Abstract
Semantically incoherent speech is a pernicious clinical feature of serious mental illness (SMI). The precise mechanisms underlying this deficit remain unclear. Prior studies have found that arousal of negative emotion exaggerates the severity of these communication disturbances; this has been coined “affective reactivity”. Recent research suggests that “cognitive reactivity” may also occur, namely reflecting reduced “on-line” cognitive resources in SMI. We tested the hypothesis that communication disturbances manifest as a function of limited cognitive resources in SMI above and beyond that associated with state affectivity. We also investigated individual differences in symptoms, cognitive ability, and trait affect that may be related to cognitive reactivity. We compared individuals with SMI (n=52) to nonpsychiatric controls (n=27) on a behavioral-based coding of communication disturbances during separate baseline and experimentally-manipulated high cognitive-load dual tasks. Controlling for state affective reactivity, a significant interaction was observed such that communication disturbances decreased in the SMI group under high cognitive-load. Furthermore, a reduction in communication disturbances was related to lower trait and state positive affectivity in the SMI group. Contrary to our expectations, limited cognitive resources temporarily relieved language dysfunction. Implications, particularly with respect to interventions, are discussed.
Keywords: serious mental illness, cognition, affect, language, communication disturbances
1.0. Introduction
Language function is severely disrupted in individuals with serious mental illness (SMI). Of particular importance, individuals with SMI frequently produce language that is semantically incoherent, often leading to the discourse structure to be obfuscated (Elvevåg et al., 2007; Hoffman et al., 1986; Perlini et al., 2012; Rubino et al., 2011). Moreover, these communication disturbances are often stable over time, medication resistant, and linked to poor functional outcome (Bowie and Harvey, 2008; Kuperberg, 2011). Despite the wealth of empirical research into the ubiquity and burden of language dysfunction in SMI, the underlying mechanism of it remains a mystery. The present study leveraged behavioral language assessments to understand the cognitive mechanisms underlying communication disturbances in SMI.
Historically, investigators have measured language function using interview-based measures such as the Scale for Assessment of Thought, Language, and Communication (TLC; Andreasen, 1986). These measures have a number of drawbacks that contribute to limited understanding of language dysfunction in SMI. For example, clinical rating scales do not account for either the statistical properties or the structure of normal language, hence complicating definitions of “abnormal” language. Moreover, these measures employ ordinal based rating systems that are inappropriate for parametric statistics, produce data that are generally insensitive to change given the limited range of response options and ambiguous operational definitions, and are imprecise for isolating specific facets of language (Alpert et al., 2002; Cohen and Elvevåg, 2014; Elvevåg et al., 2016).
Given these limitations, there have been efforts to characterize language output in SMI in an objective and quantitative “behavioral-based” manner, particularly with respect to semantic expression. Behavior-based approaches are advantageous over clinical rating scales in that they quantify language disruptions using ratio scales and are not reliant on global clinical impressions. Of note, Docherty and colleagues developed the Communications Disturbances Index (CDI; Docherty et al., 1996) to systematically code for reference errors that make the discourse structure difficult to comprehend and has also been shown to be distinct from interview based measures of disorganized speech that assess for traditional speech symptoms (e.g., tangentiality, derailment, neologisms; Andreasen, 1986).
As yet, behavioral based language assessments have had limited application for understanding the mechanisms underlying language dysfunction in SMI. Studies on the influence of emotional valence on discourse structure in patients have observed that patients produce more communication disturbances in their speech when discussing affectively negative versus positive and neutral topics (Burbridge and Barch, 2002; Docherty et al., 1994; Rubino et al., 2011). Emerging evidence also suggests that “cognitive resources”, defined in terms of attentional, working memory, and related “on-line” resources (Plass et al., 2010), are also important for understanding language dysfunction in SMI (Cohen et al., 2014; Docherty, 2005; Melinder and Barch, 2003). Extensive research from a wide range of disciplines demonstrates that humans have a limited amount of cognitive resources at any given time, and allocating resources toward one task (e.g. remembering a phone number or name, operating a vehicle) limits the resources available for other tasks, for example, effective language function (e.g., Plass et al., 2010). Thus, it is reasonable to posit that communication disturbances manifest as a function of limited cognitive resources.
Three lines of research support this notion. First, a broad array of deficits in attention, working memory, concentration and other “on-line” abilities is exhibited in individuals with SMI, and these deficits appear to be similar across SMI boundaries (Mackin and Areán, 2009; Simonsen et al., 2011; Strauss et al., 2015). Second, a number of correlational studies have observed that cognitive deficits are associated with more language impairment in schizophrenia, depression, and mania using behavioral-based procedures/technologies (Becker et al., 2012; Docherty, 2005; Radanovic et al., 2013; Rosenstein et al., 2014). Third, investigators using experimental methods have found cognitive reactivity in speech, defined in terms of increased communication disturbances resulting from experimentally-manipulated cognitive load in healthy participants (Barch and Berenbaum, 1994; Kerns, 2007). While experimental studies have examined cognitive reactivity in patients, they have failed to include control groups (Barch and Berenbaum, 1996; Melinder and Barch, 2003) or a chronic SMI group (Minor et al., 2016); the present study addresses these limitations. Importantly, we previously evaluated the cognitive reactivity of negative speech symptoms (i.e., blunted vocal affect, alogia) using the same sample and task data as the current study (Cohen et al., 2014). Utilizing computationally-derived natural speech indices, we found that pause length abnormally increased as a function of increased cognitive load for patients with SMI (Cohen et al., 2014). The current study investigates the cognitive reactivity of communication disturbances in individuals with SMI and healthy controls utilizing behavioral-based measures of language production.
