Abstract
Prior murine and human studies suggest that vascular endothelial growth factor (VEGF) contributes to endothelial cell activation and severity of illness in sepsis. Furthermore, circulating levels of soluble VEGF receptor 1 (sFLT) levels were found to increase as part of the early response to sepsis in mice. The objective of the study was to evaluate the blood levels of free VEGF-A and sFLT in patients presenting to the emergency department (ED) with suspected infection and to assess the relationship of these levels with severity of illness and inflammation. It was a prospective, observational study initiated in the ED of an urban, tertiary care, university hospital. Inclusion criteria were (1) ED patients aged 18 years or older and (2) clinical suspicion of infection. Eighty-three patients were enrolled in the study. The major findings were that (1) the mean VEGF and sFLT levels were increasingly higher across the following groups: noninfected control patients, infected patients without shock, and septic shock patients; (2) initial and 24-h VEGF levels had a significant correlation with the presence of septic shock at 24 h; (3) initial and 24-h sFLT levels correlated with Acute Physiology Age Chronic Health Evaluation II and Sepsis-related Organ Failure Assessment scores initially and at 24 h; and (4) VEGF and sFLT levels correlated with inflammatory cascade activation. This is the first report of sFLT as a potential new marker of severity in patients with sepsis. Vascular endothelial cell growth factor and its signaling axis are important in the endothelial cell response to sepsis, and further elucidation of these mechanisms may lead to advances in future diagnostic and therapeutic opportunities.
Keywords: Biomarker, sepsis, endothelium, inflammation, shock
INTRODUCTION
Sepsis is common, lethal, and costly to treat, accounting for 215,000 deaths and $16 billion of health care expenditures in the United States annually. After years of unsuccessful clinical trials, several new therapies have proven effective, including early goal-directed therapy, drotrecogin-α (recombinant human activated protein C), intensive insulin therapy, steroid supplementation, and lowYtidal volume ventilation in acute respiratory distress syndrome (1–5). A common theme inherent in these treatments is their capacity to attenuate endothelial cell activation and dysfunction. For example, early goal-directed therapy results in more rapid hemodynamic support and, thus, improved flow at the level of the endothelium; activated protein C may function in part by decreasing endothelial cell activation and apoptosis, and low-dose glucocorticoids may dampen the activity of proinflammatory transcriptional networks in the endothelium. Although a link between therapeutic efficacy and “endothelial health” remains speculative, the findings are consistent with a growing body of literature that supports an important role for the endothelium in severe sepsis [reviewed in Ref. (6)].
Vascular endothelial growth factor (VEGF) was first described as a potent stimulator of endothelial permeability (7). During the past several decades, most of the attention has focused on the role of VEGF as a proangiogenic molecule in cancer. More recently, VEGF signaling in endothelial cells was reported to induce the expression of cell adhesion molecules, the release of cytokines and chemokines, and the expression of procoagulant molecules (8–11). Vascular endothelial growth factor binds to two transmembrane receptors, Flk-1 (also known as VEGFR2 or KDR) and Flt-1 (also known as VEGFR1). Within the vessel wall, Flk-1 is selectively expressed in endothelium. Flt-1 is present on both endothelial cells and monocytes. Flt-1 is also produced as a soluble receptor, soluble Flt-1 (sFLT), via alternative splicing of the precursor mRNA (12) and functions as a decoy molecule, competing with membrane-bound Flt-1 for binding to VEGF. We recently reported that VEGF plays an important role in sepsis pathophysiology (13). Consistent with the results of previous studies in humans (14, 15), we found an association between sepsis and increased circulating levels of VEGF in mice with severe sepsis (13). The systemic administration of either exogenous sFLT (supranormal levels, 20-fold over baseline) or antibodies to the VEGF receptor Flk-1 blocked the effect of endotoxemia and cecal ligation puncture on morbidity and mortality (13). Interestingly, endotoxin challenge in mice resulted in elevated (~5-fold) circulating levels of sFLT. Together, these observations raise the interesting possibility that sFLT contributes to the systemic anti-inflammatory host response to infection.
