Abstract
Rationale: Aging is associated with reduced FEV1 to FVC ratio (FEV1/FVC), hyperinflation, and alveolar enlargement, but little is known about how age affects small airways.
Objectives: To determine if chest computed tomography (CT)-assessed functional small airway would increase with age, even among asymptomatic individuals.
Methods: We used parametric response mapping analysis of paired inspiratory/expiratory CTs to identify functional small airway abnormality (PRMFSA) and emphysema (PRMEMPH) in the SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study) cohort. Using adjusted linear regression models, we analyzed associations between PRMFSA and age in subjects with or without airflow obstruction. We subdivided participants with normal spirometry based on respiratory-related impairment (6-minute-walk distance <350 m, modified Medical Research Council ≥2, chronic bronchitis, St. George’s Respiratory Questionnaire >25, respiratory events requiring treatment [antibiotics and/or steroids or hospitalization] in the year before enrollment).
Measurements and Main Results: Among 580 never- and ever-smokers without obstruction or respiratory impairment, PRMFSA increased 2.7% per decade, ranging from 3.6% (ages 40–50 yr) to 12.7% (ages 70–80 yr). PRMEMPH increased nonsignificantly (0.1% [ages 40–50 yr] to 0.4% [ages 70–80 yr]; P = 0.34). Associations were similar among nonobstructed individuals with respiratory-related impairment. Increasing PRMFSA in subjects without airflow obstruction was associated with increased FVC (P = 0.004) but unchanged FEV1 (P = 0.94), yielding lower FEV1/FVC ratios (P < 0.001). Although emphysema was also significantly associated with lower FEV1/FVC (P = 0.04), its contribution relative to PRMFSA in those without airflow obstruction was limited by its low burden.
Conclusions: In never- and ever-smokers without airflow obstruction, aging is associated with increased FVC and CT-defined functional small airway abnormality regardless of respiratory symptoms.
Keywords: spirometry, imaging analysis, aging, geriatrics, lung function
At a Glance Commentary
Scientific Knowledge on the Subject
Hyperinflation and reductions in the FEV1/FVC ratio have been reported in association with lung aging in healthy never-smokers, although the exact mechanism for these changes has not been well defined.
What This Study Adds to the Field
Computed tomography–defined functional small airway abnormality increased with age and was associated with lower FEV1/FVC ratio, primarily because of greater FVC, even among asymptomatic subjects with FEV1/FVC ratio greater than 0.7.
Greater life expectancy has been associated with an increased prevalence of chronic diseases (1), including chronic obstructive pulmonary disease (COPD), bringing new health care needs and challenges. One of these challenges is to understand the “normal” process of aging and to separate it from “disease.” Lung aging is a complex process previously noted to involve loss of elastic recoil, air trapping (2), decreased vital capacity, increased residual volume (3), and homogeneous hyperinflation (4). These changes, considered normal age-related phenomena, are also present in emphysema-predominant COPD, although with greater severity, including actual destruction of alveolar structures and thickening of the airways. The resemblance between emphysema and age-related changes has led some authors to hypothesize that accelerated lung aging (“senile lung,” sometimes known as “senile emphysema”) is part of COPD pathogenesis (5, 6), a matter that is still intensely debated (7). Despite great interest in the changes at the level of the air sacs, less is known about the impact of aging on the peripheral airways, a major site of airflow resistance and an early target in the development of COPD (8).
New methods and algorithms of lung imaging continue to be proposed to evaluate COPD, with the goal of delineating phenotypic characteristics associated with clinical outcomes (9–11) and disease progression (12, 13). One strength of the recently introduced parametric response mapping (PRM) computed tomography (CT) analysis method is its ability to distinguish emphysematous from nonemphysematous gas trapping, with the latter termed functional small airways abnormality (PRMFSA) (14–17). Given the limited available information about the impact of aging on small airways (8, 18, 19), identifying age-related changes is an important goal with direct implications in defining lung diseases.
In this analysis, we use clinical, functional, and imaging data from participants in a well-characterized cohort to test the hypotheses that abnormalities of the functional small airways are part of normal aging and that early obstructive lung disease accelerates age-related changes. Specifically, we (1) quantified the extent of PRMFSA in never- and ever-smokers with neither physiologic nor clinical pulmonary abnormalities, (2) tested whether the extent of CT-defined abnormalities increased with aging, and (3) determined whether age-related differences in PRMFSA vary by presence of airflow obstruction. Parts of the results have been presented in abstract form at the 2016 American Thoracic Society International Conference on May 16, 2016, San Francisco, California (20).
Methods
Design and Study Participants
This study is a cross-sectional analysis of baseline data from participants in SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study), a multicenter cohort study that has enrolled 2,981 participants across four strata (never-smokers and ever-smokers without COPD, with mild/moderate COPD, and with severe COPD) with the goals of identifying new COPD subgroups and intermediate markers of disease progression. Complete details of SPIROMICS design and protocols have been reported in detail elsewhere (21). The protocols for SPIROMICS have been approved by the institutional review board at each participating institution. For the current analysis, we selected participants, both ever- and never-smokers, without airflow obstruction, based on the spirometry definition of a fixed ratio of FEV1 over FVC greater than or equal to 0.7 and FEV1% predicted greater than the lower limit of normal. Additionally, we excluded subjects with FVC below the lower limit of normal.
Participants were considered asymptomatic if they did not report any symptom or clinical marker of respiratory-related impairment (22) (chronic bronchitis symptoms [23]; dyspnea score ≥2 on the modified Medical Research Council [mMRC] scale [24]; distance walked in 6 min <350 m; St. George’s Respiratory Questionnaire total score >25 points [25]; and acute respiratory events in the year before enrollment, defined as use of antibiotic and/or steroid or hospital admission for a respiratory “flare-up”). Subjects reporting any of these complaints or markers of lung impairment were considered symptomatic. For some additional analyses, aimed at testing the age-by-disease interaction, we also included subjects with mild to moderate airflow obstruction (FEV1/FVC <0.7, and FEV1 >50% predicted).
