Abstract
Importance
While progress continues to be made in the field of stem cell regenerative medicine for the treatment of cardiovascular disease, significant barriers to clinical translation still exist that have thwarted the delivery of cell therapy to the bedside.
Objective
The purpose of this review is to summarize the major current hurdles for the clinical implementation of stem cell therapy and discuss potential strategies to overcome them.
Evidence Review
Information for this review was obtained through a search of PubMed and the Cochrane database for English language studies published between January 1, 2000 and June 15, 2016. Ten randomized clinical trials and eight systematic reviews were included in this review.
Findings
One of the major clinical hurdles facing the routine implementation of stem cell therapy is the limited and inconsistent benefit observed thus far. Reasons for this are unclear but may be due to poor cell retention and survival, as suggested by numerous preclinical studies and a handful of human studies incorporating cell fate imaging. Additional cell fate imaging studies in humans are needed to determine how these factors contribute to limited efficacy. Treatment strategies to address poor cell retention and survival are under investigation and include the following: 1) co-administering of immunosuppressive and pro-survival agents, 2) delivering cardioprotective factors packaged in exosomes rather than the cells themselves, and 3) using tissue engineering strategies to provide structural support for cells. If larger grafts are achieved using the aforementioned strategies, it will be imperative to carefully monitor the potential risks of tumorigenicity, immunogenicity, and arrhythmogenicity.
Conclusions and Relevance
Despite important achievements to date, stem cell therapy is not yet ready for routine clinical implementation. Significant research is still needed to address the clinical hurdles outlined herein before the next wave of large clinical trials is underway.
Introduction
Stem cell therapy still holds promise despite conflicting reports of efficacy from recent adult stem cell clinical trials.1–7 Like any high-risk, high-reward scientific endeavor, initial efforts are fraught with challenges, but the scientific community and general public remain optimistic that continuing effort will realize the full potential of stem cells. In this review, we outline the major clinical hurdles facing stem cell regenerative therapy and potential strategies to overcome these obstacles.
Major Clinical Hurdles for Routine Clinical Implementation
Recent clinical trials have found that transplantation of adult bone marrow mononuclear cells (BMMNCs) produces only modest benefit, ranging from an improvement of 2–5% in left ventricular ejection fraction (LVEF),4,7 a degree of change with uncertain clinical significance given the inherent variation of traditional imaging modalities. Although efficacy questions remain, these studies have confirmed that the administration of these cells appears to be safe; however, the risks of tumorigenicity, immunogenicity, and arrhythmogenicity may increase if larger grafts are achieved. In the following section, we will highlight the major clinical hurdles facing stem cell regenerative therapy, including our incomplete knowledge of cell fate post-delivery, poor cell survival and engraftment, and major safety concerns. Additional economic, regulatory, and ethical hurdles have been described in other comprehensive reviews.8
Lack of knowledge regarding the fate of cells post-delivery
One of the primary challenges of bringing stem cell therapy into the clinic is our limited knowledge of cell fate after delivery in humans. Unlike drugs whose presence in the blood can be used to correlate with response, for stem cell therapy, we need to be able to locate the cells, quantify their number, evaluate their viability, and determine whether they could integrate into the host tissue to correlate dose with benefit.
Without sufficient knowledge about cell fate after delivery, it has been difficult to interpret previous dose response studies. Of the five clinical studies evaluating the relationship between cell dose and efficacy,9–13 two studies have shown an inverse relationship,10,11 whereas the other three have shown a positive dose relationship.9,12,13 In a study of 167 patients with refractory angina who received transendocardial injection of autologous CD34+ cells, Losordo et al. observed a significant improvement in angina frequency and exercise tolerance in the low dose group compared to the high dose group (e.g., 1×105 vs. 5×105 cells per kg).10 Similarly, Hare et al. found a significantly greater increase in LVEF and reduction in infarct size in patients with ischemic cardiomyopathy (ICM) receiving transendocardial injection of only 20 million mesenchymal stem cells (MSCs) compared to those receiving higher doses of 100 and 200 million.11 By contrast, after delivering escalating doses of 5, 10, or 15 million autologous CD34+ BMMCs into the myocardium of patients with ST elevation MI via intracoronary injection, Quyyumi et al. found that patients with ≥≥10 million cells had the greatest improvement in myocardial perfusion.9 Although the reasons for these discrepant findings remain unclear, one possible explanation is that cell influx and retention at the target site might vary depending on the operators, the target patients, and even the delivery methods. However, these studies, like many others published to date, contain little information on whether these cells arrived and were retained at the site of injury, leaving many questions unanswered.
To address this limitation, Vrtovec et al. performed two sequential studies in patients with nonischemic cardiomyopathy (NICM) by imaging cell fate shortly after delivery.12,13 Patients with evidence of greater myocardial homing via intracoronary injection or higher retention rates via transendocardial injection had better improvement in LVEF. These studies demonstrated that the ability to track cells post-delivery plays a critical role in understanding the dose-response relationship and ultimately predicting overall clinical efficacy. Future studies should examine cell fate to better define the relationship between cell survival/retention and clinical outcomes.
Tracking cell fate may also help determine the optimal timing of cell delivery following MI, a period during which the tissue microenvironment might be hostile to cells and could lead to cell death.14 The two randomized controlled trials sponsored by the Cardiovascular Cell Therapy Research Network (e.g., Timing In Myocardial infarction Evaluation [TIME],15,16 and Late-TIME trials17) that were designed to evaluate whether timing of delivery affects outcome failed to show a significant benefit from cell therapy when cells were transplanted early (e.g., day 3 and 7) or late (e.g., a mean of 17 days). Neither TIME15,16 nor Late-TIME17 tracked cell fate after delivery, so it remains unclear whether the observed lack of benefit was due to poor cell retention/survival or whether timing truly had no significant effect on efficacy.