There is considerable variability in language dysfunction across individuals with SMI. Identifying individual differences that influences language function across patients may also yield understanding of the mechanisms underlying communication disturbances in SMI. The present study examined four candidate individual differences potentially related to cognitive reactivity: 1) cognitive ability; 2) state and trait affect; 3) positive symptoms; and 4) negative symptoms. It is particularly important to consider state affectivity when investigating language dysfunction in SMI. For example, arousal of negative emotions (e.g., discussing negatively valenced topics, attending to visually negative stimuli), dubbed affective reactivity, has exacerbated communication disturbances in both healthy (Docherty et al., 1998) and SMI (Burbridge and Barch, 2002; Rubino et al., 2011) samples. Similarly, Cohen and Docherty (2005) observed that arousal of positive emotions may also influence semantic coherence in a subset of a schizophrenia sample with more severe psychiatric symptomatology. To control for this potential alternate mechanism (i.e., high cognitive load may evoke negative or positive emotions in participants), we measured emotional lexical expression of negative and positive emotion in language production as our indirect measure of state affective reactivity (Pennebaker, 2001). This behavioral-based measure has been employed as an alternate method of assessing emotional experience in prior studies (Cohen et al., 2009; Minor et al., 2015; Najolia et al., 2011; St-Hilaire et al., 2008)
2.0. Methods
2.1. Participants
The patient group included 52 adults with Diagnostic and Statistical Manual of Mental Disorders 4th edition (American Psychiatric Association [APA], 1994) diagnosed schizophrenia (n = 38), unipolar major depressive or bipolar disorder (n = 14), recruited from an outpatient clinic. Diagnoses were made based on information obtained from the patients’ medical records and from a structured clinical interview (SCID-IV; First et al., 1996). Patients were also recruited based on meeting federal criteria for having an SMI, defined in terms of adults (age 18 or older) who currently, or in the past year, meet criteria for a diagnosable mental, behavioral, or emotional disorder that results in functional impairment which substantially interferes with one more major life activities (i.e., per the ADAMHA Reorganization Act and Substance Abuse and Mental Health Services Administration) Exclusion criteria included the following: a) Global Assessment of Functioning (APA, 1994) rating below 30, indicating symptom levels that could interfere with participation in the study, b) documented evidence of intellectual disability from the medical records, c) current or historical DSM-IV diagnosis of alcohol or drug abuse suggestive of severe physiological symptoms (e.g., delirium tremens, repeated loss of consciousness), and d) history of significant head trauma (requiring overnight hospitalization). All patients were clinically stable at the time of testing and were receiving pharmacotherapy under the supervision of a multi-disciplinary team. Controls (n=27) were recruited from the community using the above exclusion criteria with the exception that they be free of current and past psychotic and affective disorders (per a SCID-IV interview). A more thorough description of our patient and control groups is detailed in our previous study (Cohen et al., 2014).
2.2. Speaking tasks
Subjects were seated in front of a computer monitor and asked to perform two separate 90-second speaking tasks involving discussion of affectively neutral topics (i.e., hobbies, foods, daily routines) during which participants were encouraged to speak as much as possible (Cohen et al., 2012; Cohen et al., 2014). During a baseline, “low-load” narrative task, participants provided speech while passively watching symbols appear on the monitor. Six different visual symbols were presented at inter-stimulus intervals of 2000 ms. During a “high-load” narrative task, participants spoke while performing a one-back test. This task involved forced-choice responding (i.e., “match”, “non-match”) to stimuli when consecutively appearing visual symbols on a computer screen were identical. The visual stimuli and their presentation were identical across the two conditions. Four patients with a schizophrenia diagnoses were excluded from the present study for not responding during the cognitive task (accuracy < 10%). Participants underwent extensive training without the speech component to become familiar with the cognitive task (i.e., one-back). Feedback was offered during this practice. Order of task and speech topic was randomized. The following is an example of probe used to elicit speech: What kinds of hobbies do you have? You can discuss any hobby that you can think of, such as sports, walking, watching TV, or anything else you can think of.
2.3. Communication disturbances and cognitive reactivity
The tape-recorded interviews from the cognitive load tasks were transcribed by one research assistant and checked for accuracy by a second research assistant. A doctoral level graduate student rated the typed transcripts on communication disturbances using the Communication Disturbance Index (CDI; Docherty et al., 1996) after training to achieve adequate inter-rater reliability (ICC = 0.75) using a consensus-rated samples from an archival data set maintained by the principal investigator of this study. The rater was blind to participant group and cognitive load category. The CDI is a highly sensitive and reliable measure of communication c disturbances and rates the number of reference errors, with a reference error being any spoken word or phrase obscures the meaning of the larger communication. The dependent measure for all analyses presented below was total CDI score. The CDI has been used frequently in schizophrenia research as a measure of communication disturbances (Burbridge and Barch, 2002; Docherty, 2012). Cognitive reactivity was calculated by regressing baseline performance on high cognitive load performance and saving the standardized residual (Prochaska et al., 2008).
