Abstract
Purpose
We aimed to reveal the prognostic influence of B-cell CLL/lymphoma 2 (BCL2) on molecular subtypes of breast cancer.
Methods
We analyzed 9,468 patients with primary breast cancer. We classified molecular subtypes according to the National Comprehensive Cancer Network (NCCN) and St. Gallen guidelines, mainly on the basis of the expression of hormonal receptor (HR), human epidermal growth factor receptor 2 (HER2), and Ki-67.
Results
Regarding NCCN classification, BCL2 was a strong favorable prognostic factor in the HR(+)/HER2(–) subtype (p<0.001) and a marginally significant favorable prognosticator in the HR(+)/HER2(+) subtype (p=0.046). BCL2 had no prognostic impact on HR(–)/HER2(+) and HR(–)/HER2(–) subtypes. In relation to St. Gallen classification, BCL2 was a strong favorable prognosticator in luminal A and luminal B/HER2(–) subtypes (both p<0.001). BCL2 was a marginally significant prognosticator in the luminal B/HER2(+) subtype (p=0.046), and it was not a significant prognosticator in HER2 or triple negative (TN) subtypes. The prognostic effect of BCL2 was proportional to the stage of breast cancer in HR(+)/HER2(–), HR(+)/HER2(+), and HR(–)/HER2(–) subtypes, but not in HR(–)/HER2(+) subtype. BCL2 was not a prognostic factor in TN breast cancer regardless of epidermal growth factor receptor expression.
Conclusion
The prognostic influence of BCL2 was different across molecular subtypes of breast cancer, and it was largely dependent on HR, HER2, Ki-67, and the stage of cancer. BCL2 had a strong favorable prognostic impact only in HR(+)/HER2(–) or luminal A and luminal B/HER2(–) subtypes, particularly in advanced stages. Further investigations are needed to verify the prognostic influence of BCL2 on molecular subtypes of breast cancer and to develop clinical applications for prognostication using BCL2.
Keywords: Bcl-2, Breast neoplasms, Prognosis, Survival analysis, Triple negative breast neoplasms
INTRODUCTION
B-cell CLL/lymphoma 2 (BCL2) encodes an integral outer mitochondrial membrane protein that blocks the apoptotic death of some cells such as lymphocytes, and it is located on chromosome 18q21.3 and has 6 exons [1]. The BCL2 protein, encoded by the BCL2 gene, is the founding member of the BCL2 family of regulator proteins that regulate cell death (apoptosis), by either inducing (proapoptotic) or inhibiting (antiapoptotic) apoptosis. BCL2 is the key antiapoptotic protein and its gene is thus classified as an oncogene in general [2]. Although BCL2 was originally found in human follicular B cell lymphoma carrying the chromosomal translocation t(14,18) [3], researchers have also reported its roles in other cancers such as leukemia, breast cancer, lung cancer, prostate cancer, gastric cancer, and pancreatic cancer, among others [4,5,6,7,8].
Reports describing the prognostic role of BCL2 in patients with breast cancer have been published since 1994 [9,10,11]. Callagy et al. [12] reported that BCL2 is an independent predictor of favorable outcomes in breast cancer, particularly in the first 5 years after diagnosis. Berardo et al. [13] reported that high BCL2 expression is associated with a number of good prognostic factors and is independently associated with a better clinical outcome for patients with lymph node-positive breast carcinoma. Ali et al. [14] reported that a high Ki-67/BCL2 index is significantly associated with a decreasing likelihood of breast cancer-specific survival (BCSS) in estrogen receptor (ER)-positive breast cancer. Several meta-analyses have shown that BCL2 is an independent favorable prognostic marker in breast cancer [15,16]. Although inconsistent results have been reported, BCL2 has been considered a favorable prognostic factor in breast cancer. Previously, we also reported that BCL2 was a powerful independent prognostic factor in breast cancer and that favorable clinicopathologic features and a strong correlation with the hormonal receptor (HR) were suggested as the causes of superior survival in patients with BCL2-positive breast cancer [17].
Currently, molecular subtypes of breast cancer are widely accepted in clinical practice. Molecular subtypes are classified according to the expression of HR and human epidermal growth factor receptor 2 (HER2) by the National Comprehensive Cancer Network (NCCN) guidelines. According to the St. Gallen guidelines, additional factors are involved to classify molecular subtypes besides HR and HER2, including mainly Ki-67 and histologic grade. Compared to unselected breast cancer, the prognostic influence of BCL2 on molecular subtypes of breast cancer has rarely been reported, but the results have been inconsistent and remain controversial. Moreover, the reported results regarding the prognostic role of BCL2 in triple negative breast cancer (TNBC) have been contradictory.
Although the majority of recent studies have reported the favorable prognostic role of BCL2 in unselected breast cancer, its role in molecular subtypes of breast cancer including TNBC has rarely been reported and the results remain controversial. In the present study, we aimed to investigate the prognostic influence of BCL2 on molecular subtypes of breast cancer.
METHODS
Patients
Patients with breast cancer from Seoul National University Boramae Medical Center and Seoul National University Hospital were participants in this study. Initially, the total number of patients was 19,127, and the final number of subjects was 9,468 following the exclusion of 9,659 patients. The following patients were excluded from the study: 2,361 patients with no survival data, 296 patients with metastases at initial diagnosis, 1,884 patients diagnosed with carcinoma in situ, 160 patients diagnosed with malignant phyllodes tumor, 1,266 patients who had received neoadjuvant chemotherapy, 1,638 patients without information regarding BCL2, 64 patients less than 20 or more than 80 years of age, 32 male patients, and 1,958 patients with insufficient data for analysis. Study subjects underwent operations for primary breast cancer between July 1, 1994 and June 26, 2015. This study was approved by the Institutional Review Board (IRB) of Seoul National University Boramae Medical Center and Seoul National University Hospital (26-2015-107).
