Abstract
Adult bone marrow-derived cells can improve organ function in chronic disease models, ostensibly by the release of paracrine factors. It has, however, been difficult to reconcile this prevailing paradigm with the lack of cell retention within injured organs and their rapid migration to the reticuloendothelial system. Here, we provide evidence that the salutary antifibrotic effects of bone marrow-derived early outgrowth cells (EOCs) are more consistent with an endocrine mode of action, demonstrating not only the presence of antifibrotic factors in the plasma of EOC-treated rats but also that EOC conditioned medium (EOC-CM) potently attenuates both TGF-β- and angiotensin II-induced fibroblast collagen production in vitro. To examine the therapeutic relevance of these findings in vivo, 5/6 subtotally nephrectomized rats, a model of chronic kidney and heart failure characterized by progressive fibrosis of both organs, were randomized to receive i.v. injections of EOC-CM, unconditioned medium, or 106 EOCs. Rats that received unconditioned medium developed severe kidney injury with cardiac diastolic dysfunction. In comparison, EOC-CM-treated rats demonstrated substantially improved renal and cardiac function and structure, mimicking the changes found in EOC-treated animals. Mass spectrometric analysis of EOC-CM identified proteins that regulate cellular functions implicated in fibrosis. These results indicate that EOCs secrete soluble factor(s) with highly potent antifibrotic activity, that when injected intravenously replicate the salutary effects of the cells themselves. Together, these findings suggest that an endocrine mode of action may underlie the effectiveness of cell therapy in certain settings and portend the possibility for systemic delivery of cell-free therapy.
Keywords: Kidney, Chronic kidney disease, Congestive heart failure, Fibrosis, Early outgrowth cell, Cell therapy
Introduction
The mechanisms by which bone marrow-derived cells induce their beneficial effects has been the subject of longstanding speculation. Although previously thought to engraft and differentiate into mature parenchymal or vascular cells [1–4], the extent to which this occurs has been controversial [5–8]. Indeed, recent opinion favors a paracrine mechanism of action whereby the administered cells secrete a range of factors that initiate endogenous regenerative programs by cells in their immediate proximity [9]. It has, however, been difficult to reconcile key experimental findings with this paradigm. First, despite dramatic structural and functional improvements [10], long-term target organ retention of transferred cells is often extremely low [7, 11, 12]. Second, the in vivo administration of the ostensible paracrine factors leads to tissue effects at distances that are substantially greater than what would conventionally be considered paracrine [8]. Such discrepancies have lead to calls for a more detailed examination of the mechanisms of action of cell therapy prior to their clinical translation [13].
Compounding these reservations are the uncertainties surrounding the ultimate fate of such cells postinfusion [13]. Given the proliferative potential of certain bone marrow-derived cells, it is perhaps unsurprising that recent reports have highlighted the potential for pathologic growth of certain cell types when used clinically [14]. In the absence of a more detailed understanding of the mechanisms controlling cell homing and cell fate postinfusion, these studies have raised concerns regarding the safety of bone marrow-derived cell infusion for the treatment of chronic disease [13].
Fibrotic injury is a pivotal pathophysiologic contributor to the progression of many chronic diseases, including chronic kidney disease (CKD) and its cardiovascular complications. In CKD, progressive fibrosis of the kidney and heart leads, over time, to irreversible damage and loss of organ function. Whereas in the kidney, this injury impairs filtration and tubular cell function, in the heart, fibrosis stiffens the left ventricle and impairs its ability to relax and fill during diastole. This shared cardio-renal pathophysiology is reflected clinically in the common development of heart failure with preserved ejection fraction in CKD patients, a phenotype with a particularly poor prognosis and for which therapeutic options are extremely limited [15].
We have previously reported that bone marrow-derived early outgrowth cells (EOCs) attenuate tissue fibrosis and organ dysfunction in models of diabetic [12] and nondiabetic [11] kidney disease. In these studies, we found that i.v. and intra-arterial routes were similarly efficacious. We further found that in contrast to their near-absence in the kidney, labeled EOCs were present in abundance in the liver, spleen, and bone marrow [11, 12]. In this study, we provide evidence that the antifibrotic activity of EOCs is mediated by a novel endocrine mechanism of action, demonstrating not only the presence of circulating antifibrotic factors in the plasma of rats treated with EOCs but also that EOC-derived soluble factors potently inhibit both transforming growth factor-β (TGF-β)-and angiotensin II-induced fibroblast collagen production in vitro. We further demonstrate the therapeutic relevance of these findings in vivo, showing that infusion of EOC-derived factors alone can mimic the antifibrotic tissue protective effects of the cells themselves in the 5/6 subtotally nephrectomized (SNX) rat, a clinically relevant model of cardio-renal failure.
Materials and Methods
Isolation of EOCs and Cell Culture
EOCs were cultured as previously described [11]. Briefly, bone marrow cells were flushed from the femora and tibiae of 4-week-old male Fischer 344 rats (Charles River, Montreal, Quebec) with sterile phosphate-buffered saline (PBS). The collected cells were plated in endothelial cell culture medium-2 (with single quots, Lonza, Walkersville, MD, www.lonza.com) on human fibronectin-coated tissue culture flasks and incubated at 37°C with 5% CO2 for 7–10 days to produce EOCs.
Isolation of Mesenchymal Stem Cells and Cell Culture
Mesenchymal stem cells (MSCs) were cultured as previously described [11, 16]. Flushed bone marrow cells harvested as above were seeded in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Burlington, Ontario, Canada, http://www.invitrogen.com) on plastic tissue culture flasks, and incubated at 37°C with 5% CO2 to produce MSCs. Medium was changed every 2–3 days. Cells used for experiments were between passages 2 and 6.
Flow Cytometric Analysis
Flow cytometric analysis of EOC and MSC surface marker expression was performed as previously described [12, 17]. Cultured EOC expression of a panel of cell surface markers was examined following immunostaining with the following antibodies (1 μg of each antibody for 106 cells in a 100 μL volume for 20 minutes) and analysis using a MACS Quant flow cytometer (Miltenyi Biotech, Auburn, CA, http://www.miltenyibiotec.com): (a) Alexa Fluor 647-conjugated anti-CD34 (BD Biosciences, San Jose, CA, http://www.bdbiosciences.com), (b) VioBlue-conjugated anti-vascular endothelial growth factor 2 (VEGFR2) (BD Biosciences), (c) PE-conjugated anti-vascular endothelial (VE)-cadherin (Santa Cruz Biotechnology, Santa Cruz, CA, http://www.scbt.com), (d) Allophycocyanin (APC)-conjugated anti-CD73 (Biolegend, San Diego, CA, www.biolegend.com), (e) BV421-conjugated anti-CD90 (Biolegend), (f) Phycoerythrin (PE)-conjugated anti-CD105 (Biolegend), (g) PE-Cy7-conjugated anti-CD45 (Biolegend), and (h) Fluorescein isothiocyanate (FITC)-conjugated anti-CD11b (Biolegend). EOCs were additionally stained with fluorescein-labeled Griffonia (Bandeiraea) simplicifolia lectin I—isolectin B4 (Vector Laboratories, Burlington, Ontario, Canada, http://www.vectorlabs.com). Cultured MSCs were stained for CD73, CD90, CD105, CD45, and CD11b with the above-described antibodies. Cells incubated with irrelevant IgGs conjugated to the appropriate fluorophore were used as negative isotype controls and gating was further validated using the appropriate Fluorescence Minus One controls. Analysis was performed using MACS Quantify data acquisition and analysis software (Miltenyi Biotech).
