms by which, aerobic exercise affects cancer incidence, progression and/or metastasis. There is a strong inverse association between self-reported exercise and the primary incidence of several forms of cancer; similarly, emerging data suggest that exercise exposure following a cancer diagnosis may improve outcomes for early-stage breast, colorectal, or prostate cancer. Arguably, critical next steps in the development of exercise as a candidate treatment in cancer control require preclinical studies to validate the biological efficacy of exercise, identify the optimal “dose”, and pinpoint mechanisms of action. To evaluate the current evidence base, we conducted a critical systematic review of in vivo studies investigating the effects of exercise in cancer prevention and progression. Studies were evaluated on the basis of tumor outcomes (e.g., incidence, growth, latency, metastasis), dose-response, and mechanisms of action, when available. A total of 53 studies were identified and evaluated on tumor incidence (n=24), tumor growth (n=33) or metastasis (n=10). We report that the current evidence base is plagued by considerable methodological heterogeneity in all aspects of study design, end points, and efficacy. Such heterogeneity precludes meaningful comparisons and conclusions at present. To this end, we provide a framework of methodological and data reporting standards to strengthen the field to guide the conduct of high-quality studies required to inform translational, mechanism-driven clinical trials.
Keywords: Exercise, Physical Activity, Mouse Models, Rat Models, Cancer, Carcinogenesis, Metastasis, Mechanisms
INTRODUCTION
Structured exercise training (hereto referred to as exercise) is considered an integral component of “standard of care” therapy in primary and secondary prevention of numerous common chronic conditions (1–4). In comparison, the role of exercise has received surprisingly little attention in individuals at high-risk or following a diagnosis of cancer. Over the past two decades however, an increasing number of groups are investigating the effects of general physical activity as well as exercise in the oncology setting, a field now commonly referred to as “Exercise-Oncology” (5).
A strong body of observational evidence indicates that higher levels of self-reported exercise, physical activity, as well as cardiorespiratory fitness (i.e., objective assessment of exercise exposure) are inversely associated with the primary incidence of several forms of cancer. This evidence base is summarized by several excellent systematic reviews and meta-analyses (6–9). For example, exercise participation consistent with the national guidelines (i.e., 150 minutes of moderate-intensity or 75 minutes of vigorous-intensity exercise per week) is associated, on average, with 25%, and 30% to 40% risk reductions in breast and colon cancers, respectively compared with inactivity. There is also evidence of a linear dose-response relationship in prevention of breast and colon cancer. On this basis, the evidence for the exercise-prevention relationship is categorized as “convincing” for breast and colon cancer by several national agencies, and regarding as “encouraging” or “promising” for the prevention of prostate, lung, and endometrial cancers (10, 11). On the basis of this data, several phase II randomized controlled trials (RCTs) were initiated, predominantly in breast cancer prevention, to investigate the effects of highly-structured exercise on modulation of host-related factors (e.g., adiposity, circulating factors including sex-steroid and metabolic sex hormones, and the immune-inflammatory axis) that may underpin the exercise-cancer prevention relationship (6, 12–14). In general, exercise was associated with modest changes in markers of adiposity and select circulating factors (6, 12, 13). Whether the observed alterations are of biological or clinical importance remains to be determined, as only one trial to date has investigated whether exercise-induced changes in circulating factors occur in conjunction with changes in factors/pathways in the organ/tissue of primary interest (i.e., colon crypts) (15). Whether exercise reduces cancer incidence or modulates established surrogate markers of cancer incidence has not been investigated.
In patients with cancer, a steadily growing and ever diversifying series of studies indicate that, in general, exercise is a tolerable adjunct therapy associated with significant benefit across a wide range of symptom control variables both during and after primary adjuvant therapy (5, 16, 17). This data combined with the powerful inverse relationships with primary cancer incidence has led researchers to investigate whether exercise influences disease outcomes after diagnosis (18, 19). Although not nearly as mature as the evidence in the prevention setting, emerging observational data suggest that regular exercise exposure is associated with between a 10%–50% reduction in the risk of recurrence and cancer-specific death in patients with colorectal, breast, and prostate cancer (reviewed in (20)), compared to inactivity.
In the development of potential drug candidates, data from observational studies is insufficient to support the initiation of human trials; appropriate preclinical evidence is first required prior to human testing (21). While exercise is not a drug and exhibits a markedly different safety profile than most anticancer agents, the use of preclinical models are of critical importance to confirm biological plausibility, establish the therapeutic window of efficacy, a biologically effective dose, and identify predictors of response (22). This evidence, combined with data from epidemiological and molecular epidemiological studies facilitates the design of early “signal-seeking” clinical studies and ultimately definitive RCTs. Accordingly, we conducted a critical systematic review of in vivo pre-clinical studies across the cancer continuum (i.e., prevention through metastasis). A secondary purpose was to provide recommendations to facilitate the standardization of the conduct of in vivo exercise-oncology studies as well as directions for future research.
METHODS
Search Strategy and Inclusion Criteria
A systematic literature search was conducted using OVID MEDLINE (1950 to May 2015), PUBMED (1962 to May 2015) and WEB OF SCIENCE (1950 to May 2015) with MeSH terms and keywords related to exercise, cancer and animals. Text words were searched and appropriate MeSH terms were found (Table S1). When possible, Boolean logic was used with MeSH terms to build searches. All results and reference lists were searched manually. Peer-reviewed research articles involving animals with cancer and exposed to chronic exercise (repeated bouts of more than 3 sessions) adopting either forced (i.e., treadmill running or swimming) endurance (aerobic) training or physical activity (i.e., voluntary wheel running) paradigms were considered eligible. All types of animal models of solid tumors were considered eligible including genetically predisposed models (transgenic, genetically engineered mouse models [GEMMs]), orthotopic, subcutaneous and intravenous injections, tumor transplant, and spontaneous or carcinogen-induced solid tumor models. Studies that included multiple treatments, such as study arms with dietary restrictions, were eligible, as long as it was possible to compare sedentary (control) and exercise alone groups. Studies that evaluated pre-neoplastic lesions (e.g., aberrant crypt foci, ApcMin), or hematopoietic and ascites tumors were excluded. Only mouse and rat studies were included. Papers unavailable in English were excluded.
Study Selection and Classification
Four authors (KAA, RMP, ASB and LWJ) assessed study eligibility by reviewing the titles and abstracts of all potential citations according to the inclusion criteria. KAA, RMP, MWD and ASB extracted and interpreted data from published papers. When necessary, effort was made to contact authors and acquire publications for evaluation. Studies are summarized by type of exercise model and method of tumor initiation. Exercise characteristics are described using common prescription elements: modality, frequency, duration, intensity and total length of intervention.
Tumor initiation vs. growth
Papers were classified by the endpoints reported in the individual studies: (1) “Incidence” (tumor presence or multiplicity) (2) “Growth” (data on tumor growth, latency or survival), and (3) “Metastasis” (evaluation of tumors distinct from the primary tumor or developing following the common metastasis model of intravenous tumor cell injection). Study categorization was not always mutually exclusive; thus studies that included multiple endpoints applicable to more than one categorization were included in both.
Analysis of mechanistic findings
For evaluation of mechanistic findings, an alternative classification was applied based on the initiation of the exercise intervention relative to the time of tumor cell transplant or application of carcinogens. “Prevention” was defined as exercise initiated prior to tumor transplant/induction. “Progression” was defined as exercise initiated ≥3 days post-transplant/induction. Studies involving GEMMs were categorized as prevention studies. Distinguishing between exercise initiation and tumor cell inoculation is important because exercise-induced adaptations prior to tumor injection could ‘prime’ the host and/or tissue microenvironment, making it physiological or biologically distinct from tumor inoculation into a sedentary host. Mechanistic findings were classified as either intratumoral or systemic.
Interpretation and analysis
Studies were assessed for changes in tumor growth (as well as the related parameters of time to tumor-related endpoint, and tumor size/mass at the end of the study), incidence (tumor presence) and multiplicity (number of tumors/metastases). All study results that were reported to be “statistically significant” achieved p<0.05 according to the authors of the original manuscripts. Preliminary analysis of included studies indicated considerable methodological heterogeneity in all aspects of study design, end points, and efficacy. As such, it was not possible to compare the efficacy of exercise across cancer setting (i.e., incidence, progression, metastasis), cancer histology, exercise paradigm (i.e., forced vs. voluntary), or exercise dose.
RESULTS
A total of 426 potential citations were initially identified using search terms. After secondary screening, 53 papers were deemed eligible and underwent full review (Figure S1). Classification of papers was as follows: (1) incidence (n=24; 45.3%) (23–46), growth (n=33; 62.3%) (24, 26, 34, 37, 39, 40, 42, 44–69), and metastasis (n=10; 18.9%) (53, 55, 63, 64, 70–75).
Tumor Models and Exercise Prescription Characteristics
Included studies tested the effects of exercise on 15 different tumor types/cell lines, using six different tumor initiation methods (Table S2). Details of the exercise prescriptions are summarized in Tables 1, 2 and 3. The most common modalities included voluntary running (n=22; 41.5%) (24–28, 31, 33, 35, 42, 43, 51, 52, 55, 57, 64–66, 70, 71, 73–75) forced running (n=25; 47.2%) (26, 29, 36–41, 44–46, 48, 49, 54, 56, 58–60, 63, 67–70, 72, 74) and swimming (n=10; 18.9%) (23, 30, 32, 34, 47, 50, 53, 54, 61, 62).
Table 1.
