Abstract
BACKGROUND:
Variations in the prevalence of traditional cardiac risk factors only partially account for geographic variations in the incidence of cardiovascular disease. We examined the extent to which preventive ambulatory health care services contribute to geographic variations in cardiovascular event rates.
METHODS:
We conducted a cohort study involving 5.5 million patients aged 40 to 79 years in Ontario, Canada, with no hospital stays for cardiovascular disease as of January 2008, through linkage of multiple population-based health databases. The primary outcome was the occurrence of a major cardiovascular event (myocardial infarction, stroke or cardiovascular-related death) over the following 5 years. We compared patient demographics, cardiac risk factors and ambulatory health care services across the province’s 14 health service regions, known as Local Health Integration Networks (LHINs), and evaluated the contribution of these variables to regional variations in cardiovascular event rates.
RESULTS:
Cardiovascular event rates across LHINs varied from 3.2 to 5.7 events per 1000 person-years. Compared with residents of high-rate LHINs, those of low-rate health regions received physician services more often (e.g., 4.2 v. 3.5 mean annual family physician visits, p value for LHIN-level trend = 0.01) and were screened for risk factors more often. Low-rate LHINs were also more likely to achieve treatment targets for hypercholes-terolemia (51.8% v. 49.6% of patients, p = 0.03) and controlled hypertension (67.4% v. 53.3%, p = 0.04). Differences in patient and health system factors accounted for 74.5% of the variation in events between LHINs, of which 15.5% was attributable to health system factors alone.
INTERPRETATION:
Preventive ambulatory health care services were provided more frequently in health regions with lower cardiovascular event rates. Health system interventions to improve equitable access to preventive care might improve cardiovascular outcomes.
The Canadian Heart Health Strategy and Action Plan and similar cardiovascular health promotion efforts in other countries have been introduced to reduce the incidence of cardiovascular disease (CVD).1–3 However, major geographic disparities persist in cardiovascular hospital admission and mortality rates.4,5 Previous studies have shown that variations in the prevalence of traditional cardiac risk factors account only partially for this variation, which suggests that other factors also play a role.4 A potentially important contributing factor is the health care system. Clinical practice guidelines recommend best practices for the detection and management of traditional CVD risk factors, but rates of adherence to these guidelines may vary.6 In Canada, despite a universal health care system, geographic variation in the supply of physician services is a policy concern and may contribute to regional disparities in health care outcomes.7–10
The Cardiovascular Health in Ambulatory Care Research Team (CANHEART) “big data” initiative was established to measure and improve the cardiovascular health and quality of ambulatory cardiovascular care provided in Ontario, Canada.11 It aims to contribute to a learning health care system by linking routinely collected information from multiple population-based data sources to form a single, comprehensive database that can be used to study the delivery and outcomes of clinical CVD care in the entire population over time.11,12
In this CANHEART Regional Variations cohort study, we sought to identify patient and health system factors associated with regional variations in the incidence of major cardiovascular events. In particular, we studied the delivery of preventive health care services provided by family physicians. A better understanding of key contributing factors could lead to more targeted and effective interventions to address disparities in CVD event rates.
Methods
Data sources
As part of the CANHEART initiative, we created a population-based cohort of 5.5 million community-dwelling adults aged 40–79 years as of Jan. 1, 2008, who had resided in Ontario for at least the 2 previous years. We identified them through record linkage of 17 population-based health databases using unique, encoded identifiers, as described in detail elsewhere.11 Our cohort was restricted to primary prevention patients, defined as those with no history of a hospital stay for cardiovascular disease in the previous 20 years.
We obtained baseline demographic and risk factor information from the Ontario Registered Persons Database, the Ontario Hypertension Database, the Ontario Diabetes Database and other data sources (Appendix 1, Supplement 1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.160823/-/DC1).13,14 Information on health behaviours, ethnicity, laboratory test results and blood pressure measurements were obtained from representative samples of the Ontario population linked to the Canadian Community Health Survey; the Immigration, Refugees and Citizenship Canada Permanent Resident Database; the Dynacare Medical Laboratories database; and the Electronic Medical Record Administrative data Linked Database (EMRALD), respectively (Appendix 1, Supplements 1 and 2). Information about physician services, medication use (for patients aged ≥ 65 yr) and clinical events was obtained from population-based physician billing, drug benefits, hospital discharge and vital statistics records.
Cardiovascular health and health system indicators
A set of key primary prevention (e.g., cardiovascular health and health system) indicators were identified by a CANHEART expert panel (Appendix 1, Supplement 3). Depending on the context, the indicators were measured either before Jan. 1, 2008, or from Jan. 1, 2008, to Dec. 31, 2012, across Ontario’s 14 health service regions, known as Local Health Integration Networks (LHINs). Where possible, we modelled our indicator definitions to be consistent with the Canadian Cardiovascular Harmonized National Guidelines Endeavour (C-CHANGE) clinical practice guidelines.6
Primary outcome
Our primary outcome was the occurrence of a major CVD event during the 5-year follow-up period (2008–2012). A major CVD event was defined as hospital admission due to myocardial infarction or stroke, or cardiovascular-related death (from ischemic heart disease or cerebrovascular disease).
