Abstract
Objective
To quantify early auditory exposures in the neonatal intensive care unit (NICU) and evaluate how these are related to medical and environmental factors. We hypothesized that there would be less auditory exposure in the NICU private room, compared with the open ward.
Study design
Preterm infants born at ≤ 28 weeks gestation (33 in the open ward, 25 in private rooms) had auditory exposure quantified at birth, 30 and 34 weeks postmenstrual age (PMA), and term equivalent age using the Language Environmental Acquisition device.
Results
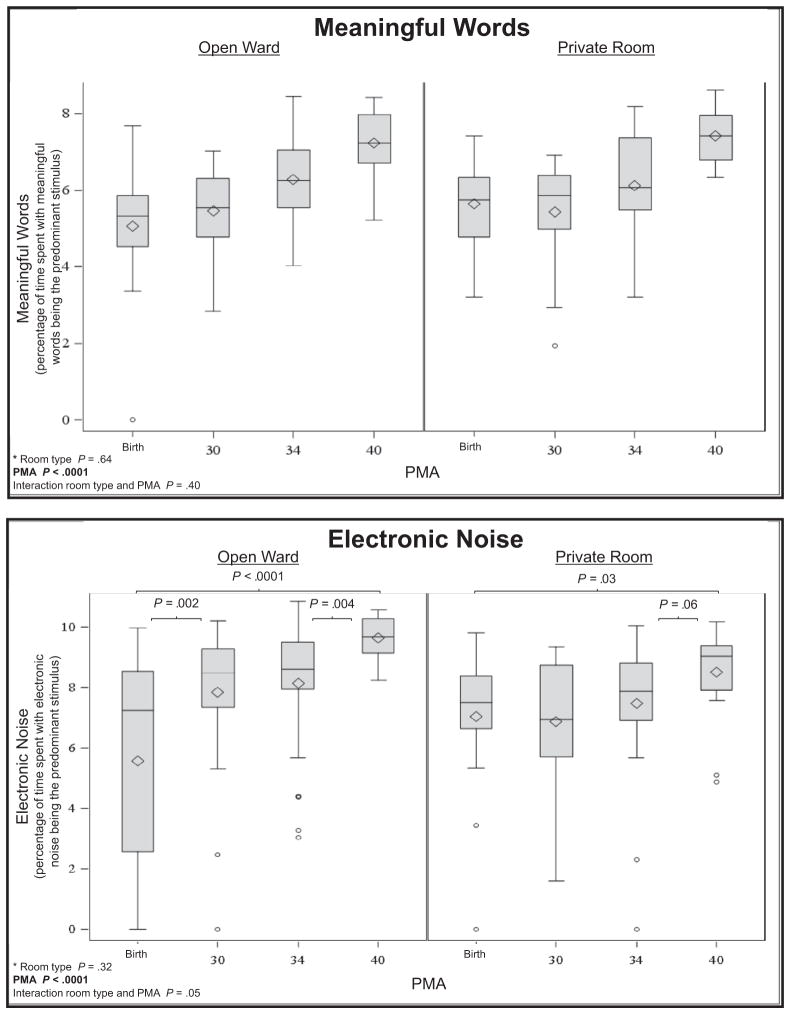
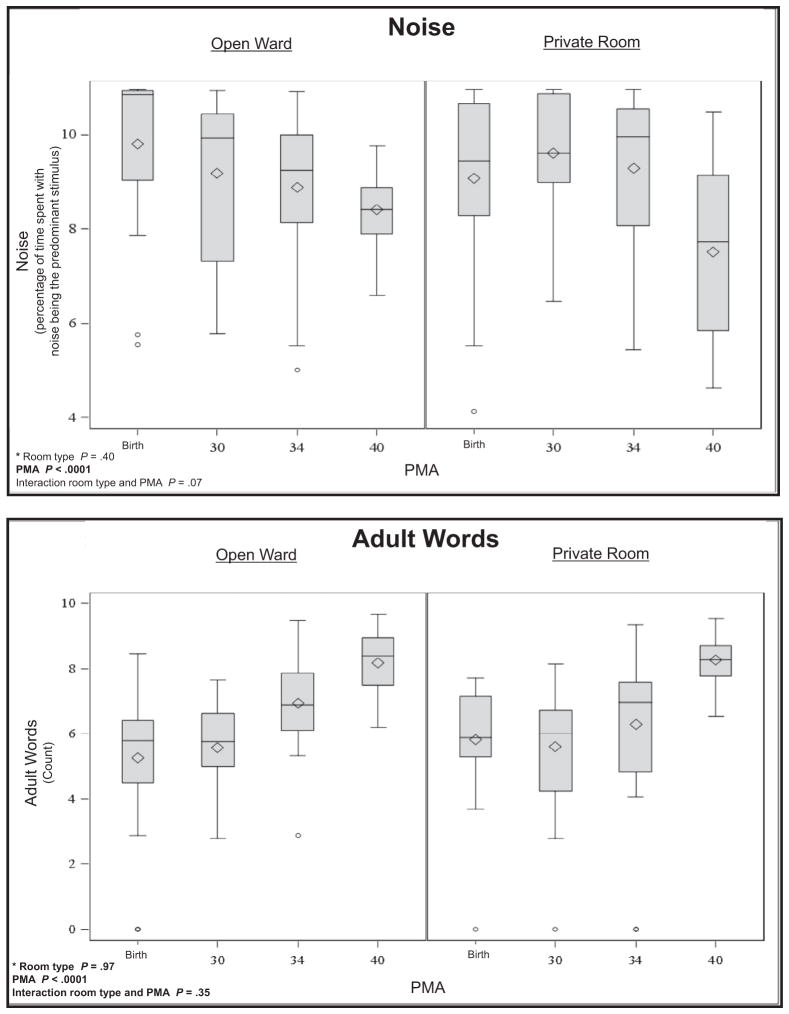
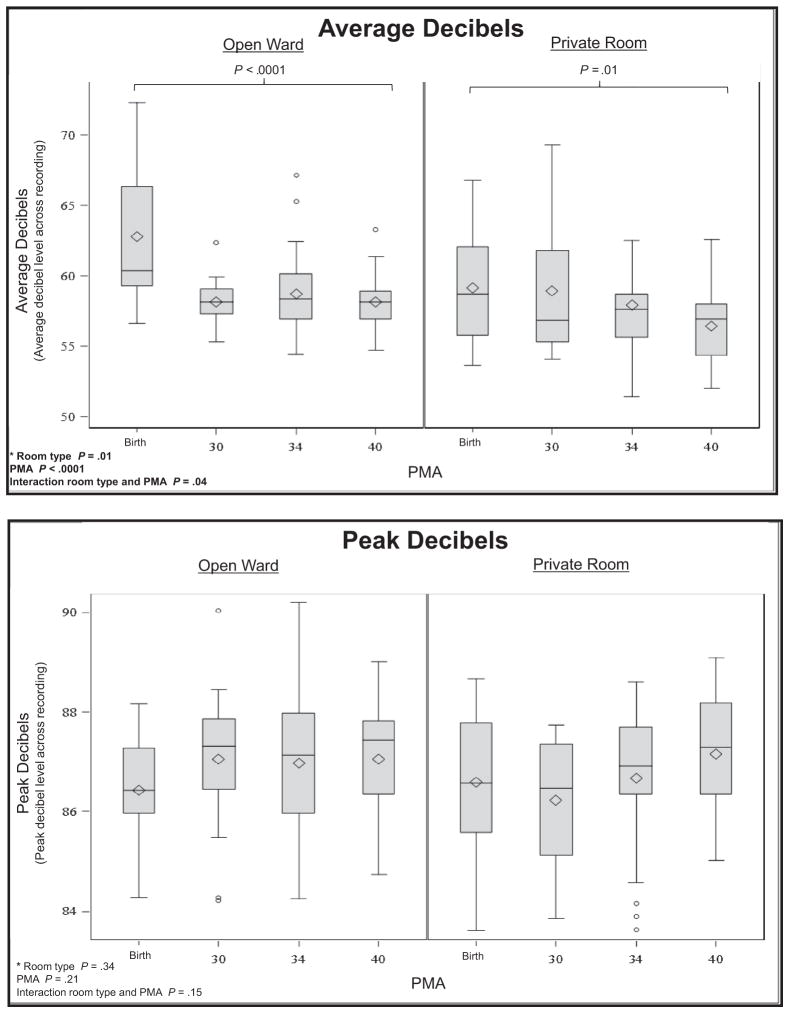
Meaningful language (P < .0001), the number of adult words (P < .0001), and electronic noise (P < .0001) increased across PMA. Silence increased (P = .0007) and noise decreased (P < .0001) across PMA. There was more silence in the private room (P = .02) than the open ward, with an average of 1.9 hours more silence in a 16-hour period. There was an interaction between PMA and room type for distant words (P = .01) and average decibels (P = .04), indicating that changes in auditory exposure across PMA were different for infants in private rooms compared with infants in the open ward. Medical interventions were related to more noise in the environment, although parent presence (P = .009) and engagement (P = .002) were related to greater language exposure. Average sound levels in the NICU were 58.9 ± 3.6 decibels, with an average peak level of 86.9 ± 1.4 decibels.