Data Collection
SPIROMICS participants provided demographic information (age, sex, race), smoking history (ever- or never-smoker, smoking within the last month, lifetime cumulative smoking in pack-years), dyspnea severity (using the mMRC), medical history, and respiratory symptoms at enrollment, using validated questionnaires. Comorbid conditions, including asthma, were considered present if the participant answered yes to the question “Have you ever been diagnosed by a physician with [condition name]?” Anthropometric data on height and weight were acquired at enrollment, following standardized protocols. SPIROMICS uses post-bronchodilator spirometry values, with spirometry performed following American Thoracic Society recommendations on a pneumotachograph spirometer, with predicted values based on Hankinson reference equations (26).
Imaging Analysis
PRM analysis of paired inspiratory and expiratory CTs was used to identify and quantify extent of functional small airway abnormality (PRMFSA) and emphysema (PRMEMPH). Briefly, the PRM method (27) consists of three key steps: (1) image acquisition per SPIROMICS protocol, (2) image processing, and (3) classification. During image processing, lung parenchyma and airways are segmented to restrict the focus of the registration process to the lungs. Deformable registration is performed to spatially align the expiration with the inspiration scan, allowing them share the same spatial geometry. Classification of voxel-by-voxel attenuation maps into discrete zones allows for quantification of global measures of normal parenchyma, PRMFSA, and PRMEMPH. Classification is determined by imposing two thresholds: −950 HU on inspiration scans, with lesser values denoting emphysema; and −856 HU on expiration scan, with lesser values denoting nonemphysematous gas trapping. These thresholds are applied to a joint histogram formed using all voxel pairs within the registered inspiration-expiration images. Three discrete classifications are identified: (1) healthy lung parenchyma, (2) PRMFSA, and (3) PRMEMPH. PRM has been validated as a technique to identify abnormality even in those without spirometric obstruction (15, 28). PRM was performed using the Imbio Lung Density Analysis software application (Imbio, LLC, Minneapolis, MN). Extent of normal parenchyma, PRMEMPH, and PRMFSA is described as percentage of the whole lung area.
Statistical Analysis
We performed descriptive statistics for demographic, clinical, and imaging variables (means with SD and proportions). Linear regression was used to identify the effect of age (in decades) on the presence of PRMFSA, in unadjusted models and models adjusted, based on previous knowledge, for covariates relevant to lung function and structure (sex, race, height, smoking status, cumulative smoking history, and history of asthma, with additional adjustment for clinical center and scanner). Initial analyses were restricted to subjects without airflow obstruction, contrasting those with versus without symptoms. In a second step, we compared the extent of PRMFSA between participants with versus without airflow obstruction and tested for airflow obstruction-by-age interactions. We performed three subgroup analyses, restricting the population to (1) never-smokers with no symptoms and no asthma; (2) participants without airflow obstruction and neither respiratory symptoms nor asthma; and (3) participants without history of wheezing before 16 years of age. Last, we tested the impact of PRMFSA on FEV1, FVC, and the FEV1/FVC ratio in models adjusted for variables known to be associated with lung function (age, sex, race, height, and emphysema percentage). All analyses were performed using Stata version 12 (Stata Corp., College Station, TX).
Results
Characteristics of Participants
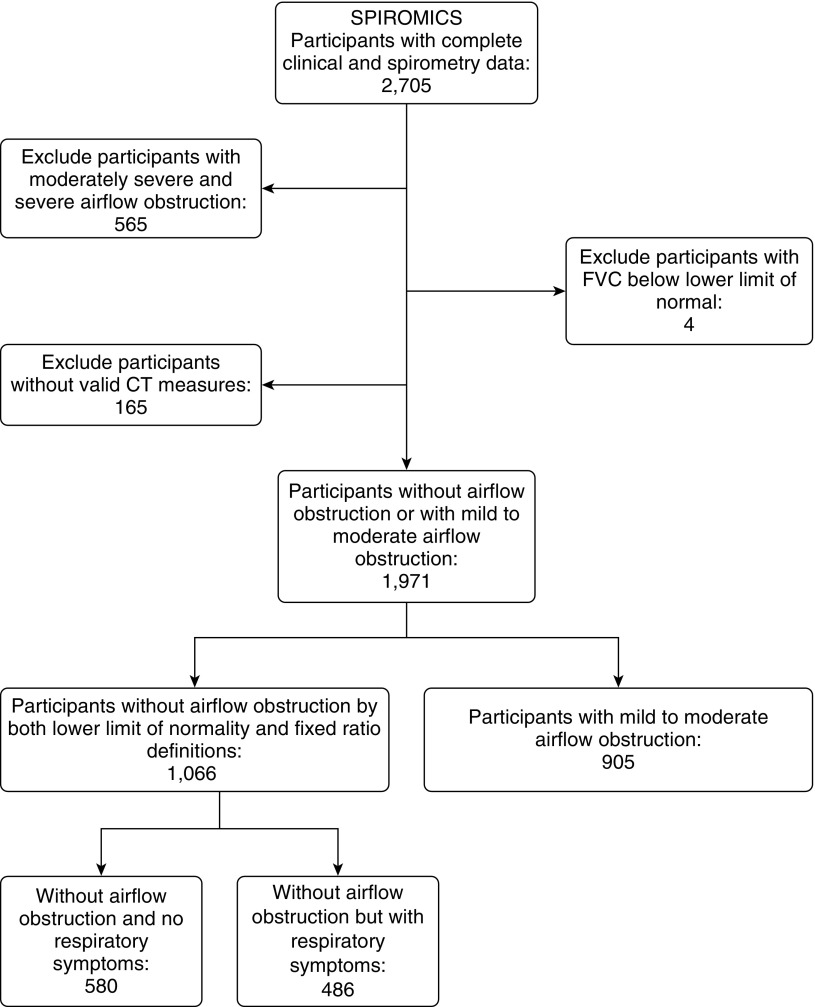
By September 2014, SPIROMICS had enrolled 2,705 subjects with complete clinical data and valid spirometry. Given our interest in normal aging and early disease, we excluded 565 participants with moderately severe or severe airflow obstruction (Global Initiative for Chronic Obstructive Lung Disease spirometry stages 3 and 4), 165 participants whose PRM measures were unavailable, and four with FVC below the lower limit of normal, leaving 1,971 subjects for the current analyses, 1,066 without airflow obstruction by both the fixed ratio and lower limit of normal and 904 subjects with obstructive spirometry, Global Initiative for Chronic Obstructive Lung Disease stages 1 and 2. The flow of participants is described in Figure 1. Among the 1,066 subjects without airflow obstruction, ever- and never-smokers, 580 were asymptomatic, based on not reporting any of five markers of symptoms or respiratory-related impairment (22) (chronic bronchitis symptoms [23], dyspnea score ≥2 on the mMRC scale [24], acute respiratory events in the year before enrollment, distance walked in 6 min <350 m, and St. George’s Respiratory Questionnaire total score >25 points [25]).