Statistical analysis
Descriptive statistics, including means and proportions, were calculated for baseline demographic characteristics and each indicator by LHIN. Incidence rates of the primary outcome were reported as events per 1000 person-years of follow-up. Performance on all indicators and outcomes were age- and sex-standardized to the 2006 Ontario census population. To examine for linear trends between each indicator and the incidence of CVD at the LHIN level, we calculated Pearson correlation coefficients and their associated p values for each indicator.
To simplify the presentation of descriptive results, we combined the 14 LHINs into 3 groups: those with low event rates (3 LHINs), medium event rates (7) and high event rates (4) based on their population’s CVD risk from an age- and sex-adjusted Cox proportional hazards model that incorporated LHIN-specific random effects (described below). We considered LHINs whose 95% confidence interval (CI) for their random effect lay entirely below (or above) 1.0 as low-rate (or high-rate) regions; remaining LHINs were deemed medium-rate regions. Because the relative ranking of LHINs by CVD event rate was similar among men and women (Appendix 1, Supplement 4), our analyses focused on combined results for men and women.
To determine the incremental impact of patient and health system factors on the occurrence of a CVD event, we fit a sequence of multivariable multilevel Cox proportional hazards models in which the effects of LHINs were assumed to follow a normal distribution and modelled as random effects (or frailty terms), and patient-level covariates modelled as fixed effects.15 To quantify the heterogeneity in CVD event rates between LHINs, we calculated the median hazard ratio (HR) from the variance of the LHIN-specific random effects.16,17 The median HR quantifies the median difference in CVD risk for an identical patient in all 14 LHINs when all possible comparisons of higher- versus lower-risk LHINs are made, as opposed to comparing individual LHINs against a single reference LHIN, as in conventional statistical methods.16
We sequentially adjusted for (a) age and sex, (b) patient factors, including traditional cardiac risk factors, neighbourhood income and ethnicity, and (c) health system factors, including lipid screening, family physician visits and periodic health examination rates, and determined the amount of variation explained by each model.18,19 We determined the proportion of variation explained by each step of adjustment by computing the percent change in the variance of the LHIN-specific random effects from an unadjusted model. We used similar models to examine the likelihood of receiving selected preventive health services after adjusting for cardiovascular risk. We used multiple imputation methods for missing data in the models (Appendix 1, Supplement 5).20
All data were analyzed at the Institute for Clinical Evaluative Sciences, Toronto, with the use of SAS version 9.3 (SAS Institute Inc.).
Ethics approval
This study was approved by the Sunnybrook Health Sciences Centre Research Ethics Board.
Results
Baseline characteristics of the study population by CVD event rate group are shown in Table 1, and by LHIN in Appendix 1, Supplement 6. Mean age was 54.6 ± 10.4 years, and 52.0% were women. Residents of low-rate LHINs were more likely than those in medium- and high-rate LHINs to be a member of an ethnic minority group, recent immigrant, have completed high school and to reside in an urban area.
Table 1:
Baseline characteristics and prevalence of cardiac risk factors, by LHIN event rate group*
| Characteristic | LHIN event rate group; % of patients† | p value for trend across LHINs | |||
|---|---|---|---|---|---|
| Low CVD event rate n = 1 683 104 |
Medium CVD event rate n = 3 019 957 |
High CVD event rate n = 841 086 |
Overall n = 5 544 147 |
||
| No. of LHINs | 3 | 7 | 4 | 14 | NA |
| Baseline characteristics | |||||
| Age as of Jan. 1, 2008, yr, mean ± SD | 54.1 ± 10.4 | 54.8 ± 10.4 | 55.2 ± 10.4 | 54.6 ± 10.4 | NA |
| Female sex | 51.9 | 52.2 | 51.7 | 52.0 | NA |
| Less than secondary school graduation‡ | 13.0 | 16.3 | 19.0 | 15.7 | < 0.001 |
| Ethnicity‡ | |||||
| White | 70.0 | 84.3 | 92.9 | 81.3 | 0.005 |
| South Asian | 6.1 | 4.8 | 0.7 | 4.6 | 0.08 |
| Chinese | 9.0 | 2.7 | 0.8 | 4.3 | 0.003 |
| Black | 3.4 | 2.7 | 0.5 | 2.6 | 0.04 |
| Other | 11.5 | 5.5 | 5.1 | 7.2 | < 0.001 |
| Immigrant ≤ 20 yr in Ontario | 25.0 | 11.1 | 3.3 | 14.1 | < 0.001 |
| Low-income neighbourhood§ | 34.1 | 39.7 | 39.1 | 37.9 | 0.3 |
| Rural or small-town residence¶ | 0.4 | 15.1 | 28.4 | 12.7 | 0.003 |
| Cardiac risk factors | |||||
| Cigarette smoker‡ | 14.9 | 18.1 | 22.2 | 17.7 | < 0.001 |
| Hypertension | 31.4 | 32.1 | 33.5 | 32.1 | 0.5 |
| Systolic blood pressure,** mmHg | 124.5 | 126.5 | 130.9 | 126.6 | 0.004 |
| Diabetes | 12.5 | 12.0 | 12.0 | 12.1 | 0.6 |
| Obesity (BMI ≥ 30)‡ | 14.5 | 19.2 | 22.4 | 18.2 | < 0.001 |
| Physically inactive‡ | 48.3 | 46.9 | 46.5 | 47.3 | 0.3 |
| Inadequate fruit and vegetable consumption (<5/d)‡ | 57.9 | 59.1 | 62.4 | 59.2 | 0.002 |
| Total cholesterol,†† mmol/L | 4.82 | 4.83 | 4.89 | 4.83 | 0.9 |
| High-density lipoprotein,†† mmol/L | 1.36 | 1.34 | 1.33 | 1.35 | 0.01 |
| Low-density lipoprotein,†† mmol/L | 2.84 | 2.85 | 2.84 | 2.84 | 0.4 |
| Framingham 10-year CVD risk,** mean % | 11.0 | 12.4 | 13.6 | 12.1 | < 0.001 |
Note: BMI = body mass index, CVD = cardiovascular disease, LHIN = Local Health Integration Network, NA = not applicable, SD = standard deviation.