Finally, understanding the fate of cells post-delivery can help identify the optimal mode of delivery. In clinical trials to date, cells have been delivered via intravenous injection, catheter-based intracoronary injection into a culprit vessel, catheter-directed transendocardial injection, or transepicardial injection at the time of cardiac surgery. The choice of delivery mode is often based on the target patient cohort and their specific disease. While an intracoronary approach may be best for patients with acute myocardial infarction (AMI), a transendocardial or intramyocardial approach is better suited for patients with chronic ICM who may have completely occluded arteries. In a preliminary study of 40 patients, Vrtovec et al. found that patients receiving transendocardial injections of CD34+ cells had higher myocardial retention rates than those receiving intracoronary injections,13 echoing findings from several preclinical studies.18,19
Few studies, however, have directly correlated cellular retention rates after intravenous, intracoronary, or intramyocardial delivery with efficacy. Replicating findings from two stem cell tracking studies performed in large animal studies,18,20 Vrtovec et al. demonstrated that patients with NICM, after treatment with transendocardial injection of BM-derived CD34+ cells, had higher myocardial cell retention rates with better ventricular function and exercise capacity compared to patients who underwent intracoronary injections.13 The study, however, was small in size (n=40) and conducted as an open-label in a single center. Larger multi-center clinical studies that incorporate cell fate imaging are needed to investigate the comparative effectiveness of these delivery methods.
Poor engraftment and survival of cells, limiting their potential efficacy
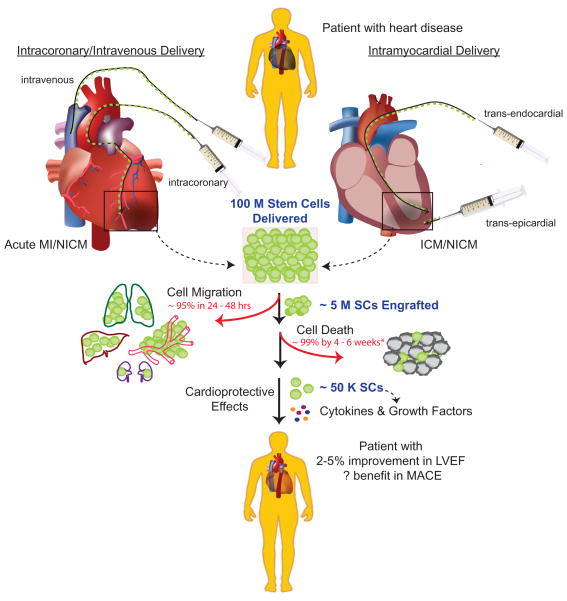
Arguably the most significant barrier for clinical translation is poor cell engraftment and survival, which has been reported in multiple small animal studies and a handful of large animal studies (including humans) incorporating stem cell fate imaging.21–23 As shown in Figure 1, once cells are delivered to the patient, a percentage of cells may arrive at the target area and engraft while others may migrate to distant organs. Those that remain in the heart, however, may or may not survive long-term to provide cardio-protection. Indeed, the handful of human studies evaluating stem cell fate show that only 5% of stem cells engraft within 24 hours, but longer-term data in humans are limited.24 Long-term data from small animal studies suggest that cells are present near the site of injury for only a short time, with the majority migrating to distant organs and then dying in situ.19,24 Taken together, these results suggest that even if a patient receives 100 million cells at the time of delivery, perhaps only ~50,000 will survive in the heart at 4–6 weeks to contribute to its repair.
Figure 1. A major clinical hurdle is poor stem cell survival and retention post-delivery.
Most trials published to date have employed intravenous, intracoronary, or intramyocardial delivery of autologous bone marrow mononuclear cells to treat both ischemic and non-ischemic heart diseases. Although a dose of 100 million cells are delivered, typically only a small fraction of cells (<5%) are retained at the site of transplantation after 24–48 hours in humans. Of those that are retained at the site of transplantation, many (~99%) do not survive beyond 4–6 weeks, as demonstrated in preclinical studies. Poor cell retention and survival likely limit improvement in LVEF and the incidence of MACE, although these issues have not been well studied (*denotes data that are only available in humans). SCs: stem cells; LVEF: left ventricular function; MACE: major adverse cardiac events.
Not surprisingly, the lack of viable cells in the infarct area after delivery has been associated with reduced efficacy in a rodent model,25 which was later confirmed in human studies.12 Although an intuitive approach, large clinical trials have yet to incorporate in vivo imaging to better correlate factors such as cell retention, engraftment, and survival in individual patients with their responses afterwards.
Risk of Tumorigenicity, Immunogenicity, and Arrhythmogenicity
Before cells reach patients, they need to undergo rigorous testing to ensure their purity, quality, and sterility. Compliance with good manufacturing practice (GMP) is mandatory before human transplantation can be performed. Once cells are safely isolated and then delivered, patients are monitored for the development of potential tumors, activation of the immune system, and/or the development of arrhythmias.
Stem cell transplantation carries a risk of tumorigenicity, which varies depending on the cell source. Adult stem cells, for example, have a low risk of tumorigenicity because they have limited growth and differentiation potential. As expected, not a single case of cancer has been reported to date from any clinical trial using adult stem cells for heart disease. It should be noted, however, that a tumor developed in a one patient with ataxia telangiectasia who received fetal neural stem cells26 and in a second patient who received autologous bone-marrow derived stem cells for the treatment of lupus nephritis.27 The risk for tumor formation is higher when transplanting cardiomyocytes derived from embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs). Fortunately, the cardiac differentiation process has improved significantly over the past decade,28 and the minimal threshold needed to form a tumor also appears to be quite high, on the order of 105 undifferentiated cells,29 in part due to poor survival of transplanted cells.
Another potential safety risk for stem cell transplantation is increased immunogenicity. Like other organ transplants, stem cell transplantation may activate the immune system, especially if cells are allogenic (i.e., donor cells are not obtained from the recipient), potentially leading to graft rejection and progressive donor cell death.30 As expected, immune activation has not been reported in large-scale clinical trials, because most patients receive stem cells harvested from their own organs (e.g., autologous cells).