Definitions of clinicopathologic parameters
Patients' ages were defined as the age at the time of diagnosis of primary breast cancer. The TNM staging was determined according to the seventh edition of the American Joint Committee on Cancer. HR status was defined as positive when immunohistochemistry test results for either the ER or progesterone receptor (PR) were positive, and defined as negative when both tests results were negative. HER2 expression was defined as negative when the immunohistochemistry results were negative or 1+, and defined as positive when the results were 3+. When the results were 2+, we defined the positivity of HER2 according to the results of the fluorescence in situ hybridization. We followed the American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of ER, PR, and HER2 [18,19]. Histologic grade was defined according to the modified Scarff-Bloom-Richardson grading system. BCL2 was defined as negative when the results of immunohistochemical stains were positive in less than 10% of observed tumor cells and the intensity of staining was weak or lower. Otherwise, BCL2 was defined as positive. A detailed description of the definition of BCL2 positivity was provided in our previous report [17]. All operations with curative intent for patients with breast cancer were classified into either breast conserving surgery or total mastectomy according to the extent of surgery for the breast tissue.
Definition of molecular subtypes according to guidelines
In the NCCN classification, breast cancers are categorized into four subtypes according to HR and HER2 positivity: HR(+)/HER2(–); HR(+)/HER2(+); HR(–)/HER2(+); and HR(–)/HER2(–) [20]. According to the St. Gallen classification, breast cancers are categorized into five subtypes: luminal A (high ER/PR, HER2 negative, Ki-67 <14%, T1/2, and N0/1); luminal B/HER2 negative (low ER/PR, HER2 negative, Ki-67 ≥20%, N2/3, T3, histologic grade 3, and extensive lymphovascular invasion); luminal B/HER2 positive (HR positive and HER2 positive); HER2 (HR negative and HER2 positive); and triple negative (TN; HR negative and HER2 negative) [21,22,23].
Statistical analyses
Data were presented as frequency and percentage for categorical variables. Pearson chi-square test was used to determine differences in clinicopathologic characteristics between pairs of groups. The Kaplan-Meier method was used for estimation of survival rates, and the log-rank test was used to determine the significance of differences between two or more survival curves. The Cox proportional hazards model was used for univariate and multivariate analysis, and the hazard ratio was calculated according to the cutoff value of a 95% confidence interval (CI). The time durations of overall survival (OS) and BCSS were defined as the time from operation to death from any cause and death from breast cancer, respectively. All statistical analyses were performed using IBM SPSS Statistics version 20.0 (IBM Corp., Armonk, USA). All tests were two-sided and we regarded the results of statistical analyses as significant when the p-value was less than 0.05.
RESULTS
Clinicopathologic characteristics according to BCL2 expression in molecular subtypes of breast cancer
The mean follow-up period was 70.47±52.16 months (range, 0–227 months) and the mean age was 49.78±10.29 years (range, 20–80 years) in the total study population of 9,468 patients. BCL2 expression was positive in 6,797 patients (71.8%) and negative in 2,671 patients (28.2%). Patients with positive BCL2 expression showed overall higher rates of the following clinicopathologic features: age less than 50 years, small tumor size (less than 2 cm), early stage (stage I), positive HR, positive ER, positive PR, negative HER2, high histologic grade (histologic grade 3), negative lymphovascular invasion, low Ki-67 index (less than 14%), and breast-conserving surgery, but not nodal positivity (Supplementary Table 1, available online). According to the NCCN classification, the number of patients classified into each subtype were 4,998 (52.8%), 899 (9.5%), 847 (8.9%), and 1,501 (15.9%) for HR(+)/HER2(–), HR(+)/HER2(+), HR(–)/HER2(+), and HR(–)/HER2(–), respectively. Within HR(+)/HER2(–) subtype, the BCL2-positive group showed higher rates of small tumor size, negative node involvement, early stage, positive ER, positive PR, low histologic grade, negative lymphovascular invasion, low Ki-67 index, and breast conserving surgery, but no difference in age at diagnosis. In HR(+)/HER2(+) and HR(−)/HER2(+) subtypes, no differences were found between the positive and negative BCL2 groups except for PR and histologic grade, respectively. In HR(−)/HER2(−) subtype, the BCL2-positive group showed higher rates of patients with age less than 50 years, low Ki-67 index, and breast conserving surgery (Table 1). BCL2 positive rates for molecular subtypes according to the NCCN and St. Gallen classifications are described in Table 2. According to the NCCN classification, the proportions of positive BCL2 expression for each subtype were 88.8%, 76.4%, 18.3%, and 39.8% in HR(+)/HER2(−), HR(+)/HER2(+), HR(−)/HER2(+), and HR(−)/HER2(−), respectively.