Figure 1.
Flow of participants in SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study). CT = computed tomography.
Overall, subjects had age 60.1 years (mean, SD, 9.9 yr), 53.3% females, and more than 24% of African American race. As expected, FEV1% predicted (mean, 96.3%; SD, 13.7%) and FEV1 absolute value (mean, 2.78 L; SD, 0.73) were within the normal range. The asymptomatic group had a statistically significant higher FEV1% predicted (99.3 vs. 92.7% among those with symptoms; P < 0.05), higher frequency of never-smokers (26.6 vs. 6.0% among those with symptoms; P < 0.05), lower frequency of current-smokers (30.1 vs. 52.2% among those with symptoms; P < 0.05) and a lower frequency of comorbidities (Table 1).
Table 1.
Characteristics of SPIROMICS Participants without Airflow Obstruction (N = 1,066)
| All Subjects | Without Respiratory Impairment* (n = 580) | With Respiratory Impairment* (n = 486) | |
|---|---|---|---|
| Demographics | |||
| Age, yr, mean (SD) | 60.1 (9.9) | 60.2 (10.0) | 59.9 (9.8) |
| Females, %† | 53.3 | 49.5 | 57.8 |
| African American, %† | 24.2 | 19.7 | 29.6 |
| Body mass index, kg/m2, mean (SD)† | 28.8 (5.1) | 28.0 (4.7) | 29.6 (5.4) |
| Smoking status | |||
| Never-smokers, %† | 17.2 | 26.6 | 6.0 |
| Former smoker, % | 42.6 | 43.3 | 41.8 |
| Currently smoking, %† | 40.2 | 30.1 | 52.2 |
| Smoking history | |||
| Pack-years smoked, mean (SD)† | 36.2 (27.3) | 30.6 (25.9) | 42.9 (27.5) |
| Respiratory function | |||
| FEV1, L, mean (SD)† | 2.78 (0.73) | 2.92 (0.71) | 2.61 (0.71) |
| FEV1 % predicted, mean (SD)† | 96.3 (13.7) | 99.3 (12.9) | 92.7 (13.8) |
| Comorbidities, % | |||
| Hypertension† | 42.4 | 37.1 | 48.8 |
| Anxiety or depression† | 32.2 | 24.7 | 41.2 |
| Gastroesophageal reflux disease† | 28.0 | 23.4 | 33.5 |
| Diabetes mellitus† | 12.5 | 7.9 | 17.9 |
| Asthma† | 15.0 | 6.2 | 25.5 |
| Coronary artery disease | 4.7 | 3.3 | 6.4 |
| Sleep apnea† | 16.4 | 14.5 | 18.7 |
| Peripheral vascular disease† | 5.6 | 2.6 | 9.3 |
Definition of abbreviation: SPIROMICS = Subpopulations and Intermediate Outcome Measures in COPD Study.
Participants were considered to be without respiratory-related impairment (21) if they did not report chronic bronchitis symptoms (22), dyspnea score greater than or equal to two on the modified Medical Research Council scale (23), distance walked in 6 minutes greater than 350 m, St. George’s Respiratory Questionnaire total score less than or equal to 25 points (24), and acute respiratory events in the year before enrollment, defined as use of antibiotic and/or steroid or hospital admission for a respiratory “flare-up.”
P less than 0.05.
Functional Small Airway Abnormalities Increase with Advancing Age
PRM mapping showed that in subjects (both ever- and never-smokers) without airflow obstruction, most of the lung parenchyma was normal, with a low PRMFSA burden (8.3 ± 8.0%) that was similar in those without symptoms (8.2 ± 7.7%) compared with those with symptoms (8.3 ± 8.4%; P = 0.81). There was also paucity of emphysematous changes (PRMEMPH 0.5 ± 1.6%). PRMFSA increased with advancing age, in univariate analysis by 3.1% (95% confidence interval [CI], 2.6–3.5%) for decade of age. This association was maintained and remained significant, at 2.7% higher per decade (95% CI, 2.2–3.2%), in multivariate models adjusted for sex, race, height, smoking status, smoking history, history of asthma, clinical center, and CT scanner model (Table 2). Importantly, the increase of PRMFSA by age was of similar magnitude among nonobstructed subjects with (2.6%; 95% CI, 1.7–3.5%) or without (2.7%; 95% CI, 2.02–3.4%) symptoms or respiratory-related impairment, in multivariate models equally adjusted.
Table 2.
Multivariate Models of the Impact of Age on Extent of CT-defined Functional Small Airway Abnormality (PRMFSA) in Subjects Free of Airflow Obstruction (N = 1,066)*
| β (95% CI) | |
|---|---|
| Demographics | |
| Age (per 10-yr increment) | 2.7 (2.2 to 3.2) |
| Female (vs. male) | −1.1 (−2.3 to 0.1) |
| African American race (vs. non-Hispanic white) | −0.4 (−1.7 to 0.8) |
| Smoking history (per 10 pack-years) | 0.1 (−0.1 to 0.3) |
| Smoking status | |
| Never-smoker | Reference |
| Former smoker | 0.3 (−1.3 to 1.9) |
| Currently smoker | −0.8 (−2.4 to 0.7) |
| History of asthma (vs. no history of asthma) | −0.4 (−1.7 to 0.9) |
Definition of abbreviations: CI = confidence interval; CT = computed tomography; FSA = functional small airway abnormality; PRM = parametric response mapping.