Acetylated Low Density Lipoprotein (LDL) Uptake
EOCs were incubated with DiI-labeled acLDL (Biomedical Technologies Inc., U.K., www.btiinc.com) for 4 hours at 37°C and fixed with 4% paraformaldehyde. Cells were imaged using an inverted epifluorescence microscope equipped with a digital camera.
Mesenchymal Lineage Differentiation Assays
EOCs and MSCs were cultured in adipogenic, osteogenic, or chondrogenic differentiation media (Invitrogen) for 1–2 weeks as previously described [18]. Medium was changed every 2–3 days. After fixation with 4% paraformaldehyde, cells were stained with oil red O, von Kossa, or Alcian blue stains to confirm successful adipogenic, osteogenic, and chondrogenic differentiation, respectively.
Phagocytosis Assays
The phagocytic activity of EOCs was assessed using a commercially available kit according to the manufacturer’s instructions (Vybrant Phagocytosis Assay Kit, Molecular Probes, Burlington, Ontario, Canada, http://probes.invitrogen.com). Cell-free medium served as a negative control. Mouse PU5-1.8 macrophages (a generous gift from Dr. John Semple) were used as a positive control.
Conditioned Medium Generation
EOC conditioned medium (EOC-CM) was generated by incubating subconfluent 10-day cultured EOCs with serum-free endothelial basal medium-2 (EBM-2, Lonza) for 24 hours after washing of the EOCs to remove any residual serum [19]. Collected EOC-CM from n ≥ 5 donor animals was then pooled and used for in vitro assays. For in vivo experiments, cell-free EOC-CM was collected and pooled as above from 30 donor animals. Prior to in vivo injection, pooled EOC-CM and EBM-2 were concentrated 10-fold using a <10 kDa cutoff centrifuge filtration column (Millipore, Billerica, MA, http://www.millipore.com), followed by filtration using a 0.45 μm filter (Millipore). 0.5 mL aliquots of concentrated EOC-CM and EBM-2 were stored at −80°C until injection.
[3H]-Proline Incorporation Assay
Following serum starvation, primary neonatal rat cardiac fibroblasts were incubated with 0.5 mL of neat EOC-CM, diluted EOC-CM, or unconditioned EBM-2 for 4 hours. Fibroblasts were then stimulated with 10−7 mol/L of angiotensin II (Sigma-Aldrich, Oakville, Canada, http://www.sigmaaldrich.com) or 20 ng/mL of TGF-β (R & D Biosystems, Minneapolis, MN, http://www.rndsystems.com) and incubated with [3H]-proline (1 μCi/ well, Amersham Biosciences, Piscataway, NJ, http://www.amer-sham.com) for 44 hours. In some experiments, fibroblasts were coincubated with rat plasma or marimastat 100 nM (Tocris Bioscience, Minneapolis, MN), a pan-matrix metalloproteinase (MMP) inhibitor. Incorporation of [3H]-proline was measured using a liquid scintillation counter (LS 6000 Beckman Instruments, Beckman Coulter, Mississauga, Canada) [11, 20–23].
Animal Experiments
All animal studies were approved by the St. Michael’s Hospital Animal Ethics Committee. In the first set of experiments, Fischer 344 rats (Charles River) of 8 weeks of age were randomized to one-step subtotal 5/6 nephrectomy (n = 6) or sham surgery (n = 3) [11]. Four weeks after surgery, SNX animals were sub-randomized to receive a single i.v. injection of 106 EOCs (n = 3) or control vehicle (n = 3). Eight weeks after surgery, plasma was collected by tail vein bleed into an EDTA-containing tube followed by centrifugation at 1,500 rpm for 5 minutes and collection of the resulting supernatant. This rat plasma was then subjected to fibroblast [3H]-proline incorporation assays as described above.
In the second set of experiments, Fischer 344 rats (Charles River) of 8 weeks of age were randomized to SNX (n = 26) or sham surgery (n = 4) [11]. Four weeks after SNX, animals were randomized to receive an infusion of: (a) 1 × 106 EOCs (tail vein injection, n = 8), (b) thrice weekly tail vein injections of 10× concentrated EOC-CM for 2 weeks (n = 9), or (c) thrice weekly tail vein injections of 10× concentrated EBM-2 (n = 9). On the day of infusion, EOCs were trypsinized, washed with PBS, and resuspended at a concentration of 106 cells per milliliter in sterile PBS. The total volume of EOC infusion for each animal was 1 mL. The total volume of each EOC-CM or EBM-2 medium injection was 0.5 mL.
Renal Functional Parameters
Body weight was determined weekly. Before surgery, and 4, 6, and 8 weeks after surgery, spot urine collections were performed for measurement of urine protein and creatinine concentration. Systolic blood pressure was measured in conscious rats using an occlusive tail-cuff plethysmograph (Powerlab, ADInstruments, CO Springs, CO) [24].
Glomerular Filtration Rate Measurement
Prior to sacrifice, rats underwent glomerular filtration rate (GFR) measurement using a modified FITC-inulin plasma clearance assay [25]. Briefly, rats were tail vein injected with 3.74 μL/g b.wt. FITC-inulin. Tail vein blood was sampled at various time points post-FITC-inulin injection. Sample fluorescence was detected with a plate reader (excitation 485 nm, emission 527 nm). GFR was calculated using a two-phase, exponential decay curve using nonlinear regression statistics as previously described [25].
Cardiac Catheterization
Cardiac catheterization and data analysis were performed as previously published [26]. In brief, following cardiac catheterization with a 2F combined conductance catheter-micro-manometer (Model SPR-838 Millar instruments, Houston, TX), data were acquired under steady state conditions and during preload reduction. The following functional parameters were measured and calculated (Millar analysis software PVAN 3.4): heart rate, dp/dt +, dp/dt −, Tau logistic, and the slope of the end diastolic pressure volume relationship (EDPVR).
Tissue Preparation and Histochemistry
At study end, the heart and kidney were immersion fixed in 10% neutral buffered formalin, embedded in cryostat matrix (Tissue-Tek, Sakura, Kobe, Japan) and flash frozen in liquid nitrogen. Formalin-fixed tissues were embedded in paraffin and sectioned before staining with picrosirius red or immunolabeling as described below.
Immunohistochemistry and Assessment of Renal Fibrosis
Glomerulosclerosis and tubulointerstitial fibrosis were assessed by examining the accumulation of type IV collagen in 30 randomly selected glomeruli and 6 random nonoverlapping 20× fields for each animal following immunostaining with a goat anti-rat type IV collagen polyclonal antibody (Southern Biotech, Birmingham, AL) [27]. Omission of primary antisera served as the negative control. Type IV collagen accumulation was analyzed from scanned images as previously described using Imagescope (Aperio, Vista, CA, www.aperio.com), a program which identifies positively stained (brown) pixels [11]. The amount of bound anti-type IV collagen antibody was calculated by dividing the number of brown pixels by the total number of pixels in the selected fields. Glomeruli were analyzed by drawing outlines around each randomly selected glomerulus. Cortical tubulo-interstitial 20× fields were analyzed by inclusion of the entire randomly selected 20× field.
Assessment of Cardiac Fibrosis
The accumulation of extracellular matrix was quantified in five random nonoverlapping subendocardial 16× fields in picrosirius red stained heart sections using Imagescope computer-assisted image analysis. The Imagescope program was used to quantify picrosirius red-stained fibrillar collagen by dividing the number of positively stained (red) pixels by the total number of pixels in each randomly selected subendocardial 16× field.