Table 1. Clinicopathologic characteristics according to BCL2 expression in molecular subtypes of breast cancer by NCCN classification.
| Characteristic | All No. (%) | HR(+)/HER2(−) (n=4,998) | HR(+)/HER2(+) (n=899) | HR(−)/HER2(+) (n=847) | HR(−)/HER2(−) (n = 1,501) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BCL2(−) No. (%) |
BCL2(+) No. (%) |
p-value* | BCL2(−) No. (%) |
BCL2(+) No. (%) |
p-value* | BCL2(−) No. (%) |
BCL2(+) No. (%) |
p-value* | BCL2(−) No. (%) |
BCL2(+) No. (%) | p-value* | ||
| Total | 9,468 (100.0) | 558 (11.2) | 4,440 (88.8) | 212 (23.6) | 687 (76.4) | 692 (81.7) | 155 (18.3) | 904 (60.2) | 597 (39.8) | ||||
| Age (yr) | 0.057 | 0.983 | 0.136 | 0.002 | |||||||||
| ≤ 50 | 5,368 (56.7) | 309 (55.4) | 2,642 (59.6) | 129 (60.8) | 418 (60.9) | 307 (44.4) | 79 (51.0) | 471 (52.2) | 360 (60.3) | ||||
| > 50 | 4,093 (43.3) | 249 (44.6) | 1,793 (40.4) | 83 (39.2) | 268 (39.1) | 385 (55.6) | 76 (49.0) | 432 (47.8) | 237 (39.7) | ||||
| Tumor size (cm) | < 0.001 | 0.733 | 0.339 | 0.236 | |||||||||
| ≤2 | 4,805 (50.8) | 227 (40.8) | 2,521 (56.9) | 99 (46.7) | 342 (49.8) | 268 (38.8) | 68 (43.9) | 374 (41.4) | 223 (37.4) | ||||
| >2 | 4,352 (46.0) | 318 (57.1) | 1,804 (40.7) | 105 (49.5) | 320 (46.6) | 392 (56.7) | 83 (53.5) | 499 (55.2) | 356 (59.6) | ||||
| Nodal positivity | 0.001 | 0.735 | 0.661 | 0.501 | |||||||||
| Negative | 6,130 (64.8) | 320 (57.3) | 2,888 (65.1) | 118 (55.7) | 403 (58.7) | 425 (61.4) | 97 (62.6) | 632 (69.9) | 420 (70.4) | ||||
| Positive | 3,189 (33.7) | 230 (41.2) | 1,491 (33.6) | 90 (42.5) | 271 (39.4) | 256 (37.0) | 57 (36.8) | 264 (29.2) | 168 (28.1) | ||||
| Stage | < 0.001 | 0.381 | 0.384 | 0.386 | |||||||||
| Stage I | 3,791 (40.0) | 176 (31.5) | 1,973 (44.4) | 71 (33.5) | 248 (36.1) | 201 (29.0) | 53 (34.2) | 315 (34.8) | 188 (31.5) | ||||
| Stage II, III | 5,358 (56.6) | 371 (66.5) | 2,354 (53.0) | 129 (60.8) | 414 (60.3) | 465 (67.2) | 98 (63.2) | 557 (61.6) | 385 (64.5) | ||||
| HR | - | - | - | - | |||||||||
| Negative | 2,601 (27.5) | 0 | 0 | 0 | 0 | 692 (100.0) | 155 (100.0) | 904 (100.0) | 597 (100.0) | ||||
| Positive | 6,813 (72.0) | 558 (100.0) | 4,440 (100.0) | 212 (100.0) | 687 (100.0) | 0 | 0 | 0 | 0 | ||||
| Estrogen receptor | < 0.001 | 0.831 | - | - | |||||||||
| Negative | 3,115 (32.9) | 59 (10.6) | 271 (6.1) | 33 (15.6) | 102 (14.8) | 692 (100.0) | 155 (100.0) | 904 (100.0) | 597 (100.0) | ||||
| Positive | 6,256 (66.1) | 495 (88.7) | 4,157 (93.6) | 179 (84.4) | 584 (85.0) | 0 | 0 | 0 | 0 | ||||
| Progesterone receptor | < 0.001 | < 0.001 | - | - | |||||||||
| Negative | 4,284 (45.2) | 284 (50.9) | 823 (18.5) | 103 (48.6) | 198 (28.8) | 692 (100.0) | 155 (100.0) | 904 (100.0) | 597 (100.0) | ||||
| Positive | 5,160 (54.5) | 274 (49.1) | 3,611 (81.3) | 107 (50.5) | 487 (70.9) | 0 | 0 | 0 | 0 | ||||
| HER2 | - | - | - | - | |||||||||
| Negative | 6,505 (68.7) | 558 (100.0) | 4,440 (100.0) | 0 | 0 | 0 | 0 | 904 (100.0) | 597 (100.0) | ||||
| Positive | 1,750 (18.5) | 0 | 0 | 212 (100.0) | 687 (100.0) | 692 (100.0) | 155 (100.0) | 0 | 0 | ||||
| Histologic grade | < 0.001 | 0.419 | 0.011 | 0.221 | |||||||||
| 1, 2 | 4,717 (49.9) | 313 (56.3) | 2,908 (65.6) | 97 (45.8) | 290 (42.3) | 135 (19.6) | 47 (30.3) | 175 (19.4) | 102 (17.1) | ||||
| 3 | 3,919 (41.4) | 209 (37.6) | 1,127 (25.4) | 101 (47.6) | 333 (48.5) | 497 (72.0) | 99 (63.9) | 674 (74.6) | 447 (74.9) | ||||
| Lymphovascular invasion | < 0.001 | 0.090 | 0.082 | 0.591 | |||||||||
| Negative | 1,240 (13.1) | 68 (12.3) | 660 (14.9) | 24 (11.4) | 49 (7.1) | 77 (11.2) | 10 (6.5) | 105 (11.6) | 63 (10.6) | ||||
| Positive | 2,667 (28.2) | 221 (39.8) | 1,238 (27.9) | 66 (31.3) | 201 (29.3) | 210 (30.4) | 41 (26.5) | 254 (28.1) | 159 (26.6) | ||||
| Ki-67 (%) | < 0.001 | 0.713 | 0.173 | 0.002 | |||||||||
| ≤ 14 | 7,827 (82.7) | 467 (83.7) | 4,067 (91.6) | 173 (81.6) | 564 (82.1) | 514 (74.3) | 106 (68.4) | 537 (59.4) | 305 (51.1) | ||||
| > 14 | 1,595 (16.8) | 85 (15.2) | 353 (8.0) | 39 (18.4) | 121 (17.6) | 174 (25.1) | 49 (31.6) | 361 (39.9) | 291 (48.7) | ||||
| Operation | < 0.001 | 0.764 | 0.878 | 0.001 | |||||||||
| BCS | 4,821 (50.9) | 259 (46.4) | 2,607 (58.8) | 75 (35.5) | 255 (37.1) | 218 (31.5) | 52 (33.5) | 442 (48.9) | 350 (58.6) | ||||
| TM | 4,559 (48.2) | 296 (53.0) | 1,780 (40.1) | 135 (64.0) | 426 (62.0) | 470 (67.9) | 102 (65.8) | 457 (50.6) | 244 (40.9) | ||||
BCL2=B-cell CLL/lymphoma 2; NCCN=National Comprehensive Cancer Network; HR=hormonal receptor; HER2=human epidermal growth factor receptor 2; BCS=breast-conserving surgery; TM=total mastectomy.
*Chi-square test.