Conclusions
Understanding the NICU auditory environment paves the way for interventions that reduce high levels of adverse sound and enhancing positive forms of auditory exposure, such as language.
The auditory environment of the preterm infant differs considerably from the environment in-utero. Intense forms of non-natural sounds in the neonatal intensive care unit (NICU), such as ventilatory support, have been reported1–4 and sound levels in the often chaotic environment may exceed the decibel recommendations of the American Academy of Pediatrics. This is important because the overstimulating environment of the NICU may have adverse effects on the growth and development of preterm infants.5–7
To increase family involvement and to decrease the risk of overstimulation, hospitals around the world are converting traditional open ward NICUs to private rooms.8 Positive benefits of private rooms reported in the scientific literature include a decreased need for medical procedures, decreased stress, improved neurobehavior, and better long-term outcomes.9–11 Despite these benefits, a prior study also showed that infants hospitalized in the presumably quieter environment of the NICU private room exhibited lower language scores at 2 years of age12 than infants hospitalized in open ward NICUs. This previous work, however, did not measure auditory exposure.
Although auditory exposure in the NICU is important for the developing preterm infant,13–17 the appropriate amount or type of auditory exposure has not been defined. The aim of this study was to measure the auditory environment in the NICU and to determine medical, environmental, and sociodemographic factors that predicted the infant’s auditory exposure in the NICU. We hypothesized that there would be less auditory exposure in the NICU private room, compared with the open ward. We explored this and other medical and environmental factors that impact the sound environment in the NICU.
Methods
The study was approved by the Washington University Human Research Protection Office, and parents provided informed consent. Language and sound were quantified longitudinally at 4 different time periods across hospitalization: within 2 weeks of birth, at 30 and 34 weeks postmenstrual age (PMA), and again at term equivalent age (between 37 and 40 weeks PMA).
Fifty-eight consecutive preterm infants born at ≤28 weeks gestation were recruited from August 2012 to October 2014. Exclusion criteria were failed hearing screening, multiple birth, and congenital anomalies. The study site was the 75-bed level IV NICU at St. Louis Children’s Hospital (St. Louis, Missouri) that has 38 traditional open ward beds and 37 private rooms. As part of routine care, infants were assigned to a bed space in either the open ward or in a private room upon admission, based on availability and staffing, and remained in the assigned room type for their entire hospitalization. Private rooms had an average area of 168 square feet and were enclosed by 3 walls, with a sliding glass door for the fourth wall. The open ward was composed of 4 large rooms (ranging from 802 to 1375 square feet) with 8–14 beds per room. The nurse to infant ratio varied, based on staffing and medical complexity, but typically was 1 nurse for every 2 infants and was similar whether an infant was in the open ward or private room. As part of the standard of care, parents were welcome in the NICU 24 hours a day Mothers were encouraged to engage in the care of their infant and to put their infants skin-to-skin when visiting. Loungers were available at the bedside in both open ward and private rooms. Volunteers were available to hold infants whose parents are unable to visit, but this was implemented inconsistently during the study time period, and volunteers typically held older infants as they approached term PMA.
Study Procedures
The Language Environmental Acquisition (LENA) (LENA Research Foundation, Boulder, Colorado) device is a digital language processor that captures environmental sound for up to 16 hours.18 The LENA has been used in previous research on infants in the NICU at 32 weeks PMA and 36 weeks PMA, justifying it as an appropriate method of capturing noise and language in the NICU.14 Before the study started, 2 methods of recording were evaluated. The quantification of sound was similar when the LENA was hanging in the infant’s bed (within a pouch that was cut out from the vest) and when the infant wore it in a vest (the recommended method). As it would be less invasive for the infant, for this study, the LENA was hung on the infant’s crib or inside the incubator within 2 feet of the infant’s ear. A sign at the bedside indicated the LENA was in use during the recordings (as required by the institutional review board). LENA recordings commenced before 10 a.m. each morning and automatically ended 16 hours later. Data from the digital language processor was downloaded into the LENA Pro software, which generates an estimate of the amount of time spent with meaningful words, distant words, electronic sounds, noise, and silence. Each outcome variable was recorded as the amount of time it was the predominant auditory stimulus in the room over a 16-hour period. In addition, the LENA software defined the number of adult words heard during each recording as well as the highest and average decibel levels in the infant’s auditory environment. The LENA quantified sound at 4 different time points: within 2 weeks of birth, at 30 and 34 weeks PMA, and at term-equivalent age (37–40 weeks PMA). When an infant was born at 28 weeks gestation, the birth recording was often the same as the 30 weeks PMA recording. The infant’s PMA on the day of each recording was recorded.
Environmental factors during the sound recordings were documented. These included room type (open ward or private room), the census in the NICU and in the infant’s room (for open wards), bed type (incubator or open crib), parental presence or absence, the number of times a parent held the infant, the number of pumps (defined as any device that delivered fluids via nasogastric or intravenous line and produced sounds upon completion or interruption), and the type of respiratory support (room air, oxygen via nasal cannula, continuous positive airway pressure, mechanical ventilation, or high-frequency oscillatory ventilation).
Infants were placed in an incubator on admission and were transitioned to a crib when they were able to maintain temperature (at approximately 1800 g). Maternal factors were collected from the medical record at the time of the infant’s birth, including age, marital status, education level (dichotomized as college education or no college education), the number of prenatal visits, delivery type, and prenatal illicit drug exposure (from toxicology reports at delivery). Social factors were collected, including the infant’s sex and race (dichotomized as African American or non-African American). Medical factors were collected from the medical record. These included estimated gestational age at birth, birth weight, head circumference at birth, Apgar scores at 1 and 5 minutes, use of prenatal or postnatal steroids, days on total parenteral nutrition, total days of breast milk feedings, breast milk at discharge, presence of moderate to severe brain injury (defined as having either a grade III–IV intraventricular hemorrhage, cystic periventricular leukomalacia, or cerebellar hemorrhage as determined by cranial ultrasound scan and/or magnetic resonance imaging), presence of patent ductus arteriosus (treated with indomethacin or surgical ligation), necrotizing enterocolitis (all stages), retinopathy of prematurity requiring surgical intervention, score on the Clinical Risk Index for Babies (CRIB),19 length of stay, days, and types of respiratory equipment (high-frequency oscillation, mechanical ventilation, continuous positive airway pressure, high humidity, nasal cannula), total oxygen hours (including time on any of the aforementioned), and oxygen requirement at 36 weeks PMA.
