In this issue of the Journal (pp. 515–529), Hong and colleagues (1) provide evidence indicating a key role for decreases in the function of the pulmonary arterial smooth muscle (PASM) mitochondrial calcium uniporter (MCU) complex in controlling many important aspects of the cancer cell–like metabolic profile associated with changes in mitochondrial function and proliferation seen in pulmonary arterial hypertension (PAH) (2, 3). Evidence is also provided for a depletion of MCU in pulmonary arteries from the rat monocrotaline pulmonary hypertension model and in PASM cells from humans with PAH. In addition, microRNAs miR-138 and miR-25 appear to be key down-regulators of MCU. The study provides novel mechanistic insights into multiple ionic, metabolic, and mitochondrial aspects of the role of these regulatory systems in the progression of PAH, including new potential therapeutic targeting of MCU expression through anti-miRs.
The roles for changes in intracellular calcium in controlling vascular contractile function and remodeling of pulmonary arteries that participate in the development and progression of pulmonary hypertension are well established (4). However, the role of mitochondrial calcium and the function of MCU in vascular physiology and pathophysiology remain rather poorly understood. A study (5) of mice depleted of MCU revealed an alteration in the phosphorylation of pyruvate dehydrogenase in skeletal muscle, which resembled a change in PAH extensively studied by Archer and colleagues (2) and by Sutendra and Michelakis (3). On the basis of their work, alterations in the phosphorylation of pyruvate dehydrogenase appear to be controlling a cancer cell–associated Warburg-type shift in energy metabolism from aerobic mitochondrial metabolism to glycolysis that appears to suppress apoptosis and promote PASM proliferation associated with vascular remodeling in PAH (2, 3).
The study by Hong and colleagues (1) demonstrates these changes in PASM from the monocrotaline-induced rat model of PAH. Decreases in MCU are associated with increased cytosolic calcium and decreased mitochondrial calcium in both the rat monocrotaline model and in PASM cells from humans with PAH. Multiple approaches modulating the expression and inhibition of MCU provide new evidence for its potentially normal role in attempting to attenuate increases in cytosolic calcium and to maintain mitochondrial calcium–regulated processes under conditions that potentially promote PAH development. These experiments also document how the function of MCU is lost by inhibition or depletion, and how this loss of MCU coordinates the promotion of processes linked to PASM migration, proliferation, and resistance to apoptosis through relationships shown in Figure 1. Moreover, conditions promoting increased or restored MCU function are demonstrated to reverse key aspects of these PAH-associated processes. Although these results clearly highlight an important role for the MCU transporter in controlling intracellular calcium and in the pathogenesis of PAH, observations in this study raise many additional questions, which remain to be investigated. For example, how does the function of MCU influence well-established roles for calcium in eliciting and/or maintaining PASM cell contraction, and in regulating mitotic and migratory aspects of PASM cells suggested in this and other (6) studies. Besides, intracellular calcium can accumulate in PASM cells through increased voltage-gated and receptor-operated cation channel function, elevated calcium release/leakage from internal stores, decreased sequestration by the sarcoplasmic reticulum, or by diminished removal from the cells. Thus, a question arises concerning whether an increase in intracellular calcium through any one of these pathways can stimulate PASM cells to switch from a contractile to cancer cell–like phenotype. In addition, it remains to be determined whether chelating intracellular calcium without regulating MCU levels in PASM cells under PAH conditions reverses the acquired cancer cell–like proliferative phenotype to a normal contractile state. Thus, the results of the study by Hong and colleagues (1) may initiate new avenues of mechanistic research in the PAH field.
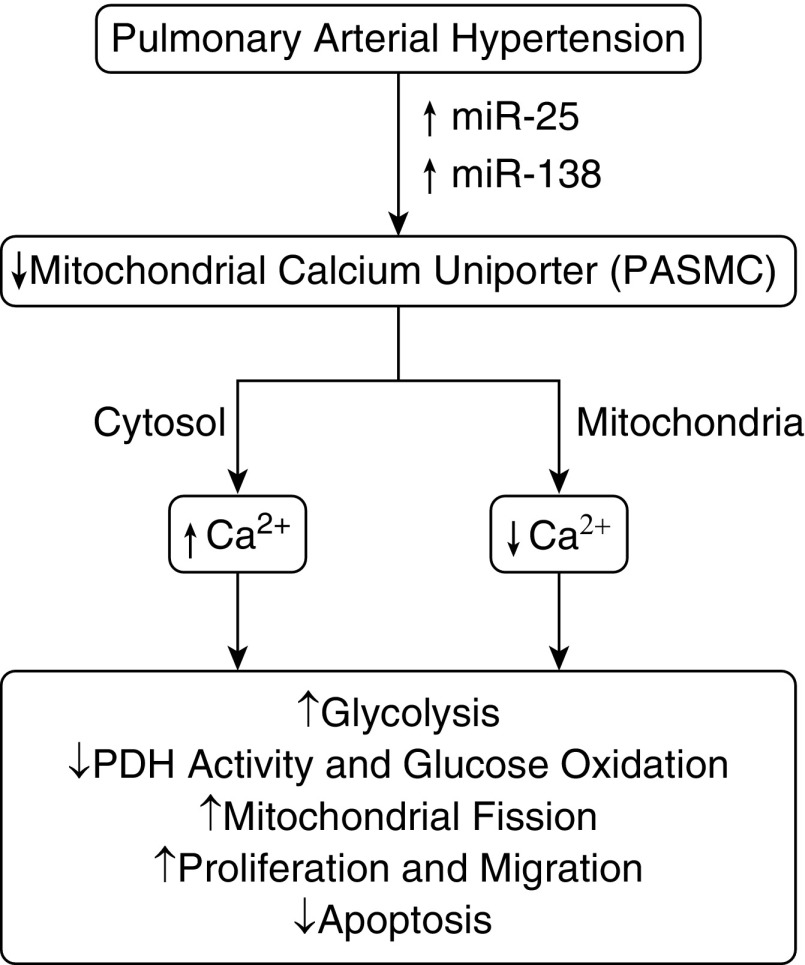
Figure 1.