Values for cardiac risk factors are age- and sex-standardized to the 2006 Ontario census population.
Unless stated otherwise.
Estimates are from a study subpopulation of 68 067 individuals linked to the 2005–2012 Canadian Community Health Surveys and 101 117 individuals linked to the 2001–2012 (for education and ethnicity) Canadian Community Health Surveys; they exclude on-reserve Indigenous populations. Estimates are weighted with the use of Statistics Canada survey weights.
Defined as neighbourhood income quintile 1 or 2, where quintile 1 has the lowest income.
Based on Statistics Canada 2006 census population and defined as community size <10 000.
Estimates are from a study subpopulation of individuals linked to the Electronic Medical Record Administrative data Linked Database (EMRALD) (n = 35 790 for baseline systolic blood pressure, n = 64 381 for Framingham risk).
Estimates are from a study subpopulation of 1 389 072 individuals with results in the Dynacare Medical Laboratories database.
Cardiac risk factors
The prevalence of traditional cardiac risk factors is reported in Table 1 and Appendix 1, Supplement 6. Compared with low-rate regions, residents of high-rate regions were more likely to smoke, be obese, have a higher mean systolic blood pressure, and have suboptimal daily consumption of fruits and vegetables (all p < 0.01). In contrast, baseline prevalence of diabetes and mean total and low density lipoprotein (LDL) cholesterol levels were similar across LHINs.
Health system factors
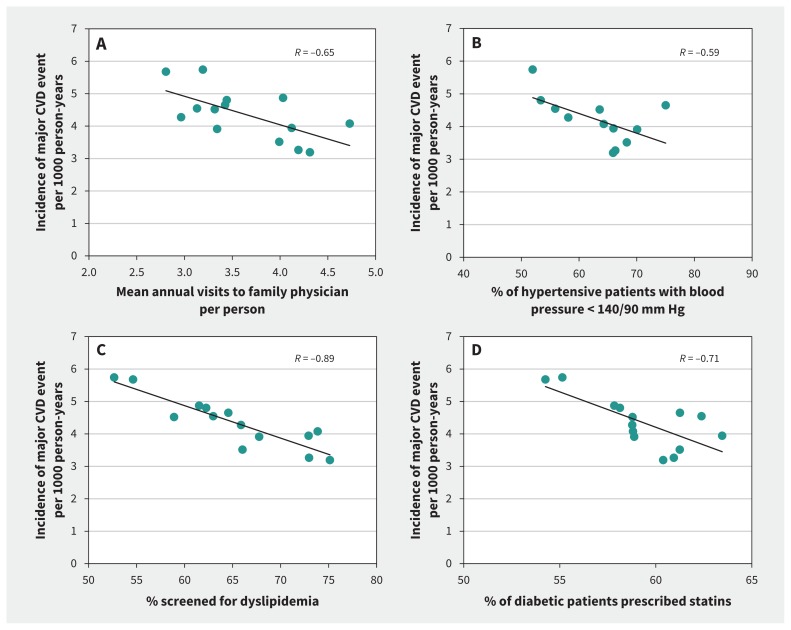
Performance on cardiovascular health system indicators is shown in Table 2 by event rate group, and by LHIN in Figure 1 and Appendix 1, Supplement 7. In all LHINs, almost 90% of individuals visited a family physician in 2006–2007. Individuals living in low-rate LHINs had a higher mean annual number of visits (4.2 v. 3.6 and 3.5 in the medium- and high-rate LHINs, respectively; R = −0.65, p = 0.01 for LHIN-level trend). Similar trends were observed for visits to a specialist (R = −0.74, p = 0.003), the proportion of residents who received at least 1 periodic health examination between 2005 and 2007 (R = −0.93, p < 0.001), and dyslipidemia and diabetes screening rates (both R = −0.89, p < 0.001). Multivariable risk-adjusted models predicting annual visits to a family physician, dyslipidemia screening and having a periodic health exam showed similar gradients by CVD event rate (Appendix 1, Supplement 8).