The models were additionally adjusted for height, clinical center, and scanner.
Given that air sac dilation resembling emphysema has been described with aging, we also tested the burden of PRMEMPH and its association with age in those without airflow obstruction, using similar models to those used to investigate airway abnormalities. We found that the extent of PRMEMPH is low, ranging from 0.01% at age 40–50 years to 0.4% at age 70–80 years. Moreover, among subjects without airflow obstruction, the increase in PRMEMPH (per 10 yr of age) was quite low at 0.1% (95% CI, 0–0.2%) (see Table E1 in the online supplement).
To understand better the impact of smoking on PRMFSA among subjects without airflow obstruction and to test whether smoking history alters the association between age and PRMFSA, we also compared never-, former-, and current-smokers in models additionally adjusted for demographics, history of asthma, clinical center, and CT scanner model. Not only did the results not change the age and PRMFSA associations, but we also found a limited contribution of smoking history and intensity to the presence of FSA in these subjects with normal post-bronchodilator spirometry (see Table E2).
Associations of Age with Functional Small Airway Abnormalities Are Maintained in Subgroup Analyses
Based on the different frequency of asthma between never- and ever-smokers, and between subjects with and without symptoms, we tested if PRMFSA increased with advancing age in subgroups excluding subjects with respiratory symptoms and those with asthma. We found that the associations detected among never-smokers without respiratory symptoms were of similar magnitude after additionally excluding subjects with history of asthma (2.8; 95% CI, 1.7–3.9). Similarly, restricting the analyses to subjects without airflow obstruction, never- and ever-smokers, without symptoms showed similar associations (2.7; 95% CI, 2.0–3.4) to analyses further restricted to exclude participants with asthma (2.7; 95% CI, 2.0–3.5). Exclusion of participants reporting wheezing early in life (at age ≤15 yr) the age and PRMFSA association was still maintained, both in the group of never-smokers without airflow obstruction (2.4; 95% CI, 1.3–3.6) and among never- and ever-smokers without airflow obstruction (2.7; 95% CI, 2.1–3.2) (see Table E3).
Functional Small Airway Abnormalities Impact FEV1 and FVC
Next, in subjects without airflow obstruction, never- and ever-smokers, we analyzed the associations between PRMFSA and FEV1 or FVC, in models adjusted for age, sex, race, height, smoking history, smoking status, history of asthma, and PRMEMPH. We found that an increase in PRMFSA of 1% was not associated with a significant change in FEV1 (β = 1.9 ml; 95% CI, −1.6 to 5.5 ml; P = 0.35). However, PRMFSA significantly impacted FVC, with a 1% increase in PRMFSA being associated with 9.4-ml greater (95% CI, 5.0–13.7 ml) FVC. As a consequence of this differential impact on FEV1 and FVC, an increase of 1% in PRMFSA has a net effect of decreasing the FEV1/FVC ratio by 0.15% (95% CI, −0.19 to −0.10%).
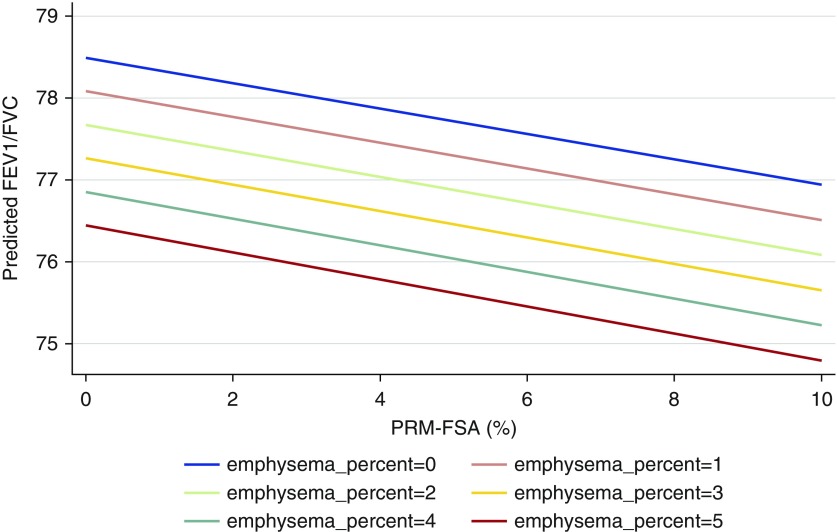
In the same models, increasing age was associated with decreases in both FEV1 and FVC, but there was no interaction between age and PRMFSA. The effect of PRMEMPH is not to be dismissed: in our analysis, a 1% increase in PRMEMPH was associated with a 19-ml lower FEV1, no effect on FVC, and a 0.41% lower FEV1/FVC. Nevertheless, among participants without airflow obstruction, the higher burden of PRMFSA (mean, 8.3%) compared with a low emphysema burden (Figure 2), with almost 99% nonobstructed participants had emphysema less than or equal to 5%, makes the PRMEMPH contribution of lesser importance in this group of individuals.
Figure 2.
Changes in FEV1/FVC ratio across varying levels of computed tomography–defined small airway abnormality (parametric response mapping with functional small airways abnormality [PRMFSA]) and emphysema among subjects without airflow obstruction. Increasing percentage of PRMFSA (x-axis) is associated with decreasing FEV1/FVC ratio (y-axis), an effect that persists at any degree of emphysema severity, as shown by the similar slope of the lines describing the burden of emphysema.