Tumor Incidence Studies
| METHODS | RESULTS | ||||||
|---|---|---|---|---|---|---|---|
| Study | Reference | Rodent Model |
Tumor Type / Induction model |
Exercise Protocol | Tumor Incidence Results | ||
| Exercise Modality | Exercise Prescription Freq/week | Dur | Intens | Length |
Exercise Initiation | |||||
| Andrianopoulos, 1987 |
25 | 5w old male Sprague- Dawley rats |
Intestinal / DMH, i.p. q1w for 6w |
Voluntary wheel running |
Wheel running | 1w prior to first injection | -Tumors present in 18/20 SED rats and 6/11 EX rats |
| Reddy, 1988 | 33 | Male F344 rats |
Intestinal / Subcutaneous AOM 15mg/kg BW, q1wx2w at 7 wk of age. |
Voluntary wheel running |
Wheel running for 38 weeks | 4d post-AOM | -EX ↓ incidence and multiplicity adenocarcinomas, and liver foci. of colon and |
| Sugie, 1992 | 35 | 5w old F-344 rats |
Hepatocellular / 15 mg/kg AOM s.c. q1w for 2w |
Voluntary wheel running |
38w | 4d post-injection. | -Liver tumors noted in 7% of AOM treated SED only. |
| Ikuyama, 1993 | 28 | Jc1:Wistar rats |
Hepatoma / 0.0177 g/day/kg BW dietary 3'- Me-DAB for 35 weeks. |
Voluntary wheel running |
Wheel running for 62 weeks, using food as a reward for achieving specified distances |
17w prior to dietary 3'-Me- DAB |
-65% reduction in tumor incidence. |
| Zhu, 2008 | 42 | 21d old female Sprague- Dawley rats |
Mammary / 25 or 50 mg/kg MNU, i.p. |
Voluntary wheel running |
Voluntary wheel running for 8w | 1w post-injection | -84.5% incidence vs 98.1%, (SED vs. EX) |
| Esser, 2009 | 27 | C3(1)Tag mice |
Prostate / Transgenic mouse model |
Voluntary wheel running |
Wheel running for 10 weeks Data analyzed based on mice that ran >5K or <5K a day |
10w of age | -18% (>5K) and 55% (<5K) of animals with high grade neoplasia at 20 weeks -57% reduction in incidence of high grade neoplasia in >5K vs <5K mice |
| Alessio, 2009 | 24 | 3w old female Sprague- Dawley rats |
Spontaneous tumors | Voluntary wheel running or activity box |
Wheel: every other day, 24h access throughout animals’ life Activity box (PA): 1h in large activity box twice a week for life. |
Spontaneous tumors, animals followed for life. |
-During weeks 60–120, 38% of EX rats were tumor-bearing animals (vs. 42% PA rats and 54% SED rata). -At week 88, tumor multiplicity was 0.69 for EX animals, 0.75 for PA, and 0.96 for SED. |
| Colbert, 2009 | 26 | Female heterozygous (p53+/−): MMTV-Wnt- 1 transgenic mice |
Mammary / Transgenic | Voluntary wheel running and forced treadmill running |
Treadmill running: TREX 1: 5×|45min|20m/min at 5% grade|until completion TREX 2: 5×|45min|24m/min at 5% grade|until completion Voluntary wheel running with 24 hour access. |
11w of age | -Tumor incidence ↑ in wheel running mice by 32% |
| Mann, 2010 | 31 | 21d old female Sprague- Dawley rats |
Mammary / 50 mg/kg MNU i.p. |
Voluntary non- motorized and motorized wheel running |
Voluntary non-motorized and motorized (40m/min) wheel running |
1w post-injection | -96%, 74% and 70% incidence in controls, non-motorized and motorized mice, respectively |
| Zhu, 2012 | 43 | 21d old female Sprague- Dawley rats |
Mammary / 50 mg/kg MNU i.p. |
Voluntary wheel running at a fixed daily distance |
Three levels: WR-High: maximum 3500m/d WR-Low: maximum 1750m/d. SED control. |
1w post-injection | -97% tumor incidence in controls, 80% tumor incidence in WR-High -Cannot compare WR-High and WR-Low, because dietary energy restriction was applied to WR-Low only |
| Thorling, 1993 | 36 | 5w old male Fischer rats |
Intestinal / 15mg/kg AOM s.c. on Days 1, 4 and 8. |
Forced treadmill running |
5×|2km/day|7m/min|38w. First week was acclimatization. |
3d after last injection | -EX ↓ colon neoplasia incidence, 53% vs 78% in |
| Woods, 1994 | 40 | 6w old male C3H/HeN mice |
Mammary / 2.5 ×105 mammary SCA-1 cells s.c. in the back. |
Forced treadmill running |
Treadmill running: Moderate: 7×|30min|18m/min at 5% grade|1w. Exhaustive: 7×|varied|18m/min for 30min, then 3m/min↑ every 30min until exhausted|1w |
3d prior to injection. | -Increase in tumor incidence at Day 7 in both EX groups, but no differences in any subsequent time points. |
| Whittal, 1996 | 38 | 21d old female Sprague- Dawley Rats |
Mammary / 50 mg/kg NMU i.p. at 50d of age |
Forced treadmill running |
Progressive training to 5×|60min|18 m/min at 15% grade|4w |
21d of age (29d prior to injection) |
-Incidence/multiplicity at 24w post-NMU: 58 tumors in SED rats, 33 tumors in EX rats -No significant difference in latency or incidence |
| Whittal-Strange, 1998 |
39 | 21d old female Sprague- Dawley rats |
Mammary / 37.5mg/kg NMU i.p. at 50d of age, (1 day after last bout of EX) |
Forced treadmill running |
Progressive training to 5×|60min|18 m/min at 15% grade|4w |
21d of age (29d prior to injection) |
-Incidences of carcinomas, high grade and low grade tumors were 29.2%, 10.4%, and 25% in SED, and 38%, 14.3% and 21.4% in EX |
| Westerlind, 2003 |
37 | 21d old female Sprague- Dawley rats |
Mammary / 25 or 50 mg/kg MNU, i.p. |
Forced treadmill running |
Week 1: 5×|10–15min|30m/min|1w. Week 2–9: 5×|30min|23– 25m/min|8w |
1w post-injection | -↑ Latency in EX (35.8d vs. 33.1d). -No difference in median tumor-free survival time was observed in the EX versus sham-EX (SHAM), nor were there any differences in multiplicity at either a high or moderate dose of MNU |
| Zielinski, 2004 | 44 | 6–8w old female BALB/c mice |
Neoplastic Lymphoid cells / 2×107 EL-4 cells s.c. in the back behind the neck |
Forced treadmill running |
7×|3h or until volitional fatigue|gradually increasing speed, 20–40m/min, 5% grade|5–14d |
First session immediately before injection. |
-EX ↓ tumor appearance |
| Zhu, 2009 | 41 | 21d old female Sprague- Dawley rats |
Mammary / 50 mg/kg MNU, i.p. |
Forced wheel running | Motorized running wheel; details not provided |
1w post-injection | -66.7% and 92.6% incidence in EX and SED |
| Kato, 2011 | 29 | 5w old male Fischer 344 rats |
Renal / 5mg/kg BW Fe- NTA i.p. once a day for 7 days, then 10mg/kg Fe-NTA 3×/wk for 11w. |
Forced treadmill running |
Short-term: 15m|8m/min, 0%|12w. Long-term: 15m|8m/min, 0%|12w; then 5×|30m|8m/min, 0%|12w for a total of 24w training Exercise continued until 40 weeks, but at lower intensity to account for the decline in rat health. |
Training done 15m before each injection |
-No differences in number of rats with nodules, nodules/rat or mean area of nodules. -Short-term EX ↑ rats with microcarcinomas, - Long-term EX ↓ rats cinomas, karyomegalic cells and degenerative tubules compared to with microcar short-term. |
| Malicka, 2015 | 45 | 4w old female Sprague Dawley rats |
Mammary / 180 mg/kg MNU i.p. |
Forced treadmill running |
Low intensity (LIT): 5×|10– 35min|0.48–1.34 km/h|12w Moderate intensity (MIT): 5×|10– 35min|0.6–1.68 km/h|12w High intensity (HIT): 5×|10– 35min|0.72–2.0 km/h|12w |
Immediately after MNU injection |
-Incidence 64%, 67%, 40% and 43% in SED, LIT, MIT and HIT groups, respectively (Not significant) -Multiplicity: Rats with tumors had an average of 2.4, 1.6, 1 and 1 tumors in SED, LIT, MIT and HIT groups, respectively (Statistics not performed.) |
| Piguet, 2015 | 46 | 7–9w old male AlbCrePten flox/flox mice |
Hepatocellular carcinoma / Transgenic |
Forced treadmill running |
5w acclimation period followed by: 5×|60m|12.5m/min|27w |
7–9w of age | -Tumor incidence 100% in SED vs. 71% in EX |
| Lunz, 2008 | 30 | 11w old male Wistar Rats |
Intestinal / 4 s.c. injections DMH 3 days apart. |
Forced swimming with 0%, 2% or 4% BW load |
Week 1–2: 5×|5–20min|- load|2w Week 3–5: 5×|5–20min|+ load|3w Week 6– 35: 5×|20min|+load|30w |
24h post first injection | -No difference in tumor incidence -Aerobic swimming training with 2% body weight of load protected against the DMH-induced preneoplastic colon lesions, but not against tumor development in the rat |
| Paceli, 2012 | 32 | Adult male Balb/c mice |
Lung / 1.5mg/kg BW urethane i.p. twice, 2 days apart |
Forced swimming | Aerobic: 4×|20m|--|19w Week 1: 10m/d to 50m in 5 days Anaerobic: 3×|20m (2m swimming/2m resting)|progressive loading of 5– 20%BW|20w |
Within a week after injection | -No significant effects of aerobic training on lung cancer incidence. -Aerobic training resulted in 8 lung nodules per animal vs 52 in the control. Not significant. -Median control nodules was 2.0, median aerobic control nodules was 0.0. Not significant. |
| Abdalla, 2013 | 23 | 8w old female Balb/c mice |
Mammary / 1mg/ml DMBA p.o. once weekly for 6 weeks |
Forced swimming | 5×|45m|--|8 weeks Water temperature 30+/−4°C |
Same as tumor initiation | -EX ↓ tumor incidence |
| Sáez Mdel, 2007 |
34 | 50d old female Sprague- Dawley rats |
Mammary / 5mg/w DMBA gastric intubation for 4w |
Forced swimming | 30m/d, 5d/w for 38–65d. | 1d after appearance of first tumor |
-No difference in survival time or tumor multiplicity |
EX = exercise or activity groups; SED = Sedentary controls; NMU = nitrosomethylurea; MNU= 1-methyl-1-nitrosourea; AOM= azoxymethane; DMBA= 7,12-dimethylbenz(a)anthracene; DMH= 1,2-dimethylhydrazine; 3’-Me-DAB= 3’-methyl-4-dimethylaminoazobenzene; Fe-NTA= ferric nitrilotriacetate; s.c.= sub-cutaneous; i.v.= intravenous
Table 2.
Tumor Growth Studies
| METHODS | RESULTS | ||||||
|---|---|---|---|---|---|---|---|
| Study | Reference | Rodent Model | Tumor Type / Induction model |
Exercise Protocol | Tumor Progression Results | ||
| Exercise Modality | Exercise Prescription Freq/week | Dur | Intens | Length |
Exercise Initiation | |||||
| Daneryd, 1995 | 51 | Female Wistar Furth rats |
Leydig cell / 1.5mm3 injection of Leydig cell sarcoma (LTW) |
Voluntary wheel running |
13d | Immediately after injection | -EX ↓ tumor volume by 34%. |
| Daneryd, 1995 | 52 | Female Wistar Furth rats |
Leydig cell / 1.5mm3 injection of Leydig cell sarcoma (LTW) or Nitrosoguanine- induced adenocarcinoma s.c. into each flank |
Voluntary wheel running |
32d | Immediately after injection | - 13.4g vs 16.4g EX vs SED final LTW weights |
| Zhu, 2008 | 42 | 21d old female Sprague-Dawley rats |
Mammary / 25 or 50 mg/kg MNU, i.p. |
Voluntary wheel running |
Voluntary wheel running for 8w | 1w post-injection | -Tumor weight 0.62g vs. 1.16g (SED vs. EX) |
| Jones, 2010 | 57 | 3–4w old female athymic mice |
Mammary / 1×106 MDA-MB-231 cells, injected orthotopically |
Voluntary wheel running |
Voluntary wheel running for 41– 48d |
2d post-implant | No change in primary tumor growth (EX ↑21%) |
| Yan, 2011 | 64 | 3w old male C57BL/6 mice |
Lung / 2.5×105/50 µl/mouse Lewis lung carcinoma cells s.c. lower dorsal region |
Voluntary wheel running |
Primary tumors excised at 1cm diameter, access to wheels continued for additional 2w. |
9w before tumor implantation | -No difference in tumor cross-sectional area and tumor volume. |
| Jones, 2012 | 55 | 6–8w old male C57BL/6 mice |
Prostate / 5×105 mouse prostate C-1 cells, orthotopically |
Voluntary wheel running |
Wheel running for 8 weeks | 14d after tumor transplant | -Primary tumor growth was comparable between groups |
| Goh, 2014 | 66 | 18m old Balb/cBy mice |
Mammary / 1×104 cells in 4th mammary fat pad |
Voluntary wheel running |
Voluntary wheel running for 90 days |
60 days prior to tumor transplant, followed by 30 days post-transplant |
-Inverse relationship between distance run and final tumor mass |
| Betof, 2015 | 65 | Female Balb/c or female C57Bl/6 mice |
Mammary / 5×105 4T1-luc or 2.5×105 E0771 cells in dorsal mammary fat pad |
Voluntary wheel running |
Voluntary wheel running beginning either 9 weeks prior to tumor transplant, or at the time of tumor transplant |
SS: SED before and after transplant RS: EX for 9 weeks prior to transplant; SED after transplant SR: SED before transplant; EX after transplant RR: EX 9 weeks prior to transplant, continuing after transplant |
- Growth rates of SS and RS were similar, and growth rates of SR and RR were similar - EX slowed tumor growth compared to SED (both tumor models) |
| Alessio, 2009 | 24 | 3w old female Sprague-Dawley rats |
Spontaneous tumors | Voluntary wheel running or activity box |
Wheel: every other day, 24h access throughout animals’ life. Activity box (PA): 1h in large activity box twice a week for life. |
Spontaneous tumors, animals followed for life. |
- EX ↓tumor growth rate |
| Colbert, 2009 | 26 | Female heterozygous (p53+/−): MMTV-Wnt-1 transgenic mice |
Mammary / Transgenic |
Voluntary wheel running and forced treadmill running |
Treadmill running: TREX 1: 5×|45min|20m/min at 5% grade|until completion TREX 2: 5×|45min|24m/min at 5% grade|until completion Voluntary wheel running with 24 hour access. |
11w of age | - Time to tumor size of 1.5cm: 24.8d, 13.8d, and 19.5d in control, TREX1 and TREX2 animals. - Treadmill running led to faster tumor growth, no difference due to voluntary wheel running - Treadmill running ↓ survival |
| Newton, 1965 | 60 | 45d old Sprague- Dawley rats |
Carcinoma / Equal volumes of Walker- 256 cells s.c. into the right flank. |
Forced treadmill running |
Pre-Tumor: 50h over 5d at 950ft/hr. Post-Tumor: 138h over 10d at 950 ft/hr. |
5d before tumor implantation +/− 4d after tumor implantation |
-EX ↓ final tumor weight vs SED control. -Early life manipulation + EX ↓ final tumor weight vs EX alone and SED. |
| Uhlenbruck, 1991 |
63 | BALB/c mice | Sarcoma / 2.4×104 L- 1 cells s.c. |
Forced treadmill running |
7×|distances of 200m/400m/800m|0.3m/s|2w |
4w before and 2w after injection | -200m group ↓ tumor weight |
| Woods, 1994 | 40 | 6w old male C3H/HeN mice |