Table 2:
Use of physician services, risk factor screening and risk factor management, by LHIN event rate group*
| Indicator | LHIN event rate group; % of patients† | p value for trend across LHINs | |||
|---|---|---|---|---|---|
| Low CVD event rate | Medium CVD event rate | High CVD event rate | Overall | ||
| Physician services and risk factor screening | |||||
| Visited a family physician (2006–2007) | 86.4 | 87.5 | 86.4 | 87.0 | 0.2 |
| Annual visits to a family physician (2006–2007), no. per person | 4.2 | 3.6 | 3.5 | 3.8 | 0.01 |
| Periodic health examination (2005–2007) | 63.8 | 56.2 | 48.5 | 57.4 | < 0.001 |
| Visited a specialist (2006–2007) | 60.0 | 57.8 | 55.8 | 58.1 | 0.004 |
| Annual visits to a specialist (2006–2007), no. per person | 1.8 | 1.6 | 1.4 | 1.6 | 0.003 |
| Dyslipidemia screening (2005–2007) | 71.9 | 67.3 | 58.0 | 67.3 | < 0.001 |
| Diabetes screening (2005–2007) | 73.6 | 69.9 | 60.4 | 69.6 | < 0.001 |
| Risk factor management‡ | |||||
| Prescribed statins | 31.4 | 31.9 | 31.3 | 31.7 | 0.4 |
| LDL ≤ 2 mmol/L among statin users | 51.8 | 53.5 | 49.6 | 52.6 | 0.03 |
| Among individuals with hypertension | |||||
| Prescribed ≥ 2 antihypertensive medications | 54.8 | 56.1 | 56.3 | 55.8 | 0.07 |
| Controlled blood pressure§ | 67.4 | 65.0 | 53.3 | 64.4 | 0.04 |
| Annual visits to a family physician for hypertension, no. per person (2008–2012) | 1.1 | 0.9 | 0.8 | 1.0 | 0.005 |
| Among individuals with diabetes | |||||
| Prescribed any antiglycemic medication | 70.9 | 70.1 | 74.1 | 70.8 | 0.03 |
| Prescribed any ACE inhibitor or ARB | 67.2 | 67.7 | 69.4 | 67.7 | 0.1 |
| Prescribed statins | 60.8 | 60.4 | 57.2 | 60.2 | 0.004 |
| HbA1C ≤ 7% | 58.2 | 59.6 | 60.8 | 59.3 | 0.7 |
Note: ACE = angiotensin-converting enzyme, ARB = angiotensin II receptor blocker, CI = confidence interval, CVD = cardiovascular disease, HbA1C = glycated hemoglobin, LDL = low-density lipoprotein, LHIN = Local Health Integration Network.
Values are age- and sex-standardized to the 2006 Ontario census population.
Unless stated otherwise.
Prescribed medications are in the 100 days before Jan. 1, 2008 among ≥ 65 year olds on Jan. 1, 2007 (n = 984 101). Antihypertensive medications are among individuals prescribed at least 1 antihypertensive medication (n = 460 090). Medications and HbA1c ≤ 7% among individuals with diabetes are restricted to those with laboratory confirmed diabetes or a history of prescriptions for antiglycemic medications before Jan. 1, 2008 (n = 64 163). LDL ≤ 2 mmol/L and HbA1c ≤ 7% are based on the latest result available between 2008 and 2012. LDL ≤ 2 mmol/L is the target in Canadian Dyslipidemia Guidelines.6
Defined as < 140/90 mm Hg based on the average of the 3 most recent measurements during 2008–2012 among hypertensive patients in the Electronic Medical Record Administrative data Linked Database (EMRALD).
Figure 1:
Association between mean annual visits to family physician per person (A), proportion of hypertensive patients with controlled blood pressure (B), proportion of patients screened for dyslipidemia (C) and statin use among diabetic patients (D) and the incidence of a major cardiovascular (CVD) event per 1000 person-years across health regions (Local Health Integration Networks [LHINs]) in Ontario, Canada. Each dot represents a LHIN.
Related to risk factor management, patients with hypertension living in low-rate LHINs made more annual visits to a family physician for hypertension during the follow-up period (R = −0.70, p = 0.005) and had higher rates of blood pressure control (≤ 140/90 mm Hg) than those living in medium- and high-rate regions (R = −0.59, p = 0.04). Overall, 31.7% of Ontarians 65 years of age or older were receiving statins, with similar rates across LHINs. Statin use was higher among those with diabetes living in low-rate LHINs than in other health regions (R = −0.71, p = 0.004). The mean proportion of statin users achieving treatment targets was also higher in low-rate LHINs than in high-rate regions (R = −0.57, p = 0.03), whereas mean rates of glycemic control (glycated hemoglobin ≤ 7%) among individuals with diabetes were similar (p = 0.7).