Statistical Analyses
For variables of meaningful words, distant words, electronic sounds, noise, and adult word count, a log transformation of the sound measurements were used to stabilize the variance. We tested for room type, PMA, and room type by PMA interaction using a mixed random effects repeated measures ANOVA model (with infant within room type as the random effect). When the room type by PMA interaction was significant (P < .05), the effect of PMA, stratified by the room type, was explored. When the interaction was not significant, we investigated the relationships of PMA and sound as well as the relationships between room type and sound using main effects. The relationships between medical, sociodemographic, and environmental factors were investigated using Pearson correlation coefficients. Because of a large number of relationships explored, a strict P value of .01 was considered significant when identifying predictors of auditory exposure.
Results
Fifty-eight infants were enrolled (33 in open ward and 25 in private rooms). Ten infants expired before discharge (6 in open ward and 4 in private rooms). Data obtained from deceased infants were included in the analyses.
There were 52 sound recordings at birth, 50 recordings at 30 weeks PMA, 48 recordings at 34 weeks PMA, and 44 recordings at term equivalent age. Infants who died did not have recordings at birth (n = 4), 30 weeks (n = 8), 34 weeks (n = 10), and term PMA (n = 10). Two infants whose birth and 30-week recordings overlapped did not have recordings at birth. Four infants were discharged before the term recording. Table I shows the characteristics of the 48 infants (27 in open ward and 21 in private room) who were enrolled into the study and discharged from the NICU.
Table I.
Sample characteristics of discharged infants
| Baseline factors (total n = 48*) | Range | N (%), median (IQR) or mean (SD) |
|---|---|---|
| Maternal age, y | 16–40 | 27.1 (5.8) |
| Married marital status | 24 (44%) | |
| Maternal education, college education | 23 (43%) | |
| Number of prenatal visits (n = 47) | 0–10 | 3.8 (2.3) |
| Vaginal delivery | 22 (41%) | |
| Illicit drug use | 4 (7%) | |
| Social factors | ||
| Infant sex, female | 20 (37%) | |
| Infant race, African American | 30 (56%) | |
| Medical factors | ||
| EGA, wk | 23–28 | 25.7 (1.4) |
| Birth weight, g | 510–1310 | 854.8 (183.8) |
| Head circumference at birth, cm | 20.8–29.5 | 23.4 (1.5) |
| Use of prenatal steroids | 40 (74%) | |
| Use of postnatal steroids | 19 (35%) | |
| Total parenteral nutrition, d | 8–136 | 32.2 (31.1) |
| Breast milk feeding initiated | 45 (94%) | |
| Total days on breast milk (n = 46) | 0–121 | 51.2 (30.3) |
| Breast milk feeding at discharge (n = 46) | 13 (28%) | |
| Presence of moderate to severe brain injury† | 10 (21%) | |
| Patent ductus arteriosus (n = 47)‡ | 33 (70%) | |
| Necrotizing enterocolitis (n = 45)‡ | 10 (22%) | |
| Retinopathy of prematurity (n = 45)‡ | 9 (20%) | |
| CRIB score§ | 1–13 | 5.3 (3.7) |
| Length of stay, d (n = 45) | 62–265 | 120.1 (42.9) |
| Days of high-frequency oscillatory ventilation (n = 45) | 0–22 | 0 (0–3) |
| Number of days ventilated (n = 45) | 0–116 | 9 (1–36.5) |
| Continuous positive airway pressure, d | 0–1776 | 19 (7–39) |
| Days on high humidity | 0–78 | 27 (14.5–41.5) |
| Days on nasal cannula | 0–480 | 19 (11–33.5) |
| Infant was receiving oxygen at 28 d (n = 45) | 44 (98%) | |
| Infant was receiving oxygen at 36 wk (n = 44) | 36 (82%) | |
| Total oxygen, h (n = 45) | 528–6360 | 2550.9 (1220.8) |
| Environmental factors | ||
| Total NICU census at time of term recording | 42–88 | 60 (55.3–71.8) |
| Room type, single patient room | 21 (44%) | |
Infants who were enrolled and discharged from the study NICU.
Moderate to severe brain injury was defined as a grade III–IV intraventricular hemorrhage, cystic periventricular leukomalacia, or cerebellar hemorrhage as measured by cranial ultrasound scan and/or magnetic resonance imaging.
Presence of patent ductus arteriosus treated with indomethacin or surgical ligation; necrotizing enterocolitis (all stages); retinopathy of prematurity requiring surgical intervention.
Higher CRIB scores indicate more medical compromise at admission to the NICU. CRIB scores ranged from 0 to 24.
Table II shows the quantification of auditory exposure across all time points for open wards and private rooms. Average sound levels in the NICU were 58.9 ± 3.6 decibels, with an average peak level of 86.9 ± 1.4 decibels.
Table II.
Types of sound environment by room type
| Room type | Mean (h:min) ± SD | Difference between rooms (h:min) or decibels or number of words | |
|---|---|---|---|
| Amount of time spent with meaningful language (in a 16-h period) | |||
| Birth (n = 52) | Private room | 0:08 ± 0:09 | 0:02 |
| Open ward | 0:06 ± 0:07 | ||
| 30 wk (n = 50) | Private room | 0:06 ± 0:05 | 0 |
| Open ward | 0:06 ± 0:05 | ||
| 34 wk (n = 48) | Private room | 0:17 ± 0:19 | 0:01 |
| Open ward | 0:16 ± 0:21 | ||
| Term (n = 44) | Private room | 0:35 ± 0:25 | 0:04 |
| Open ward | 0:31 ± 0:23 | ||
| Amount of time spent with distant language (in a 16-h period) | |||
| Birth (n = 52) | Private room | 1:44 ± 1:27 | 0:17 |
| Open ward | 1:27 ± 1:31 | ||
| 30 wk (n = 50) | Private room | 0:36 ± 0:29 | −0:56 |
| Open ward | 1:32 ± 1:27 | ||
| 34 wk (n = 48) | Private room | 0:57 ± 0:46 | −0:14 |
| Open ward | 1:11 ± 0:45 | ||