The above data suggest that VEGF plays an important pathophysiological role in sepsis, and that VEGF and sFLT are potential markers for sepsis severity. It follows that these molecules represent potentially novel diagnostic and therapeutic targets in severe sepsis and septic shock. However, VEGF data in humans are currently limited to two small studies (14, 15), and we are aware of no reports that characterize sFLT in human sepsis. Thus, the overall goal of the current study is to describe the behavior of VEGF and sFLT in a group of patients presenting to the emergency department (ED) with suspected infection.
MATERIALS AND METHODS
Design
This was a prospective cohort study of a convenience sample of adult patients (age, 18 years or older) presenting to the ED with suspected infection. Suspected infection was defined as a clinical suspicion of an infectious etiology as determined by the treating clinician. The setting was Beth Israel Deaconess Medical Center, an urban, academic teaching hospital. A broad definition of infection was applied and then subsequently categorized as described below. A convenience sample of noninfected controls was assembled by identifying patients who met the following criteria: (1) aged 18 years or older and (2) absence of suspected infection as determined by the ED clinicians, and subsequent chart review.
Procedures
Subjects received serial blood draws at 0, 3, 6, and 24 h, as available. In cases where the subject was discharged before completion of blood draws, the study was truncated at that point. The primary times for analysis were at times 0 and 24 h. If a patient was discharged before 24 h or a sample was unavailable at 24 h, the closest marker value before that time was carried forward for analysis. Plasma was assayed for free VEGF-A (which we will refer to as VEGF), sFLT, placental growth factor (PlGF), IL-1α (which we will refer to as IL-1) and IL-6 using Quantikine enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, Minn), and for total VEGF using a human VEGF enzyme-linked immunosorbent assay kit (Accucyte, Rockville, Md). This study was approved by our Institutional Human Use Committee and informed consent was obtained.
Severity of illness and septic shock
The primary markers of severity of illness (Acute Physiology Age Chronic Health Evaluation [APACHE] II score) and organ dysfunction (Sepsis-related Organ Failure Assessment [SOFA] score) were assessed upon enrollment in the ED and at 24 h. Acute Physiology Age Chronic Health Evaluation II scores for initial ED presentation and at 24 h were based on worst vital signs initially in the ED or during the first 24 h, respectively (16). Sepsis-related Organ Failure Assessment score was designed to identify morbidity and individualizes the dysfunction or failure of each organ system (17).
Patients were stratified into three groups: (1) noninfected controls, (2) suspected infection without the presence of shock (systolic blood pressure < 90 mmHg or pressor dependence after 20–30 mL/kg fluid challenge), and (3) septic shock, suspected infection plus shock. These were categorized by each individual time point; thus, if a patient presented with shock, but it resolved at 24 h, the patient was classified as septic shock at the 0-h measure and suspected infection without shock at 24 h.
Activation of the inflammatory cascade was assessed using the previously established markers of the body's inflammatory response (IL-1 and IL-6).
Statistical methods
The markers and baseline demographics were categorized and presented as means with standard deviations, medians with interquartile range, or proportions, as appropriate. We analyzed VEGF and sFLT in noninfected controls, patients with suspected infection without septic shock, and those with septic shock at 0 and 24 h. We performed both a one-way ANOVA and a median test to compare the means and medians for the three groups. Next, we created a Spearman-rank correlation matrix showing the association with each of the markers with severity of illness score for the following comparisons: marker time 0, severity score time 0; marker time 0, severity score by time 24 h; and marker at 24 h, severity score by 24 h. This shows the general trends in association with severity of illness on a univariate basis. We also examined the correlation of VEGF and sFLT with inflammation as indicated by IL-1 and IL-6 over time along with septic shock. To further assess interactions within the VEGF axis (VEGF and sFLT), we divided VEGF and sFLT into dichotomous groups at their medians and categorized the values as low or high to assess the association of the combination of VEGF and sFLT levels with severity of illness. We then compared the mean APACHE-II and SOFA scores for the high and low groups using a t test. To assess the potential of sFLT and VEGF levels to identify patients who are anticipated to have a worsening course, we stratified the patients into two groups: those whose SOFA scores worsened by two points or greater and those whose scores either did not worsen or improve. The mean values were plotted, and a linear mixed-effects model was used to estimate the mean difference of VEGF and sFLT between these two groups. The model assumed a compound symmetry for the variance-covariance matrix of within-person measurements of VEGF (or sFLT), and the estimation was done via restricted maximum likelihood. We used linear contrast to estimate the mean difference at baseline and 24 h. Finally, potential confounders defined a priori (malignancy, diabetes, end-stage renal disease, and mild renal disease) were added and retained if they reached significance α (0.05).