Additional evidence comes from preclinical studies that imaged cell fate after transplantation into immunodeficient mice after transplantation of allogeneic cells.22,32 Some cells survived longer than others because they were less immunogenic (e.g., MSCs and iPSC derivatives),19 although majority of transplanted cells died within 4–6 weeks of transplantation. Administration of adjuvant agents including immunosuppressants and pro-survival agents mitigated graft rejection, reduced cell death, and improved left ventricular function,33,34 providing further evidence that regulating the immune response may be one of the keys to improving efficacy.
Perhaps the most feared complication of transplantation is the development of life-threatening arrhythmias due to possible enhanced automaticity or development of re-entry circuit induced by stem cells. Evidence for a pro-arrhythmia effect in trials using BMMNCs has been scant, but evaluation has not been rigorous. In one of the earliest trials, Wollert et al. found no evidence of arrhythmia on 24-hour Holter monitoring or on electrophysiological testing.35 Limited survival of implanted stem cells and the fact that favorable effects of adult stem cells are mediated by mechanisms other than direct cardiomyocyte differentiation, however, might account for this favorable safety profile.
Future research using cardiomyocytes derived from ESCs or iPSCs will need to incorporate more extensive surveillance with event monitors, insertable long-term recorders, microvolt T-wave alternans, and invasive electrophysiological studies, especially because these cells have been shown to exhibit heterogeneous electrophysiological phenotypes, immature action potentials, immature gap junctions, and spontaneous automaticity in cell culture.36 Similarly, more careful monitoring will also be required if sizeable grafts are achieved using other cell sources.
Potential Strategies for Addressing Clinical Hurdles
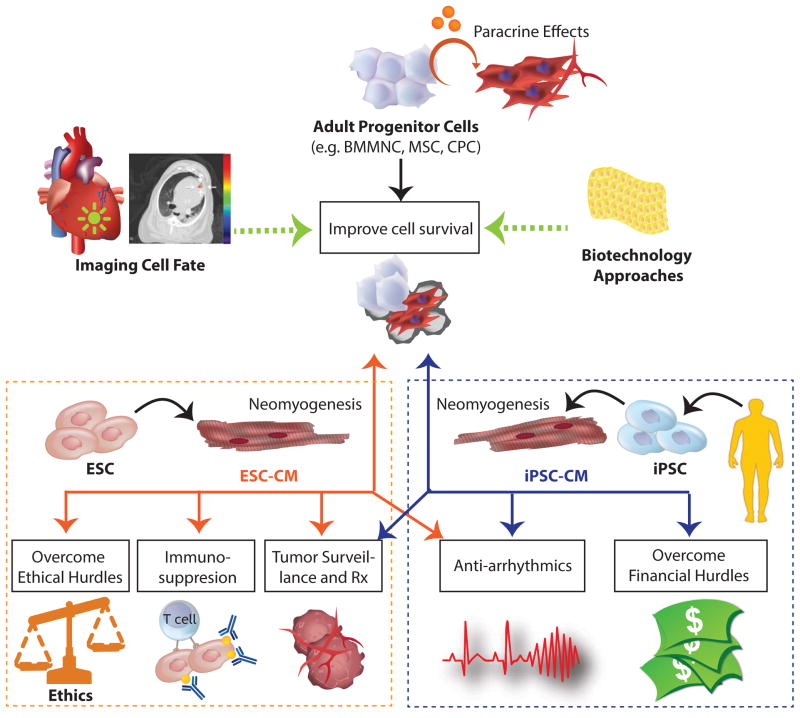
Overcoming the clinical hurdles outlined above will require a multi-faceted approach that centers on the incorporation of advanced imaging techniques to track cell fate. Additional strategies to address these limitations include the use of pluripotent stem cell (PSCs)--which includes ESCs and iPSCs--that can potentially contribute to cardiomyogenesis and novel tissue engineering approaches to improve cell survival (Figure 2). While improving stem cell survival will be crucial for addressing efficacy concerns for all cell types, tackling safety as well as ethical, regulatory, and economic hurdles may be more challenging for the clinical implementation of PSCs.8
Figure 2. Potential solutions to clinical hurdles faced by the implementation of adult progenitor cells and pluripotent stem cells.
Unlike adult stem cells, pluripotent stem cells such as ESCs and iPSCs can be differentiated into cardiomyocytes, which can generate new myocardial tissues. However, when cell death is pervasive, the extent of “neomyogenesis” and their subsequent functional output is debatable. Nevertheless, imaging technologies can be used to better identify strategies that can improve cell survive, engraftment, and efficacy. Pluripotent stem cells also face additional hurdles. ESCs require immunosuppression and face difficult regulatory and ethical challenges, whereas iPSCs may not be economically feasible (yet). Both cell types will likely require antiarrhythmic therapy if large grafts are achieved. ESCs: embryonic stem cells, iPSCs: induced pluripotent stem cells, Rx: treatment.