Quantitative Real-Time Polymerase Chain Reaction
RNA was isolated from snap-frozen kidney tissue and reverse transcribed. Real-time quantitative polymerase chain reaction (PCR) was performed to determine the relative expression levels of α (1) type I and α (1) type IV collagen, in comparison to Rpl13a, a housekeeping transcript. All primers were purchased from Applied Biosystems (Burlington, Ontario, Canada, http://www.appliedbiosystems.com). Experiments were performed in triplicate. Data analyses were performed using the Applied Bio-systems Comparative CT method. All values were referenced to the mRNA transcript levels of the housekeeper gene Rpl13a.
Trypsin digestion, Mass spectroscopy, and Data Analysis
Ten micrograms of EOC-CM samples were trypsin digested, acidified to pH 2 with formic acid, and loaded on to a LTQ-Orbitrap XL (Thermo Scientific, www.thermoscientific.com) mass spectrometer using a nanoelectrospray ionization source (Proxeon Biosystems, www.proxeon.com) as previously described [28]. XCalibur RAW files were searched against the nonredundant IPI.Rat v.3.85 database, and the search results were loaded into Scaffold (v.2.06, Proteome Software Inc., www.proteomesoftware.com) and an additional database search with the X!Tandem (Global Proteome Machine, www.thegpm.org) matching algorithm was performed. Identified proteins were explored using Ingenuity Pathway Analysis (version 9.0).
Statistical Analysis
All data are shown as mean ± SEM unless otherwise stated. A minimum number of three independent experiments were performed for all in vitro experiments. Differences between groups were analyzed by ANOVA with post hoc Fisher’s protected least significant difference. All statistics were performed using Graph-Pad Prism 5.00 (GraphPad Software, San Diego, CA, www.graphpad.com) or SPSS 15.0 (SPSS, Chicago, IL, www.ibm.com/software/analytics/SPSS). A change was considered statistically significant if p < .05.
Results
EOCs Are a Heterogeneous Bone Marrow-Derived Cell Population with Endothelial and Monocyte/ Macrophage-Like Properties
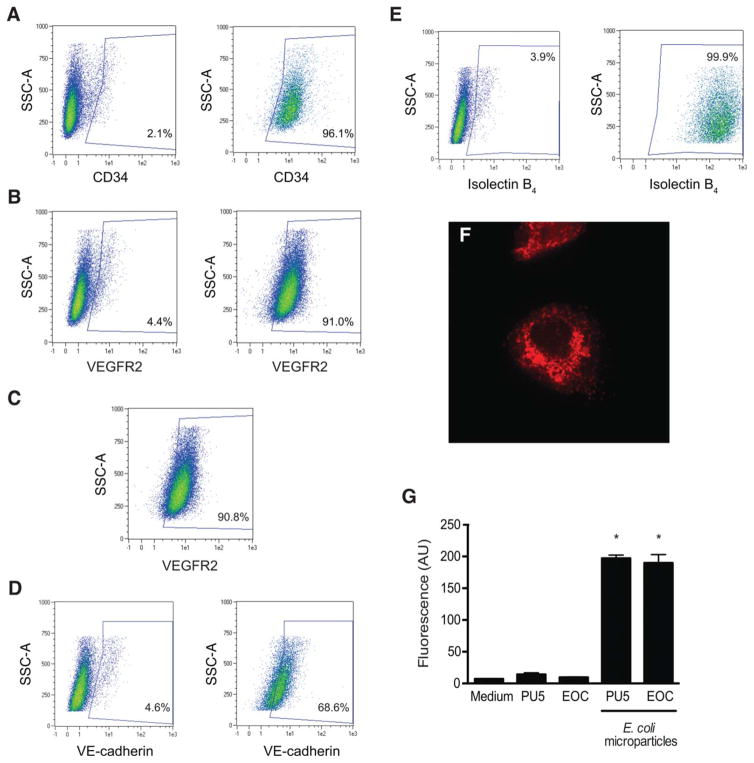
Flow cytometric analysis demonstrated that EOCs are a heterogeneous population of cells enriched in their expression of hematopoietic (CD34) and endothelial (lectin binding, VEGFR2, VE-cadherin) markers (Fig. 1A–1E) [11, 17]. Functionally, as previously shown, EOCs displayed features of both endothelial cells and macrophages, demonstrating the ability to uptake acetylated LDL [29, 30] and the capacity to ingest fluorescein-labeled E. coli microparticles (Fig. 1F–1G) [31].
Figure 1.
Early outgrowth cells (EOCs) are a heterogeneous bone marrow-derived cell population with endothelial and monocyte/macrophage-like properties. EOCs were characterized with a set of flow cytometric and functional assays. (A–C): Expression of commonly measured EOC surface markers was analyzed by flow cytometry after staining with the following antibodies: (A) Alexa Fluor 647-conjugated anti-CD34 and (B) VioBlue-conjugated anti-VEGFR2. Left panels: Unstained cells. Right panels: Antibody-stained cells. The percentage of positive cells for a given area is listed on each plot. (C): Single positive CD34+ cells were gated as shown in panel (A) and examined for VEGFR2 expression. (D, E): To assess for expression of additional endothelial surface markers, EOCs were stained with (D) PE-conjugated anti-VE-cadherin and (E) fluorescein-conjugated Griffonia (Bandeiraea) simplicifolia lectin I—isolectin B4. Left panels: Unstained cells. Right panels: Stained cells. (F): EOCs were incubated with DiI-conjugated acetylated LDL (red) for 30 minutes and then imaged with an inverted epifluorescence microscope equipped with a digital camera. Original magnification: ×10. Representative image from n = 3 independent experiments. (G): EOCs were incubated with fluorescently labeled E. coli microparticles using a commercially available phagocytosis assay kit. After washing and quenching of uningested fluorescent microparticles, fluorescence was read by a plate reader. PU5-1.8 mouse macrophages were used as a positive control. *, p < .05 versus medium alone. Results are representative of a minimum of n = 6 replicates per condition. Abbreviations: AU, arbitrary units; SSC-A, side scatter; VEGFR, vascular endothelial growth factor receptor 2; VE, vascular endothelium.
As MSCs, another bone marrow-derived cell population, have been previously shown to release soluble renoprotective factors [32], we specifically examined whether EOCs met previously published consensus definition criteria for MSCs [33]. While EOCs expressed high levels of MSC antigens such as CD73, CD90, and CD105, they also expressed significant levels of cell surface proteins that are not found on MSCs, such as CD45 and CD11b (Supporting Information Table 1). Furthermore, unlike MSCs, EOCs failed to differentiate into mesenchymal lineage cells, suggesting that MSCs are not a cellular constituent of our EOC populations (Supporting Information Fig. 1).
CM from EOCs Displays Potent Antifibrotic Activity In Vitro
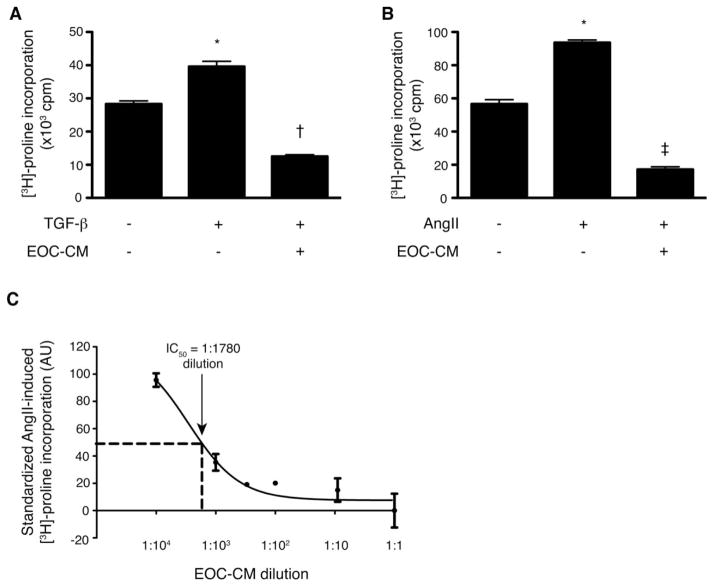
Given that glomerulosclerosis and tubulo-interstitial fibrosis are key determinants of kidney disease progression [34], our studies focused on the antifibrotic activity of the EOC secretome. To assess the ability of EOC-derived factors to inhibit fibrosis, we generated EOC-CM by incubating EOCs with serum-free EBM-2 medium for 24 hours to test in a fibroblast collagen production assay system. EOC-CM significantly attenuated fibroblast [3H]-proline incorporation, a robust marker of collagen production [11, 20–23], induced by stimulation with either TGF-β or angiotensin II, two key profibrotic cytokines upregulated in the chronically injured kidney and heart (Fig. 2A, 2B). As MMPs have been previously identified in EOC-CM [35], we next tested whether the antifibrotic effect of our EOC-CM could be inhibited by marimastat, a broad spectrum MMP inhibitor. Blockade of MMP activity did not attenuate the inhibitory effects of EOC-CM on TGF-β-induced fibroblast collagen production (Supporting Information Fig. 2), suggesting that the antifibrotic activity of EOC-CM is mediated at least in part by factors other than MMPs.