Cardiovascular events
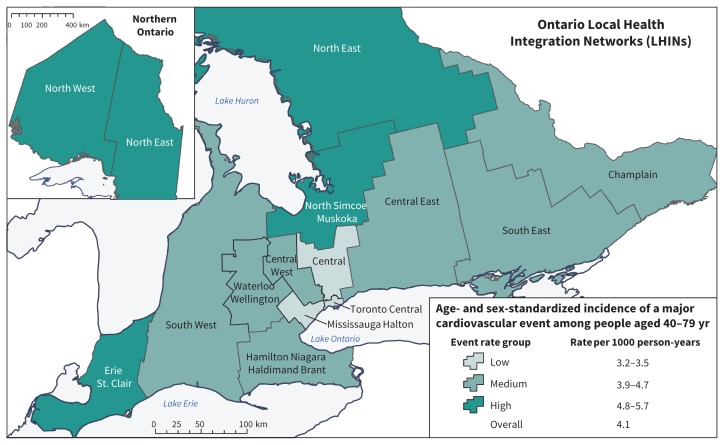
Overall, 103 280 CVD events were identified over 27 million years of patient follow-up between 2008 and 2012 (mean duration of follow-up 4.9 ± standard deviation 0.6 yr). The age- and sex-standardized incidence rate of major CVD events varied nearly twofold, from a low of 3.2 events per 1000 person-years in the Central LHIN to a high of 5.7 events per 1000 person-years in the North West and North East LHINs (Figure 2; Appendix 1, Supplements 4 and 9). The 3 LHINs with the lowest incidence (Central, Mississauga Halton and Toronto Central) were urban communities of the Greater Toronto Area. The 2 regions with the highest incidence were in northern Ontario, the least densely populated area of Ontario.
Figure 2:
Incidence of major cardiovascular events across health service regions (Local Health Integration Networks [LHINs]) in Ontario, by event rate group, 2008–2012.
Multilevel models
Table 3 shows the results from sequential multilevel modelling adjusted for demographic characteristics, risk factors and health system factors. Hazard ratios by LHIN are shown in Appendix 1, Supplement 10. Adjustment for differences in age, sex and traditional cardiac risk factors explained 33.2% of the variation in CVD incidence between health regions. Additional adjustment for socioeconomic status and ethnic composition accounted for a further 25.8% of the variation, and adjustment for health system factors accounted for an additional 15.5%. The median HR between LHINs also decreased proportionately, from an unadjusted median HR of 1.22 (95% CI 1.10–1.31) to 1.11 (1.05–1.15) in the fully adjusted model, which indicated a median relative difference of 11% in the hazard of events when comparing a higher-rate LHIN with a lower-rate LHIN after adjustment for all of these factors.
Table 3:
Sequential proportional hazards models for the occurrence of a major CVD event*
| Variable | Model; median hazard ratio (95% CI) | |||||
|---|---|---|---|---|---|---|
| Unadjusted | Age, sex | + traditional risk factors | + neighbourhood income quintile | + ethnicity | + health system factors | |
| Median hazard ratio for LHIN effects | 1.22 (1.10–1.31) | 1.19 (1.09–1.26) | 1.18 (1.08–1.25) | 1.18 (1.08–1.24) | 1.14 (1.06–1.19) | 1.11 (1.05–1.15) |
| Female sex (v. male) | 0.47 (0.47–0.48) | 0.55 (0.54–0.56) | 0.54 (0.54–0.55) | 0.54 (0.54–0.55) | 0.55 (0.55–0.56) | |
| Age, yr (v. 40–49) | ||||||
| 50–59 | 2.16 (2.12–2.21) | 2.12 (2.08–2.17) | 2.13 (2.09–2.17) | 2.10 (2.06–2.14) | 2.17 (2.13–2.22) | |
| 60–69 | 3.80 (3.73–3.88) | 3.56 (3.49–3.64) | 3.58 (3.50–3.65) | 3.48 (3.40–3.55) | 3.67 (3.59–3.74) | |
| 70–79 | 7.79 (7.64–7.94) | 7.08 (6.92–7.24) | 7.06 (6.91–7.22) | 6.90 (6.75–7.06) | 7.21 (7.05–7.38) | |
| Smoking | 2.07 (1.99–2.15) | 2.02 (1.95–2.11) | 2.01 (1.93–2.09) | 2.00 (1.92–2.09) | ||
| Total cholesterol, per 1-mmol/L increase | 1.12 (1.11–1.13) | 1.12 (1.11–1.13) | 1.12 (1.11–1.13) | 1.11 (1.10–1.12) | ||
| HDL cholesterol, per 1-mmol/L increase | 0.61 (0.59–0.63) | 0.62 (0.60–0.64) | 0.62 (0.60–0.64) | 0.62 (0.60–0.65) | ||
| Hypertension | 1.51 (1.49–1.54) | 1.51 (1.49–1.53) | 1.50 (1.48–1.52) | 1.56 (1.54–1.58) | ||
| Diabetes | 1.54 (1.52–1.57) | 1.53 (1.50–1.55) | 1.53 (1.50–1.55) | 1.55 (1.53–1.58) | ||
| Neighbourhood income quintile (v. 5 [highest]) | ||||||
| 1 (lowest) | 1.29 (1.27–1.32) | 1.33 (1.30–1.36) | 1.28 (1.26–1.31) | |||
| 2 | 1.16 (1.13–1.18) | 1.18 (1.16–1.21) | 1.15 (1.13–1.18) | |||
| 3 | 1.11 (1.08–1.13) | 1.12 (1.10–1.15) | 1.11 (1.08–1.13) | |||
| 4 | 1.07 (1.05–1.09) | 1.08 (1.06–1.10) | 1.07 (1.05–1.09) | |||
| Ethnicity (v. white long-term resident) | ||||||
| East Asian | 0.43 (0.41–0.45) | 0.44 (0.42–0.46) | ||||
| Black | 0.72 (0.67–0.77) | 0.73 (0.68–0.78) | ||||
| Latin American | 0.75 (0.69–0.81) | 0.76 (0.70–0.82) | ||||
| South Asian | 0.93 (0.90–0.96) | 0.93 (0.90–0.96) | ||||
| Southeast Asian | 0.63 (0.59–0.68) | 0.64 (0.59–0.69) | ||||
| West Asian or Arab | 0.69 (0.65–0.74) | 0.70 (0.65–0.75) | ||||
| White Eastern European | 0.85 (0.81–0.90) | 0.84 (0.80–0.89) | ||||
| White Western European | 0.69 (0.64–0.74) | 0.67 (0.63–0.73) | ||||
| Dyslipidemia screening (2005–2007) | 0.83 (0.81–0.84) | |||||
| Visited a family physician (2006–2007) | 1.14 (1.12–1.17) | |||||
| Periodic health examination (2005–2007) | 0.75 (0.74–0.76) | |||||