Table 2. BCL2 expression for molecular subtypes according to NCCN classification and St. Gallen classifications.
| Characteristic | All No. (%) | BCL2 expression | p-value† | |||
|---|---|---|---|---|---|---|
| No No. (%) |
Yes No. (%) |
p-value* | OS | BCSS | ||
| Total | 9,468 (100.0) | 2,671 (28.2) | 6,797 (71.8) | < 0.001 | < 0.001 | |
| NCCN | < 0.001 | |||||
| HR(+)/HER2(−) | 4,998 (52.8) | 558 (11.2) | 4,440 (88.8) | < 0.001 | < 0.001 | |
| HR(+)/HER2(+) | 899 (9.5) | 212 (23.6) | 687 (76.4) | 0.046 | 0.002 | |
| HR(−)/HER2(+) | 847 (8.9) | 692 (81.7) | 155 (18.3) | 0.256 | 0.436 | |
| HR(−)/HER2(−) | 1,501 (15.9) | 904 (60.2) | 597 (39.8) | 0.104 | < 0.001 | |
| Unknown | 1,223 (12.9) | 305 (24.9) | 918 (75.1) | < 0.001 | < 0.001 | |
| St. Gallen | < 0.001 | |||||
| Luminal A | 2,717 (28.7) | 232 (8.5) | 2,485 (91.5) | < 0.001 | < 0.001 | |
| Luminal B/HER2(-) | 1,762 (18.6) | 273 (15.5) | 1,489 (84.5) | < 0.001 | < 0.001 | |
| Luminal B/HER2(+) | 899 (9.5) | 212 (23.6) | 687 (76.4) | 0.046 | 0.002 | |
| HER2 | 847 (8.9) | 692 (81.7) | 155 (18.3) | 0.256 | 0.436 | |
| TN | 1,501 (15.9) | 904 (60.2) | 597 (39.8) | 0.104 | < 0.001 | |
| Unknown | 1,742 (18.4) | 358 (20.6) | 1,384 (79.4) | < 0.001 | < 0.001 | |
BCL2=B-cell CLL/lymphoma 2; NCCN=National Comprehensive Cancer Network; OS=overall survival; BCSS=breast cancer-specific survival; HR=hormonal receptor; HER2=human epidermal growth factor receptor 2; TN=triple negative.
*Chi-square test; †Log-rank test.
Prognostic influence of BCL2 on molecular subtypes of breast cancer
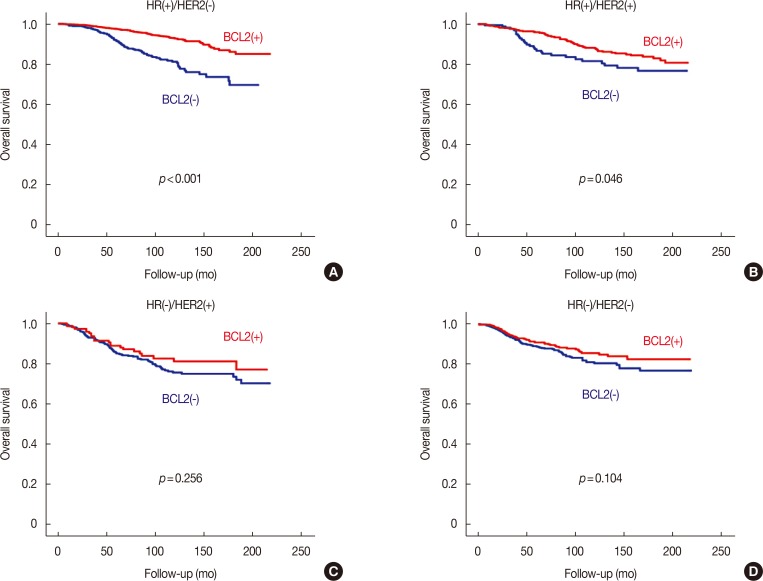
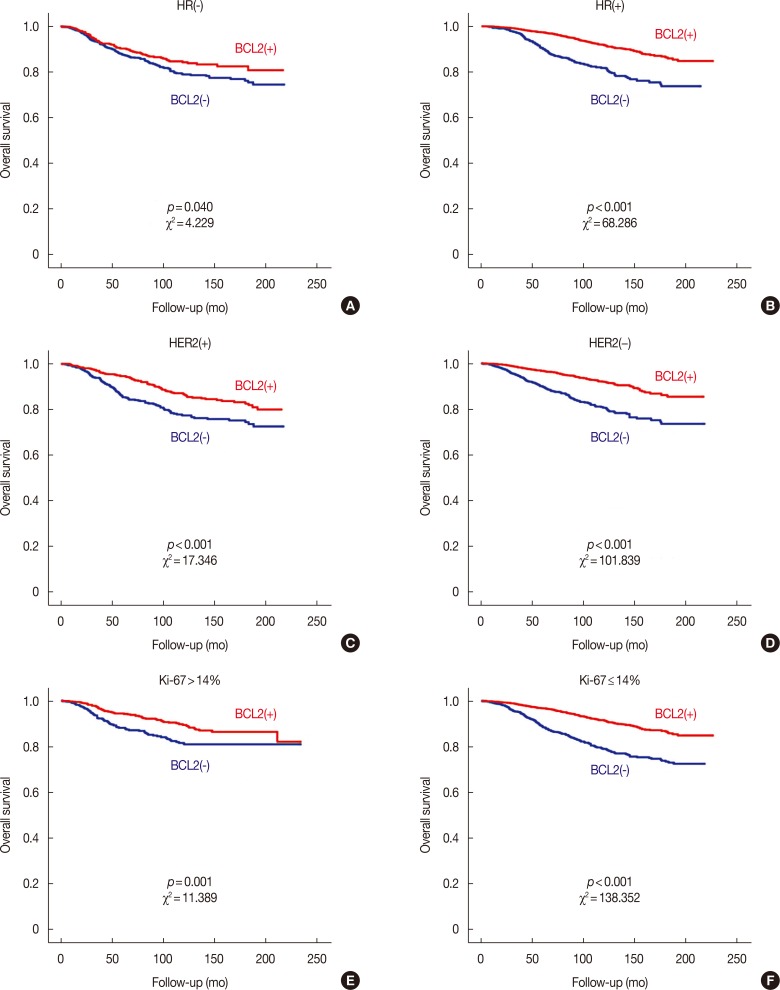
BCL2 was a significant prognostic factor in all patients with breast cancer in terms of both OS and BCSS (log-rank test, both p<0.001) (Supplementary Figure 1, available online). In subgroup analyses, BCL2 was also a significant prognosticator in all subgroups with the exception of the subgroup with negative lymphovascular invasion in terms of both OS and BCSS (Supplementary Table 2, available online). The Cox proportional hazards model showed that BCL2 was a significant prognostic factor not only in univariate analysis but also in multivariate analysis in terms of both OS (hazard ratio, 0.589; 95% CI, 0.494–0.702; p<0.001) and BCSS (hazard ratio, 0.253; 95% CI, 0.179–0.358; p<0.001) (Supplementary Table 3, available online). Regarding NCCN classification, BCL2 was a powerful prognostic factor in the HR(+)/HER2(−) subtype (log-rank test, p<0.001) but its prognostic influence was marginally significant in the HR(+)/HER2(+) subtype (log-rank test, p=0.046) in terms of OS. BCL2 was not a significant prognosticator in HR(−)/HER2(+) and HR(−)/HER2(−) subtypes (Figure 1). Regarding the St. Gallen classification, BCL2 was a significant prognostic factor in luminal A and luminal B/HER2(−) subtypes and the unknown group (log-rank test, all p<0.001). BCL2 was marginally significant in luminal B/HER2(+) subtype, and not a significant prognosticator in HER2 or TN subtypes (Supplementary Figure 2, available online). BCL2 was also not a significant prognosticator in epidermal growth factor receptor positive (EGFR[+]) TNBC, and EGFR(−) TNBC (Supplementary Figure 3, available online). As the NCCN and St. Gallen classification largely determine categories according to HR, HER2, and Ki-67, we analyzed the prognostic effect of BCL2 according to these factors. BCL2 had a more powerful effect in the HR(+), HER2(−), and Ki-67 low groups (Figure 2).
