Abstract
Physical exercise induces transient upregulation of the pro-oxidative mediators peroxisome proliferator-activated receptor-δ (PPARδ), silent information regulator of transcription (sirtuin)-1 (SIRT1), PPARγ coactivator 1α (PGC-1α), and PGC-1β in skeletal muscle. To determine the role of the cytokine IL-15 in acute postexercise induction of these molecules, expression of these factors after a bout of exhaustive treadmill running was examined in the gastrocnemius muscle of untrained control and IL-15–knockout (KO) mice. Circulating IL-15 levels increased transiently in control mice after exercise. Control mice, but not IL-15–KO mice, upregulated muscle PPARδ and SIRT1 protein after exercise, accompanied by a complex pattern of mRNA expression for these factors. However, in exhaustive exercise, control mice ran significantly longer than IL-15–KO mice. Therefore, in a second experiment, mice were limited to a 20-minute run, after which a similar pattern of induction of muscle PPARδ and SIRT1 protein by control mice only was observed. In a separate experiment, IL-15–KO mice injected systemically with recombinant IL-15 upregulated muscle PPARδ and SIRT1 mRNA within 30 minutes and also exhibited increased muscle PPARδ protein levels by 3 hours. After exercise, both control and IL-15–KO mice downregulated IL-15 receptor-α (IL-15Rα) mRNA, whereas IL-15Rα–deficient mice exhibited constitutively elevated circulating IL-15 levels. These observations indicate IL-15 release after exercise is necessary for induction of PPARδ and SIRT1 at the protein level in muscle tissue and suggest that exercise releases IL-15 normally sequestered by the IL-15Rα in the resting state. These findings could be used to develop an IL-15–based strategy to induce many of the metabolic benefits of physical exercise.
Endurance exercise is a well-documented treatment and preventive measure for metabolic disorders such as obesity and insulin resistance (1, 2) as well as for age-associated muscle loss (3). The positive effects of endurance exercise on metabolism are largely due to stimulation of fat oxidation in skeletal muscle and other metabolically responsive tissues (1, 2). Recent evidence (1, 2) indicates that the benefits of exercise on metabolism and skeletal muscle are mediated by induction of the pro-oxidative intracellular mediators peroxisome proliferator-activated receptor-δ (PPARδ), silent information regulator of transcription (sirtuin)-1 (SIRT1), and PPARγ coactivator 1α (PGC-1α), and PGC-1β. Individual bouts of exercise can transiently increase expression of these factors at the mRNA and protein levels in skeletal muscle (4–6); however, the acute signals from exercising muscle that induce expression of PPARδ, SIRT1, PGC-1α, and PGC-1β have not been completely characterized.
IL-15 is a cytokine that is highly expressed in skeletal muscle (7). Transgenic mice with constitutively elevated circulating IL-15 levels exhibit increased exercise endurance, increased insulin sensitivity, an oxidative metabolic phenotype, and elevated expression of PPARδ, SIRT1, PGC-1α, and PGC-1β in skeletal muscle tissue (8–10). In human subjects, negative correlations between IL-15 expression and adiposity have been observed (11, 12). Moreover, in humans, circulating IL-15 levels increase within 10 minutes after an exercise session (13, 14). These findings suggested that IL-15 release from exercising skeletal muscle tissue may induce the pro-oxidative mediators PPARδ, SIRT1, PGC-1α, and PGC-1β in skeletal muscle. The present study used control and IL-15–knockout (KO) mice undergoing a single session of exhaustive exercise to test the hypothesis that IL-15 is necessary for acute postexercise upregulation of the aforementioned pro-oxidative mediators in skeletal muscle. Expression of some of these factors after nonexhaustive exercise and after systemic injection of recombinant IL-15 was also examined.
IL-15 can signal through heterotrimeric receptor complexes comprising IL-15 receptor-α (IL-15Rα), IL-2Rβ, and IL-2Rγ (also called γc); however, IL-15 can also signal through heterodimeric IL-2Rβ/IL-2Rγ receptor complexes lacking the IL-15Rα subunit (15). Because IL-15 has an inefficient signal sequence, IL-15 secretion is believed to be mediated by intracellular binding of IL-15 to the IL-15Rα, followed by transport to the cell membrane (15). It has been proposed that IL-15 can thereby signal by surface-associated transpresentation, or be released by cleavage of surface-associated IL-15Rα to generate soluble IL-15Rα/IL-15 complexes (15). Postexercise changes in muscle IL-15 and IL-15Rα mRNA expression in control and IL-15–KO mice and baseline circulating levels of IL-15 in IL-15Rα–KO mice were also examined in this study to gain an initial understanding of whether such mechanisms could play a role in postexercise IL-15 release from skeletal muscle.
Materials and Methods
Animals and husbandry
Animal procedures were approved by the VA Puget Sound Institutional Animal Care and Use Committee and complied with the Institute for Laboratory Animal Research Guide for the Care and Use of Laboratory Animals. Male IL-15–KO (16) and corresponding control mice, both on a C57BL/6 background, were obtained from Taconic Farms. Mice were obtained at 6 weeks of age, maintained on standard mouse chow (irradiated PicoLab Mouse Diet 20; LabDiet) with food and water ad libitum, on a 12-hour light, 12-hour dark cycle, at 21°C ± 3°C in groups of 2 to 3 per cage. Mice were used at 4 months of age without previous exercise training. In one experiment, circulating IL-15 levels were determined in male IL-15Rα–KO mice and their respective controls (17) on a B6129SF2/J background, obtained from The Jackson Laboratory at 5 weeks of age and maintained as above until use at 4 months of age.
Body composition
Body composition was evaluated on conscious mice by quantitative magnetic resonance using an EchoMRI-4in1/700 rodent body composition analyzer (Echo Medical Systems). Total body fat and lean body mass were quantified based on the averages of triplicate measures for each animal with coefficients of variation of <1.2% and <1% for fat and lean mass, respectively.
Exercise protocols
To assess exercise endurance, untrained control and IL-15–KO mice were subjected to a single run-to-exhaustion trial. On the day of the trial, mice were acclimated to the testing room for 1 to 2 hours and then placed individually in a treadmill (Eco 3/6, Columbus Instruments; inclination +5°) not equipped with a shock grid and acclimated to the apparatus and motor sound for 5 minutes. The belt was turned to a slow speed (10 m/min), raised gradually to 15 to 17 m/min within the initial 10 minutes, and the speed held constant thereafter, as described previously (8). Mice generally ran until reaching exhaustion; mice that stopped running were encouraged to resume by gentle tapping of hindquarters with a tongue depressor. Mice that stopped running 3 times in succession were deemed to have run to exhaustion and exhibited behavioral signs of exhaustion such as labored breathing and splayed posture. The total amount of time spent running at all speeds was recorded. Because of genotypic differences in running endurance, in a second exercise protocol, control and IL-15–KO mice were subjected to the exercise regimen described above but removed from the treadmill after 20 minutes of running. Mice were anesthetized for terminal blood collection and subsequent postmortem tissue collection at the intervals indicated in the text and figures (immediately after exercise, 30 minutes after exercise, and 3 hours after exercise). Sample sizes were 9 to 13 exercised mice per genotype per time point. Sedentary mice were acclimated to the treadmill apparatus but did not undergo the running experiment and were anesthetized for blood and tissue collection immediately thereafter. For both sedentary and exercised mice, the immediate time points occurred approximately 5 minutes after the mice were removed from the apparatus (the time required for anesthesia before blood collection); tissue collection took approximately 15 minutes per animal to complete.
Serum IL-15 determination
Serum IL-15 levels in resting control mice were determined 2 weeks before treadmill running experiments by blood sampling from the submandibular vein under isoflurane anesthesia. This interval allowed complete recovery of blood volume, in compliance with National Institutes of Health animal use regulations. After running (or exposure to the treadmill for sedentary mice), animals were deeply anesthetized with pentobarbital (80 mg/kg, ip) and blood collected by cardiac puncture (exsanguination), followed by tissue collection for biochemical analyses. Blood was collected similarly from sedentary control mice, and blood was collected from sedentary and exercised IL-15–KO mice at each time point to control for physiological stress from the procedure; however, due to immunoreactivity with truncated IL-15 species, serum IL-15 values were not determined for IL-15–KO mice. Serum was separated and stored frozen at 20°C, and IL-15 protein levels were assessed with a Bio-Rad mouse IL-15 kit (with a sensitivity of 6.6 pg/mL and intra-assay coefficient of variation of 6%) using a Bio-Plex protein array instrument (Bio-Rad).
Acute IL-15 injection
To assess the acute effects of systemic IL-15 injection, IL-15–KO mice were administered 150 ng recombinant mouse IL-15 (R&D Systems) in 25 μL saline vehicle containing 0.1% ultrapure BSA (Jackson ImmunoResearch Laboratories) sc and compared with IL-15–KO mice that received an equal volume of saline vehicle only. Mice were anesthetized for collection of blood and tissue as described above at 30 minutes and 3 hours after injection.