Mammary / 2.5 ×105 mammary SCA-1 cells s.c. in the back. |
Forced treadmill running |
Treadmill running: Moderate: 7×|30min|18m/min at 5% grade|1w. Exhaustive: 7×|varied|18m/min for 30min, then 3m/min↑ every 30min until exhausted|1w |
3d prior to injection. | No difference in growth rate of tumor size at time of euthanasia (two weels) |
| Whittal-Strange, 1998 |
39 | 21d old female Sprague-Dawley rats |
Mammary / 37.5mg/kg NMU i.p. at 50d of age, (1 day after last bout of EX) |
Forced treadmill running |
Progressive training to 5×|60min|18 m/min at 15% grade|4w |
21d of age (29d prior to injection) | -Tumor growth rate at 22w post-NMU: 0.043g/day in SED vs. 0.107g/day in EX -Final tumor weight: (3.2g in SED vs. 1.2g in EX |
| Bacurau, 2000 | 49 | 12w old male Wistar rats |
Carcinoma / 2×107 Walker-256 cells s.c. in the flank |
Forced treadmill running |
5×|60min|60% of VO2 peak|10w | 8w prior to injection | -Day 15 tumor weight 1.82% vs 19% of BW (EX vs SED) -EX prolonged survival by 1.9 fold |
| Westerlind, 2003 |
37 | 21d old female Sprague-Dawley rats |
Mammary / 25 or 50 mg/kg MNU, i.p. |
Forced treadmill running |
Week 1: 5×|10–15min|30m/min|1w. Week 2–9: 5×|30min|23– 25m/min|8w |
1w post-injection | -↑ Latency in EX (35.8d vs. 33.1d). -No difference in median tumor-free survival time was observed in the EX versus sham-EX (SHAM), nor were there any differences in multiplicity at either a high or moderate dose of MNU |
| Zielinski, 2004 | 44 | 6–8w old female BALB/c mice |
Neoplastic lymphoid cells / 2×107 EL-4 cells s.c. in the back behind the neck |
Forced treadmill running |
7×|3h or until volitional fatigue|gradually increasing speed, 20–40m/min, 5% grade|5–14d |
First session immediately before injection. |
-No difference in tumor density |
| Jones, 2005 | 56 | 3–4w old female athymic mice |
Mammary / Flank injection of 5×106 MDA-MB-231 cells |
Forced treadmill running |
5d/w|10m/min for 10min up to 18m/min for 45 min|0% grade|8w |
14d post-injection | -No change in tumor growth. |
| Bacurau, 2007 | 48 | 8w old male Wistar rats |
Carcinoma / 2×107 Walker-256 cells s.c. in the flank |
Forced treadmill running |
5×|30min|85% of VO2 max|10w | 8w prior to injection | -Survival: 16d for SED, 45d for EX -EX tumors were 6.9% of final body mass vs. 17.33% for SED control. |
| Lira, 2008 | 58 | Male Wistar rats | Carcinoma / 2×107 Walker-256 cells s.c. in the flank |
Forced treadmill running |
5×|60min|60–65% of VO2 peak|10w 2w pre-training period: rats ran progressively from 15 to 60min at 10m/min. Running was increased to 20m/min for two weeks after injection. |
8w prior to injection | -Tumor weight: 17.2g in SED; 1.9g in EX |
| Murphy, 2011 | 59 | 4w old C3(1)SV40Tag mice |
Mammary / Transgenic (tumors began developing at 12w of age). |
Forced treadmill running |
6×|60m|20m/min at 5%|20w | 4w of age | -Tumor volume: ↓ in EX at 21 and 22w. |
| Gueritat, 2014 | 69 | 10–12w old Copenhagen rats |
Prostate / surgical s.c. implantation of R3327 Dunning AT1 tumor fragment |
Forced treadmill running |
5×|15–60m|20–25 m/min|5w | 15d after tumor implantation | - EX rats had smaller tumors at 14 and 21 days compared to SED controls - Tumor doubling time was significantly slower in EX vs. SED (6.19d vs. 8.81d) |
| Shalamzari, 2014 |
67 | 4–6w old Balb/c mice |
Mammary / 1×106 MC4-L2 s.c. in the flank |
Forced treadmill running |
-|20–40min|6–20 m/min|15w | RTR: Sedentary before and after transplant RTE: EX after tumor transplant only ETR: EX before tumor transplant only ETE: EX before and after transplant 9w before tumor transplant, and/or 6w after transplant |
- ETE had significantly slower growth compared to RTR - No difference in final tumor volume of RTE and ETR groups |
| Aveseh, 2015 | 68 | 5w old female Balb/c mice |
Mammary / 1.2×106 MC4-L2 cells in right dorsal mammary fat pad |
Forced treadmill running |
7×|20–55min|10–20 m/min|7w | 10d after tumor transplant | -EX decreased tumor volume |
| Malicka, 2015 | 45 | 4w old female Sprague Dawley rats |
Mammary / 180 mg/kg MNU i.p. |
Forced treadmill running |
Low intensity (LIT): 5×|10– 35min|0.48–1.34 km/h|12w Moderate intensity (MIT): 5×|10– 35min|0.6–1.68 km/h|12w High intensity (HIT): 5×|10– 35min|0.72–2.0 km/h|12w |
Immediately after MNU injection | -No difference in final volume of total tumor volume |
| Piguet, 2015 | 46 | 7–9w old male AlbCrePten flox/flox mice |
Hepatocellular carcinoma / Transgenic |
Forced treadmill running |
5w acclimation period followed by: 5×|60m|12.5m/min|27w |
7–9w of age | -EX decreased total volume of liver tumors |
| Hoffman, 1962 | 54 | Wistar rats | Carcinoma / 2mL Walker 256 cell suspension s.c. into the right thigh. |
Continuous running on a 20ft runway + swimming + revolving drum |
21d of EX, all EX did all 3 modalities each day: Runway: continuous running on 20ft runway, duration and intensity not clear Swimming: increasing 20min/day to 4h/day Revolving drum: 5.4 mi in 12h |
Immediately after injection | -97% inhibition of tumor growth. -Tumor weight ↓ in EX group |
| Gershbein, 1974 | 53 | Holtzman rats | Carcinosarcoma / Walker-256 tumor i.m. into both hindlimbs. |
Forced swimming | 10×|15min|--|10d | Immediately after injection. | -EX ↓ tumor size. -No change in survival rates. |
| Baracos, 1989 | 50 | Sprague-Dawley rats |
Hepatoma / 20uL Morris hepatoma 777 s.c. |
Forced swimming | Low: 5×|5min/d, increased by 5min/d for 3w. Medium: 5×|10min/d, increased by 10min/d for 3w. High: 5×|15min/d, increased by 15min/d for 3w. |
2 Groups - 3w of swimming, tumor transplant, then 3w additional swimming. -3w of swimming beginning 3d post-transplant |
-EX ↓ final tumor weight, both groups. |
| Radak, 2002 | 61 | Adult female hybrid BDF1 mice |
Solid leukemia / 5×106 P-388 lymphoid leukemia cells s.c. |
Forced swimming | 5×|60min|--|10w ET: training terminated at tumor implantation. EC: training continued for 18d after tumor implantation. |
10w before injection. | -EC animals had slower tumor growth than ET and control, (endpoints were ~1.24cm3 vs. 2cm3 and 2.4cm3) |
| SáezMdel, 2007 | 34 | 50d old female Sprague-Dawley rats |
Mammary / 5mg/w DMBA gastric intubation for 4w |
Forced swimming | 30m/d, 5d/w for 38–65d. | 1d after appearance of first tumor | -EX ↑ tumor growth by 200%. |
| Almeida, 2009 | 47 | 7w old male Swiss mice |
Carcinoma / 2×106 Ehrlich tumor cells s.c. in the dorsum. |
Forced swimming | 5×|60min|50/80% max test|6w. Progressive load test began after 1w: load increased by 2% BW every 3min until exhaustion. |
4w before injection | -50% workload ↓ tumor weight and tumor volume (0.18mg/g and 0.11mm3) vs control (0.55mg/g and 0.48mm3) -Tumor volume and weight were 270% and 280% ↑ in SED mice. |
| Sasvari, 2011 | 62 | Adult female BDF1 mice |
Sarcoma / 5×106 S- 180 cells s.c. injected. |
Forced swimming | 5×|60m|--|10w ETT: cells injected after EX. ETC: EX (10w), cells injected, EX (18 additional days) |
10w before tumor implantation. | -ETC ↓ final tumor weight vs. SED control. |
EX = exercise or activity groups; SED = Sedentary controls; NMU = nitrosomethylurea; MNU= 1-methyl-1-nitrosourea; AOM= azoxymethane; DMBA= 7,12-dimethylbenz(a)anthracene; DMH= 1,2-dimethylhydrazine; 3’-Me-DAB= 3’-methyl-4-dimethylaminoazobenzene; Fe-NTA= ferric nitrilotriacetate; s.c.= sub-cutaneous; i.v.= intravenous
Table 3.
Tumor Metastasis Studies
| METHODS | RESULTS | |||||||
|---|---|---|---|---|---|---|---|---|
| Study | Reference | Rodent Model | Tumor Type / Induction model |
Exercise Protocol | Tumor Metastasis Results | |||
| Exercise Modality | Exercise Prescription Freq/week | Dur | Intens | Length |
Exercise Initiation | ||||||
| Naturally occurring | Gershbein, 1974 | 53 | Holtzman rats | Carcinosarcoma / Walker-256 tumor i.m. into both hindlimbs. |
Forced swimming | 10×|15min|--|10d | Immediately after injection. | -Older EX rats ↑ lower abdominal and inguinal secondary tumor nodules. |
| Yan, 2011 | 64 | 3w old male C57BL/6 mice |
Lung / 2.5×105/50 µl/mouse Lewis lung carcinoma cells s.c. lower dorsal region |
Voluntary wheel running |
Tumors excised at 1cm diameter, access to wheels continued for additional 2w. |
9w before tumor implantation | -Trend to inverse relationship between running distance and metastatic tumor yield | |
| Jones, 2012 | 55 | 6–8w old male C57BL/6 mice |
Prostate / 5×105 mouse prostate C-1 cells, orthotopically |
Voluntary wheel running |
Wheel running for 8 weeks | 14d after tumor transplant | -EX ↓ tumor nodal involvement by 36%, metastases weight by 88% and number of metastases by 34% (None were significant) |
|
| Artificial (intravenous injection of tumor cells) | Uhlenbruck, 1991 |
63 | BALB/c mice | Sarcoma / 2.4×104 L-1 cells i.v. |
Forced treadmill running |
7×|predetermined distances of 200–850m|0.3–0.5m/s|4–6w |
4w before injection, followed by either rest or an additional 2w running |
-EX decreased lung tumor multiplicity -Differences in running prescriptions make it difficult to determine if exercise cessation after tumor cell transplant was different from continuous exercise |
| MacNeil, 1993a | 74 | 4–5w old male C3H/He mice |
Transformed fibroblasts / 1.5×106 CIRAS 1 tumor cells i.v. via tail vein. |
Forced treadmill and voluntary wheel running |
Treadmill: 5×|30min|15m/min, 0%|9w. Voluntary Wheel: 24h access to wheel for 9w. All animals remained SED after injection for 3w. |
9w before injection | -No significant effect of activity on lung tumor retention. -Significant but weak correlation of distance run and multiplicity of lung metastases. -Median number of lung metastases per animal was greater in EX mice (21 vs 10 SED control). |
|
| MacNeil, 1993b | 73 | C3H/He mice | Transformed fibroblasts / 3×105 CIRAS1 cells i.v. |
Voluntary wheel running |
3–12w. | 9w prior to and/or 3w after injection |
-EX had no effect on lung tumor density -EX prior to tumor injection ↑ incidence in the lowest tertile of tumor distribution vs SED controls. |
|
| Hoffmann- Goetz, 1994a |
71 | C3H/He-bg2J/+ and C3H/HeJ mice |
Transformed fibroblasts / 5×105 CIRAS 1 or CIRAS 3 radiolabeled transformed fibroblast cells i.v. via tail vein. |
Voluntary wheel running |
24h access to running wheel for 9w. |
9w before injection | -EX ↓ retention of radioactivity in lungs 5min and 30min post-injection. | |
| Hoffman- Goetz, 1994b |
70 | 3w old female BALB/c mice |
Mammary / Lateral tail vein injection of 1×104 MMT 66 cells |
Forced treadmill running or voluntary wheel running |
Treadmill running: 5×|30min|18m/min at 0%| 8w or 3w. Groups: WS: wheel mice, 24h access for 8w, then SED for 3w. TS: treadmill mice, then SED for 3w. TT/WT: Continuous EX for 11w total. ST or SW: SED for 8w then treadmill (ST) or wheel (SW) for 3w. SS: SED for 11w total. |
8w prior or 36h after injection for 3w |
-No EX effect on number of lung tumors. -TT tended to have ↑ tumor multiplicity. -ST and SW tended to have ↓ tumor multiplicity. |
|
| Jadeski, 1996 | 72 | 4–9w old C3H/He-bg2J/+ and C3H/HeJ mice |
Transformed fibroblasts / 5×105 CIRAS 1 or CIRAS 3 transformed fibroblast cells i.v. via tail vein. |
Forced treadmill running |
5×|30min|20m/min, 0%|9w Acclimatization period of 1 week. |
9w before injection | -EX ↓ tumor cell lung retention (44.2 vs 52.8% control). -EX ↓ lung retention of the less aggressive CIRAS 1, no difference in CIRAS 3 cells. -EX did not alter final tumor lung metastases numbers, (subgroup evaluated 3w after EX and injection). |
|
| Yan, 2011 | 64 | 3w old male C57BL/6 mice |
Melanoma / 0.75×105/200 µl/mouse B16BL/6 cells in lateral tail vein |
Voluntary wheel running |
Melanoma: 24h access to wheels, continued 2w post-implantation. |
9w before tumor implantation | -No difference in number of lung metastasis | |
| Higgins, 2014 | 75 | 6w old male scid-beige mice |
Lung / 5×105 A549- luc-C8 cells i.v. via tail vein |
Voluntary wheel running |
28d Mice averaged 600–1200 m/day |
After lung tumors were detectable | - EX decreased tumor volume, as measured by bioluminescent imaging | |
EX= exercise or activity groups; SED= Sedentary controls; i.v.= intravenous
Effect of exercise on tumor outcomes
In incidence studies, 58% reported that exercise inhibited tumor initiation or multiplicity (23–25, 27, 28, 31, 33, 35, 36, 41, 43–46), 8% reported that exercise accelerated tumor incidence (26, 42), and 3% found null effects (29, 30, 32, 34, 37–40). In the growth category, exercise was associated with tumor inhibition in 64% of studies (24, 39, 47–54, 58–63, 65–69), while 21% (37, 40, 44, 55–57, 64) and 9% (26, 34, 42) of studies reported null and accelerated tumor growth, respectively. Finally, in the tumor metastasis category, three studies utilized models in which metastases arose from a primary tumor; of these, two reported non-significant inhibition of tumor growth (55, 64), while the other found accelerated tumor growth with exercise (53). Eight studies utilized intravenously injected tumor cell model of metastases. MacNeil and Hoffman-Goetz found that exercise did not alter retention of lung tumor cells, but increased the median number of lung metastases (74). Conversely, Jadeski and Hoffman-Goetz reported that exercise decreased retention of lung tumor cells, but had no effect on the number of lung metastases (72). One additional paper reported no difference in tumor cell retention in the lungs (71), whereas three papers reported no effect of exercise on lung metastasis (64, 70, 73). Two papers reported that exercise inhibited lung metastasis multiplicity (63) or total volume (75).