| Term (n = 44) | Private room | 0:35 ± 0:57 | −0:39 |
| Open ward | 1:14 ± 0:49 | ||
| Amount of time spent with electronic sounds (in a 16-h period) | |||
| Birth (n = 52) | Private room | 0:57 ± 1:11 | 0:01 |
| Open ward | 0:56 ± 1:36 | ||
| 30 wk (n = 50) | Private room | 0:50 ± 0:58 | −1:07 |
| Open ward | 1:57 ± 2:01 | ||
| 34 wk (n = 48) | Private room | 1:25 ± 1:33 | −1:59 |
| Open ward | 3:24 ± 4:23 | ||
| Term (n = 44) | Private room | 2:31 ± 2:10 | −2:50 |
| Open ward | 5:19 ± 3:16 | ||
| Amount of time spent with noise (in a 16-h period) | |||
| Birth (n = 52) | Private room | 6:35 ± 6:04 | −3:58 |
| Open ward | 10:33 ± 6:24 | ||
| 30 wk (n = 50) | Private room | 6:42 ± 5:51 | 0:54 |
| Open ward | 5:48 ± 5:12 | ||
| 34 wk (n = 48) | Private room | 6:33 ± 5:32 | 2:13 |
| Open ward | 4:20 ± 4:33 | ||
| Term (n = 44) | Private room | 2:02 ± 3:09 | 0:25 |
| Open ward | 1:37 ± 1:15 | ||
| Amount of time spent in silence (in a 16-h period) | |||
| Birth (n = 52) | Private room | 6:35 ± 5:01 | 3:36 |
| Open ward | 2:59 ± 4:03 | ||
| 30 wk (n = 50) | Private room | 7:45 ± 5:22 | 1:08 |
| Open ward | 6:37 ± 4:25 | ||
| 34 wk (n = 48) | Private room | 6:48 ± 5:20 | 0 |
| Open ward | 6:48 ± 4:26 | ||
| Term (n = 44) | Private room | 10:17 ± 3:59 | 3:00 |
| Open ward | 7:17 ± 2:55 | ||
| Average decibels | |||
| Birth (n = 52) | Private room | 58.9 ± 4.2 | −4.3 |
| Open ward | 63.2 ± 4.4 | ||
| 30 wk (n = 50) | Private room | 58.9 ± 5.0 | 0.7 |
| Open ward | 58.2 ± 1.4 | ||
| 34 wk (n = 48) | Private room | 58.1 ± 4.2 | −0.7 |
| Open ward | 58.8 ± 2.9 | ||
| Term (n = 44) | Private room | 56.1 ± 2.5 | −2.0 |
| Open ward | 58.1 ± 1.9 | ||
| Highest decibel stimulus | |||
| Birth (n = 52) | Private room | 87.0 ± 1.3 | 0.5 |
| Open ward | 86.5 ± 1.1 | ||
| 30 wk (n = 50) | Private room | 86.0 ± 1.4 | −1.0 |
| Open ward | 87.0 ± 1.3 | ||
| 34 wk (n = 48) | Private room | 86.9 ± 1.3 | −0.2 |
| Open ward | 87.1 ± 1.6 | ||
| Term (n = 44) | Private room | 87.3 ± 1.2 | 0.2 |
| Open ward | 87.1 ± 1.3 | ||
| Number of spoken words around the infant | |||
| Birth (n = 52) | Private room | 639.1 ± 671.4 | 61.0 |
| Open ward | 578.1 ± 892.2 | ||
| 30 wk (n = 50) | Private room | 696.2 ± 835.5 | 252.2 |
| Open ward | 444.0 ± 451.9 | ||
| 34 wk (n = 48) | Private room | 2110.7 ± 3030.9 | −241.1 |
| Open ward | 2351.8 ± 3349.8 | ||
| Term (n = 44) | Private room | 4987.2 ± 3721.4 | −198.3 |
| Open ward | 5185.5 ± 4162.3 | ||
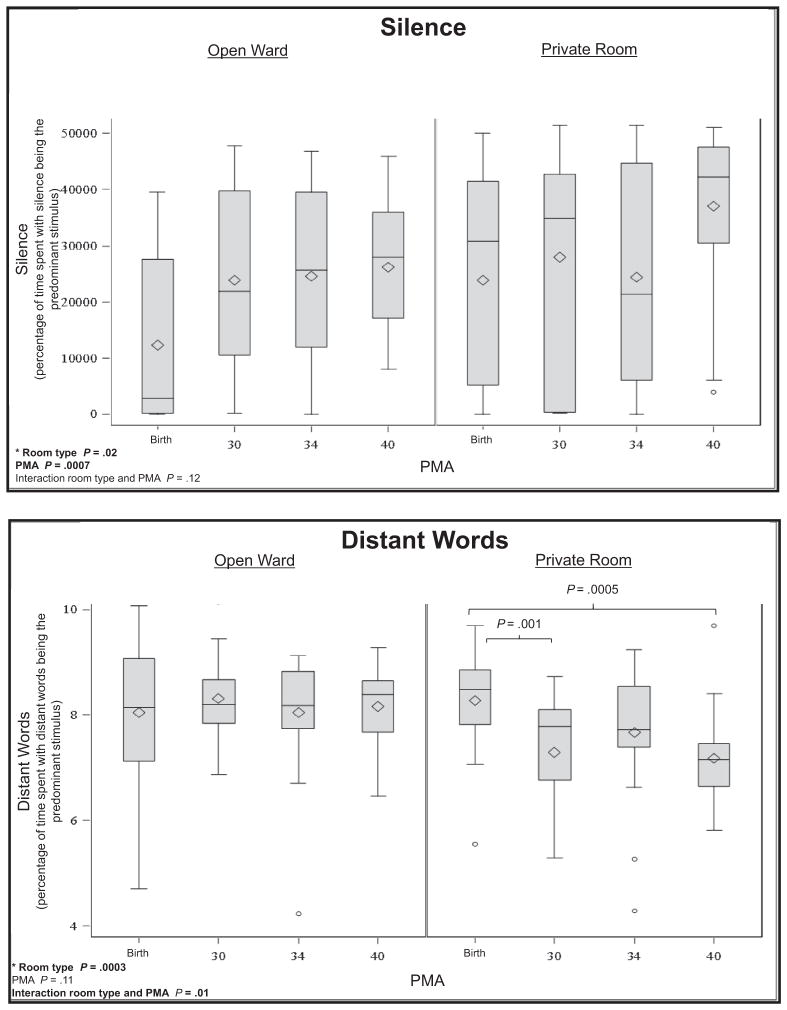
There were significant ANOVA interactions between PMA and room type for distant words (P = .01) and average decibels (P =.04), indicating that changes in auditory exposure across PMA were different for infants in private rooms compared with infants in the open ward (Figure). In private rooms, there were significant decreases in distant words from birth to 30 weeks PMA (P = .001) and between birth and term (P = .0005), whereas there were no significant differences in distant words across PMA in the open wards. In the private room, on average there were 33 minutes less distant language recorded at term compared with at birth. There were significant decreases in average decibels for infants in the open ward (P < .0001) and in private room (P = .01) between birth and term equivalent age. Average sound levels decreased by 5.2 decibels at term compared with birth in the open ward and 2.8 decibels at term compared with birth in the private room. There were no interactions of room type and PMA for meaningful language, adult words, electronic sound, silence, and noise.
Figure.
Auditory exposure across hospitalization in different NICU room types. *Main ANOVA effects for room type and PMA as well as interaction P values are provided at the bottom of each panel. Contrasts that are significant are reported within the figure when the interaction is significant (P < .05). The main effects of room type and PMA apply when the interaction is not significant.
In the ANOVA, there were significant main effects of PMA with meaningful language (P < .0001; average of 26 minutes more in a 16-hour period at term compared with birth), number of adult words (P < .0001; 4490 more words in a 16-hour period at term compared with birth), and electronic noise (P < .0001; 4 hours 2 minutes more electronic sound in a 16-hour period at term compared with birth). Silence increased (P = .0007; 4 hours 4 minutes more silence in a 16-hour period at term compared with at birth), and noise decreased (P < .0001; 1 hour 39 minutes more noise in a 16-hour period at term compared with at birth) across PMA. There were no differences in peak decibels across PMA.
The only main effect related to room type was silence. Compared with the open ward, there was significantly more silence in the private room (P = .02), with an average of 1.9 hours more silence in the private room in a 16-hour period. There were no other main effects related to room type.
Correlations at all 4 time points between medical, social, and environmental factors and auditory exposure are shown in Table III. Medical interventions, such as mechanical ventilation (P < .0001) were related to more noise in the environment, although parent presence (P = .009) and holding of the infant (P = .002) were related to greater adult word exposure. Table III lists all correlations with a P value of <.01. Unlisted correlations were not significant.
Table III.