Analysis of mRNA expression
Gastrocnemius muscles were dissected, weighed, equilibrated in RNAlater (QIAGEN) with storage at 20°C, and homogenized into Trizol (Life Technologies) followed by RNA precipitation. Resuspended RNA was treated with deoxyribonuclease, purified on RNeasy spin columns (QIAGEN), and treated with DNA removal buffer, and 1.5 μg was reverse transcribed into cDNA (RT2 First Stand Kit; QIAGEN). Reaction quality was assessed using hypoxanthine phosphoribosyltransferase 1 (HPRT1). Real-time PCR was performed using RT2 qPCR primers from QIAGEN as specified in Table 1. For determination of total IL-15 mRNA expression, primers directed toward the 3′-untranslated region (UTR) of IL-15 were used, whereas for determination of the putatively secreted long signal peptide (LSP) isoform of IL-15 (15), primers directed at the 5′-UTR of IL-15 were used. Primers with comparable efficiencies approaching 100% were used with RT2 Real-Time SYBR Green Fluor qPCR Master Mix (QIAGEN). Cycle threshold (CT) values were set identically for all plates, and sample CT values were normalized to HPRT1 CT; relative abundance of specific mRNA species was calculated as 2−ΔCT, and fold difference (used for presentation of multiple mRNA species with large differences in abundance) between control and IL-15–KO was calculated as 2−ΔΔCT (18). IL-15 mRNA was not determined for IL-15–KO mice due to amplification of truncated, nonfunctional IL-15 transcripts.
Table 1.
Real-Time PCR Primers for This Study
| Gene Target | RefSeq Accession No.a | Reference Positionb | QIAGEN/SABiosciences Catalog No. |
|---|---|---|---|
| HPRT1 | NM_013556.2 | 902 | 330001/PPM03559E |
| IL-15 (3′-UTR) | NM_008357.1 | 982 | 330001/PPM66757A |
| IL-15 (5′-UTR, LSP) | NM_008357.1 | 355 | 330001/PPM03022B |
| IL-15Rα | NM_008358.1 | 251 | 330001/PPM04202A |
| MHC I (SO) | NM_080728.2 | 5968 | 330001/PPM67019A |
| MHC IIa (FOG) | NM_001039545.2 | 4523 | 330001/PPM28209A |
| MHC IIb (FG) | NM_010855.2 | 5348 | 330001/PPM24856A |
| MHC IIx (FO) | NM_030679.1 | 2109 | 330001/PPM41612B |
| PPARδ | NM_011145.3 | 1182 | 330001/PPM03365F |
| PGC-1α | NM_008904.2 | 2980 | 330001/PPM03360E |
| PGC-1β | NM_133249.2 | 2931 | 330001/PPM05522B |
| SIRT1 | NM_019812.2 | 2179 | 330001/PPM05054A |
Abbreviations: FO, fast/oxidative; FOG, fast/oxidative-glycolytic; SO, slow/oxidative.
Sequence used for primer design.
Position of amplicon within gene (base pairs from 5′-end).
Western blot analyses
Antibodies used in Western blotting are summarized in Table 2. Antitubulin was used as a control for loading and transfer efficiency. Gastrocnemius muscles were frozen in liquid N2 and stored at −80°C until homogenization in Bio-Plex lysis buffer (Bio-Rad) containing protease inhibitors. Soluble protein fractions were assayed for protein content by the BCA method (19). Equal amounts of protein were loaded onto SDS-PAGE gels, separated, and blotted onto 0.45-μm nitrocellulose (Bio-Rad) using transfer buffer without SDS. Membranes were blocked and incubated with primary antibodies diluted to 1 μg/mL, rinsed, and incubated with horseradish peroxidase-conjugated secondary antibodies (1:1000–2500; Santa Cruz Biotechnology). Bands were visualized using luminol (Santa Cruz) on autoradiography film (ISCBioExpress) and digitized using UN-SCAN-IT gel version 5.1 (Silk Scientific). Background-corrected signal (density × area) for each band was normalized to tubulin. PGC-1α and PGC-1β were distinguished by relative mobility (20).
Table 2.
Antibodies Used in Western Blotting for This Study
| Peptide/Protein Target | Antigen Sequence | Name of Antibody | Manufacturer Catalog No. | Species in Which Raised, Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|---|
| SIRT1 | Synthetic peptide ATRQELTDV NYPSDKS (with C-terminal added lysine) corresponding to C-terminal amino acids 722–737 of mouse SIRT1 | Anti-SIRT1 | Abcam ab12193 | Polyclonal in rabbit | 1:2000 |
| PPARβ/δ | Peptide mapping at the N terminus of PPARβ/δ of mouse origin | PPARβ (K-20) | Santa Cruz Biotechnology sc-1987 | Polyclonal in goat | 1:200 |
| α-Tubulin | Amino acids 149–448 of α-tubulin of human origin; reacts with mouse, rat, and human | α-Tubulin (B-7) | Santa Cruz Biotechnology sc-5286 | Monoclonal in mouse | 1:1000 |
| PGC-1 | Amino acids 1–300 mapping near the N terminus of PGC-1 of human origin; reacts with mouse, rat, and human PGC-1α/β | PGC-1 (H-300) | Santa Cruz Biotechnology sc-13067 | Polyclonal in rabbit | 1:200 |
Statistical procedures
Statistical analyses were performed using SigmaPlot version 11.0 software (Systat Software) using one- or two-way ANOVA, with post hoc pairwise multiple comparisons performed by Holm-Sidak or Dunn's method where ANOVA revealed significant differences. For two-way ANOVAs, the overall significance of genotype and treatment (exercise and interval after exercise), and interactions of these factors, are reported in the figures. For most parameters, there were 9 to 13 mice per group. The Pearson product-moment correlation test was used to determine the relationship and significance between body weight and run-to-exhaustion time for each genotype (n = 25 control and 24 IL-15–KO mice). Significant differences (P < .05) are noted in the tables and figures.
Results
Body composition in control and IL-15–KO mice
Consistent with previous reports (12), IL-15–KO mice were significantly heavier and displayed significantly more fat compared with control mice (Table 3). IL-15–KO mice also displayed increased lean body mass and greater gastrocnemius muscle mass (Table 3). However, gastrocnemius mass normalized to total body mass or to lean body mass did not differ significantly in the 2 genotypes (Table 3).
Table 3.
Body Composition in Control and IL-15–Deficient (KO) Mice
| Parameter | Control (n = 25) | IL-15–KO (n = 45) | ANOVA P Value |
|---|---|---|---|
| BM, g | 34.82 ± 0.58 | 39.10 ± 0.76 | .001 |
| Fat mass, g | 9.65 ± 0.46 | 13.13 ± 0.49 | .001 |
| LBM, g | 23.31 ± 0.29 | 24.39 ± 0.21 | .01 |
| Gastrocnemius mass (pair), mg | 258.78 ± 3.93 | 272.63 ± 5.61 | .05 |
| Gastrocnemius mass (pair), % LBM | 1.11 ± 0.02 | 1.12 ± 0.02 | NS |
| Gastrocnemius mass (pair), % BM | 0.75 ± 0.01 | 0.71 ± 0.02 | NS |
Abbreviations: BM, body mass; LBM, lean BM; NS, not significant.