Two methods were used to account for potential differences in the amount of speech elicited by group membership and different cognitive load conditions (e.g., low-load vs. high load). First, reference errors were calculated as the number of errors per 100 words of speech to control for differences in verbosity. Second, a repeated-measures ANOVA did not reveal a significant group by condition interaction for speech production (i.e., word count), though we did observe significant condition effects such that patient and control speech decreased from the low load (CON: M = 228.10, SD = 64.85; SMI: M = 175.69, SD = 67.64) to the high load condition (CON: M = 173.31, SD = 55.49; SMI: M = 123.04, SD = 62.05). However, we entered change in speech production as a covariate in our ANCOVA model; controlling for speech production did not change our results.
2.4. Lexical affect and state affective reactivity
The typed transcripts were then analyzed using the Linguistic Inquiry and Word Count program (LIWC; Pennebaker, 2001), which contains separate positive and negative emotional categories that are comprised of words related to emotional states (e.g., angry, happy, friendly). The LIWC software generated percentage scores, which accounts for total words spoken, for positive and negative word used during each cognitive load condition. Higher percentages indicate more frequent word use. Construct validity of the LIWC program in examining emotional expression has been previously supported (Kahn et al., 2007). State affective reactivity was computed by regressing baseline performance on high cognitive load performance and saving the standardized residual (Prochaska et al., 2008).
2.5. Symptoms ratings
The Scale for the Assessment of Positive Symptoms (SAPS; Andreason, 1984) was used to measure positive symptoms. Global domains reflecting hallucinations, delusions, bizarre behavior, and formal thought disorder were computed. The Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1984) was used to measure negative symptoms global scores. Global domains reflecting affective flattening, alogia, avolition-apathy, and anhedonia-asociality were computed. Preliminary diagnoses and ratings were made by one of four doctoral-level students who were trained to criterion (ICC > 0.70).
2.6. Trait affect
The Positive Emotion and Negative Emotion subscales of the Positive and Negative Affect Schedule (PANAS; Watson et al., 1988) was used to measure trait affect. The PANAS asks participants to rate the extent to which they have experienced positive and negative affective states during the prior week on a scale from 1 (“very slightly or not at all”) to 5 (“extremely”).
2.7. Cognitive ability
Basic cognitive ability was measured using the Brief Assessment of Cognition in Schizophrenia (BACS; Keefe et al., 1999), a battery assessing executive functions, psychomotor speed, attention, verbal memory and working memory. Due to potential circularity in examining speech as a function of verbal fluency, the verbal fluency score was excluded.
2.8. Analyses
The analyses were conducted in three steps. First, we examined potential demographic, affective, and cognitive differences between the SMI and control groups that might inform subsequent analyses. Second, we compared the SMI and control groups on communication disturbances for the baseline and high-load tasks using repeated-measures ANCOVAs; we simultaneously entered positive and negative state affectivity as the covariates. We predicted significant group, condition, and interaction effects such that a) all subjects would show an inclination of communication disturbances as a function of increasing cognitive load, b) patients overall would show more communication disturbances, and c) the speech of patients would show a more dramatic inclination of communication disturbances under high cognitive load compared to controls. Third, different from our previous study (Cohen et al., 2014), we sought to determine the degree to which individual differences in positive and negative symptoms, state and trait affect, and cognitive abilities were related to changes in communication disturbances (i.e., cognitive reactivity) from the baseline to high-load condition within the SMI group. We hypothesized that higher negative affect and poorer cognitive abilities would be significantly correlated with greater cognitive reactivity. All analyses in this study were two-tailed and all variables were normally distributed (skew < 1.5). Extreme scores (>3.5 SD) were trimmed (i.e., replaced with values 3.5 SD).
3. Results
3.1. Demographic variables
Table 1 presents descriptive statistics for demographic, clinical, affective, and cognitive variables. Education and GAF scores were significantly different between the patient and control groups (all p’s < 0.05), but there were no other significant differences between demographic variables. As expected, patient and healthy control groups significantly differed on all cognitive abilities (p’s < 0.05) and trait negative affect (p < 0.05). Patients meeting criteria for schizophrenia versus those without did not significantly differ in demographic variables, affect, cognitive ability, performance on the dual-load task, or negative symptom ratings. With the exception of bizarre behavior ratings, patients with schizophrenia had more severe positive symptoms including hallucinations, delusions, and thought disorder (p’s < 0.05). The patient groups did not significantly differ in communication disturbances in the baseline or high-load condition.
Table 1.