In Established Airflow Obstruction Age Has Greater Impact on the Extent of Small Airway Abnormalities
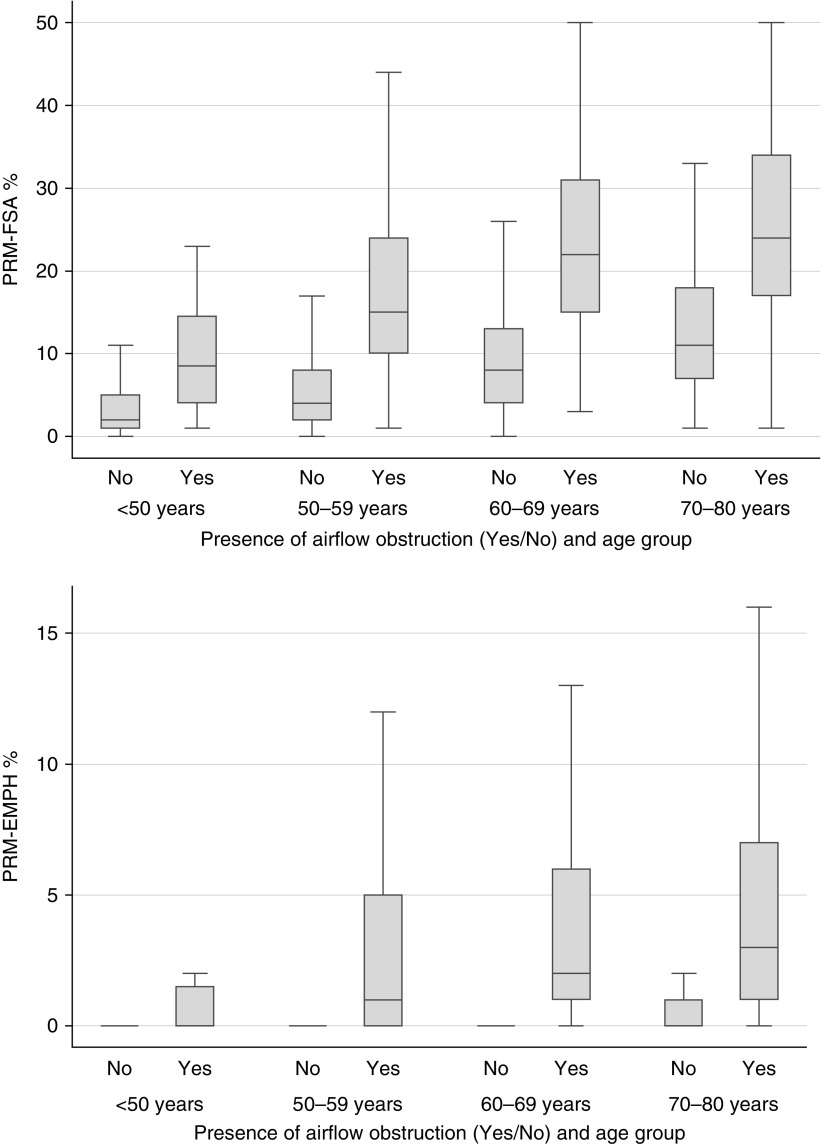
Finally, we compared the impact of age on PRMFSA between the group of 1,066 SPIROMICS participants without airflow obstruction and 905 participants with mild to moderate airflow obstruction (FEV1/FVC <0.7 and FEV1 >50% predicted), including never- and ever-smokers whose demographic and clinical characteristics are presented in Table E4. We found that the extent of PRMFSA is significantly greater (P = 0.002 for the interaction term) at any age among those with airflow obstruction (Figure 3; see Figure E1), indicating that the contribution of aging to greater PRMFSA is different in presence of established obstruction. Thus, in models adjusted for FEV1% predicted, sex, race, height, smoking status, smoking history, history of asthma, clinical center, and scanner, there was a 4.2% higher PRMFSA per decade of age (95% CI, 3.3–5.1%) among subjects with obstruction, versus only a 2.7% higher PRMFSA per decade of age (95% CI, 2.2–3.2%) among those without airflow obstruction (see Figure E2). We also found a significant interaction between age and airflow obstruction in determining the extent of PRMEMPH (P = 0.006 for the interaction term), although the difference per decade of age was of lower magnitude (see Figure E3).
Figure 3.
Distribution of parametric response mapping–determined small airway abnormality (PRMFSA, top) and emphysema (PRMEMPH, bottom) by age and presence of airflow obstruction. At any age, those without airflow obstruction (represented by group No) have significantly less small airway abnormality (y-axis, top) and emphysema (y-axis, bottom) than subjects of similar age with mild airflow obstruction (Yes).
Discussion
In this investigation of a well-characterized group of never- and ever-smokers, our cross-sectional analysis of imaging data using the PRM technique identified several novel findings about lung aging. First, in subjects without airflow obstruction, a modest degree of PRMFSA was associated with greater age, regardless of the presence of symptoms or respiratory-related impairment, an association maintained in different subgroups by history of smoking or asthma. Second, we found a significant association between airway abnormality (as defined by PRMFSA) and increased FVC. Third, we also demonstrate greater effect of age on the extent of PRMFSA among subjects with airflow obstruction, such that for the same degree of airflow obstruction, older subjects have greater small airway abnormality.
Hence, our data support the hypotheses that in this cohort (1) early events of lung aging in subjects without airflow obstruction or respiratory symptoms are represented by imaging abnormalities related with small airways, with minimal extent of emphysematous changes; (2) in older subjects without respiratory disease, nonclinically significant signs of small airway disease, which can be detected by PRM analysis, can bias spirometry values toward a spurious obstruction, when defined by the fixed FEV1/FVC ratio method (29); and (3) that the impact of age on imaging-derived markers of small airway abnormality is particularly relevant among subjects who have airflow obstruction compatible with a diagnosis of COPD.
With a growing number of older adults needing medical care, understanding age-related changes to the lungs is needed to differentiate between healthy aging and disease, in particular when a new diagnostic method becomes available. With advancing age, respiratory physiologic studies (3, 30, 31) show a loss of elastic recoil, hyperinflation and air trapping, and linearly increased residual volume and decreased vital capacity. Probably the best known effect of normal aging of the lungs is progressive enlargement of the alveolar ducts and distal alveoli resembling emphysematous changes (31). Despite the paucity of inflammatory infiltration of the airways or changes in collagen and elastin content in the healthy elderly (32), it has become customary to label (erroneously) this process as “senile emphysema,” whereas a more precise term might be “senile lung” changes (7, 18). We found that the burden of CT-defined emphysema, as determined by PRMEMPH, was low in our selected group of participants with normal spirometry, even among those with respiratory-related impairment. Using a novel method to quantify emphysema noninvasively in a large sample, we provide new evidence to the long-lasting discussion about the interpretation of emphysema-like changes in the healthy elderly, and suggest that in the absence of toxic exposures and concurrent specific susceptibility factors (7), emphysema development is not an age-related condition.