Running endurance and IL-15 expression
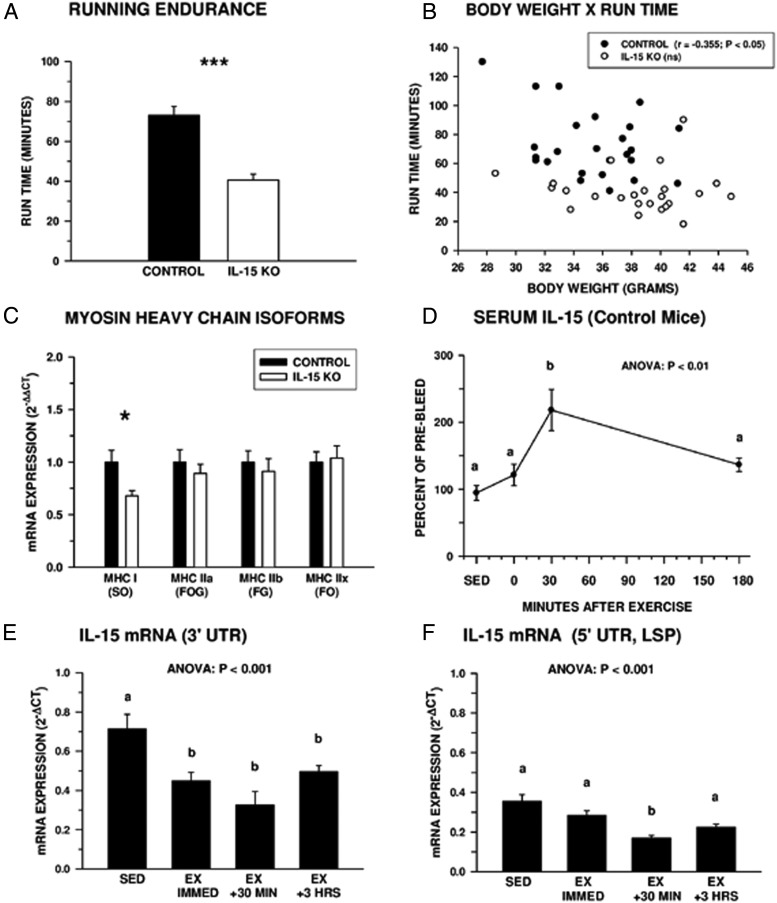
Untrained control and IL-15–KO mice were subjected to a run-to-exhaustion test that revealed IL-15–KO mice displayed significant deficits in running endurance compared with controls (Figure 1A). Body weight was negatively correlated with running endurance in control mice (r = −0.355; P < .05), but there was no correlation between these 2 variables in IL-15–KO mice (Figure 1B). This suggests the reduced exercise endurance of IL-15–KO mice was not due to increased weight load. Myosin heavy chain (MHC) mRNA isoform expression was examined in the gastrocnemius muscle, used by rodents in running (Figure 1C). IL-15–KO mice expressed significantly lower levels of the MHC I isoform characteristic of slow/oxidative muscle fibers used in endurance exercise, whereas expression of other MHC isoforms (IIa, IIb, and IIx) was not different in the 2 genotypes.
Figure 1.
Running endurance and IL-15 expression. A, Control mice ran longer than IL-15–KO mice in a run-to-exhaustion test. *, Significant difference between control and IL-15—KO mice at P < .001 by one-way ANOVA; n = 25 control and 24 IL-15–KO mice. B, Correlation between body weight and run-to-exhaustion time. Body weight and run time were negatively correlated for control but not IL-15–KO mice; n = 25 control and 24 IL-15–KO. C, Expression of mRNA isoforms of MHC in gastrocnemius muscles of control and IL-15–KO mice, reflecting muscle fiber type distribution, as follows: MHC I, slow/oxidative (SO) fibers; MHC IIa, fast/oxidative-glycolytic fibers (FOG); MHC IIb, fast/glycolytic (FG) fibers; MHC IIx, fast/oxidative (FO) fibers. Data represent fold difference in expression between genotypes (2−ΔΔCT) for each isoform. *, Significant difference between control and IL-15–KO at P < .05 by one-way ANOVA; n = 6 mice per group. D, Circulating IL-15 levels peak 30 minutes after exercise in control mice. Due to high variation in baseline IL-15 levels, data are expressed as mean percentage of individual baseline sample taken 2 weeks before the exercise session; n = 5–14 mice per time point, including unexercised sedentary (SED) mice. E and F, Expression of total (E) and the putatively secreted LSP isoform (F) of IL-15 mRNA decreased in the gastrocnemius muscle after exercise in control mice; n = 6–12 mice per time point including the SED group. In D–F, bars or data points with different superscripts are significantly different at P < .05 by one-way ANOVA. Bars or data points in all panels except B represent means ± SEM; in B, data points represent values for individual mice. Due to immunoreactivity with, and amplification of, truncated nonfunctional IL-15 species, serum IL-15 (D) and IL-15 mRNA (E and F) values were not determined for IL-15–KO mice.
In control mice, serum IL-15 levels increased significantly by 30 minutes after exhaustive exercise and returned to near baseline by 180 minutes (Figure 1D), in a pattern similar to that observed after treadmill exercise in human subjects (14). Expression of both total IL-15 mRNA and the putatively secreted LSP isoform of IL-15 mRNA (15) in gastrocnemius muscle was decreased by exhaustive exercise (Figure 1, E and F) indicating postexercise increases in circulating IL-15 are not accompanied by transcriptional activation of muscle IL-15 mRNA.
Postexercise induction of PPARδ and SIRT1
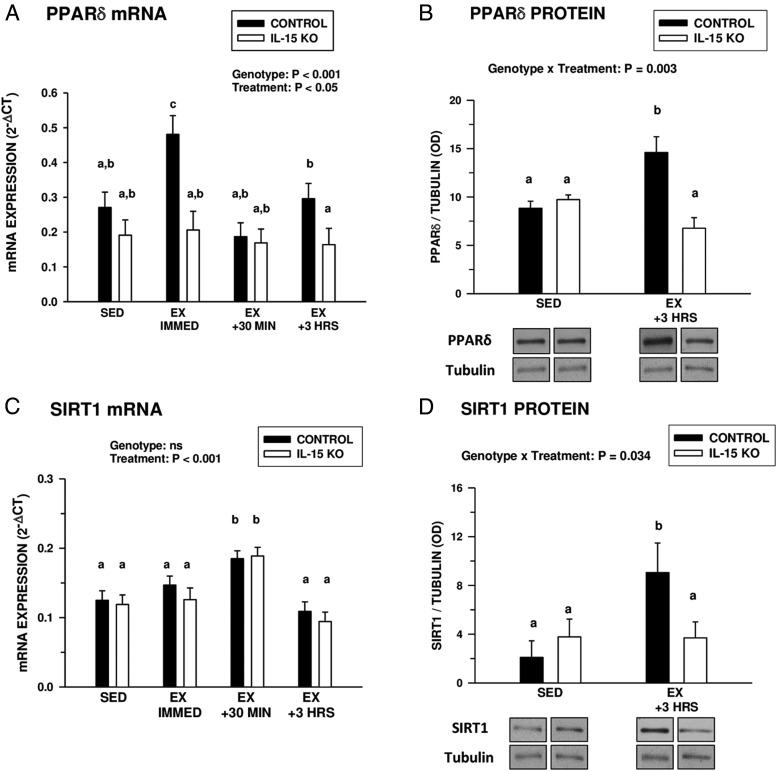
Baseline expression of PPARδ mRNA and protein in the gastrocnemius was not different in control and IL-15–KO mice (Figure 2, A and B). Immediately after an acute bout of exhaustive exercise, untrained control mice exhibited an immediate and transient increase in PPARδ mRNA expression (Figure 2A). In contrast, IL-15–KO mice did not exhibit changes in PPARδ mRNA expression at any time point examined up to 3 hours after exercise (Figure 2A). Likewise, control mice exhibited increased PPARδ protein levels in the gastrocnemius muscle 3 hours after exhaustive exercise, whereas IL-15–KO mice did not exhibit this increase (Figure 2B). Control and IL-15–KO mice exhibited no differences in baseline SIRT1 mRNA expression, but both groups exhibited a significant increase in SIRT1 mRNA expression 30 minutes after exercise (Figure 2C). However, control mice, but not IL-15–KO mice, showed increased gastrocnemius SIRT1 protein levels 3 hours after exercise (Figure 2D).
Figure 2.
Postexhaustive exercise induction of PPARδ and SIRT1. A and C, PPARδ (A) and SIRT1(C) gastrocnemius muscle mRNA expression in control and IL-15–KO sedentary (SED) mice at different time points (immediately [IMMED], 30 minutes, and 3 hours) after exhaustive exercise (EX). B and D, PPARδ (B) and SIRT1 (D) protein levels (normalized to tubulin) in the gastrocnemius muscles of SED control and IL-15–KO mice and at 3 hours after exhaustive exercise (EX +3 HRS). Representative Western blots are shown below the respective graphs in B and D; bands shown for each species are compiled from different regions of the same blot. In each panel, the overall significance of genotype, treatment, or the interaction of these 2 factors, determined by two-way ANOVA is shown; bars with different superscripts are significantly different at P < .05. Sample sizes were 6 SED and 9 to 13 EX mice per time point in each genotype. Bars represent means ± SEM.