Infant and maternal factors related to auditory exposure; concurrent environmental factors related to auditory exposure
| Type of sound related to infant and maternal factors Pearson correlation coefficients P value for correlation
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Meaningful words | Distant words | Electronic | Silence | Noise | Adult word count | Peak decibels | Average decibels | |
| Estimated gestational age | 0.21 | .07 | .09 | .19 | −.14 | .18 | −.004 | −.004 |
| .003 | .31 | .18 | .008 | .049 | .014 | .96 | .96 | |
| Birthweight | .23 | .11 | .10 | .28 | −.16 | .23 | −.10 | −.08 |
| .003 | .13 | .17 | <.0001 | .03 | .001 | .15 | .31 | |
| Breastmilk at discharge | .12 | .02 | .03 | .20 | −.10 | .05 | .08 | −.15 |
| .10 | .76 | .66 | .006 | .16 | .52 | .25 | .08 | |
| PDA | −.20 | −.13 | −.15 | −.24 | .13 | −.18 | −.03 | −.04 |
| .006 | .08 | .04 | .0008 | .07 | .01 | .73 | .63 | |
| Days on high frequency oscillator | −.14 | −.08 | −.09 | −.25 | .26 | −.10 | .11 | .08 |
| .06 | .31 | .23 | .0008 | .0003 | .18 | .13 | .33 | |
| Cesarean delivery | −.11 | .09 | −.05 | −.23 | .13 | −.11 | .05 | .06 |
| .14 | .24 | .47 | .002 | .07 | .13 | .51 | .50 | |
|
| ||||||||
|
Type of sound related to concurrent environmental factors Pearson correlation coefficients P value for correlation
|
||||||||
| Meaningful words | Distant words | Electronic | Silence | Noise | Adult word count | Peak decibels | Average decibels | |
|
| ||||||||
| Bed type, incubator | −.55 | .16 | −.41 | −.24 | .43 | −.57 | −.07 | .20 |
| <.0001 | .026 | <.0001 | .0008 | <.0001 | <.0001 | .35 | .02 | |
| Any pumps present | −.28 | −.003 | −.11 | −.15 | .13 | −.22 | −.08 | .11 |
| <.0001 | .96 | .12 | .03 | .08 | .002 | .31 | .23 | |
| Number of pumps | −.32 | .04 | −.28 | −.34 | .32 | −.30 | .04 | .30 |
| <.0001 | .59 | .0003 | <.0001 | <.0001 | .0001 | .69 | .002 | |
| CPAP | −.085 | .16 | .02 | −.02 | −.19 | −.12 | −.02 | .05 |
| .24 | .03 | .82 | .80 | .008 | .09 | .76 | .55 | |
| Mechanical vent | −.43 | −.16 | −.24 | −.40 | .41 | −.30 | −.07 | .23 |
| <.0001 | .03 | .0008 | <.0001 | <.0001 | <.0001 | .40 | .009 | |
| Oscillator | −.17 | −.12 | −.17 | −.22 | .18 | −.21 | .08 | .002 |
| .02 | .10 | .02 | .002 | .01 | .003 | .29 | .98 | |
| Nasal cannula | .26 | .03 | .047 | .20 | .06 | .17 | −.02 | −.25 |
| .0002 | .67 | .51 | .004 | .37 | .02 | .77 | .004 | |
| Parent present | .18 | −.05 | .12 | .14 | −.19 | .19 | .005 | −.03 |
| .013 | .53 | .09 | .05 | .008 | .009 | .95 | .75 | |
| Parent held infant | .29 | −.006 | .24 | .16 | −.19 | .27 | .13 | −.25 |
| .0008 | .95 | .005 | .07 | .03 | .002 | .15 | .02 | |
| Number of times held | .42 | .15 | .26 | .12 | −.32 | .40 | .13 | −.38 |
| <.0001 | .15 | .01 | .28 | .003 | <.0001 | .15 | .003 | |
| Private room | .07 | −.22 | −.04 | .18 | −.04 | −.002 | −.01 | −.14 |
| .35 | .002 | .54 | .01 | .56 | .98 | .88 | .09 | |
CPAP, continuous positive airway pressure; PDA, patent ductus arteriosus.
Table IV (available at www.jpeds.com) provides descriptions of sound in each room type based on whether parents visited on the day of the recording.
Table IV.
Language and sound in each room type, based on whether parents were visiting on the day of recording (in h:min)
| Meaningful words
| |||||
|---|---|---|---|---|---|
| Birth
|
34 wk PMA
|
||||
| Open ward n = 29 | Private room n = 23 | Open ward n = 24 | Private room n = 20 | ||
| Parent present on day of recording n = 37 | 0:05 ± 0:04 | 0:08 ± 0:08 | Parent present on day of recording n = 32 | 0:16 ± 0:20: | 0:20 ± 0:21 |
| Parent not present on day of recording n = 15 | 0:07 ± 0:14 | 0:07 ± 0:08 | Parent not present on day of recording n = 16 | 0:15 ± 0:23 | 0:09 ± 0:12 |
|
| |||||
|
30 wk PMA
|
Term
|
||||
| Open ward n = 27 | Private room n = 23 | Open ward n = 24 | Private room n = 20 | ||
|
| |||||
| Parent present on day of recording n = 29 | 0:05 ± 0:05 | 0:07 ± 0:04 | Parent Present on day of recording n = 32 | 0:30 ± 0:19 | 0:38 ± 0:27 |
| Parent not present on day of recording n = 21 | 0:06 ± 0:05 | 0:04 ± 0:03 | Parent not present on day of recording n = 12 | 0:34 ± 0:30 | 0:26 ± 0:13 |
|
| |||||
|
Distant words
| |||||
|
Birth
|
34 wk PMA
|
||||
| Open ward n = 29 | Private room n = 23 | Open ward n = 26 | Private room n = 22 | ||
|
| |||||
| Parent present on day of recording n = 37 | 1:32 ± 1:36 | 1:56 ± 1:23 | Parent present on day of recording n = 32 | 1:01 ± 0:42 | 0:47 ± 0:42 |
| Parent not present on day of recording n = 15 | 1:04 ± 1:12 | 1:25 ± 1:34 | Parent not present on day of recording n = 16 | 1:28 ± 0:46 | 1:17 ± 0:51 |
|
| |||||
|
30 wk PMA
|
Term
|
||||
| Open ward n = 27 | Private room n = 23 | Open ward n = 24 | Private room n = 20 | ||
|
| |||||
| Parent present on day of recording n = 29 | 1:59 ± 1:55 | 0:39 ± 0:29 | Parent present on day of recording n = 32 | 1:00 ± 0:33 | 0:23 ± 0:16 |
| Parent not present on day of recording n = 21 | 1:09 ± 0:51 | 0:26 ± 0:25 | Parent not present on day of recording n = 12 | 1:47 ± 1:05 | 1:10 ± 1:52 |
|
| |||||
|
Electronic sounds
| |||||
|
Birth
|
34 wk PMA
|
||||
| Open ward n = 29 | Private room n = 23 | Open ward n = 26 | Private room n = 22 | ||
|
| |||||
| Parent present on day of recording n = 37 | 1:03 ± 1:44 | 0:53 ± 0:51 | Parent present on day of recording n = 32 | 3:34 ± 4:27 | 1:32 ± 1:44 |
| Parent not present on day of recording n = 15 | 0:30 ± 0:48 | 1:04 ± 1:37 | Parent not present on day of recording n = 16 | 2:48 ± 4:28 | 1:12 ± 1:09 |
|
| |||||
|
30 wk PMA
|
Term
|
||||
| Open ward n = 27 | Private room n = 23 | Open ward n = 24 | Private room n = 20 | ||
|
| |||||
| Parent present on day of recording n = 29 | 2:11 ± 1:52 | 0:41 ± 0:47 | Parent present on day of recording n = 32 | 6:19 ± 3:06 | 2:30 ± 2:04 |