RESULTS
There were 83 patients enrolled in the study. The baseline demographics, comorbid conditions, results of overall marker assay, and severity of illness are shown (Table 1). The mean age for patients was 61 ± 17.8 years. There were 17 (20%) patients in septic shock on presentation. The overall hospital mortality rate was 5%. There were 31 patients who were discharged before having their 24-h levels measured or who were unavailable for their 24-h draw, in which case markers assayed from the closest point were inputted for the analyses. This was done to avoid biasing against less severely ill samples by disallowing those who were eligible for ED discharge.
Table 1.
Demographics and baseline characteristics for suspected infection patients
| Mean (SD) | Median (Q1–Q3) | |
|---|---|---|
| Age | 61.2 (17.8) | 64 (47–76) |
| APACHE | 11.7 (7.9) | 12 (5–17) |
| SOFA | 1.9 (2.6) | 1 (0–3) |
| IL-1 | 378 (686) | 174 (57–359) |
| IL-6 | 407 (1028) | 98 (30–308) |
| VEGF | 139 (126) | 108 (59–177) |
| sFLT | 420 (370) | 308 (206–516) |
| Total VEGF | 9917 (6915) | 8,746 (4,879–13,931) |
| PIGF | 20.8 (13.4) Frequency (%) | 17.6 (11.1–26.4) |
| Malignancy | 16 (19) | |
| Diabetes | 31 (37) | |
| End-stage renal disease | 12 (14) | |
| ARDS (72 h) | 3 (3) | |
| In-hospital mortality | 4 (5) | |
| Clinical condition on presentation | ||
| Suspected infection without shock | 66 (80) | |
| Septic shock | 17 (20) |
ARDS indicates adult respiratory distress syndrome.
The initial mean levels of sFLT increased comparing patients stratified by the following groups: noninfected control patients (159 ± SD 79 ng/dL), suspected infection without shock (386 ± 365 ng/dL), and suspected infection with septic shock (551 ± 371 ng/dL) (P < 0.01) (Fig. 1A). At 24 h, the mean levels of sFLT were higher, comparing the means of 159 ± 79 ng/dL in noninfected control patients (0-h levels carried forward), to 369 ± 329 ng/dL in suspected infection without shock, to 601 ± 247 ng/dL in suspected infection with septic shock (P < 0.01) (Fig. 1A). The VEGF levels for the same groups were 58 ± 38, 132 ± 112, and 166 ± 171 ng/dL, respectively, on the initial draw, and for the 24-h draw, 58 ± 38 (0-h controls carried forward), 109 ± 105, and 304 ± 277 ng/dL (Fig. 1B). These means and medians were all found to be different between the three groups by one-way ANOVA and the test for the median (P < 0.01) total VEGF (as opposed to free VEGF) and PlGF levels, which were similar between all groups (data not shown). We also assessed our study group post hoc for the actual presence of infection and found three patients where infection was identified on enrollment, but the subsequent course led to an alternative diagnosis. The VEGF levels were similar to the control groups for these three patients at baseline (67, 72, 29 ng/dL) and at 24 h (29, 67, 117 ng/dL), as were the sFLT levels (146, 266, 268 and 211, 266, 339 ng/dL, respectively); however, because this was a prospectively defined cohort, they were included for all analyses as “suspected infection.”
Fig. 1. A, The sFLT levels (pg/dL) for noninfected controls, patients with suspected infection but no shock, and with suspected septic shock at 0 and 24 h are shown.