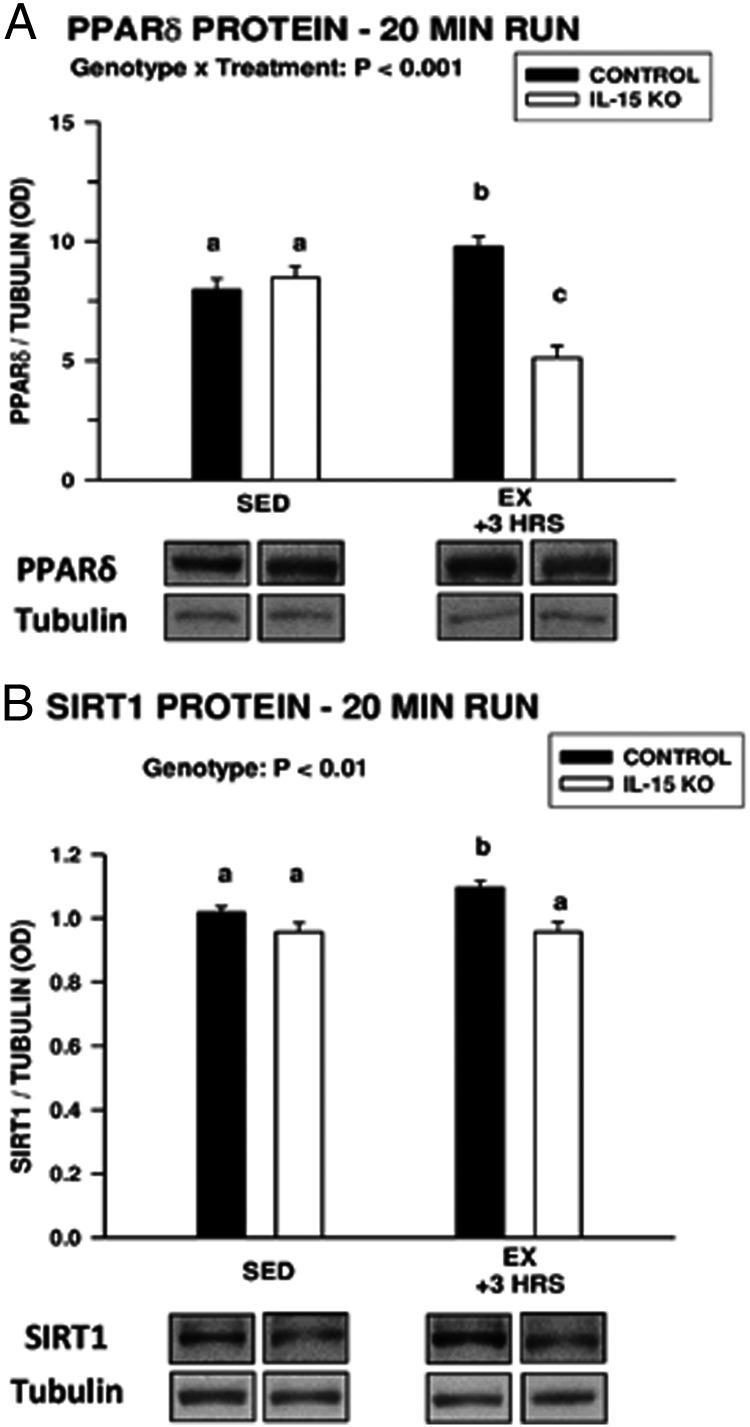
These observations suggest that after exhaustive exercise, IL-15 is necessary for induction of PPARδ mRNA and protein and for induction of SIRT1 protein. However, a confounding factor is that control mice ran almost twice as long as IL-15–KO mice before reaching exhaustion (Figure 1A). Therefore, control and IL-15–KO mice were exercised for 20 minutes and removed from the treadmill. Three hours after the abbreviated exercise session, control mice exhibited small but significant increases in PPARδ and SIRT1 protein expression, whereas such increases were not exhibited by IL-15–KO mice (Figure 3). These observations indicate IL-15, rather than prolonged exercise, is required for increases in muscle PPARδ and SIRT1 protein levels after exercise in control mice.
Figure 3.
A and B, Gastrocnemius muscle PPARδ (A) and SIRT1 (B) protein levels in sedentary mice (SED) and mice exercised for 20 minutes (EX) and analyzed 3 hours later. In each panel, the overall significance of genotype, treatment, or the interaction of these 2 factors determined by two-way ANOVA is shown; bars with different superscripts are significantly different at P < .05. Sample sizes were 6 to 9 mice in each genotype per treatment group. Bars represent means ± SEM. Representative Western blots are shown below the respective graphs; bands shown for each species are compiled from different regions of the same blot.
Postexercise induction of PGC-1α and PGC-1β
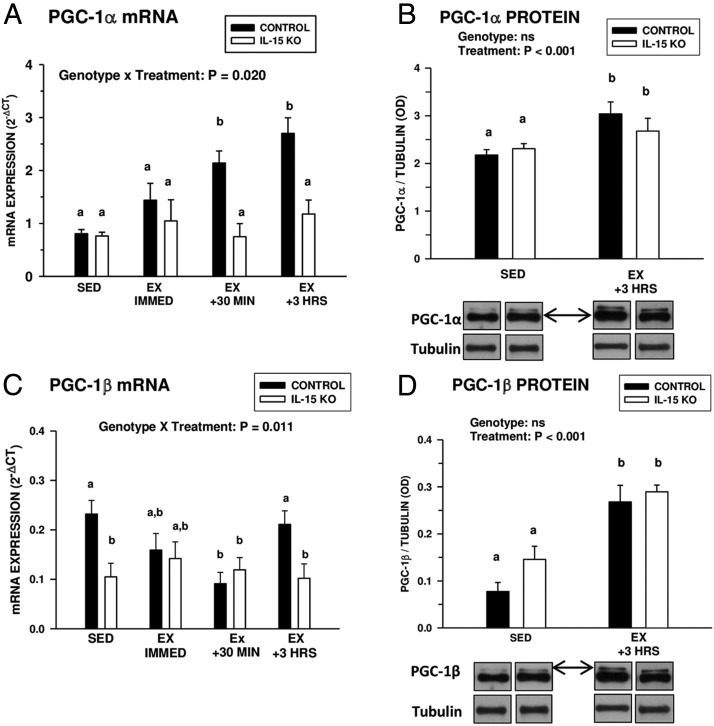
Baseline levels of PGC-1α expression in the gastrocnemius muscle were similar in control and IL-15–KO mice (Figure 4A). After exhaustive exercise, control mice exhibited a progressive increase in gastrocnemius PGC-1α mRNA expression, which was not exhibited by IL-15–KO mice (Figure 4A). However, both control and IL-15–KO mice exhibited increases in gastrocnemius PGC-1α protein levels by 3 hours after exercise (Figure 4B), suggesting a nontranslationally mediated stimulation or stabilization of this factor. Baseline gastrocnemius PGC-1β mRNA expression was significantly lower in IL-15–KO mice compared with controls (Figure 4C). After exercise, control, but not IL-15–KO mice, downregulated gastrocnemius muscle PGC-1β mRNA at 30 minutes after exercise. However, similar to the pattern with PGC-1α, gastrocnemius PGC-1β protein levels were significantly increased in both genotypes 3 hours after exercise (Figure 4D). These findings indicate that IL-15 is not necessary for acute postexercise induction of PGC-1α and PGC-1β at the protein level.
Figure 4.
Postexhaustive exercise induction of PGC-1α and PGC-1β. A and C, PGC-1α (A) and PGC-1β (C) mRNA expression in gastrocnemius muscles of control and IL-15–KO sedentary mice (SED) or at different time points (immediately [IMMED], 30 minutes, and 3 hours [HRS]) after exhaustive exercise (EX). B and D, PGC-1α (B) and PGC-1β (D) protein levels (normalized to tubulin) in the gastrocnemius muscles of SED control and IL-15–KO mice and at 3 hours after exhaustive exercise (EX +3 HRS). Representative Western blots are shown below the respective graphs in B and D; bands shown for each species are compiled from different regions of the same blot. PGC-1α and PGC-1β were detected using a pan-PGC-1 antibody and distinguished by relative mobility, with arrows indicating the species of PGC-1. In each panel, the overall significance of genotype, treatment, or the interaction of these two factors, determined by two-way ANOVA, is shown; bars with different superscripts are significantly different at P < .05. Sample sizes were 6 SED and 9 to 13 EX mice per time point in each genotype. Bars represent means ± SEM.