Figure 2.
Cell-free EOC-CM demonstrates potent antifibrotic activity in vitro. Cultured fibroblasts were preincubated with EOC-CM for 4 hours prior to stimulation with TGF-β 10 ng/mL or angiotensin II 10−7 mol/L. EOC-CM significantly attenuated both (A) TGF-β- and (B) angiotensin II-stimulated [3H]-proline incorporation, a robust marker of collagen production (n = 3 replicates per condition for each panel. Each EOC-CM replicate represents an aliquot from pooled EOC-CM collected from n ≥ 5 animals). (C): EOC-CM was next serially diluted as shown and tested in the same fibroblast collagen production assay, demonstrating significant inhibition of [3H]-proline incorporation even with significant EOC-CM dilution (n = 3–6 replicates per dilution. Each replicate represents an aliquot from pooled EOC-CM collected from n ≥ 5 animals). The measured IC50 was equivalent to a 1:1,780 dilution of EOC-CM. The results in panel (C) are standardized with the effects of serum-free endothelial basal medium-2 (EBM-2) set as 0 and the effects of AngII stimulation set as 100. *, p < .05 versus serum-free EBM-2 medium; †, p < .05 versus TGF-β stimulation; ‡, p < .05 versus AngII stimulation. Abbreviations: AngII, angiotensin II. AU, arbitrary units; cpm, counts per minute; EOC-CM, early outgrowth cell-conditioned media; IC50, concentration of EOC-CM leading to a 50% reduction in anti-fibrotic activity when compared with undiluted EOC-CM; TGF-β, transforming growth factor β.
To test the potency of the antifibrotic factor(s) released by EOCs, we serially diluted EOC-CM and compared the activity of the dilutions with neat EOC-CM and serum-free medium in this same fibroblast assay system. Less than 50% of the antifibrotic activity of EOC-CM was maintained even with 1:1,000 dilution, with the calculated IC50 being a 1:1,780 dilution (Fig. 2C).
Rats Treated with EOC Infusion Have Detectable Circulating Antifibrotic Factors in Their Plasma
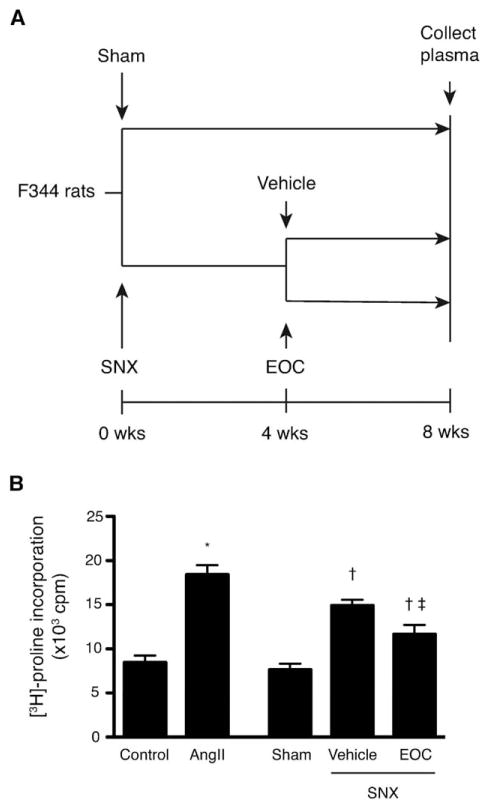
To determine whether this antifibrotic activity was detectable in the in vivo setting, we treated SNX rats, a model of progressive CKD which develops robust renal and cardiac fibrosis similar to patients with CKD, with a single i.v. injection of 106 EOCs or vehicle 4 weeks after surgery. Eight weeks after surgery, we collected plasma from each group of animals (Fig. 3). At this time point, EOC-treated animals are protected against the cardio-renal fibrosis and organ dysfunction that untreated SNX animals develop, despite minimal EOC retention within either organ and accumulation of these cells in the liver and spleen [11]. We analyzed the antifibrotic activity of the collected plasma using the fibroblast [3H]-pro-line incorporation assay, reasoning that EOC-derived factors with endocrine antifibrotic activity should be detectable in plasma of EOC-treated SNX animals (Fig. 3). Whereas plasma from sham-operated rats did not stimulate fibroblast [3H]-proline incorporation in the presence of angiotensin II, plasma from vehicle-treated SNX rats induced a significant increase in collagen production. In contrast, plasma from SNX animals that had been treated with 106 EOCs 4 weeks prior induced a significantly reduced fibrogenic response (Fig. 3), suggesting the presence of circulating factors in EOC-treated rats that reduce fibroblast collagen production.
Figure 3.
The plasma of rats injected with EOCs contains detectable circulating antifibrotic factors. (A): Fischer 344 rats were subjected to SNX or sham surgery at t = 0. Four weeks after surgery, SNX rats were randomized to receive an i.v. injection of 106 EOCs or vehicle. Eight weeks after surgery, a time point at which EOC injection has been previously shown to inhibit the renal and cardiac fibrosis that develops in untreated SNX animals [11], plasma from each treatment group was collected. (B): Collected plasma was then assayed in the [3H]-proline incorporation assay to test for effects on fibroblast collagen production in the setting of AngII. *, p < .05 versus serum-free endothelial basal medium-2 medium; †, p < .05 versus sham rat plasma; ‡, p < .05 versus vehicle-treated SNX rat plasma. n = 3 animals per condition. Abbreviations: AngII, angiotensin II; cpm, counts per minute; EOC, early outgrowth cell; SNX, subtotal nephrectomy.
CM from EOCs Attenuates Renal Dysfunction when Infused into the SNX Rat
Having demonstrated the potent antifibrotic activity of EOC-CM in vitro, and the presence of circulating anti-fibrotic factors in EOC-treated rats, we next examined whether i.v. administration of EOC-CM would also mimic the antifibrotic effects of EOC infusion when administered to 5/6 SNX rats, a model of progressive cardio-renal fibrosis leading to CKD and heart failure. Eight weeks after surgery, SNX rats developed a marked reduction in GFR, a marker of kidney function, accompanied by an increase in urinary protein excretion and systolic blood pressure, when compared with sham-operated animals (Table 1). As previously reported [11], infusion of 106 EOCs via a single tail vein injection resulted in an improvement in a range of renal functional parameters compared with serum-free medium-treated SNX rats, including a higher GFR, reduced urinary protein excretion, and attenuated blood pressure rise (Table 1). Thrice weekly injections of EOC-CM alone for 2 weeks similarly protected renal function, as reflected by a higher GFR compared with serum-free medium-treated SNX rats (Table 1). EOC-CM injections also significantly attenuated the rise in urinary protein excretion seen in SNX rats, while inducing a nonsignificant reduction in systolic blood pressure (Table 1).