Effects on the intratumoral microenvironment
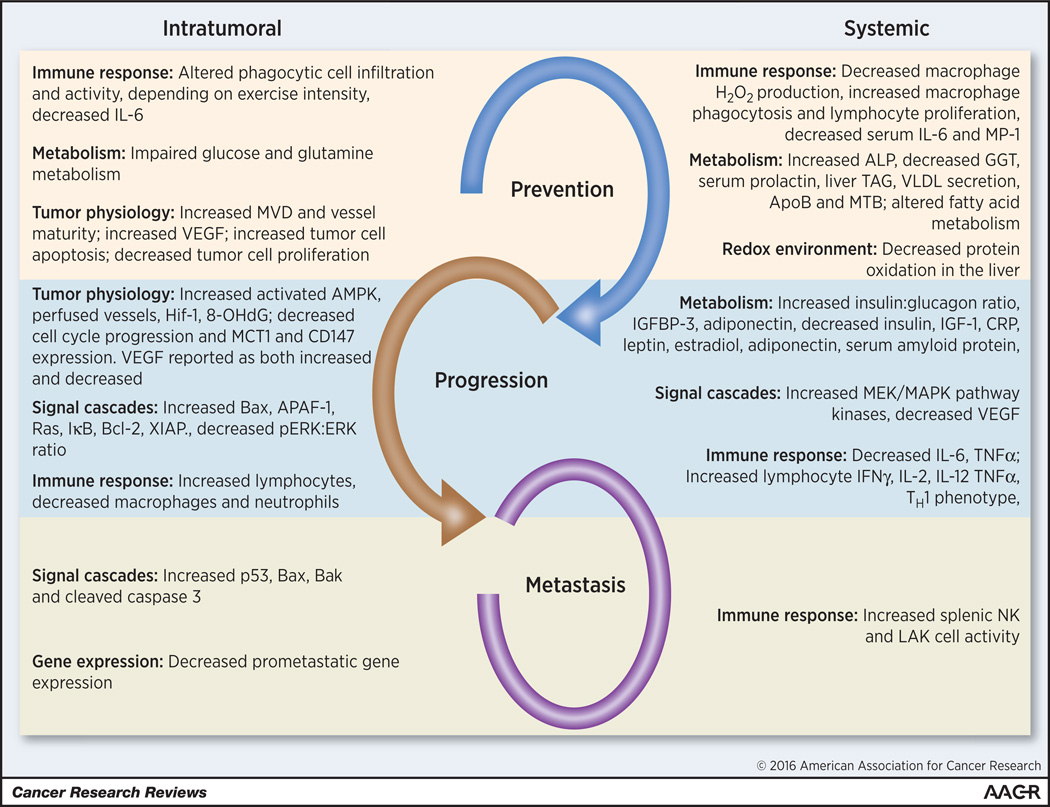
Mechanistic findings are summarized in Table 4 and Figure 1. Studies were classified as prevention (n=20; 37.7%), progression (n=26; 49.1%) or metastasis (n=7; 13.2%). Eight prevention studies (40%) reported significant effects of exercise in modulation of local immune response, tumor metabolism and tumor physiology/angiogenesis. Ten progression studies (38.5%) examined mechanisms underlying the effects of exercise on tumor progression (32, 41, 42, 44, 52, 55, 57, 61, 68, 69). The mechanisms examined included multiple different factors/pathways such as apoptosis, perfusion and immune cell infiltration. Only one metastasis study examined changes in tumor biology: Higgins et al. found that exercise favored a pro-apoptotic environment (75), reflecting changes in immune cell populations/function, tumor physiology and signaling cascades.
Table 4.
Mechanistic results
| Study | Reference | Tumor type | Exercise Modality | Mechanistic findings | |||
|---|---|---|---|---|---|---|---|
| Intratumoral | Systemic | Potential Implications | |||||
| Prevention | Ikuyama, 1993 | 28 | Carcinogen induced hepatoma | Voluntary wheel running |
-None reported. | -EX ↓ gamma-glutamyl transpeptidase and ↑ ALP. | Reduced liver damage |
| Alessio, 2009 | 24 | Spontaneous tumors | Voluntary wheel running |
-None reported. | -Mean serum prolactin levels were ↓ in exercising rats and ↑ in SED rats at 24 and 52w of age. |
Prolactin has been associated with tumor growth | |
| Goh, 2014 | 66 | Orthotopic mammary tumor | Voluntary wheel running |
-Inverse correlation between distance run and mitotic index within tumors |
-EX increased VO2 and respiratory exchange rate | Decreased tumor cell proliferation | |
| Betof, 2015 | 65 | Orthotopic mammary tumor | Voluntary wheel running |
-EX increased colocalization of desmin and CD31 and decreased tumor hypoxia and necrosis -EX increased expression of Fas, caspase 8 and cleaved caspase 3 |
-None reported | Increased microvessel density and maturity, which leads to improved tumor perfusion; increased tumor cell apoptosis |
|
| Woods, 1994 | 40 | s.c. mammary tumor transplant | Forced treadmill running |
-Moderate EX: ↑ phagocytic cells -Exhaustive EX: ↓ total and highly phagocytic cells |
-None reported | Increased tumoricidal immune response | |
| Bacurau, 2000 | 48 | s.c. carcinoma transplant | Forced treadmill running |
-None reported | -EX ↓ glucose consumption and production, and lactate production and ↑ glutamine consumption of macrophages -EX ↓ H2O2 production by macrophages, and ↑ phagocytosis. -EX ↑ lymphocyte proliferation |
Increased tumoricidal immune response | |
| Bacurau, 2007 | 49 | s.c. carcinoma transplant | Forced treadmill running |
-EX impairs tumor cell glucose and glutamine metabolism. |
-EX, non-tumor ↑ plasma corticosterone vs. control. -EX prevents tumor-induced reduction in body weight and food intake, activation of glutamine metabolism in macrophages and lymphocytes. |
Modulation of physiology | |
| Lira, 2008 | 58 | s.c. carcinoma transplant | Forced treadmill running |
-None reported. | -EX ↓ liver triacylglycerol (TAG) content compared to SED. -SED tumor animals had ↑ serum and liver TAG, and ↓ rate of VLDL secretion, apoB expression and microsomal TAG transfer protein compared to control. |
Associated with reduced cachexia | |
| Murphy, 2011 | 59 | Transgenic mammary tumors | Forced treadmill running |
-None reported. | -↓ spleen weight compared to wild-type mice. -No difference in spleen weight due to EX. -EX ↓ plasma IL-6 and MP-1. |
Increased tumoricidal immune response | |
| Kato, 2011 | 29 | Carcinogen-induced renal tumors | Forced treadmill running |
-None reported. | -Long-term EX and Fe-NTA ↓ renal brown droplets compared to SED, ↑ adrenal weight compared to both other groups. |
Brown droplets could reflect renal damage caused by carcinogen applied. Increases in adrenal weight have been linked to psychological stress. |
|
| Shalamzari, 2014 | 67 | s.c. mammary tumor transplant | Forced treadmill running |
- EX after tumor transplant decreased tumor IL-6 and VEGF - IL-6 and VEGF levels in ETR mice were not different from RTR (sedentary) mice. |
-None reported | Reduced inflammation and subsequent angiogenesis | |
| Piguet, 2015 | 46 | Transgenic hepatocellular carcinoma |
Forced treadmill running |
- EX decreased proliferation (Ki67 staining) in tumor nodules >15mm3. |
- EX altered gene expression of fatty acid metabolism pathways |
Altered lipogenesis. Some lipogenesis pathways are prognostic indicators following HCC surgical removal |
|
| Malicka, 2015 | 45 | Carcinogen-induced mammary tumor |
Forced treadmill running |
-EX increased TUNEL positive cells | - None reported | ||
| Almeida, 2009 | 47 | s.c. carcinoma transplant | Swimming | -50% workload ↓ macrophage infiltration and neutrophil accumulation. | -80% workload induced cardiac hypertrophy vs. 50%. | Altered immune response; future analysis of macrophage subsets would be beneficial to the field |
|
| Sasvari, 2011 | 62 | s.c. sarcoma transplant | Swimming | -None reported. | -EX ↓ oxidative modification of protein in the liver as measured by protein carbonyls. |
Reduced oxidative stress or improved anti-oxidant activity |
|
| Progression | Daneryd, 1995 | 51 | Carcinogen-induced or s.c. leydig cell transplant |
Voluntary wheel running |
-None reported | - EX normalized insulin sensitivity compared to SED - EX ↑ skeletal muscle metabolism - EX attenuated ↓ of reverse triiodothyronine secretion by the thyroid - EX ↑ insulin:glucagon ratio - EX ↓ corticosterone |
Reflects metabolic adaptations to prevent hypo- or hyperglycemia, which may develop in cancer patients. |
| Daneryd, 1995 | 52 | s.c. leydig cell transplant | Voluntary wheel running |
-EX 1.31-fold ↑ in tumor cell energy charge and uric acid content |
-None reported. | Uric acid has since been shown to stimulate dendritic cell activation, and is elevated in tumors with anti- tumor immune responses |
|
| Zhu, 2008 | 42 | Carcinogen-induced mammary tumor |
Voluntary wheel running |
-EX activated AMPK -EX ↓ VEGF -EX ↓ Bcl-2, X-linked inhibitor of apoptosis pathway (XIAP) while ↑ Bax, apoptosis peptidase-activating factor-1 |
↓ Insulin, IGF-1, CRP, leptin and estradiol ↑ Corticosterone |
EX increases cell metabolism and skews the BCL-2 family member protein profile pro-apoptotic. |
|
| Jones, 2010 | 57 | Orthotopic mammary tumor | Voluntary wheel running |
-EX ↑ perfused vessels and HIF-1 protein levels | -None reported | Improved tumor perfusion, oxygenation | |
| Yan, 2011 | 64 | s.c. lung tumor transplant | Voluntary wheel running |
-None reported. | -T umor presence ↑ plasma VEGF, PDGF-AB, MCP-1. -Running: ↓ plasma insulin and leptin, ↑ adiponectin, ↑ plasma VEGF. |
VEGF expression has been linked to worse outcome in patients, but could instead represent improved microvessel density and vascular normalization in tumors. |
|
| Jones, 2012 | 55 | Orthotopic prostate tumor | Voluntary wheel running |
-EX ↑vascularization, stabilization of HIF-1a → 40% ↑regulation of VEGF -Expression of prometastatic genes was significantly modulated in exercising animals with a shift toward reduced metastasis |
-↑ activity of protein kinases within MEK/MAPK and PI3/mTOR |
Improved tumor perfusion/oxygenation | |
| Mann, 2010 | 31 | Carcinogen-induced mammary tumor |
Voluntary non- motorized or motorized wheel running |
-None reported | -Induced citrate synthase activity | Reflects training response | |
| Zhu, 2012 | 43 | Carcinogen-induced mammary tumor |
Voluntary wheel running at a fixed daily distance |
-None reported | -↓ Insulin-like growth factor-1 (IGF-1), insulin, interleukin- 6, serum amyloid protein, TNF-α, and leptin -↑ IGF-binding protein 3 (IGFBP-3) and adiponectin |
Increased tumoricidal immune response | |
| Westerlind, 2003 | 37 | Carcinogen-induced mammary tumor |
Forced treadmill running |
-None reported | - ↑heart and soleus weights | Reflects training response | |
| Zielinski, 2004 | 44 | s.c. neoplastic lymphoid cell transplant |
Forced treadmill running |
-No difference in the fluid content of tumor -EX ↓ in vessel density -EX ↓ inflammatory cells (macrophages and neutrophils) but increased lymphocytes |
-None reported | Altered immune response; future analysis of macrophage subsets would be beneficial to the field |
|
| Zhu, 2009 | 41 | Carcinogen-induced mammary tumor |
Forced wheel running | -Apoptosis induced via mitochondrial pathway in EX -Cell cycle progression suppressed at G(1)/S transition in EX -EX ↓ blood vessel density. |
-None reported | EX promotes tumor cell apoptosis and prevents proliferations and angiogenesis |
|
| Gueritat 2014 | 69 | s.c. prostate tumor transplant | Forced treadmill running |
- EX decreased tumor cell proliferation (Ki67 staining), p- ERK/ERK ratio and 8-OHdG - No difference in tumor SOD, protein carbonylation or lipid oxidattion |
-None reported | ERK phosphorylation is increased by oxidative stress | |
| Aveseh, 2015 | 68 | Orthotopic mammary tumor | Forced treadmill running |
- EX shifted tumor lactate dehydrogenase expression towards the LDH1 isoenzyme - EX decreased tumor monocarboxylate transporter 1 (MCT1) and CD147 expression. |
-None reported | A shift towards LDH-1 may signal reduced lactate concentrations in the tumor. Lack of MCT1 may result in the tumor cells utilizing glucose instead of lactose, and eventually starving the tumor cells. CD147 is required for MCT1 expression |
|
| Radak, 2002 | 61 | s.c. solid leukemia transplant | Swimming | -EC tumors had ↑ Ras protein compared to control. -EC tumors had ↑ I-kappaB protein than ET and control. |
-None reported | May be associated with lymphocyte proliferation and activity, but additional analyses are needed |
|
| Abdalla, 2013 | 23 | Carcinogen-induced mammary tumor |
Swimming | -None reported. | -Physical activity ↑ lymphocytes producing IFN-γ, IL-2, IL- 12, and TNF-α. -Physical activity promoted immune system polarization toward an antitumor Th1 response pattern profile. |
Increased tumoricidal immune response | |
| Metastasis | MacNeil, 1993a | 74 | i.v. injection of transformed fibroblasts |
Forced treadmill or voluntary wheel running |
-None reported. | -Wheel group ↑ splenic NK response vs control. | Increased tumoricidal immune response |
| Hoffmann-Goetz, 1994a |
71 | i.v. injection of transformed fibroblasts |
Voluntary wheel running |
-None reported. | -Running ↓recovery of radioactivity from liver, spleen and kidney at 30min and 90min post-injection. |
May reflect decreased retention of circulating tumor cells in organs, thereby lowering the risk of metastasis. |
|
| Hoffman-Goetz, 1994b |
70 | i.v. injection of mammary tumor cells |
Forced treadmill running or voluntary wheel running |
-None reported | -Pre-tumor EX ↑ LAK cell activity. -NK activity lower in animals that stopped EX at tumor injection. |
Increased tumoricidal immune response | |
| Jadeski, 1996 | 72 | i.v. injection of transformed fibroblasts |
Forced treadmill running |
-None reported | -EX ↑ citrate synthase activity in the soleus. | Indicates a training effect developed | |
| Higgins, 2014 | 75 | i.v. injection of lung tumor cells | Voluntary wheel running |
-EX tumors had increased levels of p53, Bax and Bak. -EX tumors had increased staining for cleaved caspase 3 |
-EX increased serum BUN and decreased ALT, but both were still within normal ranges |
Supports increased apoptosis of tumor cells | |
Figure 1.