Model showing how down-regulation of the mitochondrial calcium uniporter by microRNA (miR)-25 and miR-138 in pulmonary arterial hypertension (PAH) could alter mitochondrial and extramitochondrial calcium (cytosol) levels in ways that drive metabolic changes in pulmonary arterial smooth muscle cells potentially linked to processes contributing to PAH-associated vascular remodeling. PASMC = pulmonary arterial smooth muscle cell; PDH = pyruvate dehydrogenase.
MCU is targeted by miR-25 (7), and this study showed that up-regulation of miR-25 and miR-138 decreased MCU expression and activity. Interestingly, increasing miR-138 and/or miR-25 restored MCU expression and ameliorated monocrotaline-induced pulmonary hypertension. Although these findings are intriguing, miRs have multiple targets (8) and their down- or up-regulation can affect the expression of multiple proteins in cells. Because miR-138 and miR-25 regulated MCU in PASM cells, this raises a possibility that multiple pathways exist in regulating the expression of MCU. Therefore, an RNA-sequencing analysis with in silico analysis in future could be beneficial to provide insight concerning whether regulation of MCU is sufficient or whether it works in conjunction with other effector targets of miR-138 or miR-25 to modulate intracellular calcium in the PASM cells of patients with PAH and experimental models.
PAH is a two-hit disease, and therefore it is likely that MCU requires other partners such as factors/pathways to dampen signaling that provokes PAH. In this study, the authors have reported mostly end-point measurements, and from these data it is not clear whether down-regulation of MCU is a trigger for PAH or a contributor to the development and maintenance/progression of PAH. In this regard, a time course study of MCU down-regulation and the development of PAH would have been helpful to interpret these results. Moreover, do MCU knockout mice spontaneously develop pulmonary hypertension or do they develop severe pulmonary hypertension when challenged with a second hit? Measurement of PAH and pulmonary artery structure in MCU knockout mice will answer these questions and will be important in establishing the significance of the role of mitochondrial calcium regulation through MCU in the pathogenesis of PAH, as elegantly demonstrated in the current study.
Footnotes
Supported by NIH grants R01HL115124 and R01HL129797 (M.S.W.).
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hong Z, Chen K-H, DasGupta A, Potus F, Dunham-Snary K, Bonnet S, Tian L, Fu J, Breuils-Bonnet S, Provencher S, et al. MicroRNA-138 and microRNA-25 down-regulate mitochondrial calcium uniporter, causing the pulmonary arterial hypertension cancer phenotype. Am J Respir Crit Care Med. 2017;195:515–529. doi: 10.1164/rccm.201604-0814OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation. 2010;121:2045–2066. doi: 10.1161/CIRCULATIONAHA.108.847707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutendra G, Michelakis ED. The metabolic basis of pulmonary arterial hypertension. Cell Metab. 2014;19:558–573. doi: 10.1016/j.cmet.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Yuan JX, Aldinger AM, Juhaszova M, Wang J, Conte JV, Jr, Gaine SP, Orens JB, Rubin LJ. Dysfunctional voltage-gated K+ channels in pulmonary artery smooth muscle cells of patients with primary pulmonary hypertension. Circulation. 1998;98:1400–1406. doi: 10.1161/01.cir.98.14.1400. [DOI] [PubMed] [Google Scholar]
- 5.Pan X, Liu J, Nguyen T, Liu C, Sun J, Teng Y, Fergusson MM, Rovira II, Allen M, Springer DA, et al. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol. 2013;15:1464–1472. doi: 10.1038/ncb2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuhr FK, Smith KA, Song MY, Levitan I, Yuan JX. New mechanisms of pulmonary arterial hypertension: role of Ca2+ signaling. Am J Physiol Heart Circ Physiol. 2012;302:H1546–H1562. doi: 10.1152/ajpheart.00944.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marchi S, Lupini L, Patergnani S, Rimessi A, Missiroli S, Bonora M, Bononi A, Corrà F, Giorgi C, De Marchi E, et al. Downregulation of the mitochondrial calcium uniporter by cancer-related miR-25. Curr Biol. 2013;23:58–63. doi: 10.1016/j.cub.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasquinelli AE. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet. 2012;13:271–282. doi: 10.1038/nrg3162. [DOI] [PubMed] [Google Scholar]