| Parent not present on day of recording n = 21 | 1:47 ± 2:10 | 1:16 ± 1:23 | Parent not present on day of recording n = 12 | 2:54 ± 2:22 | 2:33 ± 2:44 |
|
| |||||
|
Noise
| |||||
|
Birth
|
34 wk PMA
|
||||
| Open ward n = 29 | Private room n = 23 | Open ward n = 26 | Private room n = 22 | ||
|
| |||||
| Parent present on day of recording n = 37 | 10:02 ± 6:35 | 3:35 ± 5:22 | Parent present on day of recording n = 32 | 3:24 ± 3:50 | 5:47 ± 5:42 |
| Parent not present on day of recording n = 15 | 12:32 ± 5:44 | 10:48 ± 4:38 | Parent not present on day of recording n = 16 | 6:06 ± 5:28 | 8:11 ± 5:12 |
|
| |||||
|
30 wk PMA
|
Term
|
||||
| Open ward n = 27 | Private room n = 23 | Open ward n = 24 | Private room n = 20 | ||
|
| |||||
| Parent present on day of recording n = 29 | 5:50 ± 5:49 | 6:15 ± 5:48 | Parent present on day of recording n = 32 | 1:31 ± 1:09 | 1:44 ± 2:55 |
| Parent not present on day of recording n = 21 | 5:55 ± 4:51 | 7:59 ± 6:21 | Parent not present on day of recording n = 12 | 1:52 ± 1:33 | 2:55 ± 4:02 |
|
| |||||
|
Silence
| |||||
|
Birth
|
34 wk PMA
|
||||
| Open ward n = 29 | Private room n = 23 | Open ward n = 26 | Private room n = 22 | ||
|
| |||||
| Parent present on day of recording n = 37 | 3:17 ± 4:10 | 9:09 ± 4:30 | Parent present on day of recording n = 32 | 7:34 ± 4:31 | 7:33 ± 5:17 |
| Parent not present on day of recording n = 15 | 1:47 ± 3:38 | 2:37 ± 2:40 | Parent not present on day of recording n = 16 | 5:22 ± 4:07 | 5:11 ± 5:29 |
|
| |||||
|
30 wk PMA
|
Term
|
||||
| Open ward n = 27 | Private room n = 23 | Open ward n = 24 | Private room n = 20 | ||
|
| |||||
| Parent present on day of recording n = 29 | 5:54 ± 4:36 | 8:17 ± 5:27 | Parent present on day of recording n = 32 | 6:37 ± 2:46 | 10:44 ± 3:39 |
| Parent not present on day of recording n = 21 | 7:12 ± 4:20 | 6:15 ± 5:17 | Parent not present on day of recording n = 12 | 8:53 ± 2:49 | 8:56 ± 5:05 |
|
| |||||
|
Peak decibels
| |||||
|
Birth
|
34 wk PMA
|
||||
| Open ward n = 29 | Private room n = 23 | Open ward n = 26 | Private room n = 22 | ||
|
| |||||
| Parent present on day of recording n = 37 | 86.7 ± 1.1 | 86.8 ± 1.3 | Parent present on day of recording n = 32 | 87.1 ± 1.8 | 86.7 ± 1.4 |
| Parent not present on day of recording n = 15 | 86.0 ± 1.2 | 87.3 ± 1.3 | Parent not present on day of recording n = 16 | 87.0 ± 1.2 | 87.2 ± 1.3 |
|
| |||||
|
30 wk PMA
|
Term
|
||||
| Open ward n = 27 | Private room n = 23 | Open ward n = 24 | Private room n = 20 | ||
|
| |||||
| Parent present on day of recording n = 29 | 87.1 ± 1.6 | 86.0 ± 1.4 | Parent present on day of recording n = 32 | 87.3 ± 1.4 | 87.5 ± 1.1 |
| Parent not present on day of recording n = 21 | 86.9 ± 1.0 | 86.2 ± 1.5 | Parent not present on day of recording n = 12 | 86.6 ± 1.1 | 86.7 ± 1.5 |
|
| |||||
|
Average decibels
| |||||
|
Birth
|
34 wk PMA
|
||||
| Open ward n = 29 | Private room n = 23 | Open ward n = 26 | Private room n = 22 | ||
|
| |||||
| Parent present on day of recording n = 37 | 63.7 ± 4.6 | 58.3 ± 3.0 | Parent present on day of recording n = 32 | 58.6 ± 2.8 | 58.3 ± 5.2 |
| Parent not present on day of recording n = 15 | 60.7 ± 3.3 | 59.8 ± 5.6 | Parent not present on day of recording n = 16 | 59.0 ± 3.2 | 57.5 ± 1.1 |
|
| |||||
|
30 wk PMA
|
Term
|
||||
| Open ward n = 27 | Private room n = 23 | Open ward n = 24 | Private room n = 20 | ||
|
| |||||
| Parent present on day of recording n = 29 | 58.6 ± 1.5 | Parent present on day of recording n = 32 | 58.1 ± 1.9 | 56.4 ± 2.7 | |
| Parent not present on day of recording n = 21 | 58.0 ± 1.4 | Parent not present on day of recording n = 12 | 57.9 ± 2.2 | 55.4 ± 2.1 | |
|
| |||||
|
Number of adult words in 16-h period
| |||||
|
Birth
|
34 wk PMA
|
||||
| Open ward n = 29 | Private room n = 23 | Open ward n = 26 | Private room n = 22 | ||
|
| |||||
| Parent present on day of recording n = 37 | 504.2 ± 454.8 | 847.6 ± 727.7 | Parent present on day of recording n = 32 | 2445.5 ± 3221.3 | 2783.0 ± 3470.7 |
| Parent not present on day of recording n = 15 | 861.3 ± 1850.9 | 314.8 ± 429.8 | Parent not present on day of recording n = 16 | 2174.8 ± 3775.9 | 670.1 ± 703.5 |
|
| |||||
|
30 wk PMA
|
Term
|
||||
| Open ward n = 27 | Private room n = 23 | Open ward n = 24 | Private room n = 20 | ||
|
| |||||
| Parent present on day of recording n = 29 | 440.6 ± 536.1 | 822.7 ± 923.8 | Parent present on day of recording n = 32 | 4727.1 ± 3216.7 | 5634.5 ± 3959.5 |
| Parent not present on day of recording n = 21 | 446.7 ± 391.6 | 338.0 ± 364.3 | Parent not present on day of recording n = 12 | 6298.9 ± 6064.6 | 3045.2 ± 2150.7 |
Discussion
The key findings of this study are that there was less auditory exposure in private rooms compared with the open ward, with an average of 1.9 hours more silence in a 16-hour period in the private room compared with the open ward. Infants in both types of rooms were exposed to seemingly very little meaningful language, and total language exposure did not differ across the 2 room types. More language exposure was observed with infants whose parents were present and engaged in holding. Medical equipment (such as high-frequency oscillator ventilation and oxygen delivered via a nasal cannula) was associated with more noise exposure. Medically fragile infants were exposed to more sound, especially during the initial, medically complicated early weeks of their NICU stay. Auditory exposure decreased and language exposure increased as infants grew closer to their due dates. Although auditory exposure may have potential benefits for the preterm infant in the NICU, the average sound levels in the NICU in this study exceeded the American Academy of Pediatrics recommendations.