The Use of Pluripotent Stem Cells
As discussed above, one major hurdle for the routine clinical implementation of stem cell therapy is that only modest, if any, efficacy was achieved in adult stem cell trials. Because this lack of significant efficacy may be related more to poor cell survival and retention post-delivery than the inability of these cells to repair myocardial injury, jettisoning this approach in favor of using PSCs may be premature.37 Nevertheless, PSCs are an attractive alternative because, unlike adult stem cells, they can be differentiated into cardiomyocytes, endothelial cells, and other reparative cells with the potential to restore functional myocardium.38,39 Although a recent study has shown that ESC-derived cardiomyocytes can develop extensive grafts in non-human primates,40 results from this study were criticized because: 1) only variable benefit in LVEF was achieved; 2) only a small number of animals that had small infarcts were included; 3) an incomplete evaluation of pump function and electrical properties was performed; and 4) a high incidence of ventricular arrhythmias was observed,41 which contradicted earlier observations from the same group.42 The underlying cause for the high arrhythmia rate is unclear, but may be related to the high number of cells injected (i.e., 1 billion), the large size of the grafts that developed, as well as the presence of a prominent non-cardiac cell population. Although findings from this study raises concerns about the use of ESC- and iPSC-derivatives in humans, a Phase I/II study led by Menasche et al. has reported no adverse arrhythmias associated with the first transplantation of ESC-derived cardiac progenitor cells embedded in a fibrin patch that is sutured directly onto the epicardium, a strategy which may reduce myocardial irritation and, thus, arrhythmia risk.43
PSCs and their derivatives may also have a greater risk of tumorigenicity because of possible contamination from residual undifferentiated cells, culture-acquired mutations (e.g., karyotypic abnormalities, copy number variants, as well as the loss of heterozygosity and phenotypic features),44,45 and the risk of de-differentiation after transplantation.46–48 Strategies to minimize the risk of oncogenic transformation include the development of safer methods for reprogramming and use of efficient and reproducible differentiation protocols.39,49–53 In our experience with rodent and porcine models (unpublished data), we have not seen tumor formation following the injection of cardiomyocytes derived from ESCs or iPSCs. Long-term monitoring of potential tumor development is needed in the future. This may involve a combination of magnetic resonance imaging (MRI) and serum biomarkers, which was recently shown to have greater sensitivity in the detection of stem cell derived tumors than MRI or echocardiography alone.54 Additional imaging with positron emission tomography (PET) can potentially differentiate between ESC-derived tumors, which express higher levels of αvβ3 integrin than other tumors.55
Incorporating Molecular Imaging in Cardiac Stem Cell Trials
In addition to the lack of significant clinical improvement, large randomized controlled clinical trials to date have failed to show a consistent benefit across different trials and across different patients within the same trial, raising questions about the reasons behind these perplexing findings.2,16,56–59 Perhaps in patients who showed no detectable improvement, transplanted cells never arrived to the area of injury or died shortly after delivery. Although this may be a plausible explanation, whether or how variations in cell retention or survival affect efficacy remains unknown because the majority of randomized controlled clinical trials did not image cell fate.
While the details of how to perform stem cell fate imaging is beyond the scope of this review, we will briefly review the fundamentals of this approach and refer the reader to other more comprehensive reviews.19,24 To follow the spatiotemporal distribution of cells following delivery, stem cells must be labeled with a molecular probe emitting signals that can be detected by an appropriate imaging modality. In small animals, in vivo serial imaging of cell fate can be performed using small animal imaging systems (e.g., bioluminescence imaging [BLI] and microPET) over weeks or months, because cells can be labeled with reporter genes that integrate into their genome and generate signals in the presence of reporter probes as long as the cells are viable. Emitted signals are then detected by cameras with high spatial resolution (BLI: ~3–5 mm; microPET: ~1–2 mm) and imaging sensitivity (BLI: ~10−15 to 10−17 mol/L probe; microPET: ~10−11 to 10−12 mol/L probe), enabling a lower detection limit of ≥≥103 and ≥≥104 for BLI and microPET, respectively.60 The resolution and sensitivity of BLI, however, is depth-dependent, and thus cannot be used in large animals and humans.
In humans, however, stem cell imaging is currently limited by the sensitivity of clinical imaging systems. Even clinical PET, the most sensitive modality available for human imaging, has a relatively inferior spatial resolution (e.g., 6–10 mm) and imaging sensitivity (e.g., 10−11 to 10−12 mol/L) compared to small animal imaging systems, resulting in a lower detection limit of ≥≥104 cells.60 Clinical stem cell fate imaging is also limited by the short half-lives of radiolabelled probes (≤3 days) that are used for direct cell labeling. As technology advances, longer-term imaging of stem cell fate will likely be feasible in humans using reporter genes, as reported by Yaghoubi et al., who demonstrated that successful tracking of cytolytic T cells labeled with a viral PET reporter gene in a patient with gliobastoma.61 Concerns over issues of immune activation of non-human reporter genes and the risk of oncogenic transformation secondary to random insertions into the genome, however, have impeded further application of this approach in humans. The development of humanized reporter genes and techniques for site-specific integration of these reporter genes may circumvent these safety issues.62
Immunosuppressive Therapy, Pro-survival Agents and Exosomes
Several hypotheses have emerged to explain the potential causes of poor cell survival and engraftment that have contributed to the limited efficacy found in previous trials. One notable theory is that the hostile tissue microenvironment, characterized by ischemia and inflammation, promotes cell death. Inflammation and ischemia, however, can be modulated by the delivery of local immunosuppressive therapy, pro-survival agents, and exosomes that reduce inflammatory cytokines, promote angiogenesis, and decrease apoptosis. As shown in two independent small animal studies,33,34 immunosuppressive therapy can decrease cell death in immunocompetent mice. Other studies have shown that co-administration of pro-survival factors63 or pro-survival miRNA cocktail64 can significantly enhance cell survival. Alternatively, cells can be genetically engineered to express genes to potentially enhance survival and biological function.65,66 Finally, recent studies have suggested that cardioprotective factors, including miRNAs released by stem cells, packaged into exosomes can be potentially delivered as stand alone therapy.67,68 Importantly, the local delivery of these agents enables the generation of a microenvironment that is less hostile to cells while avoiding unintended systemic effects seen in systemic immunosuppression regimens.