Postulated intratumoral and systemic mechanisms underlying exercise oncology across the cancer continuum. Note that few reports combined measurements in tumor with systemic changes. The link between intratumoral changes and systemic changes is largely unknown.
Effects on systemic (host) pathways
Correlative systemic pathways examined are summarized in Table 4. Ten prevention (24, 28, 29, 40, 46–49, 59, 62), seven progression (23, 31, 37, 42, 51, 55, 64, 76) and five metastasis studies (70–72, 74, 75) reported significant effects of exercise on changes in systemic effectors, predominantly changes in factors involved in immune surveillance or metabolism.
DISCUSSION
The findings of this critical review indicate that the current evidence base is plagued by heterogeneity in all aspects of study methodology and data reporting. Unfortunately, such heterogeneity precludes rigorous evaluation of essential questions such as the biologically effective exercise dose to modulate specific tumor pathways or inhibit tumor growth, the effects of manipulating exercise intensity or duration or the differential impact of exercise across tumor subtypes (22). Nevertheless, our review did permit identification of the most salient methodological issues that prudent investigators may consider when designing in-vivo pre-clinical exercise-oncology studies. These aspects are described in the proceeding sections and summarized in Table 5.
Table 5.
Recommendations for Preclinical Exercise Oncology Research
| Concern | Recommendation |
|---|---|
| Use of xenograft models in immune deficit animals |
Orthotopic implantation of syngeneic tumor cell lines or induction of orthotopic tumors via transgenic or chemical methods in immune competent animals |
| Poor description of exercise intervention characteristics |
Describe frequency, intensity, duration, and progression, as appropriate. Avoid vague terms such as “exercise to exhaustion”. Confirmation of ‘training’ effect via muscle fiber or mitochondrial function analysis |
| Handling of control (sedentary) animals | Handling, social interaction, and environment should be similar to animals randomized to exercise conditions. This includes differences in cage size and social housing. If possible, animals should be acclimated to exercise, or introduced to the activity gradually. |
| Tail vein models of metastasis | Consider using orthotopic implantation of syngeneic tumor cell lines or transgenic models that spontaneously develop metastasis. However, tail vein models of metastasis may still be useful for assessing the effects of exercise at later time points in the metastatic cascade. |
| Lack of assessment of systemic and molecular mechanisms |
Investigate effects on systemic mechanisms (metabolic and sex hormones, inflammation, immunity, and products of oxidation) postulated to underlie effects of exercise on tumorigenesis as well as potential mediating molecular mechanisms (e.g., cell signaling pathways, angiogenesis, metabolism, migration). Findings should be validated by the use of knock-out/knock-in transgenic animals. |
| Lack of assessment of tumor biology beyond tumor incidence, weight, or volume |
Report on other common markers of the neoplastic phenotype (e.g., apoptosis, proliferation, microvessel density, necrosis, angiogenesis) |
| Lack of concern regarding the psychological differences between voluntary physical activity vs. forced exercise |
Comprehensive study on hypothalamic- pituitary- adrenal axis activation in response to different exercise prescriptions and the effects that associated hormones have on tumor progression/prevention in sedentary controls. |
Heterogeneity in Exercise Prescription
The components of the exercise prescription being investigated in preclinical studies should mirror, as closely as possible, exercise prescription parameters tested in human studies (22). Key parameters include: (1) modality/paradigm (i.e., forced vs. voluntary), (2) dose (i.e., intensity, session duration, frequency of training sessions/week, and length of treatment), and (3) schedule (i.e., time of initiation).
Exercise Modality (Forced vs. Voluntary Paradigms)
A foremost decision is the use of forced versus voluntary exercise paradigms. An often overlooked key point is that voluntary wheel running is a model of physical activity whereas forced paradigms are a model of exercise training (i.e., structured and purposeful physical activity). Observational (epidemiological) studies typically measure both physical activity and exercise, whereas efficacy-based clinical trials largely examine highly structured exercise training. The decision of which exercise modality is selected should depend on whether the investigators envisage the study findings directly informing clinical translation or plausibility/mechanisms of a clinical observation.
An important caveat to consider in all exercise paradigms is the degree of associated negative stress, as evidenced by exercise-induced increases in serum corticosterone and fecal corticosterone metabolites (77, 78), as observed in both voluntary and forced exercise paradigms. Furthermore, voluntary running may result in “addictive behavior” with negative effects on the hypothalamic-pituitary-adrenal (HPA) axis and animal health (79). Human studies have taught us that exercise induces spikes in cortisol at the beginning of exercise and immediately after bout of exercise ceases (80). Stress-induced activation of the HPA axis may accelerate tumor growth (81), and have diverse effects on the immune response (reviewed in (82)), thereby confounding the efficacy of exercise on tumor growth characteristics. HPA-mediated changes on the immune response could contribute to discordance between studies with respect to immune function. For example Zhu et al. reported a decrease in TNF-α (43) whereas Abdalla et al. reported an increase (23).
Though changes in glucocorticoids have been seen using all exercise modes and intensities, such considerations may be especially important when investigating the effects of high-intensity or high-volume exercise treatment doses. This is relevant because, as reviewed here, studies have tested the effects of exhaustive exercise doses (40) (e.g., forced swimming for four hours followed by 12 hours of physical activity (54)), or forced swimming of animals loaded with external weights (30, 32, 47). The latter exercise paradigms may have additional safety and ethical concerns. Indeed, one study investigating the effect of forced swimming reported four animal deaths due to drowning (30). Researchers should also be cognizant of the impact of the exercise on the animals’ natural circadian rhythms. Fuss et al. demonstrated that voluntary wheel running occurs predominantly during the animals’ dark cycle (78), therefore it would be prudent to conduct exercise training during the dark cycle. Regardless of exercise prescription, corticosterone analysis would be helpful to include in all study designs; such levels could be correlated with tumor growth end points. In this review, three studies analyzed systemic corticosterone levels (42, 48, 51); of these, two studies reported increases in corticosterone levels whereas the other study reported a decrease. Interestingly, tumor growth was inhibited in all studies.
Exercise Dose (Intensity, Session Duration, Frequency of Training, and Length of Treatment)
Exercise at different intensities confers remarkably different physiological and gene expression adaptations in mammals (83), however a key question is whether these differences translate into differential effects on tumor outcomes. In clinical trials, the efficacy of different exercise intensities varying from 50% to 100% of a patient-derived physiological parameter (e.g., age-predicted maximum heart rate, measured exercise capacity) have been investigated. Such an approach permits personalized exercise prescriptions, which is important because exercise abilities (and therefore appropriate exercise intensity) vary considerably in patients with cancer (83). Animal-specific exercise prescriptions are challenging to implement in pre-clinical studies, but could be important, as findings reviewed here suggest that exercise intensity may differentially modulate tumor end points. For example, using a transgenic model of p53+/− MMTV-Wnt-1, Colbert et al. found that exposure to forced treadmill training of different intensities accelerated tumor growth rate compared to sedentary controls, with the highest tumor multiplicity in the lowest intensity group (26). Conversely, Almeida et al. reported diminishing returns in the context of increasing exercise intensity using two different swimming intensities (50% or 80% of maximal workload; CT50 and CT80, respectively, defined using a maximum load test conducted one week prior to exercise initiation) (47). CT50 caused significant tumor growth inhibition whereas CT80 was ineffective. Using a model of forced treadmill running, Woods et al. compared the effects of “moderate” vs “exhaustive” exercise prescriptions, with no differences in mammary tumor growth or overall incidence (40). In contrast, Malicka et al. reported a trend towards a negative correlation between exercise intensity (varied by progressively adjusting the speed of the treadmill [low: 0.48–1.34 km/h, moderate: 0.6–1.68 km/h, high: 0.72–2.0 km/h]) and tumor incidence and multiplicity, as well as an increase in tumor cell apoptosis across all exercise prescriptions, compared to controls (45). Kato et al. examined duration of prescription of forced treadmill running using a nitrilotriacetate-induced renal carcinoma model. Rats were assigned to exercise exposure for either 12 weeks or 40 weeks (29). Twelve weeks of exercise increased microcarcinoma incidence and multiplicity compared to sedentary controls with no differences (compared to control) in 40 weeks of exercise.
Schedule of Exercise Exposure
A critical question is whether exercise exposure prior to diagnosis improves disease outcomes compared to inactivity (prior to diagnosis). Similarly, do previously sedentary patients initiating exercise after diagnosis have superior outcomes compared to those remaining sedentary or decreasing exercise levels after diagnosis? Exploratory findings from epidemiological studies suggest that timing or initiation of exercise exposure could be important (84). Two studies have empirically investigated this question: Betof et al. and Shalamzari et al. found that exercise initiated after tumor transplantation (equivalent to post-diagnosis setting) inhibited tumor growth, independent of exposure to exercise prior to transplantation (65, 67). Similarly, Radak et al. found slower tumor growth with exercise pre- as well as post-tumor implantation in comparison with no exercise after transplant or sedentary control (61). Such findings stress the importance of maintaining exercise post-diagnosis. Finally, whether the effects of exercise are different across the steps in the metastatic cascade (altered adhesion [invasion]; intravasation; survival in the circulation; extravasation, and seeding at a distant site [metastatic colonization] (85)) has not been investigated. Addressing this question has significant clinical implications, and further research in this area is warranted.
Common Methodological/Experimental Weaknesses
Several salient methodological issues across studies were identified (see Table 5). Of these, two common issues that require particular attention regarded selection of appropriate in vivo tumor models, and lack of mechanistic studies.
In Vivo Tumor Models
Preclinical oncology studies have generally focused on subcutaneous tumor implantation models. These models permit monitoring of tumor growth kinetics but do not accurately recapitulate the tissue microenvironment of the ectopic (orthotopic) or distant organ or mimic the natural evolution of cancer. In addition, blood flow to subcutaneous tumors may not reflect that of orthotopic tumors. As the benefits/risks of using subcutaneous models are beyond the scope of this review, here we will simply caution investigators against using subcutaneous tumors. This model is not a perfect surrogate for spontaneously occurring tumors.