| Explained LHIN-level variation, % | – | 26.3 | 33.2 | 35.8 | 59.0 | 74.5 |
Note: CI = confidence interval, CVD = cardiovascular disease, HDL = high-density lipoprotein, LHIN = Local Health Integration Network.
From 2-level hierarchical Cox proportional hazards models with the median hazard ratio derived from the effect of individual LHINs modelled as random effects (frailty terms) and all other variables modelled as fixed effects. The median hazard ratio indicates the median difference in CVD risk for an identical patient in all 14 LHINs when all possible comparisons of higher-versus lower-risk LHINs are made. LHIN-specific hazard ratios are provided in Appendix 1, Supplement 10 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.160823/-/DC1). Ethnicity is based on validated algorithms using surname for the entire study population, as well as country of birth and mother tongue among immigrants.
An online tool to explore various cardiovascular health and health system indicators determined in this study is available at www.canheart.ca/eatlas.
Interpretation
The CANHEART initiative was designed to facilitate the study of cardiovascular health and delivery of ambulatory preventive health care to the adult population in all regions of Ontario, Canada. We found an inverse association between LHIN-level CVD event rates and health system factors, such as number of ambulatory care visits to family physicians, and rates of dyslipidemia and diabetes screening, controlled blood pressure among patients with hypertension and statin use among people with diabetes. Residents of high-rate regions were the least likely to receive certain preventive services, even though they had the highest rates of smoking and obesity and the lowest rates of dietary intake of fruits and vegetables. Cumulatively, 74.5% of the regional variation could be explained, of which 15.5% was due to health system factors.
Our study suggests that, even in a country with a universal health insurance system, higher rates of preventive health care contribute to lower rates of CVD events at a regional level.21 Our findings provide new information that health system factors may be important contributors to regional variations in CVD event rates. Although their medical needs were greater, residents of LHINs with the highest CVD event rates had the fewest family physician visits per year. The lower numbers of visits may reflect differences in health-seeking behaviour between residents of different LHINs, but they may also reflect differences in access to care. Ontario has had long-standing challenges in recruiting and retaining primary care physicians to northern and more rural parts of the province, where wait times for urgent primary care visits are the longest and physician shortages the greatest.9 More frequent visits provide family physicians more opportunities to deliver preventive care such as early screening for risk factors, encouraging lifestyle changes (e.g., diet and exercise), and titrating medical therapy (e.g., statins) to treatment targets if lifestyle interventions are ineffective.22,23 Periodic health examinations, which typically focus on preventive care, were conducted most frequently in the regions with low CVD event rates. Although lower numbers of family physician visits may be explained in part by greater numbers of visits with nurses and nurse practitioners in high-rate LHINs, we did not have data to examine this possibility.
Our analyses also suggest that differences in the ethnic composition of residents of LHINs contributed to regional variations in CVD incidence. Ontario is one of the world’s most ethnically diverse jurisdictions. A relatively high proportion of individuals in ethnic minority groups and recent immigrants live in health regions with the lowest CVD event rates, specifically LHINs in the Greater Toronto Area. Previous analyses of the CANHEART database have shown a “healthy immigrant” effect, with CVD event rates that were 30% lower among recent immigrants than among long-term residents of Ontario.24 East Asian immigrants (predominantly ethnic Chinese) were at lowest risk, and South Asian immigrants at highest risk, albeit with rates similar to long-term residents. In the current study, the lowest CVD rates were observed in the LHIN with the highest relative proportion of Chinese residents, whereas the 2 LHINs with the highest event rates had the lowest proportion of recent immigrants.