Little regarding aging of the distal airways has been previously described. A previous report suggests that numbers of small airways may be reduced in subjects with senile lung (18). The paucity of information contrasts with growing evidence of the role of peripheral airways as an early target of physiologic changes in COPD (33), and early evidence of increasing resistance of the peripheral airways with advancing age (8, 34). More recently, studies using thin-section CT imaging have reported more frequent bronchial wall thickening in older adults, compared with their younger counterparts, independent of smoking history (35). Boudewijn and colleagues described an association between increasing age and more PRMFSA, in a study of 49 current- and 47 never-smokers, mean age 40 years (19). We confirmed the age and PRMFSA association, and extend those findings by examining a larger population of more advanced age, even without respiratory impairment.
Our results are important to the venerable and ongoing controversy over the different methods and thresholds currently used to identify and define abnormal spirometric values compatible with airflow obstruction, most commonly the fixed ratio and the lower limit of normal methods. Comparisons of both methods in older adults have shown a rate of discordance as high as 54% (36–38). It has been argued that some degree of hyperinflation caused by age-related changes of emphysema contributes to an age-related FEV1 decline, resulting in greater overdiagnosis of obstruction when using the fixed ratio definition. In the current sample of subjects without clinically evident lung impairment, we have demonstrated that small airway abnormalities have greater impact on FVC, with minimal changes on FEV1, and that the resulting lower ratio could be a function of a change in the denominator (FVC), not just in the numerator (FEV1). A plausible interpretation of our findings is that the fixed ratio definition overestimates obstruction in the healthy elderly primarily because of the impact of “silent” small airway abnormalities on the expiratory measures captured by spirometry, not just the consequence of senile emphysema-like changes. Furthermore, comparison of our PRMFSA and PRMEMPH results, and especially their disparate changes in groups of different ages, implies that small airway changes, rather than senile emphysema-like changes, are the main contributors to this effect.
The finding of greater PRMFSA extent among subjects with clinical disease and obstructive physiology, compared with those free of clinical and spirometric definitions of COPD, points toward a crucial role of small airways in the development and progression of tobacco-related obstructive lung disease (33, 34). Among participants in the current study, the impact of aging was both present and magnified among those with obstructive disease, resulting in even greater PRMFSA. Conversely, the finding of little to no difference in abnormal airway burden between smokers and nonsmokers of the same age before the development of overt obstruction agrees with current concept of COPD susceptibility. These novel findings suggest that the pathologic mechanisms involved in COPD are not just the result of exposure and accelerated aging (39, 40), but instead that COPD progression results from “accelerated aging” only among susceptible individuals (4, 41). Whether such susceptibility in turn stems entirely from genetic predisposition, or instead might result in part from stochastic interactions between the immune system and an individual’s personal history and temporal order of respiratory infections, requires further study.
Our work is subject to several limitations. First, there was no tissue confirmation of the PRMFSA measures, making it impossible to distinguish air sacs dilation from actual destruction. Whether there are intrinsic age-related structural abnormalities in the peripheral airways, or if the imaging findings of PRMFSA represent age-related changes in lung elasticity, remains unknown and the evidence provided by this study cannot provide a final answer to that question; studies to validate PRM measurements histologically are under way. Second, SPIROMICS participants are volunteers, not a representative population sample, raising questions about subtle self-selection bias. Against that possibility is our demonstration of similar outcomes regardless of the presence of respiratory impairment, which we defined robustly by inclusion of several measures of patient-reported symptoms and outcomes, and the similar findings when controlling for the effect of asthma and early life wheezing. The cross-sectional nature of our analyses is a current limitation; longitudinal analysis of this cohort would greatly extend its power. Nevertheless, published work demonstrating an association between PRMFSA and prospectively assessed lung function decline (28) supports the validity of this metric. Major strengths of the analysis include the large number of participants, use of well-defined criteria to judge presence or absence of abnormal lung function and lung impairment, and detailed analytic models with inclusion of most factors known to be associated with lung function and morphology.
Conclusions
Analysis of paired inspiratory and expiratory chest CTs using PRM showed that functional small airway abnormality increases with age, about 2% per decade after the age of 50 years, even in never-smokers with normal lung function and free of respiratory symptoms. This abnormality results in a decreased FEV1/FVC ratio, caused mostly by a relative increase in the FVC. Among individuals with mild to moderate COPD, aging is associated with significantly greater imaging-based evidence of functional small airway abnormality.
Footnotes
Supported by funding from National Institutes of Health (NIH) NHLBI 3R01HL122438-02S1 and K23 HL128936 (C.H.M.); by funding from NIH NHLBI grant K01HL118714 and the Brigham and Women’s Hospital Minority Faculty Career Development Award (A.A.D.); by Merit Review Award I01 CX000911 from the Clinical Research and Development Service, Department of Veterans Affairs (J.L.C.); by funding from NIH SBIR grant R44HL008837 (C.J.G.); by National Institute of Aging grant 5K08AG031837 and by the Claude D. Pepper Older Americans Independence Center at the University of Michigan (C.T.C.); and by funding from NIH NHLBI grant R01HL122438-01 (M.K.H.). SPIROMICS (Subpopulations and Intermediate Outcomes in COPD Study) is funded by contract from the NHLBI (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN2682009000019C, HHSN268200900020C). The funding agencies did not have any role on the analysis and interpretation of the findings, or in the drafting or approval of the manuscript.