Finally, using autologous cell sources, such as patient-specific BMMNCs, MSCs or iPSC derivatives as opposed to allogenic cell sources, may potentially reduce immune-mediated cell death and potentially avoid the use of immunosuppressive agents. Human iPSCs, for example, can be obtained by reprogramming adult somatic cells from a patient, differentiating them into cardiomyocytes, and transplanting them into the same patient.8 Before iPSCs from patients with inherited cardiomyopathies could be used, however, the genetic defect within the iPSC must be first corrected by genome editing using techniques such as zinc finger nuclease (ZFN), transcription activator-like effector nuclease (TALEN), and clustered regularly-interspaced short palindromic repeats (CRISPR)/Cas9.69 Because using autologous derivatives from iPSCs may be economically infeasible, it is not surprising that only one trial, which uses autologous iPSC-derived retinal pigment epithelial cells to treat patients with macular degeneration, has been initiated to date.70
Tissue Engineering Approaches: Injectable Scaffolds, Myocardial Patches, and Engineered Heart Muscle
Cell death may also result from the delivery of isolated cells that are not anchored to extracellular matrix, which may increase programmed cell death, commonly referred to as “anoikis”. Embedding cells in biomaterials, however, can potentially reduce anoikis by providing structural and mechanical support for cells to survive and mature in the host myocardium.71 For example, cells can be delivered in an injectable scaffold made of fibrin,43 hydrogel,72 or other biomaterials whose topology (e.g., pore/channel and structures) can facilitate vascularization within the implant and minimize fibrosis.73 To improve cell affinity, these synthetic and natural biomaterials have been modified with cell adhesive molecules (e.g. collagen, fibronectin, and laminin.74 These scaffolds have also been loaded with small molecules and trophic factors to provide a multifaceted approach to improve cell survival. Scaffolds have been built to continually release oxygen,75 pro-angiogenic cytokines,76 anti-apoptotic agents,77 and extracellular matrix proteins.78
More recently, efforts have been put into the creation of functional heart tissue constructs using ESC- or iPSC-derived cardiomyocytes in the form of a myocardial patch, cardiomyocyte sheet, or engineered heart muscle (EHM), which can be implanted directly onto the myocardium.79,80 In one study, tissue constructs prepared from neonatal cardiomyocytes were preconditioned using a cyclic stress system and then sutured onto the infarcted rat myocardium, resulting in improvement in LVEF.81 More recently, EHM and cell sheet technologies have moved into large animal models,82 showing great promise for re-muscularization of the infarcted heart.
Importantly, we have already seen early phase clinical trials testing the safety and efficacy of biomaterials or extracellular matrix products in the setting of acute MI or HF. As discussed previously, Menasche et al. initiated a Phase I/IIa trial to evaluate the efficacy and safety of delivering ESC-derived cardiac progenitor cells embedded in a fibrin scaffold, with a planned enrollment of 6 patients (https://clinicaltrials.gov/ct2/show/NCT02057900).43 LoneStar Heart Inc. (Laguna Hills, CA) recently reported successful one-year results of their randomized clinical trial on the efficacy of Algisyl, a biocompatible, inert hydrogel that can potentially be used to embed cells for more effective transplantation.83 Similarly, in a randomized, double blind, placebo-controlled study, Bellerophon Therapeutics, Inc. (Warren, NJ) reported that its bio-absorbable alginate hydrogel product designed to mitigate the adverse remodeling could be safely administered in patients after MI.84 Finally, Anker et al. reported significant improvement in exercise capacity estimated by measurements of maximal oxygen consumption in HF patients treated with alginate hydrogel directly injected into the heart compared with control patients.85 Studies such as these four early-stage clinical trials are paving the way for incorporating tissue-engineering strategies to improve the clinical translation of stem cell regenerative therapy.
Conclusion
Although the last two decades have seen great strides in stem cell therapy, significant clinical hurdles remain. Arguably the most difficult challenge in translating this therapy into routine clinical use is the inconsistent and relatively limited efficacy observed in adult stem cell clinical trials to date. The inability to determine cell fate and survival in humans has been a significant obstacle to understanding the mechanisms of the variable efficacy. The incorporation of cell fate imaging in clinical trials may help address these significant hurdles. Furthermore, concerns over tumorigenicity, immunogenicity, and arrhythmogenicity exist with the use of different stem cell sources. A multifaceted approach will be vital in addressing these issues to enable successful clinical translation of stem cell therapies.
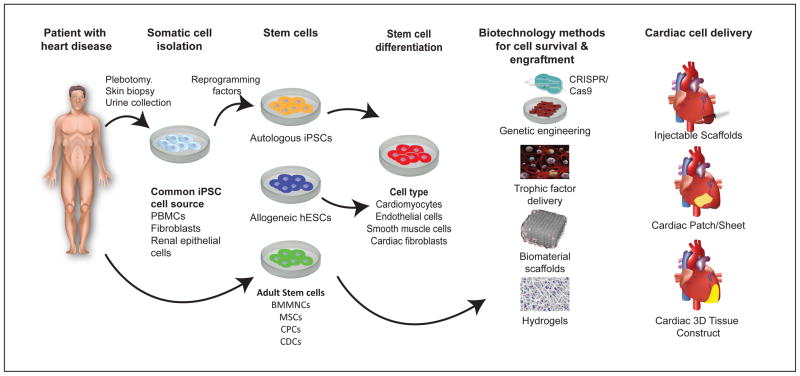
Figure 3. Schematic of how tissue engineering can be incorporated into stem cell therapy.
Patients with heart disease can be treated with different cell sources, including autologous iPSCs, allogenic ESCs, and adult stem cells. Human iPSCs are generated by reprogramming adult somatic cells that are isolated from blood, skin or renal epithelial cells into the pluripotent state, and then differentiating them into cells that can repair the heart (e.g., cardiomyocytes, endothelial cells, smooth muscle cells, or fibroblasts). Similarly, ESCs can be differentiated into cell derivatives (e.g., cardiomyocytes, endothelial cells, smooth muscle cells, cardiac fibroblasts, etc.). By contrast, adult stem cells such as bone marrow mononuclear cells, mesenchymal stem cells, cardioprogenitor cells, and cardiosphere-derived cells do not require reprogramming or differentiation. To improve their survival post-transplantation, these cells can be genetically altered to express pro-survival genes using CRISPER/Cas9, transplanted together with trophic factors, or embedded in scaffolds or hydrogels. These cells together with their adjuvant agents can be delivered as an injectable scaffold, patch, or 3D tissue construct to further improve cellular retention and efficacy.
Acknowledgments
We thank Blake Wu and Karim Sallam for critical reading of the manuscript. We thank funding support from American Heart Association 13EIA14420025, Burroughs Wellcome Foundation Innovation in Regulatory Science Award, National Institutes of Health (NIH) R01 HL113006, NIH R01 HL130020, NIH R01 Hl126527, California Institute of Regenerative Medicine (CIRM) DR2A-05394 and RT3-07798 (JCW), and Stanford Cardiovascular Institute Seed Grant (PKN).