Figure 1. Overall survival curves according to the expression of B-cell CLL/lymphoma 2 (BCL2) in each subtype regarding National Comprehensive Cancer Network classification. Survival curves in HR(+)/HER2(−) (A), HR(+)/HER2(+) (B), HR(−)/HER2(+) (C), and HR(−)/HER2(−) (D) subtypes.
HR=hormonal receptor; HER2=human epidermal growth factor receptor 2.
Figure 2. Overall survival curves according to the expression of B-cell CLL/lymphoma 2 (BCL2) in the subgroups regarding the expression of HR, HER2, and Ki-67. Survival curves in HR(−) (A), HR(+) (B), HER2(+) (C), HER2(−) (D), Ki-67 >14% (E), and Ki-67 ≤14% (F) subgroups.
HR=hormonal receptor; HER2=human epidermal growth factor receptor 2.
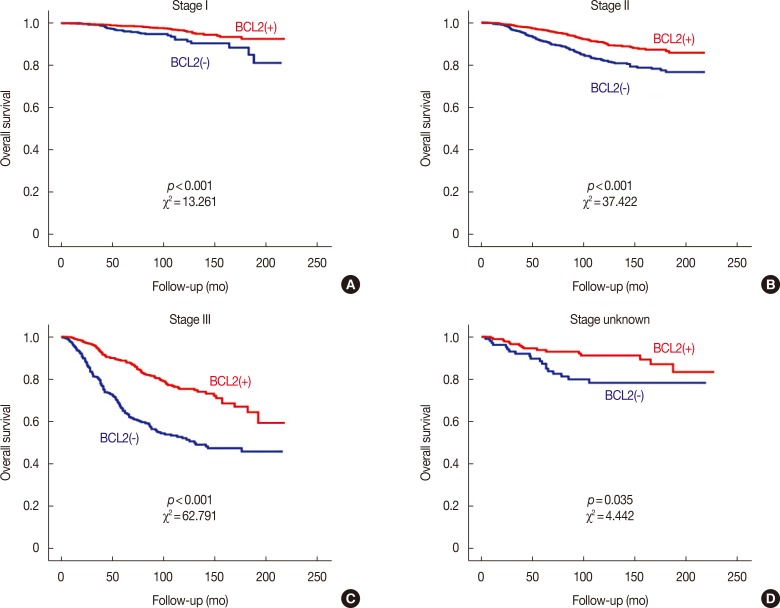
Prognostic influence of BCL2 according to stage of breast cancer
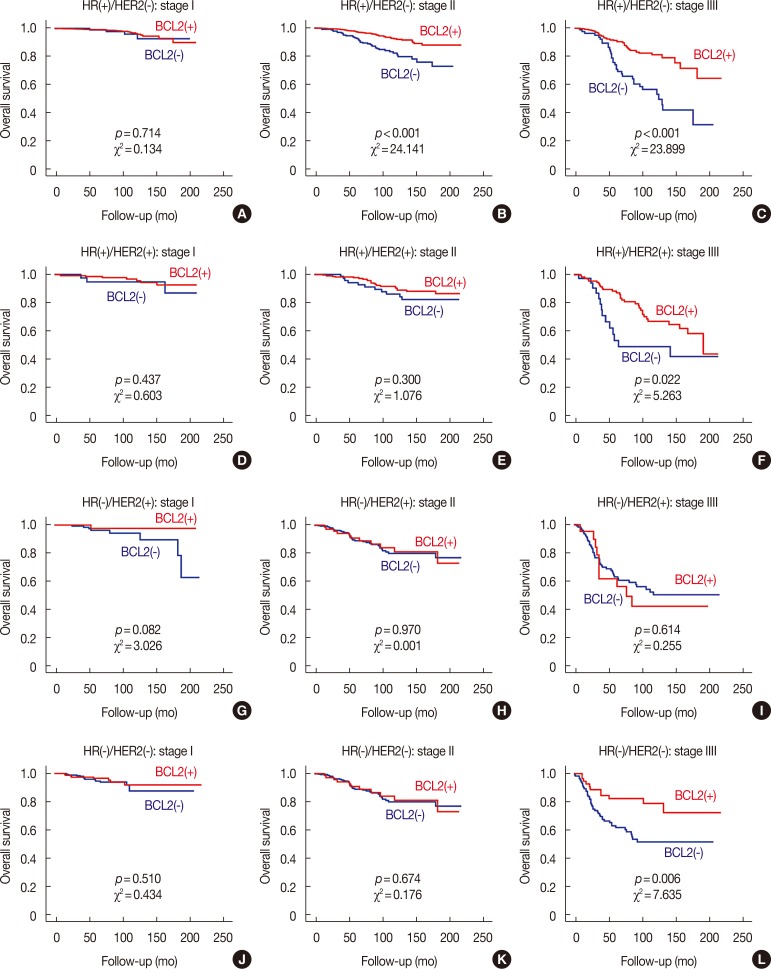
We found that the prognostic influence of BCL2 was associated with the stage of breast cancer; the prognostic effect of BCL2 increased as the stage of breast cancer advanced (Figure 3). We further analyzed the relationship between molecular subtypes and stages according to NCCN classification. In the HR(+)/HER2(−) subtype, although BCL2 was not a significant prognosticator in stage I, it was a significant prognosticator in stage II, and it was more significant in stage III. In HR(+)/HER2(+) and HR(−)/HER2(−) subtypes, BCL2 was only a significant prognosticator in stage III. In HR(−)/HER2(+) subtype, BCL2 was not a significant prognosticator in any stages (Figure 4).