The majority of studies reviewed herein investigated the effects of exercise on tumor growth characteristics in the primary organ site. In contrast, studies of metastasis, the primary cause of cancer-related death, has received limited attention to date. Additionally, 73% of the metastasis studies we reviewed used an intravenous (tail-vein) injection of tumor cells as a model of metastasis which evaluates the ability of tumor cells to survive in circulation and colonize an organ but does not enable investigation of the early steps in the metastatic cascade.(86) Indeed, three studies reported on tumor cell retention in the lungs following intravenous injection (71, 72, 74), with two studies reporting a decrease in tumor cell retention in exercising animals, but no change in the final number of metastases. Interestingly, MacNeil et al. reported that exercise did not affect tumor cell retention in the lung. These three studies differed with respect to type of exercise and number of tumor cells injected; thus no meaningful comparisons can be made between them. The impact of the methodological differences can only be evaluated by completing the studies-side-by-side, comparing all combinations of exercise/tumor inoculation models.
An alternative, and arguably more appropriate model of metastasis, is surgical excision of the primary tumor which stimulates spontaneous metastasis, predominantly to the lungs; Only one study to date (Yan et al.) has adopted this model to examine exercise effects (64); they reported a trend towards an inverse relationship between distance run and metastatic burden. Conversely, Jones et al. examined the metastasis response in a prostate cancer model by quantifying the mass of “non-contiguous external masses that were grossly visible independent from the primary prostate tissue.” In this model, exercise was not associated with a significant reduction in the number or weight of metastases (55).
Emerging technology has provided researchers with a considerable number of in vivo models beyond cell line xenografts, such as patient-derived xenografts (PDXs), syngeneic allografts, and genetically-engineered mouse models (GEMMs) (87), as well as non-rodent models (e.g., zebrafish, Drosophila). The advantages and disadvantages of each model have been reviewed elsewhere (88–91). Ultimately, no one in vivo model will be appropriate to address all exercise-oncology questions, with model selection being contingent on the scientific question at hand as well as translational importance.
The Importance of Appropriate Control Groups
Consideration of the nature of control (non-exercising) groups should not be overlooked. To the extent possible, control animals should be exposed to the same variables as exercise counterparts including aspects related to housing, transportation to different facilities, or procedures to reinforce exercise behavior (i.e., prodding, shock). Taken even further, animals could be housed in cages with locked wheels, placed on stationary treadmills, or made to stand in very shallow pools of water, depending upon the exercise regimens used. We stress that exposing control and experimental mice to different housing or environmental conditions is a study weakness. For example, one study housed control animals in unusually small cages (5 inches in diameter and 6 inches high) to restrict activity (54), whereas exercise treatment animals were not confined. The differences in housing size and daily movement could have either induced a stress response or altered the animals’ resting metabolism, thereby affecting tumor growth. We advise researchers reviewing extant publications for planning of their own exercise oncology studies to consider whether controls were properly handled before modeling their own work off of previous studies.
Mechanistic Studies/Analyses
The majority of studies reviewed here examined the effects of exercise on tumor growth characteristics as evaluated by tumor volume or growth rates. While such end points are clearly important, subtle but important modulations of intratumoral physiological or biological alterations can be masked. For instance, our group observed differences in perfusion and expression of key factors regulating metabolism and hypoxia, despite comparable primary tumor growth rates between exercised and sedentary animals (57). Elegant studies by McCollough et al. demonstrated that exercise improved blood flow and oxygen delivery to orthotopic prostate tumors in rats, but not to the normal prostate tissue in either tumor-bearing or control rats (92). These changes were reflected by decreased hypoxia within the tumor during exercise. While in-depth explications of the systemic or local molecular mechanisms underpinning the exercise-tumor prevention/progression relationship remains in its infancy, studies such as this one may set the precedent for future mechanistic studies in this field. The current working hypothesis is that exercise modulates tumor progression via modulation of the host -tumor interaction (19). Tumor progression is regulated by complex, multifaceted interactions between the systemic milieu (host), tumor microenvironment, and cancer cells (93). The microenvironments of primary and metastatic tumors are subject to modulation by systemic and local growth/angiogenic factors, cytokines, hormones, and resident cells (94, 95). Factors such as hepatocyte growth factor (HGF), tumor necrosis factor (TNF), interleukin (IL)-6, insulin, and leptin (96–98) have already been associated with higher risk of recurrence and cancer-specific mortality in a number of solid malignancies.(99) Clearly, manipulation of such factors by physical activity could alter aspects of the cancer continuum (100).
As reviewed here, multiple systemic factors are perturbed by exercise, including metabolism, inflammatory-immune, and reactive oxygen species-mediated pathways (101). The breadth of these factors likely contributes to the pleiotropic benefits of exercise in health and disease (102, 103) and likely the potential antitumor effects of exercise. (19) Notably, most cancer studies investigate single pathways in isolation, without consideration of overlap/synergism between pathways. Because the host/tumor interaction is modulated by numerous host-related factors and multiple pathways (100), an ideal study approach would be to investigate exercise effects on multiple pathways simultaneously. This would fill in the missing gaps of the “multi-targeted” effects of exercise. A recent analysis of pre-clinical exercise oncology studies by Pedersen et al. reported that only 30.7% evaluated systemic changes in animals (104). In addition, intratumoral signaling, and changes in tumor vascularity were examined by only 29.5% and 6.8%, respectively (104).
Future Recommendations
With a view towards future studies, we encourage the exercise-oncology field to consider certain guidelines for preclinical exercise-oncology research (see Table 5). However, we realize that it is impractical for all exercise-oncology experiments to standardize all study procedures. Nevertheless, it may behoove the field to develop a means of quantifying the exercise prescription applied, which will then permit comparisons between studies. Such an approach is readily used in the fields of radiation oncology (i.e., the biologically equivalent dose [BED], which allows comparison of different dose/fractionation schemes (105)) and hyperthermia (i.e., the cumulative equivalent minutes at 43°C [CEM43], which standardizes the thermal killing effect of hyperthermia, regardless of variations in heating efficiencies between tumors (106)). Establishing a biological equivalent exercise dose (BEED) would facilitate comparisons across studies as well as provide an opportunity to test elements of prescriptions such as different schedules, timing, and duration.
Exercise investigations should also strive to adopt the experimental procedures and models being utilized in the general tumor biology literature. Indeed, it appears that more recent studies reviewed here have started to utilize clinically-relevant tumor models, favoring transgenic mice and orthotopic tumors over carcinogen-induction. The general cancer field is also starting to favor the use of patient-derived xenograft (PDX) models. PDX have certain weaknesses, including increased heterogeneity, and a requirement for immunocompromised mice, but could represent an important step towards personalized medicine. Furthermore, PDXs do not accrue additional mutations through in vitro culture and tend to be slower growing than murine-derived tumor lines. Delayed growth curves may highlight slight changes in tumor growth following manipulations of exercise dose. GEM models should also be considered, especially in mechanistic studies. A second trend in cancer biology is use of biomarkers. The discovery of blood, imaging, and/or genomic biomarker(s) to predict or monitor exercise response is of obvious importance.
Finally, researchers must be cognizant of clinical care, and design studies that reflect the clinical scenario. For example, the vast majority of the current literature investigates the effects of exercise as monotherapy. The majority of clinical scenarios would be applying exercise as an adjuvant therapy to surgery, radiation, chemotherapy, or immunotherapies. As such, it is important for the next generation of preclinical studies aiming to study tumor progression to evaluate the interaction between exercise and the pharmacodynamic or pharmacokinetic activity of conventional and novel therapies to guide the design and interpretation of clinical studies. We advise researchers to proceed with caution and carefully include all possible controls groups, because the additional therapies could introduce new confounding factors that complicate data interpretations Conversely, studies on tumor incidence may be strengthened by eliminating additional factors such as concomitant manipulation of dietary composition.
Conclusion
A sound foundation of basic and translational studies will optimize the therapeutic potential of exercise on symptom control and clinical outcomes across the cancer continuum. Despite its importance, we found that the current evidence base is plagued by considerable methodological heterogeneity in all aspects of study design, end points, and efficacy thereby precluding meaningful comparisons and conclusions. To this end, we have provided an overview of methodological and data reporting standards that we hope will set the platform for the next generation of preclinical studies required for the continued development and progression of exercise-oncology research.
Supplementary Material
Figure 2.
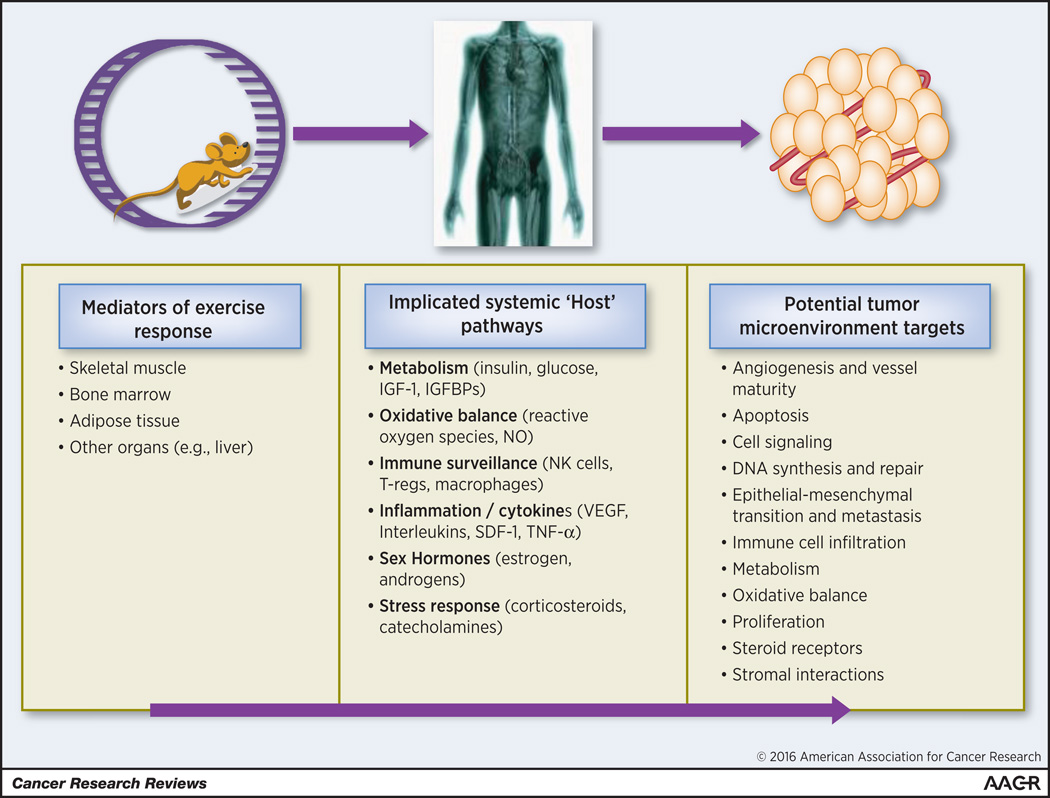
Hypothesized pathways by which endurance exercise may impact tumor progression and metastasis. Key host tissues such as skeletal muscle, adipose tissue, bone marrow, and the liver mediate the effect of exercise on a variety of systemic pathways. Exercise-induced alterations in systemic and circulating factors, in turn, influences ligand availability in the tumor microenvironment which alters cellular signaling modulating the hallmarks of cancer.
Acknowledgments
LWJ is supported in part by grants from the National Cancer Institute, AKTIV Against Cancer, and the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748). MWD is supported by NIH CA40355.