References
- 1.Pavo N, Charwat S, Nyolczas N, et al. Cell therapy for human ischemic heart diseases: critical review and summary of the clinical experiences. J Mol Cell Cardiol. 2014;75:12–24. doi: 10.1016/j.yjmcc.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 2.Jeevanantham V, Butler M, Saad a, Abdel-Latif a, Zuba-Surma EK, Dawn B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation. 2012;126:551–568. doi: 10.1161/CIRCULATIONAHA.111.086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wollert KC, Drexler H. Cell therapy for the treatment of coronary heart disease: a critical appraisal. Nature Reviews. Cardiology. 2010;7:204–215. doi: 10.1038/nrcardio.2010.1. [DOI] [PubMed] [Google Scholar]
- 4.Fisher Sheila A, Brunskill Susan J, Doree C, Mathur A, Taggart David P, Martin-Rendon E. Stem cell therapy for chronic ischaemic heart disease and congestive heart failure. Cochrane Database of Systematic Reviews. 2014 doi: 10.1002/14651858.CD007888.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Martin-Rendon E, Brunskill SJ, Hyde CJ, Stanworth SJ, Mathur A, Watt SM. Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J. 2008;29(15):1807–1818. doi: 10.1093/eurheartj/ehn220. [DOI] [PubMed] [Google Scholar]
- 6.Golpanian S, Schulman IH, Ebert RF, et al. Concise review: review and perspective of cell dosage and routes of administration from preclinical and clinical studies of stem cell therapy for heart disease. Stem Cells Translational Medicine. 2016;5(2):186–191. doi: 10.5966/sctm.2015-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher SA, Zhang H, Doree C, Mathur A, Martin-Rendon E. Stem cell treatment for acute myocardial infarction. The Cochrane Database of Systematic Reviews. 2015;9:CD006536. doi: 10.1002/14651858.CD006536.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neofytou E, O’Brien CG, Couture LA, Wu JC. Hurdles to clinical translation of human induced pluripotent stem cells. J Clin Invest. 2015;125(7):2551–2557. doi: 10.1172/JCI80575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quyyumi AA, Waller EK, Murrow J, et al. CD34(+) cell infusion after ST elevation myocardial infarction is associated with improved perfusion and is dose dependent. American Heart Journal. 2011;161(1):98–105. doi: 10.1016/j.ahj.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 10.Losordo DW, Henry TD, Davidson C, et al. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circulation Research. 2011;109:428–436. doi: 10.1161/CIRCRESAHA.111.245993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308(22):2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vrtovec B, Poglajen G, Lezaic L, et al. Effects of intracoronary CD34+ stem cell transplantation in nonischemic dilated cardiomyopathy patients: 5-year follow-up. Circulation Research. 2013;112:165–173. doi: 10.1161/CIRCRESAHA.112.276519. [DOI] [PubMed] [Google Scholar]
- 13.Vrtovec B, Poglajen G, Lezaic L, et al. Comparison of transendocardial and intracoronary CD34+ cell transplantation in patients with nonischemic dilated cardiomyopathy. Circulation. 2013;128(11 Suppl 1):S42–49. doi: 10.1161/CIRCULATIONAHA.112.000230. [DOI] [PubMed] [Google Scholar]
- 14.Swijnenburg RJ, Govaert JA, van der Bogt KE, et al. Timing of bone marrow cell delivery has minimal effects on cell viability and cardiac recovery after myocardial infarction. Circulation. Cardiovascular Imaging. 2010;3(1):77–85. doi: 10.1161/CIRCIMAGING.109.872085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Traverse JH, Henry TD, Pepine CJ, et al. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. JAMA. 2012;308(22):2380–2389. doi: 10.1001/jama.2012.28726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Traverse JH, Henry TD, Pepine CJ, et al. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. JAMA. 2013;18:1199–1216. doi: 10.1001/jama.2012.28726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Traverse JH, Henry TD, Ellis SG, et al. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. JAMA. 2011;306(19):2110–2119. doi: 10.1001/jama.2011.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou D, Youssef EA, Brinton TJ, et al. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 2005;112(9 Suppl):I150–156. doi: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen PK, Lan F, Wang Y, Wu JC. Imaging: guiding the clinical translation of cardiac stem cell therapy. Circulation Research. 2011;109(8):962–979. doi: 10.1161/CIRCRESAHA.111.242909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freyman T, Polin G, Osman H, et al. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27(9):1114–1122. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- 21.Cao F, Lin S, Xie X, et al. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation. 2006;113(7):1005–1014. doi: 10.1161/CIRCULATIONAHA.105.588954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Z, Wu JC, Sheikh AY, et al. Differentiation, survival, and function of embryonic stem cell derived endothelial cells for ischemic heart disease. Circulation. 2007;116(11 Suppl):I46–54. doi: 10.1161/CIRCULATIONAHA.106.680561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu JC, Chen IY, Sundaresan G, et al. Molecular imaging of cardiac cell transplantation in living animals using optical bioluminescence and positron emission tomography. Circulation. 2003;108(11):1302–1305. doi: 10.1161/01.CIR.0000091252.20010.6E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen PK, Riegler J, Wu JC. Stem cell imaging: from bench to bedside. Cell Stem Cell. 2014;14(4):431–444. doi: 10.1016/j.stem.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Narsinh KH, Lan F, et al. Early stem cell engraftment predicts late cardiac functional recovery: preclinical insights from molecular imaging. Circulation. Cardiovascular Imaging. 2012;5(4):481–490. doi: 10.1161/CIRCIMAGING.111.969329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amariglio N, Hirshberg A, Scheithauer BW, et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Medicine. 2009;6(2):e1000029. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thirabanjasak D, Tantiwongse K, Thorner PS. Angiomyeloproliferative lesions following autologous stem cell therapy. J Am Soc Nephrol. 2010;21(7):1218–1222. doi: 10.1681/ASN.2009111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burridge PW, Sharma A, Wu JC. Genetic and epigenetic regulation of human cardiac reprogramming and differentiation in regenerative medicine. Annu Rev Genet. 2015;49:461–484. doi: 10.1146/annurev-genet-112414-054911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee AS, Tang C, Cao F, et al. Effects of cell number on teratoma formation by human embryonic stem cells. Cell Cycle. 