Preterm infants may spend months in the NICU during a critical period of brain development.20,21 Previous research has demonstrated that auditory exposure during the final trimester of pregnancy may be necessary for brain development and optimal outcomes and, therefore, equally important in the weeks spent in the NICU for the preterm infant.22 During this important period of development, substantial changes occur in response to the auditory environment. The connections between the cochlea and the brainstem are established by 24–25 weeks gestation, and between the temporal lobe and the auditory cortex (non-primary) as early as 30–31 weeks gestation.23 Auditory evoked potentials are evident by the 28th week of gestation, and there is a marked reduction in response as neural pathways mature from 28 through 34 weeks gestation.24 It has also been proposed that experiencing mother’s voice before birth is critical for typical brain development25,26 and that the timed auditory input of low-frequency sounds experienced in utero followed by the addition of higher frequency sounds experienced ex utero is important for normal auditory development.27
Our group has reported greater impairment in language outcomes, as well as differences in early brain development, among infants hospitalized in private rooms in a NICU with low parent visitation and holding rates.12 The present study builds on these findings by defining the auditory environment in the 2 different room types and identifying a plausible mechanism for the observation of poorer language outcome in infants in the private rooms. At term equivalent age, there was an average of 3 hours more silence in a 16-hour period in the private room (Table II), which is roughly 30% more silence than in the open ward. The finding of decreased auditory exposure in this study is consistent with other studies reporting diminished sound in the NICU private room.3,28
Although we were unable to demonstrate differences in language exposure across the 2 room types in the current study, previous work has defined the importance of language exposure for the development of preterm infants. Preterm infants exposed to more language demonstrate more vocalizations before NICU discharge and higher scores on tests of language abilities in early childhood.14 Infants with mothers with low communication skills also have been shown to have relatively poor language skills in early childhood.29 More language exposure in the NICU is associated with better developmental outcome, and more auditory exposure in the NICU environment has been associated with better language and motor outcomes.14,17 Correlations between parent presence and holding and language exposure were identified in the current study. These findings could indicate the need for interventions that help inform and support parents in engaging with their infants. In addition, these findings are a potential explanation for the positive outcomes of infants cared for in private rooms in a NICU with high rates of parent engagement.9 Another recent report showed that good parent involvement in either room type was related to better cognitive and language outcomes.30 These data lend support to targeting interventions towards parents, who can significantly alter the early experiences of preterm infants in the NICU. This in turn may have a lasting positive impact on the development of the child.
Although sound and language exposure have substantial benefits for the infant, too much noise can also be harmful. The American Academy of Pediatrics recommends that sound levels in the NICU not exceed 45 decibels.1 This recommendation is based on findings of hearing loss in children who were cared for in the NICU as well as changes in the behavioral and physiological responses of infants exposed to high levels of noise in the NICU.31–33 Similar to studies from other NICUs, the average sound levels in this study exceeded American Academy of Pediatrics recommendations by nearly 20 decibels.34,35
Early medical interventions impact the auditory environment. Specifically, mechanical ventilation, high-frequency oscillatory ventilation and oxygen delivery via nasal cannula were associated with increased noise exposure. Increased decibel exposure caused by high-frequency ventilation has been found to increase the likelihood of progressive hearing loss in infants who are already at risk for hearing impairments because of oxygen deprivation.36,37 The effects of medical interventions on sound levels and noise exposure were most prominent in the early weeks of hospitalization. The ideal NICU environment may consist of positive language and environmental sound as well as protection from loud medical equipment, but more work is needed to assess the effects of sound, including only sound levels that are within the American Academy of Pediatrics recommendations.
The limitations of the current study include the relatively small sample size and a cohort of medically fragile premature infants. This study was exploratory study and did not employ a randomized design. Further, the analyses relied on multiple comparisons of outcome, which increases the risk of a type I error. However, we used a strict P value of .01 to identify predictive variables because of multiple analyses conducted. Several limitations result from use of the LENA device to characterize the sound environment. The LENA relies on an algorithm of sound, which labels the most prevalent sound at a given point in time, thus, making it impossible to distinguish multiple sounds happening concurrently. In addition, the LENA cannot distinguish music from other electronic noise (ie, alarms), which complicates the interpretation of electronic noise as an outcome measure. The device was also used at 4 specific time points, which may not have been representative of the entire NICU stay. Because there was a sign at the bedside indicating that recordings were taking place, this could have impacted healthcare professional and parent behavior. Findings from this study may not be generalized to the overall NICU population, as the infants recruited for this study were part of a cerebral monitoring study and represented a more medically complex and fragile population. In addition, this study was conducted in an urban NICU, with families who had high rates of social challenges that may have limited their engagement in the NICU. Findings may be different in settings in which parents have the resources and education that supports their full involvement in care within the NICU, and this is important when determining the implications of these findings. Careful attention to a NICU’s size, culture, and parental involvement is important when putting research findings into context. Despite these limitations, the study sets the stage for further inquiries into the auditory environment of the NICU.
The auditory environment is modifiable, and more research is needed to identify the optimal sensory environment for preterm infants to support neurodevelopment. Further research is needed to define the optimal sound environment, especially in private room NICUs with the potential for complete auditory abatement. As the optimal auditory environment is defined, it will be important to develop strategies to help infants when families have limited time with their infants. Defining the optimal auditory exposure ultimately may be beneficial for family engagement in the NICU. Additional research is needed to identify possible modifications to medical equipment to decrease current NICU sound levels and to assess the effect of such interventions. Although early sound may be critical, there are also many influences on outcomes, which can include inherent language ability, maternal education, and environmental influences post-NICU discharge. Studying the complex contribution of prematurity, medical illness, genetics, and auditory exposures during childhood will be important for future efforts to improve outcomes in preterm infants.
Acknowledgments
We thank Carolyn Baum, Katie Bogan, Jeffrey Neil, Sarah Oberle, Jessica Roussin, Chris Smyser, David Van Essen, the CORRT advisory team; Rebecca Armitage, Hayley Chrzastowski, Kelsey Dewey, Felicia Foci, Tess Greene, Rachel Harris, Elizabeth Heiny, Odo Nwabara, Katie Ross, Justin Ryckman, Gabriel Blenden, Kimberly Schwaegael-Wiskamp, Tiffany Rounsville, and Elaine Ward.
Supported by the National Institute of Health Comprehensive Opportunities for Rehabilitation Research Training (K12 HD055931), the Barnes-Jewish Hospital Foundation, and the Washington University Institute of Clinical and Translational Sciences Clinical and Translational Funding Program (National Institutes of Health/National Center for Advancing Translational Sciences UL1 TR000448), and Eunice Kennedy Shriver National Institute of Child Health and Human Development (U54 HD087011) to the Intellectual and Developmental Disabilities Research Center at Washington University. T.I. served as a member of the Editorial Board of The Journal of Pediatrics (2007–2015).
Glossary
- CRIB
Clinical Risk Index for Babies
- LENA
Language Environmental Acquisition
- NICU
Neonatal intensive care unit
- PMA
Postmenstrual age
Footnotes
The other authors declare no conflicts of interest.