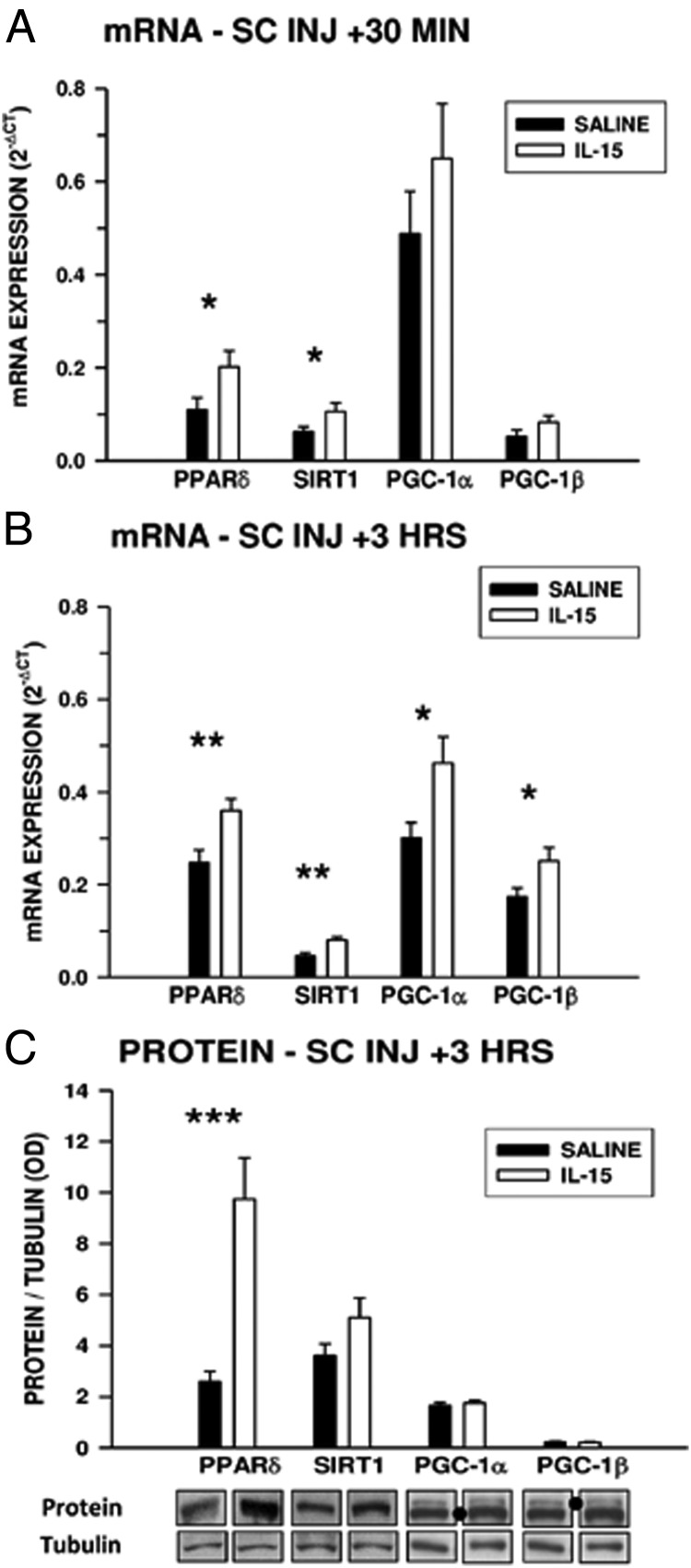
Effects of acute IL-15 injection on pro-oxidative mediators
To determine whether IL-15–KO mice could respond to acute increases in circulating IL-15 by upregulating muscle PPARδ and SIRT1, mice were injected sc with 150 ng recombinant murine IL-15 or saline vehicle and assessed 30 minutes and 3 hours after injection. At 30 minutes, gastrocnemius muscles in IL-15–KO mice injected with IL-15 exhibited significantly (P < .05) higher expression of PPARδ and SIRT1 mRNA compared with saline-injected mice, whereas expression of PGC-1α and PGC-1β mRNA was not significantly different (Figure 5A). At 3 hours after injection, mRNA coding for all 4 of these factors was significantly higher in gastrocnemius muscles in mice injected with IL-15 than in mice that received saline (Figure 5B). At 3 hours after injection, levels of PPARδ protein in gastrocnemius muscles from IL-15–injected mice were significantly higher than in saline-injected mice, whereas protein levels of the other pro-oxidative mediators were not different (Figure 5C). These findings indicate that IL-15–KO mice are capable of responding to exogenous IL-15 with acute induction of PPARδ mRNA and protein.
Figure 5.
Effects of IL-15 injection on acute expression of pro-oxidative mediators in gastrocnemius muscles of IL-15–KO mice. A and B, Expression of PPARδ, SIRT1, PGC-1α, and PGC-1β mRNA in gastrocnemius muscles of IL-15–KO mice 30 minutes (A) or 3 hours (B) after SC injection of 150 ng IL-15 or saline vehicle (SC INJ). C, PPARδ, SIRT1, PGC-1α, and PGC-1β protein levels in gastrocnemius muscles of IL-15–KO mice 3 hours after injection of IL-15 or saline vehicle. For C, representative bands are compiled from different regions of the same Western blot for each species. PGC-1α and PGC-1β were detected using a pan-PGC-1 antibody and distinguished by relative mobility, with dots indicating the species of PGC-1. *, Significant difference between IL-15–injected and saline-injected mice for each parameter, determined by one-way ANOVA (*, P < .05; **, P < .01; ***, P < .001); n = 7 saline-treated and 8 IL-15–treated mice per time point.
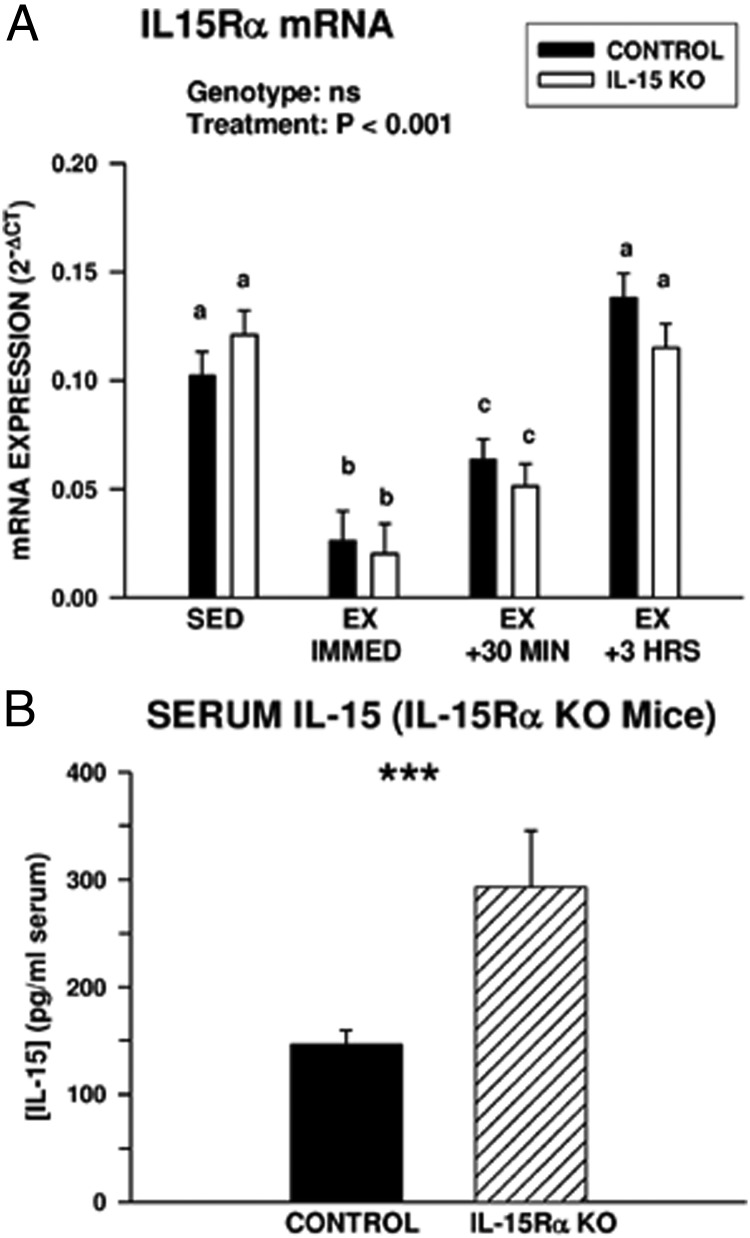
Exercise is associated with downregulation of the IL-15Rα
Postexercise increases in circulating IL-15 were not associated with increases in expression of total IL-15 mRNA or the secreted (LSP) IL-15 mRNA isoform in muscle (Figure 1), indicating IL-15 may be released from muscle tissue by a mechanism that does not involve transcriptional activation of IL-15. Immediately after exercise, expression of mRNA coding for IL-15Rα in the gastrocnemius decreased significantly in both control and IL-15–KO mice (Figure 6A). The association of decreased expression of this receptor subunit with exercise suggested that in the resting state, the IL-15Rα may act to sequester IL-15. In support of this idea, resting IL-15Rα KO mice displayed approximately 2-fold higher concentrations of serum IL-15 compared with controls (Figure 6B).
Figure 6.
Postexercise muscle expression of IL-15Rα and circulating IL-15 levels in IL-15Rα–KO mice. A, IL-15Rα mRNA expression in gastrocnemius muscles of sedentary (SED) control and IL-15–KO mice and at intervals after exhaustive exercise (EX). The overall significance of genotype and treatment, determined by two-way ANOVA, is shown; bars with different superscripts are significantly different at P < .05. Sample sizes were 6 SED and 9 to 13 EX mice per time point in each genotype. B, Serum IL-15 levels in control and IL-15Rα–KO mice. *, Significant difference at P < .001 by one-way ANOVA; n = 12 control and 15 IL-15Rα–KO mice. Bars in both panels represent means ± SEM.