Our results are consistent with those reported in other studies, which have shown that variations in the prevalence of traditional risk factors explain part but not all of the geographic variation in CVD incidence.25–30 In the British Regional Heart Study, traditional risk factors explained as much as 50% of differences in CVD risk among 7335 men living in 24 towns, with smoking being the most important factor.29 An ecologic study in China found that variation in hypertension prevalence across provinces was the most important factor contributing to geographic variation in stroke mortality.26 The British Women’s Heart and Health Study found that, in addition to traditional risk factors, variations in statin use contributed to regional variation in CVD incidence among 7173 women living in 23 towns.27
Limitations
Our study has some important limitations. Although we studied several key health indicators and system factors, we did not have complete information on all indicators for the entire study population (e.g., medication use by those less than 65 years old and blood pressure control among people with hypertension) or some health system factors (e.g., nurse practitioner visits, hospital-based laboratory tests and privately funded health care services) to enable their inclusion in our multilevel models. However, we reported the LHIN-level performance on these indicators from analyses conducted on subsamples of the study population where possible, which have been shown to be representative of the entire population.11 We also examined individual exposure to health system factors over only a few years, whereas cumulative lifetime exposure to preventive health care may be more important.
Conclusion
The CANHEART Regional Variations cohort study showed that regional variations in CVD-related health behaviours, ethnic composition and ambulatory preventive care were associated with regional variations in the incidence of CVD events in Ontario. Residents of regions with the greatest burden of cardiac disease were paradoxically less likely to receive certain preventive health services. In a system designed to facilitate equitable access to health care, interventions to improve rates of preventive care in high-rate regions might improve cardiovascular outcomes.
Acknowledgements
Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information (CIHI). The authors thank IMS Brogan Inc. for use of its Drug Information Database.
See related article at www.cmaj.ca/lookup/doi/10.1503/cmaj.170116
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Jack Tu and Anna Chu contributed to the study concept and design. Anna Chu, Laura Maclagan and Prosanta Mondal performed the data analyses. All of the authors interpreted the results. Jack Tu and Anna Chu drafted the manuscript, and all of the authors revised it critically for important intellectual content, approved the final version to be published and agreed to be accountable for all aspects of the work.
Funding: The study was supported by the Institute for Clinical Evaluative Sciences (ICES), an operating grant from the Institute of Circulatory and Respiratory Health, Canadian Institutes of Health Research (ICRH–CIHR) Chronic Diseases Team (grant no. TCA 118349), a CIHR foundation grant (grant no. FDN-143313), a CIHR operating grant (grant no. MOP-111035) and a Canadian Vascular Network (CVN) seed grant. The CVN is supported by grants from the CIHR–ICRH, and the Institute of Aging in partnership with and supported by Hypertension Canada. ICES is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC).
Jack Tu is supported by a Tier 1 Canada Research Chair in Health Services Research and an Eaton Scholar Award from the Department of Medicine, University of Toronto. Peter Austin, Dennis Ko, Gillian Booth, Douglas Lee, Moira Kapral and David Alter are supported by Career Investigator Awards; Harindra Wijeysundera by a Clinician Scientist Award; Clare Atzema by a New Investigator Award; and Jacob Udell by a National New Investigator and Ontario Clinician Scientist Award, all from the Heart and Stroke Foundation. Gillian Booth is also supported by the Department of Medicine at the University of Toronto. Douglas Lee is also supported by a Clinician Scientist Award from CIHR and the Ted Rogers Chair in Heart Function Outcomes. David Alter is also funded by a Chair in Cardiovascular and Metabolic Rehabilitation, University Health Network–Toronto Rehabilitation Institute, University of Toronto. Karen Tu is supported by a Research Scholar Award from the Department of Family and Community Medicine, University of Toronto.
Disclaimer: This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The analyses, opinions, results and conclusions reported in this article are those of the authors and are independent from ICES, the funding sources and CIHI. No endorsement by ICES, the Ontario MOHLTC, CVN, CIHR or CIHI is intended or should be inferred.
Members of the Cardiovascular Health in Ambulatory Care Research Team (CANHEART): The complete list of team members is available in Appendix 2 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.160823/-/DC1).