Descriptive statistics for demographic, clinical, affective, and cognitive variables for the control and serious mental illness (SMI) groups.
| Variable | Control (n = 27) | SMI (n = 52) | Statistic | ||
|---|---|---|---|---|---|
|
|
|
|
|||
| % | % | X2 | |||
| Females | 59% | 39% | 3.10 | ||
| Ethnicity (% Caucasian) | 52% | 58% | 0.25 | ||
| Psychiatric Disorders (by history) | |||||
| Major depressive episode | 0% | 43% | |||
| Manic episode | 0% | 26% | |||
| Psychotic episode | 0% | 74% | |||
| M | SD | M | SD | t | |
|
| |||||
| Age (years) | 40.82 | 12.57 | 41.42 | 12.07 | −0.21 |
| Education (years) | 14.48 | 2.46 | 11.52 | 1.84 | 6.03* |
| Highest Level of Parental Education | 14.29 | 2.73 | 13.08 | 3.48 | 1.45 |
| GAF | 84.36 | 5.42 | 47.38 | 9.79 | 16.56* |
| Number of Hospitalizations | 7.22 | 10.27 | |||
| Performance on Dual-load Task | |||||
| High-load accuracy % | 0.71 | 0.20 | 0.56 | 0.21 | 3.11* |
| High-load reaction time | 633.57 | 328.85 | 959.54 | 440.12 | 3.38* |
| Cognitive Performance | |||||
| Working Memory | 21.13 | 4.18 | 15.06 | 5.38 | 4.85* |
| Psychomotor | 70.33 | 14.16 | 47.06 | 19.28 | 5.23* |
| Executive Functioning | 16.29 | 5.34 | 11.29 | 5.71 | 3.58* |
| Verbal Memory | 46.75 | 10.63 | 27.90 | 11.53 | 6.46* |
| Attention | 59.58 | 15.23 | 32.58 | 15.49 | 7.01* |
| Trait Affect | |||||
| Positive | 35.92 | 4.45 | 35.05 | 8.64 | 0.49 |
| Negative | 15.52 | 4.09 | 23.43 | 9.03 | 4.27* |
| Positive Symptoms | |||||
| Hallucinations | 1.52 | 1.58 | |||
| Delusions | 1.44 | 1.51 | |||
| Bizarre behavior | 0.65 | 1.01 | |||
| Formal thought disorder | 1.71 | 1.59 | |||
| Negative Symptoms | |||||
| Flat affect | - | - | 1.36 | 1.55 | - |
| Alogia | - | - | 1.59 | 1.58 | - |
| Avolition | - | - | 1.65 | 1.32 | - |
| Anhedonia | - | - | 0.64 | 1.16 | - |
p < 0.05
3.2. Group comparisons on communication disturbances
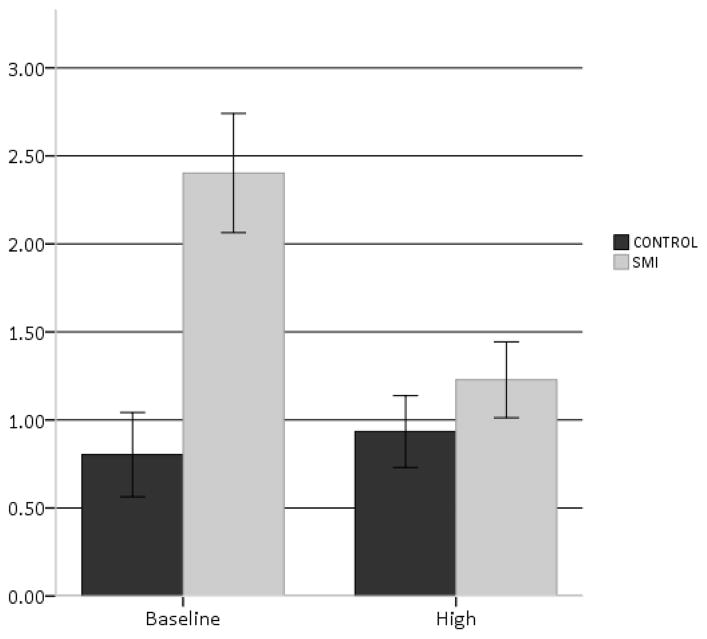
Results for the repeated measures ANCOVA examining communication disturbances across groups and cognitive load conditions are presented in Table 2. While SMI was significantly associated with more communication disturbances, significant condition effects were observed such that increased cognitive demands were associated with fewer reference errors, F(1,73) = 5.05, p = 0.03. A significant interaction effect was also observed for reference errors, F(1,73) = 5.30, p = 0.02. Post-hoc analyses of the interaction, using dependent t-tests, revealed that there a dramatic decrease in communication disturbances for patients versus healthy controls as the cognitive demands of the task increased (see Fig. 1). Taken together, increased cognitive resources led to changes in communication disturbances for the SMI group controlling for affective reactivity, but in the opposite direction as hypothesized. Lastly, a repeated measure ANCOVA restricted to the schizophrenia patients (i.e., excluding patients with unipolar and bipolar disorder) did not change our results. We also did not observe significant order effects.
Table 2.
Group comparisons on communication disturbances with F values for repeated-measures analysis of covariance
| CON (n = 27) | SMI (n = 52) | F-values | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| M | SD | M | SD | Condition | Group | Interaction | |
| Baseline Condition | 0.80 | 1.24 | 2.40 | 2.44 | 5.05* | 7.67* | 5.30* |
| High Load Condition | 0.93 | 1.06 | 1.23 | 1.56 | |||
Note. CON = control; SMI = serious mental illness
p < 0.05
Fig. 1.
Comparison of communication disturbances for baseline and high-load condition for control and serious mental illness (SMI) groups.