Discussion
Endurance exercise induces transient upregulation of the pro-oxidative intracellular mediators PPARδ, SIRT1, PGC-1α, and PGC-1β in skeletal muscle (4–6), molecules involved in stimulating fat oxidation, energy expenditure, and exercise endurance (1–3). The mechanisms by which these molecules are induced by exercise have not been identified. This study investigated the role of IL-15 in acute induction of PPARδ, SIRT1, PGC-1α, and PGC-1β in the gastrocnemius muscle after exercise using control and IL-15–KO mice. After exercise, IL-15–KO mice upregulated PGC-1α and PGC-1β protein but did not upregulate PPARδ and SIRT1 protein. Our findings indicate that IL-15 is required for postexercise skeletal muscle induction of PPARδ and SIRT1 protein, but not for postexercise induction of PGC-1α and PGC-1β protein. Additionally, IL-15–KO mice injected systemically with IL-15 upregulated PPARδ protein in the gastrocnemius muscle within 3 hours, indicating IL-15 is sufficient for induction of this factor.
Similar to human exercise physiology protocols (21), all mice in the run-to-exhaustion protocol ran to their individual limit of endurance. However, control mice exhibited significantly greater running endurance compared with IL-15–KO mice. On average, control mice weighed less than IL-15–KO mice, and body weight in control mice was negatively correlated with running endurance, consistent with studies showing mice selected for high running activity exhibit reduced body mass and a more oxidative MHC isoform profile (22). In IL-15–KO mice, body weight did not correlate with running endurance, and several individual IL-15–KO mice with low body weight nevertheless exhibited reduced endurance. These observations are consistent with studies in humans and 4-legged mammals, indicating that body weight is not a major determinant of the metabolic costs of running (23). Previous studies detected no obvious histological differences in skeletal muscles of control and IL-15–KO mice (16). However, the present study found a significant decrease in expression of MHC I mRNA, characteristic of the slow/oxidative muscle fibers used in endurance exercise. This finding is consistent with the finding that transgenic mice with constitutive, muscle-specific overexpression of IL-15 exhibited markedly increased running endurance and a pro-oxidative shift in skeletal muscle MHC isoform expression (8). Therefore, the deficit in exercise endurance in IL-15–KO mice may be due to developmental effects of IL-15 on muscle fiber type. This, in turn, may reduce systemic lipid oxidation, resulting in greater fat deposition in IL-15–KO mice. These observations are consistent with previous studies showing overexpression of IL-15 in transgenic mice reduces adiposity (8–10).
The significantly reduced mean running endurance of IL-15–KO mice compared with control mice nevertheless introduced a confounding factor into this protocol. To obviate this concern, a second experiment involved treadmill exercise of all mice for 20 minutes. This exercise protocol resulted in similar, but smaller, differences between control and IL-15–KO mice regarding induction of PPARδ and SIRT1 protein expression, indicating IL-15, rather than exercise duration or fatigue, is required for increases in these 2 mediators after exercise. Moreover, both control and IL-15–KO mice exhibited post—exhaustive-exercise induction of PGC-1α and PGC-1β at the protein level and of SIRT1 at the mRNA level despite differences in duration of the exhaustive running bouts between the 2 genotypes. These findings are in agreement with the above-referenced study (8) demonstrating transgenic mice that constitutively oversecreted IL-15 from skeletal muscle tissue exhibited elevated expression of muscle PPARδ and SIRT1 and increased exercise endurance. The reduced exercise endurance of IL-15–KO mice shown here is consistent with significantly lower baseline levels of gastrocnemius muscle PGC-1β mRNA observed in sedentary IL-15–KO mice in this study, because PGC-1β overexpression induces a more oxidative muscle phenotype and increases exercise endurance (24).
This study examined gastrocnemius muscle PPARδ, SIRT1, PGC-1α, and PGC-1β expression at the mRNA level as well as at the protein level. The dependence of PPARδ mRNA and protein expression on IL-15 was consistent in both the run-to-exhaustion and IL-15 injection experiments, with PPARδ mRNA induction observed within 30 minutes of systemic IL-15 injection and increased PPARδ protein observed within 3 hours of IL-15 injection. Similarly, PPARδ mRNA induction in control, but not IL-15–KO mice, was observed immediately after exhaustive exercise, with a decline to baseline by 30 minutes, whereas elevations in PPARδ protein were observed within 3 hours of exhaustive exercise. However, the other 3 factors examined in this study did not exhibit a similar concordance of IL-15–dependent mRNA and protein expression. SIRT1 mRNA was induced 30 minutes after exercise in both control and IL-15–KO mice. However, control, but not IL-15–KO mice, exhibited elevated levels of SIRT1 protein by 3 hours after exercise. Conversely, postexercise PGC-1α mRNA induction was restricted to control mice, whereas both control and IL-15–KO mice exhibited increases in PGC-1α protein levels after exhaustive exercise. Additionally, PGC-1β mRNA was not induced in either genotype, yet both control and IL-15–KO mice exhibited elevated PGC-1β protein levels 3 hours after exhaustive exercise. These observations are consistent with published studies that have established a complex interplay among these factors that includes both transcriptional and posttranscriptional regulatory pathways (1, 2). These include acetylation, methylation, and phosphorylation of PGC-1α (2) and phosphorylation, sumoylation, methylation, and nitrosylation of SIRT1 (25). SIRT1 activates PGC-1α by deacetylation (2, 25) but is apparently not required for postexercise PGC-1α deacetylation (26). Another study suggested that SIRT1, but not PGC-1α, is required for the short-term metabolic adaptations to exercise (6). Therefore, multiple and redundant pathways may regulate expression of these metabolic regulators after exercise. Indeed, calcineurin, calmodulin-dependent kinase, p38MAPK, and AMP-activated kinase signaling have all been implicated in postexercise induction of PGC-1α (1). Moreover, constitutively high expression of PGC-1α appears to stimulate IL-15 mRNA expression in muscle tissue (27), whereas prolonged IL-15 overexpression stimulates all 4 of the pro-oxidative factors examined here (8). Because of these interactions, conclusions from acute vs prolonged exercise protocols and/or acute vs chronic IL-15 stimulation may be only broadly comparable, and it is not surprising that mRNA and protein levels of the 4 pro-oxidative factors examined did not always correlate.
Several studies have demonstrated that PPARδ can stimulate transcription of both SIRT1 and PGC-1α (20, 28, 29). We observed muscle PPARδ mRNA upregulation in control mice immediately after exhaustive exercise, with a decline thereafter. Induction of SIRT1 and PGC-1α mRNA was not observed until 30 minutes after exhaustive exercise, a temporal pattern that suggests the early, IL-15–dependent postexercise rise in PPARδ mRNA induced PPARδ protein expression, which in turn could have induced PGC-1α mRNA expression. Moreover, systemic injection of IL-15 induced PPARδ expression at both the mRNA and protein levels, with a later rise in PGC-1α and PGC-1β mRNA expression. This study therefore indicates that IL-15 is both necessary and sufficient to induce muscle PPARδ mRNA and protein. Induction of PPARδ may be the primary mechanism underlying IL-15 action after exercise, because myogenic cell culture studies have suggested that the metabolic effects of IL-15 are PPARδ-dependent (30). Indeed, transgenic mice that constitutively overexpress IL-15 and those that overexpress PPARδ are metabolically similar, displaying increased lipid oxidation, increased exercise endurance, and an oxidative skeletal muscle phenotype (8, 31).
This study also examined the appearance of IL-15 in serum after exhaustive exercise in control mice and the potential role of the IL-15Rα in IL-15 release from muscle. We observed that serum IL-15 levels increased significantly by 30 minutes after exercise and returned to baseline thereafter. The pattern of postexercise changes in serum IL-15 in our mouse study is similar to that described for human subjects, except that serum IL-15 levels peaked at 10 minutes after exercise in humans (14), which may be due to biomechanical differences between mice and humans. Several studies with much later postexercise blood sampling times have not found increases in circulating IL-15, whereas others have shown increases after prolonged training (32–35).