If a patient was in shock at 0 h but resolved at 24 h, they were reclassified as not having shock at the 24-h mark. The mean levels are denoted by the black lines for each category. When the ANOVA test is applied to each draw hour (0- and 24-h groups, with control values from 0 h used as a reference group), there is a significant difference found for the means across the groups (P < 0.01); similarly, the medians were also found to be statistically different across the groups. (P < 0.01). B, The VEGF levels (pg/dL) for noninfected controls, patients with suspected infection but no shock, and with suspected septic shock at 0 and 24 h are shown. If a patient was in shock at 0 h but resolved at 24 h, they were reclassified as not having shock at the 24-h mark. The mean levels are denoted by the black lines for each category. When the ANOVA test is applied to each draw hour (0- and 24-h groups, with control values from 0 h used as a reference group), there is a significant difference found between the means across the groups (P < 0.01); similarly, the medians were also found to be statistically different across the groups (P < 0.01).
Next, we assessed the association between (1) the initial level of the markers with the initial severity measures (APACHE-II, SOFA, and presence of septic shock), (2) the initial markers with 24-h severity measures, and (3) the association of the marker at 24 h with the severity measures at 24 h (Table 2). This matrix demonstrates that initial VEGF levels were significantly associated with the initial APACHE-II score. Additionally, initial and 24-h VEGF levels had a significant correlation with the presence of septic shock at 24 h. The initial and 24-h sFLT levels were well correlated with all measures except for 24-h sFLT with 24-h septic shock. Both total VEGF and PlGF showed little correlation with any of the severity measures (data not shown). This is not surprising because the total VEGF includes VEGF bound to endogenous proteins that is essentially inactive as opposed to free VEGF that is available to bind and activate the endothelium. Initial IL-1 levels were well correlated with both initial and 24-h measures; however, at 24 h, IL-1 levels had no correlation with the measures of interest, further bolstering its role as an early marker in the inflammatory cascade. Finally, initial IL-6 levels showed good correlation with all of the initial measures (except SOFA score) and had a stronger correlation with subsequent severity measures at 24 h. The 24-h IL-6 level also showed a substantial correlation with all severity of illness measures at the 24-h point.
Table 2.
Correlation of VEGF, sFLT, IL-1, and IL-6 with severity of illness
| Time = 0 h |
Time = 24 h |
|||||
|---|---|---|---|---|---|---|
| APACHE-II | SOFA | Septic shock | APACHE-II | SOFA | Septic shock | |
| VEGF | ||||||
| Corr. Coeff. | 0.28 | 0.16 | 0.11 | 0.18 | 0.09 | 0.24 |
| P – value | 0.01 | 0.15 | 0.33 | 0.13 | 0.43 | 0.03 |
| sFLT | ||||||
| Corr. Coeff. | 0.29 | 0.27 | 0.18 | 0.28 | 0.32 | 0.27 |
| P – value | 0.01 | 0.01 | 0.1 | 0.02 | 0.01 | 0.01 |
| IL-1 | ||||||
| Corr. Coeff. | 0.41 | 0.41 | 0.33 | 0.47 | 0.49 | 0.51 |
| P – value | 0.001 | 0.001 | 0.01 | 0.001 | 0.001 | 0.001 |
| IL-6 | ||||||
| Corr. Coeff. | 0.22 | 0.11 | 0.18 | 0.4 | 0.42 | 0.46 |
| P – value | 0.04 | 0.31 | 0.1 | 0.001 | 0.0002 | <0.0001 |
| VEGF | * | * | * | 0.26 | 0.1 | 0.37 |
| Corr. Coeff. | ||||||
| P – value | 0.03 | 0.4 | 0.001 | |||
| sFLT | ||||||
| Corr. Coeff. | * | * | * | 0.25 | 0.27 | 0.17 |
| P – value | 0.04 | 0.02 | 0.13 | |||
| IL-1 | ||||||
| Corr. Coeff. | * | * | * | 0.14 | 0.02 | 0.07 |
| P – value | 0.26 | 0.86 | 0.56 | |||
| IL-6 | ||||||
| Corr. Coeff. | * | * | * | 0.34 | 0.39 | 0.47 |
| P – value | 0.005 | 0.001 | 0.001 | |||
The correlation coefficients (Corr. Coeff.) for VEGF, sFLT, IL-1, and IL-6 with the severity of illness measures of APACHE-II, SOFA, and the presence of septic shock are shown.