2009;8(16):2608–2612. doi: 10.4161/cc.8.16.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pearl JI, Kean LS, Davis MM, Wu JC. Pluripotent stem cells: immune to the immune system? Sci Transl Med. 2012;4(164):164ps125. doi: 10.1126/scitranslmed.3005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs. autologous bone marrow – derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swijnenburg RJ, Schrepfer S, Cao F, et al. In vivo imaging of embryonic stem cells reveals patterns of survival and immune rejection following transplantation. Stem Cells and Development. 2008;17(6):1023–1029. doi: 10.1089/scd.2008.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swijnenburg RJ, Schrepfer S, Govaert JA, et al. Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(35):12991–12996. doi: 10.1073/pnas.0805802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pearl JI, Lee AS, Leveson-Gower DB, et al. Short-term immunosuppression promotes engraftment of embryonic and induced pluripotent stem cells. Cell Stem Cell. 2011;8(3):309–317. doi: 10.1016/j.stem.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: The BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 36.Chen HS, Kim C, Mercola M. Electrophysiological challenges of cell-based myocardial repair. Circulation. 2009;120(24):2496–2508. doi: 10.1161/CIRCULATIONAHA.107.751412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.King A. Stem cells. Could it be TIME to abandon BMCs? Nat Rev Cardiol. 2013;10(1):8. doi: 10.1038/nrcardio.2012.170. [DOI] [PubMed] [Google Scholar]
- 38.Lian X, Hsiao C, Wilson G, et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proceedings of the National Academy of Sciences of the United States of America. 2012 doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burridge PW, Matsa E, Shukla P, et al. Chemically defined generation of human cardiomyocytes. Nat Methods. 2014 doi: 10.1038/nmeth.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chong JJH, Yang X, Don CW, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson ME, Goldhaber J, Houser SR, Puceat M, Sussman MA. Embryonic stem cell-derived cardiac myocytes are not ready for human trials. Circulation Research. 2014;115(3):335–338. doi: 10.1161/CIRCRESAHA.114.304616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shiba Y, Fernandes S, Zhu WZ, et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489(7415):322–325. doi: 10.1038/nature11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menasche P, Vanneaux V, Hagege A, et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur Heart J. 2015;36(30):2011–2017. doi: 10.1093/eurheartj/ehv189. [DOI] [PubMed] [Google Scholar]
- 44.Lund RJ, Narva E, Lahesmaa R. Genetic and epigenetic stability of human pluripotent stem cells. Nature Reviews. Genetics. 2012;13(10):732–744. doi: 10.1038/nrg3271. [DOI] [PubMed] [Google Scholar]
- 45.Peterson SE, Loring JF. Genomic instability in pluripotent stem cells: implications for clinical applications. The Journal of Biological Chemistry. 2014;289(8):4578–4584. doi: 10.1074/jbc.R113.516419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker DE, Harrison NJ, Maltby E, et al. Adaptation to culture of human embryonic stem cells and oncogenesis in vivo. Nat Biotechnol. 2007;25(2):207–215. doi: 10.1038/nbt1285. [DOI] [PubMed] [Google Scholar]
- 47.Lee AS, Tang C, Rao MS, Weissman IL, Wu JC. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med. 2013;19(8):998–1004. doi: 10.1038/nm.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mayshar Y, Ben-David U, Lavon N, et al. Identification and classification of chromosomal aberrations in human induced pluripotent stem cells. Cell Stem Cell. 2010;7(4):521–531. doi: 10.1016/j.stem.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 49.Jia F, Wilson KD, Sun N, et al. A nonviral minicircle vector for deriving human iPS cells. Nat Methods. 2010;7(3):197–199. doi: 10.1038/nmeth.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Churko JM, Burridge PW, Wu JC. Generation of human iPSCs from human peripheral blood mononuclear cells using non-integrative Sendai virus in chemically defined conditions. Methods in Molecular Biology. 2013;1036:81–88. doi: 10.1007/978-1-62703-511-8_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diecke S, Lisowski L, Kooreman NG, Wu JC. Second generation codon optimized minicircle (CoMiC) for nonviral reprogramming of human adult fibroblasts. Methods in Molecular Biology. 2014;1181:1–13. doi: 10.1007/978-1-4939-1047-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Warren L, Manos PD, Ahfeldt T, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7(5):618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burridge PW, Keller G, Gold JD, Wu JC. Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell. 2012;10(1):16–28. doi: 10.1016/j.stem.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riegler J, Ebert A, Qin X, et al. Comparison of magnetic resonance imaging and serum biomarkers for detection of human pluripotent stem cell-derived teratomas. Stem Cell Reports. 2016 doi: 10.1016/j.stemcr.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao F, Li Z, Lee A, et al. Noninvasive de novo imaging of human embryonic stem cell-derived teratoma formation. Cancer Research. 2009;69(7):2709–2713. doi: 10.1158/0008-5472.CAN-08-4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perin EC, Willerson JT, Pepine CJ, et al. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. JAMA. 2012;307(16):1717–1726. doi: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fisher Sa, Doree C, Mathur A, Martin-Rendon E. Meta-analysis of cell therapy trials for patients with heart failure. Circulation Research. 2015;116:1361–1377. doi: 10.1161/CIRCRESAHA.116.304386. [DOI] [PubMed] [Google Scholar]