Table 1.
Renal parameters at 8 weeks after surgery
| Sham | SNX-EBM-2 | SNX-EOC-CM | SNX-EOC | |
|---|---|---|---|---|
| N | 4 | 9 | 9 | 8 |
| Body weight (g) | 194 ± 2 | 202 ± 2 | 199 ± 3 | 191 ± 4 |
| Glomerular filtration rate (μL/min per g body weight) | 4.36 ± 0.06 | 1.30 ± 0.18* | 1.89 ± 0.10* | 2.10 ± 0.32* |
| Urinary protein:creatinine ratio (mg/mmol) | 36 ± 1 | 1974 ± 283* | 1059 ± 280* | 630 ± 177† |
| Systolic blood pressure (mm Hg) | 112 ± 8 | 201 ± 9* | 180 ± 8* | 163 ± 9* |
p < .05 vs. sham-operated animals.
p < 0.05 vs. SNX-EBM-2 animals.
Abbreviations: SNX-EOC-CM, subtotally nephrectomized rat treated with cell-free early outgrowth cell-derived conditioned medium; SNX-EBM-2, subtotally nephrectomized rat treated with serum-free EBM-2 medium; SNX-EOC, subtotally nephrectomized rat treated with early outgrowth cells.
CM from EOCs Attenuates Renal Fibrosis
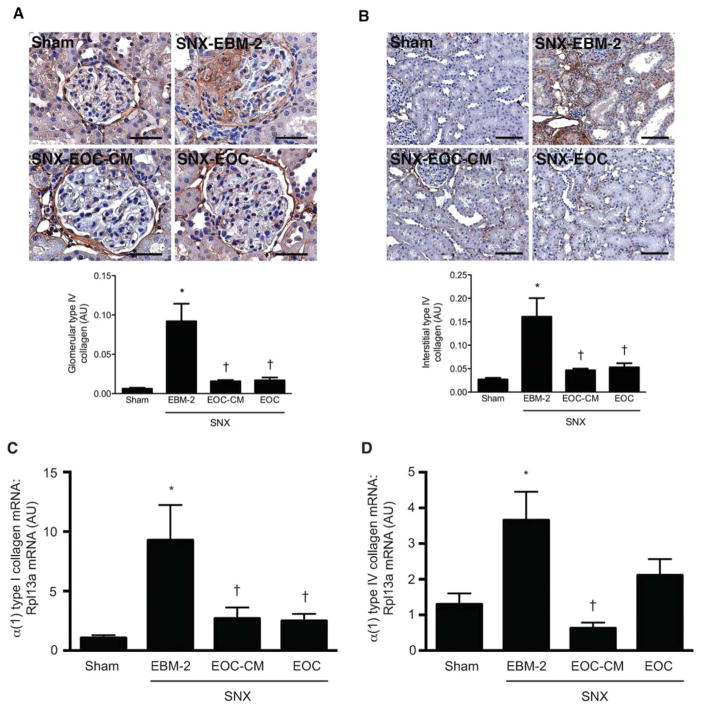
We next examined the effects of EOC-CM injections on renal structure. Eight weeks after surgery, SNX rats developed significant kidney fibrosis, as evidenced by a markedly increased deposition of type IV collagen, one of the principal extracellular matrix proteins in the injured kidney, in both the glomeruli (Fig. 4A) and the surrounding tubulointerstitium (Fig. 4B). In contrast, both EOC and EOC-CM injections significantly reduced glomerular and tubulointerstitial type IV collagen deposition to levels seen in sham-operated animals (Fig. 4A, 4B). Quantitative analysis of type I and type IV collagen mRNA levels in snap-frozen kidney tissue demonstrated similar findings. Whereas EBM-2-treated SNX rat kidneys exhibited significantly increased levels of α (1) type I and α (1) type IV collagen mRNA, both EOC and EOC-CM treatment were associated with reduced levels of these transcripts (Fig. 4C, 4D).
Figure 4.
Both EOC and EOC-CM injections significantly reduce renal fibrosis in the SNX rat 4 weeks post-therapy initiation. (A, B): Kidney sections were prepared 8 weeks after surgery and immunostained with an antibody specific for type IV collagen. (A): Representative glomerular images with quantitative analysis below. Original magnification ×400. Scale bars = 50 μm. (B): Representative cortical interstitial images with quantitative analysis below. Original magnification ×160. Scale bars = 100 μm. (C, D): RNA isolated from snap-frozen kidney tissue was reverse transcribed and analyzed via quantitative real-time polymerase chain reaction for levels of α (1) type I collagen (C) and α (1) type IV collagen mRNA (D). All values are referenced to levels of Rpl13a, a housekeeper transcript, and are expressed relative to sham-operated animal values. n = 4–9 animals per condition. *, p < .05 versus sham-operated animals; †, p < .05 versus SNX-EBM-2 animals. Abbreviations: AU, arbitrary units; EBM-2, endothelial basal medium-2; EOC-CM, early outgrowth cell conditioned media; SNX, subtotal nephrectomy.
CM from EOCs Attenuates Cardiac Dysfunction when Infused into the SNX Rat
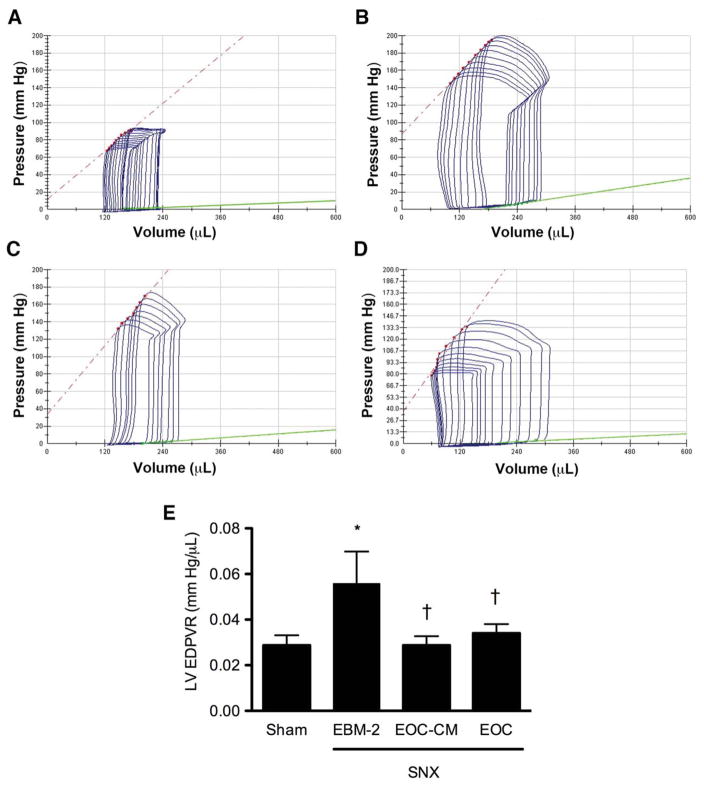
Given the close association between chronic kidney injury and cardiac dysfunction, and the morbidity and mortality associated with heart failure in CKD [36], we next examined cardiac function in SNX rats in the four treatment groups prior to sacrifice. Similar to many human patients with CKD, SNX animals treated with serum-free medium demonstrated reduced left ventricular (LV) chamber compliance, as evidenced by an elevated LV EDPVR. In contrast, SNX animals treated with either EOC or EOC-CM injections demonstrated improved left ventricular chamber compliance, as manifested by a reduced LV EDPVR (Fig. 5). Other parameters of cardiac function, including heart rate and systolic function, were unaffected by either SNX surgery or treatment with EOCs or EOC-CM (Supporting Information Table 2).