Author Contributions: C.H.M., A.A.D., C.M., C.P., N.M., and C.T.C. contributed to conceiving and writing the manuscript, data analysis, and clinical interpretation of the data. J.L.C., C.B.C., R.E.K., R.P., P.G.W., E.R.B., N.N.H., R.G.B., G.J.C., E.A.K., E.A.H., B.D.R., C.J.G., F.J.M., and M.K.H. contributed to acquisition of the data, conceiving and writing the manuscript, data analysis, and clinical interpretation of the data. All authors reviewed and approved the final draft of the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201604-0871OC on August 26, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Divo MJ, Martinez CH, Mannino DM. Ageing and the epidemiology of multimorbidity. Eur Respir J. 2014;44:1055–1068. doi: 10.1183/09031936.00059814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee KW, Chung SY, Yang I, Lee Y, Ko EY, Park MJ. Correlation of aging and smoking with air trapping at thin-section CT of the lung in asymptomatic subjects. Radiology. 2000;214:831–836. doi: 10.1148/radiology.214.3.r00mr05831. [DOI] [PubMed] [Google Scholar]
- 3.Brozek J. Age differences in residual lung volume and vital capacity of normal individuals. J Gerontol. 1960;15:155–160. doi: 10.1093/geronj/15.2.155. [DOI] [PubMed] [Google Scholar]
- 4.Fukuchi Y. The aging lung and chronic obstructive pulmonary disease: similarity and difference. Proc Am Thorac Soc. 2009;6:570–572. doi: 10.1513/pats.200909-099RM. [DOI] [PubMed] [Google Scholar]
- 5.Faner R, Rojas M, Macnee W, Agustí A. Abnormal lung aging in chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;186:306–313. doi: 10.1164/rccm.201202-0282PP. [DOI] [PubMed] [Google Scholar]
- 6.Ito K, Barnes PJ. COPD as a disease of accelerated lung aging. Chest. 2009;135:173–180. doi: 10.1378/chest.08-1419. [DOI] [PubMed] [Google Scholar]
- 7.Teramoto S, Ishii M. Aging, the aging lung, and senile emphysema are different. Am J Respir Crit Care Med. 2007;175:197–198, author reply 198. doi: 10.1164/ajrccm.175.2.197. [DOI] [PubMed] [Google Scholar]
- 8.Niewoehner DE, Kleinerman J. Morphologic basis of pulmonary resistance in the human lung and effects of aging. J Appl Physiol. 1974;36:412–418. doi: 10.1152/jappl.1974.36.4.412. [DOI] [PubMed] [Google Scholar]
- 9.Martinez CH, Chen YH, Westgate PM, Liu LX, Murray S, Curtis JL, Make BJ, Kazerooni EA, Lynch DA, Marchetti N, et al. COPDGene Investigators. Relationship between quantitative CT metrics and health status and BODE in chronic obstructive pulmonary disease. Thorax. 2012;67:399–406. doi: 10.1136/thoraxjnl-2011-201185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grydeland TB, Dirksen A, Coxson HO, Eagan TM, Thorsen E, Pillai SG, Sharma S, Eide GE, Gulsvik A, Bakke PS. Quantitative computed tomography measures of emphysema and airway wall thickness are related to respiratory symptoms. Am J Respir Crit Care Med. 2010;181:353–359. doi: 10.1164/rccm.200907-1008OC. [DOI] [PubMed] [Google Scholar]
- 11.Diaz AA, Bartholmai B, San José Estépar R, Ross J, Matsuoka S, Yamashiro T, Hatabu H, Reilly JJ, Silverman EK, Washko GR. Relationship of emphysema and airway disease assessed by CT to exercise capacity in COPD. Respir Med. 2010;104:1145–1151. doi: 10.1016/j.rmed.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han MK, Kazerooni EA, Lynch DA, Liu LX, Murray S, Curtis JL, Criner GJ, Kim V, Bowler RP, Hanania NA, et al. COPDGene Investigators. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology. 2011;261:274–282. doi: 10.1148/radiol.11110173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirby M, Pike D, Sin DD, Coxson HO, McCormack DG, Parraga G. COPD: do imaging measurements of emphysema and airway disease explain symptoms and exercise capacity? Radiology. 2015;277:872–880. doi: 10.1148/radiol.2015150037. [DOI] [PubMed] [Google Scholar]
- 14.Galbán CJ, Han MK, Boes JL, Chughtai KA, Meyer CR, Johnson TD, Galbán S, Rehemtulla A, Kazerooni EA, Martinez FJ, et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med. 2012;18:1711–1715. doi: 10.1038/nm.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boes JL, Hoff BA, Bule M, Johnson TD, Rehemtulla A, Chamberlain R, Hoffman EA, Kazerooni EA, Martinez FJ, Han MK, et al. Parametric response mapping monitors temporal changes on lung CT scans in the subpopulations and intermediate outcome measures in COPD Study (SPIROMICS) Acad Radiol. 2015;22:186–194. doi: 10.1016/j.acra.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pompe E, van Rikxoort EM, Schmidt M, Rühaak J, Estrella LG, Vliegenthart R, Oudkerk M, de Koning HJ, van Ginneken B, de Jong PA, et al. Parametric response mapping adds value to current computed tomography biomarkers in diagnosing chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191:1084–1086. doi: 10.1164/rccm.201411-2105LE. [DOI] [PubMed] [Google Scholar]
- 17.Galban CJ, Boes JL, Bule M, et al. Parametric response mapping as an indicator of bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20:1592–1598. doi: 10.1016/j.bbmt.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verbeken EK, Cauberghs M, Mertens I, Clement J, Lauweryns JM, Van de Woestijne KP. The senile lung. Comparison with normal and emphysematous lungs. 1. Structural aspects. Chest. 1992;101:793–799. doi: 10.1378/chest.101.3.793. [DOI] [PubMed] [Google Scholar]