- 58.Gyöngyösi M, Wojakowski W, Lemarchand P, et al. Meta-Analysis of Cell-based CaRdiac stUdiEs (ACCRUE) in patients with acute myocardial infarction based on individual patient data. Circulation Research. 2015;116:1346–1360. doi: 10.1161/CIRCRESAHA.116.304346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Delewi R, Hirsch A, Tijssen JG, et al. Impact of intracoronary bone marrow cell therapy on left ventricular function in the setting of ST-segment elevation myocardial infarction: a collaborative meta-analysis. European Heart Journal. 2014;35:989–998. doi: 10.1093/eurheartj/eht372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen IY, Wu JC. Cardiovascular molecular imaging: focus on clinical translation. Circulation. 2011;123(4):425–443. doi: 10.1161/CIRCULATIONAHA.109.916338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yaghoubi SS, Jensen MC, Satyamurthy N, et al. Noninvasive detection of therapeutic cytolytic T cells with 18F-FHBG PET in a patient with glioma. Nat Clin Pract Oncol. 2009;6(1):53–58. doi: 10.1038/ncponc1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y, Zhang WY, Hu S, et al. Genome editing of human embryonic stem cells and induced pluripotent stem cells with zinc finger nucleases for cellular imaging. Circulation Research. 2012;111(12):1494–1503. doi: 10.1161/CIRCRESAHA.112.274969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Laflamme MA, Chen KY, Naumova AV, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25(9):1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 64.Hu S, Huang M, Nguyen PK, et al. Novel microRNA prosurvival cocktail for improving engraftment and function of cardiac progenitor cell transplantation. Circulation. 2011;124(11 Suppl):S27–34. doi: 10.1161/CIRCULATIONAHA.111.017954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang X, Zhao T, Huang W, et al. Hsp20-engineered mesenchymal stem cells are resistant to oxidative stress via enhanced activation of Akt and increased secretion of growth factors. Stem Cells. 2009;27(12):3021–3031. doi: 10.1002/stem.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fischer KM, Cottage CT, Wu W, et al. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase. Circulation. 2009;120(21):2077–2087. doi: 10.1161/CIRCULATIONAHA.109.884403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ibrahim AG, Cheng K, Marban E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports. 2014;2(5):606–619. doi: 10.1016/j.stemcr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gray WD, French KM, Ghosh-Choudhary S, et al. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circulation Research. 2015;116(2):255–263. doi: 10.1161/CIRCRESAHA.116.304360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gaj T, Gersbach CA, Barbas CF., 3rd ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31(7):397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kamao H, Mandai M, Okamoto S, et al. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Reports. 2014;2(2):205–218. doi: 10.1016/j.stemcr.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tzatzalos E, Abilez OJ, Shukla P, Wu JC. Engineered heart tissues and induced pluripotent stem cells: Macro- and microstructures for disease modeling, drug screening, and translational studies. Advanced Drug Delivery Reviews. 2016;96:234–244. doi: 10.1016/j.addr.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seif-Naraghi SB, Singelyn JM, Salvatore MA, et al. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Sci Transl Med. 2013;5(173):173ra125. doi: 10.1126/scitranslmed.3005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Madden LR, Mortisen DJ, Sussman EM, et al. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(34):15211–15216. doi: 10.1073/pnas.1006442107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu J, Marchant RE. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev Med Devices. 2011;8(5):607–626. doi: 10.1586/erd.11.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oh SH, Ward CL, Atala A, Yoo JJ, Harrison BS. Oxygen generating scaffolds for enhancing engineered tissue survival. Biomaterials. 2009;30(5):757–762. doi: 10.1016/j.biomaterials.2008.09.065. [DOI] [PubMed] [Google Scholar]
- 76.Chung HJ, Kim JT, Kim HJ, et al. Epicardial delivery of VEGF and cardiac stem cells guided by 3-dimensional PLLA mat enhancing cardiac regeneration and angiogenesis in acute myocardial infarction. J Control Release. 2015;205:218–230. doi: 10.1016/j.jconrel.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 77.Huang L, Ma W, Ma Y, Feng D, Chen H, Cai B. Exosomes in mesenchymal stem cells, a new therapeutic strategy for cardiovascular diseases? International Journal of Biological Sciences. 2015;11(2):238–245. doi: 10.7150/ijbs.10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rao RR, Peterson AW, Ceccarelli J, Putnam AJ, Stegemann JP. Matrix composition regulates three-dimensional network formation by endothelial cells and mesenchymal stem cells in collagen/fibrin materials. Angiogenesis. 2012;15(2):253–264. doi: 10.1007/s10456-012-9257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Riegler J, Tiburcy M, Ebert A, et al. Human engineered heart muscles engraft and survive long term in a rodent myocardial infarction model. Circ Res. 2015;117(8):720–730. doi: 10.1161/CIRCRESAHA.115.306985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sawa Y, Yoshikawa Y, Toda K, et al. Safety and efficacy of autologous skeletal myoblast sheets (TCD-51073) for the treatment of severe chronic heart failure due to ischemic heart disease. Circ J. 2015;79(5):991–999. doi: 10.1253/circj.CJ-15-0243. [DOI] [PubMed] [Google Scholar]
- 81.Zimmermann WH, Melnychenko I, Wasmeier G, et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12(4):452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 82.Shudo Y, Miyagawa S, Fukushima S, et al. Novel regenerative therapy using cell-sheet covered with omentum flap delivers a huge number of cells in a porcine myocardial infarction model. J Thorac Cardiovasc Surg. 2011;142(5):1188–1196. doi: 10.1016/j.jtcvs.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 83.Mann DL, Lee RJ, Coats AJ, et al. One-year follow-up results from AUGMENT-HF: a multicentre randomized controlled clinical trial of the efficacy of left ventricular augmentation with Algisyl in the treatment of heart failure. Eur J Heart Fail. 2016;18(3):314–325. doi: 10.1002/ejhf.449. [DOI] [PubMed] [Google Scholar]
- 84.Rao SV, Zeymer U, Douglas PS, et al. A randomized, double-blind, placebo-controlled trial to evaluate the safety and effectiveness of intracoronary application of a novel bioabsorbable cardiac matrix for the prevention of ventricular remodeling after large ST-segment elevation myocardial infarction: Rationale and design of the PRESERVATION I trial. Am Heart J. 2015;170(5):929–937. doi: 10.1016/j.ahj.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 85.Anker SD, Coats AJ, Cristian G, et al. A prospective comparison of alginate-hydrogel with standard medical therapy to determine impact on functional capacity and clinical outcomes in patients with advanced heart failure (AUGMENT-HF trial) Eur Heart J. 2015;36(34):2297–2309. doi: 10.1093/eurheartj/ehv259. [DOI] [PMC free article] [PubMed] [Google Scholar]