Figure 5.
Both EOC and EOC-CM injections significantly improve left ventricular chamber compliance in the SNX rat 4 weeks post-therapy initiation. 8 weeks after surgery, animals were subjected to invasive cardiac catheterization for cardiac functional analysis. The slope of the green line indicates the left ventricular end-diastolic pressure-volume relationship (LV EDPVR), an index of LV chamber compliance. (A–D): Representative pressure-volume loops. (A): Sham animal. (B): SNX-EBM-2 animal. (C): SNX-EOC-CM animal. (D): SNX-EOC animal. (E): Quantitative analysis of LV EDPVR. *, p < .05 versus sham-operated animals; †, p < .05 versus SNX-EBM-2 animals. Abbreviations: EBM-2, endothelial basal medium-2; EOC-CM, early outgrowth cell conditioned media; SNX, subtotal nephrectomy.
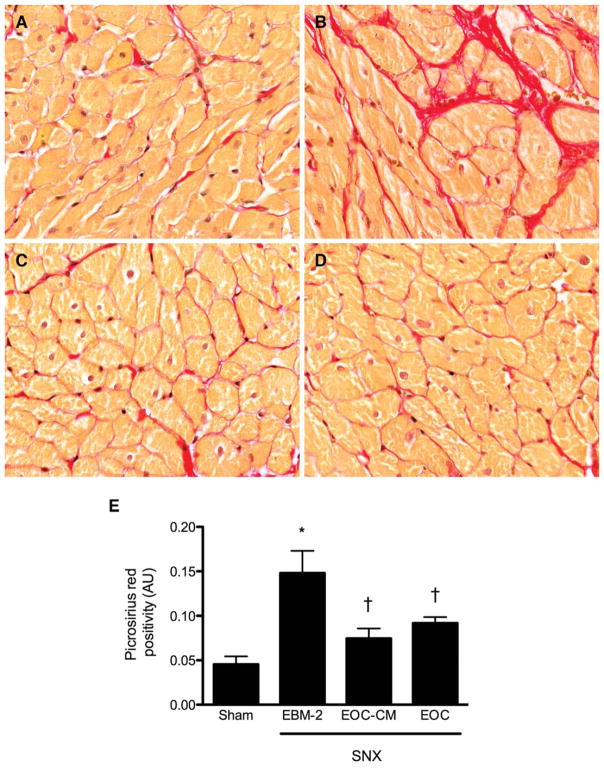
CM from EOCs Attenuates Cardiac Structural Injury
One of the principal determinants of impaired cardiac relaxation is interstitial cardiac fibrosis. We therefore examined the effects of EOC or EOC-CM injection on this parameter 8 weeks after surgery. Whereas SNX animals treated with control serum-free medium developed marked interstitial fibrosis compared to sham-operated controls, SNX animals treated with either EOC or EOC-CM injections were partially protected (Fig. 6).
Figure 6.
Both EOC and EOC-CM injections attenuate cardiac injury in the SNX rat 4 weeks post-therapy initiation. Subendocardial heart sections were prepared 8 weeks after surgery and stained with picrosirius red for assessment of interstitial fibrosis. (A–D): Representative cardiac interstitial images. Original magnification ×160. (A): Sham animal. (B): SNX-EBM-2 animal. (C): SNX-EOC-CM animal. (D): SNX-EOC animal. (E): Quantitative analysis of subendocardial interstitial fibrosis. *, p < .05 versus sham-operated animals; †, p < .05 versus SNX-EBM-2 animals. Abbreviations: AU, arbitrary units; EBM-2, endothelial basal medium-2; EOC-CM, early outgrowth cell conditioned media; SNX, subtotal nephrectomy.
Mass Spectroscopic Analysis of EOC-CM
Given the potent antifibrotic activity of EOC-CM that we observed in vitro and in vivo, we next examined the protein composition of EOC-CM via mass spectroscopy. With this approach, 251 unique proteins were identified (Supporting Information Table 3). Categorization by Ingenuity Pathway Analysis indicated that a number of EOC-CM proteins have identified roles in the control of multiple fundamental cellular processes implicated in connective tissue function (Supporting Information Table 4).
Discussion
While bone marrow-derived cell therapy exerts potent tissue protective effects in multiple chronic disease models, the controversies surrounding the mechanism of action of these cells, coupled with concerns regarding cell fate postinfusion, have hampered their translation into clinical practice. We have previously demonstrated that EOCs, a heterogeneous bone marrow-derived cell population with endothelial and monocyte/macrophage-like features, exert potent renoprotective effects when infused into models of diabetic [12] and nondiabetic [11] kidney disease, despite minimal retention in the injured kidney and rapid migration to the reticulo-endothelial system. One of the most dramatic effects of EOC infusion was a decrease in pathologic extracellular matrix deposition within the injured kidney. In this report, we provide evidence that the antifibrotic effects of EOCs are mediated by a novel endocrine mechanism of action, demonstrating that the plasma of EOC-treated rats contains circulating factors with antifibrotic activity, and that cultured EOCs secrete soluble factors that potently inhibit fibroblast collagen production in vitro and diminish fibrosis and preserve cardio-renal function in a clinically relevant rodent model of CKD associated with heart failure when infused as a cell-free preparation in vivo.
The mechanisms by which bone marrow-derived cells induce their beneficial effects remain speculative. Although previously thought to engraft into newly forming vessels where they differentiate into mature endothelial cells [1–4], the extent to which this occurs has been controversial [5–8]. Indeed, most recent reports document a lack of significant long-term cell retention in target organs, despite dramatic structural and functional improvements [10]. Consistent with these findings, a paracrine mode of action has been proposed, whereby the administered cells secrete locally active factors that assist in tissue repair [37]. In support of this hypothesis, intracardiac injection of CM from hypoxia-exposed MSCs has been shown to improve ventricular function following experimental myocardial infarction [38]. Similarly, local administration of EOC-CM, also derived from cells exposed to hypoxia, led to therapeutic angiogenesis in a hind-limb ischemia model [39]. However, whether these cell-derived factors would still mediate their observed benefits following systemic administration by a simple i.v. injection was not examined in these studies. Furthermore, whether the CM in these studies exerted their benefit by local diffusion or whether they instead entered the draining venous and lymphatic vessels to mediate their effects systemically is unknown.
Whether by paracrine effects or via direct incorporation, administration close to the site of injury has been a central, and often limiting, tenet of the cell therapy paradigm. Accordingly, strategies for cell administration in human studies have endeavored to maximize local delivery to injured tissue, using highly invasive intracoronary and transmyocardial approaches to do so [40]. Here we show for the first time that EOCs secrete antifibrotic factor(s) with remarkable potency, the activity of which can be detected in the plasma of rats injected with EOCs. Taken together, our results suggest that at least in some circumstances, local delivery may not be necessary. Our results are in line with two recent studies which demonstrated that systemic administration of soluble factors released by MSCs, another type of bone marrow-derived cell, can lead to renoprotective effects in both acute and chronic kidney disease models [8, 32]. Interestingly, van Koppen et al. [32] demonstrated in one of these studies that repeated injections of human embryonic stem cell-derived MSC CM slightly reduced renal fibrosis in a rat model of CKD. However, the antifibrotic effects of MSC therapy appear to be less potent than those of EOCs, as a direct head-to-head comparison of EOC and MSC CM found that MSC-derived factors only partially attenuated TGF-β-induced fibroblast collagen production, whereas EOC-CM completely abolished the stimulatory effect of TGF-β [11].