References
- 1.Frieden TR, Berwick DM. The “Million Hearts” initiative — preventing heart attacks and strokes. N Engl J Med 2011; 365: e27. [DOI] [PubMed] [Google Scholar]
- 2.Krska J, du Plessis R, Chellaswamy H. Implementation of NHS Health Checks in general practice: variation in delivery between practices and practitioners. Prim Health Care Res Dev 2016;17:385–92. [DOI] [PubMed] [Google Scholar]
- 3.Smith ER. The Canadian Heart Health Strategy and Action Plan. Can J Cardiol 2009;25:451–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filate WA, Johansen HL, Kennedy CC, et al. Regional variations in cardiovascular mortality in Canada. Can J Cardiol 2003;19:1241–8. [PubMed] [Google Scholar]
- 5.Obisesan TO, Vargas CM, Gillum RF. Geographic variation in stroke risk in the United States: region, urbanization, and hypertension in the Third National Health and Nutrition Examination Survey. Stroke 2000;31:19–25. [DOI] [PubMed] [Google Scholar]
- 6.Tobe SW, Stone JA, Walker KM, et al. Canadian Cardiovascular Harmonized National Guidelines Endeavour (C-CHANGE): 2014 update. CMAJ 2014;186: 1299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alter DA, Stukel TA, Newman A. The relationship between physician supply, cardiovascular health service use and cardiac disease burden in Ontario: supply–need mismatch. Can J Cardiol 2008;24:187–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Supply, distribution and migration of physicians in Canada, 2014. Ottawa: Canadian Institute for Health Information; 2015. [Google Scholar]
- 9.Quality in primary care: setting a foundation for monitoring and reporting in Ontario. Toronto: Health Quality Ontario; 2015. [Google Scholar]
- 10.Tu K, Gong Y, Austin PC, et al. An overview of the types of physicians treating acute cardiac conditions in Canada. Can J Cardiol 2004;20:282–91. [PubMed] [Google Scholar]
- 11.Tu JV, Chu A, Donovan LR, et al. The Cardiovascular Health in Ambulatory Care Research Team (CANHEART): using big data to measure and improve cardiovascular health and health care services. Circ Cardiovasc Qual Outcomes 2015;8:204–12. [DOI] [PubMed] [Google Scholar]
- 12.Friedman CP, Wong AK, Blumenthal D. Achieving a nationwide learning health system. Sci Transl Med 2010;2:57cm29. [DOI] [PubMed] [Google Scholar]
- 13.Hux JE, Ivis F, Flintoft V, et al. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care 2002;25:512–6. [DOI] [PubMed] [Google Scholar]
- 14.Tu K, Campbell NR, Chen ZL, et al. Accuracy of administrative databases in identifying patients with hypertension. Open Med 2007;1:e18–26. [PMC free article] [PubMed] [Google Scholar]
- 15.Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model. New York: Springer Science/Business Media New York; 2000. [Google Scholar]
- 16.Austin PC, Wagner P, Merlo J. The median hazard ratio: a useful measure of variance and general contextual effects in multilevel survival analysis. Stat Med 2016. November 25 [Epub ahead of print] 10.1002/sim.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bengtsson T, Dribe M. Quantifying the family frailty effect in infant and child mortality by using median hazard ratio (MHR). Hist Methods 2010;43:15–27. [Google Scholar]
- 18.Girotra S, van Diepen S, Nallamothu BK, et al. Regional variation in out-of-hospital cardiac arrest survival in the United States. Circulation 2016;133:2159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldstein H, Browne W, Rasbash J. Multilevel modelling of medical data. Stat Med 2002;21:3291–315. [DOI] [PubMed] [Google Scholar]
- 20.Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q 2005;83:457–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lugomirski P, Guo H, Boom NK, et al. Quality of diabetes and hyperlipidemia screening before a first myocardial infarction. Can J Cardiol 2013;29:1382–7. [DOI] [PubMed] [Google Scholar]
- 23.Virani SS, Woodard LD, Landrum CR, et al. Institutional, provider, and patient correlates of low-density lipoprotein and non-high-density lipoprotein cholesterol goal attainment according to the Adult Treatment Panel III guidelines. Am Heart J 2011;161:1140–6. [DOI] [PubMed] [Google Scholar]
- 24.Tu JV, Chu A, Rezai MR, et al. The incidence of major cardiovascular events in immigrants to Ontario, Canada: the CANHEART Immigrant study. Circulation 2015;132:1549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crombie IK, Smith WC, Tavendale R, et al. Geographical clustering of risk factors and lifestyle for coronary heart disease in the Scottish Heart Health study. Br Heart J 1990;64:199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He J, Klag MJ, Wu Z, et al. Stroke in the People’s Republic of China: I. Geographic variations in incidence and risk factors. Stroke 1995;26:2222–7. [PubMed] [Google Scholar]
- 27.Lawlor DA, Bedford C, Taylor M, et al. Geographical variation in cardiovascular disease, risk factors, and their control in older women: British Women’s Heart and Health study. J Epidemiol Community Health 2003;57:134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levene LS, Baker R, Bankart MJG, et al. Association of features of primary health care with coronary heart disease mortality. JAMA 2010;304:2028–34. [DOI] [PubMed] [Google Scholar]
- 29.Morris RW, Whincup PH, Lampe FC, et al. Geographic variation in incidence of coronary heart disease in Britain: the contribution of established risk factors. Heart 2001;86:277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez F, Wang Y, Naderi S, et al. Community-level cardiovascular risk factors impact geographic variation in cardiovascular disease hospitalizations for women. J Community Health 2013;38:451–7. [DOI] [PubMed] [Google Scholar]