Figure 3. Overall survival curves according to the expression of B-cell CLL/lymphoma 2 (BCL2) in each stage. Survival curves in stage I (A), stage II (B), stage III (C), and stage unknown (D) subgroups.
Figure 4. Overall survival curves according to the expression of B-cell CLL/lymphoma 2 (BCL2) in each subtype classified by each stage. Survival curves for HR(+)/HER2(−) in stage I (A), stage II (B), stage III (C), for HR(+)/HER2(+) in stage I (D), stage II (E), stage III (F), for HR(−)/HER2(+) in stage I (G), stage II (H), stage III (I), and for HR(−)/HER2(−) in stage I (J), stage II (K), stage III (L) subgroups.
HR=hormonal receptor; HER2=human epidermal growth factor receptor 2.
DISCUSSION
We found that BCL2 was a strong prognostic factor in the HR(+)/HER2(−) subtype and luminal A and luminal B/HER2(−) subtypes. BCL2 had a marginal prognostic effect on the HR(+)/HER2(+) subtype and luminal B/HER2(+) subtypes. BCL2 was not a prognosticator in HR(−)/HER2(+) or HER2 subtype, or HR(−)/HER2(−) or TN subtype. We also found that the effect of BCL2 on molecular subtypes was more prominent in advanced stages and that BCL2 became a significant prognosticator in stage III for HR(+)/HER2(+) and HR(−)/HER2(−) subtypes as well as HR(+)/HER2(−) subtype, although it was still not a prognostic factor in HR(−)/HER2(+) subtype. BCL2 was not a prognostic factor in TNBC regardless of the expression of EGFR.
Although there have been rare previous reports on the prognostic role of BCL2 in molecular subtypes of breast cancer published to date, the results have been inconsistent and controversial. Dawson et al. [16] reported that BCL2 is an independent indicator of favorable prognosis for all types of early-stage breast cancer including TNBC. Although they reported that BCL2 was a favorable prognostic factor regardless of ER, PR, and HER2, BCL2 was not analyzed according to the combination of HR(+) and HER2(−). Seong et al. [24] reported that BCL2 expression was an independent, favorable prognostic factor only in breast cancer patients with the HR(+)/HER2(−) subtype and that BCL2 was not a significant prognostic factor in the other subtypes, including HR(+)/HER2(+), HR(−)/HER2(+), and HR(−)/HER2(−) subtypes.
In the present study, the prognostic influence of BCL2 in unselected breast cancer patients was largely consistent with that reported in previous studies [12,13,14,15,16,25,26], including our previous report [17]. BCL2 was a powerful favorable prognostic factor in terms of both OS and BCSS, and the BCL2-positive group showed more favorable clinicopathologic features than the BCL2-negative group in all subgroups, with the exception of nodal positivity alone. In subgroup analyses, BCL2 was a favorable prognosticator in all subgroups, with the exception of the negative lymphovascular invasion subgroup, and BCL2 was a significant prognostic factor in both univariate and multivariate analyses. As the HR(+)/HER2(−) subtype comprises 60.6% of all patients with breast cancer, excluding an unknown subtype, the strong prognostic effects of BCL2 in unselected breast cancer patients is supposed to be largely re-flected by that of the HR(+)/HER2(−) subtype even though BCL2 was found to have no prognostic effect in the HR(−)/HER2(+) subtype (10.3%) and the HR(−)/HER2(−) subtype (18.2%) in the present study. The favorable clinicopathologic features that were observed in unselected breast cancers were also found in the HR(+)/HER2(−) subtype only. As previously reported, favorable clinicopathologic features and strong correlation with HR were suggested as the causes of superior survival in patients with BCL2-positive breast cancer [17] and these factors could partly explain the causality between the expression of BCL2 and the prognoses in molecular subtypes in breast cancer.
The prognostic influence of BCL2 was more prominent with more advanced stage from stage I to stage III (Figure 3). In stage I, BCL2 had no significant prognostic impact on any subtypes, even HR(+)/HER2(−) subtype. In stage II, BCL2 was a significant prognosticator only in HR(+)/HER2(−) subtype. In stage III, BCL2 became a significant prognosticator in all subtypes except for HR(−)/HER2(+) subtype. In HR(+)/HER2(−) subtype, prognostic effects of BCL2 were more prominent in stage III than stage II (Figure 4). As mentioned above, the prognostic effect of BCL2 is presumed to be dependent on the expression of HR and HER2; BCL2 did not become a significant prognosticator even in stage III for HR(−)/HER2(+) subtype, although it did become a significant prognostic factor in stage III for HR(+)/HER2(+) and HR(−)/HER2(−) subtypes. In the earlier stages, as the prognostic impact of the stage itself might be stronger, the prognostic influence of BCL2 might be less prominent. With the same hypothesis, the prognostic role of BCL2 could be more prominent in the advanced stage for each molecular subtype.
In the present study, BCL2 was not a significant prognosticator in unselected TNBC in terms of OS (Figure 1, Supplementary Figure 1). However, it was a significant favorable prognostic factor in stage III TNBC in terms of OS (Figure 4) and in unselected TNBC in terms of BCSS (data not shown). BCL2 was not a significant prognostic factor in EGFR(+) or EGFR(−) TNBC. Although the prognostic role of BCL2 in TNBC has been recently reported, the results have been highly inconsistent to date. Dawson et al. [16] reported that BCL2 is an independent favorable prognostic indicator for all types of early-stage breast cancer including TNBC. Abdel-Fatah et al. [27] reported that negative BCL2 expression was associated with increased risk of death and recurrence in TNBC. Bouchalova et al. [28] reported that although BCL2 was a significant prognostic factor in some studies, it was not a significant prognosticator overall in TNBC according to a meta-analysis [28]. Choi et al. [29] reported that although BCL2 expression was not associated with any clinicopathologic parameters and did not affect patient survival in TNBC, BCL2 expression showed a significant association with worse OS and disease free survival in the nonbasal (claudin-low; CK5/6– and EGFR–) subgroup of TNBC [29]. Bouchalova et al. [30] reported that high BCL2 expression is a significant independent predictor of poor outcomes in TNBC patients treated with anthracycline-based adjuvant chemotherapy, and high EGFR protein expression is associated with poor BCSS in patients with TNBC treated with anthracycline-based chemotherapy, especially in basal-like (core; CK5/6+ and/or EGFR+) TNBC [30]. Further investigations will be required to elucidate the prognostic association between BCL2 and TNBC.