3.3. Communication disturbances, symptoms, affect, and cognitive ability
Correlations among study variables within the SMI group are presented in Table 3. Significant correlations were observed for positive trait affect and cognitive reactivity such that less positive affect was associated with less semantically disturbed (i.e., more coherent) speech from baseline to the high load condition, r(49) = 0.40, p = 0.008. Similarly, cognitive reactivity was significantly correlated with state affective reactivity in positive emotion words, r(49) = 0.29, p = 0.04, indicating that an reduction in communication disturbances under cognitive load was related to a decrease in state positive affectivity. Cognitive abilities were not significantly related to cognitive reactivity, although the relationship between executive functioning and cognitive reactivity was a statistical trend, r(49) = −0.28, p = 0.09. Negative affect and psychiatric symptoms were not significantly related to cognitive reactivity across conditions.
Table 3.
Correlations between cognitive reactivity and positive and negative symptoms, trait affect, and cognitive ability in SMI group.
| Cognitive Reactivity | |
|---|---|
| Positive symptoms | |
| Hallucinations | 0.02 |
| Delusions | −0.20 |
| Bizarre Behavior | −0.13 |
| Thought Disorder | −0.08 |
| Negative symptoms | |
| Flat affect | −0.16 |
| Alogia | −0.14 |
| Avolition | −0.19 |
| Anhedonia | −0.09 |
| Affect | |
| Trait positive emotion | 0.40* |
| Trait negative emotion | −0.16 |
| State affective reactivity Positive – emotion words | .029* |
| State affective reactivity Negative – emotion words | −0.07 |
| Cognitive Ability | |
| Working memory | −0.02 |
| Psychomotor | −0.02 |
| Executive functioning | −0.28† |
| Verbal memory | −0.01 |
| Attention | −0.17 |
p < 0.10
p < 0.05
4. Discussion
The primary aim of this study was to examine how cognitive resources influence language production in SMI using highly sensitive behavioral technologies. Three notable findings emerged. First, congruent with the literature (Barch and Berenbaum, 1997; Docherty et al., 1996; Rubino et al., 2011), individuals with SMI produced more disorganized speech than healthy controls. Second, using an experimentally-based dual task, SMI versus controls showed a more pronounced decline in communication disturbances from under cognitive load condition (i.e., cognitive reactivity); even after accounting for state reactivity in affect. Third, correlation analyses provided evidence for a link between cognitive reactivity and state and trait positive affect in SMI, suggesting that individual differences in positive emotions may mitigate how speech disorder changes as a function of cognitive load. Correlational analyses revealed little evidence for a relationship between cognitive reactivity and cognitive ability. The present findings were not simply the result of patients producing less speech under cognitive load as both groups demonstrated a similar reduction, the CDI accounts for the word count.
Contrary to expectations, cognitive reactivity was observed such that depletion of cognitive resources was associated with a decrease in communication disturbances in patients; and interestingly, a relatively negligible increase for nonpsychiatric controls. Lack of direction aside, significant interaction effects indicate that cognitive resources play an important role in communication disturbances for patients but not controls, and this occurs above and beyond the influences of state affect. While prior studies have failed to find a significant effect of cognitive load on communication disturbances in chronic schizophrenia and early-stage psychosis (Melinder and Barch, 2003; Minor et al., 2016), the current findings are novel and add to the existing literature on the influential role of cognitive processes on semantically incoherent speech. Several possible explanations warrant mention for the present findings. First, it could be that the introduction of cognitive load influenced the semantic memory network in SMI. Collins and Loftus (1975) proposed a spreading activation theory which states that when a node in the semantic network is activated, it automatically activates similar nodes, thus facilitating more efficient processing of related concepts. Some researchers have postulated that the semantic network within SMI is diffusely connected with increased automaticity of loosely related concepts (Sitnikova et al., 2009). Moreover, these abnormal semantic networks may help produce disorganized speech and behavior in SMI. It is thus possible that depletion of cognitive resources and accompanying restricted attention led to decreased engagement of the semantic network, therefore leading to more typical semantic associations of related concepts.
Interestingly, we also found that less trait and state positive affect was associated with more coherent speech in SMI. The links here are difficult to explain, though the fact that similar relationships were observed for both self-reported trait and lexically expressed emotion suggests that these findings were not spurious. It is also worth noting that negative affect can constrict the attentional field (Lazar et al., 2012), which may further reduce semantic spreading within the semantic network under states of high cognitive load. Also, it may be that that limited cognitive resources may decrease semantically incoherent speech by preventing rumination and worry (i.e., providing distraction) in individuals with low positive affect (i.e., anhedonia). Generally speaking, cognitive ability was not related to changes in communication disturbances following cognitive load. While there is robust research to suggest that patients with working memory and attentional deficits show increased communication disturbances (Docherty, 2012), our results indicate that cognitive reactivity is orthogonal to the presence of cognitive deficits. Moreover, using the same task data and sample as our present study (Cohen et al., 2014), we previously reported that depletion of cognitive resources adversely affected only one aspect of speech (e.g., pause length) in SMI patients more so than controls. Accordingly, other aspects of speech production (e.g., word count, pause length, utterance length) and content (e.g., idea density, vocabulary density) were not uniquely influenced by limited cognitive resources in SMI patients compared to controls. Given our surprising findings from the present study, future research would benefit by understanding how cognition is linked to different components of speech production and content, and how these components are mechanistically similar and different from each other.