Although other studies have demonstrated the renoprotective effects of bone marrow-derived cell CM, no studies have examined the potency of antifibrotic factor(s) that might be found in this CM or sought to analyze its composition to identify these factors. In recognition of the potential clinical applicability of EOC-derived antifibrotic factor(s), we performed in vitro EOC-CM dose-response studies, followed by a systematic analysis of its composition. In these dose-response studies, we demonstrated that EOC-CM was able to potently suppress fibroblast collagen production, with an IC50 approaching a dilution of 1:2,000. By comparison, CM from Leydig tumor cells that causes humoral hypercalcemia of malignancy by secreting parathyroid hormone related protein, loses its bone resorptive activity with only a fourfold dilution [41]. Accordingly, the potency of the antifibrotic activity of EOC CM suggested to us that systemic administration of these factors, in contrast to local delivery, might be a viable therapeutic strategy. To confirm this hypothesis, we injected EOCs into animals with CKD and demonstrated the presence of antifibrotic activity in the plasma of EOC-treated animals 4 weeks after infusion, as compared to vehicle-treated controls. Given that in CKD fibrotic injury is a key contributor to cardio-renal dysfunction, our findings suggest that i.v. delivery of EOC-derived factors may represent a clinically relevant, multiorgan treatment for CKD and associated cardiac diastolic dysfunction. Furthermore, in the context of our and other groups’ previous findings that infused EOCs are not retained in significant numbers in injured tissue but rather localize to reticulo-endothelial organs such as the liver, spleen, and bone marrow [7, 11], our results suggest that EOCs may exert their antifibrotic effects from these distant locations via the secretion of circulating factors that act via a novel endocrine mechanism of action.
Having demonstrated the potent antifibrotic activity of EOC-CM in vitro, and having completed the proof-of-principle studies illustrating the in vivo antifibrotic effects of intravenously administered EOC-CM, we next performed an unbiased proteomic analysis of EOC-CM composition. As with proteomic analyses of similar cell types, our study demonstrated that the secretome of EOCs includes in excess of 250 proteins (Supporting Information Table 3) [35]. Ingenuity Pathway Analysis of these proteins demonstrated that many of the identified proteins are involved in critical cellular functions relevant to fibrosis, supporting the concept that the EOC secretome is enriched in factors that potently regulate this process. Notably, the secretome did contain several proteins with known antifibrotic activity, including the matrix degrading enzymes metalloproteinases 2, 3, 14, and 19 as well as the TGF-β neutralizing proteoglycan, biglycan (Supporting Information Table 4). Importantly, MMP inhibition failed to block the attenuating effects of EOC-CM on TGF-β-induced fibroblast collagen production (Supporting Information Fig. 2), suggesting that non-MMP factors contribute to the antifibrotic activity of EOC-CM.
Aside from the MMPs and biglycan, relatively little is known about antifibrotic factors and their mechanisms of action, so that several other proteins with such activity are also likely to be present. Accordingly, substantial further work will be required to both characterize and identify the specific factor(s) responsible. In this regard, a number of challenges must be overcome. First, it is important to note that our mass spectrometry data, as with all proteomic analyses, is dependent upon protein concentration, and thus favors the identification of abundant, stable proteins. Thus, it is possible that some unstable or low concentration bio-active proteins in EOC-CM might not have been detected by the spectrometer. While the potency of the antifibrotic activity of EOC-CM would suggest that proteins present at very low concentrations are likely not significant contributors, we cannot exclude this possibility. Second, while the factors responsible for the antifibrotic actions of EOCs may be proteins, it is also conceivable that they might, alternatively, be amines or lipids that would not be detected using microarray or proteomic-based approaches. Moreover, rather than single factors, it is also possible that a particular combination or a particular ratio of multiple factors might be required to elicit a full therapeutic effect. Thus, while the precise identification of the factor(s) responsible for the organ protective effects noted in this study remains a long-term aim, the large number of potential candidates and combinations indicates that considerably more research will be required to ultimately achieve this goal. With these challenges in mind, we first sought to compile, in an unbiased manner, as complete a list of proteins as possible to serve as a basis for future studies that will seek to identify the specific factor(s) responsible for the antifibrotic activity of EOC-CM.
The enthusiasm for adult-derived cell therapies, based largely on studies conducted in syngeneic experimental animals, has been tempered by the substantially less impressive results using autologous cells in humans, possibly as a consequence of disease-related dysfunction [42]. While allogeneic cells, particularly the ostensibly nonimmunogenic MSCs, provide a possible alternative, the latter were found to be less effective than EOCs as a treatment for experimental cardio-renal disease [11]. Accordingly, the use of a cell-free preparation derived from healthy donor cells may be advantageous. However, whether the cells required to manufacture this product would need to be obtained directly from bone marrow in humans or whether circulating cells with a high secretory profile such as CD34+ or CD133+ cells may provide an alternative source, remains to be determined.
Conclusions
In summary, we provide evidence that the antifibrotic activity of bone marrow-derived EOCs is mediated by a novel endocrine mechanism of action, in contrast to the prevailing belief that bone marrow-derived cells mediate their beneficial effects through paracrine pathways. The potency of these endocrine factors suggests the potential for a more clinically feasible, EOC-based, cell-free strategy for the treatment of chronic fibrotic diseases such as the cardio-renal injury observed in chronic kidney disease.
Supplementary Material
Acknowledgments
We thank C. Botelho, B. Stead, M. Mitchell, K. Ladak, C. Lee, and M. McKeever for their technical support of the animal studies. This research was supported in part by the Canada Research Chair Program, the Canadian Institutes of Health Research, and the Heart and Stroke Foundation of Canada. These data were presented, in part, at the 2010 Annual Scientific Meeting of the American Society of Nephrology and was supported by grants from the CIHR (CCT 83030, MOP 97912), Physicians’ Services Incorporated Foundation (PSI 09-42), and the Heart and Stroke Foundation of Canada. Dr. Yuen was sponsored by postdoctoral fellowships from KRESCENT, the Canadian Society of Transplantation fellowship, and CIHR. He currently holds a KRESCENT New Investigator Award. D.K. was supported by a Queen Elizabeth II/Heart and Stroke Foundation of Ontario Graduate Scholarship in Science and Technology. Dr. A.A. is a Canadian Diabetes Association Clinician Scientist. Dr. E.D. is the Hold’em for Life Chair in Prostate Cancer Biomarkers. Dr. P.M. is the Keenan Chair in Medical Research. Dr. R.G. is the Canadian Research Chair in Diabetes Complications.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
Author contributions: D.A.Y.: conception and design, collection and/or assembly of data, data analysis and interpretation, and manuscript writing; K.A.C.: data analysis and interpretation and manuscript writing; Y.Z., K.T., and G.K.C.S.: collection and/or assembly of data and data analysis and interpretation; S.L.A.: collection and/or assembly of data; D.K.C.S., I.B., and H.K.: data analysis and interpretation; A.A. and P.A.M.: conception and design and data analysis and interpretation; E.D.: conception and design and provision of mass spectroscopy services; R.E.G.: conception and design, financial support, data analysis and interpretation, and manuscript writing.