The present study showed the prognostic role of BCL2 in each molecular subtype and each stage of breast cancer in a relatively large group of subjects. Clinically, BCL2 could be considered one of the important prognosticators in HR(+)/HER2(−) subtype. In the advanced stage, the application of BCL2 as a prognosticator could be expanded to not only HR(+)/HER2(−), but also HR(+)/HER2(+) and HR(−)/HER2(−) subtypes. Nevertheless, this study has several limitations. First, as we could not collect sufficient information on adjuvant therapies including chemotherapy, endocrine therapy, trastuzumab therapy, and radiation therapy, we were unable to analyze their effects in the current study. Second, we could not analyze the impact of BCL2 on the subgroups of TNBC such as core type and claudin-low type because of limited data regarding CK5/6. Third, we were unable to analyze the influence of BCL2 according to the percentage or intensity of immunohistochemical staining for BCL2 because we could only collect final interpreted results regarding BCL2 immunohistochemistry. It is our hope that additional investigations could further clarify our results, and we expect that it will be possible to verify the mechanism of action by which BCL2 influences the prognosis of breast cancer in the near future.
In conclusion, the prognostic influence of BCL2 was different across molecular subtypes of breast cancer, and it was dependent on the expression of HR, HER2, and Ki-67, as well as stage. BCL2 had a strong prognostic impact in HR(+)/HER2(−) and luminal A and luminal B/HER2(−) subtypes, particularly in advanced stages, but it had no effect in HR(–)/HER2(+) or HER2 subtypes, even in advanced stages. BCL2 had an intermediate effect in HR(+)/HER2(+) and luminal B and HR(−)/HER2(−) or TN subtypes, and BCL2 was a significant prognostic factor for these subtypes only in stage III tumors. BCL2 had no prognostic impact in TNBC regardless of EGFR expression. Although favorable clinicopathologic features of the HR(+)/HER2(−) subtype could explain the majority of causality for the prognostic role of BCL2, further investigations are needed to verify the prognostic influence of BCL2 on molecular subtypes of breast cancer and to develop clinical applications of BCL2 for prognostication in each molecular subtype of breast cancer.
ACKNOWLEDGMENTS
We appreciate Michael Kim for the editing of this manuscript.
Footnotes
This research was supported by Korean Breast Cancer Foundation.
CONFLICT OF INTEREST: The authors declare that they have no competing interests.
SUPPLEMENTARY MATERIALS
Clinicopathologic characteristics according to BCL2 expression in all breast cancer patients
Subgroup survival analyses according to BCL2 expression in all breast cancer patients
Univariate analysis and multivariate analysis in terms of overall survival and breast cancer-specific survival
Survival curves according to the expression of B-cell CLL/lymphoma 2 (BCL2) in all breast cancer patients. Survival curves in terms of overall survival (A) and breast cancer-specific survival (B).
Overall survival curves according to the expression of B-cell CLL/lymphoma 2 (BCL2) in each subtype regarding St. Gallen classification. Survival curves in luminal A (A), luminal B/HER2(−) (B), luminal B/HER2(+) (C), HER2 (D), triple negative (TN) (E), and unknown (F) subtypes. HER2=human epidermal growth factor receptor 2.
Overall survival curves according to the expression of B-cell CLL/lymphoma 2 (BCL2) by the expression of epidermal growth factor receptor (EGFR) in triple negative subtype. Survival curves in in EGFR(+) (A) and EGFR(−) (B) subgroups.
References
- 1.BCL2, apoptosis regulator. National Center for Biotechnology Information. [Accessed February 8th, 2017]. http://www.ncbi.nlm.nih.gov/gene/596.
- 2.Siddiqui WA, Ahad A, Ahsan H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: an update. Arch Toxicol. 2015;89:289–317. doi: 10.1007/s00204-014-1448-7. [DOI] [PubMed] [Google Scholar]
- 3.Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984;226:1097–1099. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- 4.Tzifi F, Economopoulou C, Gourgiotis D, Ardavanis A, Papageorgiou S, Scorilas A. The role of BCL2 family of apoptosis regulator proteins in acute and chronic leukemias. Adv Hematol. 2012;2012:524308. doi: 10.1155/2012/524308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin B, Paesmans M, Berghmans T, Branle F, Ghisdal L, Mascaux C, et al. Role of Bcl-2 as a prognostic factor for survival in lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer. 2003;89:55–64. doi: 10.1038/sj.bjc.6601095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao L, Yu N, Guo T, Hou Y, Zeng Z, Yang X, et al. Tissue biomarkers for prognosis of prostate cancer: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23:1047–1054. doi: 10.1158/1055-9965.EPI-13-0696. [DOI] [PubMed] [Google Scholar]
- 7.Cheng H, Wang X, Li T, Chen L. Bcl-2 expression and patient survival in gastric cancer: a systematic review of the literature with meta-analysis. Med Oncol. 2015;32:389. doi: 10.1007/s12032-014-0389-6. [DOI] [PubMed] [Google Scholar]
- 8.Bold RJ, Hess KR, Pearson AS, Grau AM, Sinicrope FA, Jennings M, et al. Prognostic factors in resectable pancreatic cancer: p53 and bcl-2. J Gastrointest Surg. 1999;3:263–277. doi: 10.1016/s1091-255x(99)80068-7. [DOI] [PubMed] [Google Scholar]