Footnotes
Conflict of Interest: The authors report no conflicts of interest
REFERENCES
- 1.Booth FW, Gordon SE, Carlson CJ, Hamilton MT. Waging war on modern chronic diseases: primary prevention through exercise biology. Journal of applied physiology. 2000;88(2):774–787. doi: 10.1152/jappl.2000.88.2.774. [DOI] [PubMed] [Google Scholar]
- 2.Giannuzzi P, Mezzani A, Saner H, Bjornstad H, Fioretti P, Mendes M, et al. Physical activity for primary and secondary prevention. Position paper of the Working Group on Cardiac Rehabilitation and Exercise Physiology of the European Society of Cardiology. European journal of cardiovascular prevention and rehabilitation : official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2003;10(5):319–327. doi: 10.1097/01.hjr.0000086303.28200.50. [DOI] [PubMed] [Google Scholar]
- 3.Hawley JA, Hargreaves M, Joyner MJ, Zierath JR. Integrative biology of exercise. Cell. 2014;159(4):738–749. doi: 10.1016/j.cell.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 4.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2006;174(6):801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones LW, Alfano CM. Exercise-oncology research: past, present, and future. Acta oncologica. 2013;52(2):195–215. doi: 10.3109/0284186X.2012.742564. [DOI] [PubMed] [Google Scholar]
- 6.Friedenreich CM, Woolcott CG, McTiernan A, Ballard-Barbash R, Brant RF, Stanczyk FZ, et al. Alberta physical activity and breast cancer prevention trial: sex hormone changes in a year-long exercise intervention among postmenopausal women. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(9):1458–1466. doi: 10.1200/JCO.2009.24.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynch BM, Friedenreich CM, Winkler EA, Healy GN, Vallance JK, Eakin EG, et al. Associations of objectively assessed physical activity and sedentary time with biomarkers of breast cancer risk in postmenopausal women: findings from NHANES (2003–2006) Breast cancer research and treatment. 2011;130(1):183–194. doi: 10.1007/s10549-011-1559-2. [DOI] [PubMed] [Google Scholar]
- 8.Gammon MD, Schoenberg JB, Britton JA, Kelsey JL, Coates RJ, Brogan D, et al. Recreational physical activity and breast cancer risk among women under age 45 years. American journal of epidemiology. 1998;147(3):273–280. doi: 10.1093/oxfordjournals.aje.a009447. [DOI] [PubMed] [Google Scholar]
- 9.Bernstein L, Henderson BE, Hanisch R, Sullivan-Halley J, Ross RK. Physical exercise and reduced risk of breast cancer in young women. Journal of the National Cancer Institute. 1994;86(18):1403–1408. doi: 10.1093/jnci/86.18.1403. [DOI] [PubMed] [Google Scholar]
- 10.Byers T, Nestle M, McTiernan A, Doyle C, Currie-Williams A, Gansler T, et al. American Cancer Society guidelines on nutrition and physical activity for cancer prevention: Reducing the risk of cancer with healthy food choices and physical activity. CA: a cancer journal for clinicians. 2002;52(2):92–119. doi: 10.3322/canjclin.52.2.92. [DOI] [PubMed] [Google Scholar]
- 11.Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA: a cancer journal for clinicians. 2012;62(1):30–67. doi: 10.3322/caac.20140. [DOI] [PubMed] [Google Scholar]
- 12.Friedenreich CM, Neilson HK, O'Reilly R, Duha A, Yasui Y, Morielli AR, et al. Effects of a High vs Moderate Volume of Aerobic Exercise on Adiposity Outcomes in Postmenopausal Women: A Randomized Clinical Trial. JAMA oncology. 2015 doi: 10.1001/jamaoncol.2015.2239. [DOI] [PubMed] [Google Scholar]
- 13.Irwin ML, Yasui Y, Ulrich CM, Bowen D, Rudolph RE, Schwartz RS, et al. Effect of exercise on total and intra-abdominal body fat in postmenopausal women: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2003;289(3):323–330. doi: 10.1001/jama.289.3.323. [DOI] [PubMed] [Google Scholar]
- 14.McTiernan A, Ulrich CM, Yancey D, Slate S, Nakamura H, Oestreicher N, et al. The Physical Activity for Total Health (PATH) Study: rationale and design. Medicine and science in sports and exercise. 1999;31(9):1307–1312. doi: 10.1097/00005768-199909000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Campbell KL, McTiernan A, Li SS, Sorensen BE, Yasui Y, Lampe JW, et al. Effect of a 12-month exercise intervention on the apoptotic regulating proteins Bax and Bcl-2 in colon crypts: a randomized controlled trial. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16(9):1767–1774. doi: 10.1158/1055-9965.EPI-07-0291. [DOI] [PubMed] [Google Scholar]
- 16.Mishra SI, Scherer RW, Geigle PM, Berlanstein DR, Topaloglu O, Gotay CC, et al. Exercise interventions on health-related quality of life for cancer survivors. The Cochrane database of systematic reviews. 2012;8:CD007566. doi: 10.1002/14651858.CD007566.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitz KH, Holtzman J, Courneya KS, Masse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2005;14(7):1588–1595. doi: 10.1158/1055-9965.EPI-04-0703. [DOI] [PubMed] [Google Scholar]
- 18.Jones LW, Dewhirst MW. Therapeutic properties of aerobic training after a cancer diagnosis: more than a one-trick pony? Journal of the National Cancer Institute. 2014;106(4) doi: 10.1093/jnci/dju042. dju042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Betof AS, Dewhirst MW, Jones LW. Effects and potential mechanisms of exercise training on cancer progression: a translational perspective. Brain, behavior, and immunity. 2013;30(Suppl):S75–S87. doi: 10.1016/j.bbi.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. Journal of the National Cancer Institute. 2012;104(11):815–840. doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorski DH. Integrative oncology: really the best of both worlds? Nature reviews Cancer. 2014;14(10) doi: 10.1038/nrc3822. [DOI] [PubMed] [Google Scholar]
- 22.Jones LW. Precision Oncology Framework for Investigation of Exercise as Treatment for Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015 doi: 10.1200/JCO.2015.62.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdalla DR, Murta EF, Michelin MA. The influence of physical activity on the profile of immune response cells and cytokine synthesis in mice with experimental breast tumors induced by 7,12-dimethylbenzanthracene. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation. 2013;22(3):251–258. doi: 10.1097/CEJ.0b013e3283592cbb. [DOI] [PubMed] [Google Scholar]
- 24.Alessio HM, Schweitzer NB, Snedden AM, Callahan P, Hagerman AE. Revisiting influences on tumor development focusing on laboratory housing. Journal of the American Association for Laboratory Animal Science : JAALAS. 2009;48(3):258–262. [PMC free article] [PubMed] [Google Scholar]
- 25.Andrianopoulos G, Nelson RL, Bombeck CT, Souza G. The influence of physical activity in 1,2 dimethylhydrazine induced colon carcinogenesis in the rat. Anticancer research. 1987;7(4B):849–852. [PubMed] [Google Scholar]
- 26.Colbert LH, Westerlind KC, Perkins SN, Haines DC, Berrigan D, Donehower LA, et al. Exercise effects on tumorigenesis in a p53-deficient mouse model of breast cancer. Medicine and science in sports and exercise. 2009;41(8):1597–1605. doi: 10.1249/MSS.0b013e31819f1f05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esser KA, Harpole CE, Prins GS, Diamond AM. Physical activity reduces prostate carcinogenesis in a transgenic model. The Prostate. 2009;69(13):1372–1377. doi: 10.1002/pros.20987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikuyama T, Watanabe T, Minegishi Y, Osanai H. Effect of voluntary exercise on 3'-methyl-4-dimethylaminoazobenzene-induced hepatomas in male Jc1:Wistar rats. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine (New York, NY) 1993;204(2):211–215. doi: 10.3181/00379727-204-43655. [DOI] [PubMed] [Google Scholar]
- 29.Kato T, Kawaguchi H, Miyoshi N, Aoyama K, Komatsu M, Horiuchi M, et al. Effect of habitual exercise on renal carcinogenesis by ferric nitrilotriacetate. Environmental health and preventive medicine. 2011;16(4):232–238. doi: 10.1007/s12199-010-0191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lunz W, Peluzio MC, Dias CM, Moreira AP, Natali AJ. Long-term aerobic swimming training by rats reduces the number of aberrant crypt foci in 1,2-dimethylhydrazine-induced colon cancer. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica [et al] 2008;41(11):1000–1004. doi: 10.1590/s0100-879x2008001100009. [DOI] [PubMed] [Google Scholar]
- 31.Mann PB, Jiang W, Zhu Z, Wolfe P, McTiernan A, Thompson HJ. Wheel running, skeletal muscle aerobic capacity and 1-methyl-1-nitrosourea induced mammary carcinogenesis in the rat. Carcinogenesis. 2010;31(7):1279–1283. doi: 10.1093/carcin/bgq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paceli RB, Cal RN, dos Santos CH, Cordeiro JA, Neiva CM, Nagamine KK, et al. The influence of physical activity in the progression of experimental lung cancer in mice. Pathology, research and practice. 2012;208(7):377–381. doi: 10.1016/j.prp.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Reddy BS, Sugie S, Lowenfels A. Effect of voluntary exercise on azoxymethane-induced colon carcinogenesis in male F344 rats. Cancer research. 1988;48(24 Pt 1):7079–7081. [PubMed] [Google Scholar]
- 34.Saez Mdel C, Barriga C, Garcia JJ, Rodriguez AB, Ortega E. Exercise-induced stress enhances mammary tumor growth in rats: beneficial effect of the hormone melatonin. Molecular and cellular biochemistry. 2007;294(1–2):19–24. doi: 10.1007/s11010-005-9067-5. [DOI] [PubMed] [Google Scholar]
- 35.Sugie S, Reddy BS, Lowenfels A, Tanaka T, Mori H. Effect of voluntary exercise on azoxymethane-induced hepatocarcinogenesis in male F344 rats. Cancer letters. 1992;63(1):67–72. doi: 10.1016/0304-3835(92)90091-9. [DOI] [PubMed] [Google Scholar]
- 36.Thorling EB, Jacobsen NO, Overvad K. Effect of exercise on intestinal tumour development in the male Fischer rat after exposure to azoxymethane. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation. 1993;2(1):77–82. doi: 10.1097/00008469-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 37.Westerlind KC, McCarty HL, Schultheiss PC, Story R, Reed AH, Baier ML, et al. Moderate exercise training slows mammary tumour growth in adolescent rats. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation. 2003;12(4):281–287. doi: 10.1097/00008469-200308000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Whittal KS, Parkhouse WS. Exercise during adolescence and its effects on mammary gland development, proliferation, and nitrosomethylurea (NMU) induced tumorigenesis in rats. Breast cancer research and treatment. 1996;37(1):21–27. doi: 10.1007/BF01806628. [DOI] [PubMed] [Google Scholar]
- 39.Whittal-Strange KS, Chadan S, Parkhouse WS. Exercise during puberty and NMU induced mammary tumorigenesis in rats. Breast cancer research and treatment. 1998;47(1):1–8. doi: 10.1023/a:1005838721890. [DOI] [PubMed] [Google Scholar]
- 40.Woods JA, Davis JM, Kohut ML, Ghaffar A, Mayer EP, Pate RR. Effects of exercise on the immune response to cancer. Medicine and science in sports and exercise. 1994;26(9):1109–1115. [PubMed] [Google Scholar]
- 41.Zhu Z, Jiang W, McGinley JN, Thompson HJ. Energetics and mammary carcinogenesis: effects of moderate-intensity running and energy intake on cellular processes and molecular mechanisms in rats. Journal of applied physiology. 2009;106(3):911–918. doi: 10.1152/japplphysiol.91201.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Z, Jiang W, Sells JL, Neil ES, McGinley JN, Thompson HJ. Effect of nonmotorized wheel running on mammary carcinogenesis: circulating biomarkers, cellular processes, and molecular mechanisms in rats. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17(8):1920–1929. doi: 10.1158/1055-9965.EPI-08-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu Z, Jiang W, Zacher JH, Neil ES, McGinley JN, Thompson HJ. Effects of energy restriction and wheel running on mammary carcinogenesis and host systemic factors in a rat model. Cancer prevention research. 2012;5(3):414–422. doi: 10.1158/1940-6207.CAPR-11-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zielinski MR, Muenchow M, Wallig MA, Horn PL, Woods JA. Exercise delays allogeneic tumor growth and reduces intratumoral inflammation and vascularization. Journal of applied physiology. 2004;96(6):2249–2256. doi: 10.1152/japplphysiol.01210.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malicka I, Siewierska K, Pula B, Kobierzycki C, Haus D, Paslawska U, et al. The effect of physical training on the N-methyl-N-nitrosourea-induced mammary carcinogenesis of Sprague-Dawley rats. Experimental biology and medicine (Maywood, NJ) 2015 doi: 10.1177/1535370215587532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piguet AC, Saran U, Simillion C, Keller I, Terracciano L, Reeves HL, et al. Regular exercise decreases liver tumors development in hepatocyte-specific PTEN-deficient mice independently of steatosis. Journal of hepatology. 2015;62(6):1296–1303. doi: 10.1016/j.jhep.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 47.Almeida PW, Gomes-Filho A, Ferreira AJ, Rodrigues CE, Dias-Peixoto MF, Russo RC, et al. Swim training suppresses tumor growth in mice. Journal of applied physiology. 2009;107(1):261–265. doi: 10.1152/japplphysiol.00249.2009. [DOI] [PubMed] [Google Scholar]
- 48.Bacurau AV, Belmonte MA, Navarro F, Moraes MR, Pontes FL, Jr, Pesquero JL, et al. Effect of a high-intensity exercise training on the metabolism and function of macrophages and lymphocytes of walker 256 tumor bearing rats. Experimental biology and medicine (Maywood, NJ) 2007;232(10):1289–1299. doi: 10.3181/0704-RM-93. [DOI] [PubMed] [Google Scholar]