The correlation of the markers at 24 h with initial severity of illness was not assessed.
We also examined the correlation between VEGF and sFLT with the markers of the inflammatory cascade, IL-1 and IL-6, at 0 and 24 h. The VEGF levels were significantly correlated with IL-1 levels at 0 (r = 0.42; P < 0.01) and 24 h (r = 0.38; P < 0.01). Interestingly, VEGF initially had little correlation with IL-6 initially (r = 0.04; P = 0.7) but developed a strong correlation by 24 h (r = 0.49; P < 0.01). Initial sFLT correlated significantly with IL-1 (0.31 and 0.47; P < 0.01) and IL-6 (0.43 and 0.25; P < 0.01) at 0 and 24 h, respectively. Thus, both VEGF and sFLT seemed to correlate with markers of the inflammatory response.
To explore the influence of levels of VEGF and sFLT in combination, we considered the different VEGF-sFLT combinations (low VEGF–low sFLT, high VEGF–low sFLT, low VEGF–high sFLT, high VEGF–high sFLT) by dichotomizing the marker levels at their median values. There was a trend toward increasing APACHE-II and SOFA score when there are elevated levels of VEGF or sFLT, with the highest severity of illness scores occurring when both values are elevated (Table 3). The APACHE-II and SOFA scores of patients with both high VEGF and high sFLT were significantly higher than patients with low levels of each (P < 0.01 for all comparisons). Additionally, the plots in Figure 2 show a distribution toward higher initial VEGF and sFLT levels in combination as stratified by the noninfected controls, suspect infection without shock, and septic shock.
Table 3.
SOFA and APACHE-II scores by VEGF and sFLT groups
| Marker = 0 h, outcome = 0 h |
Marker = 0 h, outcome = 24 h |
Marker = 24 h, outcome = 24 h |
||||
|---|---|---|---|---|---|---|
| Categories (mean, SD) | APACHE-II | SOFA | APACHE-II | SOFA | APACHE-II | SOFA |
| Low VEGF, low sFLT | 7.7 (7.7) | 1.0 (1.7) | 12.9 (11.2) | 2.0 (2.8) | 12.6 (9.3) | 2.1 (2.5) |
| High VEGF, low sFLT | 13.0 (6.3) | 1.8 (2.5) | 17.6 (9.1) | 2.9 (2.8) | 17.4 (10.9) | 2.4 (3.4) |
| Low VEGF, high sFLT | 15.7 (4.1) | 2.3 (3.0) | 19.3 (7.1) | 3.6 (3.8) | 20.1 (11.8) | 4.8 (4.4) |
| High VEGF, high sFLT | 15.3 (8.0) | 3.5 (3.3) | 21.0 (12.5) | 5.0 (4.3) | 24.2 (9.7) | 5.3 (3.8) |
Patients were stratified by whether they were more or less than the median levels of VEGF or sFLT and grouped. The APACHE-II and SOFA scores for the high VEGF–high sFLT group were significantly higher than the low VEGF–low sFLT group (P < 0.01 for all comparisons).
Fig. 2. Actual VEGF and sFLT values plotted simultaneously for patients upon ED presentation in the different categories of controls, suspected infection without shock and infection with shock.
The distribution seems to favor higher levels of VEGF and sFLT, with increasing severity going from controls to suspected infection without shock and suspected infection with shock.