Findings from this study hold several interesting and important treatment implications. To date, language dysfunction has been treatment resistant (Bowie and Harvey, 2008). Our findings suggest that cognitive resources may be manipulated in some fashion to reduce communication disturbances in SMI. From a therapeutic perspective, interventions that involve systematic methods aimed at cognitive functioning (e.g., cognitive remediation) may prove fruitful for ameliorating communication disturbances. However, our current findings does not lend to easy interpretations for treatment implementations. The results suggest that cognitive reactivity can be deliberately manipulated to reduce semantically incoherent speech. For example, it may be beneficial for individuals with SMI to participate in cognitively taxing activities through distraction or other dual-task exercises in order to decrease communication disturbances. Alternatively, mental fatigue might also improve communication coherence. It is highly important to note that these treatment implications are based on preliminary findings and that other investigators have failed to find cognitive reactivity (i.e., a reduction in communication disturbances following cognitive load) in SMI populations (Melinder and Barch, 2003; Minor et al., 2016). Thus, more research on the cognitive mechanisms underlying communication disturbances is needed in order to inform proper treatment implications. Our findings of a link between positive affect and cognitive reactivity suggest that emotion may also be important in understanding state communication disturbances.
Several study strengths are worthy of mention. First, we investigated the cognitive underpinnings of language dysfunction in SMI using a novel experimental paradigm. Second, we examined language dysfunction and cognitive resources in SMI in a manner is that consistent with an RDoC approach (Insel et al., 2010). Our results of similar cognitive abilities and performance on the dual-task across our patient groups is consistent with previous findings (Barch, 2009; Barch and Sheffield, 2014; Simonsen et al., 2011; Strauss et al., 2015). While it is somewhat surprising that patients with schizophrenia did not differ in communication disturbances than patients without, it may be the case that patients with bipolar disorder and major depressive disorder who meet federal criteria for SMI–as in our study–exhibit similar communication disturbances as those with schizophrenia. Therefore, language functioning may differ as a function of severity of illness rather than diagnostic criteria. Other strength includes the use of objective and behavioral based approaches to characterize language output in SMI. Several limitations warrant discussion. First, a direct measurement of state affect was not used. While LIWC has been utilized to assess positive and negative affectivity in previous studies (Najolia et al., 2011; St-Hilaire et al., 2008), future research could benefit by more directly measuring self-reported affect. Another limitation concerns the nature of the speech conditions themselves. We did not employ a true “baseline” measure of speech, as even the baseline condition may have been cognitively taxing to some degree. Also, though participants discussed neutrally valenced topics, narratives may have differed in some other meaningful way (e.g., arousal). Finally, the sample size in this study was modest and we did not account for the potential confound of medications effects; future studies should replicate findings in SMI samples adequately powered and controlling for the possible influence of medications.
To conclude, this study offers evidence that cognitive processes play a critical role in language dysfunction in SMI. Though a number of studies have found correlational links between poor cognitive ability and increased communication disturbances, our experimental findings indicate that limited cognitive resources can temporarily relieve language dysfunction and suggest possible intervention routes. After attempting to control for the role of affective systems in language dysfunction, we nonetheless observed that positive affect modulates the relationship between communication disturbances and cognitive load. These findings highlight the need to understand how cognitive and affective systems affect language.
Highlights.
Behavioral language measures were used to understand communication disturbances in SMI.
Reduced cognitive resources may temporarily relieve communication disturbances.
This cognitive reactivity appears to be related lower positive affectivity in SMI.
Acknowledgments
We would like to thank those who participated in the study, lab members for their help in processing and collecting data, and the National Institute of Mental Health (R03MH092622).
Funding
Funding for this study was provided by grant R03MH092622 from the National Institute of Mental Health; the funding agency had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflicts of interest
There are no conflicts of interest.
Contributors
Thanh P. Le conducted the literature searches, performed data analyses, and wrote the bulk of the manuscript. Gina M. Najolia and Kyle S. Minor helped manage data collection and data interpretation. Alex S. Cohen was the primary investigator for this project, designed the study, and aided in data interpretation. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alpert M, Shaw RJ, Pouget ER, Lim KO. A comparison of clinical ratings with vocal acoustic measures of flat affect and alogia. J Psychiatr Res. 2002;36:347–53. doi: 10.1016/s0022-3956(02)00016-x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Author; Washington, DC: 1994. [Google Scholar]