IL-15 was deleted in all tissues in the IL-15–KO mouse model used here (16). Therefore, this study could not determine whether the postexercise increases in circulating IL-15 in control mice were due to release from skeletal muscle, other tissues, or both. Because IL-15 is highly expressed in skeletal muscle tissue and muscle contraction occurs during exercise, it has been presumed that such increases in serum IL-15 are due to release from skeletal muscle (3, 13, 14, 34). However, neither our study nor any published literature has established this in a rigorous fashion, which may require arterial/venous sampling from contracting muscle tissue. IL-15 is expressed by many tissues, and systemically expressed IL-15 has been demonstrated to exert effects on several metabolically active tissues such as adipose tissue and liver, in addition to skeletal muscle (12, 15, 35, 36). Moreover, IL-15 has important effects on immune function and inflammation (15), which can in turn impact skeletal muscle metabolism and the responses to exercise (34). Therefore, we cannot rule out the possibility that the effects of IL-15 on postexercise expression of pro-oxidative mediators are indirect and not due to IL-15 release by skeletal muscle, or are due to immune cells resident within muscle tissue. However, one study showed that serum levels of the inflammatory cytokines IL-6 and TNF-α did not differ between control and IL-15–KO mice (12), suggesting that systemic inflammation was not altered in this model. Moreover, several studies, including immunohistochemical localization and analysis of skeletal myogenic cell lines, showed that IL-15 is expressed robustly at both protein and mRNA levels in skeletal muscle fibers in the absence of inflammatory cells (32, 36; reviewed in Ref. 35). Additionally, numerous studies have established a relationship between physical exercise and IL-15 expression and between endurance exercise capacity and IL-15 and/or its specific receptor, the IL-15Rα (8, 13, 14, 32, 33, 37–39; reviewed in Ref. 35). IL-15 can exert hypertrophic actions on cultured skeletal muscle cells (40) and inhibit muscle wasting in rat models of cancer cachexia (41). These observations indicate IL-15 plays a role in skeletal muscle metabolism and suggest that muscle fibers are at least one source of IL-15, acting in an autocrine or paracrine fashion within muscle tissue and possibly contributing to circulating IL-15 levels. Additional studies are needed to investigate this possibility. Nevertheless, our study shows that IL-15, whatever the source, is required for acute postexercise induction of muscle PPARδ and SIRT1 at the protein level but is not required for postexercise induction of PGC-1α and PGC-1β.
In this study, we also examined acute postexercise changes in muscle IL-15 and IL-15Rα mRNA expression. Our results suggest a model that involves release of IL-15 normally sequestered by membrane-bound IL-15Rα in muscle but does not preclude release of IL-15 from other tissues. Postexercise increases in circulating IL-15 were not associated with increases in expression of total or secreted IL-15 mRNA isoforms, indicating IL-15 may be released from muscle tissue by a mechanism that does not involve transcriptional activation of IL-15. This is consistent with other studies that have found little correlation between changes in muscle IL-15 mRNA and protein after exercise (32). IL-15 lacks an efficient signal sequence (15) and is proposed to be transported to the cell surface by intracellular association with the IL-15Rα, which contains a more functional signal sequence and is not required for IL-15 signal transduction (15). It is then thought that membrane-associated IL-15/IL-15Rα complexes can signal in a juxtacrine fashion or be cleaved enzymatically to release soluble IL-15/truncated IL-15Rα complexes (15). This scenario is consistent with reports that most circulating IL-15 in mice and humans resides in such complexes (42) but does not take into account the considerably lower stability of free IL-15 compared with complexed IL-15 (43). In this regard, we found that IL-15Rα–KO mice exhibited nominally higher circulating IL-15 levels than controls, although we did not address whether complexing affected the sensitivity of the IL-15 assay. Nevertheless, the presence of measurable IL-15 in the serum of these mice indicates that IL-15 can be released by an IL-15Rα–independent mechanism and that in sedentary control mice, IL-15 may be sequestered by the IL-15Rα. Moreover, high circulating levels of IL-15 in IL-15Rα–KO mice can explain the phenotypic similarity of these mice to transgenic mice that oversecrete IL-15 into the circulation, both of which exhibit increased exercise endurance, a pro-oxidative metabolic phenotype, and increased expression of PPARδ (8, 37). We also observed that IL-15Rα mRNA was downregulated immediately after exhaustive exercise in both control and IL-15–KO mice. Therefore, this receptor downregulation cannot be attributed to negative feedback from released IL-15. Immediate downregulation of IL-15Rα by exercise may be responsible for IL-15 release from muscle tissue by an unknown mechanism, which could differ from that of other cell types. Of note, single-nucleotide polymorphisms in the human IL-15Rα gene have been associated with adiposity, metabolic parameters, and endurance exercise capacity (13, 37–39), consistent with a role for the IL-15Rα in controlling IL-15 bioavailability. Testing of this model was out of the scope of the present study and will require further investigation.
Additionally, we observed that gastrocnemius muscle PPARδ mRNA was elevated before the peak in postexercise serum IL-15 levels, suggesting that if exercise causes IL-15 release from muscle fibers, it may act in an autocrine/paracrine manner within this tissue before measurable diffusion into the circulation. Therefore, the extracellular space in exercising muscle tissue may contain much higher local concentrations of IL-15 than are measured systemically. This can explain why the immediate postexercise increases in PPARδ mRNA preceded the increase in serum IL-15 that occurred 30 minutes after exercise. These kinetics also suggest that IL-15 release commenced within muscle tissue during the exhaustive exercise bout, likely before 20 minutes on the basis of the experiment in which exercise duration was controlled. Indeed, in our study and in 2 studies using human subjects (13, 14), acute increases in serum IL-15 levels after exercise are quite modest compared with the postexercise increases in circulating IL-6 that range from 25- to 100-fold (34). These considerations suggest that IL-15 is released from preexisting stores and that the primary mode of IL-15 action on skeletal muscle may be autocrine/paracrine. Therefore, IL-15 can only provisionally be considered an exercise-induced myokine (3, 34).
In summary, this study has established that exercise induces transient increases in circulating IL-15 in mice, similar to observations in human subjects (13, 14). Moreover, this study provides evidence that IL-15 is necessary and sufficient for skeletal muscle upregulation of the pro-oxidative metabolic regulator PPARδ at the mRNA and protein levels. A more modest IL-15–dependent postexercise induction of SIRT1 was also observed. However, IL-15 was not required for acute postexercise induction of PGC-1α or PGC-1β at the protein level, suggesting that alternative pathways induced by exercise regulate these factors. PPARδ and SIRT1 exert many favorable metabolic effects that counteract obesity and insulin resistance (2). Conversely, previous studies showed that muscle and serum IL-15 levels decline with increasing age in rodents (44, 45), suggesting that decreases in IL-15 may play a role in the metabolic declines associated with advanced age. The findings reported here could be used to develop an IL-15–based strategy to induce many of the metabolic benefits of physical exercise in frail elderly individuals and other patients who are unable to exercise.
Acknowledgments
This work was supported by Merit Review BX001026 from the Department of Veterans Affairs (to L.S.Q.) and use of resources and facilities including the Rodent Metabolic and Behavioral Phenotyping Core at VA Puget Sound Health Care System, the Transgenic Resource Core at the University of Washington Nathan Shock Center of Excellence in the Basic Biology of Aging (NIA 5P30AG-013280), and the University of Washington Diabetes Endocrinology Research Center (NIH P30 DK-17047).
Present address for J.D.C.: Shin Nippon Biomedical Laboratories, Everett, Washington.
Disclosure Summary: The authors have no conflicts of interest to disclose.
Funding Statement
This work was supported by Merit Review BX001026 from the Department of Veterans Affairs (to L.S.Q.) and use of resources and facilities including the Rodent Metabolic and Behavioral Phenotyping Core at VA Puget Sound Health Care System, the Transgenic Resource Core at the University of Washington Nathan Shock Center of Excellence in the Basic Biology of Aging (NIA 5P30AG-013280), and the University of Washington Diabetes Endocrinology Research Center (NIH P30 DK-17047).
Footnotes
- CT
- cycle threshold
- HPRT1
- hypoxanthine phosphoribosyltransferase 1
- IL-15Rα
- IL-15 receptor-α
- KO
- knockout
- LSP
- long signal peptide
- MHC
- myosin heavy chain
- PGC-1α
- PPARγ coactivator 1α
- PPARδ
- peroxisome proliferator-activated receptor-δ
- SIRT1
- silent information regulator of transcription (sirtuin)-1
- UTR
- untranslated region.