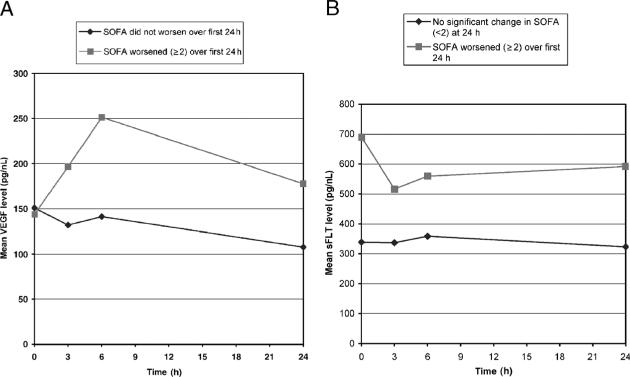
Finally, we identified patients whose 24-h SOFA score had worsened by two points or greater compared with their SOFA score on their initial presentation. The mean sFLT and VEGF levels were plotted over time (Fig. 3, A and B). Aside from the initial VEGF level, the VEGF and sFLT levels were higher in patients whose course worsened as compared with those who did not. Next, we modeled the relationship of VEGF with those who worsened their SOFA score over the first 24 h using a linear mixed-effects model. The model contained the repeated measures of VEGF for times 0, 3, 6, 24 h as the dependent variable, an indicator of whether SOFA worsened over 24 h or not, the time point indicator (0, 3, 6, 24), and the interaction of these two terms as independent variables. In the final model, we found the estimated mean VEGF of this group relative to the nonworsened group to be higher (74.9 ng/dL; SE = 36.9; P = 0.04). Similarly, we used a linear mixed-effects model to examine sFLT between these two groups and found that at both baseline and 24 h, sFLT was higher for the group with the worsened SOFA score. At baseline (342.9 ng/dL; SE = 81.1; P = 0.01) and at 24 h (211.9 ng/dL; SE = 81.6; P = 0.01) (Fig. 3B).
Fig. 3. A, This graph compares VEGF levels among patients who have a worsening of their SOFA (≥2) score as compared with those who either (1) do not worsen by more than one point or (2) improve their SOFA score.
*A linear mixed-effects model containing as independent variables an indicator of whether SOFA worsened over 24 h or not, the time point indicator (0, 3, 6, 24), and the interaction of these two terms yielded a statistically significant difference of VEGF at 24 h (75 pg/nL; SE = 36.9; P = 0.04). We tested for confounding by adding the following variables to the model: diabetes, malignancy, end-stage renal disease, and age; none of terms were significant predictors of worsening SOFA. B, This graph compares sFLT levels among patients who have a worsening of their SOFA (≥2) score as compared with those who either (1) do not worsen by more than one points or (2) improve their SOFA score.*A linear mixed-effects model containing as independent variables an indicator of whether SOFA worsened over 24 h or not, the time point indicator (0, 3, 6, 24), and the interaction of these two terms yielded a statistically significant difference of sFLT at baseline (343 pg/nL; SE = 81; P = 0.001), and at 24 h (212 pg/nL; SE = 81.6; P = 0.01). We tested for confounding by adding the following variables to the model: diabetes, malignancy, end-stage renal disease, and age; none of the terms were significant predictors of worsening SOFA.
DISCUSSION
Despite years of research and development, the treatment of patients with severe sepsis remains an important challenge. To date, therapies that inhibit one or another component of the inflammatory or coagulation pathways have had little impact on survival. Clearly, future advances in therapy will be contingent upon an improved understanding of sepsis pathophysiology. One theme that emerges from basic and preclinical research is the importance of the endothelium in mediating the host response to infection. Recent preclinical studies in mice implicate a role for VEGF signaling in the pathophysiology and diagnosis/prognosis of sepsis (13). These data raise the possibility that VEGF, and its soluble receptor sFLT, may eventually be exploited for diagnostic and/or therapeutic purposes.
The goal of the present study was to extend these observations in humans by evaluating the blood levels of free VEGF and sFLT during the early presentation of patients with suspected infection. The major findings were that (1) median sFLT and VEGF levels were higher across the following three groups: noninfected controls, suspected infection patients without shock, and suspected infection patients with shock; (2) initial VEGF levels correlated with initial APACHE-II score; (3) initial and 24-h VEGF levels had a significant correlation with the presence of septic shock at 24 h; (4) initial and 24-h sFLT levels were correlated with the presence of septic shock, APACHE-II, and SOFA scores at 0 and 24 h (except for 24-h sFLT with 24-h septic shock); and (5) VEGF and sFLT were significantly associated with inflammatory cascade activation.