- Andreasen NC. Scale for the assessment of thought, language, and communication (TLC) Schizophr Bull. 1986;12:473–82. doi: 10.1093/schbul/12.3.473. [DOI] [PubMed] [Google Scholar]
- Andreason NC. The Scale for the Assessment of Negative Symptoms (SANS) University of Iowa; Iowa City, IA: 1984. [Google Scholar]
- Andreason NC. The Scale for the Assessment of Positive Symptoms (SAPS) University of Iowa; Iowa City, IA: 1984. [Google Scholar]
- Barch DM. Neuropsychological abnormalities in schizophrenia and major mood disorders: similiarities and differences. 2009;11:313–319. doi: 10.1007/s11920-009-0045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Berenbaum H. Language generation in schizophrenia and mania: the relationships among verbosity, syntactic complexity, and pausing. J Psycholinguist Res. 1997;26:401–412. doi: 10.1023/a:1025026019107. [DOI] [PubMed] [Google Scholar]
- Barch DM, Berenbaum H. Language production and thought disorder in schizophrenia. J Abnorm Psychol. 1996;105:81–88. doi: 10.1037//0021-843x.105.1.81. [DOI] [PubMed] [Google Scholar]
- Barch DM, Berenbaum H. The relationship between information processing and language production. J Abnorm Psychol. 1994;103:241–250. doi: 10.1037//0021-843x.103.2.241. [DOI] [PubMed] [Google Scholar]
- Barch DM, Sheffield JM. Cognitive impairments in psychotic disorders: common mechanisms and measurement. World Psychiatry. 2014;13:224–32. doi: 10.1002/wps.20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker TM, Cicero DC, Cowan N, Kerns JG. Cognitive control components and speech symptoms in people with schizophrenia. Psychiatry Res. 2012;196:20–26. doi: 10.1186/1744-859X-5-S1-S229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie CR, Harvey PD. Communication abnormalities predict functional outcomes in chronic schizophrenia: differential associations with social and adaptive functions. Schizophr Res. 2008;103:240–7. doi: 10.1016/j.schres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbridge Ja, Barch DM. Emotional valence and reference disturbance in schizophrenia. J Abnorm Psychol. 2002;111:186–191. doi: 10.1037/0021-843X.111.1.186. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Docherty NM. Effects of Positive Affect on Speech Disorder in Schizophrenia [WWW Document] [accessed 5.28.16];J Nerv Ment Dis. 2005 doi: 10.1097/01.nmd.0000188963.16870.27. http://sites01.lsu.edu/faculty/asap/wp-content/uploads/sites/33/2014/11/Pos-Emo-Sp.-Do-Cohen-2005.pdf. [DOI] [PubMed]
- Cohen AS, Elvevåg B. Automated computerized analysis of speech in psychiatric disorders. Curr Opin Psychiatry. 2014;27:203–9. doi: 10.1097/YCO.0000000000000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, McGovern JE, Dinzeo TJ, Covington Ma. Speech deficits in serious mental illness: A cognitive resource issue? Schizophr Res. 2014;160:173–179. doi: 10.1016/j.schres.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Morrison SC, Brown La, Minor KS. Towards a cognitive resource limitations model of diminished expression in schizotypy. J Abnorm Psychol. 2012;121:109–118. doi: 10.1037/a0023599. [DOI] [PubMed] [Google Scholar]
- Cohen AS, St-Hilaire A, Aakre JM, Docherty NM. Understanding anhedonia in schizophrenia through lexical analysis of natural speech. Cogn Emot. 2009;23:569–586. doi: 10.1080/02699930802044651. [DOI] [Google Scholar]
- Collins AM, Loftus EF. A spreading-activation theory of semantic processing. Psychological Rev. 1975;82(6):407. [Google Scholar]
- Docherty NM. On identifying the processes underlying schizophrenic speech disorder. Schizophr Bull. 2012;38:1327–35. doi: 10.1093/schbul/sbr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty NM. Cognitive impairments and disordered speech in schizophrenia: thought disorder, disorganization, and communication failure perspectives. J Abnorm Psychol. 2005;114:269–278. doi: 10.1037/0021-843X.114.2.269. [DOI] [PubMed] [Google Scholar]
- Docherty NM, DeRosa M, Andreasen NC. Communication disturbances in schizophrenia and mania. Arch Gen Psychiatry. 1996;53:358–64. doi: 10.1001/archpsyc.1996.01830040094014. [DOI] [PubMed] [Google Scholar]
- Docherty NM, Evans IM, Sledge WH, Seibyl JP, Krystal JH. Affective reactivity of language in schizophrenia. J Nerv Ment Dis. 1994;182:98–102. doi: 10.1097/00005053-199402000-00006. [DOI] [PubMed] [Google Scholar]
- Docherty NM, Hall MJ, Gordinier SW. Affective reactivity of speech in schizophrenia patients and their nonschizophrenic relatives. J Abnorm Psychol. 1998;107:461–7. doi: 10.1037//0021-843x.107.3.461. [DOI] [PubMed] [Google Scholar]
- Elvevåg B, Cohen AS, Wolters MK, Whalley HC, Gountouna V-E, Kuznetsova KA, Watson AR, Nicodemus KK. An examination of the language construct in NIMH’s research domain criteria: Time for reconceptualization! Am J Med Genet B Neuropsychiatr Genet. 2016 doi: 10.1002/ajmg.b.32438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvevåg B, Foltz PW, Weinberger DR, Goldberg TE. Quantifying incoherence in speech: an automated methodology and novel application to schizophrenia. Schizophr Res. 2007;93:304–16. doi: 10.1016/j.schres.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. User’s Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders — Research Version. Biometrics Research, New York State Psychiatric Institute; New York, NY: 1996. [Google Scholar]