References
- 1. Benton CR, Wright DC, Bonen A. PGC-1α-mediated regulation of gene expression and metabolism: implications for nutrition and exercise prescriptions. Appl Physiol Nutr Metab. 2008;33:843–862. [DOI] [PubMed] [Google Scholar]
- 2. Matsakas A, Narkar VA. Endurance exercise mimetics in skeletal muscle. Curr Sports Med Rep. 2010;9:227–232. [DOI] [PubMed] [Google Scholar]
- 3. Arnold AS, Egger A, Handschin C. PGC-1α and myokines in the aging muscle – a mini-review. Gerontology. 2011;57:37–43. [DOI] [PubMed] [Google Scholar]
- 4. Perry CG, Lally J, Holloway GP, Heigenhauser GJ, Bonen A, Spriet LL. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol. 2010;588;4795–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dumke CL, Mark Davis J, Angela Murphy E, et al. Successive bouts of cycling stimulates genes associated with mitochondrial biogenesis. Eur J Appl Physiol. 2009;107:419–427. [DOI] [PubMed] [Google Scholar]
- 6. Suwa M, Nakano H, Radak Z, Kumagai S. Endurance exercise increases the SIRT1 and peroxisome proliferator-activated receptor γ coactivator-1α protein expressions in rat skeletal muscle. Metabolism. 2008;57:986–998. [DOI] [PubMed] [Google Scholar]
- 7. Grabstein KH, Eisenman J, Shanebeck K, et al. Cloning of a T cell growth factor that interacts with the β-chain of the interleukin-2 receptor. Science. 1994;264:965–968. [DOI] [PubMed] [Google Scholar]
- 8. Quinn LS, Anderson BG, Conner JD, Wolden-Hanson T. IL-15 overexpression promotes endurance, oxidative energy metabolism, and muscle PPARδ, SIRT1, PGC-1α, and PGC-1β expression in male mice. Endocrinology. 2013;154:232–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Quinn LS, Anderson BG, Strait-Bodey L, Stroud AM, Argilés JM. Oversecretion of interleukin-15 from skeletal muscle reduces adiposity. Am J Physiol. 2009;296:E191–E202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Quinn LS, Anderson BG, Conner JD, Pistilli EE, Wolden-Hanson T. Overexpression of IL-15 in mice promotes resistance to diet-induced obesity, increased insulin sensitivity, and markers of oxidative skeletal muscle metabolism. Int J Interferon Cytokine Med Res. 2011;3:29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nielsen AR, Hojman P, Erikstrup C, et al. Association between interleukin-15 and obesity: interleukin-15 as a potential regulator of fat mass. J Clin Endocrinol Metab. 2008;93:4486–4493. [DOI] [PubMed] [Google Scholar]
- 12. Barra NG, Reid S, MacKenzie R, et al. Interleukin-15 contributes to the regulation of murine adipose tissue and human adipocytes. Obesity. 2010;18:1601–1607. [DOI] [PubMed] [Google Scholar]
- 13. Riechman SE, Balasekaran G, Roth SM, Ferrell RE. Association of interleukin-15 protein and interleukin-15 receptor genetic variation with resistance exercise training responses. J Appl Physiol. 2004;97:2214–2219. [DOI] [PubMed] [Google Scholar]
- 14. Tamura Y, Watanabe K, Kantani T, Hyashi J, Ishida N, Kaneki M. Upregulation of circulating IL-15 by treadmill running in healthy individuals: Is IL-15 an endocrine mediator of the beneficial effects of endurance exercise? Endocr J. 2011;58:211–215. [DOI] [PubMed] [Google Scholar]
- 15. Budagian V, Bulanova E, Paus R, Bulfone-Paus S. IL-15/IL-15 receptor biology: A guided tour through an expanding universe. Cytokine Growth Factor Rev. 2006;17:259–280. [DOI] [PubMed] [Google Scholar]
- 16. Kennedy MK, Glaccum M, Brown SN, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lodolce JP, Boone DL, Chai S, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. [DOI] [PubMed] [Google Scholar]
- 18. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108. [DOI] [PubMed] [Google Scholar]
- 19. Smith PK, Krohn RI, Hermanson GT, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. [DOI] [PubMed] [Google Scholar]
- 20. Kleiner S, Nguyen-Tran V, Baré O, Huang X, Spiegelman B, Wu Z. PPARδ agonism activates fatty acid oxidation via PGC-1α but does not increase mitochondrial gene expression and function. J Biol Chem. 2009;284:18624–18633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Trimmer JK, Schwarz JM, Casazza GA, Horning MA, Rodriguez N, Brooks GA. Measurement of gluconeogenesis in exercising men by mass isotopomer distribution analysis. J Appl Physiol. 2002;93:233–241. [DOI] [PubMed] [Google Scholar]
- 22. McGillivray DG, Garland T Jr, Dlugosz EM, Chappell MA, Syme DA. Changes in efficiency and myosin expression in the small-muscle phenotype of mice selectively bred for high voluntary running activity. J Exp Biol. 2009;212:977–985. [DOI] [PubMed] [Google Scholar]
- 23. Teunissen LP, Grabowski A, Kram R. Effects of independently altering body weight and body mass on the metabolic cost of running. J Exp Biol. 2007;210:4418–4427. [DOI] [PubMed] [Google Scholar]
- 24. Arany Z, Lebrasseur N, Morris C, et al. The transcriptional coactivator PGC-1β drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab. 2007;5:35–46. [DOI] [PubMed] [Google Scholar]
- 25. Williams CB, Gurd BJ. Skeletal muscle SIRT1 and the genetics of metabolic health: therapeutic activation by pharmaceuticals and exercise. Appl Clin Genet. 2012;5:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Philp A, Chen A, Lan DM, et al. Sirtuin 1 (SIRT1) deacetylase activity is not required for mitochondrial biogenesis or peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) deacetylation following endurance exercise. J Biol Chem. 2011;286:30561–30570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boström P, Wu J, Jedrychowski MP, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schuler M, Ali F, Chambon C, et al. PGC1α expression is controlled in skeletal muscle by PPARβ, whose ablation results in fiber-type switching, obesity, and type 2 diabetes. Cell Metab. 2006;4:407–414. [DOI] [PubMed] [Google Scholar]
- 29. Okazaki M, Iwasaki Y, Nishiyama M, et al. PPARβ/δ regulates the human SIRT1 gene transcription via Sp1. Endocr J. 2010;57:403–413. [DOI] [PubMed] [Google Scholar]
- 30. Fuster G, Busquets S, Figueras M, et al. PPARδ mediates IL15 metabolic actions in myotubes: effects of hyperthermia. Int J Mol Med. 2009;24:63–68. [PubMed] [Google Scholar]
- 31. Wang YX, Zhang CL, Yu RT, et al. Regulation of muscle fiber type and running endurance by PPARδ. PLoS Biol. 2004;2:e294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nielsen AR, Mounier R, Plomgaard P, et al. Expression of interleukin-15 in human skeletal muscle effect of exercise and muscle fibre type composition. J Physiol. 2007;584:305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yeo NH, Woo J, Shin KO, Park JY, Kang S. The effects of different exercise intensity on myokine and angiogenesis factors. J Sports Med Phys Fitness. 2012;52:448–454. [PubMed] [Google Scholar]
- 34. Pedersen BK. The diseasome of physical inactivity–and the role of myokines in muscle-fat cross talk. J Physiol. 2009;587:5559–5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Quinn LS, Anderson BG. Interleukin-15, IL-15 receptor-α, and obesity: concordance of laboratory animal and human genetic studies. J Obes. 2011;2011:456347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Quinn LS, Strait-Bodey L, Anderson BG, Argilés JM, Havel PJ. Interleukin-15 stimulates adiponectin secretion by 3T3-L1 adipocytes: evidence for a skeletal muscle-to-fat signaling pathway. Cell Biol Int. 2005;29:449–457. [DOI] [PubMed] [Google Scholar]
- 37. Pistilli EE, Bogdanovich S, Garton F, et al. Loss of IL-15Rα alters endurance, fatigability, and metabolic characteristics of mouse fast skeletal muscles. J Clin Invest. 2011;121:3120–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pistilli EE, Devaney JM, Gordish-Dressman H, et al. Interelukin-15 and interleukin-15R alpha SNPs and associations with muscle, bone, and predictors of the metabolic syndrome. Cytokine. 2008;43:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Di Renzo L, Bigioni M, Bottini FG, Del Gobbo V, Premrov MG, Cianci R, De Lorenzo A. Normal weight obese syndrome: role of single nucleotide polymorphism of IL-15Rα and MTHFR 677→T genes in the relationship between body composition and resting metabolic state. Eur Rev Med Pharmacol Sci. 2006;10:235–245. [PubMed] [Google Scholar]
- 40. Quinn LS, Anderson BG, Drivdahl RH, Alvarez B, Argilés JM. Overexpression of interleukin-15 induces skeletal muscle hypertrophy in vitro: Implications for treatment of skeletal muscle wasting disorders. Exp Cell Res. 2002;280:55–63. [DOI] [PubMed] [Google Scholar]
- 41. Carbó N, López-Soriano J, Costelli P, et al. Interleukin-15 antagonizes muscle protein waste in tumour-bearing rats. Br J Cancer. 2000;83:526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bergamaschi C, Bear J, Rosati M, et al. Circulating IL-15 exists as heterodimeric complex with soluble IL-15Rα in human and mouse serum. Blood. 2012;120:e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chertova E, Bergamaschi C, Chertov O, et al. Characterization and favorable in vivo properties of heterodimeric soluble IL-15·IL-15Rα cytokine compared to IL-15 monomer. J Biol Chem. 2013;288:18093–18103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Marzetti E, Carter CS, Wohlgemuth SE, et al. Changes in IL-15 expression and death-receptor apoptotic signaling in rat gastrocnemius muscle with aging and life-long calorie restriction. Mech Aging Dev. 2009;130:272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Quinn LS, Anderson BG, Strait-Bodey L, Wolden-Hanson T. Serum and muscle interleukin-15 levels decrease in aging mice: correlation with declines in soluble interleukin-15 receptor α expression. Exp Gerontol. 2010;45:106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]