- 49.Bacurau RF, Belmonte MA, Seelaender MC, Costa Rosa LF. Effect of a moderate intensity exercise training protocol on the metabolism of macrophages and lymphocytes of tumour-bearing rats. Cell biochemistry and function. 2000;18(4):249–258. doi: 10.1002/1099-0844(200012)18:4<249::AID-CBF879>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 50.Baracos VE. Exercise inhibits progressive growth of the Morris hepatoma 7777 in male and female rats. Canadian journal of physiology and pharmacology. 1989;67(8):864–870. doi: 10.1139/y89-135. [DOI] [PubMed] [Google Scholar]
- 51.Daneryd P, Hafstrom L, Svanberg E, Karlberg I. Insulin sensitivity, hormonal levels and skeletal muscle protein metabolism in tumour-bearing exercising rats. European journal of cancer. 1995;31A(1):97–103. doi: 10.1016/0959-8049(94)00344-5. [DOI] [PubMed] [Google Scholar]
- 52.Daneryd P, Westin T, Edstrom S, Soussi B. Tumour purine nucleotides and cell proliferation in response to exercise in rats. European journal of cancer. 1995;31a(13–14):2309–2312. doi: 10.1016/0959-8049(95)00441-6. [DOI] [PubMed] [Google Scholar]
- 53.Gershbein LL, Benuck I, Shurrager PS. Influence of stress of lesion growth and on survival of animals bearing parenteral and intracerebral leukemia L1210 and Walker tumors. Oncology. 1974;30(5):429–435. doi: 10.1159/000224983. [DOI] [PubMed] [Google Scholar]
- 54.Hoffman SA, Paschkis KE, Debias DA, Cantarow A, Williams TL. The influence of exercise on the growth of transplanted rat tumors. Cancer research. 1962;22:597–599. [PubMed] [Google Scholar]
- 55.Jones LW, Antonelli J, Masko EM, Broadwater G, Lascola CD, Fels D, et al. Exercise modulation of the host-tumor interaction in an orthotopic model of murine prostate cancer. Journal of applied physiology. 2012;113(2):263–272. doi: 10.1152/japplphysiol.01575.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones LW, Eves ND, Courneya KS, Chiu BK, Baracos VE, Hanson J, et al. Effects of exercise training on antitumor efficacy of doxorubicin in MDA-MB-231 breast cancer xenografts. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11(18):6695–6698. doi: 10.1158/1078-0432.CCR-05-0844. [DOI] [PubMed] [Google Scholar]
- 57.Jones LW, Viglianti BL, Tashjian JA, Kothadia SM, Keir ST, Freedland SJ, et al. Effect of aerobic exercise on tumor physiology in an animal model of human breast cancer. Journal of applied physiology. 2010;108(2):343–348. doi: 10.1152/japplphysiol.00424.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lira FS, Tavares FL, Yamashita AS, Koyama CH, Alves MJ, Caperuto EC, et al. Effect of endurance training upon lipid metabolism in the liver of cachectic tumour-bearing rats. Cell biochemistry and function. 2008;26(6):701–708. doi: 10.1002/cbf.1495. [DOI] [PubMed] [Google Scholar]
- 59.Murphy EA, Davis JM, Barrilleaux TL, McClellan JL, Steiner JL, Carmichael MD, et al. Benefits of exercise training on breast cancer progression and inflammation in C3(1)SV40Tag mice. Cytokine. 2011;55(2):274–279. doi: 10.1016/j.cyto.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Newton G. TUMOR SUSCEPTIBILITY IN RATS: ROLE OF INFANTILE MANIPULATION AND LATER EXERCISE. Psychological reports. 1965;16:127–132. doi: 10.2466/pr0.1965.16.1.127. [DOI] [PubMed] [Google Scholar]
- 61.Radak Z, Gaal D, Taylor AW, Kaneko T, Tahara S, Nakamoto H, et al. Attenuation of the development of murine solid leukemia tumor by physical exercise. Antioxidants & redox signaling. 2002;4(1):213–219. doi: 10.1089/152308602753625979. [DOI] [PubMed] [Google Scholar]
- 62.Sasvari M, Taylor AW, Gaal D, Radak Z. The effect of regular exercise on development of sarcoma tumor and oxidative damage in mice liver. Journal of sports science & medicine. 2011;10(1):93–96. [PMC free article] [PubMed] [Google Scholar]
- 63.Uhlenbruck G, Order U. Can endurance sports stimulate immune mechanisms against cancer and metastasis? International journal of sports medicine. 1991;12(Suppl 1):S63–S68. doi: 10.1055/s-2007-1024753. [DOI] [PubMed] [Google Scholar]
- 64.Yan L, Demars LC. Effects of non-motorized voluntary running on experimental and spontaneous metastasis in mice. Anticancer research. 2011;31(10):3337–3344. [PubMed] [Google Scholar]
- 65.Betof AS, Lascola CD, Weitzel D, Landon C, Scarbrough PM, Devi GR, et al. Modulation of murine breast tumor vascularity, hypoxia and chemotherapeutic response by exercise. Journal of the National Cancer Institute. 2015;107(5) doi: 10.1093/jnci/djv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goh J, Endicott E, Ladiges WC. Pre-tumor exercise decreases breast cancer in old mice in a distance-dependent manner. American journal of cancer research. 2014;4(4):378–384. [PMC free article] [PubMed] [Google Scholar]
- 67.Shalamzari SA, Agha-Alinejad H, Alizadeh S, Shahbazi S, Khatib ZK, Kazemi A, et al. The effect of exercise training on the level of tissue IL-6 and vascular endothelial growth factor in breast cancer bearing mice. Iranian journal of basic medical sciences. 2014;17(4):231–258. [PMC free article] [PubMed] [Google Scholar]
- 68.Aveseh M, Nikooie R, Aminaie M. Exercise-induced changes in tumour LDH-B and MCT1 expression are modulated by oestrogen-related receptor alpha in breast cancer-bearing BALB/c mice. The Journal of physiology. 2015 doi: 10.1113/JP270463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gueritat J, Lefeuvre-Orfila L, Vincent S, Cretual A, Ravanat JL, Gratas-Delamarche A, et al. Exercise training combined with antioxidant supplementation prevents the antiproliferative activity of their single treatment in prostate cancer through inhibition of redox adaptation. Free radical biology & medicine. 2014;77:95–105. doi: 10.1016/j.freeradbiomed.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 70.Hoffman-Goetz L, May KM, Arumugam Y. Exercise training and mouse mammary tumour metastasis. Anticancer research. 1994;14(6B):2627–2631. [PubMed] [Google Scholar]
- 71.Hoffmann-Goetz L, MacNeil B, Arumugam Y. Tissue distribution of radiolabelled tumor cells in wheel exercised and sedentary mice. International journal of sports medicine. 1994;15(5):249–253. doi: 10.1055/s-2007-1021055. [DOI] [PubMed] [Google Scholar]
- 72.Jadeski L, Hoffman-Goetz L. Exercise and in vivo natural cytotoxicity against tumour cells of varying metastatic capacity. Clinical & experimental metastasis. 1996;14(2):138–144. doi: 10.1007/BF00121210. [DOI] [PubMed] [Google Scholar]
- 73.MacNeil B, Hoffman-Goetz L. Exercise training and tumour metastasis in mice: influence of time of exercise onset. Anticancer research. 1993;13(6A):2085–2088. [PubMed] [Google Scholar]
- 74.MacNeil B, Hoffman-Goetz L. Effect of exercise on natural cytotoxicity and pulmonary tumor metastases in mice. Medicine and science in sports and exercise. 1993;25(8):922–928. [PubMed] [Google Scholar]
- 75.Higgins KA, Park D, Lee GY, Curran WJ, Deng X. Exercise-induced lung cancer regression: mechanistic findings from a mouse model. Cancer. 2014;120(21):3302–3310. doi: 10.1002/cncr.28878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao G, Zhou S, Davie A, Su Q. Effects of moderate and high intensity exercise on T1/T2 balance. Exercise immunology review. 2012;18:98–114. [PubMed] [Google Scholar]
- 77.Roberts CJ, Stuhr KL, Hillard CJ. Swim stress differentially affects limbic contents of 2-arachidonoylglycerol and 2-oleoylglycerol. Neuroscience. 2012;204:74–82. doi: 10.1016/j.neuroscience.2011.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fuss J, Ben Abdallah NM, Vogt MA, Touma C, Pacifici PG, Palme R, et al. Voluntary exercise induces anxiety-like behavior in adult C57BL/6J mice correlating with hippocampal neurogenesis. Hippocampus. 2010;20(3):364–376. doi: 10.1002/hipo.20634. [DOI] [PubMed] [Google Scholar]
- 79.Richter SH, Gass P, Fuss J. Resting Is Rusting: A Critical View on Rodent Wheel-Running Behavior. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2014;20(4):313–325. doi: 10.1177/1073858413516798. [DOI] [PubMed] [Google Scholar]
- 80.Hackney AC, Viru A. Twenty-four-hour cortisol response to multiple daily exercise sessions of moderate and high intensity. Clinical physiology. 1999;19(2):178–182. doi: 10.1046/j.1365-2281.1999.00157.x. [DOI] [PubMed] [Google Scholar]
- 81.Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nature medicine. 2006;12(8):939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 82.Zen M, Canova M, Campana C, Bettio S, Nalotto L, Rampudda M, et al. The kaleidoscope of glucorticoid effects on immune system. Autoimmunity reviews. 2011;10(6):305–310. doi: 10.1016/j.autrev.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 83.Sasso JP, Eves ND, Christensen JF, Koelwyn GJ, Scott J, Jones LW. A framework for prescription in exercise-oncology research. Journal of cachexia, sarcopenia and muscle. 2015;6(2):115–124. doi: 10.1002/jcsm.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Irwin ML, Smith AW, McTiernan A, Ballard-Barbash R, Cronin K, Gilliland FD, et al. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(24):3958–3964. doi: 10.1200/JCO.2007.15.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mina LA, Sledge GW., Jr Rethinking the metastatic cascade as a therapeutic target. Nature reviews Clinical oncology. 2011;8(6):325–332. doi: 10.1038/nrclinonc.2011.59. [DOI] [PubMed] [Google Scholar]
- 86.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nature reviews Cancer. 2002;2(8):563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 87.Day CP, Merlino G, Van Dyke T. Preclinical mouse cancer models: a maze of opportunities and challenges. Cell. 2015;163(1):39–53. doi: 10.1016/j.cell.2015.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gonzalez C. Drosophila melanogaster: a model and a tool to investigate malignancy and identify new therapeutics. Nature reviews Cancer. 2013;13(3):172–183. doi: 10.1038/nrc3461. [DOI] [PubMed] [Google Scholar]
- 89.Gould SE, Junttila MR, de Sauvage FJ. Translational value of mouse models in oncology drug development. Nature medicine. 2015;21(5):431–439. doi: 10.1038/nm.3853. [DOI] [PubMed] [Google Scholar]
- 90.Sharpless NE, Depinho RA. The mighty mouse: genetically engineered mouse models in cancer drug development. Nature reviews Drug discovery. 2006;5(9):741–754. doi: 10.1038/nrd2110. [DOI] [PubMed] [Google Scholar]
- 91.White R, Rose K, Zon L. Zebrafish cancer: the state of the art and the path forward. Nature reviews Cancer. 2013;13(9):624–636. doi: 10.1038/nrc3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McCullough DJ, Stabley JN, Siemann DW, Behnke BJ. Modulation of blood flow, hypoxia, and vascular function in orthotopic prostate tumors during exercise. Journal of the National Cancer Institute. 2014;106(4) doi: 10.1093/jnci/dju036. dju036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487(7408):500–504. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McAllister SS, Gifford AM, Greiner AL, Kelleher SP, Saelzler MP, Ince TA, et al. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell. 2008;133(6):994–1005. doi: 10.1016/j.cell.2008.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McAllister SS, Weinberg RA. Tumor-host interactions: a far-reaching relationship. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(26):4022–4028. doi: 10.1200/JCO.2010.28.4257. [DOI] [PubMed] [Google Scholar]
- 96.Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Taylor SK, et al. Insulin- and obesity-related variables in early-stage breast cancer: correlations and time course of prognostic associations. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(2):164–171. doi: 10.1200/JCO.2011.36.2723. [DOI] [PubMed] [Google Scholar]
- 97.Sheen-Chen SM, Chen WJ, Eng HL, Chou FF. Serum concentration of tumor necrosis factor in patients with breast cancer. Breast cancer research and treatment. 1997;43(3):211–215. doi: 10.1023/a:1005736712307. [DOI] [PubMed] [Google Scholar]
- 98.Sheen-Chen SM, Liu YW, Eng HL, Chou FF. Serum levels of hepatocyte growth factor in patients with breast cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2005;14(3):715–717. doi: 10.1158/1055-9965.EPI-04-0340. [DOI] [PubMed] [Google Scholar]
- 99.Hursting SD, Berger NA. Energy balance, host-related factors, and cancer progression. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(26):4058–4065. doi: 10.1200/JCO.2010.27.9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Glass OK, Inman BA, Broadwater G, Courneya KS, Mackey JR, Goruk S, et al. Effect of aerobic training on the host systemic milieu in patients with solid tumours: an exploratory correlative study. British journal of cancer. 2015;112(5):825–831. doi: 10.1038/bjc.2014.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Joyner MJ, Green DJ. Exercise protects the cardiovascular system: effects beyond traditional risk factors. The Journal of physiology. 2009;587(Pt 23):5551–5558. doi: 10.1113/jphysiol.2009.179432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA : the journal of the American Medical Association. 2009;301(19):2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 103.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380(9838):219–229. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pedersen L, Christensen JF, Hojman P. Effects of exercise on tumor physiology and metabolism. Cancer journal. 2015;21(2):111–116. doi: 10.1097/PPO.0000000000000096. [DOI] [PubMed] [Google Scholar]
- 105.Fowler JF. 21 years of biologically effective dose. The British journal of radiology. 2010;83(991):554–568. doi: 10.1259/bjr/31372149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dewhirst MW, Viglianti BL, Lora-Michiels M, Hanson M, Hoopes PJ. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2003;19(3):267–294. doi: 10.1080/0265673031000119006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.