The underlying stimulus and the tissue source for the increased VEGF and sFLT in sepsis are unknown. It is well established that hypoxia induces the expression of VEGF in many cell types. However, other extracellular factors such as cytokines and chemokines can also increase VEGF expression. In a mouse model of endotoxemia, administration of LPS resulted in increased VEGF mRNA and protein levels in multiple organs (13). Other potential sources for VEGF include circulating leukocytes and platelets. Previous studies have demonstrated that the placenta produces sFLT. Indeed, in preeclampsia, the placenta releases higher levels of sFLT, which then binds VEGF, inhibiting its interaction with surface-bound VEGF receptors (18). To our knowledge, this is the first report of increased sFLT levels in a nonpregnant setting in humans. It remains to be determined whether the sFLT observed in septic patients represents the alternative splice form or whether it arises from protease-mediated cleavage of membrane bound Flt-1.
It is important to draw a distinction between the role of VEGF or sFLT as a pathophysiological mediator and diagnostic marker. Current evidence suggests that VEGF contributes to sepsis pathogenesis, and that circulating levels correlate with disease severity. Although increased levels of endogenous sFLT also correlate with disease severity, they may have a protective effect in sepsis by inhibiting free VEGF. Stated another way, sFLT may represent a component of the compensatory anti-inflammatory response syndrome (akin to the anti-inflammatory mediator, IL-10, whose levels also correlate with increased sepsis mortality [19]). However, a protective role for endogenous sFLT remains to be proven. Indeed, in a mouse model of endotoxemia, the increased levels of endogenous sFLT were only one fifth of the therapeutic levels achieved with exogenous administration of sFLT, suggesting that the normal host response may be insufficient to blunt VEGF-mediated signaling in sepsis.
There are two small human studies that we are aware of that have previously reported an association between circulating levels of VEGF and sepsis in humans. In a study of 18 adult patients with severe sepsis, maximal VEGF levels during the course of disease were higher in nonsurvivors compared with survivors (14). In a study of 13 pediatric patients with meningococcal infection, VEGF levels were highest in those patients with shock (15). The VEGF concentration at presentation correlated with severity of disease. In the current report, we have extended these observations by studying a larger number of patients, exploring the associations over time, and including in our analysis other members of the VEGF signaling axis. Our data add to these prior studies not only in the larger number of patients and a more generalizable population but also in the fact that we explored the associations initially and over time, that we assayed for another component of the VEGF signaling axis (i.e., sFLT), and that we compared the markers with other known mediators involved in the inflammatory response.
There are several limitations to our study. First, we used markers of severity (i.e., APACHE-II and SOFA) as opposed to an outcome such as mortality. We did not use a consecutive patient population so the study is subject to selection bias. There may have been unmeasured confounder or confounding that was not accounted for in this analysis. We did not collect all samples for all patients if they were discharged from the hospital or we were otherwise unable to collect the specimens. We were also somewhat limited by our sample size and did not perform an adjusted analysis. Finally, we did not perform duplicate measurements of the assays so there may be some degree of imprecision in our measurements.
In conclusion, we have shown that sFLT represents a potential new marker in patients with sepsis. Vascular endothelial growth factor and its signaling axis remains a promising avenue of research. Future studies are needed to determine whether VEGF and sFLT are superior to existing markers in predicting clinical course and/or monitoring therapeutic response. An improved understanding of the endothelial cell response, in particular the VEGF signaling pathway, may lead to novel diagnostic and therapeutic opportunities.
Acknowledgments
This work was supported by National Institutes of Health grants 5R01HL082927 (W.C.A.), P5O GMO76659 (N.I.S. and D.A.) and RO1 GM61992 (D.A.).
ABBREVIATIONS
- APACHE
Acute Physiology Age Chronic Health Evaluation
- ED
emergency department
- SOFA
Sepsis-related Organ Failure Assessment
- VEGF
vascular endothelial cell growth factor
Footnotes
The study was performed at Beth Israel Deaconess Medical Center, Boston, Mass.