References
- 1.Noise: a hazard for the fetus and newborn. American Academy of Pediatrics. Committee on Environmental Health. Pediatrics. 1997;100:724–7. [PubMed] [Google Scholar]
- 2.Ramm K, Mannix T, Parry Y, Gaffney MP. A comparison of sound levels in open plan versus pods in a neonatal intensive care unit. HERD. 2016 doi: 10.1177/1937586716668636. [DOI] [PubMed] [Google Scholar]
- 3.Liu WF. Comparing sound measurements in the single-family room with open-unit design neonatal intensive care unit: the impact of equipment noise. J Perinatol. 2012;32:368–73. doi: 10.1038/jp.2011.103. [DOI] [PubMed] [Google Scholar]
- 4.Lasky RE, Williams AL. Noise and light exposures for extremely low birth weight newborns during their stay in the neonatal intensive care unit. Pediatrics. 2009;123:540–6. doi: 10.1542/peds.2007-3418. [DOI] [PubMed] [Google Scholar]
- 5.Smith GC, Gutovich J, Smyser C, Pineda R, Newnham C, Tjoeng TH, et al. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann Neurol. 2011;70:541–9. doi: 10.1002/ana.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu WF, Laudert S, Perkins B, MacMillan-York E, Martin S, Graven S. The development of potentially better practices to support the neurodevelopment of infants in the NICU. J Perinatol. 2007;27:S48–74. doi: 10.1038/sj.jp.7211844. [DOI] [PubMed] [Google Scholar]
- 7.Hassanein SM, El Raggal NM, Shalaby AA. Neonatal nursery noise: practice-based learning and improvement. J Matern Fetal Neonatal Med. 2013;26:392–5. doi: 10.3109/14767058.2012.733759. [DOI] [PubMed] [Google Scholar]
- 8.White RD. The newborn intensive care unit environment of care: how we got here, where we’re headed, and why. Semin Perinatol. 2011;35:2–7. doi: 10.1053/j.semperi.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Lester BM, Hawes K, Abar B, Sullivan M, Miller R, Bigsby R, et al. Single-family room care and neurobehavioral and medical outcomes in preterm infants. Pediatrics. 2014;134:754–60. doi: 10.1542/peds.2013-4252. [DOI] [PubMed] [Google Scholar]
- 10.Morelius E, Brostrom EB, Westrup B, Sarman I, Ortenstrand A. The Stockholm Neonatal Family-Centered Care Study: effects on salivary cortisol in infants and their mothers. Early Hum Dev. 2012;88:575–81. doi: 10.1016/j.earlhumdev.2011.12.033. [DOI] [PubMed] [Google Scholar]
- 11.Ortenstrand A, Westrup B, Brostrom EB, Sarman I, Akerstrom S, Brune T, et al. The Stockholm Neonatal Family Centered Care Study: effects on length of stay and infant morbidity. Pediatrics. 2010;125:e278–85. doi: 10.1542/peds.2009-1511. [DOI] [PubMed] [Google Scholar]
- 12.Pineda RG, Neil J, Dierker D, Smyser CD, Wallendorf M, Kidokoro H, et al. Alterations in brain structure and neurodevelopmental outcome in preterm infants hospitalized in different neonatal intensive care unit environments. J Pediatr. 2014;164:52–60. e2. doi: 10.1016/j.jpeds.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webb AR, Heller HT, Benson CB, Lahav A. Mother’s voice and heartbeat sounds elicit auditory plasticity in the human brain before full gestation. Proc Natl Acad Sci USA. 2015;112:3152–7. doi: 10.1073/pnas.1414924112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caskey M, Stephens B, Tucker R, Vohr B. Importance of parent talk on the development of preterm infant vocalizations. Pediatrics. 2011;128:910–6. doi: 10.1542/peds.2011-0609. [DOI] [PubMed] [Google Scholar]
- 15.Doheny L, Hurwitz S, Insoft R, Ringer S, Lahav A. Exposure to biological maternal sounds improves cardiorespiratory regulation in extremely preterm infants. J Matern Fetal Neonatal Med. 2012;25:1591–4. doi: 10.3109/14767058.2011.648237. [DOI] [PubMed] [Google Scholar]
- 16.Filippa M, Devouche E, Arioni C, Imberty M, Gratier M. Live maternal speech and singing have beneficial effects on hospitalized preterm infants. Acta Paediatr. 2013;102:1017–20. doi: 10.1111/apa.12356. [DOI] [PubMed] [Google Scholar]
- 17.Stromswold K, Sheffield E. Neonatal intensive care unit noise & language development. Rutgers University Center for Cognitive Science Technical Report. 2004:1–15. [Google Scholar]
- 18.LENA Foundation. 2011 [Google Scholar]
- 19.The CRIB (clinical risk index for babies) score: a tool for assessing initial neonatal risk and comparing performance of neonatal intensive care units. The International Neonatal Network. Lancet. 1993;342:193–8. [PubMed] [Google Scholar]
- 20.Mewes AU, Huppi PS, Als H, Rybicki FJ, Inder TE, McAnulty GB, et al. Regional brain development in serial magnetic resonance imaging of low-risk preterm infants. Pediatrics. 2006;118:23–33. doi: 10.1542/peds.2005-2675. [DOI] [PubMed] [Google Scholar]
- 21.Engelhardt E, Inder TE, Alexopoulos D, Dierker DL, Hill J, Van Essen D, et al. Regional impairments of cortical folding in premature infants. Ann Neurol. 2015;77:154–62. doi: 10.1002/ana.24313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graven SN, Browne JV. Auditory development in the fetus and infant. Newborn Infant Nurs Rev. 2008;8:187–93. [Google Scholar]
- 23.Weitzman L, Graziani L, Duhamel L. Maturation and topography of the auditory evoked response of the prematurely born infant. Electroencephalogr Clin Neurophysiol. 1967;23:82–3. [PubMed] [Google Scholar]
- 24.Starr A, Amlie RN, Martin WH, Sanders S. Development of auditory function in newborn infants revealed by auditory brainstem potentials. Pediatrics. 1977;60:831–9. [PubMed] [Google Scholar]
- 25.DeCasper A, Spence M. Prenatal maternal speech influences newborns’ perception of speech sounds. Infant Behav Dev. 1985;9:133–50. [Google Scholar]
- 26.Fifer WP, Moon CM. The role of mother’s voice in the organization of brain function in the newborn. Acta Paediatr Suppl. 1994;397:86–93. doi: 10.1111/j.1651-2227.1994.tb13270.x. [DOI] [PubMed] [Google Scholar]
- 27.Gottleib G. Ontogenesis of sensory function in birds and mammals. In: Tobach E, Aronson L, Shaw E, editors. The biopsychology of development. New York (NY): Academic Press; 1971. pp. 66–182. [Google Scholar]
- 28.Caskey M, Tucker R, Vohr B. Language environment in a single family room NICU. Pediatric Academic Societies; Boston, MA: 2012. [Google Scholar]
- 29.Vohr B, Pierre LS, Topol D, Jodoin-Krauzyk J, Bloome J, Tucker R. Association of maternal communicative behavior with child vocabulary at 18–24 months for children with congenital hearing loss. Early Hum Dev. 2010;86:255–60. doi: 10.1016/j.earlhumdev.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Lester BM, Salisbury AL, Hawes K, Dansereau LM, Bigsby R, Laptook A, et al. 18-month follow-up of infants cared for in a single-family room neonatal intensive care unit. J Pediatr. 2016;177:84–9. doi: 10.1016/j.jpeds.2016.06.069. [DOI] [PubMed] [Google Scholar]
- 31.Winkel S, Bonding P, Larsen PK, Roosen J. Possible effects of kanamycin and incubation in newborn children with low birth weight. Acta Paediatr Scand. 1978;67:709–15. doi: 10.1111/j.1651-2227.1978.tb16248.x. [DOI] [PubMed] [Google Scholar]
- 32.Stennert E, Schulte F, Vollrath M, Brunner E, Frauenrath C. The etiology of neurosensory hearing defects in preterm infants. Arch Otorhinolaryngol. 1978;221:171–82. doi: 10.1007/BF01886292. [DOI] [PubMed] [Google Scholar]
- 33.D’Souza SW, McCartney E, Nolan M, Taylor IG. Hearing, speech, and language in survivors of severe perinatal asphyxia. Arch Dis Child. 1981;56:245–52. doi: 10.1136/adc.56.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krueger C, Wall S, Parker L, Nealis R. Elevated sound levels within a busy NICU. Neonatal Netw. 2005;24:33–7. doi: 10.1891/0730-0832.24.6.33. [DOI] [PubMed] [Google Scholar]
- 35.Darcy AE, Hancock LE, Ware EJ. A descriptive study of noise in the neonatal intensive care unit. Ambient levels and perceptions of contributing factors. Adv Neonatal Care. 2008;8:165–75. doi: 10.1097/01.ANC.0000324341.24841.6e. [DOI] [PubMed] [Google Scholar]
- 36.Lasky RE, Wiorek L, Becker TR. Hearing loss in survivors of neonatal extracorporeal membrane oxygenation (ECMO) therapy and high-frequency oscillatory (HFO) therapy. J Am Acad Audiol. 1998;9:47–58. [PubMed] [Google Scholar]
- 37.van Dommelen P, Mohangoo AD, Verkerk PH, van der Ploeg CP, van Straaten HL. Risk indicators for hearing loss in infants treated in different neonatal intensive care units. Acta Paediatr. 2010;99:344–9. doi: 10.1111/j.1651-2227.2009.01614.x. [DOI] [PubMed] [Google Scholar]