References
- 1.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Kalka C, Masuda H, Takahashi T, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kocher AA, Schuster MD, Szabolcs MJ, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 4.Murohara T, Ikeda H, Duan J, et al. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest. 2000;105:1527–1536. doi: 10.1172/JCI8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ormiston ML, Deng Y, Stewart DJ, et al. Innate immunity in the therapeutic actions of endothelial progenitor cells in pulmonary hypertension. Am J Respir Cell Mol Biol. 2010;43:546–554. doi: 10.1165/rcmb.2009-0152OC. [DOI] [PubMed] [Google Scholar]
- 6.Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease: Part II: Cell-based therapies. Circulation. 2004;109:2692–2697. doi: 10.1161/01.CIR.0000128596.49339.05. [DOI] [PubMed] [Google Scholar]
- 7.Aicher A, Brenner W, Zuhayra M, et al. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation. 2003;107:2134–2139. doi: 10.1161/01.CIR.0000062649.63838.C9. [DOI] [PubMed] [Google Scholar]
- 8.Bi B, Schmitt R, Israilova M, et al. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol. 2007;18:2486–2496. doi: 10.1681/ASN.2007020140. [DOI] [PubMed] [Google Scholar]
- 9.Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 10.Mirotsou M, Jayawardena TM, Schmeckpeper J, et al. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol. 2011;50:280–289. doi: 10.1016/j.yjmcc.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuen DA, Connelly KA, Advani A, et al. Culture-modified bone marrow cells attenuate cardiac and renal injury in a chronic kidney disease rat model via a novel antifibrotic mechanism. Plos One. 2010;5:e9543. doi: 10.1371/journal.pone.0009543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Yuen DA, Advani A, et al. Early-outgrowth bone marrow cells attenuate renal injury and dysfunction via an antioxidant effect in a mouse model of type 2 diabetes. Diabetes. 2012;61:2114–2125. doi: 10.2337/db11-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagy A, Quaggin SE. Stem cell therapy for the kidney: A cautionary tale. J Am Soc Nephrol. 2010;21:1070–1072. doi: 10.1681/ASN.2010050559. [DOI] [PubMed] [Google Scholar]
- 14.Thirabanjasak D, Tantiwongse K, Thorner PS. Angiomyeloproliferative lesions following autologous stem cell therapy. J Am Soc Nephrol. 2010;21:1218–1222. doi: 10.1681/ASN.2009111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parfrey PS, Harnett JD, Barre PE. The natural history of myocardial disease in dialysis patients. J Am Soc Nephrol. 1991;2:2–12. doi: 10.1681/ASN.V212. [DOI] [PubMed] [Google Scholar]
- 16.Ortiz LA, Gambelli F, McBride C, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuen DA, Zhang Y, Thai K, et al. Angiogenic dysfunction in bone marrow-derived early outgrowth cells from diabetic animals is attenuated by SIRT1 activation. Stem Cells Transl Med. 2012;1:921–926. doi: 10.5966/sctm.2012-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee CH, Shah B, Moioli EK, et al. CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J Clin Invest. 2010;120:3340–3349. doi: 10.1172/JCI43230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gnecchi M, Zhang Z, Ni A, et al. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ku CH, Johnson PH, Batten P, et al. Collagen synthesis by mesenchymal stem cells and aortic valve interstitial cells in response to mechanical stretch. Cardiovasc Res. 2006;71:548–556. doi: 10.1016/j.cardiores.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 21.Rhaleb NE, Peng H, Harding P, et al. Effect of N-acetyl-seryl-aspartyl-lysyl-proline on DNA and collagen synthesis in rat cardiac fibroblasts. Hypertension. 2001;37:827–832. doi: 10.1161/01.hyp.37.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Advani A, Gilbert RE, Thai K, et al. Expression, localization, and function of the thioredoxin system in diabetic nephropathy. J Am Soc Nephrol. 2009;20:730–741. doi: 10.1681/ASN.2008020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin J, Kelly DJ, Mifsud SA, et al. Tranilast attenuates cardiac matrix deposition in experimental diabetes: Role of transforming growth factor-beta. Cardiovasc Res. 2005;65:694–701. doi: 10.1016/j.cardiores.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 24.Advani A, Kelly DJ, Advani SL, et al. Role of VEGF in maintaining renal structure and function under normotensive and hypertensive conditions. Proc Natl Acad Sci USA. 2007;104:14448–14453. doi: 10.1073/pnas.0703577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horwitz EM, Gordon PL, Koo WK, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Connelly KA, Prior DL, Kelly DJ, et al. Load-sensitive measures may overestimate global systolic function in the presence of left ventricular hypertrophy: A comparison with load-insensitive measures. Am J Physiol Heart Circ Physiol. 2006;290:H1699–H1705. doi: 10.1152/ajpheart.00577.2005. [DOI] [PubMed] [Google Scholar]
- 27.Kelly DJ, Chanty A, Gow RM, et al. Protein kinase Cbeta inhibition attenuates osteopontin expression, macrophage recruitment, and tubulointerstitial injury in advanced experimental diabetic nephropathy. J Am Soc Nephrol. 2005;16:1654–1660. doi: 10.1681/ASN.2004070578. [DOI] [PubMed] [Google Scholar]
- 28.Batruch I, Lecker I, Kagedan D, et al. Proteomic analysis of seminal plasma from normal volunteers and post-vasectomy patients identifies over 2000 proteins and candidate biomarkers of the urogenital system. J Proteome Res. 2011;10:941–953. doi: 10.1021/pr100745u. [DOI] [PubMed] [Google Scholar]
- 29.Llevadot J, Murasawa S, Kureishi Y, et al. HMG-CoA reductase inhibitor mobilizes bone marrow–derived endothelial progenitor cells. J Clin Invest. 2001;108:399–405. doi: 10.1172/JCI13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teraa M, Sprengers RW, Westerweel PE, et al. Bone marrow alterations and lower endothelial progenitor cell numbers in critical limb ischemia patients. Plos One. 2013;8:e55592. doi: 10.1371/journal.pone.0055592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Koppen A, Joles JA, van Balkom BW, et al. Human embryonic mesenchymal stem cell-derived conditioned medium rescues kidney function in rats with established chronic kidney disease. Plos One. 2012;7:e38746. doi: 10.1371/journal.pone.0038746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 34.Bohle A, Mackensen-Haen S, von Gise H. Significance of tubulointerstitial changes in the renal cortex for the excretory function and concentration ability of the kidney: A morphometric contribution. Am J Nephrol. 1987;7:421–433. doi: 10.1159/000167514. [DOI] [PubMed] [Google Scholar]
- 35.Pula G, Mayr U, Evans C, et al. Proteomics identifies thymidine phosphorylase as a key regulator of the angiogenic potential of colony-forming units and endothelial progenitor cell cultures. Circ Res. 2009;104:32–40. doi: 10.1161/CIRCRESAHA.108.182261. [DOI] [PubMed] [Google Scholar]
- 36.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112–119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 37.Kumar AH, Caplice NM. Clinical potential of adult vascular progenitor cells. Arterioscler Thromb Vasc Biol. 2010;30:1080–1087. doi: 10.1161/ATVBAHA.109.198895. [DOI] [PubMed] [Google Scholar]
- 38.Gnecchi M, He H, Noiseux N, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 39.Di Santo S, Yang Z, Wyler von Ballmoos M, et al. Novel cell-free strategy for therapeutic angiogenesis: In vitro generated conditioned medium can replace progenitor cell transplantation. Plos One. 2009;4:e5643. doi: 10.1371/journal.pone.0005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdel-Latif A, Bolli R, Tleyjeh IM, et al. Adult bone marrow-derived cells for cardiac repair: A systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 41.Ibbotson KJ, D’Souza SM, Ng KW, et al. Tumor-derived growth factor increases bone resorption in a tumor associated with humoral hypercalcemia of malignancy. Science. 1983;221:1292–1294. doi: 10.1126/science.6577602. [DOI] [PubMed] [Google Scholar]
- 42.Rosenzweig A. Cardiac cell therapy–mixed results from mixed cells. N Engl J Med. 2006;355:1274–1277. doi: 10.1056/NEJMe068172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.