- Hoffman RE, Stopek S, Andreasen NC. A comparative study of manic vs schizophrenic speech disorganization. Arch Gen Psychiatry. 1986;43:831–8. doi: 10.1001/archpsyc.1986.01800090017003. [DOI] [PubMed] [Google Scholar]
- Kahn JH, Tobin RM, Massey AE, Anderson JA. Measuring emotional expression with the Linguistic Inquiry and Word Count. Am J Psychol. 2007;120:263–86. [PubMed] [Google Scholar]
- Keefe RSE. Brief Assessment of Cognition in Schizophrenia BACS Manual-A: Version 2.1. Duke University Medical Center; Durham, NC: 1999. [Google Scholar]
- Kerns JG. Experimental manipulation of cognitive control processes causes an increase in communication disturbances in healthy volunteers. Psychol Med. 2007;37:995–1004. doi: 10.1017/S0033291706009718. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR. Language in schizophrenia Part 1 : an Introduction. 2011;4:576–589. doi: 10.1111/j.1749-818X.2010.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar J, Kaplan O, Sternberg T, Lubow RE. Positive and negative affect produce opposing task-irrelevant stimulus preexposure effects. Emotion. 2012;12:591–604. doi: 10.1037/a0024867. [DOI] [PubMed] [Google Scholar]
- Mackin RS, Areán PA. Incidence and documentation of cognitive impairment among older adults with severe mental illness in a community mental health setting. Am J Geriatr Psychiatry. 2009;17:75–82. doi: 10.1097/JGP.0b013e31818cd3e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melinder MRD, Barch DM. The influence of a working memory load manipulation on language production in schizophrenia. Schizophr Bull. 2003;29:473–485. doi: 10.1093/oxfordjournals.schbul.a007020. [DOI] [PubMed] [Google Scholar]
- Minor KS, Bonfils KA, Luther L, Firmin RL, Kukla M, MacLain VR, Buck B, Lysaker PH, Salyers MP. Lexical analysis in schizophrenia: How emotion and social word use informs our understanding of clinical presentation. J Psychiatr Res. 2015;64:74–78. doi: 10.1016/j.jpsychires.2015.02.024. [DOI] [PubMed] [Google Scholar]
- Minor KS, Marggraf MP, Davis BJ, Mehdiyoun NF, Breier A. Affective Systems Induce Formal Thought Disorder in Early-Stage Psychosis. J Abnorm Psychol. 2016;125:1–7. doi: 10.1037/abn0000156. [DOI] [PubMed] [Google Scholar]
- Najolia GM, Cohen AS, Minor KS. A laboratory study of affectivity in schizotypy: Subjective and lexical analysis. Psychiatry Res. 2011;189:233–238. doi: 10.1016/j.psychres.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Pennebaker JW. Linguistic Inquiry and Word Count. Lawrence Erlbaum Associates; 2001. [Google Scholar]
- Perlini C, Marini a, Garzitto M, Isola M, Cerruti S, Marinelli V, Rambaldelli G, Ferro a, Tomelleri L, Dusi N, Bellani M, Tansella M, Fabbro F, Brambilla P. Linguistic production and syntactic comprehension in schizophrenia and bipolar disorder. Acta Psychiatr Scand. 2012;126:363–76. doi: 10.1111/j.1600-0447.2012.01864.x. [DOI] [PubMed] [Google Scholar]
- Plass JL, Moreno R, Branken R. Cognitive Load Theory. Cambridge University Press; New York, NY: 2010. [Google Scholar]
- Prochaska JJ, Velicer WF, Nigg CR, Prochaska JO. Methods of quantifying change in multiple risk factor interventions. Prev Med (Baltim) 2008;46:260–5. doi: 10.1016/j.ypmed.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radanovic M, Nunes PV, Forlenza OV, Braga Ladeira R, Gattaz WF. Cognitive-linguistic deficits in euthymic elderly patients with bipolar disorder. J Affect Disord. 2013;150:691–4. doi: 10.1016/j.jad.2013.04.035. [DOI] [PubMed] [Google Scholar]
- Rosenstein M, Diaz-Asper C, Foltz PW, Elvevåg B. A computational langauge approach to modeling prose recall in schizophrenia. Cortex. 2014;55:148–166. doi: 10.1016/j.cortex.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino IA, D’Agostino L, Sarchiola L, Romeo D, Siracusano A, Docherty NM. Referential failures and affective reactivity of language in schizophrenia and unipolar depression. Schizophr Bull. 2011;37:554–560. doi: 10.1093/schbul/sbp108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen C, Sundet K, Vaskinn A, Birkenaes AB, Engh JA, Faerden A, Jónsdóttir H, Ringen PA, Opjordsmoen S, Melle I, Friis S, Andreassen OA. Neurocognitive dysfunction in bipolar and schizophrenia spectrum disorders depends on history of psychosis rather than diagnostic group. Schizophr Bull. 2011;37:73–83. doi: 10.1093/schbul/sbp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitnikova T, Goff D, Kuperberg GR. Neurocognitive abnormalities during comprehension of real-world goal-directed behaviors in schizophrenia. J Abnorm Psychol. 2009;118:256–77. doi: 10.1037/a0015619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Hilaire A, Cohen AS, Docherty NM. Emotion word use in the conversational speech of schizophrenia patients. Cogn Neuropsychiatry. 2008;13:343–56. doi: 10.1080/13546800802250560. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Thaler NS, Vogel SJ, Sutton GP, Lee BG, Allen DN. Predicting Psychosis Across Diagnostic Boundaries : Behavioral and Computational Modeling Evidence for Impaired Reinforcement Learning in Schizophrenia and Bipolar Disorder With a History of Psychosis. J Abnorm Psychol. 2015;124:697–708. doi: 10.1037/abn0000039. dx.doi.org/10.1037/abn0000039. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]