- 9.Leek RD, Kaklamanis L, Pezzella F, Gatter KC, Harris AL. bcl-2 in normal human breast and carcinoma, association with oestrogen receptor-positive, epidermal growth factor receptor-negative tumours and in situ cancer. Br J Cancer. 1994;69:135–139. doi: 10.1038/bjc.1994.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silvestrini R, Veneroni S, Daidone MG, Benini E, Boracchi P, Mezzetti M, et al. The Bcl-2 protein: a prognostic indicator strongly related to p53 protein in lymph node-negative breast cancer patients. J Natl Cancer Inst. 1994;86:499–504. doi: 10.1093/jnci/86.7.499. [DOI] [PubMed] [Google Scholar]
- 11.Joensuu H, Pylkkänen L, Toikkanen S. Bcl-2 protein expression and long-term survival in breast cancer. Am J Pathol. 1994;145:1191–1198. [PMC free article] [PubMed] [Google Scholar]
- 12.Callagy GM, Pharoah PD, Pinder SE, Hsu FD, Nielsen TO, Ragaz J, et al. Bcl-2 is a prognostic marker in breast cancer independently of the Nottingham Prognostic Index. Clin Cancer Res. 2006;12:2468–2475. doi: 10.1158/1078-0432.CCR-05-2719. [DOI] [PubMed] [Google Scholar]
- 13.Berardo MD, Elledge RM, de Moor C, Clark GM, Osborne CK, Allred DC. bcl-2 and apoptosis in lymph node positive breast carcinoma. Cancer. 1998;82:1296–1302. [PubMed] [Google Scholar]
- 14.Ali HR, Dawson SJ, Blows FM, Provenzano E, Leung S, Nielsen T, et al. A Ki67/BCL2 index based on immunohistochemistry is highly prognostic in ER-positive breast cancer. J Pathol. 2012;226:97–107. doi: 10.1002/path.2976. [DOI] [PubMed] [Google Scholar]
- 15.Callagy GM, Webber MJ, Pharoah PD, Caldas C. Meta-analysis confirms BCL2 is an independent prognostic marker in breast cancer. BMC Cancer. 2008;8:153. doi: 10.1186/1471-2407-8-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dawson SJ, Makretsov N, Blows FM, Driver KE, Provenzano E, Le Quesne J, et al. BCL2 in breast cancer: a favourable prognostic marker across molecular subtypes and independent of adjuvant therapy received. Br J Cancer. 2010;103:668–675. doi: 10.1038/sj.bjc.6605736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang KT, Woo JW, Shin HC, Kim HS, Ahn SK, Moon HG, et al. Prognostic influence of BCL2 expression in breast cancer. Int J Cancer. 2012;131:E1109–E1119. doi: 10.1002/ijc.27539. [DOI] [PubMed] [Google Scholar]
- 18.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network. Clinical practice guildlines-v.1. 2016. [Accessed February 8th, 2017]. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 21.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, et al. Strategies for subtypes-dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, et al. Tailoring therapies-improving the management of early breast cancer: St Gallen International Expert Consensus on the primary therapy of early breast cancer 2015. Ann Oncol. 2015;26:1533–1546. doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seong MK, Lee JY, Byeon J, Sohn YJ, Seol H, Lee JK, et al. Bcl-2 is a highly significant prognostic marker of hormone-receptor-positive, human epidermal growth factor receptor-2-negative breast cancer. Breast Cancer Res Treat. 2015;150:141–148. doi: 10.1007/s10549-015-3305-7. [DOI] [PubMed] [Google Scholar]
- 25.Eom YH, Kim HS, Lee A, Song BJ, Chae BJ. BCL2 as a subtype-specific prognostic marker for breast cancer. J Breast Cancer. 2016;19:252–260. doi: 10.4048/jbc.2016.19.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen LY, Tsang JY, Ni YB, Chan SK, Chan KF, Zhang S, et al. Bcl2 and Ki67 refine prognostication in luminal breast cancers. Breast Cancer Res Treat. 2015;149:631–643. doi: 10.1007/s10549-015-3288-4. [DOI] [PubMed] [Google Scholar]
- 27.Abdel-Fatah TM, Perry C, Dickinson P, Ball G, Moseley P, Madhusudan S, et al. Bcl2 is an independent prognostic marker of triple negative breast cancer (TNBC) and predicts response to anthracycline combination (ATC) chemotherapy (CT) in adjuvant and neoadjuvant settings. Ann Oncol. 2013;24:2801–2807. doi: 10.1093/annonc/mdt277. [DOI] [PubMed] [Google Scholar]
- 28.Bouchalova K, Kharaishvili G, Bouchal J, Vrbkova J, Megova M, Hlobilkova A. Triple negative breast cancer-BCL2 in prognosis and prediction: review. Curr Drug Targets. 2014;15:1166–1175. doi: 10.2174/1389450115666141106151143. [DOI] [PubMed] [Google Scholar]
- 29.Choi JE, Kang SH, Lee SJ, Bae YK. Prognostic significance of Bcl-2 expression in non-basal triple-negative breast cancer patients treated with anthracycline-based chemotherapy. Tumour Biol. 2014;35:12255–12263. doi: 10.1007/s13277-014-2534-4. [DOI] [PubMed] [Google Scholar]
- 30.Bouchalova K, Svoboda M, Kharaishvili G, Vrbkova J, Bouchal J, Trojanec R, et al. BCL2 is an independent predictor of outcome in basal-like triple-negative breast cancers treated with adjuvant anthracycline-based chemotherapy. Tumour Biol. 2015;36:4243–4252. doi: 10.1007/s13277-015-3061-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinicopathologic characteristics according to BCL2 expression in all breast cancer patients
Subgroup survival analyses according to BCL2 expression in all breast cancer patients
Univariate analysis and multivariate analysis in terms of overall survival and breast cancer-specific survival
Survival curves according to the expression of B-cell CLL/lymphoma 2 (BCL2) in all breast cancer patients. Survival curves in terms of overall survival (A) and breast cancer-specific survival (B).
Overall survival curves according to the expression of B-cell CLL/lymphoma 2 (BCL2) in each subtype regarding St. Gallen classification. Survival curves in luminal A (A), luminal B/HER2(−) (B), luminal B/HER2(+) (C), HER2 (D), triple negative (TN) (E), and unknown (F) subtypes. HER2=human epidermal growth factor receptor 2.
Overall survival curves according to the expression of B-cell CLL/lymphoma 2 (BCL2) by the expression of epidermal growth factor receptor (EGFR) in triple negative subtype. Survival curves in in EGFR(+) (A) and EGFR(−) (B) subgroups.