The authors do not disclose any competing interests.
N.I.S., K.Y., and W.A. conceived of the study, acquired and analyzed the data, and drafted the manuscript. H.O. and K.S. assisted in performing enzyme-linked immunosorbent assay measurements. M.H., C.F., and D.A. assisted in data interpretation and manuscript preparation. L.N. assisted in data analysis. All authors read and approved the final manuscript.
REFERENCES
- 1.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 2.Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, Korach JM, Capellier G, Cohen Y, Azoulay E, Troche G, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–871. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- 3.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 4.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 5.ARDSNET Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 6.Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101:3765–3777. doi: 10.1182/blood-2002-06-1887. [DOI] [PubMed] [Google Scholar]
- 7.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 8.Kim I, Moon SO, Kim SH, Kim HJ, Koh YS, Koh GY. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E- selectin through nuclear factor-kappa B activation in endothelial cells. J Biol Chem. 2001;276:7614–7620. doi: 10.1074/jbc.M009705200. [DOI] [PubMed] [Google Scholar]
- 9.Reinders ME, Sho M, Izawa A, Wang P, Mukhopadhyay D, Koss KE, Geehan CS, Luster AD, Sayegh MH, Briscoe DM. Proinflammatory functions of vascular endothelial growth factor in alloimmunity. J Clin Invest. 2003;112:1655–1665. doi: 10.1172/JCI17712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucerna M, Mechtcheriakova D, Kadl A, Schabbauer G, Schafer R, Gruber F, Koshelnick Y, Muller HD, Issbrucker K, Clauss M, et al. NAB2, a corepressor of EGR-1, inhibits vascular endothelial growth factor-mediated gene induction and angiogenic responses of endothelial cells. J Biol Chem. 2003;278:11433–11440. doi: 10.1074/jbc.M204937200. [DOI] [PubMed] [Google Scholar]
- 11.Kuenen BC, Levi M, Meijers JC, Kakkar AK, van Hinsbergh VW, Kostense PJ, Pinedo HM, Hoekman K. Analysis of coagulation cascade and endothelial cell activation during inhibition of vascular endothelial growth factor/vascular endothelial growth factor receptor pathway in cancer patients. Arterioscler Thromb Vasc Biol. 2002;22:1500–1505. doi: 10.1161/01.atv.0000030186.66672.36. [DOI] [PubMed] [Google Scholar]
- 12.Kendall RL, Wang G, Thomas KA. Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochem Biophys Res Commun. 1996;226:324–328. doi: 10.1006/bbrc.1996.1355. [DOI] [PubMed] [Google Scholar]
- 13.Yano K, Liaw PC, Mullington JM, Shih SC, Okada H, Bodyak N, Kang PM, Toltl L, Belikoff B, Buras J, et al. Vascular endothelial growth factor is an important determinant of sepsis morbidity and mortality. J Exp Med. 2006;203:1447–1458. doi: 10.1084/jem.20060375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Flier M, van Leeuwen HJ, van Kessel KP, Kimpen JL, Hoepelman AI, Geelen SP. Plasma vascular endothelial growth factor in severe sepsis. Shock. 2005;23:35–38. doi: 10.1097/01.shk.0000150728.91155.41. [DOI] [PubMed] [Google Scholar]
- 15.Pickkers P, Sprong T, Eijk L, Hoeven H, Smits P, Deuren M. Vascular endothelial growth factor is increased during the first 48 hours of human septic shock and correlates with vascular permeability. Shock. 2005;24:508–512. doi: 10.1097/01.shk.0000190827.36406.6e. [DOI] [PubMed] [Google Scholar]
- 16.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 17.Vincent J, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart C, Suter P, Thijs L. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 18.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 19.Lehmann AK, Halstensen A, Sornes S, Rokke O, Waage A. High levels of interleukin 10 in serum are associated with fatality in meningococcal disease. Infect Immun. 1995;63:2109–2112. doi: 10.1128/iai.63.6.2109-2112.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]