- 19.Boudewijn IM, Postma DS, Telenga ED, Ten Hacken NH, Timens W, Oudkerk M, Ross BD, Galbán CJ, van den Berge M. Effects of ageing and smoking on pulmonary computed tomography scans using parametric response mapping. Eur Respir J. 2015;46:1193–1196. doi: 10.1183/09031936.00009415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez CH, Diaz AA, Curtis JL, Cooper CB, Pirozzi C, Kanner R, Paine R, III, Woodruff PG, Bleecker E, Hansel NN, et al. Functional small airway abnormalities increase with age among never and ever-smokers without airflow obstruction: an analysis of the SPIROMICS cohort. Am J Respir Crit Care Med. 2016;194:A4277. [Google Scholar]
- 21.Couper D, LaVange LM, Han M, Barr RG, Bleecker E, Hoffman EA, Kanner R, Kleerup E, Martinez FJ, Woodruff PG, et al. SPIROMICS Research Group. Design of the subpopulations and intermediate outcomes in COPD Study (SPIROMICS) Thorax. 2014;69:491–494. doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regan EA, Lynch DA, Curran-Everett D, Curtis JL, Austin JH, Grenier PA, Kauczor HU, Bailey WC, DeMeo DL, Casaburi RH, et al. Genetic Epidemiology of COPD (COPDGene) Investigators. Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med. 2015;175:1539–1549. doi: 10.1001/jamainternmed.2015.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim V, Davey A, Comellas AP, Han MK, Washko G, Martinez CH, Lynch D, Lee JH, Silverman EK, Crapo JD, et al. COPDGene® Investigators. Clinical and computed tomographic predictors of chronic bronchitis in COPD: a cross sectional analysis of the COPDGene study. Respir Res. 2014;15:52. doi: 10.1186/1465-9921-15-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93:580–586. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 25.Jones PW, Quirk FH, Baveystock CM. The St George's Respiratory Questionnaire. Respir Med. 1991;85:25–31. doi: 10.1016/s0954-6111(06)80166-6. (Suppl B) discussion 3–7. [DOI] [PubMed] [Google Scholar]
- 26.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 27.Hoff BA, Kozloff KM, Boes JL, Brisset JC, Galbán S, Van Poznak CH, Jacobson JA, Johnson TD, Meyer CR, Rehemtulla A, et al. Parametric response mapping of CT images provides early detection of local bone loss in a rat model of osteoporosis. Bone. 2012;51:78–84. doi: 10.1016/j.bone.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhatt SP, Soler X, Wang X, Murray S, Anzueto AR, Beaty TH, Boriek AM, Casaburi R, Criner GJ, Diaz AA, et al. Association between functional small airway disease and FEV1 decline in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;194:178–184. doi: 10.1164/rccm.201511-2219OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaz Fragoso CA, McAvay G, Van Ness PH, Casaburi R, Jensen RL, MacIntyre N, Gill TM, Yaggi HK, Concato J. Phenotype of normal spirometry in an aging population. Am J Respir Crit Care Med. 2015;192:817–825. doi: 10.1164/rccm.201503-0463OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turner JM, Mead J, Wohl ME. Elasticity of human lungs in relation to age. J Appl Physiol. 1968;25:664–671. doi: 10.1152/jappl.1968.25.6.664. [DOI] [PubMed] [Google Scholar]
- 31.Niewoehner DE, Kleinerman J, Liotta L. Elastic behavior of postmortem human lungs: effects of aging and mild emphysema. J Appl Physiol. 1975;39:943–949. doi: 10.1152/jappl.1975.39.6.943. [DOI] [PubMed] [Google Scholar]
- 32.Lang MR, Fiaux GW, Gillooly M, Stewart JA, Hulmes DJ, Lamb D. Collagen content of alveolar wall tissue in emphysematous and non-emphysematous lungs. Thorax. 1994;49:319–326. doi: 10.1136/thx.49.4.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, Wright AC, Gefter WB, Litzky L, Coxson HO, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365:1567–1575. doi: 10.1056/NEJMoa1106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med. 1968;278:1355–1360. doi: 10.1056/NEJM196806202782501. [DOI] [PubMed] [Google Scholar]
- 35.Copley SJ, Wells AU, Hawtin KE, Gibson DJ, Hodson JM, Jacques AE, Hansell DM. Lung morphology in the elderly: comparative CT study of subjects over 75 years old versus those under 55 years old. Radiology. 2009;251:566–573. doi: 10.1148/radiol.2512081242. [DOI] [PubMed] [Google Scholar]
- 36.Mannino DM, Sonia Buist A, Vollmer WM. Chronic obstructive pulmonary disease in the older adult: what defines abnormal lung function? Thorax. 2007;62:237–241. doi: 10.1136/thx.2006.068379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhatt SP, Sieren JC, Dransfield MT, Washko GR, Newell JD, Jr, Stinson DS, Zamba GK, Hoffman EA COPDGene Investigators. Comparison of spirometric thresholds in diagnosing smoking-related airflow obstruction. Thorax. 2014;69:409–414. doi: 10.1136/thoraxjnl-2012-202810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swanney MP, Ruppel G, Enright PL, Pedersen OF, Crapo RO, Miller MR, Jensen RL, Falaschetti E, Schouten JP, Hankinson JL, et al. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax. 2008;63:1046–1051. doi: 10.1136/thx.2008.098483. [DOI] [PubMed] [Google Scholar]
- 39.Walters MS, De BP, Salit J, Buro-Auriemma LJ, Wilson T, Rogalski AM, Lief L, Hackett NR, Staudt MR, Tilley AE, et al. Smoking accelerates aging of the small airway epithelium. Respir Res. 2014;15:94. doi: 10.1186/s12931-014-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou S, Wright JL, Liu J, Sin DD, Churg A. Aging does not enhance experimental cigarette smoke-induced COPD in the mouse. PLoS One. 2013;8:e71410. doi: 10.1371/journal.pone.0071410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rojas M, Mora AL, Kapetanaki M, Weathington N, Gladwin M, Eickelberg O Clinical Impact and Cellular and Molecular Pathways. Aging and lung disease. Ann Am Thorac Soc. 2015;12:S222–S227. doi: 10.1513/AnnalsATS.201508-484PL. [DOI] [PMC free article] [PubMed] [Google Scholar]